EVALI
EVALI is short for “E-cigarette use or Vaping Associated Lung Injury”, which was also called vaping-associated pulmonary injury (VAPI) 1, 2. EVALI is a severe lung disease related to using e-cigarette and vaping products, the first being identified during 2019 3, 4. As of February 18, 2020, all 50 states, the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands) have reported 2,807 EVALI cases or deaths to Centers for Disease Control and Prevention (CDC), including 68 (2.4%) EVALI-associated deaths 5, 4. Based on established definitions, patients with EVALI require reported use of e-cigarette, or vaping, products within 3 months of symptom onset, positive imaging findings, and an evaluation to rule out infectious causes 5. Health officials point to vitamin E acetate, an additive in some tetrahydrocannabinol (THC) containing e-cigarette or vaping products appear to be associated with the EVALI outbreak; however, no single causative agent has been identified 5. Among case associated THC-containing product samples, investigators found vitamin E acetate in 49% of the samples and at least one other potential toxicant (e.g., medium-chain triglycerides [MCT]) in 24% of the samples 6. Furthermore, trade websites have reported the addition of various diluents (e.g., vitamin E acetate and medium-chain triglycerides [MCT]) to THC-containing products to enhance their quality and appearance, provide desirable aroma or taste, and lower product cost 7.
EVALI cases were first reported to the CDC in August 2019 and rapidly increased in number thereafter, which suggests new or increased exposure to one or more toxicants from the use of e-cigarette products 4. A recent study analyzed samples from 51 EVALI cases from 16 states, vitamin E acetate was identified in bronchoalveolar lavage (BAL) fluid samples (fluid samples collected from the lungs) from 48 of the 51 EVALI patients, but not in the bronchoalveolar lavage (BAL) fluid from the healthy comparison group 7. No other toxicants were found in BAL fluid from either group, except for coconut oil and limonene (1 EVALI patient each). Vitamin E acetate usually does not cause harm when ingested as a vitamin supplement or applied to the skin. However, previous research suggests that when vitamin E acetate is inhaled, it may interfere with normal lung functioning.
Patients with e-cigarette or vaping product use associated lung injury (EVALI) can present with a wide variety of symptoms, ranging from respiratory such as cough, chest pain, shortness of breath, gastrointestinal such as abdominal pain, nausea, vomiting, diarrhea to general symptoms such as fever, chills, or weight loss. A thorough history is crucial to establishing the diagnosis focusing on the acuity of symptoms. The CDC recommends obtaining detailed information during the patient interview regarding the type of vaping device used, type of substance used, frequency of vaping, and where the e-cigarette, or vaping, products were obtained 5.
The CDC has proposed the following 4 required criteria for public health reporting of confirmed EVALI (e-cigarette or vaping product use associated lung injury) 8, 9:
- Using an e-cigarette (vaping) or dabbing during the 90 days before symptom onset;
- Having a pulmonary infiltrate, such as opacities on plain film chest radiograph or ground-glass opacities on chest CT;
- The absence of clinical evidence of a pulmonary infection on initial work-up: Minimum criteria include negative respiratory viral panel, influenza polymerase chain reaction, or rapid test if local epidemiology supports testing. All other clinically indicated respiratory infectious disease testing (e.g., urine antigen for Streptococcus pneumoniae and Legionella, sputum culture if productive cough, bronchoalveolar lavage culture when indicated, blood cultures, HIV–related opportunistic respiratory infections when appropriate) must be negative; and
- No medical record evidence of alternative plausible diagnoses (e.g., cardiac, rheumatologic, or neoplastic process).
Confirming a diagnosis of EVALI has been difficult because no single lab test for EVALI is available. Right now, doctors diagnose EVALI based on symptoms, recent use of vaping products, abnormalities found on lung scans, and no evidence of infection. Unfortunately, direct lung examination requires a bronchoscopy, which most patients are too sick to tolerate safely. Data from patients who did undergo bronchoscopy has so far failed to identify the mechanism causing lung injuries. Microbiology specimens for bacteria, viral, and fungal organisms are usually negative. When Broncho-alveolar-lavage (BAL) specimens are obtained, lipid-laden macrophages have been identified 8.
Because EVALI disease is so new, the course of the illness is unpredictable. Among the cases reported, 96% of patients required hospitalization, including some who died. Treatment is based on expert recommendations and depends on the severity of the illness. Most patients have been treated with antibiotics and/or antivirals until infection is ruled out as well as corticosteroids to help fight inflammation in the lungs 8. Patients with more severe cases will need hospitalization and, because they may be unable to breathe on their own, could be placed on a ventilator. Even patients who have less severe symptoms may need supplemental oxygen.
