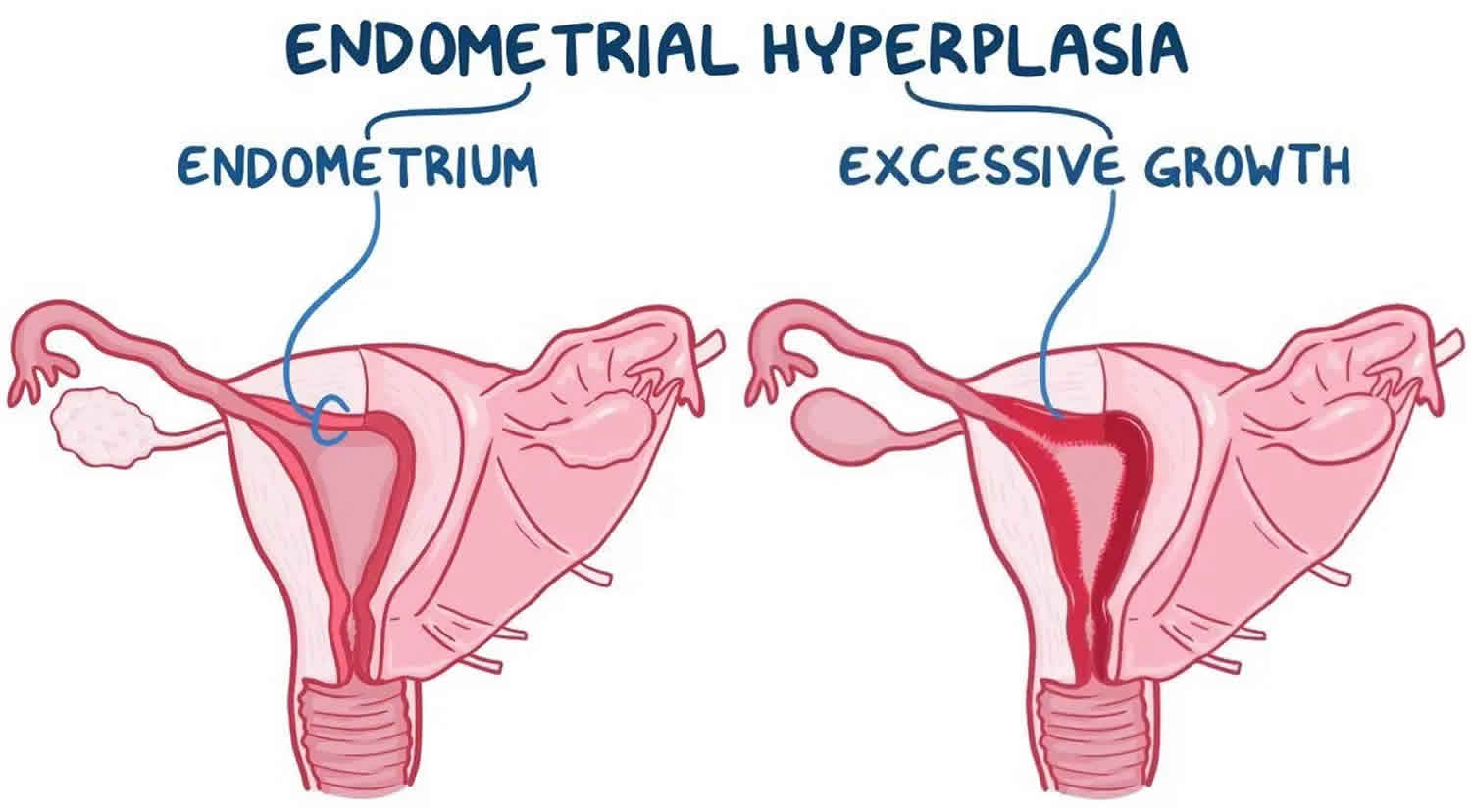
Endometrial hyperplasia
Endometrial hyperplasia is a common condition in which the endometrium (lining of the uterus) is abnormally thick. The endometrium is the lining of the uterus. The uterus is part of the female reproductive system. It is a hollow, pear-shaped, muscular organ in the pelvis, where a fetus grows. Endometrial hyperplasia is defined as a proliferation of endometrial glands of irregular size and shape that results in a greater than normal gland-to-stroma ratio 1. Endometrial hyperplasia is caused by too much exposure to exogenous or endogenous estrogen along with a relative deficiency of progesterone 2. Both estrogen and progesterone hormones play roles in the menstrual cycle. Estrogen makes the cells grow, while progesterone signals the shedding of the cells. A hormonal imbalance estrogen and progesterone hormones in can produce too many cells or abnormal cells. Endometrial hyperplasia is not cancer, but in some cases, if it’s not treated, has the propensity to develop into endometrial cancer or cancer of the uterus 3. Endometrial hyperplasia is believed to be a precursor of cancer of the uterus or endometrial carcinoma, which is the most common gynecologic cancer and the fourth most common cancer in women in the United States 4.
Endometrial hyperplasia is most frequently diagnosed in postmenopausal women, but women of any age can be at risk if they are exposed to a source of unopposed estrogen 5. Endometrial hyperplasia is increasingly seen in young women with chronic anovulation due to polycystic ovary syndrome (PCOS) or obesity 6.
Endometrial hyperplasia usually occurs after menopause, when ovulation stops and progesterone is no longer made 7. Endometrial hyperplasia can also develop during perimenopause, when ovulation may not occur regularly. There may be high levels of estrogen and not enough progesterone in other situations, including when a woman 7:
- uses medications that act like estrogen, such as tamoxifen for cancer treatment
- uses estrogen for hormone therapy and does not use progesterone or progestin if she still has a uterus
- has irregular menstrual periods, especially associated with polycystic ovary syndrome (PCOS) or infertility
- has obesity – body mass index (BMI) of 27 or more
The main symptom of endometrial hyperplasia is abnormal menstrual bleeding. However, abnormal uterine bleeding can be a symptom for many things. Your doctor can perform an exam and tests to diagnose the cause of the abnormal uterine bleeding. A transvaginal ultrasound measures your endometrium. It uses sound waves to see if the layer is average or too thick. A thick layer can indicate endometrial hyperplasia. Endometrial hyperplasia is typically diagnosed by endometrial biopsy or curettage when a woman is noted as suffering from abnormal uterine bleeding 8. Your doctor will take a biopsy of your endometrium cells to determine if cancer is present.
Treatment options for endometrial hyperplasia depend on what type you have. The most common treatment is progestin. This can be taken in several forms, including pill, shot, vaginal cream, or intrauterine device.
Atypical types of endometrial hyperplasia, especially complex, increase your risk of getting cancer. If you have these types, you might consider a hysterectomy. This is a surgery to remove your uterus. Doctors recommend this if you no longer want to become pregnant.
There are also a number of more conservative treatments for younger women who do not wish to have a hysterectomy. Your doctor will help you decide which treatment option is best for you.
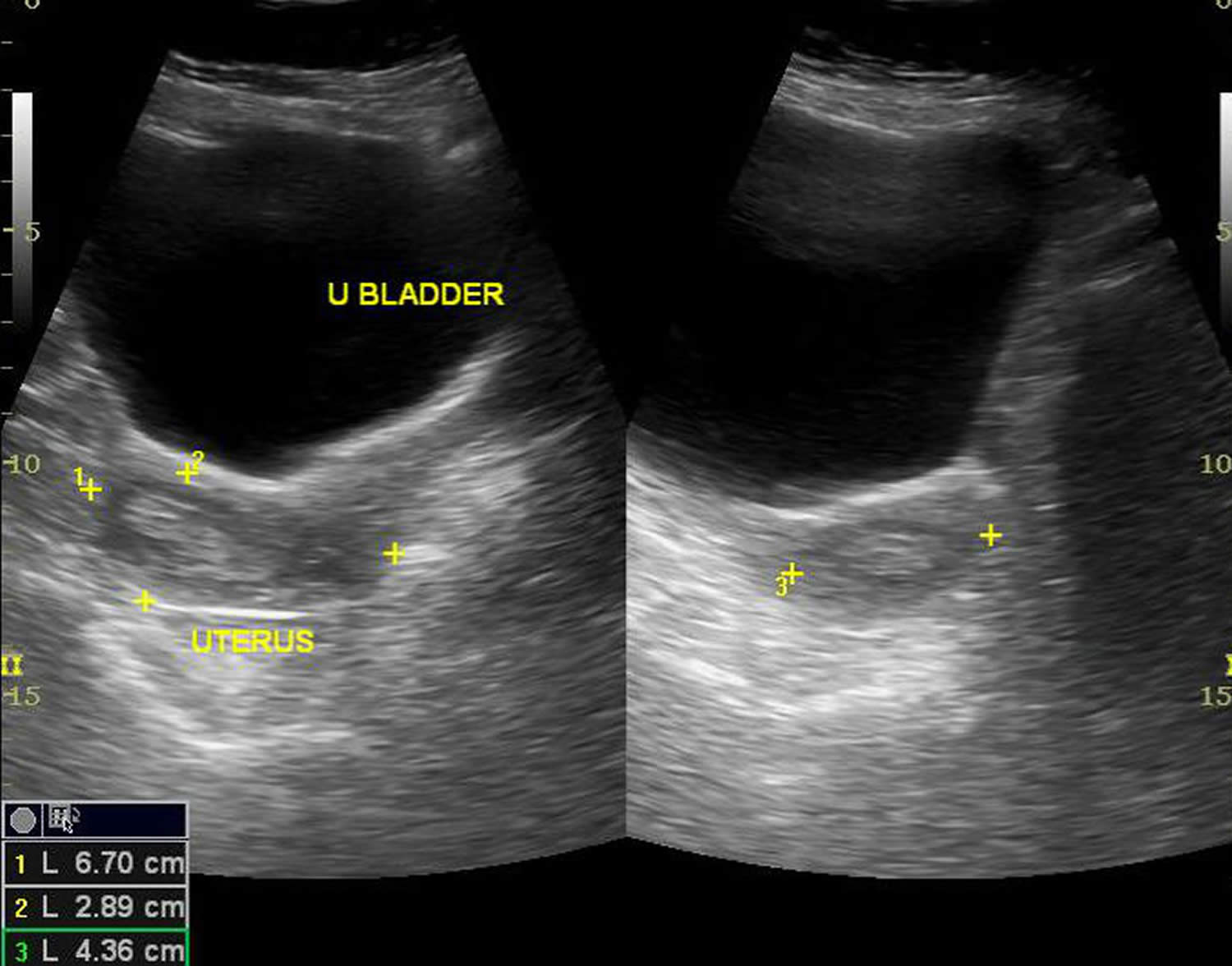
Figure 1. Endometrial hyperplasia ultrasound
Footnote: Endometrial thickness is 12 mm. Multiple cystic spaces are noted within. Solid areas are echogenic. Margins of endometrial lining was well-defined. No significant flow signals seen. Myometrium does not show focal lesion. Either of ovaries could not be localized.
