Gonococcal arthritis
Gonococcal arthritis is a bacterial arthritis that develops in approximately 42-85% of patients with disseminated gonococcal infection of Gram-negative diplococcus Neisseria gonorrhoeae from primary sexually acquired mucosal gonococcal infection 1. Gonococcal arthritis has become rare in Western countries since the introduction of effective control programmes 2, but it still needs to be recognized promptly to avoid potentially life-threatening systemic involvement, destructive changes associated with chronic arthritis and spread of the gonococcal infection. Sexually active women are predominantly affected. Gonococcal arthritis is more commonly seen in young healthy individuals although it may occur in any age group 3. Clinical features include polyarthralgia, sometimes migratory, tenosynovitis, arthritis, constitutional symptoms and skin lesions, which are mild and easily unnoticed. True arthritis occurs in less than 50% of cases 4.
Gonococcal arthritis may be classified into bacteraemic form (arthritis-dermatitis syndrome) and suppurative form, but many patients have features of both 5. In bacteraemic form also called arthritis-dermatitis syndrome, clinical features include migratory polyarthralgia (knees, elbows and ankles), moderate fever, chills, vesicular or pustular lesions, tenosynovitis (50 to 60% of cases of gonococcal arthritis) particularly extensor tendon of the hands, wrists, fingers, toes and ankles 6. Tenosynovitis and polyarthralgia may be related to autoimmune abnormalities induced by Neisseria gonorrhoeae. In suppurative form, septic arthritis (joint swelling and effusion) is frequent, it occurs in 50% of disseminated gonococcal infection patients. Suppurative gonococcal arthritis is frequently monoarticular. The most commonly involved joint are the knees, wrists, ankles and fingers. Hip involvement is rare 7. Septic discitis 8 and sternoclavicular arthritis are even rarer 9.
Risk factors for gonococcal arthritis are 10:
- Female sex,
- Pregnancy,
- Menstruations,
- Multiple sexual partners,
- Low socio-economic status,
- Intravenous drug use,
- Complement deficiency,
- HIV infection,
- Systemic lupus erythematosus (SLE),
- Gonococcus strain characteristics (Protein 1A serotype, Lack of protein II) 5
Hospitalization of gonococcal arthritis patients is recommended to confirm diagnosis, search systemic complications including endocarditis, myocarditis, hepatitis (Fitz -Hugh -Curtis syndrome), meningitis and to start antibiotic treatment 5. Neisseria gonorrhoeae is isolated from blood and synovial cultures in 50 % of gonococcal arthritis patients. When a synovial effusion is present, it should be aspirated. The leukocyte count in synovial fluid is inflammatory, usually in the range of 10 000 to 100 000 cells/mm³ 5. In patients with purulent joint effusions, synovial fluid culture is often positive with negative blood cultures 11. Gonococcal arthritis responds well to antibiotics and prognosis is good when appropriate therapy is quickly initiated. Destructive arthritis may be observed in HIV patients or in chronic infections due to inappropriate treatment. Third-generation cephalosporins are the first choice treatment 12, such as ceftriaxone, (1 g IM/IV), ceftizoxime (1g IM/IV every 8 hours) and cefotaxime (1g IV every 8 hours). If the woman is allergic to β lactam drugs, spectinomycin, 2 g IM every 12 hours may be used. Spectinomycin and ceftriaxone can be used in pregnant women. Parenteral antibiotics should be continued until symptoms have improved for 24-48 hours. Oral therapy may then be prescribed to complete 7 days of antibiotic. Cefixime 400 mg twice a day, ciprofloxacin 500 mg twice daily, can be given orally, Ciprofloxacin is contraindicated during pregnancy.
Repeat cultures of all positive sites at least 5 days after the last antibiotic dose are recommended. Infected joints should be aspirated to monitor the decrease in leukocyte count of synovial fluid. Saline lavage can also be used. Surgical treatment is exceptionally indicated 5. If chlamydial infection is identified, tetracycline or doxycycline for 7 days (not allowed in pregnant women) or azithromycin (a single 1 g oral dose) should be started. Patients should be educated about the mode of transmission of gonorrhea, tested for HIV and syphilis initially and after 4-6 weeks. Sexual partners should also be treated to prevent dissemination and gonococcal re- infection 5.
