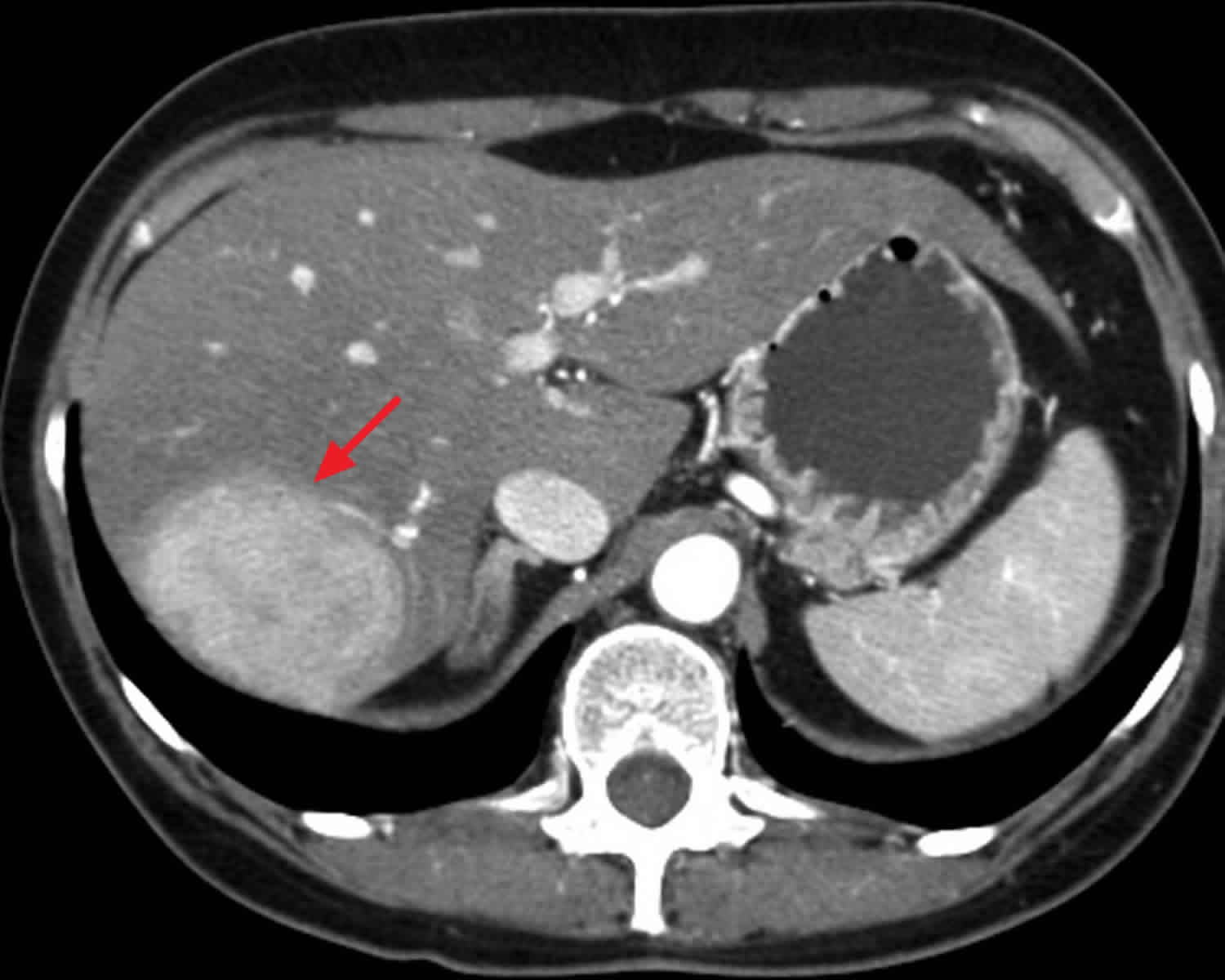
Hepatocellular adenoma
Hepatic adenoma also known as hepatocellular adenoma, is a rare but benign (non-cancerous) solid, epithelial tumors of the liver frequently associated with women of reproductive age who are taking exogenous estrogens in the form of oral contraceptive pills 1. Hepatic adenoma is also seen in patients treated with anabolic steroids for athletic enhancement, Fanconi anemia, or aplastic anemia. Hepatocellular adenoma has an estimated incidence of 3–4 per 100,000 women as compared to 1 to 1.3 cases per million among nonusers 2; this frequency is based on population research including women using oral contraceptives 3. This risk increases with more than 5 years of use. Thus, in one study, the relative risk for hepatic adenomas for women who used oral contraceptive pills for more than 109 months was calculated as 25 times that of women who used it for less than 12 months. The incidence increased with the advent of the use of oral contraceptive pills 1. A causal role for hormone activity in hepatic adenoma growth is suggested by data linking hepatic adenoma regression to the cessation of oral contraceptive pills use, and growth associated with pregnancy, making the case stronger for association with sex hormones 4. The ratio of women to men who develop hepatic adenomas is 4:1. This ratio appears to be changing because of the widespread use of anabolic drugs in sports. Other populations that exhibit this condition include those with glycogen storage disease types I and III, iron-overload associated with beta-thalassemia and hemochromatosis, and perhaps those with an endogenous imbalance of sex hormones such as Klinefelter and polycystic ovarian syndromes (PCOS). In these cases, except for PCOS, there is a male predominance, and the diagnosis is made during childhood 5.
