What is heterotopic ossification
Heterotopic ossification refers to the formation of mature, lamellar bone in muscle and soft tissues where bone normally does not usually exist. Heterotopic ossification can be conceptualized as a tissue repair process gone awry and is a common complication of trauma and surgery 1. The acquired form of heterotopic ossification is usually secondary to musculoskeletal trauma, stroke, spinal cord injury, burns, traumatic amputation, joint replacement, and traumatic brain injury (TBI). There are other genetic causes of heterotopic ossification such as fibrodysplasia ossificans progressiva, Albright’s hereditary osteodystrophy and progressive osseous heteroplasia 2.
There are 2 main categories of heterotopic ossification: traumatic (fracture, arthroplasty, muscular trauma, joint dislocation, burns) versus neurogenic (stroke, spinal cord injury, traumatic brain injury, brain tumors) 3. In total joint arthroplasty, heterotopic ossification most commonly occurs for the replacement of hips, knees, elbow, and shoulder. Chronic muscular trauma leads to what is traditionally known specifically as traumatic myositis ossificans. The most common sites for traumatic myositis ossificans are the quadriceps femoris muscle and the brachialis muscle. The most common sites for neurogenic heterotopic ossification are the hips, elbows (extensor side), shoulders, and knees. Uncommon sites of heterotopic ossification that may be encountered in a rehabilitation setting are incisions, kidneys, uterus, corpora cavernosum, and the gastrointestinal (GI) tract.
The classic presentation of non-genetic heterotopic ossification is in young adults with a clear history of local trauma or surgery 4. Approximately half of patients are in their second and third decades of life; however, a broad age distribution is present from infancy to late adulthood 5. Men are slightly more commonly affected with a sex ratio of 3:2, but it is noted that females older than 65 years old have an increased risk of developing heterotopic ossification. A history of trauma as the initiating event is present in most cases (up to 75%) 6 and unrecognized or “microtrauma” or repetitive mechanical stress is generally thought to be present in the remaining patients.
Heterotopic ossification is well documented to occur at increased frequency with certain predisposing conditions, including orthopedic surgery, most commonly hip arthroplasty (occurs in up to ∼40% of cases) 7, bone fracture or dislocation (occurs in up to ∼30% of cases 8, with elbow trauma or dislocation being a common site of involvement) 9, high‐energy extremity trauma 10, traumatic brain and spinal cord injury and other neurologic disorders (occurs in up to ∼50% of spinal cord injuries) 11 and severe burns (occurs in up to ∼20% of third‐degree burns) 12. For severe traumatic amputations, this incidence rises to above 90%) 13. In the total hip arthroplasty population, the incidence varies. In those with high-risk factors, incidence can be as high as 90% 2. Overall rates for developing heterotopic ossification in the total hip arthroplasty population is closer to 53%. The incidence of neurogenic heterotopic ossification is 10% to 20%. When differentiating between the incidence of heterotopic ossification in adult spinal cord injury patients is 20% to 30% and in adult traumatic brain injury patients is 10% to 20% 3. Heterotopic ossification in the pediatric TBI population ranges from 3% to 20% 14.