[Source 9 ]Uterus and endometrium
The uterus is a hollow, muscular organ shaped somewhat like an inverted pear. The uterus receives the embryo that develops from an oocyte fertilized in the uterine tube, and sustains its development.
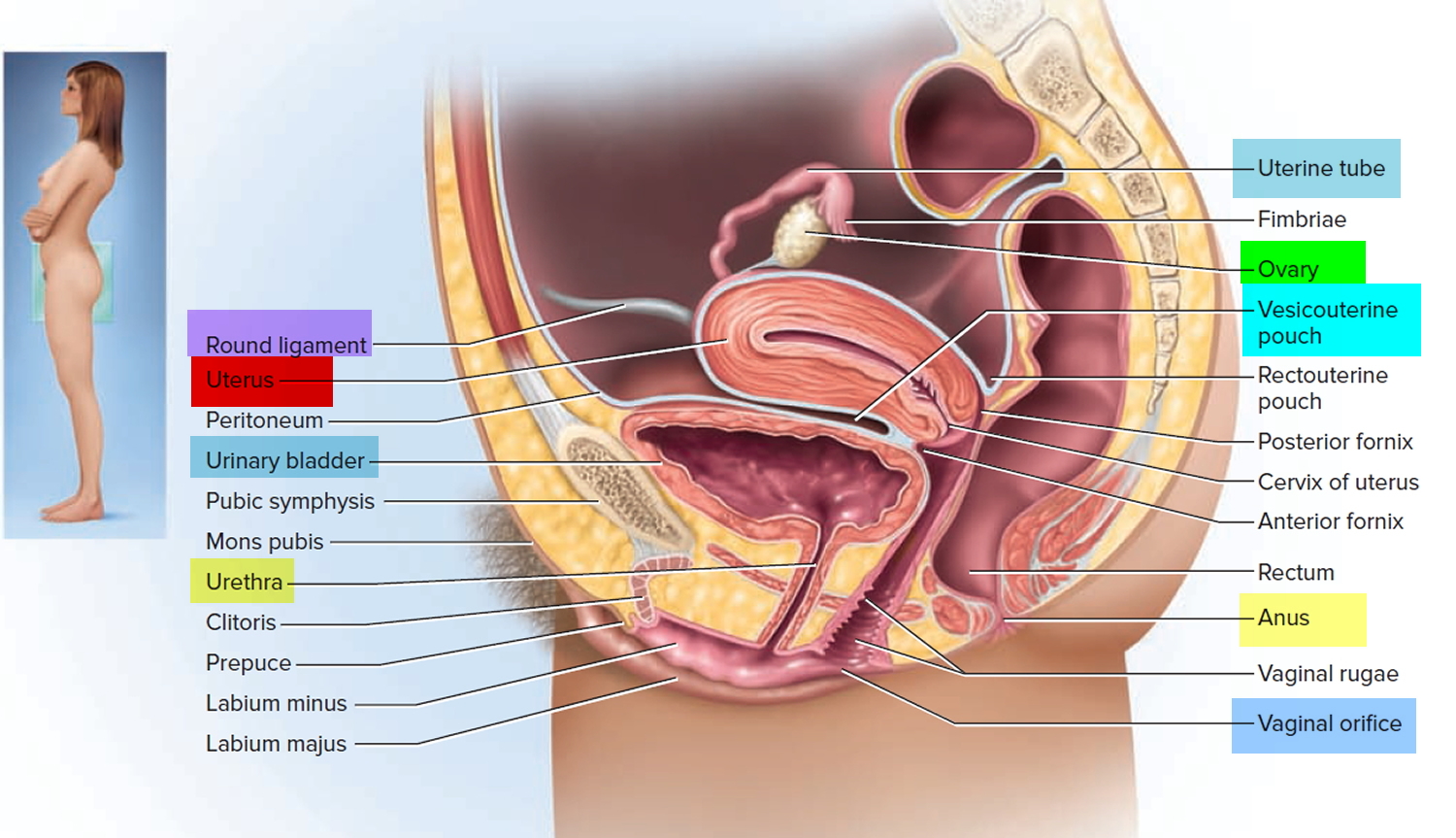
In its nonpregnant, adult state, the uterus is about 7 centimeters long, 5 centimeters wide (at its broadest point), and 2.5 centimeters in diameter. The size of the uterus changes greatly during pregnancy and it is somewhat larger in women who have been pregnant. The uterus is located medially in the anterior part of the pelvic cavity, superior to the vagina, and usually bends forward over the urinary bladder (see Figure 4).
The uterus has 2 main parts:
- The upper part of the uterus is called the body or the corpus. Corpus is the Latin word for body.
- The cervix is the lower end of the uterus that joins it to the vagina.
When people talk about cancer of the uterus, they usually mean cancers that start in the body of the uterus, not the cervix. Cervical cancer is a separate kind of cancer.
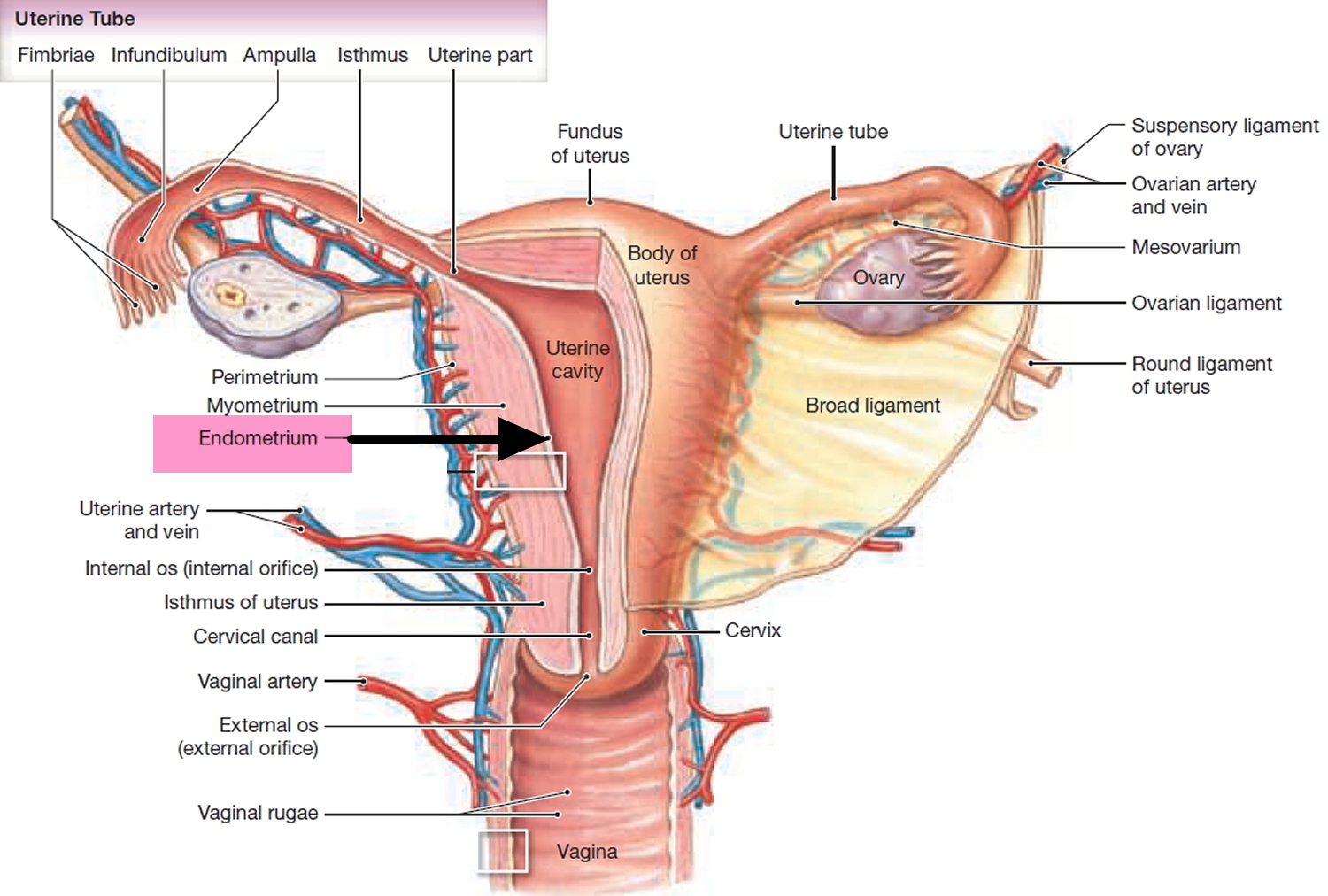
The upper two-thirds or body (corpus), of the uterus has a domeshaped top called the fundus (see Figure 2). The uterine tubes (also called Fallopian tubes) connect at the upper lateral edges of the uterus. The lower third of the uterus is called the cervix. This tubular part extends downward into the upper part of the vagina. The cervix surrounds the opening called the cervical orifice, through which the uterus opens to the vagina.