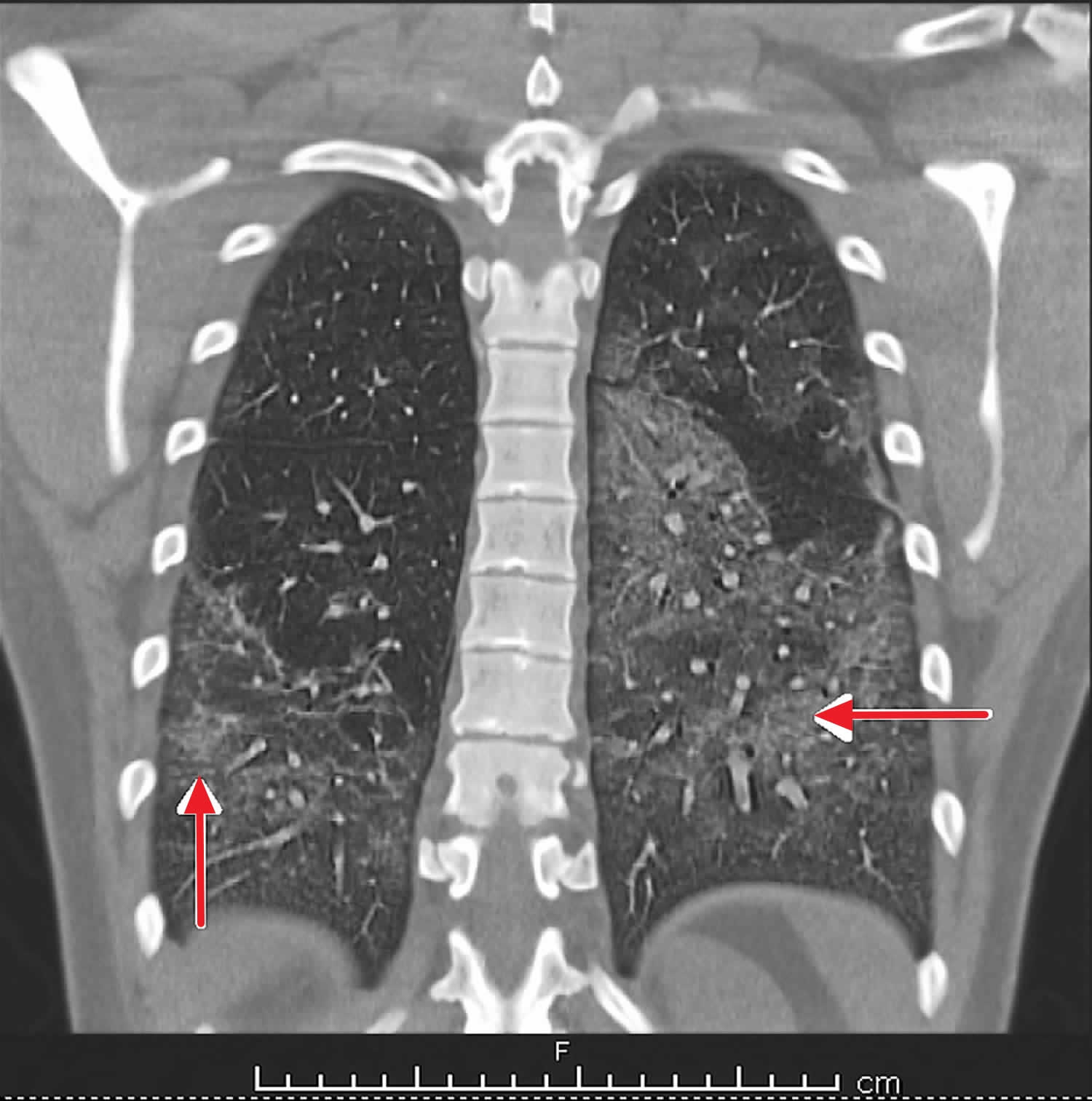
Figure 1. EVALI
Footnote: Computed tomography (CT) chest (coronal view) – bilateral lung infiltrates due to EVALI
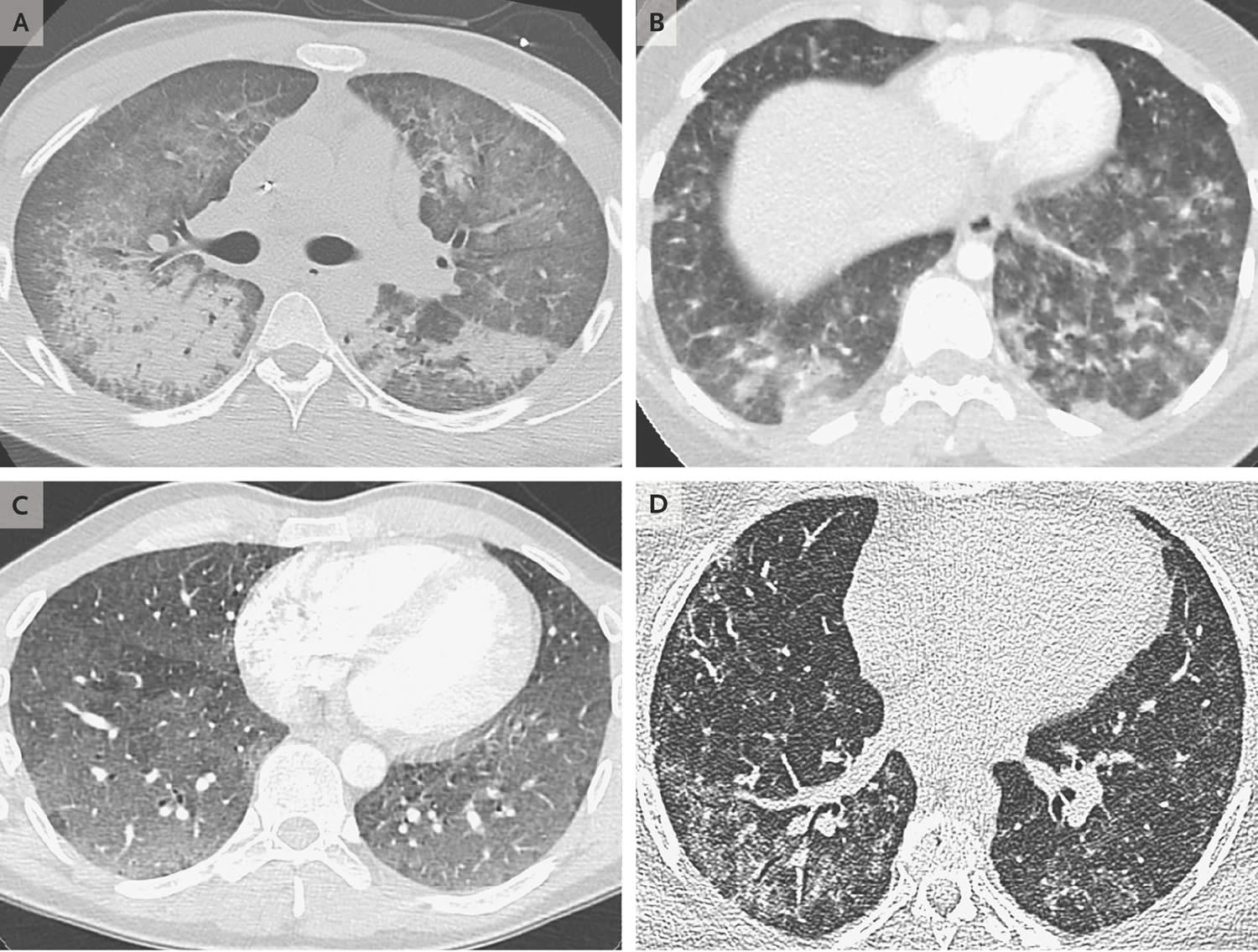
[Sourcr 10 ]Figure 2. EVALI lung (Computed Tomographic Scans of the Chest Obtained from Patients with EVALI [Vaping-Associated Lung Injury])
Footnotes: An image obtained from a 20-year-old man with diffuse alveolar damage. (Panel A) shows dependent consolidation and diffuse ground-glass opacity, with some areas of bronchial dilatation typical of diffuse alveolar damage. The patient underwent intubation on hospital day 2 but eventually recovered after receiving glucocorticoid therapy. An image obtained from a 19-year-old woman with acute eosinophilic pneumonia. (Panel B) shows diffuse nodular areas of consolidation and ground-glass opacity, with mild septal thickening and a small right pleural effusion. The findings cleared within days after the administration of glucocorticoids. An image obtained from a 35-year-old man with a pattern of hypersensitivity pneumonitis. (Panel C) shows extensive centrilobular ground-glass attenuation nodules, especially in the anterior region, and more confluent ground-glass opacity in the dependent lungs, with lobules of mosaic attenuation. The patient’s symptoms improved after cessation of vaping. An image obtained from a 49-year-old woman with giant-cell interstitial pneumonia (Panel D), which was diagnosed on the basis of findings on surgical biopsy of the lung and was attributed to cobalt in her vape pen, shows fibrosis characterized by peripheral reticulation, ground-glass opacity, and mild traction bronchiectasis. The patient’s symptoms improved after cessation of vaping.
[Source 11 ]What are E-cigarettes?
E-cigarettes also known as electronic cigarettes, include e-pens, e-pipes, e-hookah, vape pens, JUULs and e-cigars are known collectively as ENDS (electronic nicotine delivery systems) that use a battery to heat up a special liquid into an aerosol that users inhale. According to the Food and Drug Administration (FDA), e-cigarettes are devices that allow users to inhale an aerosol containing nicotine or other substances.
