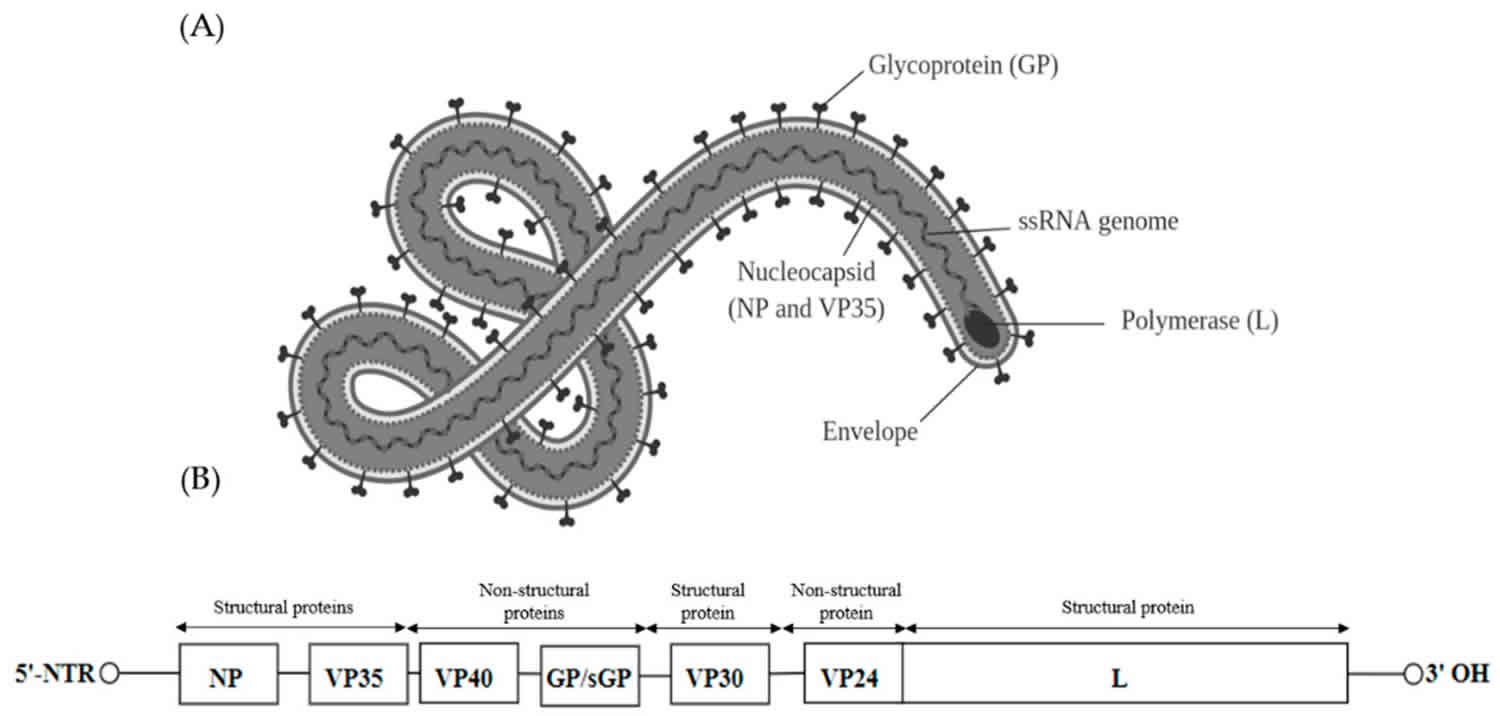
Filoviridae
Filoviruses (Ebola virus and Marburg virus) belong to a virus family called Filoviridae and can cause severe hemorrhagic fever in humans and nonhuman primates 1. So far, three genera of filoviruses or Filoviridae virus family have been identified: Cuevavirus, Marburgvirus and Ebolavirus. These further subdivide into six species: Zaire, Sudan, Tai Forest, Bundibugyo, Reston, and Bombali. Thus far, the Reston and Bombali strains are not known to cause disease in humans 2. Filoviruses are highly contagious and spread quickly via human-human contact. They are considered biosafety level 4 agents with very high mortality rates 3.
Within the genus Ebolavirus, six species of Ebolavirus have been identified: Ebola virus (species Zaire ebolavirus), Sudan virus (species Sudan ebolavirus), Taï Forest virus (species Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus), Bundibugyo virus (species Bundibugyo ebolavirus), Reston virus (species Reston ebolavirus), and Bombali virus (species Bombali ebolavirus) 4. Of these, only four (Ebola, Sudan, Taï Forest, and Bundibugyo viruses) are known to cause disease in people. Reston virus is known to cause disease in nonhuman primates and pigs, but not in people. Bombali virus was recently identified in bats, and it is unknown at this time if it causes disease in either animals or people. In contrast to the genus Ebolavirus, the genus Marburgvirus contains a single virus species (Marburg marburgvirus), albeit consisting of two distinct viruses, Marburg virus and Ravn virus 5, which are approximately 20% divergent from one another 6. The filoviruses are primarily African in origin, with the exception of Reston virus, which so far has been found only in the Philippines or in nonhuman primates originating in the Philippines. In addition, an Ebola-like filovirus, Lloviu virus, was recently identified in bats in Spain 7 and likely represents another distinct genus.
