Deep neck infection
Deep neck infection also called deep neck space infection, refers to an infection or abscess (collection of pus) located deep in the neck (deep cervical space) under the skin near blood vessels, nerves, and muscles. Deep neck abscesses occur in the potential spaces between the layers of deep cervical fascia 1. Deep neck infections include parapharyngeal abscess, peritonsillar abscess, and retropharyngeal abscess, commonly arise from an odontogenic or upper aerodigestive tract origin. Deep neck infections can be life threatening if not noticed adequately. Many vital organs are at risk of being involved and the resultant respiratory, vascular, neurologic and systemic complications endanger the life of the patient and if not noticed in a well-timed manner, lead to death rapidly. Early diagnosis and correct treatment planning can save the patient’s lives and prevent complications of disease extension and also surgical procedures that in some instances may be performed in an emergent situation with higher complication rates.
Most deep neck infections arise from foci in the mucosal surfaces of the upper aerodigestive tract or from a carious tooth 2. Less commonly, deep neck infections are the result of perforation by foreign body or thrombophlebitis 2. Usually the results of cultures are polymicrobial but as a whole, Streptococcus are the organisms most commonly cultured from deep neck abscesses 3. In an immunocompromised patient, however other uncommon organisms may be encountered 4. Despite the availability of antibiotics, deep neck infections with anaerobic germs (for example in Ludwig’s angina) still carry the potential for significant morbidity and mortality with delayed treatment 5.
Deep neck infections classically present with high fever, systemic toxicity, and local pressure effects on the respiratory, nervous, or gastrointestinal (GI) tract 6.
Although introduction of antibiotics and improvements in oral hygiene have made deep neck infections occur less frequently today than in the past, but it is important to notice the patient because deep neck infections can cause severe morbidity and also mortality 5.
Deep neck infections account for approximately 3400 hospitalizations annually in the United States 7. A study using the Kids’ Inpatient Database to determine the incidence of deep neck infections found that the incidence of retropharyngeal abscess increased significantly from 0.1/10,000 in 2000 to 0.22/10,000 in 2009 7. The incidence of peritonsillar abscess in 2009 was 0.94 cases/10,000, and that of parapharyngeal abscess was 0.14 cases/10,000 7.
The treatment and diagnosis of deep neck space infections have always been challenging due to their deep location, complex anatomy of this region, and unfamiliarity of physicians given its declining incidence in the post-antibiotic era 7.
Early diagnosis and correct treatment planning can save the patient’s life and prevent complications of disease extension. Discovery of the underlying causative factors requires imaging. The addition of CT to the initial work up provides early detection of true underlying disease.
Directed antimicrobial coverage, surgical drainage for discrete abscesses and aggressive supportive care are the main management options. Empiric regimens which are based on the expected microbiology and local resistance data should be initiated and adjusted appropriately once the organism and its sensitivities become available. The choice of antimicrobial regimens for the treatment of deep neck space infections has not yet been studied in clinical trials. Intravenous nafcillin or vancomycin plus gentamycin or tobramycin combination, ampicillin/sulbactam, or clindamycin are generally accepted initial choices. For methicillin-resistant Staphylococcus aureus (MRSA) infections, vancomycin or linezolid plus cefepime (alternates are metronidazole, imipenem, meropenem, piperacillin-tazobactam) can be used. MRSA coverage must be included as part of the initial treatment regimen for patients that are at risk for MRSA carriage