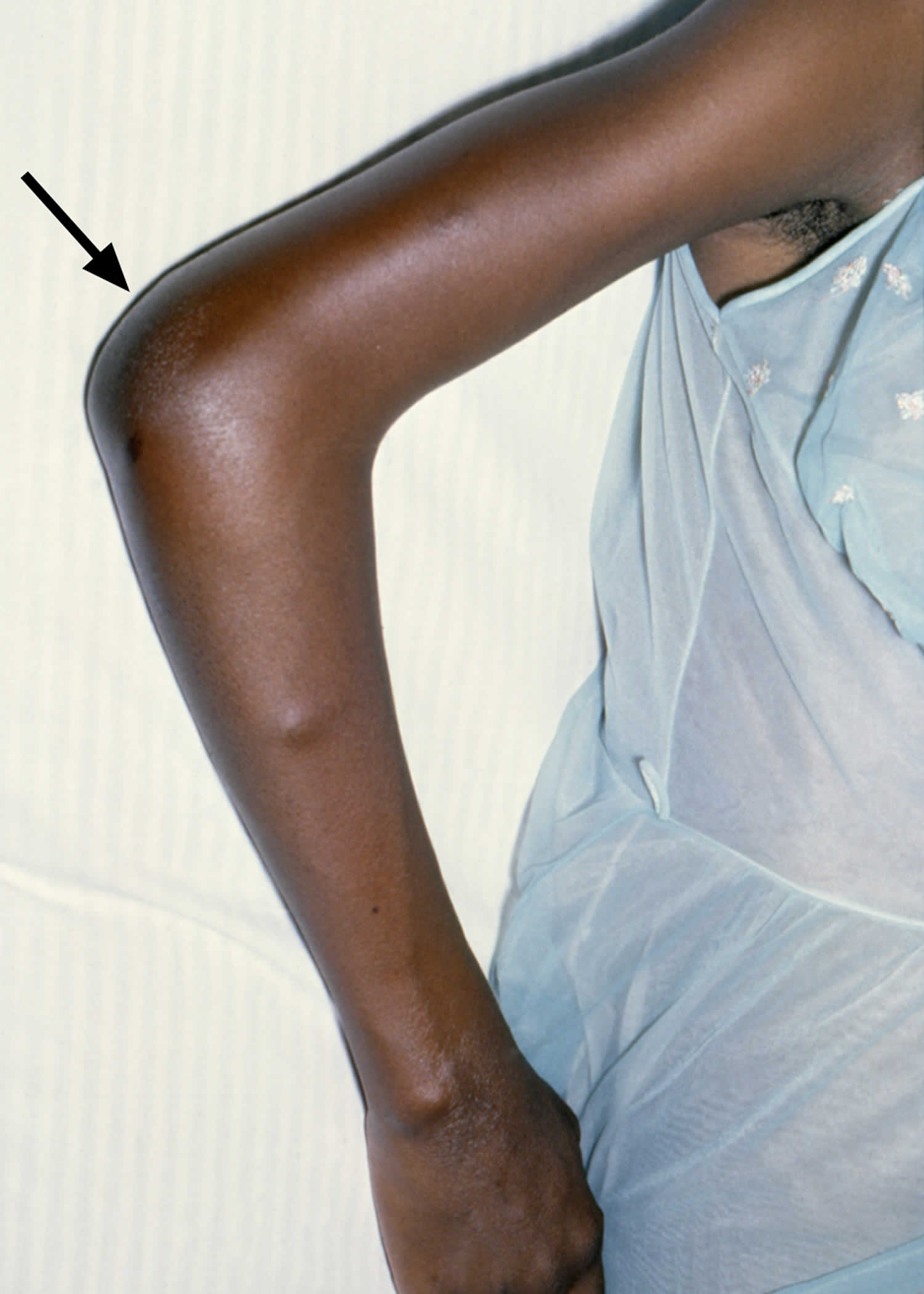
Figure 1. Gonococcal septic arthritis
Footnote: Disseminated gonococcal infection with arthritis. This image taken from a woman with disseminated gonococcal infection and right elbow arthritis.
