Dermatoglyphics
Dermatoglyphics is the study of epidermal ridge patterns and lines (fingerprints) on the skin of the fingers, palms, toes, and soles 1. Dermatoglyphics is a Greek word, “derma” means skin and “glyph” means carving 2. A dermatoglyph is literally a ‘skin carving’ or furrow in the skin 3. These ridges appear on the hands between sixth and seventh week of fetal development and are fully formed by the 21st week and remain largely unchanged after this period 4. Alterations in the patterns and counts of dermatoglyphics may be an indication of disruption to fetal development in the early- to mid-gestation period. Dermatoglyphics is the process of taking the impression of papillary ridges of fingertips for analysis 5. Papillary ridges are confined to the palms and soles and flexure surfaces of the digits. These ridges form narrow parallel or curved arrays separated by narrow furrow. The aperture of sweat ducts opens at regular intervals along the summit of each ridge.
Dermatoglyphics has been used widely in fields of anthropology, genetics, and medicine and as a valuable non-invasive diagnostic tool and early assessment of risk for certain medical conditions 6. It reflects disturbances in fetal development during early prenatal weeks 14–22 when fingerprints develop 3. Dermatoglyphic asymmetry has been used to measure developmental instability during a specific period of human fetal development. The relationship between different dermatoglyphic traits and various medical diseases have been widely evaluated, and the main hypothesis for support of this association is “if growth of the limbs is disturbed in very early fetal life, changes in the epidermal ridge configurations are likely” 3. It should be added, however, that both environmental and genetic factors do influence the development of dermatoglyphics 7.
If the growth of the limbs is disturbed in very early foetal life changes in the epidermal ridge configurations are likely. Such growth disturbances may result from environmental factors in utero, single gene mutations or chromosomal aberrations. Abnormal dermatoglyphics have been reported in many conditions resulting from the above factors. In many cases the dermatoglyphic findings are typically and specifically peculiar for particular disorders. Dermatoglyphic studies can, and have, been used in medicine as an additional diagnostic tool used to strengthen a diagnosis or diagnostic impression. The use of dermatoglyphics has the advantage of being rapid, inexpensive and the techniques used can be carried out at birth before other procedures are possible. Dermatoglyphics can be used as a screening technique for selecting patients suspected of genetic or chromosomal defects for further, more definitive studies. Detection of unusual dermatoglyphics in a particular patient may suggest a diagnosis when other manifestations of a condition are not apparent and may otherwise have been missed. Dermatoglyphic studies can also be used to verify paternity and zygosity.
Dermatoglyphic patterns can deviate from normal in a wide array of disorders 8. For example 9:
- Autosomal aneuploidy such as mongolism, trisomy 18, and trisomy 15
- Aberrations of sex chromosomes such as Turner’s syndrome, Klinefelter syndrome
- Single Gene Disorders; such as Wilson’s Disease, and Huntington’s Chorea
- Disorders with uncertain genetic transmission such as idiopathic mental retardation, congenital heart disease, psoriasis
- Exogenous influences such as thalidomide damaged infants, cerebral palsy, rubella.
Wilms’ tumor is the most common childhood renal tumor and Curró et al. 10 study showed a significantly lower incidence of radial loops and whorls in Wilms’ tumor patients. Gutjahr et al 11, showed a lower occurrence of digital arch patterns in affected cases and a slightly higher frequency of whorls in Wilms’ tumor patients. A recent systematic review revealed an association of both qualitative and quantitative dermatoglyphic traits with several kidney diseases 6. However, it was concluded that methodological issues may limit any interpretation of significant findings 6.
A lot of work has been published on the formation of the papillary ridges 12. Volar pads appear first of all in the second, third, and fourth interdigitals at about the 6th week of gestation and reach maximum size by about 12 weeks, and start regressing by the 13th week. The ridges are unalterable in the later part of pregnancy. The fetal epidermis, thin to begin with, gradually thickens through cellular proliferation and there is papillary modeling of epidermis and dermis. The ridges develop in a craniocaudal direction, those on hands being completed before those on feet. In later life also they do not change, except enlarge in size.
In 1965, Penrose 13 emphasized that in the human fetus the permanent configuration is the result of laying a carpet of parallel lines, in some way as economically as possible, over the contours presented by the fetal hand. This observation was also confirmed in nonhuman primates by Mulvihill and Smith 14. They also concluded in their study that character of fetal pad bears a relationship with final fingertips ridge patterns. According to them, fetal pads which are mound-shaped collections of mesenchymal tissue deep to the epidermis determine the ridge patterns. For example, if the fetal pad is high, the pattern formed is whorl 15.
In 1970, Popich and Smith 15 did embryological studies on crease development. They indicated that digital and palmar creases are secondary features which are related to flexion movements in the developing hand between the 7th and 14th weeks of development.