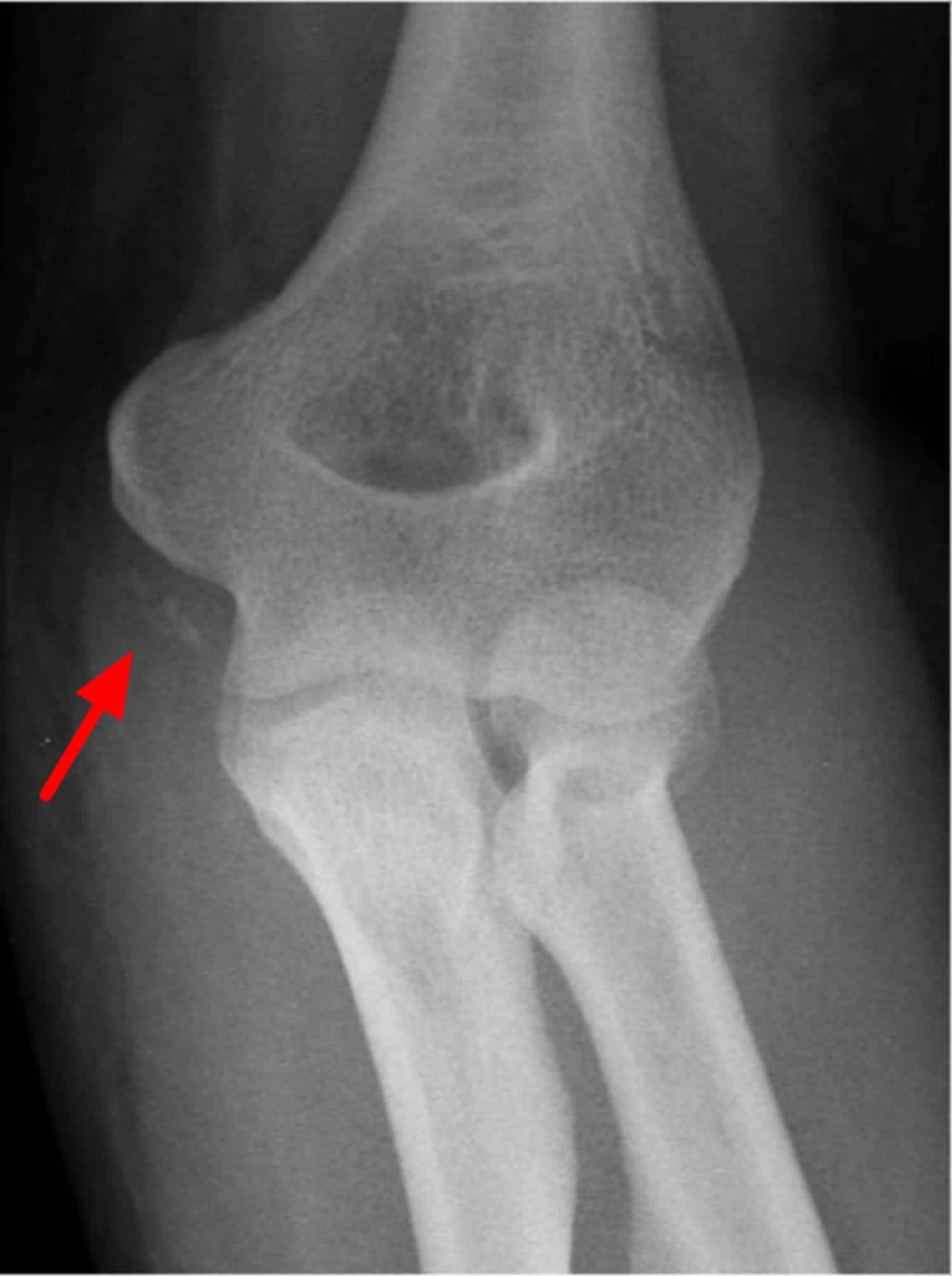
Figure 1. Heterotopic ossification elbow
Footnote: Heterotopic ossification is noted on medial aspect of elbow, representing ulnar collateral ligament ossification.
Figure 2. Heterotopic ossification hip
Footnote: Heterotopic ossification with a trabecular pattern of the calcification surrounding the right and left hip joints and proximal femur with some sclerosing and cystic regions.
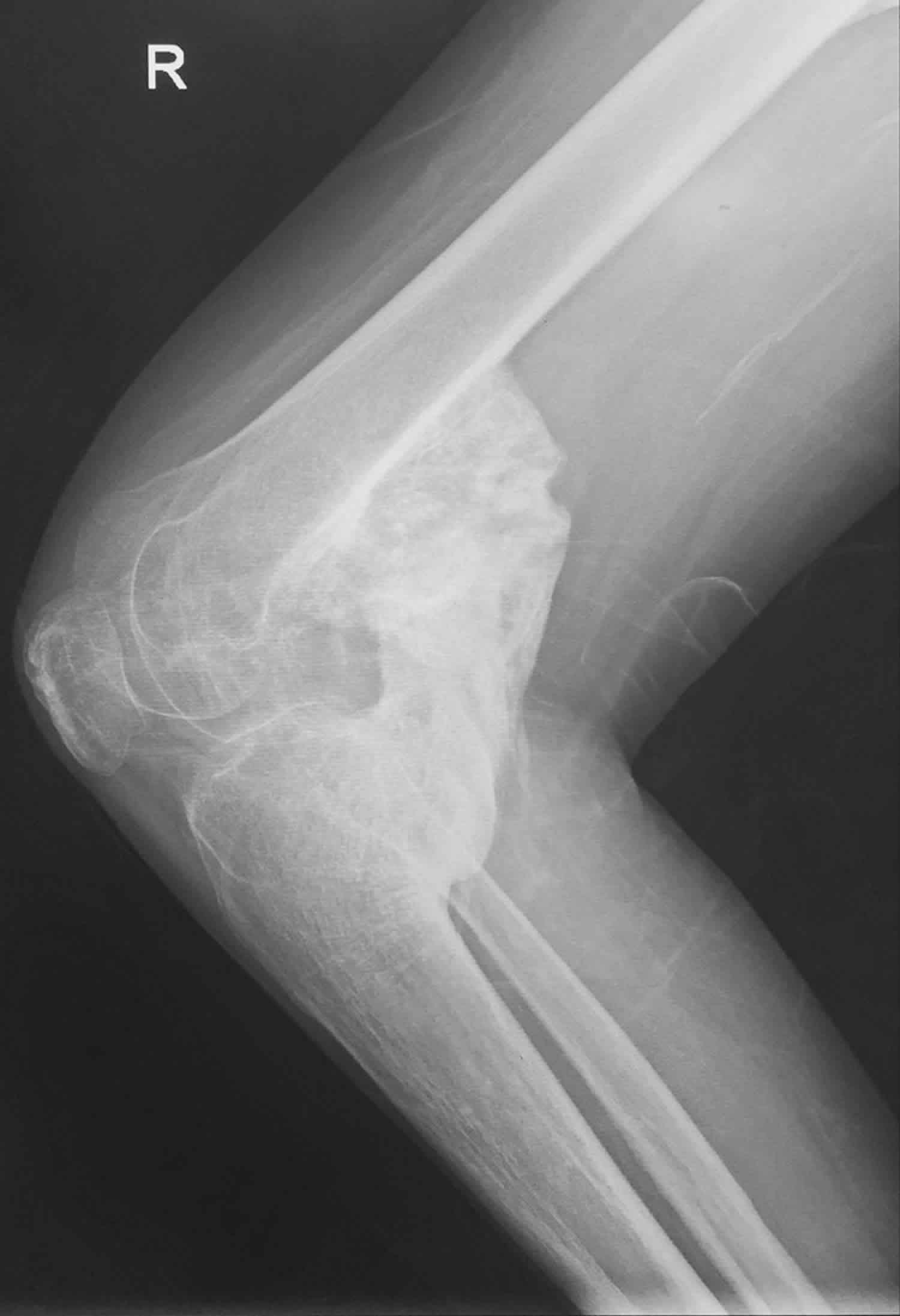
Figure 3. Heterotopic ossification knee
Footnote: Heterotopic ossification at the posterior aspect of the right knee joint, there is greater than 1cm in length of the bone island is formed.
Heterotopic ossification causes
The exact mechanism of heterotopic ossification in traumatic and neurogenic heterotopic ossification is unknown 15, but 2 common factors precede the formation of heterotopic ossification, the first being trauma or an inciting neurological event. In the spinal cord injury population, it is hypothesized that acute rehabilitation, transfer activities, and repeated microtrauma can add up during activities of daily living can cause an accumulation of mechanical stress that predisposes one to the formation of heterotopic ossification 2. Second, after trauma or neurological injury, is the tissue expression of bone morphogenic proteins. Bone morphogenic proteins stimulate mesenchymal spindle stem cells, also known as satellite cells, to migrate to the injured area and transform into fibroblasts and eventually, osteoblasts 2. It has been proposed that alkaline phosphatase also plays a role in ectopic hone formation. Alkaline phosphatase acts to suppress inhibitors of bone formation is known to be elevated in vascular smooth muscle tissue in the presence of inflammatory cytokines and macrophages 16. After migration, mesenchymal spindle cells begin to differentiate into fibroblasts which then start to secrete immature connective tissue composed of collagen and extracellular matrix. With continued tissue irritation, fibroblastic metaplasia is activated, transforming fibroblasts into chondrocytes in a similar process to endochondral ossification. Some of the chondrocytes continue to deposit collagen into the cartilage matrix while the remaining chondrocytes transform into osteoblasts. By 1 to 2 weeks, new osteoid is present within the tissue, and new bone formation starts to form within the osteoid. Osteoblasts then begin to degrade and replace the cartilage with bone. Calcium pyrophosphate within the osteoid is slowly replaced with hydroxyapatite crystals as the bone mineralizes and and starts to mature 17.