While Ebola virus infections can result in a case fatality approaching 90% 8, the case fatalities associated with other viruses of the genus Ebolavirus appear to be considerably lower. The case fatality associated with Sudan virus infections ranges from 53 to 66% 9 and that of Bundibugyo virus is estimated near 40% based on epidemiologic findings from the 2007 Uganda outbreak 10. Reston virus, while highly pathogenic for nonhuman primates, does not appear to cause disease in humans 11. In the Philippines, several abattoir workers on infected pig farms were found to be seropositive for Reston virus yet reported no clinical symptoms 12. Finally, Taï Forest virus has been described only in a single nonfatal human case 13. Marburg and Ravn virus infections can result in case fatalities ranging from approximately 20% to 90% 6.
Structurally, filoviruses obtained their name due to their macroscopic appearance as filamentous viruses, from the Latin filum for “thread,” often having a stringlike or torus-like appearance 1. However, filovirus virions (complete viral particles) may appear in several shapes, a biological features called pleomorphism. These shapes include long, sometimes branched filaments, as well as shorter filaments shaped like a “6”, a “U”, or a circle 4. Filoviruses have enveloped particles that contain a negative-sense single-stranded RNA genome with genes arranged linearly. Additionally, they have a single glycoprotein spike on the surface and four structural proteins which include the virus-encoded polymerase 2. Viral filaments may measure up to 14,000 nanometers in length, have a uniform diameter of 80 nanometers, and are enveloped in a lipid (fatty) membrane. Each virion contains one molecule of single-stranded, negative-sense RNA. New viral particles are created by budding from the surface of their hosts’ cells; however, filovirus replication strategies are not completely understood.
The first Filovirus was recognized in 1967 when a number of laboratory workers in Germany and Yugoslavia, who were handling tissues from green monkeys, developed hemorrhagic fever 4. A total of 31 cases and 7 deaths were associated with these outbreaks. The virus was named after Marburg, Germany, the site of one of the outbreaks. In addition to the 31 reported cases, an additional primary case was retrospectively serologically diagnosed.
After this initial outbreak, the virus disappeared. It did not reemerge until 1975, when a traveler, most likely exposed in Zimbabwe, became ill in Johannesburg, South Africa. The virus was transmitted there to his traveling companion and a nurse. A few sporadic cases and 2 large epidemics (Democratic Republic of Congo in 1999 and Angola in 2005) of Marburg hemorrhagic fever (Margurg HF) have been identified since that time. For information on known Marburg hemorrhagic fever cases and outbreaks, please refer to the chronological list.
Ebolavirus was first identified in 1976 when two outbreaks of Ebola hemorrhagic fever (Ebola HF) occurred in northern Zaire (now the Democratic Republic of Congo) and southern Sudan. The outbreaks involved what eventually proved to be two different species of Ebola virus; both were named after the nations in which they were discovered. Both viruses showed themselves to be highly lethal, as 90% of the Zairian cases and 50% of the Sudanese cases resulted in death.
Since 1976, Ebolavirus have appeared sporadically in Africa, with small to midsize outbreaks confirmed between 1976 and 1979. Large epidemics of Ebola hemorrhagic fever occurred in Kikwit, Democratic Republic of Congo in 1995, in Gulu, Uganda in 2000, in Bundibugyo, Uganda in 2008, and in Issiro, Democratic Republic of Congo in 2012. Smaller outbreaks were identified in Gabon, Democratic Republic of Congo, and Uganda.
Genetic investigations of filovirus outbreaks have found them to be of two general types, those that are the result of a single introduction into the human population followed by person-to-person spread and those outbreaks that result from multiple introductions followed by short chains of human transmission. During person-to-person transmission, there appears to be little molecular evolution of the virus 6. Initial introduction into the human population is often thought to result from contact with infected carcasses of nonhuman primates or other mammals or direct contact with an infected reservoir host 14. Despite numerous attempts to identify the natural reservoir(s) of the filoviruses over the past ≥30 years, only recently have bats been implicated as possible reservoirs for the ebolaviruses and marburgviruses 15. Over the past 10 years, filovirus RNA and antibodies have been detected in several bat species, but it was not until 2007 that Marburg and Ravn virus isolates were recovered from Egyptian fruit bats (Rousettus aegyptiacus) associated with a small outbreak of Marburg hemorrhagic fever in southwestern Uganda 14. In contrast to the low virus genetic diversity observed with person-to-person virus transmission during human outbreaks, potential bat reservoir populations appear to harbor genetically diverse virus populations at single geographical locations 14.
Although the filoviruses are thought to have originated quite some time ago, age estimates have ranged from a few thousand 16 to hundreds of thousands 7 to a few million 17 years ago. Further confusing the issue, some authors have presented data indicating that viruses of at least one filovirus species (Zaire ebolavirus) share a most recent common ancestor in the very recent past 18. Previous studies have attempted to estimate rates of nonsynonymous substitutions and divergence times among viruses within the Filoviridae; however, most have been limited in terms of sample size 19 or have examined only a single species, in particular, Zaire ebolavirus 18.
Ebola filoviridae
Ebola virus disease is a rare and deadly disease in people and nonhuman primates (such as monkeys, gorillas, and chimpanzees) with occasional outbreaks that occur primarily on the African continent. Ebola virus disease is caused by an infection with a group of viruses within the genus Ebolavirus 20:
- Ebola virus (species Zaire ebolavirus)
- Sudan virus (species Sudan ebolavirus)
- Taï Forest virus (species Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus)
- Bundibugyo virus (species Bundibugyo ebolavirus)
- Reston virus (species Reston ebolavirus)
- Bombali virus (species Bombali ebolavirus)
Of these, only four (Ebola, Sudan, Taï Forest, and Bundibugyo viruses) are known to cause disease in people 20. Reston virus is known to cause disease in nonhuman primates and pigs, but not in people. It is unknown if Bombali virus, which was recently identified in bats, causes disease in either animals or people 20.