Dermatoglyphics classification
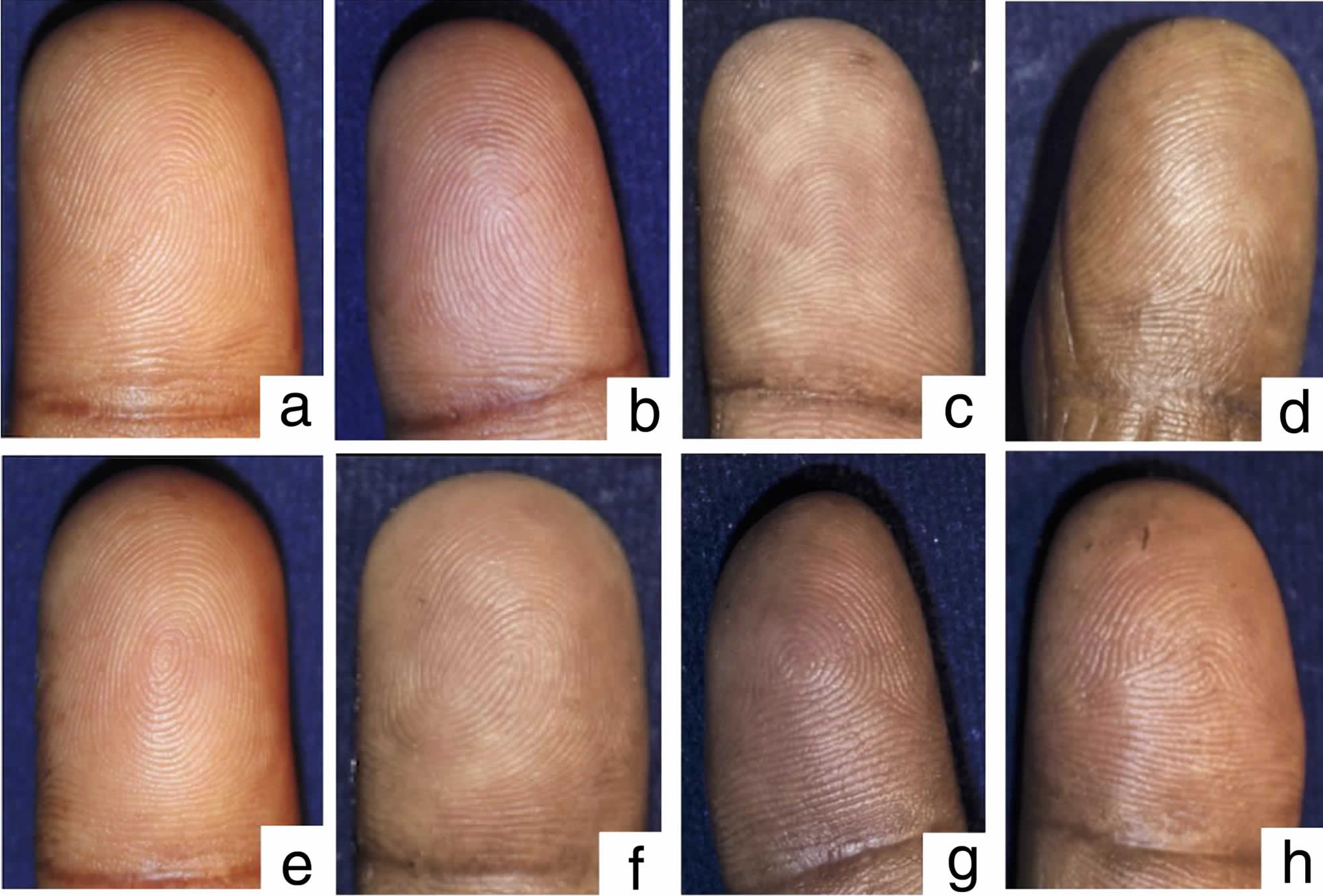
Digital fingerprint patterns were classified into eight types: ulnar loop (UL), radial loop (RL), plain arch (PA), tented arch (TA), plain whorl (PW), double loop (DL), central pocket loop (CPL), and accidental whorl (A), according to the pattern classification described by the Federal Bureau of Investigation (FBI), USA (Figure 1) 16.
Cummins and Midlo classified various pattern types on fingertips as 17:
- Arch
- Loop
- Whorl
- Composites.
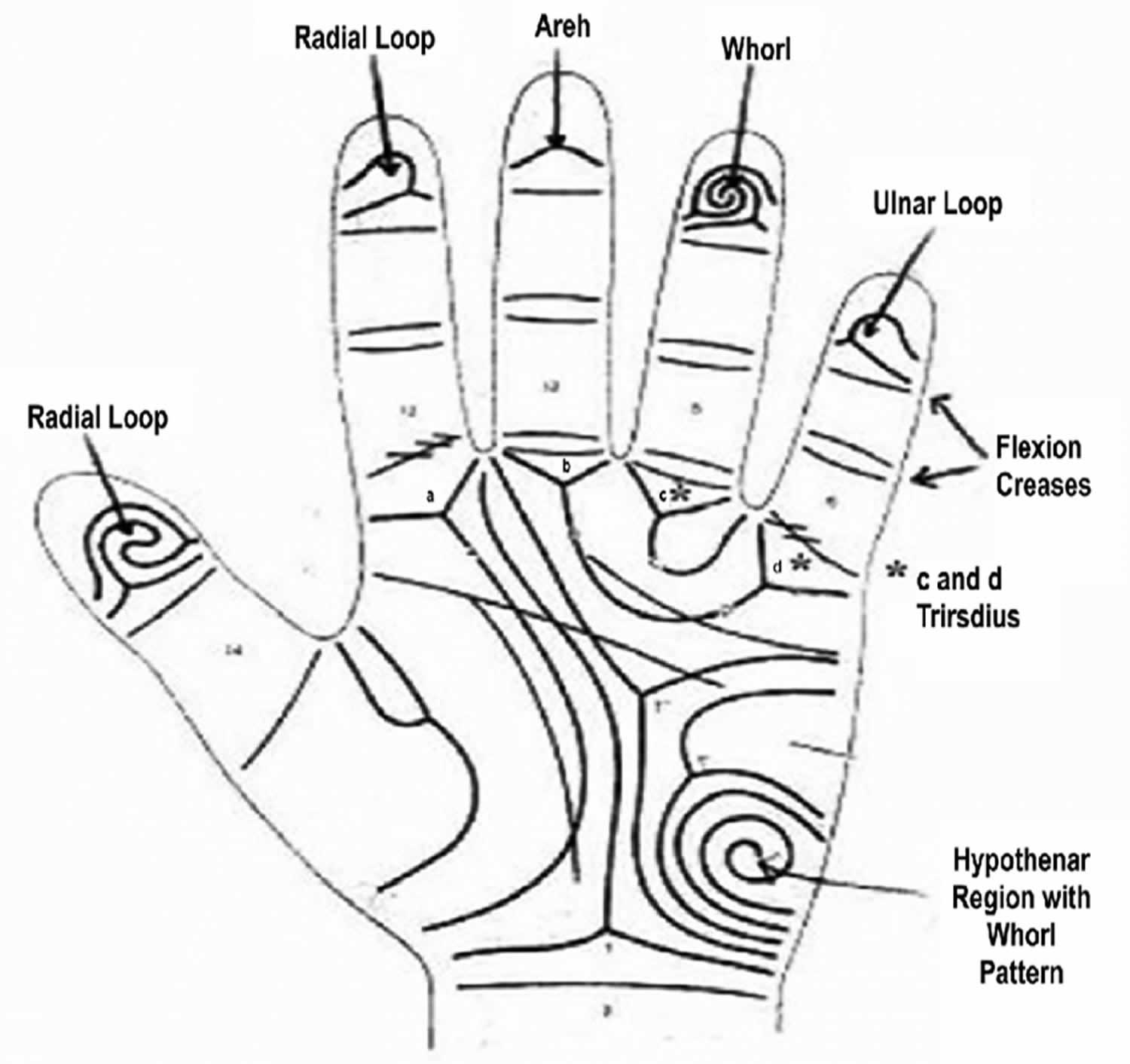
They also advocated ridge counting in biological studies and its application to various pattern types as a measure of pattern size. The palmar surface is divided into dermatoglyphic areas, which are hypothenar, thenar, and the four interdigital areas numbered I to IV. There are four digital triradii and one or two axial triradii (t) 17. A triradius (junctional area where three sets of parallel ridges meet) occurs where three ridge systems meet at a point and occurs four times on the palm, at the base of each of the four digits (a, b, c, and d). Dermatoglyphic indices include: fingertip patterns; finger ridge counts, which are the number of ridges between the center of the fingertip patterns and their corresponding triradius; palmar ridge counts, which are the number of ridges on the palm connecting two triradii; fluctuating asymmetries, which are the differences in ridge counts or pattern types between parallel structures on the left and right hands; and the ATD angle, which is the angle formed by lines