Although benign, hepatic adenomas are associated with risks of unexpected bleeding from the adenoma and malignant transformation. Elective resection is recommended in all men with hepatic adenomas and in women with hepatic adenomas greater than 5 cm, with conservative management for the straightforward, hepatic adenomas smaller lesions (<5 cm) 6. Advances in the radiologic diagnosis and subtype classifications based on molecular behavior have emerged, which provide a more systematic approach to treating patients with hepatic adenomas. Although typically singular, multiple hepatic adenomas can occur. Hepatic adenomatosis refers to the presence of ten or more tumors 7.
Hepatic adenoma key facts
- Hepatic adenomas are rare benign epithelial tumors of the liver frequently associated with women of reproductive age who are taking exogenous estrogens in the form of oral contraceptive pills.
- The classification scheme of hepatic adenomas based on molecular behavior patterns called the phenotypic-genotypic classification has been validated and is a useful classification scheme.
- HNF-1alpha type is associated with hepatocyte differentiation, mostly seen in women or with mature-onset diabetes of the young.
- Beta-catenin activated mutations that account for 15% to 20% of hepatic adenomas are most frequently associated with exposure to male hormones. Because this population has a higher risk for malignant transformation to hepatocellular carcinoma, surgical resection is the recommended treatment in this type of adenoma.
- Inflammatory hepatic adenoma is predominantly seen in women with higher body mass index, alcohol consumption, and systemic inflammatory syndrome. These tumors stain positive with serum amyloid A and with C-reactive protein.
- Discontinuation of oral contraceptive pills can lead to spontaneous regression of tumors in cases of single or multiple tumors less than 5 cm in size. Imaging surveillance is still recommended to monitor for regression.
- Surgery is recommended for all tumors greater than 5 cm in size but does not require wide margins or regional lymphadenectomy.
- Obesity, nonalcoholic steatohepatitis, and metabolic syndromes are associated with hepatic adenomatosis.