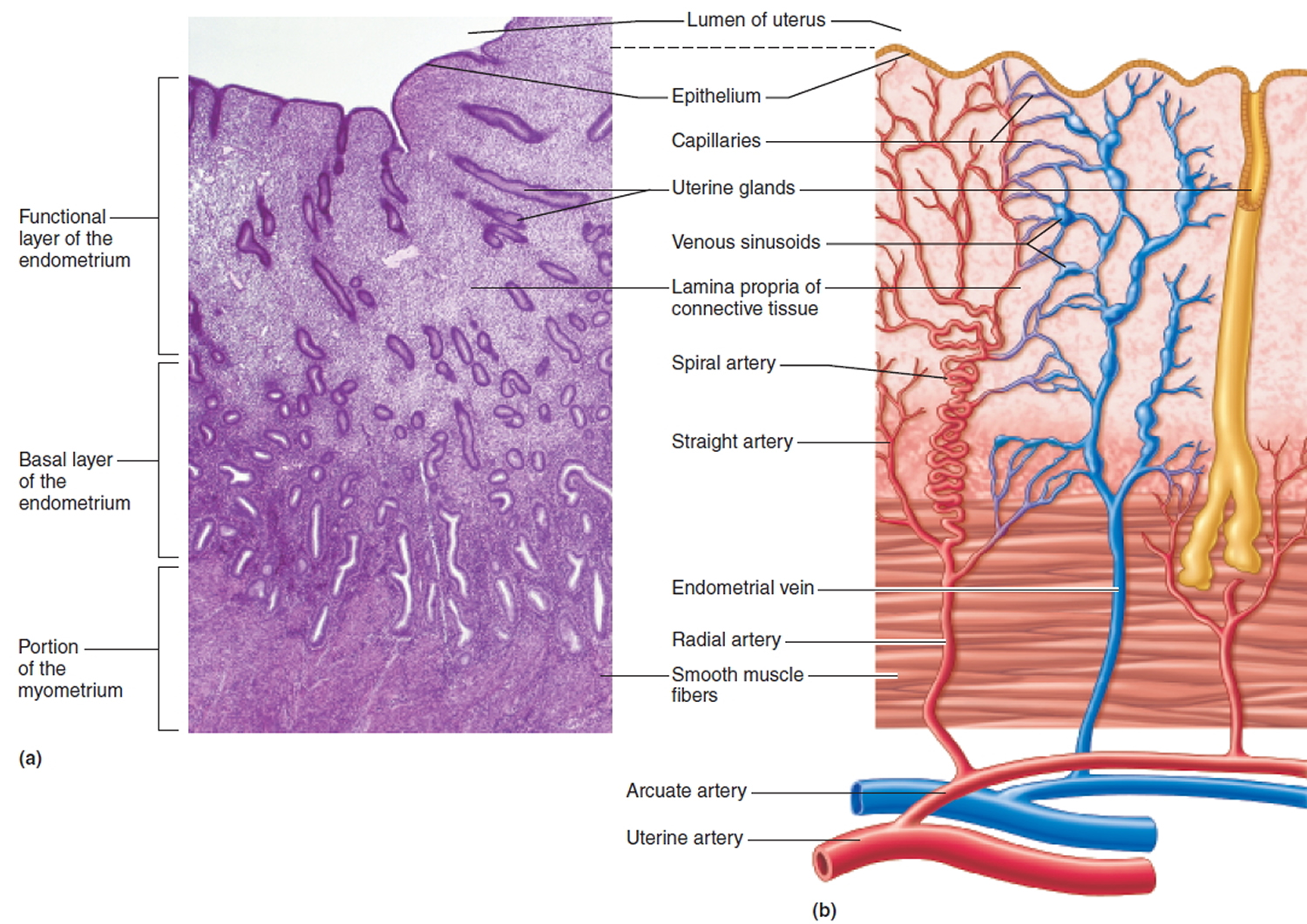
The uterine wall is thick and has 3 layers (Figure 2):
- The endometrium, the inner mucosal layer, is covered with columnar epithelium and contains abundant tubular glands (see Figure 3). During a woman’s menstrual cycle, hormones cause the endometrium to change. Estrogen causes the endometrium to thicken so that it could nourish an embryo if pregnancy occurs. If there is no pregnancy, estrogen is produced in lower amounts and more of the hormone called progesterone is made. This causes the endometrial lining to shed from the uterus and become the menstrual flow (period). This cycle repeats until menopause.
- The myometrium, a thick, middle, muscular layer, consists largely of bundles of smooth muscle cells. This thick layer of muscle is needed to push the baby out during birth. During the monthly female menstrual cycles and during pregnancy, the endometrium and myometrium change extensively.
- The perimetrium also called the serosa consists of an outer serosal layer, which covers the body of the uterus and part of the cervix.
During a woman’s menstrual cycle, hormones cause the endometrium to change. During the early part of the cycle, before the ovaries release an egg (ovulation), the ovaries produce hormones called estrogens. Estrogen causes the endometrium to thicken so that it could nourish an embryo if pregnancy occurs. If there is no pregnancy, estrogen is produced in lower amounts and more of the hormone called progesterone is made after ovulation. This prepares the innermost layer of the lining to shed. By the end of the cycle, the endometrial lining is shed from the uterus and becomes the menstrual flow (period). This cycle repeats until the woman’s goes through menopause (change of life).
The uterus is supported by the muscular floor of the pelvis and folds of peritoneum that form supportive ligaments around the organ, as they do for the ovary and uterine tube. The broad ligament has two parts: the mesosalpinx mentioned earlier and the mesometrium on each side of the uterus. The cervix and superior part of the vagina are supported by cardinal (lateral cervical) ligaments extending to the pelvic wall. A pair of uterosacral ligaments attaches the posterior side of the uterus to the sacrum, and a pair of round ligaments arises from the anterior surface of the uterus, passes through the inguinal canals, and terminates in the labia majora.
As the peritoneum folds around the various pelvic organs, it creates several dead-end recesses and pouches (extensions of the peritoneal cavity). Two major ones are the vesicouterine pouch, which forms the space between the uterus and urinary bladder, and rectouterine pouch between the uterus and rectum (see Figure 3).
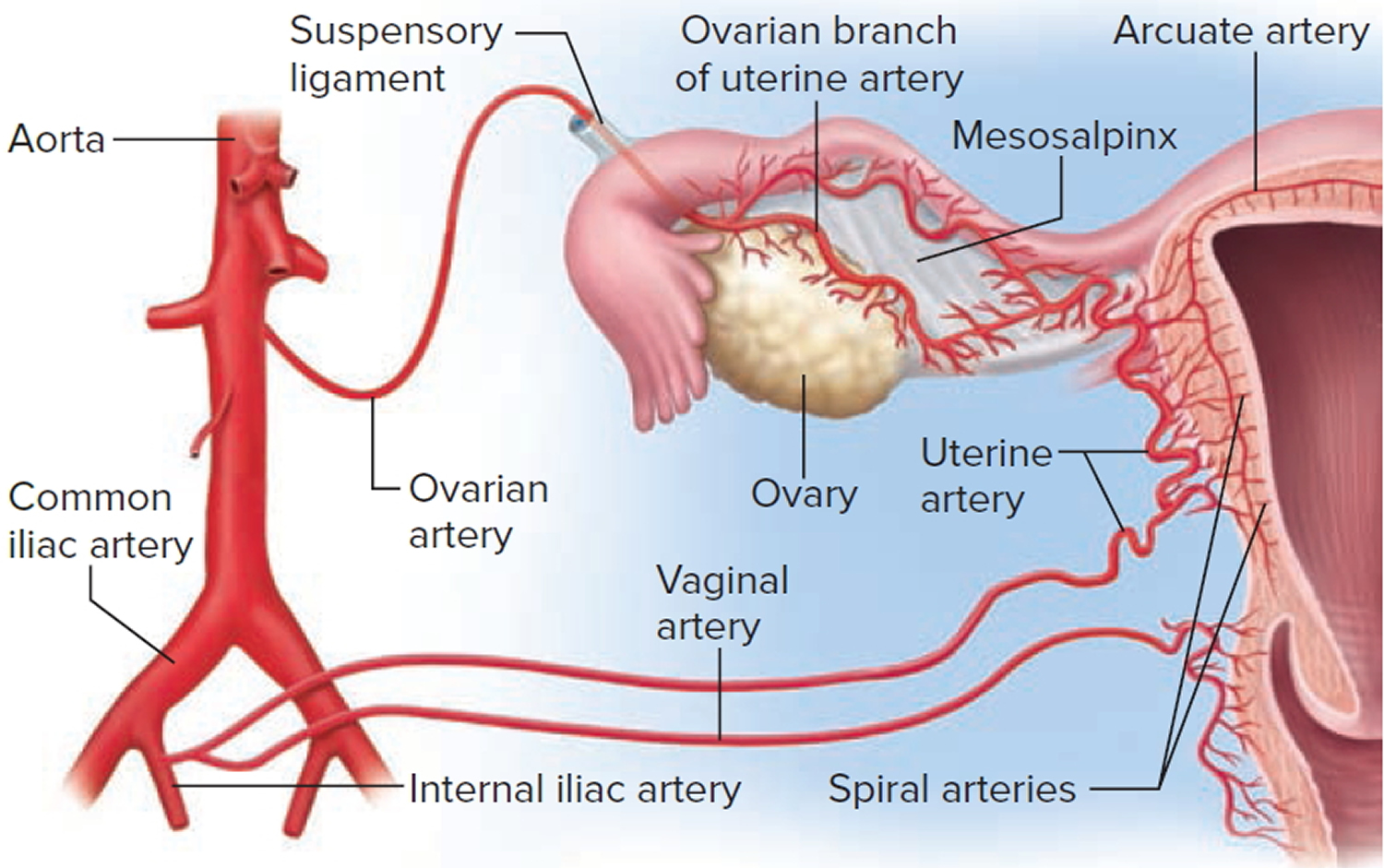
The uterine blood supply to the uterus is particularly important to the menstrual cycle and pregnancy. A uterine artery arises from each internal iliac artery and travels through the broad ligament to the uterus (Figure 5). It gives off several branches that penetrate into the myometrium and lead to arcuate arteries. Each arcuate artery travels in a circle around the uterus and anastomoses with the arcuate artery on the other side. Along its course, it gives rise to smaller arteries that penetrate the rest of the way through the myometrium, into the endometrium, and give off spiral arteries. The spiral arteries wind tortuously between the endometrial glands toward the surface of the mucosa. They rhythmically constrict and dilate, making the mucosa alternately blanch and flush with blood.