or infection, such as those with comorbid disease (for example, diabetes mellitus), history of intravenous drug use, and in communities or hospitals where there is a substantial incidence of MRSA. For the majority of deep neck infections especially parapharyngeal, retropharyngeal, or prevertebral space infections, antibiotic treatment should generally be continued for 2 to 3 weeks, and longer courses may be required when complications are present. Antibiotics can be switched to the oral route once there is significant clinical improvement and patient able to tolerate oral intake. Consultation with head and neck surgeons is recommended as surgical drainage may become necessary if there is no improvement after 48 hours of antibiotic therapy.
Nonetheless, in the acute setting, airway always comes first so patients with potential airway compromise from a deep neck space infection should have their airways adequately secured first and foremost. The use of glucocorticoids for symptomatic relief in patients with acute airway obstruction remains controversial and lacks supporting evidence.
Cervical fascia and cervical compartments
Cervical fascia
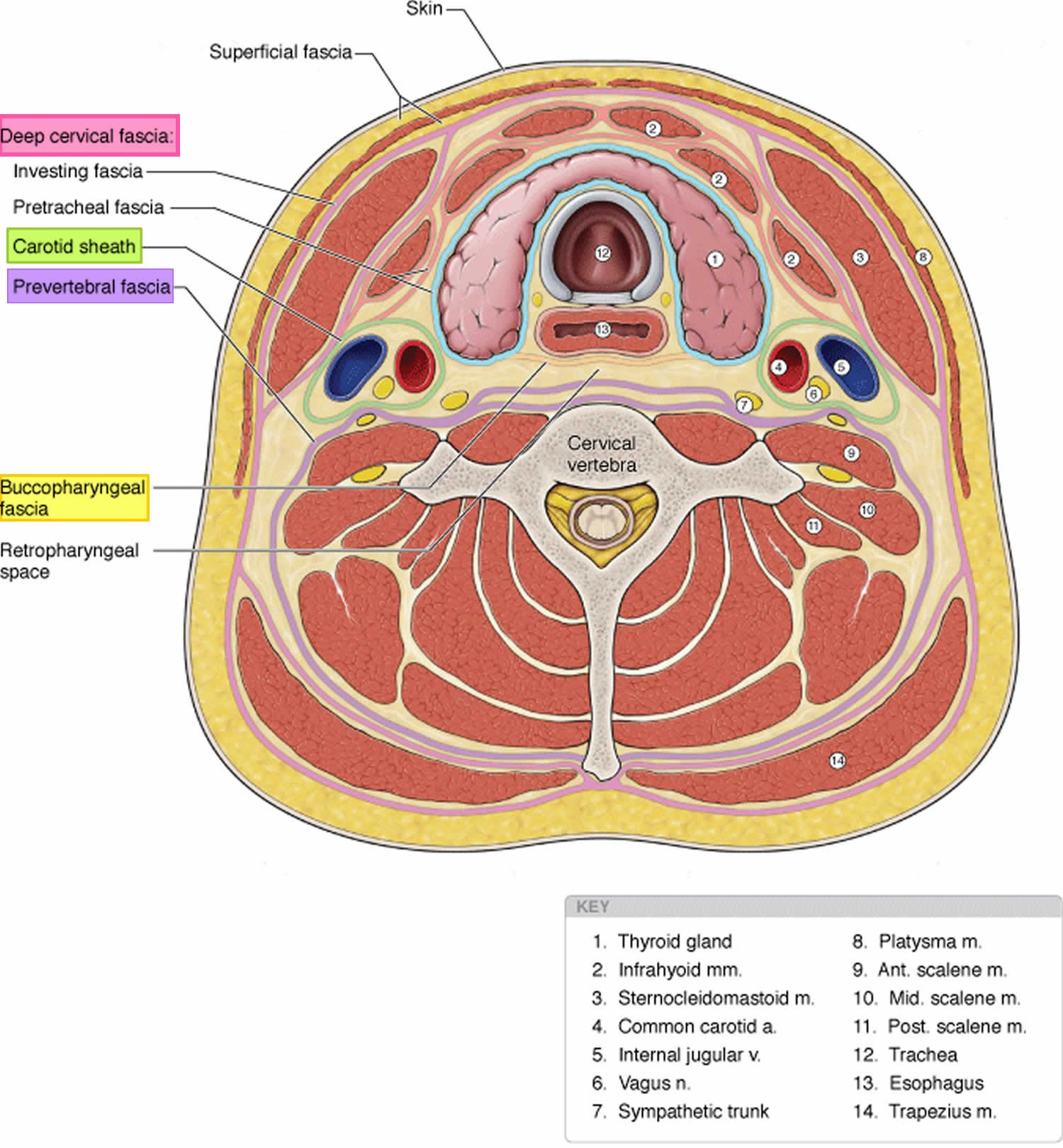
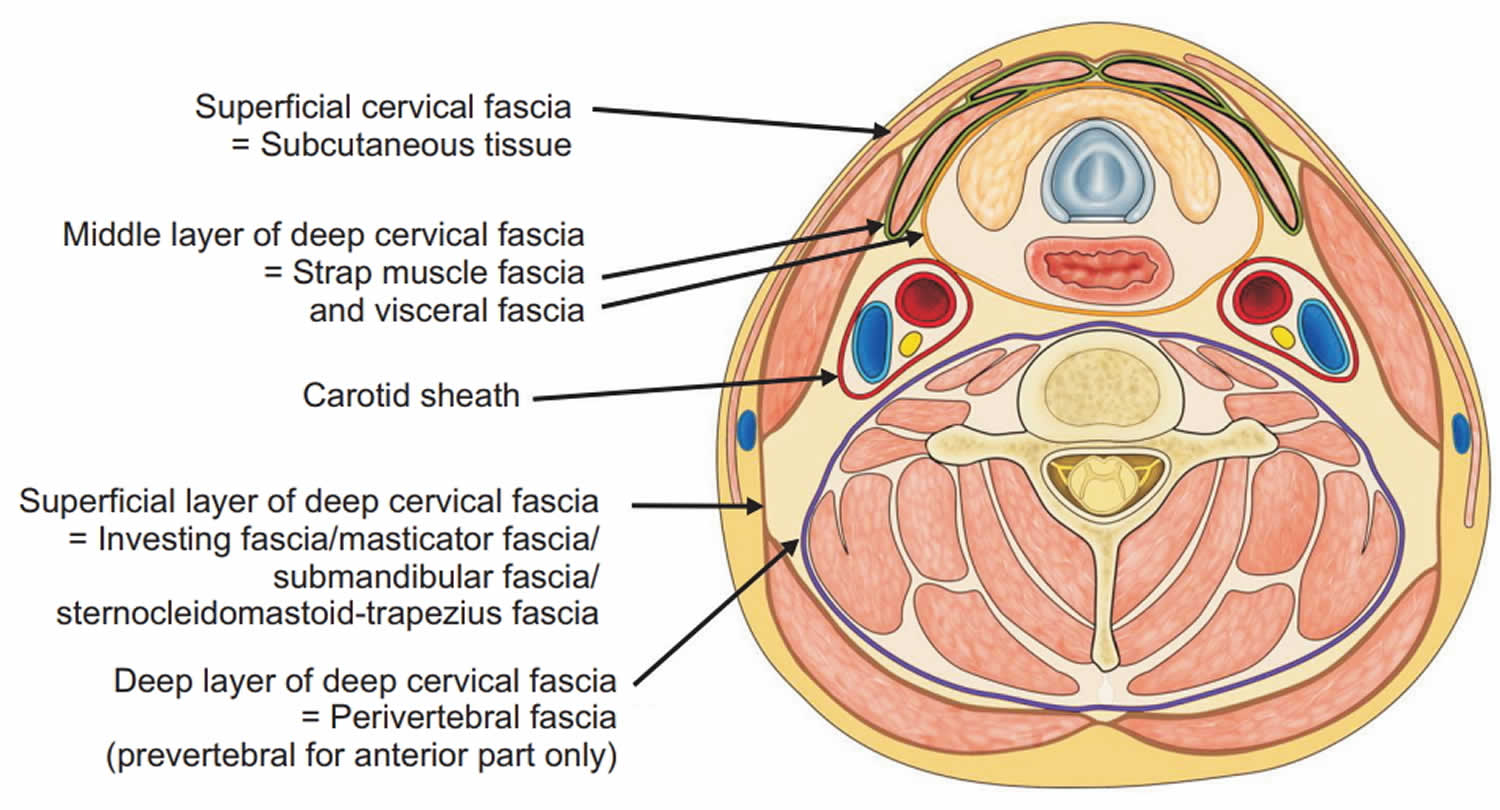
The cervical fascia can be divided into the superficial cervical fascia and deep cervical fascia.
- Superficial cervical fascia: The superficial fascia, which lies just deep to the dermis, surrounds the muscles of facial expression. It includes the superficial musculoaponeurotic system (SMAS) and extends from the epicranium to the axillae and chest. The space deep to this layer contains fat, neurovascular bundles, and lymphatics. It does not constitute part of the deep neck space system.
- Deep cervical fascia: The deep cervical fascia encloses the deep neck spaces and is further divided into 3 layers, the superficial, middle, and deep layers of the deep cervical fascia.
- The superficial layer of the deep cervical fascia is an investing fascia that surrounds the neck. It encompasses the sternocleidomastoid muscle, trapezius, muscles of mastication, and submandibular and parotid glands. It is limited superiorly by the nuchal ridge, mandible, zygoma, mastoid, and hyoid bones. Inferiorly, it is bounded by the clavicles, sternum, scapula, hyoid, and acromion. It contributes to the fascia covering the digastric muscle and to the lateral aspect of the carotid sheath. In its course from the hyoid bone to the medial table of the ramus of the mandible, it envelops the anterior belly of the digastric muscle and forms the floor of the submandibular space. Laterally, this fascia helps to define the parotid and masticator spaces.
- The middle layer of the deep cervical fascia has 2 divisions, muscular and visceral. The muscular division surrounds the strap muscles (ie, sternohyoid, sternothyroid, thyrohyoid, omohyoid) and the adventitia of the great vessels. The visceral division surrounds the constrictor muscles of the pharynx and esophagus to create the buccopharyngeal fascia and the anterior wall of the retropharyngeal space. Both the muscular and visceral divisions contribute to the formation of the carotid sheath. The middle layer also envelops the larynx, trachea, and thyroid gland. It attaches to the base of the skull superiorly and extends inferiorly as low as the pericardium via the carotid sheath.
- The deep layer of the deep cervical fascia is also subdivided into 2 divisions, prevertebral and alar. The prevertebral division adheres to the anterior aspect of the vertebral body and extends laterally to the transverse processes of the vertebrae. The alar division lies between the prevertebral division and the visceral division of the middle layer and defines the posterior border of the retropharyngeal space. It surrounds the deep neck muscles and contributes to the carotid sheath. Posteriorly, the muscular division of the middle layer of the deep cervical fascia fuses with the alar division of the deep layer of the deep cervical fascia at the level of thoracic vertebrae 1-2 (T1-T2).