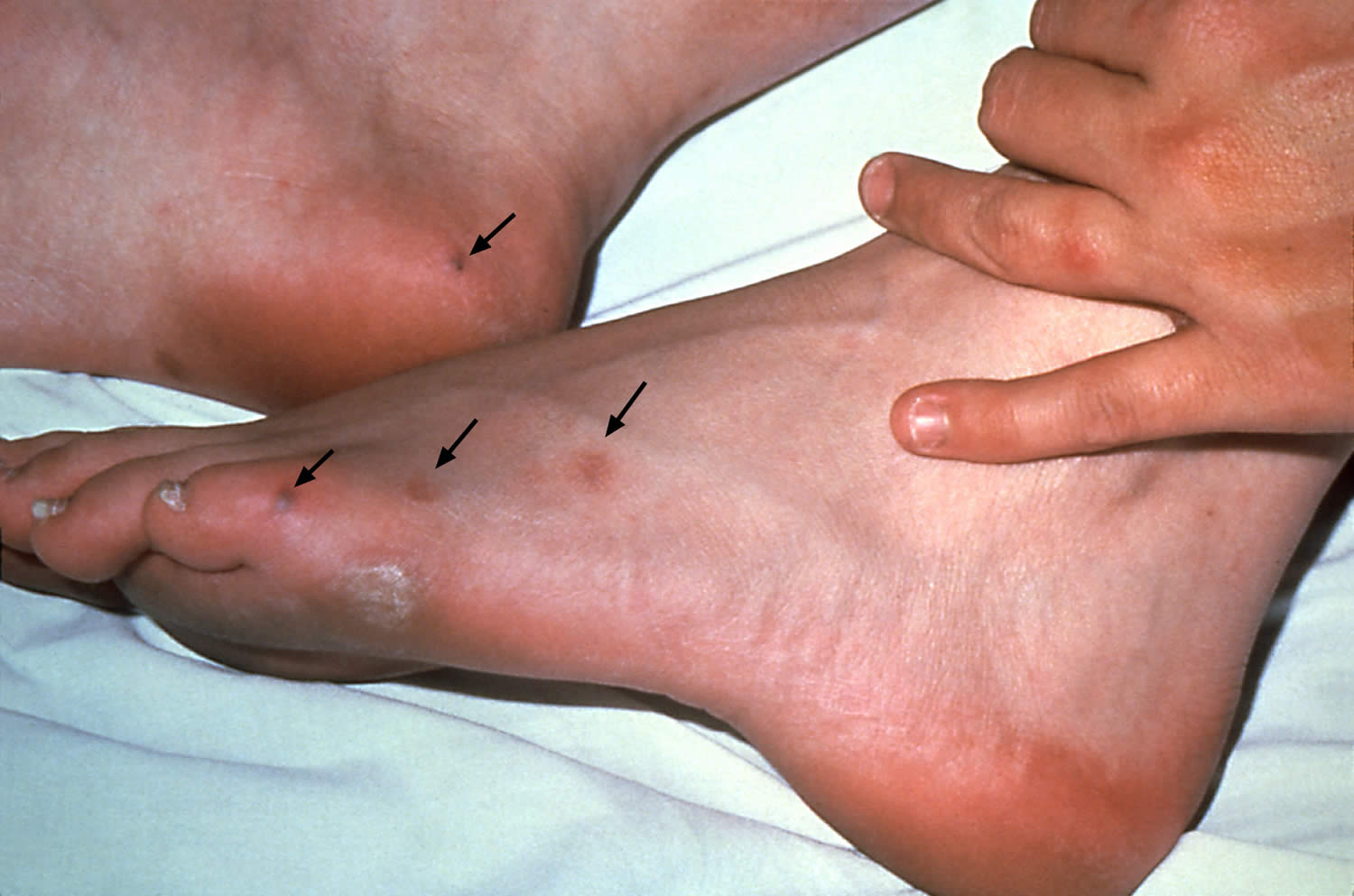
Figure 2. Gonococcal arthritis rash
Footnote: Disseminated gonococcal infection with skin Lesions. This patient had disseminated gonococcal infection including multiple cutaneous lesions on the feet (black arrows).
