What is Hartnup disease
Hartnup disease is an autosomal recessive disorder caused by the body’s inability to absorb certain protein building blocks (amino acids) from the diet. Hartnup disease is caused by impaired neutral (ie, monoaminomonocarboxylic) amino acid transport in the apical brush border membrane of the small intestine and the proximal tubule of the kidney. As a result, affected individuals are not able to use these amino acids to produce other substances, such as vitamins and proteins. Hartnup disease patients present with pellagra-like skin eruptions, cerebellar ataxia, and gross aminoaciduria 1. However, most people with Hartnup disease are able to get the vitamins and other substances they need with a well-balanced diet.
Hartnup disease is estimated to affect 1 in 30,000 individuals 2. With an overall prevalence of 1 case per 24,000 population (range, 1 case per 18,000-42,000 population), Hartnup disease ranks among the most common amino acid disorders in humans 3. Hartnup disease has been reported to occur in all ethnic groups studied to date, including those from Israel, Japan, West Africa, and India 4.
The onset of Hartnup disease is in childhood, usually in children aged 3-9 years, but it may present as early as 10 days after birth. In addition, a case of Hartnup disease presenting for the first time in an adult female, after prolonged lactation and increased physical activity, is described 3.
People with Hartnup disease have high levels of various amino acids in their urine (aminoaciduria). For most affected individuals, this is the only sign of the condition. However, some people with Hartnup disease have episodes during which they exhibit other signs, which can include skin rashes; difficulty coordinating movements (cerebellar ataxia); and psychiatric symptoms, such as depression or psychosis. These episodes are typically temporary and are often triggered by illness, stress, nutrient-poor diet, or fever. These features tend to go away once the trigger is remedied, although the aminoaciduria remains. In affected individuals, signs and symptoms most commonly occur in childhood.
Hartnup disease causes
Hartnup disease is caused by mutations in the SLC6A19 gene. This gene provides instructions for making a protein called B0AT1, which is primarily found embedded in the membrane of intestine and kidney cells. The function of this protein is to transport certain amino acids into cells. In the intestines, amino acids from food are transported into intestinal cells then released into the bloodstream so the body can use them. In the kidneys, amino acids are reabsorbed into the bloodstream instead of being removed from the body in urine. In the body, these amino acids are used in the production of many other substances, including vitamins and proteins. One particular amino acid transported by B0AT1, tryptophan, is needed to produce vitamin B3 (also known as niacin).
SLC6A19 gene mutations result in the production of a B0AT1 protein with reduced activity. As a result, specific amino acids cannot be taken in by cells and are instead removed from the body as waste. Because these amino acids are removed from the body without being used, people with this condition may be lacking (deficient) in certain amino acids and vitamins. However, individuals who are nutrient-deficient due to their diet, illness, stress, or a variety of other reasons, can develop serious signs and symptoms of this condition including rashes, cerebellar ataxia, and psychiatric symptoms. Researchers believe that many of these features are related to a deficiency of tryptophan and niacin, specifically.