drawn from the most remote triradius near the base of the palm, to triradii a and d, located close to the index and little fingers respectively. Fingerprint measuring parameters include 1) frequency of ridges in a particular pattern and 2) disposition of triradii (junctional area where three sets of parallel ridges meet). Fingerprint ridge pattern can be separated into three major types, arches, loops, and whorls. Arches have no triradii, loops have one triradii, and whorls have two or more triradii.
Uchida et al. 18, classified fingerprints into arch, loop, and whorl. Ridge count was used as a dermatoglyphic indicator. Digital triradii a, b, c, d, and axial triradius “t” were described. The position of “t” was described by measuring “atd” angle. Three major palmar flexion creases were mentioned alongwith a full or partial Simian crease present in some. They also worked on and described dermatoglyphic patterns in chromosomal abnormalities 18.
In 1971, Bali and Chaube 19 wrote on the formation of palmar creases and classified them as single radial base crease (SRBC), double radial base crease (DRBC), or triple radial base crease (TRBC).
There are three main types of fingerprint patterns:
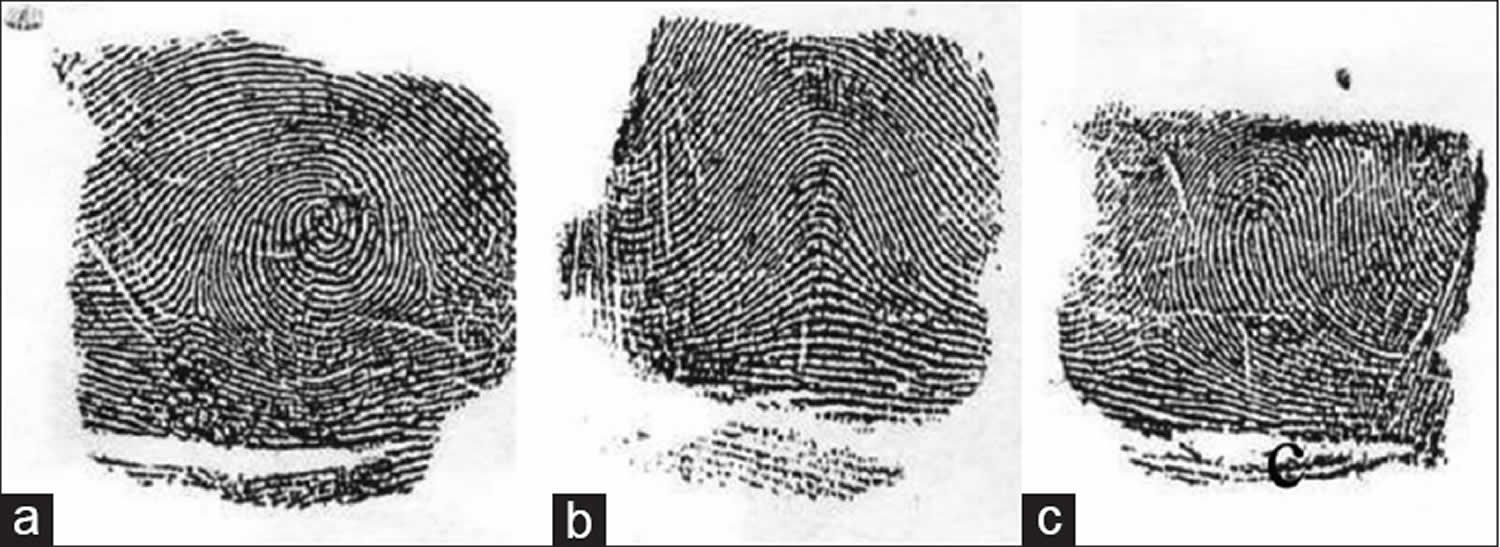
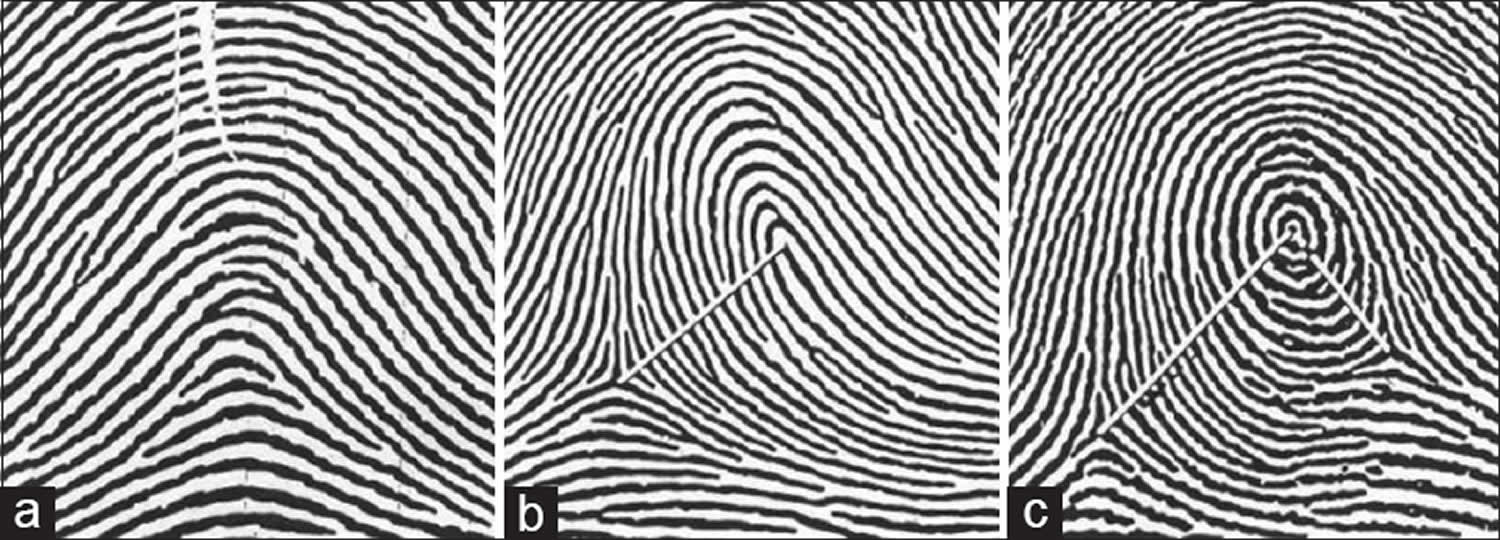
- Whorl – Whorl is distinguished by concentric design. The majority of the ridges make circuits around the core. True whorls typically possess two triradii (Figure 4A). There are also composite patterns in which two or more designs are combined in one pattern area. They have two or more triradii. They are included under whorls.