The viruses that cause Ebola virus disease are located mainly in sub-Saharan Africa. People can get Ebola virus disease through direct contact with an infected animal (bat or nonhuman primate) or a sick or dead person infected with Ebola virus 21. The U.S. Food and Drug Administration (FDA) has approved the Ebola vaccine rVSV-ZEBOV (tradename “Ervebo”) for the prevention of Ebola virus disease. The rVSV-ZEBOV vaccine has been found to be safe and protective against only the Zaire ebola virus species of ebola virus.
Ebola virus was first discovered in 1976 near the Ebola River in what is now the Democratic Republic of Congo. Since then, the virus has been infecting people from time to time, leading to outbreaks in several African countries. Scientists do not know where Ebola virus comes from. However, based on the nature of similar viruses, they believe the virus is animal-borne, with bats or nonhuman primates with bats or nonhuman primates (chimpanzees, apes, monkeys, etc.) being the most likely source. Infected animals carrying the virus can transmit it to other animals, like apes, monkeys, duikers and humans.
The virus spreads to people initially through direct contact with the blood, body fluids and tissues of animals. Ebola virus then spreads to other people through direct contact with body fluids of a person who is sick with or has died from Ebola virus disease. This can occur when a person touches these infected body fluids (or objects that are contaminated with them), and the virus gets in through broken skin or mucous membranes in the eyes, nose, or mouth. People can get the virus through sexual contact with someone who is sick with Ebola virus disease, and also after recovery from Ebola virus disease. The virus can persist in certain body fluids, like semen, after recovery from the illness.
Ebola survivors may experience side effects after their recovery, such as tiredness, muscle aches, eye and vision problems and stomach pain.
Persistence of the Ebola virus
Ebola virus can remain in areas of the body that are immunologically privileged sites after acute infection. These are sites where viruses and pathogens, like the Ebola virus, are shielded from the survivor’s immune system, even after being cleared elsewhere in the body. These areas include the testes, interior of the eyes, placenta, and central nervous system, particularly the cerebrospinal fluid. Whether the virus is present in these body parts and for how long varies by survivor. Scientists are now studying how long the virus stays in these body fluids among Ebola survivors.
During an Ebola outbreak, the virus can spread quickly within healthcare settings (such as clinics or hospitals). Clinicians and other healthcare personnel providing care should use dedicated, preferably disposable, medical equipment. Proper cleaning and disposal of instruments such as needles and syringes are important. If instruments are not disposable, they must be sterilized before using again.
Ebola virus can survive on dry surfaces, like doorknobs and countertops for several hours; in body fluids like blood, the virus can survive up to several days at room temperature. Cleaning and disinfection should be performed using a hospital-grade disinfectant.
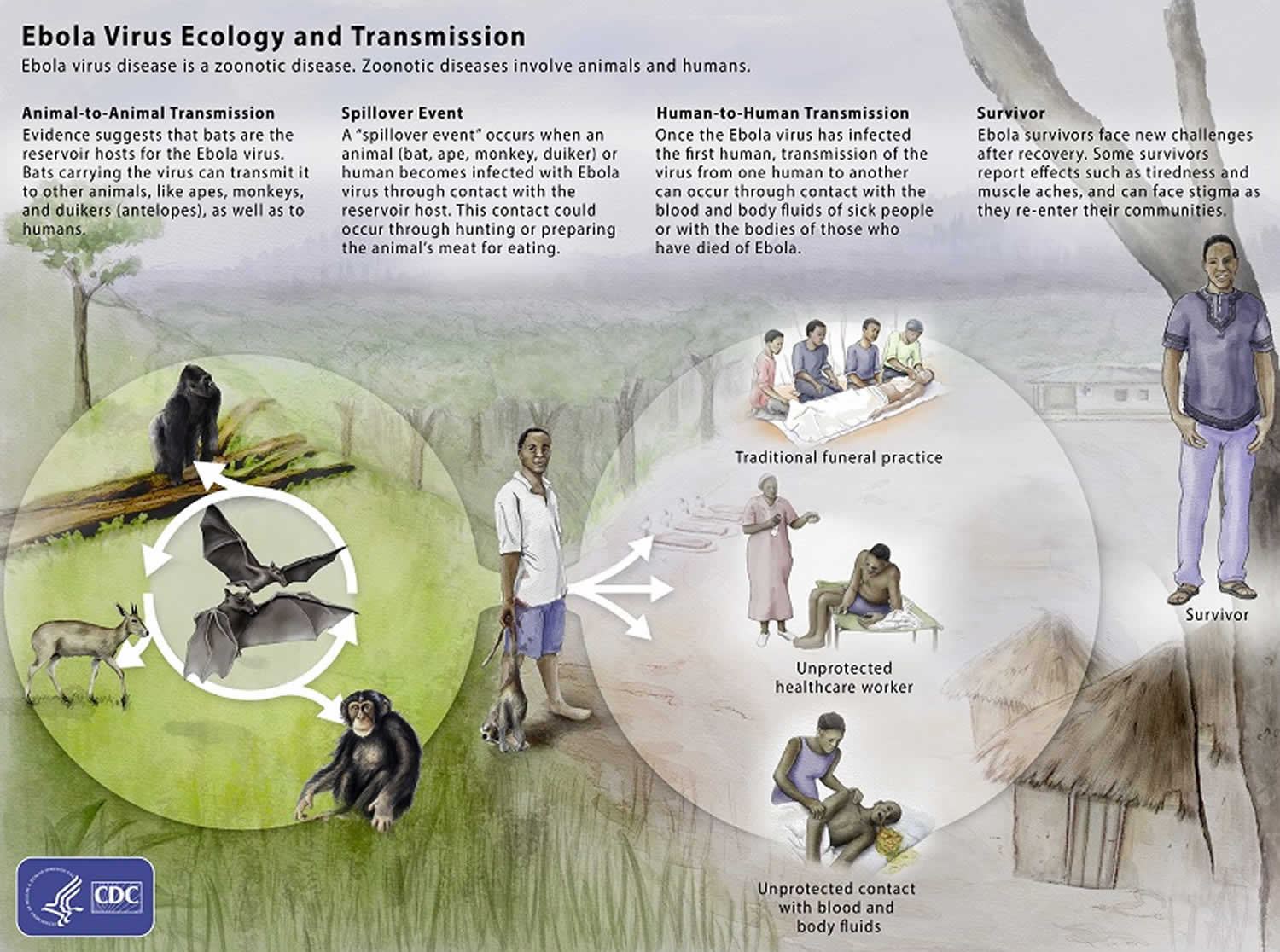
Figure 1. Ebola virus disease transmission
Ebola virus key points
- Ebola virus disease can be confused with other more common infectious diseases such as malaria, typhoid fever, meningococcemia, and other bacterial infections.