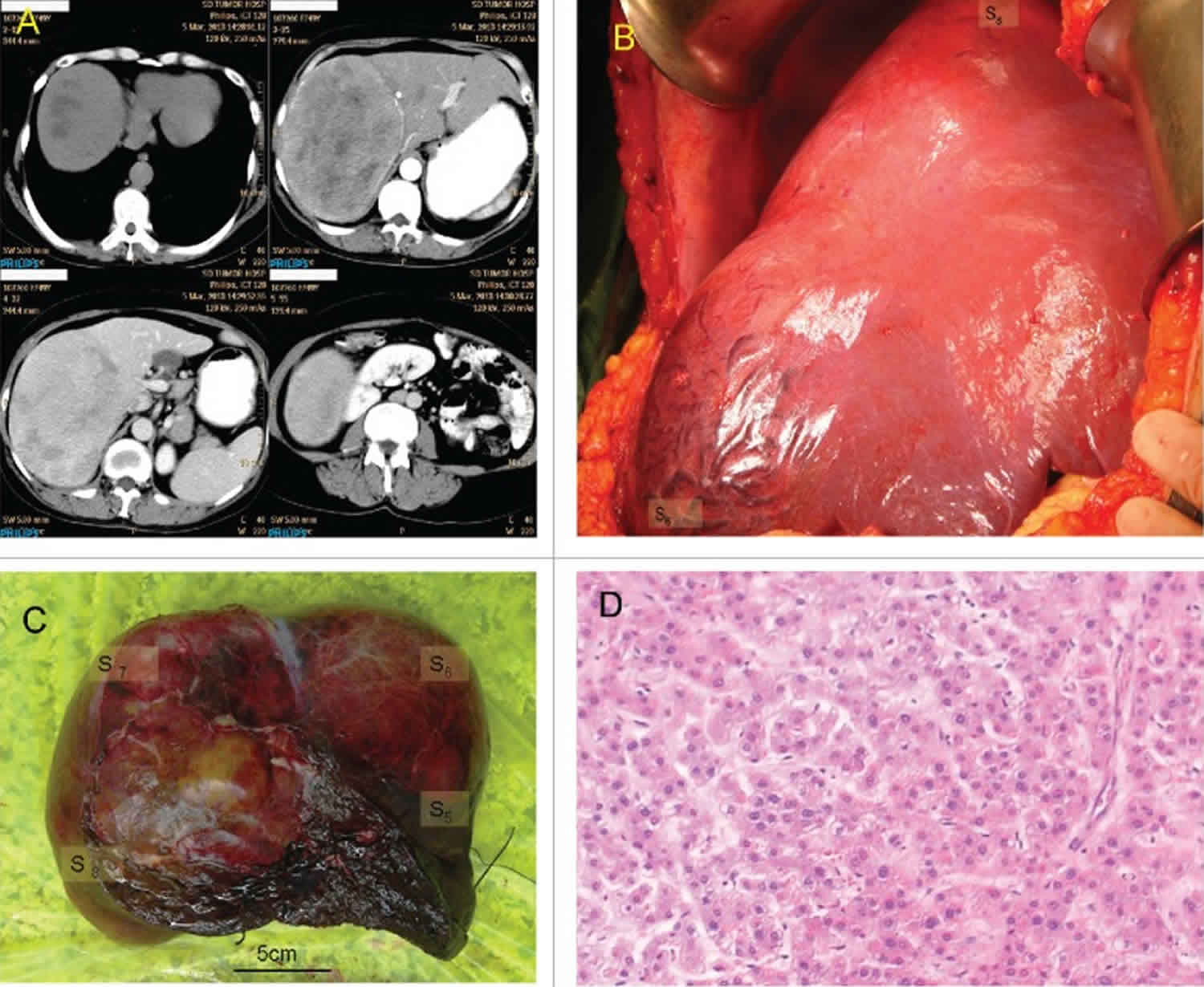
Figure 1. Hepatic adenoma
Footnote: Giant hepatic adenoma. (A) CT scan; clockwise from upper-left: Unenhanced scan, arterial phase of contrast enhanced scan, delayed phase of contrast enhanced scan, portal phase of contrast enhanced scan. (B) In situ hepatic adenoma. (C) View of resected hepatic adenoma. (D) Histology of resected hepatic adenoma.
[Source 8 ]Hepatic adenoma causes
Understanding of the molecular behavior of hepatic adenomas deepened with the discovery by Chen et al. 9 of the role of beta-catenin from the Wnt signaling pathway in the formation of hepatic adenomas in 2002. In 2007, Bioulac-Sage and associates, from Bordeaux, came up with a classification scheme for hepatic adenomas based on molecular behavior patterns called phenotypic-genotypic classification 10. Other groups have since validated this classification scheme. This scheme distributes hepatic adenomas into 4 main groups:
HNF-1alpha inactivated mutations (35% to 40%)
It involves biallelic mutations of the TCF-1 gene that encodes the hepatocyte nuclear factory family transcription factor HNF-1alpha. HNF-1alpha is associated with hepatocyte differentiation and liver development, as well as glucose and lipid metabolism. This type occurs mostly in women and is often associated with mature-onset diabetes of the young (MODY3). Ninety percent of the mutations are somatic, although those associated with MODY3 can be germline and common in patients with adenomatosis.
Beta-catenin activated mutations (15% to 20%)
In approximately 10–20% of hepatic adenomas, a β-catenin mutation is found 11. These are frequently associated with exposure to male hormones, glycogenesis, and familial adenomatous polyposis. Mild cytological or architectural abnormalities in the liver parenchyma lead to an acinar pattern. It is rarely detected in patients with steatosis and those with inflammatory changes in the liver. This group has a higher risk of malignant transformation, and the Wnt pathway has been implicated in 10% to 25% of the cases of hepatocellular carcinomas. On immunohistochemical staining, these adenomas tend to stain for glutamine synthetase rather than beta-catenin, which stains patchily.