- Arch – the ridges pass from one margin of the digit to the other with a gentle, distally bowed sweep. There is no triradius (Figure 4B).
- Loop – it possesses only one triradius. The ridges curve around only one extremity of the pattern, forming the head of the loop (Figure 4C). From the opposite extremity of the pattern, ridges flow to the margin of the digit, this extremity of the pattern may thus be described as ‘open’. According to this, loops may further be of two types:
- Ulnar loop – When the loop opens to the ulnar margin
- Radial loop – When the loop opens to the radial margin.
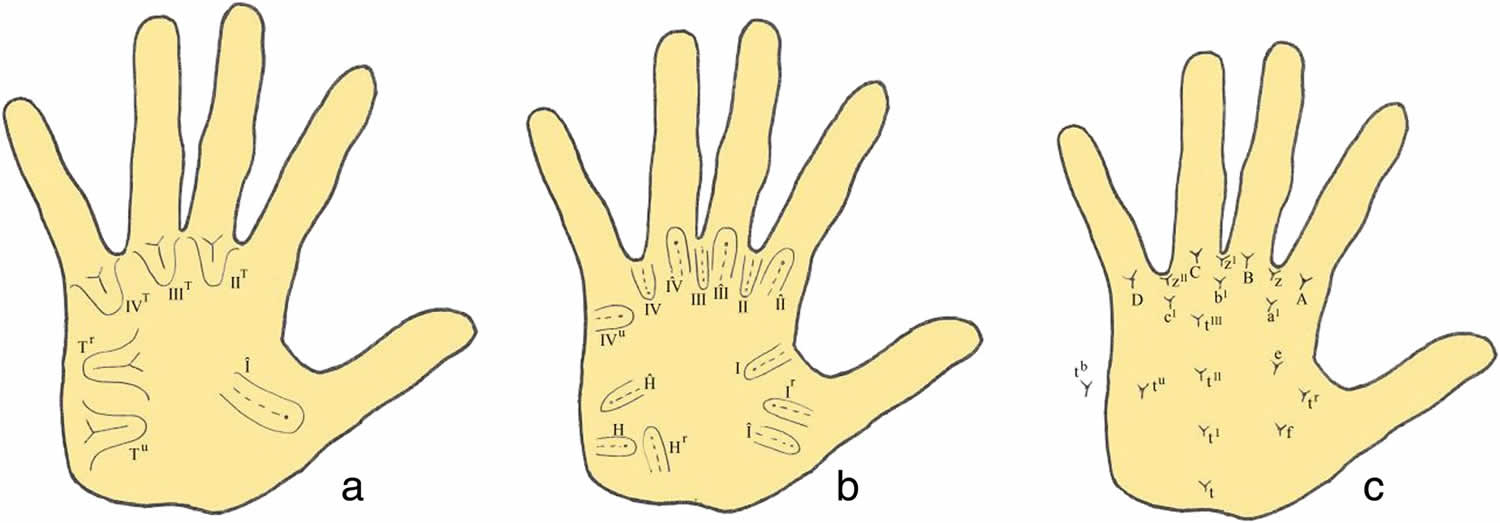
Penrose Topological palmar dermatoglyphics classification (Figure 2) 20. Loops are specified according to the configuration area in which it occurs along with the direction of its core 20. The configuration areas roughly correspond to fetal mounds 20. Loops are designated by roman numerals according to the configuration area in which they were located and to the main direction of their cores, either distal (peripheral) or proximal (central) 20. Triradii were termed according to letters of the English alphabet 20.
Open fields (O): These are configurations in which the ridges are essentially straight, and therefore, form no patterns.
Vestiges (V): They lack the sharp recurvature of ridges which distinguish true patterns. It is merely a local disarrangement of ridges.
Pattern intensity: This is the number of triradii on all the ten fingers of an individual. The value ranges from 0 to 20.