Hartnup disease inheritance pattern
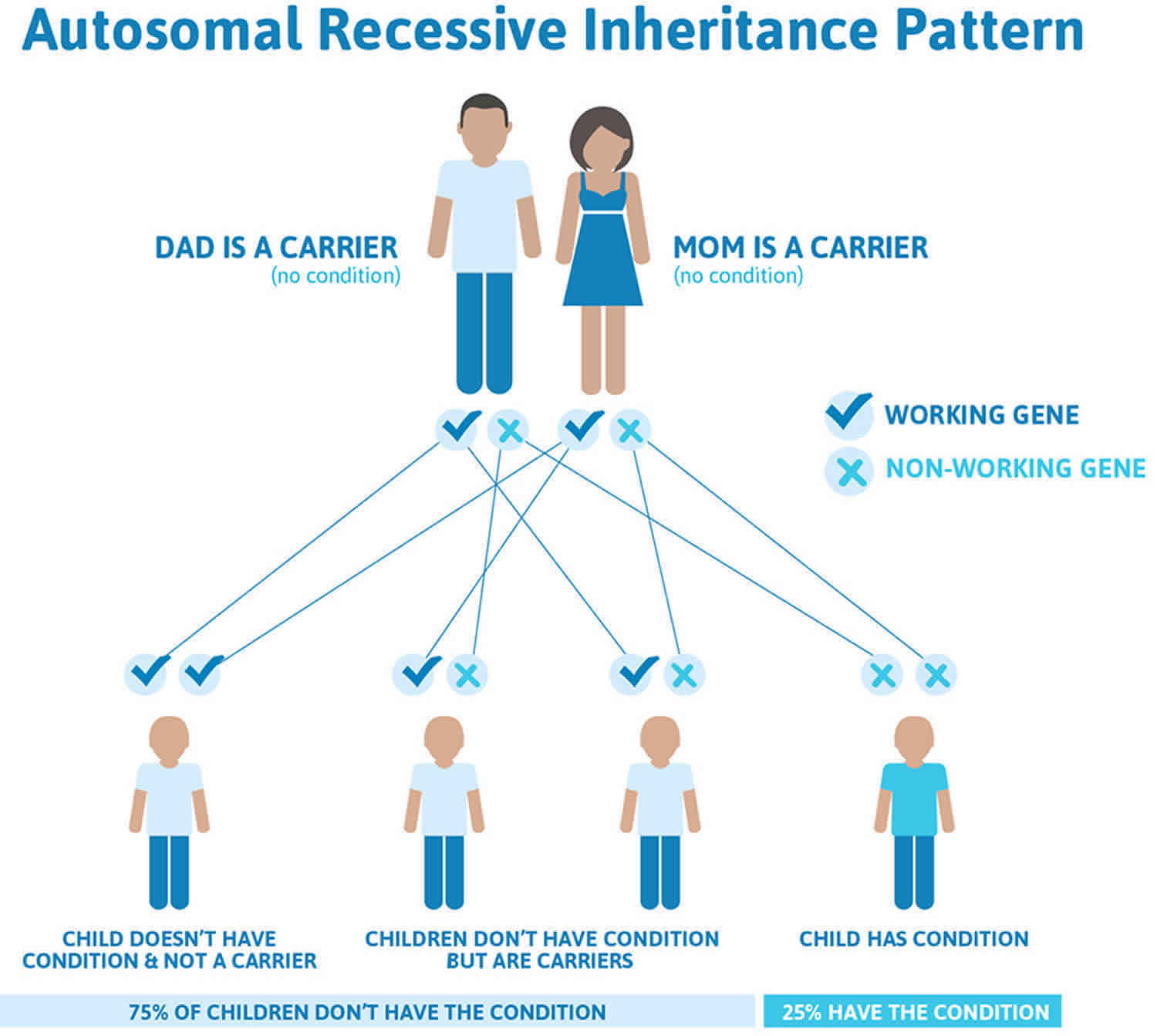
Hartnup disease is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Hartnup disease autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Hartnup disease symptoms
The symptoms of Hartnup disease vary greatly from one person to another. Exacerbations are seen most frequently in the spring or early summer after exposure to sunlight. The attacks may be provoked by a febrile illness, poor nutrition, sulfonamides, and possibly emotional stress and increased physical activity 3.
Most children with the Hartnup defect remain asymptomatic.
When symptoms do develop, they most often occur between the ages of 3-9. In rare instances, symptoms first appear in adulthood.