Unlike traditional cigarettes, e-cigarettes are generally battery-operated and use a heating element to heat e-liquid from a cartridge (usually refillable), releasing a chemical-filled aerosol.
What is in an E-cigarette?
The “e-juice” that fills the cartridges usually contains nicotine (which is extracted from tobacco), propylene glycol, flavorings and other chemicals. Studies have found that even e-cigarettes claiming to be nicotine-free contain trace amounts of nicotine. Additionally, when the e-liquid heats up, more toxic chemicals are formed.
The Food and Drug Administration (FDA) has not begun its review of any e-cigarette or its ingredients, nor has FDA issued any standards on the products, e-cigarette composition and effects vary. What researchers do know is that these toxic chemicals and metals have all been found in e-cigarettes 12:
- Nicotine – a highly addictive substance that negatively affects adolescent brain development
- Propylene glycol – a common additive in food; also used to make things like antifreeze, paint solvent, and artificial smoke in fog machines
- Carcinogens – chemicals known to cause cancer, including acetaldehyde and formaldehyde
- Acrolein – a herbicide primarily used to kill weeds, can cause irreversible lung damage
- Diacetyl – a chemical linked to a lung disease called bronchiolitis obliterans aka “popcorn lung”
- Diethylene glycol – a toxic chemical used in antifreeze that is linked to lung disease
- Heavy metals such as nickel, tin, lead
- Cadmium – a toxic metal found in traditional cigarettes that causes breathing problems and disease
- Benzene – a volatile organic compound (VOC) found in car exhaust
- Ultrafine particles that can be inhaled deep into the lungs
Research from The Johns Hopkins University on vape ingredients published in October 2021 reveals thousands of chemical ingredients in vape products, most of which are not yet identified 13. Among those the researchers could identify were several potentially harmful substances, including caffeine, three chemicals never previously found in e-cigarettes, a pesticide and two flavorings linked with possible toxic effects and respiratory irritation 13.