Gonococcal arthritis cause
Gonococcal arthritis is caused by infection with the Gram-negative diplococcus Neisseria gonorrhoeae. Neisseria gonorrhoeae is usually transmitted sexually and is an important cause of bacterial arthritis in sexually active adolescents. However, it may also be acquired perinatally during childbirth and may cause gonococcal infection in newborns 13.
Host risk factors that are associated with disseminated gonococcal infection include complement deficiencies, menstruation, pregnancy, history of pelvic surgery, and intrauterine devices (IUD). Specific strains of Neisseria gonorrhoeae have virulence factors that have been associated with dissemination. These include strains containing a specific outer membrane called the porin 1A serotype that promotes resistance, diminished host inflammatory response, and host cell invasion and strains that require specific substrates for growth. It is also hypothesized that the pathogenesis of disseminated gonococcal infection may be partly immune-mediated. This is supported by the fact that the pathogen is frequently not isolated from both the synovial fluid and blood of patients with clinical manifestations of dissemination 14.
Risk factors for gonococcal arthritis
The risk of dissemination after mucosal gonococcal infection depends on both the ability of the patient’s immune system to control the infection and the virulence of the organism 15. Factors that correlate with an increase in this risk have been identified for both the host and the organism. Host-related risk factors for disseminated infection include the following 16:
- Female sex
- Pregnancy
- Menstruation
- Systemic lupus erythematosus
- Complement deficiency
- Low socioeconomic or educational status
- IV drug use
- HIV infection
- Multiple sexual partners
Organism-related risk factors for disseminated gonococcal infection include the following 17:
- Antigenic variation of pili
- Protein Por B1A on the outer membrane – This inhibits host factor H and C4-binding protein, making host complement cascade less effective
- Lack of protein Por B1B
- Strains with nutritional requirements for arginine, hypoxanthine, and uracil (ie, AHU strains) – These are often associated with protein IA
- Colony opacity (Opa) protein-independent invasion – Opa proteins are adhesins crucial to the process of N gonorrhoeae attachment to host cells, particularly avoidance of carcinoembryonic antigen-related cell adhesion molecule-3 (CEACAM3) 18
Gonococcal arthritis symptoms
The clinical presentation of disseminated gonococcal infection is typically divided into a bacteremic form (arthritis-dermatitis syndrome) and a septic arthritis form. Approximately 60% of patients present with symptoms consistent with the bacteremic form, and the remaining 40% present with symptoms of more localized infection. Although each form presents with its own symptom complex, the overlap can be considerable. The time from initial infection to initial manifestations of disease ranges from 1 day to 3 months 19.
Bacteremic form or arthritis-dermatitis syndrome
In the bacteremic form or arthritis-dermatitis syndrome, symptoms are typically present 3-5 days before diagnosis 20. The bacteremic form of disseminated gonococcal infection usually comprises a triad of manifestations which include tenosynovitis, dermatitis, and polyarthralgia. The bacteremic form or arthritis-dermatitis syndrome is also often associated with nonspecific constitutional symptoms such as fever, chills, and body malaise. Approximately 60% developed fever in one study in women with disseminated gonococcal infection.
Migratory arthralgias are the most common presenting symptom in persons with disseminated gonococcal infection and are usually polyarticular. The arthralgias are typically asymmetric and tend to involve the upper extremities more than the lower extremities. The wrist, elbows, ankles, and knees are most commonly affected. Symptoms resolve spontaneously in 30-40% of cases or evolve into a septic arthritis in 1 or several joints.
Pain may also be due to tenosynovitis. Tenosynovitis is a unique finding in gonococcal arthritis and is usually not seen in other forms of septic arthritis. The tenosynovitis of disseminated gonococcal infection is asymmetric and may affect both large and small joints and most commonly occurs over the dorsum of the wrist and hand, as well as over the metacarpophalangeal joints, ankles, and knees. Tenosynovitis is demonstrated by tenderness along the flexor sheath and pain on a passive extension during a physical examination. Tenosynovitis usually affects multiple tendons, more commonly the fingers, wrists, toes, and ankles. Diffuse involvement of fingers can result in dactylitis 19.