Inflammatory hepatic adenomas (40% to 50%)
Predominantly seen in women with a high body mass index, alcohol consumption, and systemic inflammatory syndrome 12. Patients presenting with inflammatory hepatocellular adenoma demonstrate both serum, and lesional indicators of an active inflammatory response. It is associated with the activation of the IL-6 inflammatory pathway with dystrophic vessels (thickened wall arteries with sinusoidal dilatation called peliosis) and telangiectasia. These were formerly called “telangiectatic focal nodular hyperplasia.” In these lesions, increased expression is seen of the markers serum amyloid A (SAA) and C-reactive protein (CRP), both classic indicators of the acute phase response 13.
Unclassified hepatic adenomas (10%)
These hepatic adenomas do not stain for C-reactive protein, SAA, beta-catenin, or glutamine synthetase. They also have typical liver fatty acid-binding protein (L-FABP) staining.
Hepatic adenoma symptoms
Typically about half of patients with hepatic adenomas are asymptomatic, and the tumor is found incidentally on imaging. The remaining patients have symptoms that could range from a mild, often ill-defined abdominal pain in the right hypochondrium or epigastrium to bloating. Labs outside of reference range may include elevated alkaline phosphatase and gamma-glutamyl transferase. In case of malignant transformation to hepatocellular carcinoma (liver cancer), alpha-fetoprotein (AFP) can be elevated. Hepatomegaly with mild tenderness can be seen. The sudden presentation occurs when the hepatic adenoma ruptures, resulting in acute hemoperitoneum. An enlarged liver would be smooth but slightly tender. It presents with severe abdominal pain with hypotension and/or shock. This event is not uncommon and is often seen with oral contraceptive pill use. It is associated with a significant mortality rate. Tumors that rupture are typically greater than 5 cm, solitary, and superficially located. Often, the women are either menstruating or pregnant at that time.
Hepatic adenoma complications
- Hemorrhage
- Malignant transformation
- Shock
- Death
Intralesional bleeding
On reviewing the recent literature, van Aalten and colleagues detected evidence of hemorrhage in 27.2% of all patients (315/1160) with one or more hepatocellular adenomas, giving a 15.8% chance of hemorrhage for every hepatocellular adenoma (118/748) 14. Acute rupture and intraperitoneal bleeding were reported in 17.5% of patients. A size for the smallest hepatocellular adenomas showing hemorrhage was reported for 13 of the 28 articles reviewed; hemorrhage generally arose in the larger lesions (>5 cm), although smaller lesions could also bleed, albeit at much lower rates. These data should be interpreted with caution, as only the resected cases were included. The actual chance of bleeding in the different subtypes is likely to be significantly lower. The risk of bleeding was inconsistent across the subtypes of hepatocellular adenoma: Inflammatory hepatocellular adenoma showed a higher propensity for macroscopic hemorrhage (30%), than HNF-1alpha-hepatocellular adenoma (8%) 15 which can presumably be attributed to the larger number of venous structures, or telangiectatic changes in this subtype.
Although there may be a difference in prevalence of internal bleeding, all subtypes bear this intrinsic risk 16, which diminishes the utility of subtype classification in terms of the clinical management of this risk. Furthermore, more data are needed to prove any correlate between reduced bleeding and the HNF-1alpha-hepatocellular adenoma subtype.
Bieze and colleagues described a series of 45 patients with 195 lesions. In this cohort, there was a tendency to an enhanced risk of bleeding when the lesion was located in the left lateral liver (11/32 versus 31/163 in other regions), and showed exophytic growth (16/24 versus 9/82) 17. The latter phenomenon is probably due to the subcapsular location, with no intrinsic capsule, and minimal surrounding liver with which to prevent rupture of the hematoma into the abdominal cavity. However, no other data are available to support this theory, and preventive treatment in these cases does not appear to be warranted.
As for the clinical application of these findings, there is no evidence that supports the use of subtype classification in the stratification and management of individual patients. Moreover, size still remains the most important marker to predict those at risk for larger bleeding in follow up.
