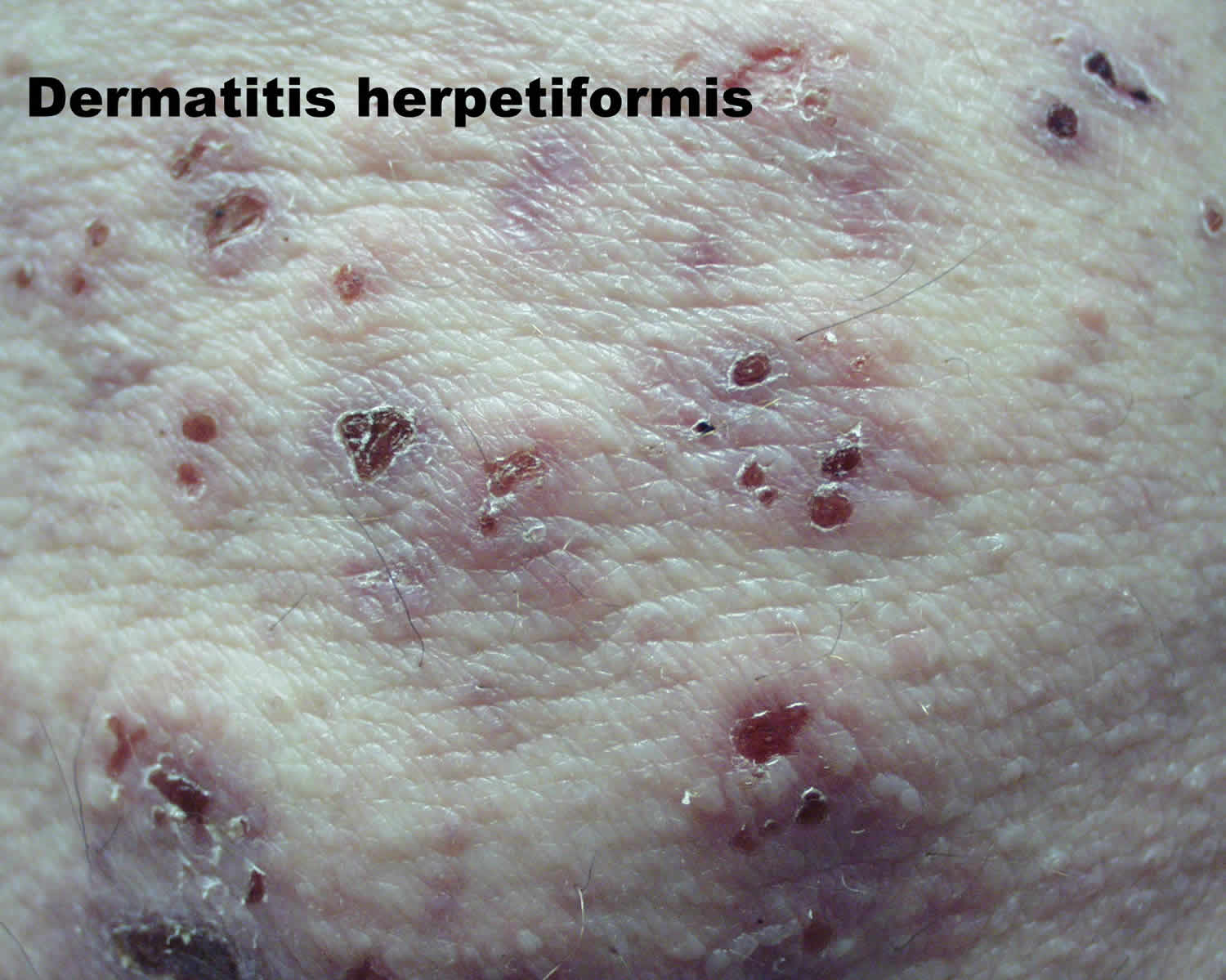
What is dermatitis herpetiformis
Dermatitis herpetiformis also known as Duhring-Brocq disease, is a common extremely itchy, red, blistery skin disease associated with celiac disease, a gluten-sensitive enteropathy 1. The name herpetiformis is derived from the tendency for blisters to appear in clusters, resembling herpes simplex. However, dermatitis herpetiformis is not due to viral infection.