Risk factors for developing heterotopic ossification are spasticity, older age, pressure ulcer, the presence of deep vein thrombosis (DVT), having a tracheostomy, long bone fractures, prior injury to the same area, edema, immobility, long-term coma, and severity of injury (trauma, spinal cord injury, traumatic brain injury, stroke). High/moderate risk factors in the total hip arthroplasty population include men with bilateral total hip arthroplasty, prior history of heterotopic ossification, ankylosing spondylitis, diffuse idiopathic hyperostosis, or Paget’s disease 18.
Heterotopic ossification histopathology
Microscopic examination of biopsy in myositis ossificans revealed an outer zone of hypercellular spindle cells and woven bone surrounding an inner zone of trapped muscle with normal osteoblasts continuing to lay down bone. To distinguish heterotopic ossification from osteosarcoma, heterotopic ossification is zonation from central immature fibrous tissue to peripheral mature bone is characteristic of heterotopic ossification. This helps distinguish heterotopic ossification from osteosarcoma, in which the central zone consists of mature bone 3. At initial injury, there will be a proliferation of hypercellular spindle cells. Cartilage and woven bone start to form 2 weeks after injury. Trabecular bone starts to form from weeks 2 to 5 with mature fatty bone marrow. After 6 weeks lamellar bone will mature 19.
Heterotopic ossification prevention
The approach to prevention involves identifying patients with high risk of developing heterotopic ossification. Routine prophylaxis is not recommended. Current recommendations for prevention of heterotopic ossification are gentle range of motion (ROM) exercises, indomethacin, etidronate, and external beam radiation, which is primarily used after joint arthroplasty. It is also important to manage risk factors, such as spasticity, in the prevention of heterotopic ossification.
Nonsteroidal anti‐inflammatory drugs (NSAIDs)
Nonsteroidal anti‐inflammatory drugs (NSAIDs) remain the most commonly utilized prophylaxis for heterotopic ossification 20. Numerous nonsteroidal anti-inflammatory drugs (NSAIDs) have demonstrated efficacy, though postoperative indomethacin has been the historical gold standard, traditionally dosed at 25 mg three times daily for up to 6 weeks after surgery 21. Numerous studies have suggested that nonsteroidal anti-inflammatory drugs (NSAIDs) may prove beneficial for indications unrelated to hip arthroplasty and acetabular fracture surgery, including hip arthroscopy, elbow trauma, and spinal cord injury 22. Despite its relatively low rate of complications, NSAID use has been associated with an increased risk for nonunion of acetabular fractures 23. Overall, the utility of NSAIDs and radiation in preventing nongenetic heterotopic ossification appear similar, though the cost associated with NSAID therapy is typically markedly less 24.
Indomethacin is the most commonly used nonsteroidal anti-inflammatory drugs (NSAIDs) used for prophylaxis 25. Others nonsteroidal anti‐inflammatory drugs (NSAIDs) that have been proven effective are meloxicam, celecoxib, rofecoxib, and ibuprofen. The recommended dose of indomethacin for prophylaxis in the total hip arthroplasty population is 75 to 100 mg per day for 7 to 14 days post-operatively. In the spinal cord injury population, 75 mg per day of indomethacin for 3 weeks is the recommended 26. Careful monitoring must be done for risk of bleeding, especially with concurrent chemoprophylaxis for venous thromboembolism. It is advised to use concurrent prophylaxis for gastrointestinal (GI) ulcers. Twenty mg per day of a selective COX-2 inhibitor can be used with fewer GI side effects compared to traditional nonsteroidal anti-inflammatory drugs (NSAIDs) 26.
Bisphosphonates
Etidronate is a bisphosphonate that has been approved for prevention of heterotopic ossification in spinal cord injuries and complication of total hip arthroplasty. For spinal cord injury associated heterotopic ossification, the recommended treatment is 20 mg/kg per day for 2 weeks then 10 mg/kg per day for 10 weeks for a total treatment period of 12 weeks. For total hip arthroplasty, recommended treatment is 20 mg/kg per day for 1 month preoperatively and then the same dosing for 3 months postoperatively 27. Despite early enthusiasm for the potential role of bisphosphonates in the prevention of neurogenic heterotopic ossification, further analysis has failed to demonstrate a clear benefit for the use of these medications in preventing heterotopic ossification 28. In fact, there is some indication that antiresorptive therapy may increase the risk of developing heterotopic ossification, or may simply delay rather than prevent the bone formation. Future therapeutic modalities such as retinoic acid receptor (RARγ) agonists 29, as well as free‐radical scavengers126 are currently under investigation, though their clinical utility remains to be elucidated.