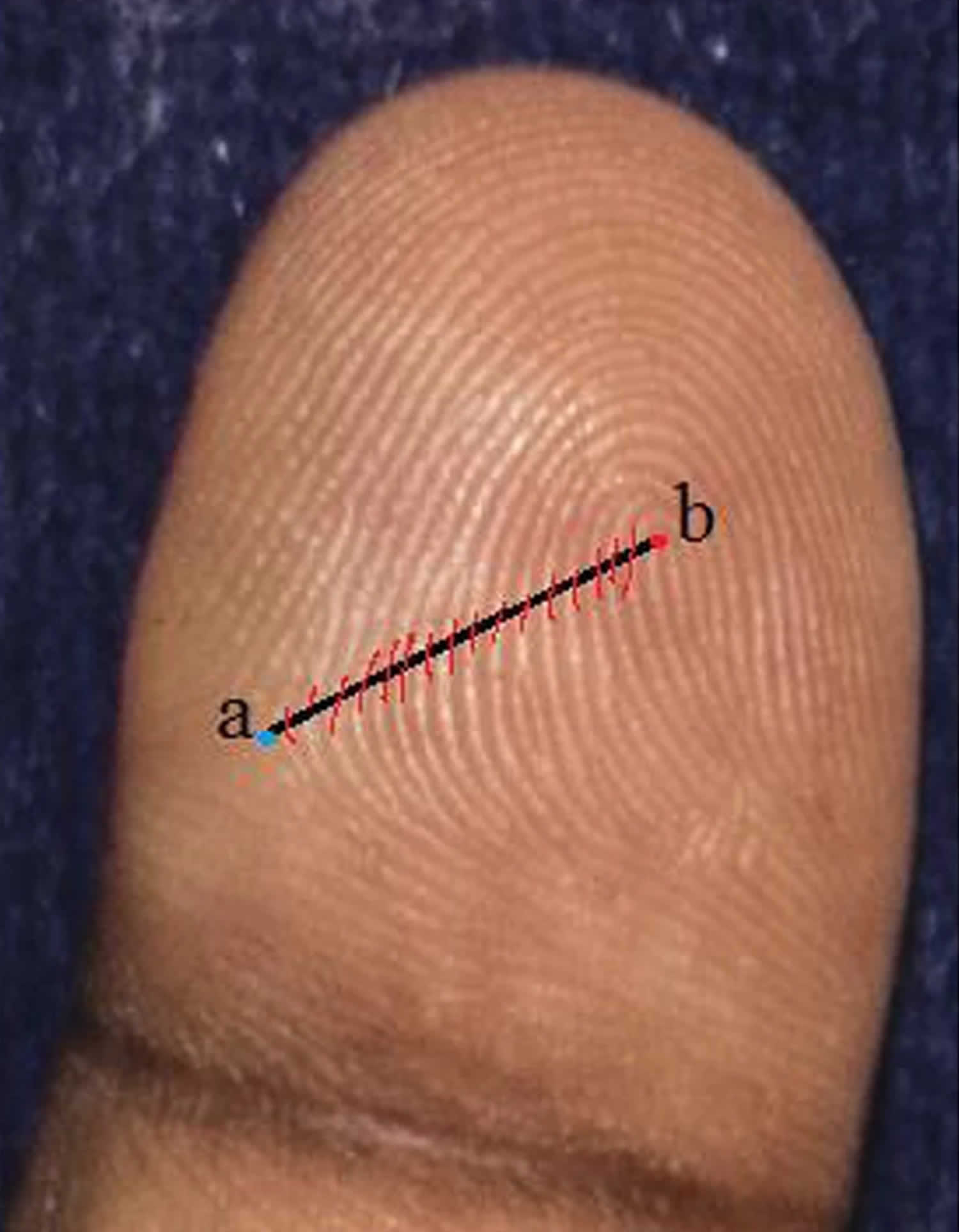
Ridge count: These are made from triradii point to point of the core. After locating the triradii point and point of the core, as outer and inner termini of the count, the line is set in position to connect them (Figures 5 and 6 below). Triradial point and print of core are not included in the count. As there are two counts in whorls, only the higher one is used. In single arches, the score is “zero.” The count on the ten fingers of each individual is then summed up to give a single value, the total ridge count.
Interdigital areas: Interdigital intervals, the clefts between digits, are numbered in sequence beginning with the interval between the thumb and index finger. The palmer surface is divisible into dermatoglyphic areas or configurational fields, which are hypothenar, thenar and the four interdigital areas numbered I to IV. Each area is a topographic unit, and there is in some palms a discrete pattern and partial boundaries formed by triradii and their radiants for each area. Characteristically, there are four “ digital triradii” located in proximal relation to the bases of digits II, III, IV, and V. In radioulnar sequence, they are named a, b, c, and d (Figure 2c). Axial triradius (t) is located at or near the proximal margin of the palms, in the interval between thenar and hypothenar eminences. The configurational area lying between digital triradii “a” and “b” is interdigital II, that between triradii “b” and “c” is interdigital III, and the area between triradii “c” and “d” is interdigital IV (Figure 2a and 2b). When a digital triradius fails or is much displaced the midpoint of the base of the corresponding digit affords a landmark separating the interdigital areas on either side. The configuration may be a true pattern (whorl or loop), a vestige or an open field. Whenever there are two patterns in an area, the one on the radial side is written first.
Palmar flexion creases: The main flexion creases-distal transverse, proximal transverse and radial longitudinal – are classified by their common point of origin as single radial base crease (SRBC), double radial base crease (DRBC) or triple radial base crease (TRBC). The double radial base crease (DRBC) can be further divided into two groups on the basis of its distal and proximal positions.
Hypothenar patterns: There are three primary true patterns in the hypothenar area: whorls, loops, and tented arches.
ATD angle: ATD angle is formed between lines drawn from the triradii at the bases of the index and little fingers to the axial triradius. The more distal the axial triradius, the larger is the angle. Positions of the axial triradii forming angles greater than 56° are designated “distal.” If more than one axial triradius is present, the most distal one is used in the analysis.