What is in E-cigarette Aerosol?
The e-cigarette aerosol that users breathe from the device and exhale can contain harmful and potentially harmful substances, including:
- Nicotine
- Ultrafine particles that can be inhaled deep into the lungs
- Flavorings such as diacetyl, a chemical linked to a serious lung disease
- Volatile organic compounds (VOCs)
- Cancer-causing chemicals
- Heavy metals such as nickel, tin, and lead 14
The aerosol that users inhale and exhale from e-cigarettes can expose both themselves and bystanders to harmful substances.
It is difficult for consumers to know what e-cigarette products contain. For example, some e-cigarettes marketed as containing zero percent nicotine have been found to contain nicotine 15.
Is vaping bad for you?
In addition to EVALI, previously published case reports have linked electronic cigarette use to a broad spectrum of pulmonary disease, including asymptomatic and incidental radiographic findings 16, lipoid pneumonia 17, acute eosinophilic pneumonia 18, hypersensitivity pneumonitis 19 and diffuse alveolar hemorrhage 20. These early case reports were likely the first evidence that electronic cigarettes do carry lung toxicity.
Potential long-term health concerns related to vaping 21:
- Humectants are additives used to produce vapor, such as propylene glycol or glycerol. Human respiratory cells exposed to humectants in lab experiments show increased inflammation and decreased survival. This raises concern about lung damage when people inhale humectants.
- Thousands of vape flavoring products have been reported. Because these are inhaled, not ingested, they are not regulated by the Flavor and Extract Manufacturers Association (FEMA). Diacetyl, which gives food a buttery or creamy flavor, is one example. Factory workers exposed to high levels of diacetyl in popcorn factories have developed lung injury known as “popcorn lung,” so it is regulated in the workplace by Occupational Safety and Health Administration (OSHA). Yet diacetyl is used in over 60% of sweet-flavored vapes, and just three to four puffs a day far exceeds exposure limits set by OSHA.
- Heating ingredients to create vapor causes their chemical components to decompose, which may also be a health hazard. For example, heating propylene glycol produces aldehydes, which expose users to five to 15 times the levels of formaldehyde vapor — a known carcinogen — found in tobacco cigarettes.
- Additionally, repeated use of refillable cartridges can cause metal heating coils to decompose, which could lead to inhaling or ingesting heavy metals. The toxic metals manganese and zinc have been isolated from used vaping devices. These can cause illness when ingested at high levels. There are also case reports of lung injury linked with cobalt in vaping liquid. This has been attributed to coil corrosion.
The bottom line on the risks of E-cigarettes 22:
- The use of e-cigarettes is unsafe for kids, teens, and young adults. Children and adults have been poisoned by swallowing, breathing, or absorbing e-cigarette liquid through their skin or eyes. Nationally, approximately 50% of calls to poison control centers for e-cigarettes are for kids 5 years of age or younger.
- Most e-cigarettes (vapes) contain nicotine. Nicotine is highly addictive drug and can harm adolescent brain development, which continues into the early to mid-20s 14, 23. Using nicotine in adolescence can harm the parts of the brain that control attention, learning, mood, and impulse control 23. Each time a new memory is created or a new skill is learned, stronger connections – or synapses – are built between brain cells. Young people’s brains build synapses faster than adult brains. Nicotine changes the way these synapses are formed. Using nicotine in adolescence may also increase risk for future addiction to other drugs 23.
- When a person is dependent on (or addicted to) nicotine and stops using it, their body and brain have to get used to not having nicotine. This can result in temporary symptoms of nicotine withdrawal.
- Nicotine withdrawal symptoms include irritability, restlessness, feeling anxious or depressed, trouble sleeping, problems concentrating, and craving nicotine 15. People may keep using tobacco products to help relieve these symptoms 24.
- Youth may turn to vaping to try to deal with stress or anxiety, creating a cycle of nicotine dependence. But nicotine addiction can be a source of stress.
- E-cigarettes can contain other harmful substances besides nicotine.
- Diacetyl: This food additive, used to deepen e-cigarette flavors, is known to damage small passageways in the lungs.
- Formaldehyde: This toxic chemical can cause lung disease and contribute to heart disease.
- Acrolein: Most often used as a weed killer, this chemical can also damage lungs.
- Young people who use e-cigarettes may be more likely to smoke cigarettes in the future.
- Youth e-cigarette and cigarette use have been associated with mental health symptoms such as depression 25, 26.
- Scientists are still learning about the long-term health effects of e-cigarettes.