Dermatitis herpetiformis is a common extraintestinal manifestation of celiac disease presenting most commonly with itchy papules and vesicles on the scalp, shoulders, buttocks, elbows and knees. Dermatitis herpetiformis is characterized by prurigo (extremely itchy papules) and vesicles on normal or reddened skin. Dermatitis herpetiformis often appear in groups or serpiginous (wavy) clusters. Blisters are often eroded and crusted due to immediate scratching. Overt gastrointestinal symptoms are rare.
The mean age at dermatitis herpetiformis diagnosis has increased significantly in recent decades and presently is 40–50 years. The dermatitis herpetiformis to celiac disease prevalence ratio is 1:8 in Finland and the United Kingdom. The annual dermatitis herpetiformis incidence rate, currently 2.7 per 100,000 in Finland and 0.8 per 100,000 in the U.K. and is the same in the USA among the white population of European descent, is decreasing, whereas the reverse is true for celiac disease. The long-term prognosis of dermatitis herpetiformis patients on a gluten-free diet is excellent, with the mortality rate being even lower than for the general population.
Two recent large dermatitis herpetiformis studies with a total of 477 and 809 patients found a prevalence of 75.3 per 100,000 in Finland 2 and 30 per 100,000 in the U.K. 3 (Table 1). In the U.K. study the prevalence of celiac disease was 240 per 100,000, i.e., eight times higher than that of dermatitis herpetiformis. The same 8:1 ratio was calculated in the Finnish study, where the national prevalence of coeliac disease was 661 per 100,000 4. Nevertheless, dermatitis herpetiformis is evidently the most common extraintestinal manifestation of celiac disease 5.
Importantly, a Finnish cohort study 2 and a U.K. register study 3 covering time periods of 30 and 20 years, respectively, showed convincingly that the annual incidence rate of dermatitis herpetiformis has decreased significantly. This decrease was from 5.2 to 2.7 per 100,000 in the Finnish study and from 1.8 to 0.8 per 100,000 in the U.K. study. The opposite was true for coeliac disease; there was a maximum of a fourfold increase in the incidence rate 3. The reason for this seems to be an increasing awareness of mild symptoms, the development of efficient serological screening tests, and the identification of special risk groups, such as family members 6. Sero-epidemiological studies suggest that in addition to the better recognition of coeliac disease, there has also been a true increase in the incidence in recent decades 7.
The decreasing incidence rate of dermatitis herpetiformis in Finland and the U.K., along with a simultaneous rapid increase in coeliac disease, fits the current hypothesis that subclinical, undiagnosed celiac disease is a prerequisite for the development of dermatitis herpetiformis. In support of this hypothesis, scientists know of patients initially diagnosed with celiac disease who did not follow or only partially followed a gluten free diet and subsequently developed dermatitis herpetiformis 8. Moreover, adult patients with dermatitis herpetiformis frequently have celiac-type dental enamel defects, which develop early in childhood as a result of malabsorption or immune alteration caused by undiagnosed celiac disease 9.
Table 1. Differences between dermatitis derpetiformis and celiac disease
| Dermatitis Herpetiformis | Coeliac Disease | |
|---|---|---|
| Gender | Slightly more males | Females predominate |
| Age at onset | Mainly adults | Children and adults |
| IgA-TG3 deposits in the skin | 100% | 0% |
| Small bowel villous atrophy | 75% | 100% * |
| IgA-TG2 deposits in the small bowel mucosa 10 | 80% | up to 100% ** |
| Prevalence in Finland and United Kingdom 3 | 75 and 30 per 100,000 | 660 and 240 per 100,000 |
| Incidence | Decreasing | Increasing |
| Response to a gluten-free diet 11 | Slow; months, in the beginning most patients need dapsone to control the rash | Rapid; days or weeks until gastro-intestinal symptoms end whereas small bowel villous atrophy may persist for many years |
| Long-term prognosis on a gluten-free diet 12 | Excellent | All-cause and lymphoma mortality may be increased |
Footnotes: TG3 = epidermal transglutaminase, TG2 = tissue transglutaminase; * Potential celiac disease with normal small bowel mucosa architecture, inflammation and positive TG2 serology also exist; ** Data still sparse.