Radiation therapy
External beam radiation is most commonly used after total joint arthroplasty. A single dose of 700 to 800 cGy (centigray) is administered up to 24 hours preoperatively or within 72 hours postoperatively 18.
Low‐dose radiation has been studied both as a prophylactic modality in heterotopic ossification occurrence (as primary prevention in high‐risk patients) or as a prophylactic modality in heterotopic ossification recurrence (as secondary prevention together with surgical excision). The prophylactic use of radiation therapy is best studied in the context of hip arthroplasty. Of the randomized controlled trials that have been performed, definitions of high‐risk patients vary but may include those patients with hypertrophic osteoarthritis, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, or prior heterotopic ossification 30. Thus, prophylaxis against heterotopic ossification occurrence (in those at high risk) and heterotopic ossification recurrence (preexisting heterotopic ossification) are often studied together. Prophylactic doses typically range from 400 to 800 cGy (centigray), and are given either 24 hours preoperatively or up to 72 hours postoperatively 30. For patients undergoing total hip arthroplasty, a single‐center randomized trial suggests that 700 cGy given postoperatively was significantly more effective at preventing heterotopic ossification (25%) than 400 cGy (42%) given postoperatively 31. Higher doses, however, have not proven to be of increased benefit for prevention 32. Furthermore, there does not appear to be a significant difference between preoperative and postoperative radiation dosing regarding efficacy or complications, with the exception that those treated more than 8 hours preoperatively or more than 72 hours postoperatively may demonstrate a greater rate of radiographic ossification after hip surgery 32.
Although the majority of controlled studies have focused upon patients undergoing surgery around the hip, the results regarding prophylactic radiation have been extended to other indications as well 33. Among spinal cord injury patients with evidence of early neurogenic heterotopic ossification, a single radiation fraction of 700 cGy limited the progression of ossification around the hips 34. After elbow trauma, however, the results are somewhat less favorable, with limited evidence to support its routine use 35. A randomized controlled trial was stopped early because of concerns over increased rates of nonunion in those receiving 700 cGy of radiation after surgical management of intra‐articular elbow fractures 36.
Among the concerns with the use of prophylactic radiation are joint stiffness and potential oncogenesis. The relatively low risk of joint stiffness after low‐dose radiation can be weighed against the decreased range of joint motion and potential ankylosis that can develop with heterotopic ossification. Regarding oncogenesis, a case‐control analysis has failed to demonstrate a significantly increased rate of malignancy in patients treated at this low dose of radiation 37. It bears noting, though, that the number of patients needed for adequate power is rarely met, and the theoretical risk of malignancy remains 38.
Corticosteroids
Although not commonly used in nongenetic heterotopic ossification, corticosteroids are used as a prophylactic modality in fibrodysplasia ossificans progressiva patients. A brief course of high‐dose corticosteroids is often used in fibrodysplasia ossificans progressiva patients within the first 24 hours of flare‐ups to reduce inflammation and tissue edema observed in early stages of the disease 39. However, the use of corticosteroids in fibrodysplasia ossificans progressiva patients is generally limited to treating flare‐ups in the major joints, the jaw, and submandibular area. Corticosteroids are not used as a chronic treatment of fibrodysplasia ossificans progressiva, so as to limit side effects associated with long‐term use 40.
Heterotopic ossification symptoms
The most common heterotopic ossification symptoma are pain around the ossification site pain and decreased range of motion (ROM). Associated features can include fever, soft tissue swelling, and poor mobility of the affected joint. Patients often complain of stiffness in their joints. Other common signs to look for are local edema, effusion, erythema, warmth, and tenderness 41 in the tissue or joint. Localized soft tissue swelling may present like a deep vein thrombosis (DVT). The patient may also present with a low-grade fever. Since spasticity is a risk factor, the patient may present with spasticity near the affected joint. Other risk factors to look for in the history are prolonged coma, tracheostomy or gastric tube, immobility, pressure ulcers and associated long bone fracture 42. The greatest risk for developing heterotopic ossification also occurs at the 3 to 4 month period post-injury.
Heterotopic ossification usually occurs 3 to 12 weeks after inciting injury (total hip arthroplasty, total knee arthroplasty, traumatic amputation, stroke, spinal cord injury, traumatic brain injury, or burn) 43, but can take up to 6 months to present.