EVALI signs and symptoms
The vast majority of EVALI patients (95%) present with respiratory symptoms of shortness of breath, chest pain, cough, and hemoptysis (coughing up blood) 8. Gastrointestinal symptoms such as abdominal pain, nausea, vomiting, and diarrhea occur in 77% of patients and can be the initial symptoms preceding respiratory symptoms 8. Symptoms usually progress in severity over one to two weeks 8 and constitutional symptoms such as fever, chills, malaise and weight loss occur in 85% of patients 27. Patients frequently exhibit tachycardia (fast heart rate), tachypnea (fast breathing), fever, and hypoxemia (a below-normal level of oxygen in your blood with pulse oximetry less than 95%) at presentation 28. The degree of respiratory failure is diverse, with up to one-third requiring intubation and mechanical ventilation 28.
Auscultation on lung exam is often normal 2. Non-specific findings of leukocytosis, elevated erythrocyte sedimentation rate and elevated liver transaminases have been reported 8. The chest radiograph is abnormal in the majority of cases revealing patchy infiltrates 11. CT of the chest commonly demonstrates basilar-predominant consolidation and ground-glass opacities, often with areas of lobular or sub pleural sparing 11.
Patients may develop severe hypoxemia and respiratory failure 24–48 hours after presenting with mild symptoms, requiring high flow oxygen and in 22% of cases mechanical ventilation for Acute Respiratory Distress Syndrome (ARDS) 8.
EVALI complications
The main serious complications of EVALI (e-cigarette or vaping product use associated lung injury) are:
- Acute respiratory distress syndrome (ARDS)
- Respiratory failure
- Need for intubation and mechanical ventilation
- Death
EVALI causes
Although the cause of EVALI remains unclear, several causes are under investigation. There are thousands of vaping products with varying ingredients, including illicit substances. Most likely, more than one specific product or substance is causing severe lung problems. No one knows why some people get EVALI and others do not, but part of this is probably due to the different ingredients they have inhaled. Of these, vitamin E acetate is by far the most recognized agent associated with EVALI (e-cigarette or vaping product use associated lung injury) 1. Supporting this is the fact that a recent study identified vitamin E acetate in bronchoalveolar lavage (BAL) fluid samples of 48 out of the 51 EVALI patients as opposed to none in the fluid samples obtained from the healthy control group 7. Vitamin E acetate was illegally being used as a diluent in multiple counterfeit, low-cost, tetrahydrocannabinol (THC) containing cartridges. Its use as a diluent in THC-based cartridges became common in 2019, coinciding with the EVALI outbreak 29. However, the possibility of other agents, including chemicals in either THC or non-THC products, implicated in the causation of EVALI disease cannot be ruled out 1.
- The most common brand associated with EVALI is Dank Vape, a brand of products containing THC, the principal psychoactive ingredient in marijuana.
- Exclusively using products with THC increases risk for EVALI. (It’s unclear whether people who used nicotine-only vapes also were exposed to vape products with THC, or whether other ingredients caused the lung injury.)
- Vitamin E acetate is strongly associated with EVALI. It is found largely in counterfeit brands (and recently in Juul products from South Korea). Vitamin E is a supplement considered safe when ingested or applied to the skin. Vitamin E acetate is an oil derivative used in vaping products as a thickener. It is found in about half of the products associated with EVALI. A recent small study found vitamin E deposits in the lung tissue of EVALI patients.
- Other chemical components, including triglycerides, plant oils, petroleum distillates, and diluent terpenes have been found in bronchoscopy specimens of EVALI patients. But none are present in all patients.
EVALI pathophysiology
The pathology of the EVALI disease is still poorly understood. A comprehensive pathophysiological basis for the lung injury seen in EVALI patients is yet to be established. Butt et al. 30, in a recent study, described a wide spectrum of histopathological findings seen in EVALI, including acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia, usually bronchiolocentric and accompanied by bronchiolitis.
Previously, studies had suggested that EVALI may represent a form of exogenous lipoid pneumonia 31. However, a recent literature review concluded that no histologic evidence of exogenous lipoid pneumonia was seen in the tissue samples. The histological findings are more likely suggestive of airway-centered chemical pneumonitis from one or more inhaled toxic substances found in the vapes. Testing for lipid-laden macrophages in bronchoalveolar lavage fluid samples using oil-red-O staining was previously thought to be a useful marker of the disease process 32. However, the current consensus is that although common, this is an essentially non-specific finding 33.
EVALI prevention
All the patients presenting with signs and symptoms of EVALI (e-cigarette or vaping product use associated lung injury) should be counseled to discontinue vaping as the risk of rehospitalization with potentially severe symptoms exists. Adults without any previous history of smoking tobacco products should not start vaping. Those patients using e-cigarettes or vapes as an alternative to cigarettes should not go back to smoking cigarettes.
CDC strongly advises against the use of all THC-containing vapes or e-cigarettes. Adults using vapes or e-cigarettes to help with tobacco smoking cessation should only buy vaping products from commercially authorized vendors. Potentially harmful ways of exposure, such as dabbing or dripping, must be avoided altogether as users who dab or drip vaping liquid directly onto the heating element are exposed to a much denser cloud of aerosol thereby increasing the risk of lung injury.