Figure 2. Uterus
Footnote: Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium.
Figure 3. Uterus location
Figure 4. Endometrium of the uterus and its blood supply
Figure 5. Blood supply to the uterus
Endometrium function
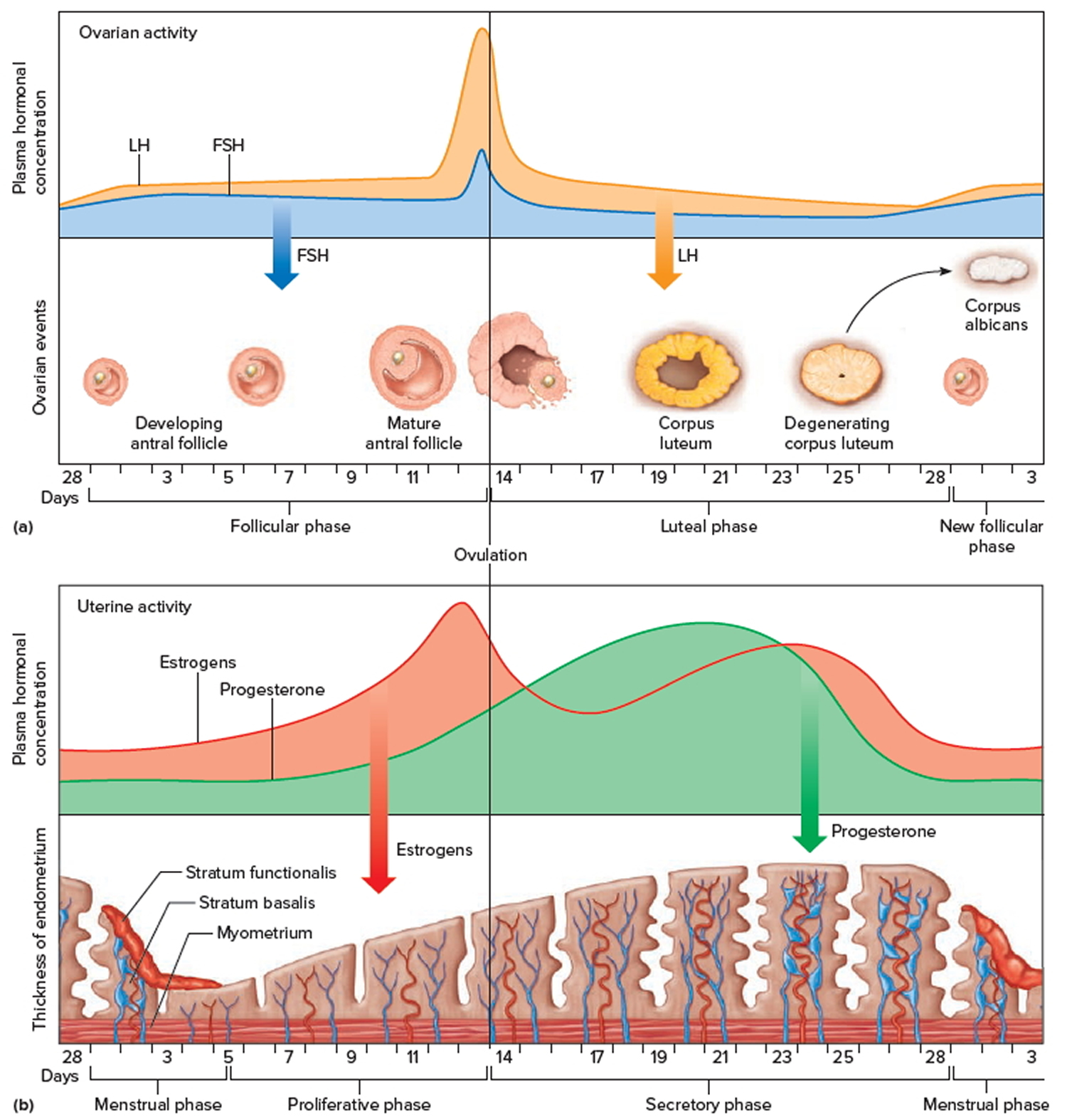
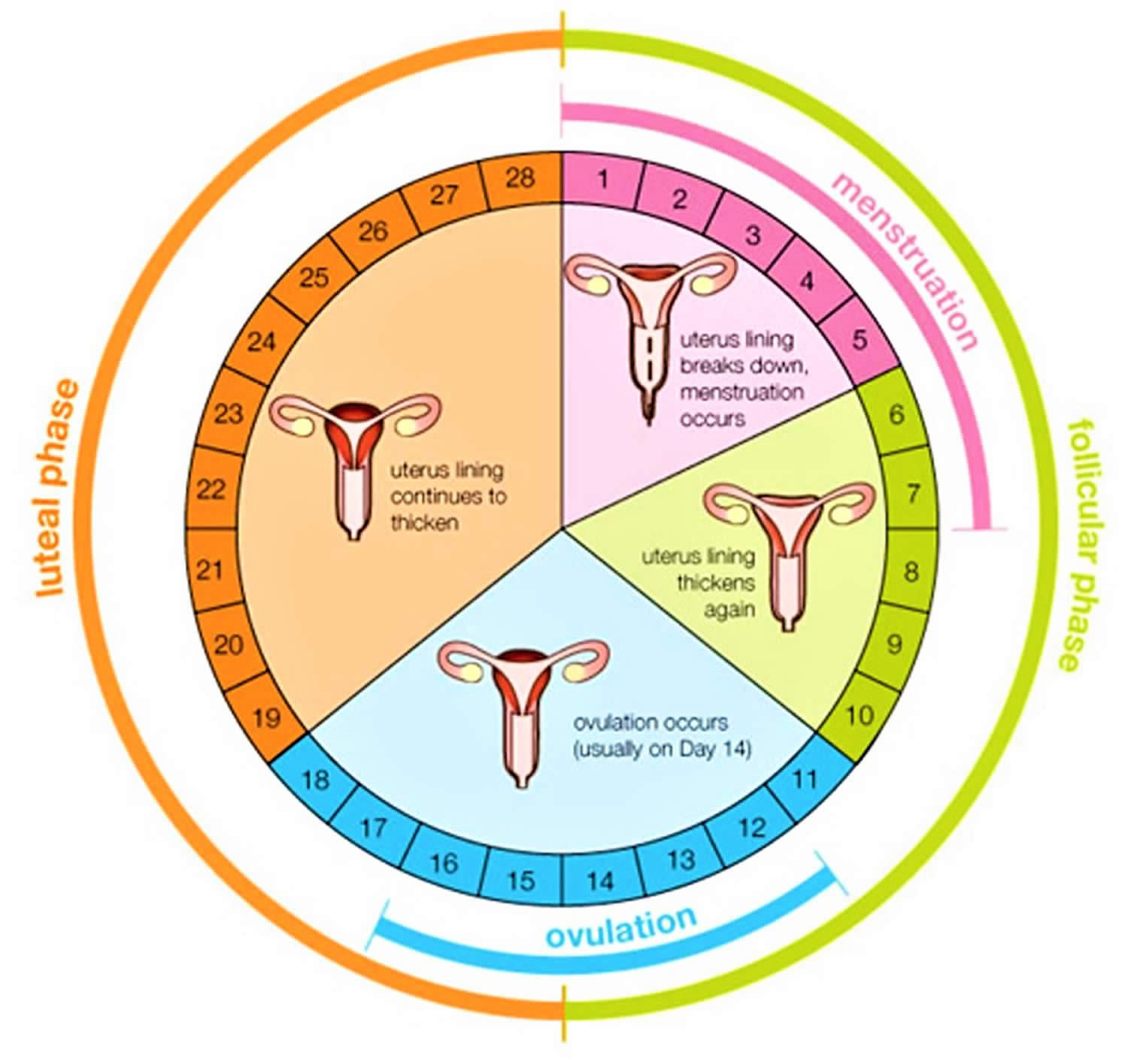
The endometrium changes throughout the menstrual cycle in response to hormones. During the first part of the cycle, the hormone estrogen is made by the ovaries. Estrogen causes the lining to grow and thicken to prepare the uterus for pregnancy. In the middle of the cycle, an egg is released from one of the ovaries (ovulation).