Deep neck spaces
Within the deep neck are 11 spaces created by planes of greater and lesser resistance between the fascial layers. There are 3 major fascial spaces (submandibular, parapharyngeal, and retropharyngeal spaces) scattered among the planes of the deep cervical fascia, which may be real or potential and may expand when pus separates layers of fascia. The deep neck spaces communicate with each other, forming avenues by which infections may spread. These spaces are described briefly.
Other spaces that have the potential to host deep neck infections are the so-called “danger” space, the prevertebral space, the pre-tracheal space, the peritonsillar space, and the parotid space. There is an abundance of lymphatic tissue and several groups of lymph nodes that are located in the neck. As the deep cervical spaces are interconnected, the spread of infection from the primary source of origin and spread through lymphatic tissues can occur to deeper spaces. Host factors such as immunocompromised state, the presence of comorbid conditions such as diabetes, trauma, or instrumentation in the area can further influence the spread of infection to deeper layers 8.
Parapharyngeal space
- The parapharyngeal space (ie, lateral pharyngeal space, pharyngomaxillary space, pterygomaxillary space, pterygopharyngeal space) occupies an inverted pyramidal area bounded by multiple components of the fascial system. The inferior limitation of this space is the lesser cornua of the hyoid bone; the entire space is situated superiorly with respect to the hyoid. The superior margin of the space is the skull base. Its medial boundary is the visceral division of the middle layer of deep cervical fascia around the pharyngeal constrictor and the fascia of the tensor and levator muscles of the velum palatini and the styloglossus. Laterally, the space is defined by the superficial layer of the deep cervical fascia that overlies the mandible, medial pterygoids, and parotid.
- The posterior border is formed by the prevertebral division of the deep layer and by the posterior aspect of the carotid sheath at the posterolateral corner. The anterior boundary is the interpterygoid fascia and the pterygomandibular raphe. The parapharyngeal space can be subdivided into compartments by a line extending from the medial aspect of the medial pterygoid plate to the styloid process.
- The internal maxillary artery, inferior alveolar nerve, lingual nerve, and auriculotemporal nerve comprise the anterior (ie, prestyloid) compartment. Infections in this compartment often give significant trismus.
- The posterior (ie, poststyloid) compartment contains the carotid sheath (ie, carotid artery, internal jugular vein, vagus nerve) and the glossopharyngeal and hypoglossal nerves, sympathetic chain, and lymphatics. It also contains the accessory nerve, which is somewhat protected from pathologic processes in this region by its position behind the sternocleidomastoid muscle.
- The parapharyngeal space connects posteromedially with the retropharyngeal space and inferiorly with the submandibular space. Laterally, it connects with the masticator space. The carotid sheath courses through this space into the chest. This space provides a central connection for all other deep neck spaces. It is directly involved by lateral extension of peritonsillar abscesses and was the most commonly affected space before the advent of modern antibiotics.
- Infections can arise from the tonsils, pharynx, dentition, salivary glands, nasal infections, or Bezold abscess (ie, mastoid abscess).
- Medial displacement of the lateral pharyngeal wall and tonsil is a hallmark of a parapharyngeal space infection. Trismus, drooling, dysphagia, and odynophagia are also commonly observed.
Retropharyngeal space
- The retropharyngeal space is sometimes considered a third medial compartment within the parapharyngeal space because the 2 communicate laterally. This space lies between the visceral division of the middle layer of the deep cervical fascia around the pharyngeal constrictors and the alar division of the deep layer of deep cervical fascia posteriorly. It extends from the skull base to the tracheal bifurcation around T2 where the visceral and alar divisions fuse. It primarily contains retropharyngeal lymphatics.
- Infection may enter this space directly, as with traumatic perforations of the posterior pharyngeal wall or esophagus, or indirectly, from the parapharyngeal space. More than 60% of retropharyngeal abscesses in children are caused by upper respiratory tract infections, whereas most infections in adults in this region are caused by trauma and foreign bodies. Other common sources of infection in the retropharyngeal space are the nose, adenoids, nasopharynx, and sinuses.
- Infections of this space may drain into the prevertebral space and follow that space into the chest. Mediastinitis and empyema ensue. Abscess in this space may push forward, occluding the airway at the level of the pharynx. It may appear as anterior displacement of one or both sides of the posterior pharyngeal wall because of involvement of lymph nodes, which are distributed lateral to the midline fascial raphe.
- Retropharyngeal lymph nodes tend to regress by about age 5 years, making infection in this space much more common in children than adults.
Prevertebral space
- The prevertebral space is located anterior to the vertebral bodies and posterior to the prevertebral division of the deep layer of the deep cervical fascia. It lies just posterior to the danger space. Laterally, it is bounded by the fusion of the prevertebral fascia with the transverse processes of the vertebral bodies. It extends from the skull base to the coccyx.
- The most common etiology of prevertebral abscesses (other than extension from other sites) is trauma, particularly iatrogenic instrumentation. Involvement of the vertebrae can lead to osteomyelitis and spinal instability.
Danger space
- The danger space is immediately posterior to the retropharyngeal space and immediately anterior to the prevertebral space, between the alar and prevertebral divisions of the deep layer of the deep cervical fascia. It extends from the skull base to the posterior mediastinum and diaphragm. Laterally, it is limited by the fusion of the alar and prevertebral division with the transverse processes of the vertebrae. Some authors consider the danger space a component of the prevertebral space.
- Infections in this region may be extensions of retropharyngeal, parapharyngeal, or prevertebral infections.
- Spread within the danger space tends to occur rapidly because of the loose areolar tissue that occupies this region. This spread can lead to mediastinitis, empyema, and sepsis.
Masticator space
- The masticator space is situated laterally to the medial pterygoid fascia and medially to the masseter muscle. It is bounded by the sphenoid bone, the posterior aspect of the mandible, and the zygomatic arch. It lies inferiorly to the temporal space and is anterolateral to the parapharyngeal space. It contains the masseter, pterygoids, ramus and body of the mandible, temporalis tendon, and the inferior alveolar vessels and nerve.
- Infection in the masticator space may spread to the parapharyngeal, parotid, or temporal space.
- Infections here may be a result of dental infections, particularly of the third mandibular molars, and have reportedly occurred from removal of suspension wires following reduction and fixation of facial fractures.
- Trismus is commonly seen in the initial presentation and may be a long-term sequela.
Submandibular space
- The submandibular space is bounded inferiorly by the superficial layer of the deep cervical fascia extending from the hyoid to the mandible, laterally by the body of the mandible, and superiorly by the mucosa of the floor of mouth.
- It is considered to have 2 subdivisions, the sublingual and submaxillary spaces, which are divided by the mylohyoid muscle. The sublingual space contains the sublingual gland, hypoglossal nerve, and Wharton duct. It is in continuity with the submaxillary space via the posterior margin of the mylohyoid muscle, around which pus can readily tract. The submaxillary division is further subdivided by the anterior belly of the digastric into a central submental compartment and a lateral submaxillary space.
- Infection in the submandibular space may be secondary to oral trauma, submaxillary or sublingual sialadenitis, or dental abscess of mandibular teeth.