Nongenetic heterotopic ossification can occur nearly anywhere in the body, but the most common areas include locations that are susceptible to trauma, such as the elbow, thigh, pelvis, and shoulder 44. The head and neck is also a well‐described location for traumatic heterotopic ossification 45. Heterotopic ossification may occur in the skin, particularly in autoimmune disorders such as dermatomyositis20 and systemic sclerosis 46. The digits are also a well‐described site for heterotopic ossification, in which case the term “fibro‐osseous pseudotumor of the digits” is also used 47. A spectrum of other distinctive reactive bony lesions of the hands and feet are also well described (including florid reactive periostitis, subungual exostosis, and bizarre parosteal osteochondromatous proliferation). All have variably overlapping histologic features with heterotopic ossification 48; however, these lesions are almost always associated with the periosteum. Heterotopic ossification classically forms without connection to the periosteum and can later fuse to the periosteum as a secondary feature. Some anatomic sites are relatively infrequently involved by heterotopic ossification , for example, the viscera in both genetic and nongenetic heterotopic ossification 39 or the diaphragm in genetic forms of heterotopic ossification 39. Understanding why these tissue sites are relatively inhospitable to heterotopic ossification formation is an interesting and unanswered question in the field.
The clinical presentation depends on the temporal stage of nongenetic heterotopic ossification development. In the early/inflammatory phase, heterotopic ossification presents with localized pain, tenderness, and swelling. During this time, heterotopic ossification is often characterized by a rapid increase in size, which may arouse clinical suspicion of a soft tissue sarcoma 49. In later stages and with gradual maturation of the bone tissue, the swelling becomes more localized, firm, and when adjacent to a joint may restrict motion. Lesions resembling heterotopic ossification have been reported within nerves 50 or the abdominal mesentery and fascia 51 and their presentation is site specific. A unifying theme of all locations is the presence of connective tissue and thus of tissue sites that may contain stromal cells with osteogenic potential.
The rare genetic causes of heterotopic ossification have a different presentation and clinical severity than the far more common nongenetic cases, and include fibrodysplasia ossificans progressiva and progressive osseous heteroplasia. Fibrodysplasia ossificans progressiva is a rare, slowly progressive disorder caused by ACVR1 mutations and initially presenting in childhood 52. Multiple congenital skeletal malformations are associated with fibrodysplasia ossificans progressiva, including most frequently an abnormal first toe 53, dysmorphologies affecting the digits of the hand 54 and malformations of the cervical spine 54. Fibrodysplasia ossificans progressiva patients eventually develop progressive, painful flares and heterotopic lesions limiting mobility and function. Biopsies should not be performed on fibrodysplasia ossificans progressiva patients because any surgical intervention leads to additional spread of heterotopic lesions. Most cases arise from a spontaneous mutation, but autosomal dominant transmission has also been described 54. Fibrodysplasia ossificans progressiva is characterized by progressive ossification of muscle, tendon, aponeuroses, and ligaments. Ossifications generally develop from cranial to caudal and axial to appendicular. Eventual peri‐articular and soft tissue ossification becomes so severe as to lead to difficulty with posture, gait, and respiration 55. Median age at death is approximately 40 years 56. The mechanisms of ACVR1/ALK2 mutations have been well documented, which includes the R206H mutation resulting in hyperactive bone morphogenetic protein (BMP) signaling and primarily endochondral ossification 57. Cells with the R206H mutation respond to Activin A with increased SMAD1/5/8 phosphorylation comparison with wild‐type cells 58. The bone formed is thought to occur through an endochondral process based on human data and animal models.
Progressive osseous heteroplasia is a more recently characterized genetic form of progressive heterotopic ossification caused by heterozygous inactivating mutations in the GNAS1 gene 59. Progressive osseous heteroplasia is an autosomal dominant disorder and can be a spontaneous/new mutation in the affected person or paternal inheritance of the mutant allele 60. Ossification in progressive osseous heteroplasia has a predilection for the skin and subcutis and appears to be primarily intramembranous, although sporadic cartilage may also be found. The molecular defect causing progressive osseous heteroplasia is the same as that causing pseudopseudohypoparathyroidism 61, which has a constellation of physical findings referred to as Albright’s hereditary osteodystrophy (Aheterotopic ossification) 60.