[Source 1]Diagnosis of dermatitis herpetiformis is easily confirmed by immunofluorescence biopsy showing pathognomonic granular immunoglobulin A (IgA) deposits in the papillary dermis 1. The majority (> 90%) of patients with dermatitis herpetiformis also have celiac disease (gluten-sensitive enteropathy). Gastrointestinal symptoms may be mild to severe; some patients remain symptom-free. Around 15–25% celiac patients have concurrent dermatitis herpetiformis. These patients tend to have a more severe intestinal pathology in comparison to those with mainly dermatitis herpetiformis.
Dermatitis herpetiformis and celiac disease are due to intolerance to the gliadin fraction of gluten found in wheat, rye and barley. Gluten triggers production of immunoglobulin A (IgA) antibodies and an autoimmune process that targets the skin and gut. In celiac disease, gluten causes intestinal inflammation resulting in diarrhea, tiredness, weight loss and abdominal discomfort. A valid hypothesis for the immunopathogenesis of dermatitis herpetiformis is that it starts from latent or manifest celiac disease in the gut and evolves into an immune complex deposition of high avidity IgA epidermal transglutaminase (TG3) antibodies, together with the TG3 enzyme, in the papillary dermis 1.
A life-long gluten-free diet is the treatment of choice for all patients with dermatitis herpetiformis. Dermatitis herpetiformis usually has good prognosis, with the majority of patients responding well to a strict gluten-free diet and dapsone (4,4-diaminodiphenylsulfone) medication. The response rate varies between individuals (days to years). Most patients initially receive dapsone medication, which can be tapered off after a mean of two years’ strict adherence to a gluten-free diet 13.
Key facts on dermatitis herpetiformis
- Very itchy!
- Favorite sites include knees, elbows, sacrum/back, posterior axillary folds and the forehead.
- Autoimmune vesicular disease caused by an allergy to gluten.
- Greater than 90% of patients with dermatitis herpetiformis by endoscopy have a subclinical gluten-sensitive enteropathy.
- Of patients with gluten-sensitive enteropathy, only 15-25% develop dermatitis herpetiformis.
- A gluten-free diet reduces all-cause mortality by 30%.
- Associations: Cryofibrinogenemia 14.
- Patients with dermatitis herpetiformis may have other autoimmune disease especially thyroid (5-11%), pernicious anemia (1-2%), type I diabetes (1-2%), collagen vascular disease and other autoimmune disorders including vitiligo, Addison disease and alopecia areata.
Clinical features of dermatitis herpetiformis
Dermatitis herpetiformis has a symmetrical distribution. The typical sites of predilection of dermatitis herpetiformis are the extensor surfaces of elbows and knees, and the buttocks (Figure 1A,B). In addition, the upper back, abdomen, scalp and face can be affected, but oral lesions are rare 15. The rash is polymorphic with small blisters (Figure 1C). These are, however, often eroded and crusted because of intense itch and scratching. Purpuric lesions may also appear on the hands and feet, however, this is rare 15. The presentation and activity of the rash varies greatly from patient to patient, but complete remission is infrequent on a normal, gluten-containing diet. Dermatitis herpetiformis lesions resolve to leave postinflammatory hypopigmentation and hyperpigmentation.
Dermatitis herpetiformis may also present initially as digital petechiae. Flat red patches, thickened plaques and wheals may occur resembling other inflammatory skin conditions such as dermatitis, scabies and papular urticaria.
Figure 1. Dermatitis herpetiformis
Footnotes: (A) Typical scratched papules and macules on the elbows and (B) on the knees. (C) Fresh small blisters on the elbow. (D) Direct immunofluorescence showing granular IgA deposits in the basal membrane zone between epidermis and dermis.