- The term Ludwig angina describes inflammation and cellulitis of the submandibular space, usually starting in the submaxillary space and spreading to the sublingual space via the fascial planes, not the lymphatics. As the submandibular space is expanded by cellulitis or abscess, the floor of the mouth becomes indurated, and the tongue is forced upward and backward, causing airway obstruction. Ludwig angina does not require the presence of a focal abscess. It typically includes bilateral involvement and manifests with drooling, trismus, pain, dysphagia, submandibular mass, and dyspnea or airway compromise caused by displacement of the tongue. This is a life-threatening condition that requires tracheostomy for airway control. Before antibiotics, the mortality rate of Ludwig angina was 50%. With modern antimicrobial and surgical therapies, the mortality rate is less than 5%.
- Submandibular space infections may spread to the parapharyngeal space or retropharyngeal space.
Carotid space
- The carotid (ie, visceral vascular) space is a potential space within the carotid sheath containing the carotid artery, internal jugular vein, vagus nerve, and sympathetic postganglionic fibers.
- Carotid space may be affected indirectly from spread of infection from the surrounding parapharyngeal space or directly by injection of drugs in those who abuse IV drugs. The resulting jugular vein thrombophlebitis sends septic emboli to the heart and lungs. The carotid artery may thrombose, form an aneurysm, or erode and rupture. Horner syndrome may occur because of involvement of the cervical sympathetics in this space.
- Treatment may include anticoagulation and possibly the ligation of involved vessels.
Pretracheal space
- The pretracheal (ie, anterior visceral) space is enclosed by the visceral division of the middle layer of the deep cervical fascia and lies immediately anterior to the trachea. It extends from the thyroid cartilage to the superior mediastinum.
- Infections here are most commonly caused by perforation of the anterior esophageal wall by endoscopic instrumentation, foreign bodies, or trauma.
- Infections cause dysphagia and odynophagia, pain, fever, and possible hoarseness and airway obstruction.
Peritonsillar space
- Peritonsillar space is bounded by the tonsil medially and the superior constrictor laterally. The anterior and posterior tonsillar pillars form the remaining borders of this space.
- Peritonsillar abscesses are the most common deep neck space abscess and represent a sequela of tonsillar infections.
- Individuals with peritonsillar abscesses typically exhibit trismus, pain, odynophagia, drooling, a “hot potato” voice, and fever. They demonstrate uvular deviation, palatal asymmetry, and displacement of the tonsil medially. Note that the tonsillar erythema and exudates may be mild despite the presence of an abscess.
- Patients who have had their tonsils removed effectively lose this space, but they can still develop peritonsillar pathology.
- Peritonsillar abscesses are most commonly managed by incision and drainage or by needle aspiration. Most commonly, interval tonsillectomy is performed 4-12 weeks after resolution of the infection.
Peritonsillar abscesses may spread to the parapharyngeal space if not addressed promptly.
Parotid space
- The parotid space is enclosed by the superficial layer of the deep cervical fascia. This is an incomplete enclosure because the superomedial aspect of the gland is not covered. This discontinuity allows communication between the parapharyngeal space and the parotid space.
- The parotid space is crossed by the external carotid artery, the posterior facial vein, and the facial nerve.
- Infections in the parotid space often occur in dehydrated, debilitated patients with poor oral hygiene who develop salivary duct obstruction.
- Pain, edema, and erythema in the region of the parotid are typically observed with fever. Trismus is a later finding.
Temporal space
- The temporal space lies between the temporalis fascia and the periosteum of the temporal bone, and it contains the internal maxillary artery and the inferior alveolar artery and nerve. The temporalis muscle effectively divides the space into a deep and superficial compartment.
- Abscesses in this region are characterized by pain and trismus and may exhibit deviation of the mandible.
- Incision and drainage may be accomplished by an approach 3 cm lateral to the lateral canthus or by a horizontal brow incision.
Figure 1. Cervical fascia and cervical compartment
Deep neck infection causes
Before the widespread use of antibiotics, 70% of deep neck space infections were caused by spread from tonsillar and pharyngeal infections. Today, tonsillitis remains the most common cause of deep neck space infections in children, whereas odontogenic origin is the most common cause in adults 9. A study by Adoviča et al 10 found that out of 263 patients hospitalized for deep neck space phlegmons and/or abscesses, 70.6% of the cases arose from dental infections.
Deep neck infections are usually polymicrobial, representing their origin from the normal flora of the oral cavity and upper respiratory tract. Streptococcus viridans is the most commonly identified organism causing deep neck space infections 7. Other organisms identified include Staphylococcus aureus, gram-negative rods, anaerobes, Mycobacterium, and fungi. The presence of risk factors such as immunocompromised state, diabetes mellitus, intravenous (IV) drug use, as well as the site of origin of infection, influences the type of causative organism.
Causes of deep neck infections include the following 11:
- Tonsillar and pharyngeal infections
- Dental infections or abscesses
- Oral surgical procedures or removal of suspension wires
- Salivary gland infection or obstruction 12
- Trauma to the oral cavity and pharynx (eg, gun shot wounds, pharynx injury caused by falls onto pencils or Popsicle sticks, esophageal lacerations from ingestion of fish bones or other sharp objects)
- Instrumentation, particularly from esophagoscopy or bronchoscopy
- Foreign body aspiration
- Cervical lymphadenitis
- Branchial cleft anomalies
- Thyroglossal duct cysts
- Thyroiditis
- Mastoiditis with petrous apicitis and Bezold abscess
- Laryngopyocele
- IV drug use 13
- Necrosis and suppuration of a malignant cervical lymph node or mass
As many as 20-50% of deep neck infections have no identifiable source.
Other important considerations include patients who are immunosuppressed because of human immunodeficiency virus (HIV) infection, chemotherapy, or immunosuppressant drugs for transplantation. These patients may have increased frequency of deep neck infections and atypical organisms, and they may have more frequent complications.
A retrospective study by Alotaibi et al 14 indicated that in patients with odontogenic infection, criteria for hospital admission based on a risk of deep neck space infection should include not just the well-known risk signs—fever, trismus, leukocytosis, swollen neck, dysphagia, dyspnea, and elevated C-reactive protein levels—but also the presence of mandibular (as opposed to maxillary) odontogenic infection and/or dental abscess. The study included a cohort of 97 patients.
Systemic lupus erythematosus (SLE) may be another etiologic factor in deep neck space infection. A retrospective study by Chang et al 15 indicated that individuals with SLE have an approximately five-fold greater risk of developing the condition.
A retrospective study by Almutairi et al 16 of 183 patients with deep neck space infection found that 93 (50.8%) had comorbidities, with diabetes and hypertension being the most prevalent (45.2% and 23.7% of comorbidities, respectively).
Deep neck space infections pathophysiology
Deep neck space infections can arise from a multitude of causes. Whatever the initiating event, development of a deep neck space infection proceeds by one of several paths, as follows:
- Spread of infection can be from the oral cavity, face, or superficial neck to the deep neck space via the lymphatic system.
- Lymphadenopathy may lead to suppuration and finally focal abscess formation.
- Infection can spread among the deep neck spaces by the paths of communication between spaces.
- Direct infection may occur by penetrating trauma.
Once initiated, a deep neck infection can progress to inflammation and phlegmon or to fulminant abscess with a purulent fluid collection. This distinction is important because the treatment of these 2 entities is very different.