Heterotopic ossification diagnosis
Laboratory Studies
Alkaline phosphatase (ALP) is historically the most commonly ordered lab, but may not be elevated early on in heterotopic ossification formation 62 It may take up to 2 weeks to be elevated and can rise to 3.5 times the normal value 10-weeks post-injury. Serum alkaline phosphatase greater than 250 has been shown to correlate with heterotopic ossification in the total hip arthroplasty population, but there isn’t a correlation between the level of alkaline phosphatase and the severity of the injury. Alkaline phosphatase can also be falsely elevated with associated long-bone injuries.
Erythrocyte sedimentation rate (ESR) is another inflammatory marker that is used. ESR greater than 35 mm/hr can indicate the development of heterotopic ossification 63. C-reactive protein is another inflammatory marker that can be elevated in early heterotopic ossification. Both are non-specific. Creatine kinase (CK) can be used to determine the severity of heterotopic ossification but is not a very specific test 3. However, one study of 18 spinal cord injured patients determined that an elevated creatine kinase may be associated with a more aggressive course of heterotopic ossification and show possible resistance to etidronate therapy 64.
Imaging studies
Plain film radiographs show circumferential bone formation around or near a joint with a radiolucent center. X-ray is specific for heterotopic ossification but not sensitive in early disease. Plain films may not be positive until 3 to 4 weeks after heterotopic ossification appears on bone scan. Therefore the triple phase bone scan is the most sensitive. A bone scan can reveal heterotopic ossification as early as 2.5 weeks after injury 3.
CT may be used to delineate the area of bone formation in preparation for surgical, but their role in evaluation/diagnosis of heterotopic ossification is not established 16. MRI may also be used but is not cost effective unless bone encompasses neurologic structures. Other imaging techniques uncommonly used are an ultrasound and 3-dimensional stereolithography.
The appearance of heterotopic ossification by computed tomography (CT) is usually characteristic, and the zonal maturation of the lesion is well appreciated on CT 48. Again, the imaging characteristics of nongenetic heterotopic ossification in an intramuscular location are best characterized. Early in heterotopic ossification progression, a low‐density mass may be the only sign of heterotopic ossification by CT. In such cases and if the diagnosis of heterotopic ossification is suspected, short‐interval follow‐up CT imaging is helpful in making the final diagnosis as peripheral ossification develops. Cross‐sectional imaging with CT is also important in preoperative planning, by improving visualization of the lesion’s relationship to important anatomic landmarks 65.
By MRI, the appearance of heterotopic ossification is variable, depending on the stage of maturation. Typically, heterotopic ossification is a well‐defined mass with heterogeneous signal, characteristically associated with diffuse surrounding perilesional edema 66. In the early phases, there may be fluid‐fluid levels due to the presence of hemorrhage. Enhancement after contrast administration can occur centrally in heterotopic ossification due to vascularity within the lesion, making the differentiation from sarcoma challenging at times. However, the developing zonal ossification pattern is important in distinguishing heterotopic ossification from a sarcoma, as identified by correlative radiograph or CT. As heterotopic ossification matures, rim enhancement after contrast administration is the predominant feature of heterotopic ossification lesions, which helps to differentiate heterotopic ossification from a soft‐tissue sarcoma 67.
Several site‐specific classification schemes exist to grade the severity of heterotopic ossification, which are particularly relevant for peri‐articular heterotopic ossification. The Brooker scale classifies hip‐associated heterotopic ossification into four classes of ascending severity (I–IV), which are related to the distance between heterotopic ossification and the hip joint 68. The Hastings and Graham classification scale for elbow joint–associated heterotopic ossification uses a three‐point functional scale (I–III) to define the degree of clinical and radiographic severity 69.
Alternative imaging modalities may also be of benefit in the detection of heterotopic ossification. Positron emission tomography (PET) may be combined with CT, either using radiolabeled fluoride (F18) or radiolabeled glucose (FDG). F18 binds hydroxyapatite and detects areas of bone formation, and may be useful in the detection of nongenetic heterotopic ossification 70 and recently in the early detection and monitoring of flare‐ups in FOP 71. FDG PET localizes to areas of increased metabolic activity and inflammation, and increased FDG avidity (although nonspecific) has been noted in cases of nongenetic heterotopic ossification 72. Single‐photon emission CT (SPECT) is a potential imaging modality for early detection of heterotopic ossification with potentially improved sensitivity 73. Although operator dependent, ultrasound can be used to detect heterotopic ossification especially in spinal cord injury patients 74. Raman spectroscopy is a novel imaging technology that has the potential to define the extent of heterotopic ossification earlier than currently available radiographic studies by detecting mineralized collagen within tissues 75. Near infrared imaging and ultrasound imaging have also been described to identify heterotopic ossification before radiographic detection 76.