[Source 1]Figure 2. Dermatitis herpetiformis (note the small vesicle)
Figure 3. Dermatitis herpetiformis face
Figure 4. Dermatitis herpetiformis scalp
Figure 5. Dermatitis herpetiformis hands
Figure 6. Dermatitis herpetiformis elbow
Who gets dermatitis herpetiformis?
Dermatitis herpetiformis predominantly affects Caucasians aged 15–40 years, but may occur in those younger or older and in other races. There is a 2:1 male-to-female ratio. More females under the age of 20 are affected than males.
There is a genetic predisposition and association with human leukocyte antigens (HLAs) DQ2 and DQ8. Some patients have a personal or family history of other autoimmune disorders including thyroid disease, pernicious anemia, type 1 diabetes, vitiligo, Addison disease and alopecia areata.
Gender and Age at Onset
Earlier studies in adults with dermatitis herpetiformis have shown male to female ratios ranging up to 2:1 15 with two recent large dermatitis herpetiformis studies finding the ratio to be close to 1:1 3. This is in sharp contrast to coeliac disease, in which females outnumber males 11 (see Table 1 above). This gender imbalance may reduce with increasing age, and it has also been absent in some coeliac disease screening studies 16. These findings suggest that gender differences between dermatitis herpetiformis and coeliac disease are perhaps not as profound as was earlier thought.
Celiac disease can be diagnosed at any age with the peak incidences being in early childhood and between 40 and 60 years of age 6. Clinical series in adults with dermatitis herpetiformis from Europe and North America have shown that the mean age at diagnosis is between 40 and 50 years 17. Like celiac disease, the oldest dermatitis herpetiformis patients have been over 80 years of age at diagnosis. In contrast to celiac disease, dermatitis herpetiformis in childhood seems to be rare; it was found in only 4% of 476 Finnish patients 18. However, differences may exist. In an Italian series comprising 159 dermatitis herpetiformis patients, 36% were below the age of 20 years 19, and a large series of 127 Hungarian children with dermatitis herpetiformis has been published 20.
A study of 477 patients collected from 1970 onwards in Finland showed a significant increase in the mean age at diagnosis 2. The increase was from 35 to 51 years in men and from 36 to 46 years in women. A similar increasing trend in the mean age at diagnosis has also been observed in recent decades in adult coeliac disease, both in Finland and elsewhere 21. One explanation for this trend may be changes in dietary habits, such as the consumption of wheat, which in Sweden has changed the appearance of childhood coeliac disease 22. In Finland, the annual consumption of wheat, rye, and other cereals per person has decreased from 150 kg to 71 kg over the past 50 years 23. A lower lifetime gluten load might thus explain the increasing age at diagnosis and perhaps also the trend towards less severe small bowel atrophy in dermatitis herpetiformis and coeliac disease 24.
Complications of dermatitis herpetiformis and celiac disease
The following conditions may affect patients with dermatitis herpetiformis, especially when it is associated with celiac disease:
- Aphthous ulcers and angular cheilitis
- Dry skin, nail and hair abnormalities
- Dental problems: thin enamel
- Neurological problems: ataxia (loss of balance), polyneuropathy, epilepsy
- Heart problems: pericarditis and cardiomyopathy
- Recurrent miscarriages (spontaneous abortion)
- Fatty liver resulting in abnormal liver function
- Non-Hodgkin lymphoma affecting the intestines or any part of the body. The risk of Non-Hodgkin lymphoma is increased among dermatitis herpetiformis patients 25. Strict adherence to gluten-free diet for more than five years seems reduces this rare but serious long-term complication 26.
Dermatitis herpetiformis symptoms
Dermatitis herpetiformis is characterized by small, clustered papules and vesicles that erupt symmetrically on the elbows, knees, buttocks, back, or scalp. The face and groin can also be involved. A burning sensation may precede lesion formation. Lesions are usually scratched off by the time a patient comes in for a physical exam, and the rash may appear as erosions and excoriations.
Patients with dermatitis herpetiformis may also experience dental enamel defects to permanent teeth, which is another manifestation of celiac disease. Less than 20 percent of people with dermatitis herpetiformis have symptoms of celiac disease 27.