EVALI diagnosis
A detailed history and physical should be performed. However, specific diagnostic criteria for EVALI have not been established as there is no single test for EVALI 8. It is important to note that EVALI is a diagnosis of exclusion. Therefore it is necessary to rule out other possible causes of lung injury such as viral pneumonia, community-acquired pneumonia, and any ongoing chronic inflammatory process that might affect the lungs. Diagnosis is mostly a process of elimination because symptoms can be similar to many other respiratory diseases. These include shortness of breath, fever and chills, cough, vomiting, diarrhea, headache, dizziness, rapid heart rate and chest pain. Oxygen saturations less than 95% are reported in 57% of cases 8. Your doctor will evaluate your history of e-cigarette use and other vaping devices.
The CDC has proposed the following 4 required criteria for public health reporting of confirmed EVALI (e-cigarette or vaping product use associated lung injury) 8, 9:
- Using an e-cigarette (vaping) or dabbing during the 90 days before symptom onset;
- Having a pulmonary infiltrate, such as opacities on plain film chest radiograph or ground-glass opacities on chest CT;
- The absence of clinical evidence of a pulmonary infection on initial work-up: Minimum criteria include negative respiratory viral panel, influenza polymerase chain reaction, or rapid test if local epidemiology supports testing. All other clinically indicated respiratory infectious disease testing (e.g., urine antigen for Streptococcus pneumoniae and Legionella, sputum culture if productive cough, bronchoalveolar lavage culture when indicated, blood cultures, HIV–related opportunistic respiratory infections when appropriate) must be negative; and
- No medical record evidence of alternative plausible diagnoses (e.g., cardiac, rheumatologic, or neoplastic process).
Probable EVALI cases
- Cases should be considered possible if they meet criteria 1, 2 and 4, but respiratory infection is identified via culture or PCR and the clinical team believes infection is not the only cause of underlying lung injury OR the minimum criteria to exclude infection are not met due to testing not having been performed and the clinical team believes the infection is not the only cause of the underlying lung injury.
EVALI cases that do not strictly meet the criteria
- EVALI cases that do not strictly meet the criteria, for example if use of e-cigarette or vaping stopped more than 30 days before symptom onset, are still of interest and should be reported.
Locally appropriate and clinically indicated diagnostic evaluation should be performed, including respiratory viral panel, influenza polymerase chain reaction or rapid test, RT-PCR for SARS-COV2, urine antigens of Streptococcus pneumoniae, and Legionella species., sputum culture, bronchoalveolar lavage, blood culture, and testing for HIV-related opportunistic infections, as this may have a similar presentation to EVALI 9.
All patients with a history of vaping product use in the last 90 days should at least get a chest x-ray, even if the symptoms are mild. Those having significant respiratory distress and low oxygen saturation (less than 95%) should be evaluated with a chest computed tomography (CT) scan to see if there are hazy spots on your lungs (called opacities) that indicate tissue damage, if the suspicion for e-cigarette or vaping product use associated lung injury (EVALI) is high. Diagnostic imaging demonstrates a variety of radiographic presentations. Plain chest radiographs commonly show hazy bilateral opacities with central and peripheral sparing. Likewise, the most common CT finding is diffuse bilateral ground-glass opacities, with a basilar predominance and sometimes subpleural or lobular sparing 34.
Laboratory evaluation should include a complete blood count (CBC) with manual white blood cell differential should be performed to detect peripheral eosinophilia, liver transaminases, and inflammatory markers (e.g., erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP]). Moreover, urine toxicology testing, with informed consent, including testing for THC, should be carried out in all patients. Microbiology specimens from sputum and blood should also be obtained. The primary role of bronchoscopy is to exclude alternative diagnoses, especially when the imaging findings are atypical and suggestive of an alternate etiology. Other potential candidates for bronchoscopy include patients with a high suspicion of infection, e.g., immunocompromised patients and those on invasive mechanical ventilation.
Eosinophils have been identified in broncho-alveolar lavage (BAL) or peripheral blood specimens in a few cases, suggesting acute eosinophilic pneumonia 18. Surgical lung biopsies reveal mild and nonspecific inflammation, acute diffuse alveolar damage, organizing pneumonia, acute fibrinous pneumonitis, chemical pneumonitis, foamy macrophages, lipoid pneumonia, and interstitial and peribronchiolar granulomatous pneumonitis 35. Lipoid pneumonia has been reported from the use of tetrahydrocannabinol (THC)-containing vaping cartridges and pens 31. Hypersensitivity pneumonia with ARDS (Acute Respiratory Distress Syndrome) was reported in an 18-year-old nicotine-only electronic cigarette user 19.
EVALI treatment
The mainstay of treatment in e-cigarette or vaping product use associated lung injury (EVALI) is supportive care. The severity of symptoms guides as to whether the patient needs a hospital admission or can be managed on an outpatient basis. Hospital admission is recommended for patients with oxygen saturations less than 95% (hypoxemia) on room air with suspected EVALI 8. It is important not to miss other causes of respiratory and gastrointestinal symptoms. Treatment with antibiotics directed against organisms causing severe community-acquired pneumonia should be started at the time of presentation. Oral or intravenous corticosteroids should be started in consultation with a pulmonologist recognizing that some underlying respiratory infections may worsen with corticosteroids 8. These patients can deteriorate very rapidly and may end up requiring assisted mechanical ventilation. The dose and duration of corticosteroids has not been established. Once the patient has clinically improved, with oxygen saturations greater than 89% on room air, they should be considered for discharge. Patients should have a follow-up visit after discharge in one to two weeks, with a chest x-ray and pulse oximetry. The long-term consequences of EVALI are unknown, so pulmonary function tests, six-minute walk pulse oximetry, and CT chest may be considered one to two months after discharge.