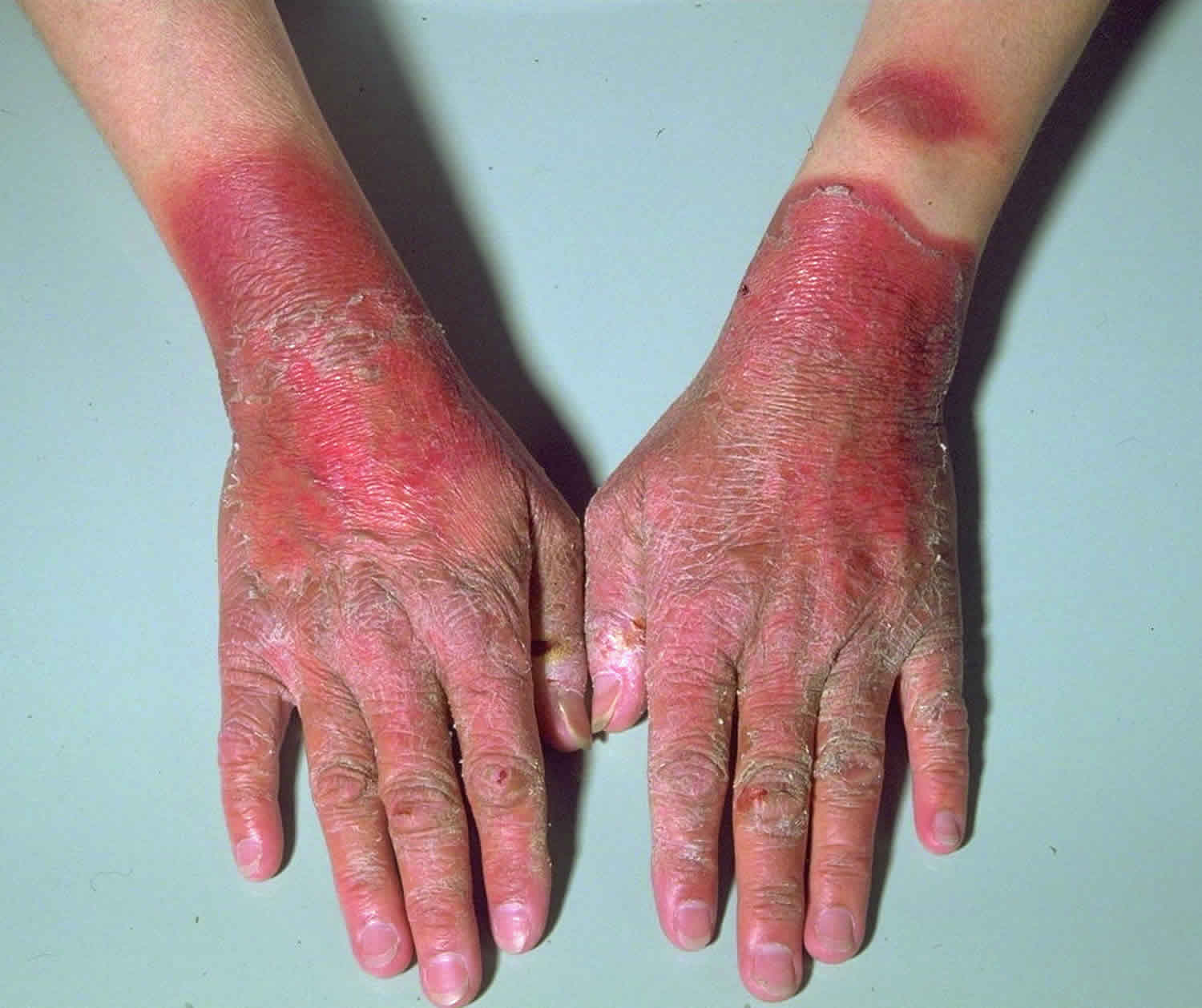
The most common symptom are red, scaly light-sensitive (photosensitive) rashes on the face, arms, extremities, and other exposed areas of skin.
A wide variety of neurological abnormalities can occur including sudden episodes of impaired muscle coordination (ataxia), an unsteady walk (gait), impaired articulation of speech (dysarthria), occasional tremors of the hands and tongue, and spasticity, a condition marked by increased muscle tone and stiffness of the muscles, particularly those of the legs.