- Gastrointestinal symptoms may develop after about 5 days to develop symptoms such as severe watery diarrhea, nausea, vomiting, and abdominal pain.
- Ebola virus enters the patient through mucous membranes, breaks in the skin, or parenterally. Healthcare personnel must prevent direct contact or splashes with blood and body fluids, contaminated equipment, and soiled environmental surfaces.
- Travelers with possible exposure to Ebola virus may need public health monitoring and movement controls depending on the risk of exposure and clinical presentation. Clinicians should contact local or state health departments for more information.
Ebola virus transmission
Scientists think people are initially infected with Ebola virus through contact with an infected animal, such as a fruit bat or nonhuman primate 22. This is called a spillover event. After that, the virus spreads from person to person, potentially affecting a large number of people.
Ebola virus spreads through direct contact (such as through broken skin or mucous membranes in the eyes, nose, or mouth) with:
- Blood or body fluids (urine, saliva, sweat, feces, vomit, breast milk, and semen) of a person who is sick with or has died from Ebola virus disease.
- Objects (such as clothes, bedding, needles, and medical equipment) contaminated with body fluids from a person who is sick with or has died from Ebola virus disease.
- Infected fruit bats or nonhuman primates (such as apes and monkeys).
- Semen from a man who recovered from Ebola virus disease (through oral, vaginal, or anal sex). The virus can remain in certain body fluids (including semen) of a patient who has recovered from Ebola virus disease, even if they no longer have symptoms of severe illness. There is no evidence that Ebola can be spread through sex or other contact with vaginal fluids from a woman who has had Ebola.
Additionally, Ebola virus is not known to be transmitted through food. However, in certain parts of the world, Ebola virus may spread through the handling and consumption of wild animal meat or hunted wild animals infected with Ebola. There is no evidence that mosquitoes or other insects can transmit Ebola virus.
When people become infected with Ebola, they do not start developing signs or symptoms right away. This period between exposure to an illness and having symptoms is known as the incubation period. A person can only spread Ebola to other people after they develop signs and symptoms of Ebola.
Patients with Ebola virus disease generally have abrupt onset of fever and symptoms typically 8 to12 days after exposure (incubation period for current outbreak has a mean of approximately 9 to 11 days). Initial signs and symptoms are nonspecific and may include elevated body temperature or subjective fever, chills, myalgias, and malaise. Because of these nonspecific symptoms, particularly early in the course of the disease, Ebola virus disease often can be confused with other more common infectious diseases such as malaria, typhoid fever, meningococcemia, and other bacterial infections (for example, pneumonia).
Patients can progress from the initial nonspecific symptoms after about 5 days to develop gastrointestinal symptoms such as severe watery diarrhea, nausea, vomiting, and abdominal pain. Other symptoms such as chest pain, shortness of breath, headache, or confusion also may develop. Patients often have conjunctival injection. Hiccups have been reported. Seizures may occur, and cerebral edema has been reported. Bleeding is not universally present but can manifest later in the course as petechiae, ecchymosis/bruising, or oozing from venipuncture sites and mucosal hemorrhage. Frank hemorrhage is less common. In the current outbreak unexplained bleeding has been reported in only 18% of patients, most often blood in the stool (about 6%). Patients may develop a diffuse erythematous maculopapular rash by day 5 to 7 (usually involving the neck, trunk, and arms) that can desquamate. Pregnant women may experience spontaneous miscarriages. The most common signs and symptoms reported from West Africa during the current outbreak from symptom-onset to the time the case was detected include: fever (87%), fatigue (76%), vomiting (68%), diarrhea (66%), and loss of appetite (65%).
Patients with fatal disease usually develop more severe clinical signs early during infection and die typically between days 6 and 16 of complications including multiorgan failure and septic shock (mean of 7.5 days from symptom onset to death during the 2014-2016 outbreak in West Africa). In nonfatal cases, patients may have fever for several days and improve, typically around day 6. Patients who survive can have a prolonged convalescence.