Dermatitis herpetiformis causes
Dermatitis herpetiformis is caused by the deposit of immunoglobulin A (IgA) in the skin, which triggers further immunologic reactions resulting in lesion formation. Dermatitis herpetiformis is an external manifestation of an abnormal immune response to gluten, in which IgA antibodies form against the skin antigen epidermal transglutaminase (transglutaminase 3 or eTAG). Epidermal transglutaminase (eTG) (transglutaminase 3 or TG3) is the major autoantigen in dermatitis herpetiformis. Tissue transglutaminase (tTG) (transglutaminase 2) is the major autoantigen in celiac disease. IgA antibodies to tissue-transglutaminase (tTG) form in the gut and cross react with epidermal-transglutaminase (e-TAG). Deposition of IgA/epidermal-transglutaminase (e-TAG) complexes in the papillary dermis lead to activation of complement and neutrophils. The average age of onset is between 20 and 40 but children and the elderly may be affected. In Finland, up to 4.4% of dermatitis herpetiformis patients have a first-degree relative with dermatitis herpetiformis and up to 6.1% have relatives with celiac disease. Thus, more than 10% have a relative with dermatitis herpetiformis or celiac disease 28.
A valid current hypothesis is that subclinical celiac disease is a prerequisite for the development of dermatitis herpetiformis 1. The reason why only some undiagnosed celiac individuals develop an itchy blistering rash with dermal IgA-TG3 deposits remains unknown 1.
Family studies show that 5 percent of first-degree relatives of a person with dermatitis herpetiformis will also have dermatitis herpetiformis. An additional 5 percent of first-degree relatives of a person with dermatitis herpetiformis will have celiac disease 29. Various other autoimmune diseases are associated with dermatitis herpetiformis, the most common being hypothyroidism.
Genetic and family studies tie dermatitis herpetiformis and celiac disease closely together. Almost every patient with dermatitis herpetiformis and celiac disease has the alleles contributing to the HLA-DQ2 or HLA-DQ8 haplotype 30. These diseases segregate in the same families 31 and even monozygotic twin pairs can be affected by dermatitis herpetiformis and coeliac disease 32.
Dermatitis herpetiformis diagnosis
A skin biopsy is the first step in diagnosing dermatitis herpetiformis. Direct immunofluorescence of clinically normal skin adjacent to a lesion shows pathognomonic granular immunoglobulin A (IgA) deposits in the upper dermis 1. Histology of lesional skin may show microabscesses containing neutrophils and eosinophils. However, histology may reveal only excoriation due to the intense itching that patients experience.
Blood tests for antiendomysial or anti-tissue transglutaminase antibodies may also suggest celiac disease. Blood tests for epidermal transglutaminase antibodies are positive in more than 90 percent of cases 33. All of these tests will become negative with prolonged adherence to a gluten-free diet.
A positive biopsy and serology confirm dermatitis herpetiformis and should be taken as indirect evidence of small bowel damage. A biopsy of the small bowel is usually not needed for dermatitis herpetiformis diagnosis. However, if clinical signs of gastrointestinal disease are evident on examination, further workup may be required 34. Whether or not intestinal damage is evident, a gluten-free diet should be implemented because the rash of dermatitis herpetiformis is gluten sensitive 29.
Skin biopsy
Skin biopsy is usually necessary to confirm dermatitis herpetiformis. Characteristic histological appearances of dermatitis herpetiformis are:
- Subepidermal blisters
- Neutrophil and eosinophil inflammatory cells in dermal papillae
- Granular IgA deposits in the dermal papillae on direct immunofluorescence
Screen for nutritional deficiencies
Patients with dermatitis herpetiformis are commonly offered the same blood tests used for patients with celiac disease to screen for nutritional deficiencies.
These include:
- Full blood count, liver function tests and serum calcium
- Iron, vitamin B12 and folate
- Thyroid function tests
Mild anaemia may be caused by iron or folic acid deficiency (or both) due to malabsorption associated with gluten-sensitive enteropathy. Thyroid function tests are usually recommended due to the association between dermatitis herpetiformis and thyroid disease.