Malignant transformation
Malignant transformation of hepatocellular adenoma to hepatocellular carcinoma (liver cancer) is rarely reported, but is an accepted risk, particularly when the diameter of the adenoma exceeds 5 cm 18. In a systematic review, Stoot and colleagues 19 reported an overall frequency of malignant transformation of 4.2% for hepatocellular adenomas (67 of 1635 hepatocellular adenomas). Only three cases showed malignant transformation for tumors <5 cm in diameter, which represented 4.4% of the total number of hepatocellular carcinomas arising from hepatocellular adenomas (3 out of 67). As suggested for the internal bleeding data, these reports should be interpreted with caution.
Hepatic adenoma diagnosis
Laboratory tests are not typically helpful in diagnosing hepatic adenomas. Alpha-fetoprotein (AFP) is usually negative. Hepatitis B and C should be checked to exclude malignant disease. A two- or threefold increase in alkaline phosphatase and g-glutamyl transferase has been seen, particularly with inflammatory hepatic adenomas. White blood cell count, fibrinogen, and C-reactive protein may be elevated as well 20.
Core needle biopsy also has limited diagnostic value, although immunohistochemical markers can be helpful in expert centers.
Ultrasonography fails to distinguish between benign and malignant tumors. Doppler can demonstrate arterial hypervascularity with vessels running along the border of the lesion in a “basket pattern.”
Dynamic magnetic resonance imaging with a hepatocyte-specific contrast agent, such as gadobenate dimeglumine, is the best modality for diagnosing hepatic adenomas 6. This method is most able to distinguish between hepatic adenomas and other benign and malignant tumors of the liver. The tumor can have a clearly defined central margin with nearly parallel vessels entering from the periphery, giving the appearance of a spoked wheel. Alternatively, it could have a tortuosity of peripheral vessels with central necrosis. A dynamic CT scan is another modality that can be useful in imaging. Equivocal imaging may have to resort to core needle biopsy for further clarification.
Hepatic adenoma treatment
Conservative management is the initial policy for all single or multiple tumors less than 5 cm in size and associated with oral contraceptive pills. Management includes cessation of oral contraceptive pills and imaging surveillance, and has been shown to cause near total to complete regression in the size of the tumors in many cases. Resumption of oral contraceptive pill use must be followed with radiologic surveillance. The optimal duration of follow-up has not been established, and surveillance has been recommended until menopause by some authors. Tumors refractory to conservative management are typically associated with obesity 21.
The majority of hepatic adenomas remain stable during pregnancy. Most tertiary centers monitor adenomas less than 5 cm serially in pregnant women every three months and during the postpartum period. They do not discourage patients with small adenomas from getting pregnant.
Surgery with resection is recommended for all male patients regardless of the tumor size and for women with tumors greater than 5 cm. Surgical resection does not require a wide margin or regional lymphadenectomy. Elective resection is associated with a mortality rate less than 1%. Emergent surgery for a ruptured hepatic adenoma with intraperitoneal bleeding has a mortality rate of 5% to 10%.
Trans-arterial embolization (TAE) is recommended for hepatic adenomas complicated by hemorrhage. Patients with intra-tumoral hemorrhage rarely present with hemodynamic instability, which allows for TAE followed by elective surgical resection of the adenoma. Trans-arterial embolization is indicated within 2 to 3 days of tumoral hemorrhage.
Radiofrequency ablation (RFA) is appropriate only for a very select patient population. It is not appropriate for hormone-sensitive tumors, underlying liver disease, surgical candidates, or those with a desire for pregnancy. It should be used to treat patients with adenomas less than 4 cm in size.
Obesity, nonalcoholic steatohepatitis, and metabolic syndromes are associated with hepatic adenomatosis. The development of complications of hemorrhage or malignant transformation are not related to the development of adenomatosis but rather to the molecular signatures. Moreover, most liver adenomatoses are typically associated with HNF-1alpha mutations which have a low risk for malignant transformations. For this reason, a liver transplant is not indicated for nonresectable hepatic adenomas as a rule. Exceptions involve men with an intrahepatic portosystemic venous shunt with nonresectable hepatic adenomas. Meanwhile, genetic counseling is recommended for patients with liver adenomatosis, particularly those associated with familial adenomatous polyposis or MODY3.