Simian line: Usually, three flexion creases are present on the palm. In some cases, however, the two distal horizontal creases are fused to form a single horizontal crease. This line is designated as a simian crease.
Figure 1. Digital fingerprint patterns
Footnote: The digital fingerprint patterns: a) Ulnar loop. b) Radial loop. c) Plain arch. d) Tented arch. e) Plain whorl. f) Double loop. g) Central pocket loop. h) Accidental whorl.
Figure 2. Palmar dermatoglyphics
Footnote: Palmar dermatoglyphics variables: a and b Loop patterns. c Triradii. Whorls are designated as “W”; loops as “L” or “l” if the pattern is small (ridge count is less than six), vestiges are ‘V’ and open fields “O.”
Figure 3. Triradii
Footnote: Whorl pattern (a) Triradii, (b) Triradii
Figure 4. Fingerprints showing different types of patterns. (a) Whorl (b) Arch (c) Loop
Ridge counting and pattern intensity
Finger ridge count was defined as the number of ridges which intersect or touch a straight line drawn from the central point of a triradius to the center or core of the adjacent pattern [11]. Two ridges that result from a bifurcation of a single epidermal ridge and both cross the straight line are counted (Figures 5 and 6). Any ridges that were close to the straight line without touching it were excluded 21. Loop patterns have one ridge count while whorls usually have two ridge counts. Arches and other similar configurations that are not true patterns have a zero ridge count. When there was a missing finger on one hand, the ridge count on the corresponding finger of the other hand was inserted based on the considerable symmetry for this trait 21. The sum of the largest ridge count on all ten fingers was defined as total ridge count (TRC) 21. On the palm, the number of ridges that crossed a straight line connecting triradii “A” and “B” was defined as the A-B ridge count (A-B RC) 21. The pattern intensity index (PII) was calculated using the formula: PII = {(2 × % whorl + % loop) ÷ 10} 22. Pattern intensity index (PII) essentially refers to the complexity of finger patterns in a specified population 23.
Figure 5. Finger ridge counting
Footnote: a) Triradius. b) Core
Figure 6. Fingerprint ridge count
Footnote: Magnified views of three basic patterns (a) No line of count in arch pattern. Ridge count score is zero. (b) White line joining centre of pattern to point of tri-radius. The number of ridges cutting the line is 13. (c) Ridge count on the left of the pattern is 17, ridge count on the right is 8
Abnormal dermatoglyphics
In clinical medicine, chromosomal anomalies such as the trisomy 13 (Patau’s syndrome), trisomy 18 (Edwards’ syndrome), trisomy 21 (Down’s syndrome) and the sex chromosomes (Turner’s syndrome X0 and Kleinfelter’s syndrome 47, XXY) and deletion of the short arm of chromosome 5 (Cri du Chat syndrome) are recognized as having abnormal dermatoglyphic patterns 24. Differences in fingerprint pattern frequencies from normal controls have also been found in leukemia 25, early onset diabetes mellitus 26, alopecia areata, atopic dermatitis 27, rubella embryopathy 28 and chronic intestinal pseudo-obstruction 29. These observations suggested that hereditary or environmental factors acting in early gestationmay have played a role in the genesis of the disease. An examination of dermatoglyphic patterns and blood pressure in an adult population concluded that fingertip whorls and a narrow palmar angle are important markers of impaired fetal development at different stages of pregnancy and that both were associated with raised blood pressure in adult life 30.
Abnormalities in the growth process which are liable to distort the alignment of dermal ridges may result from the action of abnormal genes, chromosomal aberrations, even from poisoning by a drugor from a viral infection. In some cases the cause remains unknown 31.
The characteristic patterns in an individual that deviate from the norm must be caused by the changes occurring before the completion of the fourth fetal month. Since epidermal ridge patterns form early in fetal development and remain unchanged throughout life, unusual dermatoglyphicmay indicate gene or chromosomal abnormalities consistent with a disease such as rheumatoid arthritis 32. On the right digits of males there was no significant difference in the digital patterns between idiopathic dilated cardiomyopathy and normal subjects while on the left hand, the idiopathic dilated cardiomyopathy patients showed asignificantly higher whorl pattern on the first digit than that found in the normal subjects 33.
The fingertip patterns of the patients of periodontitis were compared with those of healthy individuals.There was decreased frequencies of twinned and transversal ulnar loops on all fingers of the patients with juvenile periodontitis and a decreased frequency of double loops on all fingers and an increased frequency of radial loops on the right second digits of the patients with rapidly progressive periodontitis 34. There were increased frequencies of concentric whorls and transversal ulnar loops on all fingers of the patients with adult periodontitis and an increased frequency of triradii on the palms of the patients with juvenile periodontitis. There was anincreased frequencyof IV and H loops and tb triradii on the palms of the patients with rapidly progressive periodontitis and an increased frequency of triradiion the soles of the patients with juvenile periodontitis were found 35.
In hypospadias patients there is a significant decrease of patterns in the hypothenar area and an increase of radial arches in hypothenar area, while for other characteristics the differences are always not significant 36. Some significant distortions inducing deep clinical implications were found in epileptics 37.
True palmar patterns were increased significantly in both the sexes on all palmer areas except interdigital areas in males and thenar areas in females in autoimmune disease 34. The distal displacement of axial triradii was increased in both the sexes. The total finger ridge counts were increased significantly in both the sexes 38. No significant quantitative or qualitative differences were found between the dermatoglyphic features of asthmatic patients and those of a healthy population, except for punctate interruptions of the skin ridges that indicate pitting, a well-known manifestation of Darier’s disease 39. Whorls are highly significant statistically in both generations bronchial asthma patients as compared to control 40. Characteristic dermatoglyphic changes rank high in frequency among the variable stigmata which go to make up the syndrome of mongolism. Though they are not diagnostic alone, it is believed that these objectives, measurable and unchanging evidences of mongolism seen in handprints should be helpful in establishing an early diagnosis 41. Higher frequency of low endings of line A on both hands, and–on the left hand–significantly more patterns in the fourth interdigital area and fewer patterns in the third interdigital area. There was no association between these dermatoglyphic features and the HLA antigens (B8 and DRw3) which occurred most frequently in Systemic Lupus Erythematosus (SLE) patients 42. Higher frequency of whorls and a lower frequency of ulnar loops in the dermatoglyphic pattern in celiac disease 43.