Following ovulation, levels of another hormone called progesterone begin to increase. Progesterone prepares the endometrium to receive and nourish a fertilized egg. If pregnancy does not occur, estrogen and progesterone levels decrease. The decrease in progesterone triggers menstruation, or shedding of the lining. Once the lining is completely shed, a new menstrual cycle begins.
Figure 6. Ovarian activity during the Menstrual cycle
Footnote: Major events in the female menstrual cycle. (a) Plasma hormonal concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) affect follicle maturation in the ovaries. (b) Plasma hormonal concentrations of estrogen and progesterone influence changes in the uterine lining.
Types of endometrial hyperplasia
The types of endometrial hyperplasia vary by the amount of abnormal cells and the presence of cell changes. According to the World Health Organization endometrial hyperplasia classification system in 1994 (WHO94), endometrial hyperplasia can be divided into 4 categories 10:
- Simple endometrial hyperplasia – Increased number of endometrial glands but regular glandular architecture.
- Complex endometrial hyperplasia – Crowded irregular endometrial glands.
- Simple atypical endometrial hyperplasia – Simple hyperplasia with presence of cytologic atypia (prominent nucleoli and nuclear pleomorphism).
- Complex atypical endometrial hyperplasia – Complex hyperplasia with cytologic atypia
The World Health Organization endometrial hyperplasia classification system 1994 (WHO94) was based on the original retrospective study of 170 patients by Kurman et al 10 who found that lesions with varying degrees of complexity and presence of atypia, when left untreated for a mean of 13 years, progressed to adenocarcinoma at different rates. Simple endometrial hyperplasia was associated with a 1% rate of progression to cancer, complex endometrial hyperplasia 3% rate of progression, simple atypical endometrial hyperplasia 8% rate of progression, whereas complex atypical endometrial hyperplasia had a 29% rate of progression to cancer 10.
Not only does the concern exist for atypical endometrial hyperplasia progressing to invasive cancer if untreated, but numerous studies found coexisting carcinoma at rates ranging from 17-56% 8, 11. A prospective Gynecologic Oncology Group study found that 306 patients with preoperative biopsies that diagnosed atypical endometrial hyperplasia had concurrent invasive adenocarcinoma in 42.6% of hysterectomy specimen 12. Part of the difficulty in diagnosing concurrent carcinoma is due to lack of reproducibility in diagnosing hyperplasia, especially atypical hyperplasia versus adenocarcinoma among even expert gynecologic pathologists. Studies report only 40-69% interobserver agreement for endometrial hyperplasia or endometrial cancer 13.
Due to the poor reproducibility of diagnosis, and confusion regarding optimal clinical management, gynecologic pathologists proposed a simpler classification of endometrial hyperplasia versus endometrial intraepithelial neoplasia (EIN) using a computerized morphometric analysis. Endometrial hyperplasia is benign hyperplasia and correlates closely to simple hyperplasia, whereas endometrial intraepithelial neoplasia (EIN) is a pre-malignant condition 14. Endometrial intraepithelial neoplasia (EIN) is defined as when the volume of glandular crowding is greater than the stromal volume, the presence of cytologic alterations, a lesion larger than 1 mm, and exclusion of mimics or carcinoma 14. Classification as complex atypical hyperplasia (WHO’94) or as endometrial intraepithelial neoplasia (EIN) had similar sensitivities and negative predictive values for coexisting endometrial cancer 15. Others found the endometrial intraepithelial neoplasia (EIN) classification to be better at predicting progression to cancer. Thus in 2014 the WHO formally adopted the simplified classification of endometrial hyperplasia into 2 categories.
World Health Organization endometrial hyperplasia classification system in 2014 (Table 1) 16:
- Hyperplasia without atypia or benign endometrial hyperplasia. Hyperplasias without atypia (benign endometrial hyperplasia) exhibit no relevant genetic changes. They are benign changes and will regress again after the endocrine milieu (physiological gestagen levels) has normalized. In a few cases (1–3 %), progression to invasive disease may occur if the endocrine disorder (long-term estrogen dominance or relative or absolute gestagen deficiency) persists over the long term.
- Endometrial Intraepithelial Neoplasia (EIN) or atypical endometrial hyperplasia. Atypical endometrial hyperplasias exhibit many of the mutations typical for invasive endometrioid endometrial cancer 17. In up to 60 % of cases, patients have coexisting invasive cancer or are at extremely high risk of developing invasive cancer (see Table 1).
This reduction to 2 categories was not only due to the need to do away with the confusing multitude of terms currently in use. Rather, it reflects a new understanding of molecular genetic changes.
The American College of Obstetricians and Gynecologists and the Society of Gynecologic Oncology Committee opinion 2015 also recommend the use of endometrial intraepithelial neoplasia (EIN) schema for a more clear terminology to distinguish the two clinicopathologic categories 4, 18. Not only does this classification reflect clinical outcome, but also underlying genetic and molecular changes 19.
The implications for treatment are obvious: hyperplasias without atypia should generally be treated conservatively (normalization of the cycle through weight loss, metformin; oral contraceptives; cyclical gestagens; gestagen IUD). Preventive hysterectomy should only be considered in exceptional cases (e.g., extreme obesity without any prospect of weight loss) 20. The surgery should be done as a total hysterectomy, i.e., it must include removal of the cervix 20.
Treatment of atypical hyperplasia or endometrioid intraepithelial neoplasia should generally consist of total (not supracervical) hysterectomy 20. Conservative treatment with high-dose gestagens and close histological monitoring should only be considered in exceptional cases (when the patient wants to have children, satisfactory compliance) 21, 20.
Table 1. New World Health Organization classification of endometrial hyperplasia
| New term | Synonyms | Genetic changes | Coexistent invasive endometrial carcinoma | Progression to invasive carcinoma |
|---|---|---|---|---|
| Hyperplasia without atypia | Benign endometrial hyperplasia; simple non-atypical endometrial hyperplasia; complex non-atypical endometrial hyperplasia; simple endometrial hyperplasia without atypia; complex endometrial hyperplasia without atypia | Low level of somatic mutations in scattered glands with morphology on HE staining showing no changes | < 1 % | RR: 1.01–1.03 |
| Atypical hyperplasia or endometrioid intraepithelial neoplasia | Complex atypical endometrial hyperplasia; simple atypical endometrial hyperplasia; endometrial intraepithelial neoplasia (EIN) | Many of the genetic changes typical for endometrioid endometrial cancer are present, including: micro satellite instability; PAX2 inactivation; mutation of PTEN, KRAS and CTNNB1 (β-catenin) | 25–33 % 16 59 % 22 | RR: 14–45 |
Footnotes: RR = Relative Risk. Relative risk is a ratio of the probability of an event occurring in the exposed group versus the probability of the event occurring in the non-exposed group. Relative Risk = (Probability of event in exposed group) / (Probability of event in not exposed group) 23. For example, atypical hyperplasia or endometrioid intraepithelial neoplasia (EIN) has a Relative Risk of 14 to 45 times more likely to progress to invasive endometrial cancer in this study compared to those without atypical hyperplasia or endometrioid intraepithelial neoplasia (EIN) in the study.
[Source 19 ]Endometrial hyperplasia signs and symptoms
The most common of endometrial hyperplasia is abnormal menstrual bleeding. If you have any of the following, you should see your doctor or an obstetrician–gynecologist (ob-gyn):
- Menstrual bleeding that is heavier or longer lasting than usual.
- Menstrual cycles (amount of time between periods) that are shorter than 21 days (counting from the first day of the menstrual period to the first day of the next menstrual period).
- Menstrual bleeding between menstrual periods.
- Not having a period (pre-menopause).
- Any bleeding after menopause.
Endometrial hyperplasia causes
Endometrial hyperplasia is the result of chronic unopposed estrogenic stimulation of the endometrial tissue with a relative deficiency of the counterbalancing effects of progesterone 24. The causes of estrogen excess could be endogenous or exogenous. For example, if ovulation does not occur, progesterone is not made, and the lining is not shed. The endometrium may continue to grow in response to estrogen. The cells that make up the lining may crowd together and may become abnormal. This condition, called hyperplasia, can lead to cancer.
You are more likely to have endometrial hyperplasia if you have gone through menopause. This is because your body’s hormones and menstrual cycles change. Other risk factors for endometrial hyperplasia are:
- Long-term use of medicines that contain high levels of estrogen or chemicals that act like estrogen.
- Irregular menstrual cycles, which can be caused by infertility or polycystic ovary syndrome (PCOS).
- Obesity.
- Use of tobacco.
- First menstrual cycle at an early age.
- Going through menopause at an older age.
- Never having been pregnant.
- Family history of uterine, ovarian, or colon cancer.
Endometrial hyperplasia pathology outlines
Endometrial hyperplasia is defined as a proliferation of endometrial glands of irregular size and shape that results in a greater than normal gland-to-stroma ratio 1. This results in varying degrees of architectural complexity and cytologic atypia.
Endometrial hyperplasia results from continuous estrogen stimulation that is unopposed by progesterone 25. This can be due to endogenous estrogen or exogenous estrogenic sources. Endogenous estrogen may be caused by chronic anovulation associated with polycystic ovary syndrome (PCOS). Obesity also contributes to unopposed estrogen exposure due to chronic high levels of estradiol that result from aromatization of androgens in adipose tissue and conversion of androstenedione to estrone. Endometrial hyperplasia and cancer can also result from estradiol-secreting ovarian tumors such as granulosa cell tumors.