Skin lesions occur in up to 75% of cases and are frequently seen in the trunk and extremities and usually spares the face. The rash associated with the bacteremic form of disseminated gonococcal infection may be overlooked by patients because it is painless and nonpruritic and consists of small papular, pustular, or vesicular lesions. Other skin manifestations of disseminated gonococcal infection that have been reported are abscesses, cellulitis, petechiae, purpuric macules, necrotizing fasciitis, and vasculitis 21. Skin lesions are characteristically transient and may disappear after several days even without treatment.
Gonococcal septic arthritis form
Localized gonococcal septic arthritis symptoms begin within days to weeks of gonococcal infection 20. Patients may experience pain, redness, and swelling in one joint (monoarthritis) or sometimes multiple joints (oligo- or polyarthritis), most commonly in a knee, wrist, ankle, or elbow 19. Most patients do not present with systemic symptoms such as fever or chills.
Gonococcal arthritis complications
As a rule, complications of gonococcal arthritis are rare. When they do occur, they may include any of the following:
- Meningitis
- Permanent joint damage
- Perihepatitis
- Endocarditis
- Osteomyelitis
- Pericarditis
- Pyomyositis
- Glomerulonephritis
Gonococcal arthritis diagnosis
A high clinical suspicion is necessary for patients who present with joint pain or swelling that is concerning for septic arthritis especially in those who are sexually active and younger than 40 years old and in certain at-risk populations such as in men who have sex with men (MSM) 16. A thorough history and physical examination including an emphasis on sexual history are needed in patients with suspected gonococcal arthritis. Menstrual history and pregnancy need to be assessed in premenopausal patients. Skin and joint examination may provide information that heightens clinical suspicion especially in patients who present with the typical triad of tenosynovitis, arthralgia, and skin lesions 22.
Definitive diagnosis of disseminated gonococcal infection or gonococcal arthritis is made through the identification of the etiologic pathogen in a specimen taken from a non-mucosal site (such as blood, synovial fluid, or skin lesions). Microbiologic tests, however, are not always positive and in such cases, diagnosis is made clinically. A clinical diagnosis may be supported by evidence of Neisseria gonorrhoeae infection from specimens obtained from mucosal sites. Screening for other sexually transmitted illnesses such as HIV, syphilis, and chlamydia is often recommended since these frequently co-exist with gonococcal infection.
Two sets of blood cultures should be obtained, and a positive result helps distinguish gonococcal arthritis from other pathogens that cause septic arthritis. Positive blood cultures, however, are only positive in less than 1/3 of cases and is more frequently seen in patients who present with arthritis-dermatitis syndrome. Specimens from mucosal sites should also be obtained and are frequently helpful in patients who have a high clinical suspicion of disseminated gonococcal infection but who have sterile synovial fluid or blood specimens. Nucleic acid amplification testing (NAAT) of either urine specimens in both men and women or vaginal swabs in women is preferred, if available. The culture of a urogenital specimen is an alternative.
In patients who demonstrate joint swelling and effusion, arthrocentesis and synovial fluid analysis should be performed. Typically, a synovial fluid analysis will show white blood cell (WBC) count of 50,000 cells/mm3 or more; although, a cell count below 10,000 cells/mm³ is not uncommon. This may be associated with reduced glucose concentration and elevated lactate dehydrogenase (LDH) level but these findings are non-diagnostic and of limited value. In patients who present with localized purulent arthritis, Neisseria gonorrhoeae will be isolated in only about 50% (range of 25% to 75%) of synovial fluid specimens. This is even less in patients who present with the arthritis-dermatitis syndrome. NAAT (nucleic acid amplification testing) of synovial fluid is more sensitive (greater than 75%) than culture and should be performed if available.