Ebola virus enters the patient through mucous membranes, breaks in the skin, or parenterally and infects broad range of cell types, including monocytes, macrophages, dendritic cells, endothelial cells, fibroblasts, hepatocytes, adrenal cortical cells, and epithelial cells. After attachment to the host cell, mediated by the surface glycoprotein, the virus gets internalized via macropinocytosis. Binding efficacy of Ebola virus is related to acid sphingomyelinase and plasma membrane sphingomyelin on the host cell 23. On the viral side, attachment gets mediated via its a glycoprotein, a triplet of heterodimers each containing a binding and fusion subunit. Once internalized into the host cell, the virus travels into the late endosome where cysteine protease activates the fusogenic form of the surface glycoprotein which exposes the binding domain and results in fusion of the viral envelope with the endosome membrane releasing the viral nucleocapsid into the cytoplasm with resultant transcription, replication, and budding of new viruses. After infection begins, the virus spreads to many cell types with resultant generalized organ failure 3. Ebola virus migrates from the initial infection site to regional lymph nodes and subsequently to the liver, spleen, and adrenal gland. Although not infected by Ebola virus, lymphocytes undergo apoptosis resulting in decreased lymphocyte counts. Hepatocellular necrosis occurs and is associated with dysregulation of clotting factors and subsequent coagulopathy. Adrenocortical necrosis also can be found and is associated with hypotension and impaired steroid synthesis. Ebola virus appears to trigger a release of pro-inflammatory cytokines with subsequent vascular leak and impairment of clotting ultimately resulting in multiorgan failure and shock.
Risk factors for Ebola virus disease
- Health workers who do not use proper infection control while caring for Ebola patients, and family and friends in close contact with Ebola patients, are at the highest risk of getting sick.
- Ebola can spread when people come into contact with infected blood or body fluids.
- Ebola poses little risk to travelers or the general public who have not cared for or been in close contact (within 3 feet or 1 meter) with someone sick with Ebola.
Ebola virus prevention
In the United States, Ebola virus disease is a very rare disease that has only occurred because of cases that were acquired in other countries, eventually followed by person-to-person transmission. Ebola virus disease is most common in parts of sub-Saharan Africa, with occasional outbreaks occurring in people. In these areas, Ebola virus is believed to circulate at low rates in certain animal populations (enzootic). Occasionally people become sick with Ebola after coming into contact with these infected animals, which can then lead to Ebola outbreaks where the virus spreads between people.
When living in or traveling to a region where Ebola virus is present, there are a number of ways to protect yourself and prevent the spread of Ebola virus disease:
- Contact with blood and body fluids (such as urine, feces, saliva, sweat, vomit, breast milk, semen, and vaginal fluids) of persons who are ill.
- Contact with semen from a man who has recovered from Ebola virus disease, until testing verifies the virus is gone from the semen.
- Items that may have come in contact with an infected person’s blood or body fluids (such as clothes, bedding, needles, and medical equipment).
- Funeral or burial rituals that require handling the body of someone who died from Ebola virus disease.
- Contact with bats and nonhuman primates’ blood, fluids, or raw meat prepared from these animals (bushmeat).
- Contact with the raw meat of an unknown source.
These same prevention methods apply when living in or traveling to an area affected by an Ebola outbreak. After returning from an area affected by Ebola, monitor your health for 21 days and seek medical care immediately if you develop symptoms of Ebola virus disease.
Ebola vaccine
The U.S. Food and Drug Administration (FDA) approved the Ebola vaccine rVSV-ZEBOV (tradename “Ervebo”) on December 19, 2019. The rVSV-ZEBOV vaccine is a single dose vaccine regimen that has been found to be safe and protective against only the Zaire ebolavirus species of ebolavirus. This is the first FDA approval of a vaccine for Ebola.
Another investigational vaccine was developed and introduced under a research protocol in 2019 to combat an Ebola outbreak in the Democratic Republic of the Congo. This vaccine leverages two different vaccine components (Ad26.ZEBOV and MVA-BN-Filo) and requires two doses with an initial dose followed by a second “booster” dose 56 days later. The second vaccine is also designed to protect against only the Zaire ebolavirus species of Ebola.
Ebola virus signs and symptoms
Ebola virus symptoms may appear anywhere from 2 to 21 days after contact with the virus, with an average of 8 to 10 days. The course of the illness typically progresses from “dry” symptoms initially (such as fever, aches and pains, and fatigue), and then progresses to “wet” symptoms (such as diarrhea and vomiting) as the person becomes sicker.
Primary signs and symptoms of Ebola often include some or several of the following:
- Fever
- Aches and pains, such as severe headache, muscle and joint pain, and abdominal (stomach) pain
- Weakness and fatigue
- Gastrointestinal symptoms including diarrhea and vomiting
- Abdominal (stomach) pain
- Unexplained hemorrhaging, bleeding or bruising
Other symptoms may include red eyes, skin rash, and hiccups (late stage).
Many common illnesses can have the same symptoms as Ebola virus disease, including influenza (flu), malaria, or typhoid fever.
Ebola virus disease is a rare but severe and often deadly disease. Recovery from Ebola virus disease depends on good supportive clinical care and the patient’s immune response. Studies show that survivors of Ebola virus infection have antibodies (proteins made by the immune system that identify and neutralize invading viruses) that can be detected in the blood up to 10 years after recovery. Survivors are thought to have some protective immunity to the type of Ebola that sickened them.
Ebola virus diagnosis
Diagnosing Ebola virus disease shortly after infection can be difficult. Early symptoms of Ebola virus disease such as fever, headache, and weakness are not specific to Ebola virus infection and often are seen in patients with other more common diseases, like malaria and typhoid fever.
To determine whether Ebola virus disease is a possible diagnosis, there must be a combination of symptoms suggestive of Ebola virus disease AND a possible exposure to Ebola virus disease within 21 days before the onset of symptoms. An exposure may include contact with:
- blood or body fluids from a person sick with or who died from Ebola virus disease,
- objects contaminated with blood or body fluids of a person sick with or who died from Ebola virus disease,
- infected fruit bats and nonhuman primates (apes or monkeys), or
- semen from a man who has recovered from Ebola virus disease.