Exogenous unopposed estrogen without progesterone has been associated with increased endometrial hyperplasia and adenocarcinoma 26. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial found that unopposed estrogen exposure with 0.625 mg of conjugated equine estrogen replacement therapy increased the risk of complex hyperplasia by 22.7% and atypical hyperplasia by 11.8% over 3 years of use compared with a less than 1% increase in placebo controls 27. The risk of endometrial cancer was not increased when 2.5 mg of medroxyprogesterone acetate was used in combination with 0.625 mg of conjugated equine estrogens in 8506 women in the Women’s Health Initiative (WHI) study 28. Tamoxifen, with its estrogenic effect on the endometrium, increases the risk of endometrial hyperplasia and endometrial cancer. The risk of progression to cancer is associated with an increased duration of use 29. While unopposed estrogen in oral contraceptive pills or estrogen replacement therapy increases the risk of endometrial hyperplasia and endometrial cancer, combination oral contraceptive pills and combination hormone replacement therapy does not increase and may decrease the risk of endometrial hyperplasia and endometrial cancer 25.
However, the exact mechanism of estrogen’s role in the transformation of normal endometrium to hyperplasia and cancer is unknown 25. Genetic alterations are known to be associated with hyperplasia and type 1 endometrial cancers. Benign endometrial hyperplasia is associated with low levels of somatic mutations, whereas EIN is associated with genetic alternations similar to endometrioid endometrial cancer such as microsatellite instability, and mutations in PTEN and KRAS 30. PTEN tumor suppressor gene mutations have also been found in 55% of endometrial hyperplasia cases and 83% of endometrial hyperplasia cases once it has progressed to endometrial cancer 31.
Endometrial hyperplasia risk factors
Endometrial hyperplasia is more likely to occur in women with risk factors, including 3:
- Age (age older than 53 years)
- Early age when menstruation started (early menarche [a girl’s first period])
- Late menopause (older age at menopause)
- Nulliparity (a woman who hasn’t given birth to a child or never having been pregnant)
- Obesity: body mass index (BMI) of 27 or more
- Genetic
- Cigarette smoking
- History of certain conditions, such as diabetes mellitus, gallbladder disease, or thyroid disease.
- Anovulatory cycles: polycystic ovary syndrome (PCOS), perimenopause
- Ovarian tumors: granulosa cell tumors
- Hormone replacement therapy: estrogen-only therapy can lead to endometrial hyperplasia at even a minimal dose and is contraindicated in women with a uterus. Over the counter/ herbal preparations may have a high amount of estrogen 32
- Immunosuppression (renal graft recipients) and infection may also be involved in the development of endometrial hyperplasia 33
- Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome – women with this condition are at a greatly increased risk of endometrial hyperplasia 34.
- Family history of ovarian, colon, or uterine cancer
The independent risk factors for predicting when endometrial hyperplasia coexists with cancer include age older than 53 years, postmenopausal status, diabetes, abnormal bleeding, body mass index (BMI) of 27 or more, and atypical hyperplasia 8.
Endometrial hyperplasia prevention
You cannot prevent endometrial hyperplasia, but you can help lower your risk of endometrial hyperplasia by:
- Losing weight, if you are overweight or obese.
- Quit smoking if you smoke cigarette
- Taking a medicine with progestin (synthetic progesterone), if you already are taking estrogen, due to menopause or another condition.
- If your periods are irregular, birth control pills may be recommended. They contain estrogen along with progestin. Other forms of progestin also may be taken.
- Taking birth control or another medicine to regulate your hormones and menstrual cycle.
Endometrial hyperplasia differential diagnosis
The potential differential diagnosis for endometrial hyperplasia are conditions which can result in focal or generalized thickening of the endometrium 2:
- Endometrial cancer: Histopathological examination of the endometrial tissue can show markers of invasions in endometrial cancer.
- Endometrial polyp: Hydrosonography can be helpful in diagnosing endometrial polyp by enhancing visualization. Diagnostic hysteroscopy can confirm the presence of a polyp.
- Endometritis: Irregular endometrium and focal thickness are the hallmarks of endometritis.
Endometrial hyperplasia diagnosis
There are many causes of abnormal uterine bleeding. If you have abnormal bleeding and you are 35 or older, or if you are younger than 35 and your abnormal bleeding has not been helped by medication, your doctor may recommend diagnostic tests for endometrial hyperplasia and cancer.
A transvaginal ultrasound exam may be done to measure the thickness of the endometrium. For this test, a small device is placed in your vagina. Sound waves from the device are converted into images of the pelvic organs. If the endometrium is thick, it may mean that endometrial hyperplasia is present.
The only way to tell for certain that cancer is present is to take a small sample of tissue from the endometrium and study it under a microscope. This can be done with an endometrial biopsy, dilation and curettage (D&C), or hysteroscopy.
Endometrial hyperplasia ultrasound
Imaging the endometrium on days 5-10 of a woman’s cycle reduces the variability in endometrial thickness 35.
- Premenopausal endometrial thickness
- Normal endometrial thickness depends on the stage of the menstrual cycle, but a thickness of >15 mm is considered the upper limit of normal in the secretory phase
- Endometrial hyperplasia can be reliably excluded in patients only when the endometrium measures less than 8 mm 36
- Postmenopausal endometrial thickness of >5 mm is considered abnormal 35.
The appearance can be non-specific and cannot reliably allow differentiation between endometrial hyperplasia and endometrial carcinoma 37. Usually, there is a homogeneous smooth increase in endometrial thickness, but endometrial hyperplasia may also cause asymmetric/focal thickening with surface irregularity, an appearance that is suspicious for carcinoma. Cystic changes can also be seen in endometrial hyperplasia.
Ultrasound features that are suggestive of endometrial carcinoma as opposed to endometrial hyperplasia include 38:
- heterogeneous and irregular endometrial thickening
- polypoid mass lesion
- intrauterine fluid collection
- frank myometrial invasion
Endometrial hyperplasia biopsy
Diagnosis of endometrial hyperplasia is usually made by sampling the endometrial cavity with an endometrial biopsy in the office. Tissue sampling should be performed in women with risk factors who present with symptoms of abnormal vaginal bleeding or discharge 39. This includes women older than 35 years with abnormal bleeding, women younger than 35 years with bleeding and risk factors, women with persistent bleeding, and women with unopposed estrogen replacement, tamoxifen therapy, and hereditary nonpolyposis colorectal cancer (HNPCC) syndrome or Lynch syndrome.
In addition, a biopsy should be performed in women with atypical glandular cells Pap smear or endometrial cells in Pap smears of women older than 40 years when out of synch with menstrual cycle 40. While no evidence of improved survival has been documented, some also advocate routine screening by endometrial biopsy in asymptomatic women with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome or those on tamoxifen therapy. However, most of this high-risk population present with abnormal vaginal bleeding; thus, other experts recommend work-up only when symptoms are present.
If a patient does not tolerate an office biopsy or has cervical stenosis, endovaginal ultrasonography is an effective method to assess thickness of the endometrial echo complex and to evaluate uterine bleeding. The American College of Obstetricians and Gynecologists recommends transvaginal ultrasonography as a reasonable alternative to endometrial sampling for the evaluation of an initial episode of bleeding in a postmenopausal woman 41.
Endovaginal ultrasonography has a sensitivity of greater than 96% for ruling out endometrial carcinoma if the endometrial echo complex is less than 5 mm. Persistent bleeding despite a thin stripe still warrants tissue biopsy because of the risk of missing a type 2 cancer that is not associated with hyperplasia and thickening of the endometrial echo complex 42. If hyperplasia is diagnosed by office biopsy, one should consider D&C and hysteroscopy to more definitely rule out atypia or cancer prior to conservative medical management. This is because blind dilation and curettage (D&C) and Pipelle endometrial biopsies both sample only 50-60% of the endometrial cavity, and can miss focal lesions 43.
Endometrial hyperplasia treatment
The accurate diagnosis of endometrial hyperplasia type is vital for appropriate treatment based on risk of cancer without over or under treatment 44. Once a tissue diagnosis of endometrial hyperplasia is made, treatment depends on the type of hyperplasia, the patient’s symptoms such as severity of bleeding, surgical risks, and wish for future childbearing.
In many cases, endometrial hyperplasia can be treated with progestin (man made progesterone). Progesterone induce secretory changes in the endometrium and counterbalance the stimulatory effects of estrogen. Progestins can effectively treat endometrial hyperplasia to control bleeding and prevent progression to cancer. They can serve as prevention of recurrence in those with continued risk factors 44. Progestin is given orally, in a shot, in an intrauterine device (IUD), or as a vaginal cream. How much and how long you take it depends on your age and the type of endometrial hyperplasia you have. Treatment with progestin may cause vaginal bleeding like a period.
Multiple regimens of progestin therapy have been found effective in reversing endometrial hyperplasia, including the following 20.:
- Medroxyprogesterone acetate – MPA (Provera), 10-20 mg once daily or cyclic 12-14 days per month
- Depot Medroxyprogesterone (Depo-provera) 150 mg IM every 3 months
- Micronized vaginal progesterone (Prometrium), 100-200 mg once daily or cyclic 12-14 days per month
- Levonorgestrel-containing IUD (Mirena), 20 mcg/day 45
- Megestrol acetate (Megace), 40-200 mg per day, usually reserved for women with atypical hyperplasia
Benign endometrial hyperplasia responds well to progestins. More than 98% of women with endometrial hyperplasia treated with cyclic progestins experienced regression of the disease in 3-6 months 46. However, if you have endometrial intraepithelial neoplasia (EIN) or atypical endometrial hyperplasia changes in the lining of your uterus, the risk of endometrial cancer is increased by 42% 12. Hysterectomy may be a treatment option if you do not want another pregnancy. Talk with your doctor about the right treatment for you.