Dermatoglyphics in autism
Several studies have evaluated the association abnormal dermatoglyphics and the risk of autism. In 2013, Stošljević et al. 44 studied 182 boys with autism and 182 healthy men and reported significant differences in the distribution of both arch and whorl patterns between the patients and controls. In another study performed by Arrieta et al. 45 in Spain, children with autism displayed a significantly greater number of loop patterns and a significantly fewer number of whorl patterns than healthy controls.
In 1990, Wolman et al. 46 in America studied finger ridges in a group of 95 individuals with autism and compared the findings with those in a control group. Their findings revealed that there was no significant association with the different patterns of finger ridges between the patients and the controls. In 2003 Milicic et al. 47 in Croatia observed a significant difference in the ridge count of fingertips and palms between patients with autism and healthy controls. This significant difference was also noticed between the family members of the patients and those of the healthy controls. In another study performed in 1979 in Australia, a group of 32 children with autism and a control group of 32 healthy subjects were examined. Hartin et al. 48 established a significant difference in the distribution of dermal patterns and that of finger ridges between the 2 groups.
In a 2017 study 49, demonstrated significant differences in the loop pattern distribution of the left index finger and the arch and loop patterns of the left thumb between control subjects and patients with autism. The mean ridge counts were found to be higher in the control group for both fingers on both hands (left thumb and other fingers). No significant difference was observed between the 2 groups in the distribution of the loop, arch, and whorl patterns on the thumb and index finger of the right hand, whereas a nonsignificant difference was observed between the 2 groups in the loop pattern distribution of the index finger on the left hand 49. Furthermore, arch and loop each had a significantly higher frequency in the left thumb of the control and patient groups, respectively 49. In light of the statistical results obtained and the relationship between the distribution of the dermatoglyphic patterns along with significant differences in the ridge count between the case and control groups, it can be concluded that the patterns are associated with the risk of autism 49. However, further investigations with more informative data on the association between the genetic background of dermatoglyphic patterns and autism will help establish these patterns as a valuable diagnostic approach.
- Wijerathne, B.T.B., Meier, R.J., Salgado, S.S. et al. Qualitative and quantitative dermatoglyphics of chronic kidney disease of unknown origin (CKDu) in Sri Lanka. J Physiol Anthropol 39, 1 (2020). https://doi.org/10.1186/s40101-019-0207-0[↩]
- Gupta, R. K., & Gupta, A. K. (2013). New, Easy and Effective Method to Take Dermatog-lyphic Prints.National Journal of Medical Research, 3, 45-47.[↩]
- Blackwell D. A dermatoglyphic investigation of selected skin disorders: Durham University; 1994. http://etheses.dur.ac.uk/5536[↩][↩][↩]
- Schaumann BA, Alter M. Dermatoglyphics in medical disorders. 1st ed. Berlin, Heidelberg: Springer-Verlag; 1976. https://doi.org/10.1007/978-3-642-51620-7.[↩]
- Abue, Andrew & Christopher, Rose & Sunday, Adebisi. (2018). The Qualitative Dermatoglyphics PATTERNS in Both Hands for Males and Females in Ubang Clan Cross River State Nigeria. Advances in Anthropology. 08. 73-81. 10.4236/aa.2018.82004[↩]
- Wijerathne BTB, Meier RJ, Salgado SS, Agampodi SB. Dermatoglyphics in kidney diseases: a review. Springerplus. 2016;5:290. https://doi.org/10.1186/s40064-016-1783-7[↩][↩][↩]
- King S, Mancini-Marïe A, Brunet A, Walker E, Meaney MJ, Laplante DP. Prenatal maternal stress from a natural disaster predicts dermatoglyphic asymmetry in humans. Dev Psychopathol. 2009;21:343–53. https://doi.org/10.1017/S0954579409000364[↩]
- Alter M. Dermatoglyphic analysis as a diagnostic tool. Medicine (Baltimore) 1967;46:35-56.[↩]
- Sharma A, Sood V, Singh P, Sharma A. Dermatoglyphics: A review on fingerprints and their changing trends of use. CHRISMED J Health Res 2018;5:167-72. http://www.cjhr.org/temp/CHRISMEDJHealthRes53167-4669663_125816.pdf[↩]
- Curró V, Mastroiacovo P, Castello M, Romagnoli C, Mastrangelo R, Segni G. Palmar dermatoglyphics in Wilms’ tumor. Prog Clin Biol Res. 1982;84:385–91 http://www.ncbi.nlm.nih.gov/pubmed/6285387[↩]
- Gutjahr P, Wolffram T, Emmrich P. Dermatoglyphische Untersuchungen bei Kindern mit embryonalen Tumoren. Z Kinderheilkd. 1975;120:101–10. https://doi.org/10.1007/BF00445159[↩]
- Penrose LS. Dermatoglyphics. Sci Am 1969;221:72-84.[↩]
- Penrose LS. Dermatoglyphic topology. Nature 1965;205:544-6.[↩]
- Mulvihill JJ, Smith DW. The genesis of dermatoglyphics. J Pediatr 1969;75:579-89[↩]
- Popich GA, Smith DW. The genesis and significance of digital and palmar hand creases: Preliminary report. J Pediatr 1970;77:1017-23.[↩][↩]