The signs and symptoms of a deep neck abscess develop because of the following:
- Mass effect of inflamed tissue or abscess cavity on surrounding structures
- Direct involvement of surrounding structures with the infectious process
For example, tonsillitis may lead to peritonsillar abscess. If not treated successfully, peritonsillar abscess may spread to the lateral pharyngeal space. From there, infection spreads to the posterior pharyngeal and prevertebral spaces and into the chest. Mediastinitis and empyema may ensue, leading to death. Alternatively, infection may spread from the lateral pharyngeal space to the contents of the carotid sheath, leading to internal jugular vein thrombosis, subacute bacterial endocarditis, pulmonary emboli, carotid artery thrombosis and cerebrovascular insufficiency, or Horner syndrome. Lateral pharyngeal space abscess alone may cause airway obstruction at the level of the pharynx. Ungkanont et al 17 reviewed 117 children treated for deep neck infections during a 6-year period. The following distribution results were revealed:
- Peritonsillar infections (49%)
- Retropharyngeal infections (22%)
- Submandibular infections (14%)
- Buccal infections (11%)
- Parapharyngeal space infections (2%)
- Canine space infections (2%)
The microbiology of deep neck infections usually reveals mixed aerobic and anaerobic organisms 18, often with a predominance of oral flora. Both gram-positive and gram-negative organisms may be cultured. Group A beta-hemolytic streptococcal species (Streptococcus pyogenes), alpha-hemolytic streptococcal species (Streptococcus viridans, Streptococcus pneumoniae), Staphylococcus aureus, Fusobacterium nucleatum, Bacteroides melaninogenicus, Bacteroides oralis, and Spirochaeta, Peptostreptococcus, and Neisseria species often are found together in various combinations. Pseudomonas species, Escherichia coli, and Haemophilus influenzae are occasionally encountered.
A German study, by Cordesmeyer et al 19, of 63 patients with deep neck space infections, found Streptococcus viridans to be the most prevalent aerobic gram-positive pathogen to be isolated (26.7% of infections), with Staphylococcus epidermidis and Staphylococcus aureus each being isolated in 16.7% of infections. Among the aerobic gram-negative pathogens, Escherichia coli, Klebsiella oxytoca, and Haemophilus influenzae were the most frequently isolated. Malignancy was found in 1.6% of patients.
In a retrospective study by Shimizu et al 20 of patients with deep neck infection, bacterial cultures revealed Staphylococcus species in 60% of pediatric patients but in only 9% of adults, with Streptococcus species, on the other hand, being more common in adults (56%) than in children (27%).
A study by Asmar 21 of retropharyngeal abscess microbiology demonstrated polymicrobial culture results in almost 90% of patients. Aerobes were found in all cultures, and anaerobes were found in more than 50% of patients. Other studies have shown an average of at least 5 isolates from cultures.
May et al 22 found evidence that biofilm phenotypes may be associated with the pathogenesis of deep neck infections, as well as with the recalcitrance to treatment experienced with larger deep neck abscesses. The investigators obtained biopsy samples from abscess walls located in the deep neck spaces of 14 patients undergoing surgical drainage. Scanning electron microscopy revealed that 12 of the 14 tissue samples contained biofilm imbedded with rod- and cocci-shaped bacteria 22.
Deep neck infection symptoms
Clinical presentation of deep neck space infections is variable based on the primary site of infection, fascial plane involved, the extent of inflammation, and presence of local pressure effects and systemic complications. Most patients present with fever and neck pain. Associated symptoms such as dental pain, dysphagia, stridor, respiratory distress can provide clues regarding the affected facial plane. Predisposing factors such as immunocompromised state, recent oral/dental procedures, recent neck or oral trauma, recent neck surgery or radiation, or presence of comorbid conditions such as diabetes should be sought.
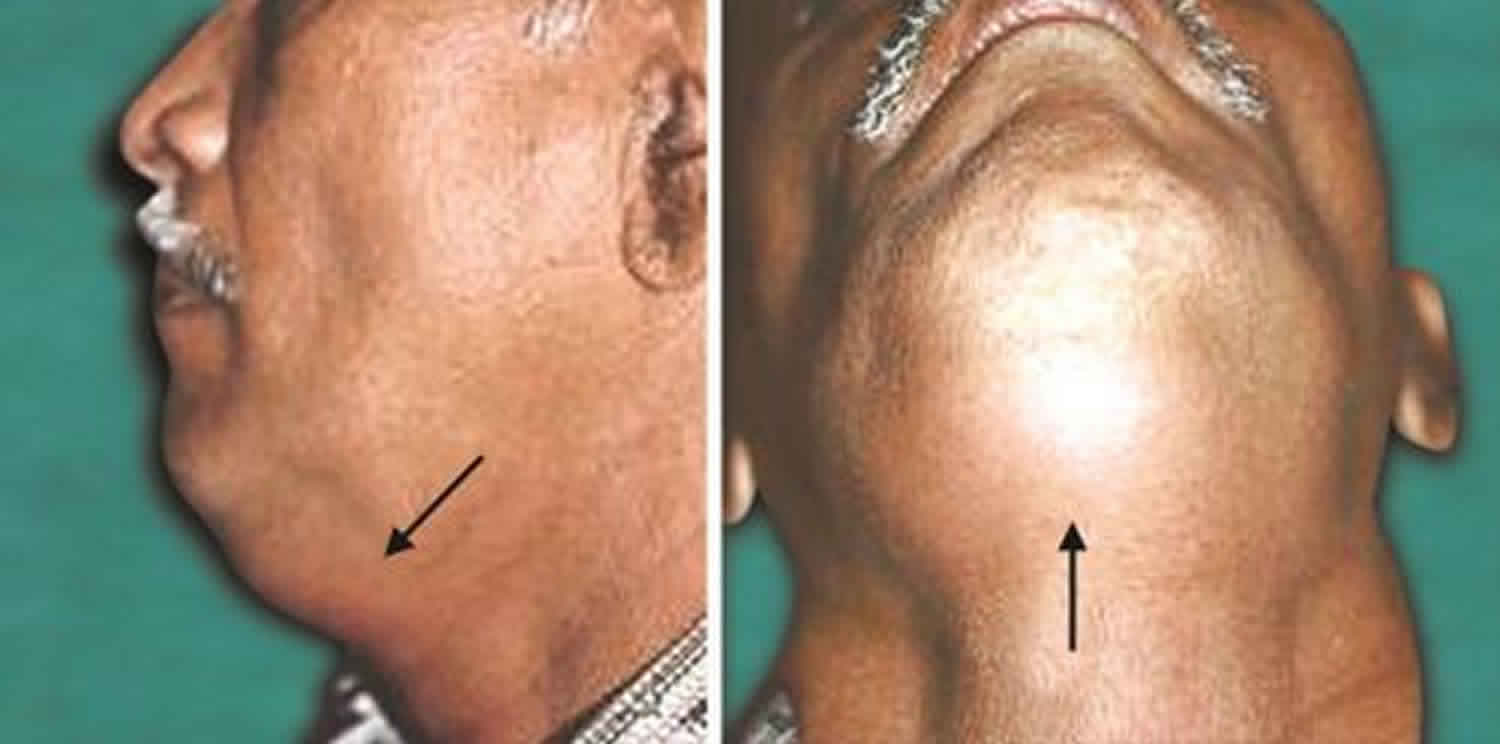
Patients are usually febrile and may appear ill and toxic. Inspection of the neck may reveal asymmetry, redness, swelling and regional lymphadenitis. Torticollis may be present. Abscesses in this area are harder to detect due to frequent absence of fluctuance due to the taut fascia, overlying muscles, and the deep location.