Interest is increasing in the use of levonorgestrel-releasing intrauterine system (LNG-IUS) as a treatment option for endometrial hyperplasia to deliver local effect with less systemic side-effects 47. A 15-year study using the levonorgestrel-releasing intrauterine system (LNG-IUS) for 34 patients with endometrial hyperplasia found regression to atrophic endometrium in 94% 48. However, this study had only 4 (12%) patients with atypical hyperplasia 48. A meta-analysis of 24 observational studies, including 1,001 cases, indicate that progestins appeared to induce a lower disease regression rate than levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of complex and atypical endometrial hyperplasia. However, a controlled clinical trial is necessary to confirm the observational findings. The authors feel data using the levonorgestrel-releasing intrauterine device (LNG-IUD) as the only treatment modality for atypical hyperplasia or endometrial cancer are still limited 49.
The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial showed a 94% normalization of complex or atypical hyperplasia in 45 women treated with progestins 27. One cohort study found that 115 patients with complex atypical hyperplasia had approximately 30% persistence or progression of disease whether treated with progestins or not. However, of 70 patients with atypical hyperplasia, 67% vs 27% had persistence or progression when not treated with progestins 50.
If the patient has not completed childbearing or is not a surgical candidate, then concurrent cancer must first be ruled out by D&C with hysteroscopy prior to medical management. Experts recommend megestrol acetate for endometrial intraepithelial neoplasia (EIN), with or without levonorgestrel IUD (intrauterine device) for patients wishing to preserve fertility or for those too ill for surgical management. Any progestin should be adequate for treatment of benign hyperplasia or for maintenance after resolution of endometrial intraepithelial neoplasia (EIN). Patient should be sampled to assess for response every 3 to 6 months for regression to normal endometrium. If there is inadequate response in 6 months, consider increasing dose or changing progestins. There is no proven protocol for selection or dosing. Continued surveillance after regression of the lesion is recommended every 6 to 12 months if risk factors persist. Repeat biopsy is also indicated for recurrent abnormal bleeding or discharge. Prevention of recurrence include use of daily or cyclic progesterone, indwelling levonorgestrel IUD, along with weight loss for obese patients.
Due to the large number of young anovulatory women diagnosed with atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) or early endometrial cancer, numerous studies have examined the outcome of fertility-sparing hormonal therapy. A meta-analysis of 24 observational studies that included a total of 151 women with atypical endometrial hyperplasia found that those who had fertility-sparing treatments had 86% regression, 26% relapse, and live birth rate of 26% 51.
Another review of complex atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) patients who underwent progestin therapy found a complete response rate of 66% 52. Median time to response was 6 months. Recurrence rate after initial response was 23%. During study follow-up time of 39 months, 41% of patients with atypical hyperplasia became pregnant 52.
The Gynecologic Oncology Group pathologic study with biopsy diagnosis of atypical hyperplasia found 42.6% concurrent endometrial carcinoma on hysterectomy specimen; 31% of these had myometrial invasion, including 10.6% with outer half myometrial invasion 12. Others also found atypical endometrial hyperplasia on office biopsy or D&C was associated with a 48-56% cancer rate on permanent pathology. Thus, if a hysterectomy is planned for treatment of atypical hyperplasia based on office endometrial biopsy, the authors recommend having a gynecologic oncologist be primary surgeon, or be available for surgical staging if needed based on frozen section of uterine specimen 53, 43. Patients should be counseled and consented for washings, hysterectomy, possible bilateral-salpingo-oophorectomy and lymph node dissection depending on intra-op exam or frozen pathology findings. If a D&C with hysteroscopy had been performed to rule out concurrent carcinoma more definitively, an oncologic surgeon is likely not needed. Due to the risk of cancer, supracervical, morcellation, or endometrial ablation is not recommended. While a simple hysterectomy is adequate for definitive treatment of hyperplasia, one can consider bilateral salpingo-oophorectomy in perimenopausal or postmenopausal women due to possibility of cancer on permanent section. Ovaries should only be removed if cancer is diagnosed in premenopausal women. They should be counseled a second surgery may be required to remove ovaries and perform lymph node staging if cancer if found on final pathology.
The need for hysterectomy to exclude concurrent myoinvasive endometrioid adenocarcinoma presents a barrier to nonsurgical management of endometrial hyperplasia. A Gynecologic Oncology Group study examined the histomorphometric 4-class rule (4C), which measures epithelial abundance, thickness, and nuclear variation as applied to diagnostic biopsies to predict myoinvasive cancer outcomes at hysterectomy 54. Women with endometrial biopsies or curettages with a community diagnosis of atypical endometrial hyperplasia were enrolled in a clinical trial in which subsequent hysterectomy was scored for endometrial adenocarcinoma, and 4C rule ability to predict cancer outcomes was measured. Qualifying biopsies were stratified into high-risk and low-risk histomorphometric subgroups 54. Assignment to a high-risk category by 4C rule was highly sensitive in predicting any (71%) or deeply (92%) myoinvasive adenocarcinoma at hysterectomy, and assignment to a low-risk group had a high negative predictive value for absence of any (90%) or deeply (99%) myoinvasive disease 54. At present, this use of histomorphometry is most suited to a centralized reference laboratory performing histomorphometry for a variety of diagnostic applications. However, in the future, formal histomorphometry of endometrial biopsies using the 4C rule may become a more common method to identify a subset of women with premalignant disease who are unlikely to have concurrent myoinvasive adenocarcinoma and therefore may qualify for nonsurgical therapy.
Endometrial hyperplasia prognosis
Several studies have shown that progestogen (synthetic forms of progesterone) therapy leads to a high rate of regression in endometrial hyperplasia without atypia (89% to 96%) 49, 45, 46. However, in the presence of endometrial intraepithelial neoplasia (EIN), there is a reduction in the success rate of progestogen therapy 12. The presence of atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) has a higher risk of progression to invasive malignancy – as high as 27.5% if not treated 55. Also, atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) has a possibility of coexistent endometrial malignancy in 43% of cases 12.
- Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004 May;59(5):368-78. doi: 10.1097/00006254-200405000-00025[↩][↩]
- Singh G, Puckett Y. Endometrial Hyperplasia. [Updated 2022 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560693[↩][↩]
- van der Meer, A.C.L. and Hanna, L.S. (2017), Development of endometrioid adenocarcinoma despite Levonorgestrel-releasing intrauterine system: a case report with discussion and review of the RCOG/BSGE Guideline on the Management of Endometrial Hyperplasia. Clin Obes, 7: 54-57. https://doi.org/10.1111/cob.12168[↩][↩]
- The American College of Obstetricians and Gynecologists Committee Opinion no. 631. Endometrial intraepithelial neoplasia. Obstet Gynecol. 2015 May;125(5):1272-1278. doi: 10.1097/01.AOG.0000465189.50026.20[↩][↩]
- Palmer, J.E., Perunovic, B. and Tidy, J.A. (2008), Endometrial hyperplasia. The Obstetrician & Gynaecologist, 10: 211-216. https://doi.org/10.1576/toag.10.4.211.27436[↩]
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a1[↩]
- Endometrial Hyperplasia. https://www.acog.org/womens-health/faqs/endometrial-hyperplasia[↩][↩]
- Chen, Y. L., Wang, K. L., Chen, M. Y., Yu, M. H., Wu, C. H., Ke, Y. M., Chen, Y. J., Chang, Y. Y., Hsu, K. F., & Yen, M. S. (2013). Risk factor analysis of coexisting endometrial carcinoma in patients with endometrial hyperplasia: a retrospective observational study of Taiwanese Gynecologic Oncology Group. Journal of gynecologic oncology, 24(1), 14–20. https://doi.org/10.3802/jgo.2013.24.1.14[↩][↩][↩]
- Endometrial hyperplasia. https://radiopaedia.org/cases/endometrial-hyperplasia?lang=us[↩]
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985 Jul 15;56(2):403-12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x[↩][↩][↩]
- Rakha E, Wong SC, Soomro I, Chaudry Z, Sharma A, Deen S, Chan S, Abu J, Nunns D, Williamson K, McGregor A, Hammond R, Brown L. Clinical outcome of atypical endometrial hyperplasia diagnosed on an endometrial biopsy: institutional experience and review of literature. Am J Surg Pathol. 2012 Nov;36(11):1683-90. doi: 10.1097/PAS.0b013e31825dd4ff[↩]