Diagnostic blood tests
Specific autoantibody tests for dermatitis herpetiformis are:
- IgA anti-endomysial antibodies
- IgA tissue transglutaminase antibody, tTG (transglutaminase 2)
- IgA epidermal transglutaminase antibodies, eTG (transglutaminase 3 or TG3). Dermatitis herpetiformis is associated with IgA antibodies directed against epidermal transglutaminase (eTG), which is not the case in coeliac disease.
- IgA and IgG deamidated gliadin peptide antibody, dGP
- IgA and IgG gliadin assay
- Total IgA level
Borderline results may be difficult to interpret.
Small intestinal biopsy
Dermatitis herpetiformis patients with abnormal blood results usually proceed to have small intestinal biopsy to confirm gluten-sensitive enteropathy. This is histologically characterized by small bowel villous atrophy. This means that instead of being highly convoluted, the lining of the intestines is smooth and flattened.
The bowel may appear normal because of treatment (gluten-free diet and/or medication), skip lesions (the sample was taken from an unaffected site) or the intestine may be unaffected by the disease.
Other tests
HLA haplotype, a set of DNA variations, testing may reveal HLA-DQ2 or HLA-DQ8. This is present in almost all patients with dermatitis herpetiformis (and celiac disease).
Dermatitis herpetiformis treatment
The sulfone dapsone (4,4-diaminodiphenylsulfone) can provide immediate relief of symptoms. For patients who cannot tolerate dapsone, sulfapyridine or sulfamethoxypyridazine may be used, although these medications are less effective than dapsone. A strict gluten-free diet is the only treatment for the underlying disease. Even with a gluten-free diet, medication therapy may need to be continued from a few months to 2 years.
The rash in dermatitis herpetiformis responds to a strict a gluten-free diet (gluten-free diet), albeit slowly, and the symptoms recur on gluten challenge 35. Therefore, a life-long gluten-free diet is the treatment of choice for all patients with dermatitis herpetiformis.
Dermatitis herpetiformis can go into remission, which is defined as absence of skin lesions and symptoms of dermatitis herpetiformis for more than 2 years while not taking sulfones or other treatments and not adhering to a gluten-free diet. Cohort studies showing dermatitis herpetiformis remission provide support for reducing sulfone therapy and weaning from a gluten-free diet in patients with well-controlled dermatitis herpetiformis 36.
Gluten-free diet
Gluten-free diet for life is strongly recommended in patients with dermatitis herpetiformis, as it:
- Reduces the requirement for medication to control dermatitis herpetiformis
- Improves associated gluten-sensitive enteropathy
- Enhances nutrition and bone density
- May reduce the risk of developing other autoimmune conditions
- May reduce the risk of intestinal lymphoma
Medication
Dapsone is the treatment of choice for dermatitis herpetiformis, as it usually reduces itch within 3 days.
- Dose varies from 25 mg to 300 mg daily
- Dapsone has potential side effects and monitoring requirements
- It may be gradually weaned off in those who have been on a stable gluten-free diet
If intolerant or allergic to dapsone, the following may be useful:
- Ultra-potent topical steroids
- Systemic steroids
- Sulfapyridine
- Rituximab
Topical steroids may be prescribed for localized areas of itching. For acute attacks of itching, e.g., in suspected cases before the DIF is back, systemic prednisone or IM kenalog can give great relief.
Sulfapyridine can be started at 1000 mg – 2000 mg/day with the usual maximum dose of 4000 mg/day. Sulphamethoxypyridaxine can be given 0.5 – 1.0 g/day with the maximum dose of 1500 mg/day. Once the symptoms are controlled, the patient should periodically attempt to taper to the lowest possible effective dose. Some patients may even be able to reduce to alternate day dosing. The use of asulfadiazine (e.g., 500 mg twice a day) may be useful in patients with deficient G6PD.
Rituximab 4 weekly infusions of 375 mg/m2 followed by maintenance azathioprine completely cleared one patient after 13 months 37.
- Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients 2018, 10(5), 602; doi:10.3390/nu10050602 http://www.mdpi.com/2072-6643/10/5/602/htm[↩][↩][↩][↩][↩][↩][↩][↩]
- Salmi, T.T.; Hervonen, K.; Kautiainen, H.; Collin, P.; Reunala, T. Prevalence and incidence of dermatitis herpetiformis: A 40-year prospective study from Finland. Br. J. Dermatol. 2011, 165, 354–359.[↩][↩][↩]
- West, J.; Fleming, K.M.; Tata, L.J.; Card, T.R.; Crooks, C.J. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: Population-based study. Am. J. Gastroenterol. 2014, 109, 757–768.[↩][↩][↩][↩][↩]
- Virta, L.; Kaukinen, K.; Collin, P. Incidence and prevalence of diagnosed coeliac disease in Finland: Results of effective case finding in adults. Scand. J. Gastroenterol. 2009, 44, 933–938.[↩]
- Leffler, D.A.; Green, P.H.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–571.[↩]
- Tack, G.J.; Verbeek, W.H.; Schreurs, M.W.; Mulder, C.J. The spectrum of celiac disease: Epidemiology, clinical aspects and treatment. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 204–213.[↩][↩]
- Kang, J.Y.; Kang, A.H.; Green, A.; Gwee, K.A.; Ho, K.Y. Systematic review: Worldwide variation in the frequency of coeliac disease and changes over time. Aliment. Pharmacol. Ther. 2013, 38, 226–245.[↩]
- Salmi, T.T.; Hervonen, K.; Kurppa, K.; Collin, P.; Kaukinen, K.; Reunala, T. Coeliac disease evolving into dermatitis herpetiformis in patients adhering to normal or gluten-free diet. Scand. J. Gastroenterol. 2015, 50, 387–392.[↩]
- Aine, L.; Mäki, M.; Reunala, T. Coeliac-type dental enamel defects in patients with dermatitis herpetiformis. Acta Derm. Venereol. 1992, 72, 25–27.[↩]
- Salmi, T.T.; Hervonen, K.; Laurila, K.; Collin, P.; Mäki, M.; Koskinen, O.; Huhtala, H.; Kaukinen, K.; Reunala, T. Small bowel transglutaminase 2-specific IgA deposits in dermatitis herpetiformis. Acta Derm. Venereol. 2014, 94, 393–397.[↩]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743.[↩][↩]
- Hervonen, K.; Alakoski, A.; Salmi, T.T.; Helakorpi, S.; Kautiainen, H.; Kaukinen, K.; Pukkala, E.; Collin, P.; Reunala, T. Reduced mortality in dermatitis herpetiformis: A population-based study of 476 patients. Br. J. Dermatol. 2012, 167, 1331–1337.[↩]
- Garioch, J.J.; Lewis, H.M.; Sargent, S.A.; Leonard, J.N.; Fry, L. 25 years’ experience of a gluten-free diet in the treatment of dermatitis herpetiformis. Br. J. Dermatol. 1994, 131, 541–545.[↩]
- JEADV 2016;30;517[↩]
- Bolotin, D.; Petronic-Rosic, V. Dermatitis herpetiformis. Part I. Epidemiology, pathogenesis, and clinical presentation. J. Am. Acad. Dermatol. 2011, 64, 1017–1024.[↩][↩][↩]
- Vilppula, A.; Kaukinen, K.; Luostarinen, L.; Krekelä, I.; Patrikainen, H.; Valve, R.; Mäki, M.; Collin, P. Increasing prevalence and high incidence of celiac disease in elderly people: A population-based study. BMC. Gastroenterol. 2009, 9, 49.[↩]
- Mansikka, E.; Salmi, T.; Kaukinen, K.; Collin, P.; Huhtala, H.; Reunala, T.; Hervonen, K. Diagnostic delay in dermatitis herpetiformis in a high-prevalence area. Acta Derm. Venereol. 2018, 98, 195–199.[↩]
- Hervonen, K.; Salmi, T.T.; Kurppa, K.; Kaukinen, K.; Collin, P.; Reunala, T. Dermatitis herpetiformis in children: A long-term follow-up study. Br. J. Dermatol. 2014, 171, 1242–1243.[↩]