Proximal deep neck infections (peritonsillar, parapharyngeal, parotid and submandibular) infections and abscesses tend to present with a sore throat and sometimes with trismus. Trismus occurs due to the local pressure on the muscles of mastication or the trigeminal nerve. A physical exam may reveal neck or lower facial swelling, local erythema, tenderness, and regional lymphadenitis. Lateral displacement of the uvula suggests peritonsillar abscess, whereas medial displacement of the pharyngeal wall is strongly suggestive of parapharyngeal space infection. Local pressure may result in dysphagia or odynophagia and may have associated inflammation in the cricoarytenoid joints. If the vagus nerve is affected, dysphonia and hoarseness can occur. Infections in the submandibular space may occur after spreading from dental abscesses, sublingual or submaxillary salivary glands or oral infections following trauma. Cellulitis in this space is also known as Ludwig’s angina that can lead to airway obstruction. Ludwig’s angina presents with drooling, inability to swallow, trismus, and induration of the floor of the mouth.
Retropharyngeal space infections are more common in infants and young children and usually are preceded by an upper respiratory infection. They often present with dysphagia, drooling and stridor and can lead to airway compromise or spread into the chest to cause mediastinitis. Infections in the danger space (located posterior to the retropharyngeal space and anterior to prevertebral space) that has loose areolar tissue facilitates the rapid spread of infection to surrounding regions and often presents with complications such as mediastinitis, empyema, and sepsis.
A study by Shimizu et al 20 found that in patients with deep neck infection, lymphadenitis was the most common preceding illness in children (73%), while in adults, it was the least common antecedent disease (7%).
Physical examination should focus on determining the location of the infection, the deep neck spaces involved, and any potential functional compromise or complications that may be developing. A comprehensive head and neck examination should be performed, including examination of the dentition and tonsils. The most consistent signs of a deep neck space infection are fever, elevated white blood cell (WBC) count, and tenderness. Other signs and symptoms largely depend on the particular spaces involved and include the following:
- Asymmetry of the neck and associated neck masses or lymphadenopathy, which is present in almost 70% of pediatric retropharyngeal abscesses according to a study by Thompson and colleagues
- Medial displacement of the lateral pharyngeal wall and tonsil caused by parapharyngeal space involvement
- Trismus caused by inflammation of the pterygoid muscles
- Torticollis and decreased range of motion of the neck caused by inflammation of the paraspinal muscles
- Fluctuance that may not be palpable because of the deep location and the extensive overlying soft tissue and muscles (eg, sternocleidomastoid muscle)
- Possible neural deficits, particularly of the cranial nerves (eg, hoarseness from true vocal cord paralysis with carotid sheath and vagal involvement), and Horner syndrome from involvement of the cervical sympathetic chain
- Regularly spiking fevers (may suggest internal jugular vein thrombophlebitis and septic embolization)
- Tachypnea and shortness of breath (may suggest pulmonary complications and warn of impending airway obstruction)
Deep neck infection complications
Deep neck infections have many severe life-threatening potential complications. Deep neck infections that are not treated or are inadequately treated, those that extend to other deep neck spaces, and those that are complicated by a delay in diagnosis and treatment are at particular risk of complications, including the following:
- Lateral pharyngeal space infections can spread to the carotid sheath and cause septic thrombophlebitis (Lemierre syndrome) and erosion 7.
- Retropharyngeal or danger space infections can spread to the mediastinum and cause acute mediastinitis that may further spread and cause empyema and pericarditis. Respiratory failure can occur from an obstructed airway and spread into the systemic circulation can result in sepsis and intracranial infections.
- Airway obstruction from compression of the trachea
- Aspiration is particularly due to perforation of a retropharyngeal abscess with drainage of pus into the airway. Aspiration may occur spontaneously or during endotracheal intubation.
- Vascular complications (ie, thrombosis of the internal jugular vein, carotid artery erosion and rupture)
- Neurologic deficits: Cranial nerve dysfunction or dysfunction of the autonomic nerves in the neck can lead to problems such as hoarseness from involvement of the vagus in the carotid sheath or Horner syndrome from involvement of the sympathetic chain.
- Septic emboli: These emboli can lead to pulmonary, brain, or joint seeding and resultant abscesses.
- Septic shock
- Necrotizing cervical fasciitis: This is a fulminant infection involving necrosis of the connective tissue that spreads via fascial planes. It has particularly high morbidity and mortality rates.
- Osteomyelitis due to local spread to bones of the spine, mandible, or skull base
- Grisel syndrome (ie, inflammatory torticollis causing cervical vertebral subluxation)
Several studies have looked at factors that may cause an increase in the risk of complications from deep neck space infections 23. One study by Huang et al 24 found a higher risk of complications in females, patients with neck swelling, and patients with associated respiratory symptoms. Another study by Huang et al suggests that diabetes and the presence of other underlying systemic diseases significantly increases the risk of complications. This finding was supported in a study by Chen et al 25, who found that not only did diabetes correlate with a higher complication rate but that it was also associated with a more severe clinical course involving more than one deep neck space and a longer hospitalization.
Using multivariate statistical analysis of 282 cases of deep neck infection, Staffieri et al 26 concluded that in patients with such infections, the involvement of more than 1 neck space was the only significant independent prognostic factor for related complications. According to the study, the following factors were associated with long hospital stays:
- Presence of comorbidities
- Nonodontogenic sites of origin
- Leukocyte counts above 11.0 cells × 109/L at presentation
- Need for both medical and surgical treatments
Deep neck infection diagnosis
A high index of suspicion is important when diagnosing a deep neck space infection. A careful history and physical examination are critical to the workup. In addition, tests, including the following, may be useful in the workup of a patient in whom a deep neck space infection is suspected.
Laboratory studies
- Blood chemistries
- Complete blood cell count (CBC)
- Clotting profile (particularly important in patients who require surgical drainage)
- Blood cultures (may be indicated in septic patients)
- Abscess cultures with Gram stains (critical to direct antimicrobial therapy)
A complete blood count (CBC) usually shows leukocytosis, and chemistry may reveal evidence of dehydration if patient’s intake is poor. A blood culture should be obtained if the patient is septic, and cultures should be obtained of any purulent discharge in the affected region.
Imaging studies
The gold standard imaging modality to diagnose the source and extent of the deep neck infection is computed tomography (CT) or magnetic resonance imaging (MRI) of the neck with intravenous contrast.
Lateral neck radiography
- These tests may reveal soft tissue swelling in the prevertebral region. Lateral neck radiographs can also demonstrate radiopaque foreign bodies, subcutaneous air, air fluid levels, and erosion of the vertebral bodies.
- Prevertebral soft tissue thickening greater than 7 mm over C2 or greater than 14 mm in children and 22 mm in adults over C6 is highly suggestive of a retropharyngeal process.
Plain radiography of the neck in children may suggest retropharyngeal abscess when the prevertebral soft tissue shadow is greater than 7 mm at the C2 level or greater than 14 mm at C6 level. Among adults, the soft tissue shadow is greater than 22 mm at C6 level. Additionally, plain x-rays may reveal foreign body or subcutaneous air when present.
Mandible series
- When a dental source of the infection is suggested, a Panorex can help evaluate the patient for a dental abscess.