About 5% of hepatic adenomas are at risk for malignant transformation to hepatocellular carcinoma. Patients with Fanconi anemia and those with androgen treatment are at particular risk. The Wnt and beta-catenin pathways are particularly associated with malignant transformation. For this reason, surgical resections are typically recommended for this subtype.
Pregnancy
Women with hepatocellular adenoma who are pregnant, or wish to become pregnant, should be closely monitored for hepatocellular adenoma size (with ultrasound or MRI) during their pregnancy, due to the tendency of the lesion to grow, especially during the third trimester when high levels of estrogens are present 4. Hormone-induced growth, and possible rupture, may result in potentially lethal complications for the mother and unborn child. Treatment of hepatocellular adenoma during pregnancy may be indicated when the lesion shows signs of growth or bleeding, however specific figures for the risk of hepatocellular adenoma complication during pregnancy are not yet available.
Whether some subtypes are more prone to complications during pregnancy is not known, mainly because the majority is diagnosed non-invasively.
The choice of follow up, surgery, radiofrequency ablation (RFA) or transcatheter arterial embolization (TAE) for the treatment of hepatocellular adenomas in pregnancy is often a matter of debate. Surgery of lesions located at the periphery of the liver can be performed safely within the first or second trimester, and will usually be indicated by the size of the lesion, and its change in size. Radiation exposure or exposure to iodinated contrast media during radiofrequency ablation or transcatheter arterial embolization may be contraindicated during the early phase of pregnancy, with the treatment of smaller lesions not being indicated. Given the increased risk of hemorrhage in larger hepatocellular adenomas (>5 cm), or when a previous pregnancy was complicated by either minor or major bleeding, we currently advocate a preemptive treatment strategy before pregnancy, as proposed by Broker and colleagues 22. Whenever a hepatocellular adenoma is discovered during pregnancy, the second trimester is the optimal moment for invasive treatment, if indicated, as anesthesia is well tolerated at this stage, and the fetus is not yet so large as to interfere with liver surgery 23.
Hepatic adenoma prognosis
Complete resolution of hepatocellular adenoma is atypical.
The risk of malignant transformation exists and is as high as 8-13% in previous studies, but a recent systematic review revealed a risk of malignant transformation of 4.2% 24.
The risk of malignant transformation remains even after contraceptive or steroid use has been discontinued.
Morbidity and mortality
Nearly 20-25% of cases have right upper quadrant pain, and 30-40% experience hemorrhage (one third within the mass, two thirds into the abdomen).
In a systematic review including a total of 1176 patients, the overall frequency of hemorrhage was 27.2%. Hemorrhage occurred in 15.8% of all hepatocellular adenoma lesions. Rupture and intraperitoneal bleeding were reported in 17.5% of patients 25.
Although a tumor size of 5 cm is the standard for resection owing to the increased risk of hemorrhage and malignant transformation, multiple case series have reported hemorrhage in sizes less than 5 cm, even as small as 1 cm 26. The risk, though, appears to be minimal. The risk of hemorrhage appears to be related to size and not related to the number of lesions. Multiple studies did not find a difference between patients with a single or multiple hepatocellular adenomas 27.
The mortality rate associated with an acute hemorrhage into the peritoneum may be as high as 25-30% in patients with large tumors (>5 cm).
The risk of malignant transformation is not completely known and may be as high as 13% based on small studies. A recent systematic review incorporating all reports on malignant degeneration of hepatocellular adenoma into hepatocellular carcinoma showed an overall risk of 4.2%, with only 4.4% of these malignant transformations occurring in lesions less than 5 cm in diameter 24.
Hepatic adenoma combined with chronic hepatitis B infection could increase the risk of malignant transformation, but more studies are needed 28.
Pregnancy has been associated with hepatic adenoma, and rupture of the adenoma during pregnancy has been associated with high rates of maternal and fetal mortality.