When discharging patients with EVALI, it is crucial to ascertain the patient’s clinical stability as dictated by stable oxygenation and exercise tolerance for 24 to 48 hours prior to planned discharge. These patients should follow-up with their primary care provider or pulmonologist within 48 hours. Furthermore, follow-up testing with spirometry and chest x-ray may be required for some patients as recommended by the pulmonologist.
EVALI prognosis
E-cigarette or vaping product use associated lung injury (EVALI) is a potentially fatal disease, with 68 deaths reported so far 4. A significant number of patients may end up requiring non-invasive or invasive mechanical ventilation. A recent study of 98 EVALI patients showed that as many as 76% of the cases needed supplemental oxygen, 22% required non-invasive ventilation and 26% required intubation and mechanical ventilation 28. Poor prognostic indicators include age more than 35 years, comorbidities that compromise pulmonary reserve, and patients presenting with resting oxygen saturation less than 95% 36. These patients can rapidly deteriorate and end up developing acute respiratory distress syndrome (ARDS).
- Zulfiqar H, Rahman O. Vaping Associated Pulmonary Injury. [Updated 2022 Jan 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560656[↩][↩][↩]
- Salzman GA, Alqawasma M, Asad H. Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic. Mo Med. 2019 Nov-Dec;116(6):492-496. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6913849[↩][↩]
- CDC, States Update Number of Cases of Hospitalized E-cigarette, or Vaping, Product Use Associated Lung Injury (EVALI). https://www.cdc.gov/media/releases/2019/s1206-e-cigarette-vaping-product-hospitalized-update.html[↩]
- Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html[↩][↩][↩][↩]
- Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-cigarette, or Vaping, Product Use–Associated Lung Injury — United States, November 2019. MMWR Morb Mortal Wkly Rep 2019;68:1081-1086. DOI: http://dx.doi.org/10.15585/mmwr.mm6846e2[↩][↩][↩][↩]
- Lung Injuries Associated with Use of Vaping Products. https://www.fda.gov/news-events/public-health-focus/lung-injuries-associated-use-vaping-products[↩]
- Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med. 2020 Feb 20;382(8):697-705. doi: 10.1056/NEJMoa1916433[↩][↩][↩]
- Siegel DA, Jatlaoui TC, Koumans EH, et al; Lung Injury Response Clinical Working Group; Lung Injury Response Epidemiology/Surveillance Group. Update: Interim Guidance for Health Care Providers Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use Associated Lung Injury – United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019 Oct 18;68(41):919-927. doi: 10.15585/mmwr.mm6841e3[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Schier JG, Meiman JG, Layden J, et al; CDC 2019 Lung Injury Response Group. Severe Pulmonary Disease Associated with Electronic-Cigarette-Product Use – Interim Guidance. MMWR Morb Mortal Wkly Rep. 2019 Sep 13;68(36):787-790. doi: 10.15585/mmwr.mm6836e2. Erratum in: MMWR Morb Mortal Wkly Rep. 2019 Sep 27;68(38):830.[↩][↩][↩]
- Adhikari R, Koritala T, Gotur R, et al. (February 24, 2021) EVALI – E-Cigarette or Vaping Product Use-Associated Lung Injury: A Case Report. Cureus 13(2): e13541. doi:10.7759/cureus.13541[↩]
- Henry TS, Kanne JP, Kligerman SJ. Imaging of Vaping-Associated Lung Disease. N Engl J Med. 2019 Oct 10;381(15):1486-1487. https://www.nejm.org/doi/10.1056/NEJMc1911995[↩][↩][↩]
- About Electronic Cigarettes (E-Cigarettes). https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html[↩]
- Tehrani MW, Newmeyer MN, Rule AM, Prasse C. Response to Letter to the Editor Regarding Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography-High-Resolution Mass Spectrometry. Chem Res Toxicol. 2022 Jan 17;35(1):1-2. https://pubs.acs.org/doi/10.1021/acs.chemrestox.1c00253[↩][↩]
- Marynak KL, Gammon DG, Rogers T, Coats EM, Singh T, King BA. Sales of Nicotine-Containing Electronic Cigarette Products: United States, 2015. Am J Public Health. 2017 May;107(5):702-705. doi: 10.2105/AJPH.2017.303660[↩][↩]
- Smoking Cessation: A Report of the Surgeon General. https://www.cdc.gov/tobacco/sgr/2020-smoking-cessation/index.html[↩][↩]
- Flower M, Nandakumar L, Singh M, Wyld D, Windsor M, Fielding D. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017 Apr 3;5(3):e00230. doi: 10.1002/rcr2.230. Erratum in: Respirol Case Rep. 2017 May 08;5(4):e00242.[↩]
- McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012 Apr;141(4):1110-1113. doi: 10.1378/chest.11-1334[↩]
- Arter ZL, Wiggins A, Hudspath C, Kisling A, Hostler DC, Hostler JM. Acute eosinophilic pneumonia following electronic cigarette use. Respir Med Case Rep. 2019 Mar 18;27:100825. doi: 10.1016/j.rmcr.2019.100825[↩][↩]
- Sommerfeld CG, Weiner DJ, Nowalk A, Larkin A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome From E-Cigarette Use. Pediatrics. 2018 Jun;141(6):e20163927. doi: 10.1542/peds.2016-3927[↩][↩]
- Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse Alveolar Hemorrhage Induced by Vaping. Case Rep Pulmonol. 2018 Jun 7;2018:9724530. doi: 10.1155/2018/9724530[↩]
- EVALI: New information on vaping-induced lung injury. https://www.health.harvard.edu/blog/evali-new-information-on-vaping-induced-lung-injury-2020040319359[↩]
- Quick Facts on the Risks of E-cigarettes for Kids, Teens, and Young Adults. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/Quick-Facts-on-the-Risks-of-E-cigarettes-for-Kids-Teens-and-Young-Adults.html[↩]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P et al. Change in mental health after smoking cessation: systematic review and meta-analysis BMJ 2014; 348 :g1151 doi:10.1136/bmj.g1151[↩][↩][↩]
- Colliding Crises: Youth Mental Health and Nicotine Use. https://truthinitiative.org/sites/default/files/media/files/2021/09/Mental-Health-and-Nicotine-Report.pdf[↩]
- Lechner WV, Janssen T, Kahler CW, Audrain-McGovern J, Leventhal AM. Bi-directional associations of electronic and combustible cigarette use onset patterns with depressive symptoms in adolescents. Prev Med. 2017 Mar;96:73-78. doi: 10.1016/j.ypmed.2016.12.034[↩]
- Obisesan OH, Mirbolouk M, Osei AD, et al. Association Between e-Cigarette Use and Depression in the Behavioral Risk Factor Surveillance System, 2016-2017. JAMA Netw Open. 2019;2(12):e1916800. doi:10.1001/jamanetworkopen.2019.16800[↩]
- Winnicka L, Shenoy MA. EVALI and the Pulmonary Toxicity of Electronic Cigarettes: A Review. J Gen Intern Med. 2020 Jul;35(7):2130-2135. doi: 10.1007/s11606-020-05813-2[↩]
- Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin – Final Report. N Engl J Med. 2020 Mar 5;382(10):903-916. doi: 10.1056/NEJMoa1911614[↩][↩][↩]
- Cherian SV, Kumar A, Estrada-Y-Martin RM. E-Cigarette or Vaping Product-Associated Lung Injury: A Review. Am J Med. 2020 Jun;133(6):657-663. doi: 10.1016/j.amjmed.2020.02.004[↩]
- Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, Khoor A, Mira-Avendano I, Patel A, Larsen BT. Pathology of Vaping-Associated Lung Injury. N Engl J Med. 2019 Oct 31;381(18):1780-1781. doi: 10.1056/NEJMc1913069[↩]
- Davidson K, Brancato A, Heetderks P, Mansour W, Matheis E, Nario M, Rajagopalan S, Underhill B, Wininger J, Fox D. Outbreak of Electronic-Cigarette-Associated Acute Lipoid Pneumonia – North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019 Sep 13;68(36):784-786. doi: 10.15585/mmwr.mm6836e1[↩][↩]
- Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, Aberegg SK. Pulmonary Lipid-Laden Macrophages and Vaping. N Engl J Med. 2019 Oct 10;381(15):1488-1489. doi: 10.1056/NEJMc1912038[↩]
- Cecchini MJ, Mukhopadhyay S, Arrossi AV, Beasley MB, Butt YM, Jones KD, Pambuccian S, Mehrad M, Monaco SE, Saqi A, Smith ML, Tazelaar HD, Larsen BT. E-Cigarette or Vaping Product Use-Associated Lung Injury: A Review for Pathologists. Arch Pathol Lab Med. 2020 Dec 1;144(12):1490-1500. doi: 10.5858/arpa.2020-0024-RA[↩]
- Kligerman S, Raptis C, Larsen B, Henry TS, Caporale A, Tazelaar H, Schiebler ML, Wehrli FW, Klein JS, Kanne J. Radiologic, Pathologic, Clinical, and Physiologic Findings of Electronic Cigarette or Vaping Product Use-associated Lung Injury (EVALI): Evolving Knowledge and Remaining Questions. Radiology. 2020 Mar;294(3):491-505. doi: 10.1148/radiol.2020192585[↩]
- Mukhopadhyay S, Mehrad M, Dammert P, Arrossi AV, Sarda R, Brenner DS, Maldonado F, Choi H, Ghobrial M. Lung Biopsy Findings in Severe Pulmonary Illness Associated With E-Cigarette Use (Vaping). Am J Clin Pathol. 2020 Jan 1;153(1):30-39. doi: 10.1093/ajcp/aqz182[↩]
- Lilly CM, Khan S, Waksmundzki-Silva K, Irwin RS. Vaping-Associated Respiratory Distress Syndrome: Case Classification and Clinical Guidance. Crit Care Explor. 2020 Feb 24;2(2):e0081. doi: 10.1097/CCE.0000000000000081[↩]