Obtaining skin lesion specimens is usually not done because of the difficulty of sampling and its very poor yield. The culture of skin lesions is often negative. NAAT of skin specimens may be slightly more sensitive, but this is not widely available.
Gonococcal arthritis treatment
Initial management in the inpatient setting is recommended for patients with a presumptive diagnosis of disseminated gonococcal infection or gonococcal arthritis. Ceftriaxone as a parenteral therapy, either given intravenously or intramuscularly, is the preferred initial antibiotic of choice. Intravenous administration of 1 gm every 24 hours is usually preferred in patients presenting with purulent arthritis. A single dose of 1 gram of azithromycin by mouth is usually added to cover potential coinfection with Chlamydia trachomatis. Alternative antimicrobial agents include third-generation cephalosporin such as cefotaxime and ceftizoxime, which are given as 1 gm every 8 hours. A dose of 100 mg of doxycycline twice a day for 7 days is an alternative to azithromycin 23.
Widespread resistance to oral cephalosporins such as cefixime has been documented. Oral cephalosporins are therefore not recommended as initial therapy. Although not always possible, the microbiologic diagnosis should be attempted, and antimicrobial susceptibility testing should be performed to guide antibiotic therapy. After 1 to 2 days of clinical improvement with ceftriaxone as parenteral therapy, a seven-day course of antibiotics may be completed with daily intramuscular ceftriaxone given at 250 mg daily. Patients with septic arthritis or those who are immunocompromised or slower to respond to treatment may require a longer course of therapy (7 to 14 days). De-escalation to oral antibiotic therapy (such as cefixime, fluoroquinolones, or penicillin) may be done only in patients who do not have septic arthritis and only if culture and susceptibility results are available. However, not all laboratories are capable of performing Neisseria gonorrhoeae culture and sensitivity testing. In cases wherein the microbiologic diagnosis is not established, the course of therapy should be completed with a parenteral cephalosporin.
Partners of patients diagnosed with disseminated gonococcal infection within 2 months should be contacted and treated whenever possible. Patients with recurrent disseminated gonococcal infection or gonococcal arthritis will require further evaluation for complement deficiency.
Patients with a history of beta-lactam allergy are often able to tolerate ceftriaxone. Due to the absence of effective alternative parenteral agents, patients who have a history of IgE mediated hypersensitivity reaction to beta-lactams may undergo either skin testing or graded challenge testing. Patients who test positive for these tests should undergo desensitization. Those with a history of severe, non-IgE mediated reaction will require consultation with an Infectious Disease specialist.
Patients presenting with localized purulent arthritis should undergo joint drainage, either arthroscopically or through repeated joint aspirations until there is evidence of response such as the resolution of fever, leukocytosis, joint pain, and effusions. Open surgical drainage may be necessary if aspiration is not adequate.
Gonococcal arthritis prognosis
For patients with septic arthritis resulting from gonococcal infection, proper antibiotic treatment and joint drainage typically leads to full recovery. For patients with more severe manifestations of disseminated gonococcal infection, the prognosis varies, depending on complications or comorbidities. For example, patients with acute endocarditis may require valve surgery and can expect to undergo at least 4-6 weeks of antibiotic therapy. Disseminated gonococcal infection-associated morbidity has decreased dramatically in the postantibiotic era. Complications are rare (1-3% of cases) 19.