Laboratory findings at admission may include leukopenia frequently with lymphopenia followed later by elevated neutrophils and a left shift. Platelet counts often are decreased in the 50,000 to 100,000 range. Amylase may be elevated, reflecting pancreatic involvement (inflammation/infection). Hepatic transaminases are elevated with aspartate aminotransferase (AST) exceeding alanine aminotransferase (ALT); these values may peak at more than 1,000 IU/L. Proteinuria may be present. Prothrombin (PT) and partial thromboplastin times (PTT) are prolonged and fibrin degradation products are elevated, consistent with disseminated intravascular coagulation (DIC).
If a person shows signs of Ebola virus disease and has had a possible exposure, he or she should be isolated (separated from other people) and public health authorities notified. Blood samples from the patient should be collected and tested to confirm infection. Ebola virus can be detected in blood after onset of symptoms. It may take up to three days after symptoms start for the virus to reach detectable levels.
Polymerase chain reaction (PCR) is one of the most commonly used diagnostic methods because of its ability to detect low levels of Ebola virus. PCR methods can detect the presence of a few virus particles in small amounts of blood, but the ability to detect the virus increases as the amount of virus increases during an active infection. When the virus is no longer present in great enough numbers in a patient’s blood, PCR methods will no longer be effective. Other methods, based on the detection of antibodies Ebola virus disease case produces to an infection, can then be used to confirm a patient’s exposure and infection by Ebola virus.
A positive laboratory test means that Ebola infection is confirmed. Public health authorities will conduct a public health investigation, including identifying and monitoring all possibly exposed contacts.
Ebola virus treatment
Clinical management of Ebola virus disease should focus on supportive care of complications, such as hypovolemia, electrolyte abnormalities, hematologic abnormalities, refractory shock, hypoxia, hemorrhage, septic shock, multiorgan failure, and DIC.
Symptoms of Ebola virus disease are treated as they appear. When used early, basic interventions can significantly improve the chances of survival. These include:
- Providing fluids and electrolytes (body salts) through infusion into the vein (intravenously).
- Offering oxygen therapy to maintain oxygen status.
- Using medication to support blood pressure, reduce vomiting and diarrhea and to manage fever and pain.
- Treating other infections, if they occur.
Recommended care includes volume repletion, maintenance of blood pressure (with vasopressors if needed), and maintenance of oxygenation, pain control, nutritional support, and treatment of secondary bacterial infections and pre-existing comorbidities. Large volumes of intravenous fluids are often required to correct dehydration due to diarrhea and vomiting. Some patients may develop profound third-spacing of fluids due to vascular leak. Some organizations suggest the addition of broad-spectrum antimicrobials, particularly in patients with evidence of septic shock. Infection prevention and control measures are a critical part of clinical management. Consider all bodily fluids and clinical specimens as potentially infectious.
Antiviral drugs
There is currently no antiviral drug licensed by the U.S. Food and Drug Administration (FDA) to treat Ebola virus disease in people.
During the 2018 eastern Democratic Republic of the Congo outbreak, four investigational treatments were initially available to treat patients with confirmed Ebola. For two of those treatments, called regeneron (REGN-EB3) and mAb114, overall survival was much higher. In clinical trials, patients receiving regeneron (REGN-EB3) and mAb114 had a greater chance of survival compared to patients receiving other treatments under investigation. The overall survival for those receiving either mAb114 or REGN-EB3 was approximately 70% and for those presenting with a low viral load it was approximately 90%. These two antiviral drugs currently remain in use for patients with confirmed Ebola.
Regeneron (REGN-EB3) is a three monoclonal antibody combination/cocktail intended for use in all patients. mAb114 is a monoclonal antibody molecule that flags the Ebola virus (Zaire ebolavirus) for action by the immune system. mAb114 is intended for use in infected adults and children who are at early stages of infection symptoms.
Drugs that are being developed to treat Ebola virus disease work by stopping the virus from making copies of itself.
Marburg virus
Marburg hemorrhagic fever is a rare but severe hemorrhagic fever which affects both humans and non-human primates. Marburg hemorrhagic fever is caused by Marburg virus, a genetically unique zoonotic (animal-borne) RNA virus of the filovirus family. The case-fatality rate for Marburg hemorrhagic fever is between 23-90% 24.
Marburg virus was first recognized in 1967, when outbreaks of hemorrhagic fever occurred simultaneously in laboratories in Marburg and Frankfurt, Germany and in Belgrade, Yugoslavia (now Serbia). Thirty-one people became ill, initially laboratory workers followed by several medical personnel and family members who had cared for them. Seven deaths were reported. The first people infected had been exposed to imported African green monkeys or their tissues while conducting research. One additional case was diagnosed retrospectively.
The reservoir host of Marburg virus is the African fruit bat, Rousettus aegyptiacus. Fruit bats infected with Marburg virus do not to show obvious signs of illness. Primates (including humans) can become infected with Marburg virus, and may develop serious disease with high mortality. Further study is needed to determine if other species may also host the virus.
This Rousettus bat is a sighted, cave-dwelling bat widely distributed across Africa. Given the fruit bat’s wide distribution, more areas are potentially at risk for outbreaks of Marburg hemorrhagic fever than previously suspected. The virus is not known to be native to other continents, such as North America.
Marburg hemorrhagic fever typically appears in sporadic outbreaks throughout Africa; laboratory confirmed cases have been reported in Uganda, Zimbabwe, the Democratic Republic of the Congo, Kenya, Angola, and South Africa. Many of the outbreaks started with male mine workers working in bat-infested mines. The virus is then transmitted within their communities through cultural practices, under-protected family care settings, and under-protected health care staff. It is possible that sporadic, isolated cases occur as well, but go unrecognized.