- Particular attention should be given to the second and third mandibular molars because the apices of these teeth extend below the mylohyoid line, giving them access to the submandibular space.
Chest radiography
- A chest x-ray is indicated if there is suspicion for mediastinitis, pneumomediastinum, or empyema.
- To evaluate the mediastinum, check for subcutaneous air or pneumomediastinum, displacement of the air stripe, or concurrent pneumonia suggesting aspiration.
CT scanning
CT scans with contrast are the gold standard in evaluation of deep neck infections. The importance of CT scanning is highlighted in a study by Crespo et al, who found that clinical examination alone underestimated the extent of deep neck space infections in 70% of patients. CT scans indicate the location, boundaries, and relation of infection to surrounding neurovascular structures. Abscesses are seen as low-density lesions with rim enhancement, occasional air fluid levels, and loculations (see the images below). A study by Kirse and Roberson also notes the association between irregularity of the abscess wall on CT as predictive of pus within the cavity 27. CT scans are fast, relatively inexpensive, and fairly widely available today. CT scanning of the chest may be helpful if extension into the mediastinum is suspected.
Ultrasound
Ultrasound is useful for relatively superficial infections to differentiate between phlegmon and abscesses but is not adequate for deep infections.
Ultrasounds do not reveal anatomic details but can help distinguish between phlegmon and abscess, give information about the condition of surrounding vessels, and guide fine-needle aspiration (FNA) attempts.
Arteriography
This may be helpful when carotid, jugular, or innominate involvement is suggested.
Deep neck infection treatment
Airway
The airway is the first priority of treatment 28. Addressing the airway may involve observation, endotracheal or nasotracheal intubation, tracheostomy, or cricothyroidotomy for emergent situations 29.
Even in experienced hands, attempted oral or nasal endotracheal intubation in a patient with a deep neck space infection or abscess may be extremely difficult. The larynx and vocal cords may be difficult to visualize because of swollen pharyngeal walls and laryngeal displacement. Instrumentation can cause additional swelling. The potential exists for abscess rupture with intubation leading to aspiration, acute airway obstruction, or death. Other factors (eg, tracheal deviation, external airway compression, trismus, cervical spine rigidity) can produce difficulty with intubation.
Patients presenting with impending respiratory distress should undergo a tracheostomy while under local anesthesia to secure a safe airway. A tracheostomy is safer, more conservative, and preferable to the development of respiratory compromise. Tracheostomy should be performed before any attempts at surgical drainage in these patients.
Cultures
- Obtain cultures whenever possible to help direct antimicrobial therapy.
- This may involve cultures of the neck, abscess fluid, and blood.
Volume and metabolic resuscitation
- Initiate these procedures in all patients with deep neck infection.
- Identify and address metabolic derangements during resuscitation.
- Address attention to other concurrent medical problems (eg, diabetes) early in the course of treatment.
Intravenous antibiotics
- Choose parenteral antibiotics to cover the most likely organisms.
- Initiate empiric regimens before culture results are obtained based on the local resistance patterns and most common etiologies.
- Cover gram-positive and gram-negative organisms and aerobic and anaerobic bacteria, including beta-lactamase–producing organisms 30.
- Modify antibiotics according to culture and sensitivity results.
- A review of deep neck infections by Broughton indicated that 50% of deep neck infections can be managed nonsurgically in patients with small fluid collections and no respiratory compromise. Other studies by Plaza 31 and McClay 32 support the option of primary nonsurgical management for selected deep neck space abscesses, reserving surgical drainage for patients who do not improve within 48 hours of initiation of broad-spectrum intravenous antibiotics. Most of these studies focus on retropharyngeal and parapharyngeal space abscesses.
- A review of current practices by Lalakea and Messner 33 indicated that 60% of pediatric otolaryngologists recommend a trial of IV antibiotics before incision and drainage in selected pediatric patients with retropharyngeal abscesses. According to this review, clindamycin, ampicillin/sulbactam, and cefuroxime are currently the most commonly used antibiotics.
- IV antibiotics are administered until the patient is clinically improving and has been afebrile for at least 48 hours. After completion of an IV course of antibiotics, oral antibiotics are given.
Surgical therapy
Incision and drainage
Incision and drainage is the cornerstone of therapy for the treatment of deep neck space abscesses. Establish a secure airway before initiating any surgical procedure.
Perform incision and drainage for any frank abscess in patients with impending complications because of abscess formation and in patients with no improvement after 48-72 hours of IV antibiotics.
Most deep neck spaces require a transcervical approach to facilitate adequate exposure of the abscess and for protection of the surrounding neurovascular structures 34. A study by Cable et al 35 describes the successful use of image-guided surgical drainage of medial parapharyngeal space abscesses in the pediatric population to help localize infections in areas that are otherwise difficult to reach. A study by Dabirmoghaddam et al 36 indicated that ultrasonographically guided drainage of deep neck space abscesses leads to shorter hospital stays than does incision and drainage, with the mean hospital stay for the ultrasonography patients in the study being 5.47 days, compared with 9.70 days for those who were treated with incision and drainage.
Approach retropharyngeal abscesses by a transoral route when the abscess is small and focal. This approach requires attention to the airway to prevent aspiration of pus once the abscess cavity is entered.
Quinsy tonsillectomy or tonsillectomy performed with infection in the peritonsillar space is controversial treatment for peritonsillar abscesses. Historically, tonsillectomy during acute infection was avoided because of concern about increased risk of postoperative hemorrhage. Several recent studies, such as those by Ungkanont et al and Dodds and Maniglia, suggest no increased morbidity from this procedure 17.
Many approaches are possible to the deep neck spaces. Description of the surgical incisions and technique of drainage is beyond the scope of this article. Every approach used must ensure adequate exposure and access to allow drainage without compromising surrounding structures. Abscess cavities should be copiously irrigated, débrided, and left open with a drain or packing to prevent reaccumulation. Once an abscess has been entered, cultures should be obtained to help direct antimicrobial therapy.
Needle aspiration
Fine needle aspiration (FNA) may be used in patients with small, easily reachable abscesses or in patients who are too unstable to undergo general anesthesia. This procedure may require the assistance of CT scanning or ultrasound guidance. It may provide preliminary culture specimens before formal incision and drainage.
Deep neck infection prognosis
Prognosis is variable depending on the immunological status of the host and severity and location of the infection. Mortality rate rates range between 1% to 25% 7.
Patients treated for deep neck infections can be expected to fully recover as long as the infection is treated properly and in a timely manner. Patients whose treatment is delayed can expect a greater number of complications and a prolonged course of recovery. Once a deep neck infection has fully resolved, no particular predisposition exists for recurrence.