- Shreenath AP, Kahloon A. Hepatic (Hepatocellular) Adenoma. [Updated 2019 Apr 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513264[↩][↩]
- Bioulac-Sage P., Balabaud C., Zucman-Rossi J. (2010) Focal nodular hyperplasia, hepatocellular adenomas: past, present, future. Gastroenterol Clin Biol 34: 355–358.[↩]
- Baum J., Bookstein J., Holtz F., Klein E. (1973) Possible Association between benign hepatomas and oral contraceptives. Lancet 2:926–929.[↩]
- Cobey F., Salem R. (2004) A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg 187: 181–191.[↩][↩]
- Brady PC, Missmer SA, Laufer MR. Hepatic Adenomas in Adolescents and Young Women with Endometriosis Treated with Norethindrone Acetate. J Pediatr Adolesc Gynecol. 2017 Jun;30(3):422-424.[↩]
- Thomeer MG, Broker M, Verheij J, et al. Hepatocellular adenoma: when and how to treat? Update of current evidence. Therap Adv Gastroenterol. 2016;9(6):898–912. doi:10.1177/1756283X16663882 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5076773[↩][↩]
- Tsilimigras DI, Rahnemai-Azar AA, Ntanasis-Stathopoulos I, Gavriatopoulou M, Moris D, Spartalis E, Cloyd JM, Weber SM, Pawlik TM. Current Approaches in the Management of Hepatic Adenomas. J. Gastrointest. Surg. 2019 Jan;23(1):199-209.[↩]
- Zhang C, Shi X, Zhao L. Synchronous giant hepatic adenoma in siblings-A case report and brief literature review. Cancer Biol Ther. 2016;17(7):727–731. doi:10.1080/15384047.2016.1177682 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4970524[↩]
- Genetic mutation in hepatic adenoma: Seeing is believing. Journal of Hepatology 45 (2006) 767–769 https://www.journal-of-hepatology.eu/article/S0168-8278(06)00492-2/pdf[↩]
- Jiang K, Al-Diffhala S, Centeno BA. Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification. Cancer Control. 2018 Jan-Mar;25(1):1073274817744625[↩]
- Van Aalten S., Verheij J., Terkivatan T., Dwarkasing R., de Man R., Ijzermans J. (2011b) Validation of a liver adenoma classification system in a tertiary referral centre: implications for clinical practice. J Hepatol 55: 120–125.[↩]
- Bioulac-Sage P., Laumonier H., Couchy G., Le Bail B., Sa Cunha A., Rullier A., et al. (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50: 481–489.[↩]
- Bioulac-Sage P., Balabaud C., Bedossa P., Scoazec J., Chiche L., Dhillon A., et al. (2007a) Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol 46: 521–527.[↩]
- Van Aalten S., de Man R., IJzermans J., Terkivatan T. (2012) Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg 99: 911–916.[↩]
- Dokmak S., Paradis V., Vilgrain V., Sauvanet A., Farges O., Valla D., et al. (2009) A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137: 1698–1705.[↩]
- Ronot M., Bahrami S., Calderaro J., Valla D., Bedossa P., Belghiti J., et al. (2011) Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53: 1182–1191.[↩]
- Bieze M., Phoa S., Verheij J., van Lienden K., van Gulik T. (2014) Risk factors for bleeding in hepatocellular adenoma. Br J Surg 101: 847–855.[↩]
- Grazioli L., Olivetti L., Mazza G., Bondioni M. (2013) MR imaging of hepatocellular adenomas and differential diagnosis dilemma. Int J Hepatol 2013: 374170.[↩]
- Stoot J., Coelen R., de Jong M., Dejong C. (2010) Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 12: 509–522.[↩]
- He MN, Lv K, Jiang YX, Jiang TA. Application of superb microvascular imaging in focal liver lesions. World J. Gastroenterol. 2017 Nov 21;23(43):7765-7775.[↩]
- Strauss E, Ferreira Ade S, França AV, Lyra AC, Barros FM, Silva I, Garcia JH, Parise ER. Diagnosis and treatment of benign liver nodules: Brazilian Society of Hepatology (SBH) recommendations. Arq Gastroenterol. 2015 Dec;52 Suppl 1:47-54.[↩]
- Broker M., Ijzermans J., van Aalten S., De Man R., Terkivatan T. (2012) The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol 2012: 725735[↩]
- Parangi S., Levine D., Henry A., Isakovich N., Pories S. (2007) Surgical Gastrointestinal Disorders During Pregnancy. Am J Surg 193: 223–232.[↩]
- Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010 Oct. 12(8):509-22.[↩][↩]
- van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012 Jul. 99(7):911-6.[↩]
- Cho SW, Marsh JW, Steel J, et al. Surgical management of hepatocellular adenoma: take it or leave it?. Ann Surg Oncol. 2008 Oct. 15(10):2795-803.[↩]
- Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009 Nov. 137(5):1698-705.[↩]
- Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011 Jan. 26(1):28-35.[↩]