Cases of Marburg hemorrhagic fever have occurred outside Africa, such as during the 1967 outbreak, but are infrequent. In 2008, a Dutch tourist developed Marburg hemorrhagic fever after returning to the Netherlands from Uganda, and subsequently died. Also in 2008, an American traveler developed Marburg hemorrhagic fever after returning to the US from Uganda and recovered. Both travelers had visited a well-known cave inhabited by fruit bats in a national park. See the History of Outbreaks table for a chronological list of known cases and outbreaks.
Marburg virus transmission
It is unknown how Marburg virus first transmits from its animal host to humans; however, for the 2 cases in tourists visiting Uganda in 2008, unprotected contact with infected bat feces or aerosols are the most likely routes of infection.
After this initial crossover of virus from host animal to humans, transmission occurs through person-to-person contact. This may happen in several ways: direct contact to droplets of body fluids from infected persons, or contact with equipment and other objects contaminated with infectious blood or tissues.
In previous outbreaks, persons who have handled infected non-human primates or have come in direct contact with their fluids or cell cultures have become infected. Spread of the virus between humans has occurred in close environments and direct contacts. A common example is through caregivers in the home or in a hospital (nosocomial transmission).
Risk of exposure to Marburg virus
People who have close contact with African fruit bats, humans patients, or non-human primates infected with Marburg virus are at risk.
Historically, the people at highest risk include family members and hospital staff who care for patients infected with Marburg virus and have not used proper barrier nursing techniques. Particular occupations, such as veterinarians and laboratory or quarantine facility workers who handle non-human primates from Africa, may also be at increased risk of exposure to Marburg virus.
Exposure risk can be higher for travelers visiting endemic regions in Africa, including Uganda and other parts of central Africa, and have contact with fruit bats, or enter caves or mines inhabited by fruit bats.
Marburg virus prevention
Preventive measures against Marburg virus infection are not well defined, as transmission from wildlife to humans remains an area of ongoing research. However, avoiding fruit bats, and sick non-human primates in central Africa, is one way to protect against infection.
Measures for prevention of secondary, or person-to-person, transmission are similar to those used for other hemorrhagic fevers. If a patient is either suspected or confirmed to have Marburg hemorrhagic fever, barrier nursing techniques should be used to prevent direct physical contact with the patient. These precautions include wearing of protective gowns, gloves, and masks; placing the infected individual in strict isolation; and sterilization or proper disposal of needles, equipment, and patient excretions.
Marburg hemorrhagic fever is a very rare human disease. However, when it occurs, it has the potential to spread to other people, especially health care staff and family members who care for the patient. Therefore, increasing awareness in communities and among health-care providers of the clinical symptoms of patients with Marburg hemorrhagic fever is critical. Better awareness can lead to earlier and stronger precautions against the spread of Marburg virus in both family members and health-care providers. Improving the use of diagnostic tools is another priority. With modern means of transportation that give access even to remote areas, it is possible to obtain rapid testing of samples in disease control centers equipped with Biosafety Level 4 laboratories in order to confirm or rule out Marburg virus infection.
Marburg virus signs and symptoms
After an incubation period of 5-10 days, symptom onset is sudden and marked by fever, chills, headache, and myalgia. Around the fifth day after the onset of symptoms, a maculopapular rash, most prominent on the trunk (chest, back, stomach), may occur. Nausea, vomiting, chest pain, a sore throat, abdominal pain, and diarrhea may then appear. Symptoms become increasingly severe and can include jaundice, inflammation of the pancreas, severe weight loss, delirium, shock, liver failure, massive hemorrhaging, and multi-organ dysfunction.
Because many of the signs and symptoms of Marburg hemorrhagic fever are similar to those of other infectious diseases such as malaria or typhoid fever, clinical diagnosis of the disease can be difficult, especially if only a single case is involved.
Marburg virus diagnosis
Many of the signs and symptoms of Marburg hemorrhagic fever are similar to those of other more frequent infectious diseases, such as malaria or typhoid fever, making diagnosis of the disease difficult. This is especially true if only a single case is involved.
However, if a person has the early symptoms of Marburg hemorrhagic fever and there is reason to believe that Marburg hemorrhagic fever should be considered, the patient should be isolated and public health professionals notified. Samples from the patient can then be collected and tested to confirm infection.
Antigen-capture enzyme-linked immunosorbent assay (ELISA) testing, polymerase chain reaction (PCR), and IgM-capture ELISA can be used to confirm a case of Marburg hemorrhagic fever within a few days of symptom onset. Virus isolation may also be performed but should only be done in a high containment laboratory with good laboratory practices. The IgG-capture ELISA is appropriate for testing persons later in the course of disease or after recovery. In deceased patients, immunohistochemistry, virus isolation, or PCR of blood or tissue specimens may be used to diagnose Marburg hemorrhagic fever retrospectively.
Marburg virus treatment
There is no specific treatment for Marburg hemorrhagic fever. Supportive hospital therapy should be utilized, which includes balancing the patient’s fluids and electrolytes, maintaining oxygen status and blood pressure, replacing lost blood and clotting factors, and treatment for any complicating infections.
Experimental treatments are validated in non-human primates models, but have never been tried in humans.