- Septic arthritis. García-Arias M, Balsa A, Mola EM. Best Pract Res Clin Rheumatol. 2011 Jun; 25(3):407-21.[↩]
- Li R, Gossman WG. Arthritis, Gonococcal. 2018 Jan. 5 (2):211-23.[↩]
- Tuttle CS, Van Dantzig T, Brady S, Ward J, Maguire G. The epidemiology of gonococcal arthritis in an Indigenous Australian population. Sex Transm Infect. 2015 Nov;91(7):497-501.[↩]
- Gonococcal arthritis. Best Pract Res Clin Rheumatol. 2003 Apr;17(2):201-8. https://doi.org/10.1016/S1521-6942(02)00125-0[↩]
- Gonococcal arthritis. Bardin T. Best Pract Res Clin Rheumatol. 2003 Apr; 17(2):201-8.[↩][↩][↩][↩][↩][↩]
- Destructive septic arthritis of the sternoclavicular joint due to Neisseria gonorrhoeae. Guillot X, Delattre E, Prati C, Wendling D. Joint Bone Spine. 2012 Oct; 79(5):519-20.[↩]
- Gonococcal septic arthritis of the hip. Lee AH, Chin AE, Ramanujam T, Thadhani RI, Callegari PE, Freundlich B. J Rheumatol. 1991 Dec; 18(12):1932-3.[↩]
- An unusual presentation of gonococcal arthritis in an HIV positive patient. Strongin IS, Kale SA, Raymond MK, Luskin RL, Weisberg GW, Jacobs JJ. Ann Rheum Dis. 1991 Aug; 50(8):572-3.[↩]
- Gonorrhoea infection presenting in pregnancy with septic arthritis of the sternoclavicular joint. O’Leary AJ, Tejura H, Latibeaudiere M, Edwards G. J Obstet Gynaecol. 2006 May; 26(4):373-4.[↩]
- El Mezouar I, Tahiri L, Lazrak F, Berrada K, Harzy T. Gonococcal polyarthritis with sternoclavicular joint involvement in pregnant woman: a case report. Pan Afr Med J. 2014;17:242. Published 2014 Mar 31. doi:10.11604/pamj.2014.17.242.4181 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4145263[↩]
- Disseminated gonococcal infection: a prospective analysis of 49 patients and a review of pathophysiology and immune mechanisms. O’Brien JP, Goldenberg DL, Rice PA. Medicine (Baltimore). 1983 Nov; 62(6):395-406.[↩]
- Trends in susceptibility of Neisseria gonorrhoeae to ceftriaxone from 1985 through 1991. Schwebke JR, Whittington W, Rice RJ, Handsfield HH, Hale J, Holmes KK. Antimicrob Agents Chemother. 1995 Apr; 39(4):917-20.[↩]
- Rouanes N, Sanchez R, Cazanave C. [A case of gonococcal arthritis: Diagnostic difficulties and usefulness of synovial fluid PCR]. Rev Med Interne. 2018 Jan;39(1):54-56.[↩]
- Mamane W, Falcone MO, Doursounian L, Nourissat G. [Isolated gonococcal tenosynovitis. Case report and review of literature]. Chir Main. 2010 Oct;29(5):335-7.[↩]
- Gonococcal arthritis. https://emedicine.medscape.com/article/333612-overview[↩]
- Li R, Hatcher JD. Gonococcal Arthritis. [Updated 2018 Nov 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470439[↩][↩]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta: U.S. Department of Health and Human Services; 2017.[↩]
- Roth A, Mattheis C, Muenzner P, Unemo M, Hauck CR. Innate recognition by neutrophil granulocytes differs between Neisseria gonorrhoeae strains causing local or disseminating infections. Infect Immun. 2013 Jul. 81(7):2358-70.[↩]
- Dalla Vestra M, Rettore C, Sartore P, Velo E, Sasset L, Chiesa G, et al. Acute septic arthritis: remember gonorrhea. Rheumatol Int. 2008 Nov. 29(1):81-5.[↩][↩][↩][↩]
- Balsa A, Martin-Mola E. Infectious arthritis I: bacterial arthritis. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH. Rheumatology. 6th ed. Philadelphia, PA: Mosby Elsevier; 2015. 1013-28.[↩][↩]
- Beatrous SV, Grisoli SB, Riahi RR, Matherne RJ, Matherne RJ. Cutaneous manifestations of disseminated gonococcemia. Dermatol Online J. 2017 Jan 15. 23 (1).[↩]
- Carlin E, Urban C, Sidle J, Cirilli A, Larson J, Richman M, Dexeus D. Gonococcal Tenosynovitis Diagnosed with the Aid of Emergency Department Bedside Ultrasound. J Emerg Med. 2018 Jun;54(6):844-848.[↩]
- Maharaj R, Mody GM. The rarity of gonococcal arthritis in association with HIV infection. J Infect Dev Ctries. 2014 Sep 12;8(9):1222-7.[↩]