- Motahari SJ, Poormoosa R, Nikkhah M, Bahari M, Shirazy SM, Khavarinejad F. Treatment and prognosis of deep neck infections. Indian J Otolaryngol Head Neck Surg. 2015;67(Suppl 1):134-137. doi:10.1007/s12070-014-0802-7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4298604[↩]
- Nusbaum AO, Som PM, Rothschild MA, Shugear JM. Recurrence of deep neck infection. Arch Otolaryngol. 1999;125(12):1379–1382. doi: 10.1001/archotol.125.12.1379.[↩][↩]
- Huang TT, Tseng FY, Yeh TH, Hsu CJ, Cen YS. Factors affecting the bacteriology of deep neck infection: a retrospective study of 128 patients. Acta Otolaryngol. 2006;126(4):396–401. doi: 10.1080/00016480500395195[↩]
- Lin C-H, Chou J-C, Lin T-L, Lou P-J. Spontaneous resolution of internal jugular vein thrombosis in a salmonella neck abscess patient. J Laryngol Otol. 1999;113(12):1122–1124. doi: 10.1017/S0022215100158086[↩]
- Schondorf J, Jungehulsing M, Brochhagen HG, Pluisch F, Schultes A, Eckel H. Infection of deep soft tissues of the neck in intravenous drug abuse. Laryngorhinootologie. 2000;79(3):171–173. doi: 10.1055/s-2000-290[↩][↩]
- McDonnough JA, Ladzekpo DA, Yi I, Bond WR, Ortega G, Kalejaiye AO. Epidemiology and resource utilization of ludwig’s angina ED visits in the United States 2006-2014. Laryngoscope. 2019 Sep;129(9):2041-2044.[↩]
- Almuqamam M, Gonzalez FJ, Kondamudi NP. Deep Neck Infections. [Updated 2020 May 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513262[↩][↩][↩][↩][↩][↩][↩]
- Li RM, Kiemeney M. Infections of the Neck. Emerg Med Clin North Am. 2019;37(1):95-107. doi:10.1016/j.emc.2018.09.003[↩]
- Conrad DE, Parikh SR. Deep Neck Infections. Infect Disord Drug Targets. 2012 Feb 17.[↩]
- Adovica A, Veidere L, Ronis M, Sumeraga G. Deep neck infections: review of 263 cases. Otolaryngol Pol. 2017 Oct 30. 71 (5):37-42.[↩]
- Deep Neck Infections. https://emedicine.medscape.com/article/837048-overview[↩]
- Favaretto N, Fasanaro E, Staffieri A, et al. Deep neck infections originating from the major salivary glands. Am J Otolaryngol. 2015 Jul-Aug. 36 (4):559-64.[↩]
- Daramola OO, Flanagan CE, Maisel RH, Odland RM. Diagnosis and treatment of deep neck space abscesses. Otolaryngol Head Neck Surg. 2009 Jul. 141(1):123-30.[↩]
- Alotaibi N, Cloutier L, Khaldoun E, Bois E, Chirat M, Salvan D. Criteria for admission of odontogenic infections at high risk of deep neck space infection. Eur Ann Otorhinolaryngol Head Neck Dis. 2015 Nov. 132 (5):261-4.[↩]
- Chang GH, Su YC, Lin KM, et al. Deep Neck Infection in Systemic Lupus Erythematosus Patients: Real-World Evidence. Sci Rep. 2020 Mar 5. 10 (1):4133.[↩]
- Almutairi DM, Alqahtani RM, Alshareef N, Alghamdi YS, Al-Hakami HA, Algarni M. Deep Neck Space Infections: A Retrospective Study of 183 Cases at a Tertiary Hospital. Cureus. 2020 Feb 1. 12 (2):e6841.[↩]
- Ungkanont K, Yellon RF, Weissman JL, et al. Head and neck space infections in infants and children. Otolaryngol Head Neck Surg. 1995 Mar. 112(3):375-82.[↩][↩]
- Celakovsky P, Kalfert D, Smatanova K, et al. Bacteriology of deep neck infections: analysis of 634 patients. Aust Dent J. 2015 Jun. 60 (2):212-5.[↩]
- Cordesmeyer R, Kauffmann P, Markus T, et al. Bacterial and histopathological findings in deep head and neck infections: a retrospective analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017 Feb 20.[↩]
- Shimizu Y, Hidaka H, Ozawa D, et al. Clinical and bacteriological differences of deep neck infection in pediatric and adult patients: Review of 123 cases. Int J Pediatr Otorhinolaryngol. 2017 Aug. 99:95-9.[↩][↩]
- Asmar BI. Bacteriology of retropharyngeal abscess in children. Pediatr Infect Dis J. 1990 Aug. 9(8):595-7.[↩]
- May JG, Shah P, Sachdeva L, et al. Potential role of biofilms in deep cervical abscess. Int J Pediatr Otorhinolaryngol. 2014 Jan. 78(1):10-3.[↩][↩]
- Kauffmann P, Cordesmeyer R, Troltzsch M, Sommer C, Laskawi R. Deep neck infections: A single-center analysis of 63 cases. Med Oral Patol Oral Cir Bucal. 2017 Sep 1. 22 (5):e536-41.[↩]
- Huang TT, Liu TC, Chen PR. Deep neck infection: analysis of 185 cases. Head Neck. 2004 Oct. 26(10):854-60.[↩]
- Chen MK, Wen YS, Chang CC. Deep neck infections in diabetic patients. Am J Otolaryngol. 2000 May-Jun. 21(3):169-73.[↩]
- Staffieri C, Fasanaro E, Favaretto N, et al. Multivariate approach to investigating prognostic factors in deep neck infections. Eur Arch Otorhinolaryngol. 2014 Feb 13.[↩]
- Kirse DJ, Roberson DW. Surgical management of retropharyngeal space infections in children. Laryngoscope. 2001 Aug. 111(8):1413-22.[↩]
- Marioni G, Staffieri A, Parisi S, Marchese-Ragona R, Zuccon A, Staffieri C, et al. Rational diagnostic and therapeutic management of deep neck infections: analysis of 233 consecutive cases. Ann Otol Rhinol Laryngol. 2010 Mar. 119(3):181-7.[↩]
- Tapiovaara L, Back L, Aro K. Comparison of intubation and tracheotomy in patients with deep neck infection. Eur Arch Otorhinolaryngol. 2017 Oct. 274 (10):3767-72.[↩]
- Raffaldi I, Le Serre D, Garazzino S, et al. Diagnosis and management of deep neck infections in children: the experience of an Italian paediatric centre. J Infect Chemother. 2015 Feb. 21 (2):110-3.[↩]
- Plaza Mayor G, Martínez-San Millán J, Martínez-Vidal A. Is conservative treatment of deep neck space infections appropriate?. Head Neck. 2001 Feb. 23(2):126-33.[↩]
- McClay JE, Murray AD, Booth T. Intravenous antibiotic therapy for deep neck abscesses defined by computed tomography. Arch Otolaryngol Head Neck Surg. 2003 Nov. 129(11):1207-12.[↩]
- Lalakea Ml, Messner AH. Retropharyngeal abscess management in children: current practices. Otolaryngol Head Neck Surg. 1999 Oct. 121(4):398-405.[↩]
- Hah YM, Jung AR, Lee YC, Eun YG. Risk factors for transcervical incision and drainage of pediatric deep neck infections. J Pediatr Surg. 2018 Apr. 53 (4):666-70.[↩]
- Cable BB, Brenner P, Bauman NM, Mair EA. Image-guided surgical drainage of medial parapharyngeal abscesses in children: a novel adjuvant to a difficult approach. Ann Otol Rhinol Laryngol. 2004 Feb. 113(2):115-20.[↩]
- Dabirmoghaddam P, Mohseni A, Navvabi Z, Sharifi A, Bastaninezhad S, Safaei A. Is ultrasonography-guided drainage a safe and effective alternative to incision and drainage for deep neck space abscesses?. J Laryngol Otol. 2017 Mar. 131 (3):259-63.[↩]