There have been reports of delayed cognitive development and, in rare instances, mild intellectual disability in some children. It is, however, unclear whether these symptoms are related to Hartnup disorder or incidentally occurred in the same individual and were therefore attributed to Hartnup disorder. Similarly, seizures, fainting, trembling, lack of muscle tone (hypotonia), headaches, dizziness and/or vertigo, and delays in motor development have been observed but may be unrelated. Some affected individuals may experience psychiatric abnormalities including emotional instability such as rapid mood changes, depression, confusion, anxiety, delusions, and/or hallucinations.
Some children experience growth delays and may be shorter than would be expected based upon age and gender (short stature). In some instances, the eyes may be affected and individuals may experience double vision (diplopia), involuntary rhythmic movements of the eyes (nystagmus), and droopy upper eyelids (ptosis).
Diarrhea may precede or follow an episode of this disorder. Some adults with Hartnup disease have been reported whose initial symptom was the onset of seizures during adulthood. Heartburn has been reported in adults with the disorder.
Skin
Photosensitivity occurs.The skin reddens after exposure to sunlight. Further exposures lead to the development of dry, scaly, well-marginated eruptions, sometimes resembling chronic eczema. This eruption preferentially affects the forehead, the cheeks, the periorbital regions, the dorsal surfaces of the hands, and other light-exposed areas.
Lesions on the face may resemble the malar rash of lupus erythematosus.
A vesiculobullous eruption with exudation may occur.
Skin changes leave long-lasting hypopigmentation and/or hyperpigmentation, which are intensified with further sunlight exposure.
One case with widespread cutaneous eruption resembling acrodermatitis enteropathica was described 5, as well as a patient with manifestations of kwashiorkor and acrodermatitis enteropathica but with normal zinc levels, which led to the search for other metabolic disorders, and Hartnup disorder was confirmed 6.
In two patients with celiac disease, Hartnup disease was found in treatment-refractory celiac disease. In both patients, exfoliative erythroderma of malnutrition developed and it resolved after a high-protein and gluten-free diet was instituted 7.
Central nervous system
Mental development is normal in most patients, but mental retardation (intelligence quotient of 50-70) is described in a few patients 8. Of 1087 patients screened for the detection of inherited metabolic diseases from the Alexandra Institute for persons with mental retardation in Cape Town, Hartnup disease was found in only 1 patient. [34]
Neurologic symptoms may vary and are fully reversible. Intermittent cerebellar ataxia, a wide-based gait, spasticity, delayed motor development, and tremulousness are the most frequent findings. Headaches and hypotonia may also occur 3. Late-onset seizures and adult-onset Hartnup disease with neurologic manifestations as the first signs have been described, with pellagralike skin lesions developing after the neurologic symptoms 9.
Other symptoms
Ocular manifestations include double vision, nystagmus, photophobia, and strabismus 10.
Gingivitis, stomatitis, and glossitis suggest niacin deficiency 8.
Diarrhea occasionally precedes or follows attacks of the disease 8.
Short stature has been described. Wilcken et al 11 found that of 14 patients with Hartnup disorder who were observed for 8 years, 10 had height percentiles less than the midparent height percentiles, while 4 had percentiles equal to or above the midparent percentiles.
Hartnup disease complications
Hartnup disease complications are as follows 12:
- Severe central nervous system (CNS) involvement may rarely lead to death in the first years of life.
- Psychotic episodes and delirium are described in a minority of patients.
- Mild mental retardation is described in only a few patients.
- Long-lasting hypopigmentation and/or hyperpigmentation of the skin are seen with repeated exposures to sunlight, which should be avoided by using proper photoprotection.
Hartnup disease diagnosis
Due to the variability of symptoms, unambiguous diagnosis can only be made through urine analysis. Pediatricians can request this analysis from selected pathology centers. The test is based on the detection of elevated amino acids in the urine by chromatography.
Molecular genetic testing can confirm a diagnosis of Hartnup disease in some cases. Molecular genetic testing can detect genetic alterations in the SLC19A6 gene known to cause the disorder, but usually is not necessary to obtain a diagnosis.