- Kerper M, Puckett Y. Filovirus. [Updated 2020 Jun 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544258[↩][↩]
- Ponce L, Kinoshita R, Nishiura H. Exploring the human-animal interface of Ebola virus disease outbreaks. Math Biosci Eng. 2019 Apr 11;16(4):3130-3143.[↩][↩]
- Malvy D, McElroy AK, de Clerck H, Günther S, van Griensven J. Ebola virus disease. Lancet. 2019 Mar 02;393(10174):936-948.[↩][↩]
- Filoviridae. https://www.cdc.gov/vhf/virus-families/filoviridae.html[↩][↩][↩]
- Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB. 2010. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch. Virol. 155:2083–2103.[↩]
- Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA, Hartman AL, Comer JA, Zaki SR, Stroher U, Gomes DA Silva F, del Castillo F, Rollin PE, Ksiazek TG, Nichol ST. 2006. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 80:6497–6516.[↩][↩][↩]
- Negredo A, Palacios G, Vazquez-Moron S, Gonzalez F, Dopazo H, Molero F, Juste J, Quetglas J, Savji N, de la Cruz Martinez M, Herrera JE, Pizarro M, Hutchison SK, Echevarria JE, Lipkin WI, Tenorio A. 2011. Discovery of an ebolavirus-like filovirus in Europe. PLoS Pathog. 7:e1002304. doi:doi:10.1371/journal.ppat.1002304[↩][↩]
- Sanchez A, Geisbert TW, Feldmann H. 2007. Filoviridae: Marburg and Ebola Viruses, p 1409–1448. In Knipe DM, Howley PM (ed), Fields virology. Lippincott Williams and Wilkins, Philadelphia, PA.[↩]
- Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, Lee WF, Spiropoulou CF, Ksiazek TG, Lukwiya M, Kaducu F, Downing R, Nichol ST. 2004. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J. Virol. 78:4330–4341.[↩]
- MacNeil A, Farnon EC, Wamala J, Okware S, Cannon DL, Reed Z, Towner JS, Tappero JW, Lutwama J, Downing R, Nichol ST, Ksiazek TG, Rollin PE. 2010. Proportion of deaths and clinical features in Bundibugyo Ebola virus infection, Uganda. Emerg. Infect. Dis. 16(12):1969–1972.[↩]
- Barrette RW, Xu L, Rowland JM, McIntosh MT. 2011. Current perspectives on the phylogeny of Filoviridae. Infect. Genet. Evol. 11(7):1514–1519.[↩]
- Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, Rollin PE, Towner JS, Shieh W-J, Batten B, Sealy TK, Carrillo C, Moran KE, Bracht AJ, Mayr GA, Sirios-Cruz M, Catbagan DP, Lautner EA, Ksiazek TG, White WR, McIntosh MT. 2009. Discovery of swine as a host for the Reston ebolavirus. Science 325:204–206.[↩]
- Le Guenno B, Formenty P, Wyers M, Gounon P, Walker F, Boesch C. 1995. Isolation and partial characterization of a new strain of Ebola virus. Lancet 345:1271–1274.[↩]
- Towner JS, Amman BR, Sealy TK, Carroll SAR, Comer JA, Kemp A, Swanepoel R, Paddock CD, Balinandi S, Khristova ML, Formenty PBH, Albarino CG, Miller DM, Reed ZD, Kayiwa JT, Mills JN, Cannon DL, Greer PW, Byaruhanga E, Farnon EC, Atimnedi P, Okware S, Katongole-Mbidde E, Downing R, Tappero JW, Zaki SR, Ksiazek TG, Nichol ST, Rollin PE. 2009. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 5:e1000536. doi:doi:10.1371/journal.ppat.1000536[↩][↩][↩]
- Swanepoel R, Smit SB, Rollin PE, Formenty P, Leman PA, Kemp A, Burt FJ, Grobbelaar AA, Croft J, Bausch DG, Zeller H, Leirs H, Braack LEO, Libande ML, Zaki S, Nichol ST, Ksiazek TG, Paweska JT, International Scientific and Technical Committee for Marburg Hemorrhagic Fever Control in the Democratic Republic of the Congo. 2007. Studies of reservoir hosts for Marburg virus. Emerg. Infect. Dis. 13:1847–1851.[↩]
- Wertheim JO, Kosakovsky Pond SL. 2011. Purifying selection can obscure the ancient age of viral lineages. Mol. Biol. Evol. 28:3355–3365.[↩]
- Taylor DJ, Dittmar K, Ballinger MJ, Bruenn JA. 2011. Evolutionary maintenance of filovirus-like genes in bat genomes. BMC Evol. Biol. 11:336.[↩]
- Wittmann TJ, Biek R, Hassanin A, Rouquet P, Reed P, Yaba P, Pourrut X, Real LA, Gonzalez JP, Leroy EM. 2007. Isolates of Zaire ebolavirus from wild apes reveal genetic lineage and recombinants. Proc. Natl. Acad. Sci. U. S. A. 104:17123–17127.[↩][↩]
- Suzuki Y, Gojobori T. 1997. The origin and evolution of Ebola and Marburg viruses. Mol. Biol. Evol. 14:800–806.[↩]
- What is Ebola Virus Disease? https://www.cdc.gov/vhf/ebola/about.html[↩][↩][↩]
- Ebola (Ebola Virus Disease). https://www.cdc.gov/vhf/ebola/transmission/index.html[↩]
- Ebola (Ebola Virus Disease) Transmission. https://www.cdc.gov/vhf/ebola/transmission/index.html[↩]
- Wynne JW, Todd S, Boyd V, Tachedjian M, Klein R, Shiell B, Dearnley M, McAuley AJ, Woon AP, Purcell AW, Marsh GA, Baker ML. Comparative Transcriptomics Highlights the Role of the Activator Protein 1 Transcription Factor in the Host Response to Ebolavirus. J. Virol. 2017 Dec 01;91(23).[↩]
- Outbreaks Chronology: Marburg Hemorrhagic Fever. https://www.cdc.gov/vhf/marburg/outbreaks/chronology.html [↩]