- Antiga, E.; Verdelli, A.; Calabri, A.; Fabbri, P.; Caproni, M. Clinical and immunopathological features of 159 patients with dermatitis herpetiformis: An Italian experience. G. Ital. Dermatol. Venereol. 2013, 148, 163–169.[↩]
- Dahlbom, I.; Korponay-Szabó, I.R.; Kovács, J.B.; Szalai, Z.; Mäki, M.; Hansson, T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 140–146.[↩]
- Dominguez Castro, P.; Harkin, G.; Hussey, M.; Christopher, B.; Kiat, C.; Liong Chin, J.; Trimble, V.; McNamara, D.; MacMathuna, P.; Egan, B.; et al. Changes in presentation of celiac disease in Ireland from the 1960s to 2015. Clin. Gastroenterol. Hepatol. 2017, 15, 864–871.[↩]
- Carlsson, A.; Agardh, D.; Borulf, S.; Grodzinsky, E.; Axelsson, I.; Ivarsson, S.A. Prevalence of celiac disease: Before and after a national change in feeding recommendations. Scand. J. Gastroenterol. 2006, 41, 553–558.[↩]
- Finnish National Nutrition Council. Action Programme for Implementing National Nutrition Recommendations; Edita Prima: Helsinki, Finland, 2003.[↩]
- Mansikka, E.; Hervonen, K.; Salmi, T.T.; Kautiainen, H.; Kaukinen, K.; Collin, P.; Reunala, T. The decreasing prevalence of severe villousa atrophy in Dermatitis herpetiformis: A 45-year experience in 393 patients. J. Clin. Gastroenterol. 2017, 51, 235–239[↩]
- Grainge, M.J.; West, J.; Solaymani-Dodaran, M.; Card, T.R.; Logan, R.F. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: A cohort study. Aliment. Pharmacol. Ther. 2012, 35, 730–739.[↩]
- Lewis, H.M.; Reunala, T.L.; Garioch, J.J.; Leonard, J.N.; Fry, J.S.; Collin, P.; Evans, D.; Fry, L. Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br. J. Dermatol. 1996, 135, 363–367.[↩]
- Alonzo-Llamazares J, Gibson LE, Rogers RS. Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. International Journal of Dermatology. 2007;46(9):910–919.[↩]
- BJD 1996;134;394[↩]
- Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Medicine 2012, 10:13 https://doi.org/10.1186/1741-7015-10-13[↩][↩]
- Balas, A.; Vicario, J.L.; Zambrano, A.; Acuna, D.; Garcıa-Novo, D. Absolute linkage of celiac disease and dermatitis herpetiformis to HLA-DQ. Tissue Antigens 1997, 50, 52–56.[↩]
- Hervonen, K.; Hakanen, M.; Kaukinen, K.; Collin, P.; Reunala, T. First-degree relatives are frequently affected in coeliac disease and dermatitis herpetiformis. Scand. J. Gastroenterol. 2002, 37, 51–55.[↩]
- Hervonen, K.; Karell, K.; Holopainen, P.; Collin, P.; Partanen, J.; Reunala, T. Concordance of dermatitis herpetiformis and celiac disease in monozygous twins. J. Investig. Dermatol. 2000, 115, 990–993.[↩]
- Rose C, Armbruster FP, Ruppert J, Igl B-W, Zillikens D, Shimanovich I. Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. Journal of the American Academy of Dermatology. 2009;61(1):39–43.[↩]
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis: part II. Diagnosis, management, and prognosis. Journal of the American Academy of Dermatology. 2011;64(6):1027–1033[↩]
- Leonard, J.; Haffenden, G.; Tucker, W.; Unsworth, J.; Swain, F.; McMinn, R.; Holborow, J.; Fry, L. Gluten challenge in dermatitis herpetiformis. N. Engl. J. Med. 1983, 308, 816–819.[↩]
- Paek SY, Steinberg SM, Katz SI. Remission in dermatitis herpetiformis: a cohort study. Archives of Dermatology. 2011;147(3):301–305.[↩]
- JAMADerm 2017;153;315[↩]