- Trimble, C.L., Kauderer, J., Zaino, R., Silverberg, S., Lim, P.C., Burke, J.J., II, Alberts, D. and Curtin, J. (2006), Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer, 106: 812-819. https://doi.org/10.1002/cncr.21650[↩][↩][↩][↩][↩]
- Zaino, R.J., Kauderer, J., Trimble, C.L., Silverberg, S.G., Curtin, J.P., Lim, P.C. and Gallup, D.G. (2006), Reproducibility of the diagnosis of atypical endometrial hyperplasia. Cancer, 106: 804-811. https://doi.org/10.1002/cncr.21649[↩]
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a2[↩][↩]
- Salman, M. C., Usubutun, A., Boynukalin, K., & Yuce, K. (2010). Comparison of WHO and endometrial intraepithelial neoplasia classifications in predicting the presence of coexistent malignancy in endometrial hyperplasia. Journal of gynecologic oncology, 21(2), 97–101. https://doi.org/10.3802/jgo.2010.21.2.97[↩]
- Zaino R, Carinelli S G, Ellenson L H, Lyon: WHO Press; 2014. Tumours of the uterine Corpus: epithelial Tumours and Precursors; pp. 125–126.[↩][↩]
- Cancer Genome Atlas Research Network, Kandoth, C., Schultz, N., Cherniack, A. D., Akbani, R., Liu, Y., Shen, H., Robertson, A. G., Pashtan, I., Shen, R., Benz, C. C., Yau, C., Laird, P. W., Ding, L., Zhang, W., Mills, G. B., Kucherlapati, R., Mardis, E. R., & Levine, D. A. (2013). Integrated genomic characterization of endometrial carcinoma. Nature, 497(7447), 67–73. https://doi.org/10.1038/nature12113[↩]
- Baak, J. P., Mutter, G. L., Robboy, S., van Diest, P. J., Uyterlinde, A. M., Orbo, A., Palazzo, J., Fiane, B., Løvslett, K., Burger, C., Voorhorst, F., & Verheijen, R. H. (2005). The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer, 103(11), 2304–2312. https://doi.org/10.1002/cncr.21058[↩]
- Emons, G., Beckmann, M. W., Schmidt, D., Mallmann, P., & Uterus commission of the Gynecological Oncology Working Group (AGO) (2015). New WHO Classification of Endometrial Hyperplasias. Geburtshilfe und Frauenheilkunde, 75(2), 135–136. https://doi.org/10.1055/s-0034-1396256[↩][↩]
- Trimble, C. L., Method, M., Leitao, M., Lu, K., Ioffe, O., Hampton, M., Higgins, R., Zaino, R., Mutter, G. L., & Society of Gynecologic Oncology Clinical Practice Committee (2012). Management of endometrial precancers. Obstetrics and gynecology, 120(5), 1160–1175. https://doi.org/10.1097/aog.0b013e31826bb121[↩][↩][↩][↩][↩]
- https://www.awmf.org/leitlinien/detail/ll/032-034OL.html[↩]
- Antonsen SL, Ulrich L, Høgdall C. Patients with atypical hyperplasia of the endometrium should be treated in oncological centers. Gynecol Oncol. 2012 Apr;125(1):124-8. doi: 10.1016/j.ygyno.2011.12.436[↩]
- Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015 Jul;76(7):e857-61. doi: 10.4088/JCP.15f10150[↩]
- Parkash V, Fadare O, Tornos C, McCluggage WG. Committee Opinion No. 631: Endometrial Intraepithelial Neoplasia. Obstet Gynecol. 2015 Oct;126(4):897. doi: 10.1097/AOG.0000000000001071[↩]
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a3[↩][↩][↩]
- Lethaby A, Suckling J, Barlow D, Farquhar CM, Jepson RG, Roberts H. Hormone replacement therapy in postmenopausal women: endometrial hyperplasia and irregular bleeding. Cochrane Database Syst Rev. 2004;(3):CD000402. doi: 10.1002/14651858.CD000402.pub2. Update in: Cochrane Database Syst Rev. 2009;(2):CD000402[↩]
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996 Feb 7;275(5):370-5. doi: 10.1001/jama.1996.03530290040035[↩][↩]
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM; Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. 2003 Oct 1;290(13):1739-48. doi: 10.1001/jama.290.13.1739[↩]
- Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004 Aug;94(2):256-66. doi: 10.1016/j.ygyno.2004.03.048[↩]
- Lancaster JM, Powell CB, Chen LM, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015 Jan;136(1):3-7. doi: 10.1016/j.ygyno.2014.09.009. Epub 2014 Sep 17. Erratum in: Gynecol Oncol. 2015 Sep;138(3):765.[↩]
- Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000 Mar;13(3):295-308. doi: 10.1038/modpathol.3880051[↩]
- Furness, S., Roberts, H., Marjoribanks, J., & Lethaby, A. (2012). Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. The Cochrane database of systematic reviews, 2012(8), CD000402. https://doi.org/10.1002/14651858.CD000402.pub4[↩]
- Kaminski P, Bobrowska K, Pietrzak B, Bablok L, Wielgos M. Gynecological issues after organ transplantation. Neuro Endocrinol Lett. 2008 Dec;29(6):852-6.[↩]
- Niskakoski A, Pasanen A, Porkka N, Eldfors S, Lassus H, Renkonen-Sinisalo L, Kaur S, Mecklin JP, Bützow R, Peltomäki P. Converging endometrial and ovarian tumorigenesis in Lynch syndrome: Shared origin of synchronous carcinomas. Gynecol Oncol. 2018 Jul;150(1):92-98. doi: 10.1016/j.ygyno.2018.04.566[↩]
- Endometrial hyperplasia. https://radiopaedia.org/articles/endometrial-hyperplasia-1?lang=us[↩][↩]
- Park, Y. R., Lee, S. W., Kim, Y., Bae, I. Y., Kim, H. K., Choe, J., & Kim, Y. M. (2019). Endometrial thickness cut-off value by transvaginal ultrasonography for screening of endometrial pathology in premenopausal and postmenopausal women. Obstetrics & gynecology science, 62(6), 445–453. https://doi.org/10.5468/ogs.2019.62.6.445[↩]
- Jorizzo JR, Chen MY, Martin D, Dyer RB, Weber TM. Spectrum of endometrial hyperplasia and its mimics on saline hysterosonography. AJR Am J Roentgenol. 2002 Aug;179(2):385-9. doi: 10.2214/ajr.179.2.1790385[↩]
- Gupta A, Desai A, Bhatt S. Imaging of the Endometrium: Physiologic Changes and Diseases: Women’s Imaging. Radiographics. 2017 Nov-Dec;37(7):2206-2207. doi: 10.1148/rg.2017170008[↩]
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a7[↩]
- Siebers AG, Verbeek AL, Massuger LF, Grefte JM, Bulten J. Normal appearing endometrial cells in cervical smears of asymptomatic postmenopausal women have predictive value for significant endometrial pathology. Int J Gynecol Cancer. 2006 May-Jun;16(3):1069-74. doi: 10.1111/j.1525-1438.2006.00578.x[↩]
- ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018 May;131(5):e124-e129. doi: 10.1097/AOG.0000000000002631[↩]
- Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006 Apr;101(1):120-5. doi: 10.1016/j.ygyno.2005.09.042[↩]
- Suh-Burgmann E, Hung YY, Armstrong MA. Complex atypical endometrial hyperplasia: the risk of unrecognized adenocarcinoma and value of preoperative dilation and curettage. Obstet Gynecol. 2009 Sep;114(3):523-529. doi: 10.1097/AOG.0b013e3181b190d5[↩][↩]
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a8[↩][↩]
- BUTTINI, M.J., JORDAN, S.J. and WEBB, P.M. (2009), The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: An Australian study and systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology, 49: 316-322. https://doi.org/10.1111/j.1479-828X.2009.00981.x[↩][↩]
- Gambrell RD Jr. Progestogens in estrogen-replacement therapy. Clin Obstet Gynecol. 1995 Dec;38(4):890-901. doi: 10.1097/00003081-199538040-00023[↩][↩]
- Orbo, A., Vereide, A., Arnes, M., Pettersen, I., & Straume, B. (2014). Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG : an international journal of obstetrics and gynaecology, 121(4), 477–486. https://doi.org/10.1111/1471-0528.12499[↩]
- Scarselli G, Bargelli G, Taddei GL, Marchionni M, Peruzzi E, Pieralli A, Mattei A, Buccoliero AM, Fambrini M. Levonorgestrel-releasing intrauterine system (LNG-IUS) as an effective treatment option for endometrial hyperplasia: a 15-year follow-up study. Fertil Steril. 2011 Jan;95(1):420-2. doi: 10.1016/j.fertnstert.2010.07.1044[↩][↩]
- Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010 Dec;203(6):547.e1-10. doi: 10.1016/j.ajog.2010.07.037[↩][↩]
- Reed, S. D., Voigt, L. F., Newton, K. M., Garcia, R. H., Allison, H. K., Epplein, M., Jordan, D., Swisher, E., & Weiss, N. S. (2009). Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstetrics and gynecology, 113(3), 655–662. https://doi.org/10.1097/AOG.0b013e318198a10a[↩]
- Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012 Oct;207(4):266.e1-12. doi: 10.1016/j.ajog.2012.08.011[↩]
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012 May;125(2):477-82. doi: 10.1016/j.ygyno.2012.01.003[↩][↩]
- Morotti M, Menada MV, Moioli M, Sala P, Maffeo I, Abete L, Fulcheri E, Menoni S, Venturini P, Papadia A. Frozen section pathology at time of hysterectomy accurately predicts endometrial cancer in patients with preoperative diagnosis of atypical endometrial hyperplasia. Gynecol Oncol. 2012 Jun;125(3):536-40. doi: 10.1016/j.ygyno.2012.02.011[↩]
- Mutter, G. L., Kauderer, J., Baak, J. P., Alberts, D., & Gynecologic Oncology Group (2008). Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: a Gynecologic Oncology Group study. Human pathology, 39(6), 866–874. https://doi.org/10.1016/j.humpath.2007.09.023[↩][↩][↩]
- Lacey, J. V., Jr, Sherman, M. E., Rush, B. B., Ronnett, B. M., Ioffe, O. B., Duggan, M. A., Glass, A. G., Richesson, D. A., Chatterjee, N., & Langholz, B. (2010). Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 28(5), 788–792. https://doi.org/10.1200/JCO.2009.24.1315[↩]