Hartnup disease treatment
Individuals with Hartnup disease who do not develop symptoms will usually not require any treatment. Symptomatic episodes that do affect some people with Hartnup disease can be reduced or avoided by maintaining good nutrition including a high protein diet, avoiding excess exposure the sun, and avoiding certain drugs such as sulphonamide drugs. Supplementing the diet with nicotinamide or niacin is also of benefit in preventing Hartnup disease episodes.
In some instances, during a symptomatic episode, treatment with nicotinamide may be recommended.
According to the medical literature, at least one individual showed an improvement of symptoms after treatment with the compound L-tryptophan ethyl ester, which restored tryptophan levels in both the serum and cerebrospinal fluid.
Other treatment is symptomatic and supportive. Genetic counseling may be helpful for affected families.
Hartnup disease prognosis
Hartnup disease is manifested by a wide clinical spectrum. Most patients remain asymptomatic, but, in a minority of patients, skin photosensitivity and neurologic and psychiatric symptoms may have a considerable influence on quality of life. Rarely, severe CNS involvement may lead to death. Mental retardation and short stature have been described in a few patients. Malnutrition and a low-protein diet are the primary factors that contribute to morbidity 3.
Attacks become less frequent with increasing age 11.
Maternal Hartnup disease does not influence the outcome of pregnancy. Placental transport of free amino acids may not be reduced in maternal Hartnup disorder 13.
- Bröer A, Cavanaugh JA, Rasko JE, Bröer S. The molecular basis of neutral aminoacidurias. Pflugers Arch. 2006 Jan. 451(4):511-7. [↩]
- Hartnup disease. https://ghr.nlm.nih.gov/condition/hartnup-disease[↩]
- Levy H. Hartnup Disorder. Scriver CR, Beaudet A L, Sly WS, Valle D. The metabolic and molecularbases of inherited disease. New York: McGraw-Hill; 2001. 4957-4969.[↩][↩][↩][↩][↩]
- Hartnup disease. https://emedicine.medscape.com/article/1115549-overview[↩]
- Seyhan ME, Selimoglu MA, Ertekin V, Fidanoglu O, Altinkaynak S. Acrodermatitis enteropathica-like eruptions in a child with Hartnup disease. Pediatr Dermatol. 2006 May-Jun. 23(3):262-5.[↩]
- Orbak Z, Ertekin V, Selimoglu A, Yilmaz N, Tan H, Konak M. Hartnup disease masked by kwashiorkor. J Health Popul Nutr. 2010 Aug. 28(4):413-5.[↩]
- Ciecierega T, Dweikat I, Awar M, Shahrour M, Libdeh BA, Sultan M. Severe persistent unremitting dermatitis, chronic diarrhea and hypoalbuminemia in a child; Hartnup disease in setting of celiac disease. BMC Pediatr. 2014 Dec 20. 14:311.[↩]
- Camargo SM, Bockenhauer D, Kleta R. Aminoacidurias: Clinical and molecular aspects. Kidney Int. 2008 Apr. 73(8):918-25.[↩][↩][↩]
- Cheon CK, Lee BH, Ko JM, Kim HJ, Yoo HW. Novel mutation in SLC6A19 causing late-onset seizures in Hartnup disorder. Pediatr Neurol. 2010 May. 42(5):369-71.[↩]
- Scriver CR, Mahon B, Levy HL, et al. The Hartnup phenotype: Mendelian transport disorder, multifactorial disease. Am J Hum Genet. 1987 May. 40(5):401-12.[↩]
- Wilcken B, Yu JS, Brown DA. Natural history of Hartnup disease. Arch Dis Child. 1977 Jan. 52(1):38-40.[↩][↩]
- Milovanovic D, Djukic A, Stepanovic R, Pekovic D, Vranjesevic D. [Hartnup disease (report of 2 cases in one family)]. Srp Arh Celok Lek. 2000 Mar-Apr. 128(3-4):97-103.[↩]
- Mahon BE, Levy HL. Maternal Hartnup disorder. Am J Med Genet. 1986 Jul. 24(3):513-8.[↩]