- United States. Federal Bureau of Investigation. United States Department of Justice: The Science of Fingerprints Classification and Uses; 2006.[↩]
- Cummins H, Midlo C. Fingerprints, Palms and Soles: An Introduction to Dermatoglyphics. New York: Dover Publication Inc.; 1961.[↩][↩]
- Uchida AI, Soltan HC. Evaluation of dermatoglyphics in medical genetics. Pediatr Clin N Am 1963;10:409-22.[↩][↩]
- Bali RS, Chaube R. On the formulation of palmer creases. Z Morph Anthrop 1971;63:121-30.[↩]
- Penrose LS, Loesch D. Topological classification of palmar dermatoglyphics. J Ment Defic Res. 1970;14:111–28. https://doi.org/10.1111/j.1365-2788.1970.tb01106.x[↩][↩][↩][↩][↩]
- Loesch DZ. Quantitative dermatoglyphics: classification, genetics, and pathology. London: Oxford University Press; 1983.[↩][↩][↩][↩]
- Wijerathne BTB, Rathnayake GK, Adikari SC, Amarasinghe S, Abhayarathna PL, Jayasena AS. Sexual dimorphism in digital dermatoglyphic traits among Sinhalese people in Sri Lanka. J Physiol Anthropol. 2013;32:27. https://doi.org/10.1186/1880-6805-32-27[↩]
- Schaumann BA, Alter M. Dermatoglyphics in medical disorders. 1st ed. Berlin, Heidelberg: Springer-Verlag; 1976. https://doi.org/10.1007/978-3-642-51620-7[↩]
- Stough TR and Seely JR (1969). Dermatoglyphics in medicine. Clinical Pediatrics832–41.[↩]
- Verbov JL (1970). Dermatoglyphs in leukaemia.Journal of Medical Genetics 7125–31.[↩]
- Shield JPH, Wadsworth EJK, Hobbs K and Baum JD (1995). Dermatoglyphics, fetal growth, and insulin-dependent diabetes in children under 5 years. Archives of Disease in Childhood 72159–60.[↩]
- Vera M, Cabrera E and Guell R (1995). Dermatoglyphics in insulin dependent diabetic patients with limited joint mobility. Acta Diabetologica 3278–81.[↩]
- Purvis-Smith SG (1968). Dermatoglyphics in adults with congenital rubella. Lancet 2141–3.[↩]
- Pulliam TJ and Schuster MM (1995).Congenital markers for chronic intestinal obstruction. American Journal of Gastroenterology 90922–6.[↩]
- Godfrey KM, Barker DJP, Peace J, Cloke J andOsmond C (1993). Relation of fingerprints and shape of the palm to fetal growth and adult blood pressure. BMJ 307405–8.[↩]
- Holt SB and Penrose LS (1968). The genetics of dermal ridges. Springfield, IL: Charles C Thomas.[↩]
- Sayee Rajangam, Roopa Ravindranth, Shubha RS, Nagesh HV and Job Johnson (2008). Dermatoglyphics-Quantitative Analysis in Rheumatoid Arthritis. Kamla-Raj, Anthropologist 10(3) 233-235.[↩]
- Oladipo GS, Olotu EJ, Fawehinmi HB, Okoh PD and Iboroma AD (2007). Dermatoglyphics in idiopathic (primary) dilated cardiomyopathy in South,Southern Nigeria Scientific Research and Essay 2(10) 416-420.[↩]
- Kaur, Jeewandeep & Pal, Arvinder & Pal Singh Batra, Arvinder. (2013). ROLE OF DERMATOGLYPHICS IN MEDICAL DISORDERS. Indian Journal of Fundamental and Applied Life Sciences. 3. 536-539.[↩][↩]
- Atasu M, Kuru B, Firatli E and Meric H (2005). Dermatoglyphic findings in periodontal diseases, International Journal of Anthropology 20(1-2)63-75.[↩]
- Floris G (1993). Dermatoglyphics in cases of hypospadias: New data. International Journal of Anthropology 8(1)39-41.[↩]
- Ţarcal A and Barabolski C(2002). Contribution to dermatoglyphic diagnosis of epilepsy. The Journal of Preventive Medicine 10(2) 28-34.[↩]
- Singh PK, Pandey SS and Singh G (1987). Dermatoglyphics in auto-immune dermatoses. Indian Journal of Dermatology 32(1) 15-8.[↩]
- Lubovitz OS, Trattner A, Katznelson MBM and Sandbank M (2007). Dermatoglyphics in Darters disease. International Journal of Dermatology 33(9) 626–627.[↩]
- Gupta UK and Prakash S (2003). Dermatoglyphics: a study of finger tip patterns in bronchial asthma and its genetic disposition. Kathmandu University Medical Journal 1(4) 267-271.[↩]
- Cummins H, Talley C and Platou RV (1950). Palmar Dermatoglyphics in Mongolisms. Pediatrics 5(2) 241-248[↩]
- Vormittag W, Weninger M, Scherak O and Kolarz G(1981). Dermatoglyphics and Systemic Lupus Erythematosus. Scandinavian Journal of Rheumatology10(4) 296-298.[↩]
- Tahan S, Medeiros EH and Wehba J (1997). Dermatoglyphic patterns in celiac disease [Article in Portuguese] Arquivos de Gastroenterologia 34(3)196-204.[↩]
- Stosljevic M, Adamovic M. Dermatoglyphic characteristics of digito-palmar complex in autistic boys in serbia. Vojnosanit Pregl. 2013;70:386–90. doi: 10.2298/VSP1304386S[↩]
- Arrieta MI. Dermatoglyphic analysis of autistic Basque children. Am J Med Genet. 1991;41:533. doi: 10.1002/ajmg.1320410433[↩]
- Wolman SR, Campbell M, Marchi ML, Deutsch SI, Gershon TD. Dermatoglyphic study in autistic children and controls. J Am Acad Child Adolesc Psychiatry. 1990;29:878–84. doi: 10.1097/00004583-199011000-00006[↩]
- Milicic J, Bujas Petkovic Z, Bozikov J. Dermatoglyphs of digito-palmar complex in autistic disorder: family analysis. Croat Med J. 2003;44:469–76.[↩]
- Hartin PJ, Barry RJ. A comparative dermatoglyphic study of autistic, retarded, and normal children. J Autism Dev Disord. 1979;9:233–46.[↩]
- Kazemi M, Fayyazi-Bordbar MR, Mahdavi-Shahri N. Comparative Dermatoglyphic Study between Autistic Patients and Normal People in Iran. Iran J Med Sci. 2017;42(4):392-396. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523047[↩][↩][↩][↩]