Heterotopic ossification staging
Staging is most often used for surgical planning and is not used much in rehabilitation management. Brooker Classification System, used for severity of heterotopic ossification in the hip, is the most commonly used staging system 43, which uses plain film radiograph to evaluate the presence and size of bone deposition visually. A simplified version has been proposed called the “Della Valle” classification system 77. None of the most commonly used rating systems take into account patient function or range of motion (ROM), so a modified Brooker classification scale has been proposed that take these factors into account.
Brooker classification divides the extent of heterotopic ossification formation in the hip into 4 classes:
- Class 1: Islands of bone within the soft tissues around the hip
- Class 2: Bone spurs that originate from the pelvis or proximal end of the femur, leaving at least 1 cm between opposing bone surfaces
- Class 3: Bone spurs originating from the pelvis or proximal end of the femur, reduced space between opposing bone surfaces to less than 1 cm
- Class 4: Ankylosis of the hip
The “Della Valle” classification system 77:
- Grade A: Absence of heterotopic ossification (or if bone is present, it may be greater than or equal to 1 island of bone of less than 1 cm in length
- Grade B: Presence of greater than or equal to 1 islands of bone at least 1 cm in length with 1 cm distance between opposing surfaces of bone
- Grade C: Bone spurs arising from the pelvis or femur with less than 1 cm between opposing surfaces or bone ankylosis
Heterotopic ossification treatment
Current treatment recommendations consist of mobilization with range of motion (ROM) exercises, indomethacin, etidronate, and surgical resection 16. Early treatment with a passive range of motion exercises should be implemented once the presence of heterotopic ossification is confirmed to prevent ankylosing of joints. Absolute treatment consists of surgical resection of mature bone once the heterotopic ossification has fully matured. This can be 12 to 18 months after the initial presentation 78. Surgical consultation with an orthopedic surgeon is warranted only if there will be an improvement in function as demonstrated by mobility, transfers, hygiene, and activities of daily living.
Indomethacin and etidronate are also used to help arrest bone formation in heterotopic ossification, but efficacy in the traumatic brain injury population has not been clearly proven. The most effective treatment option in thetraumatic brain injury (TBI) population is surgical resection. In the spinal cord injury population, the most effective nonsteroidal anti-inflammatory drug (NSAID) treatment regiments are either Rofecoxib 25 mg per day for 4 weeks or indomethacin 75 mg daily for 3 weeks 27.
Heterotopic ossification physical therapy
It is unclear whether physical therapy plays a significant role in the development or mitigation of heterotopic ossification, and conflicting thoughts are reflected in the literature among burn and spinal cord injury patients about early range‐of‐motion exercises 79. For patients with contusions to the thigh, a quadriceps stretching regimen has been proposed as a method for improving the time to return to full activity and potentially preventing heterotopic ossification 80. In the authors’ clinical experience, burn surgeons will often comment on the increasing incidence of heterotopic ossification in those patients who receive overly aggressive passive range‐of‐motion exercises on the elbow in an attempt to prevent skin contracture. Direct comparison trials are lacking, and the applicability of stretching or range‐of‐motion exercises for other risk factors for ossification are questionable. In patients who have developed maturing heterotopic ossification, clinical management differs based on clinician preferences. For example, many recommend against passive exercises, which could exacerbate inflammation and possibly heterotopic ossification, while others recommend a physical therapy regimen to improve range of motion and limit contractures.
Heterotopic ossification surgery
The natural history of heterotopic ossification is to fully ossify into mature bone over time. Many patients with heterotopic ossification report pain, painful motion, restricted motion, or prominent bone that can lead to pressure sores or impaired hygiene. Although the focal discomfort may improve after the inflammatory stage abates, patients with persistent symptoms have few management options other than operative intervention 20. Patients without significant symptoms, however, can be managed nonoperatively.
Surgical resection for nongenetic heterotopic ossification is ideally performed after the osseous maturation is complete, which is typically by 6 months after the initiation of heterotopic ossification. Excision before 6 months may be associated with an increased risk of recurrence of heterotopic ossification 81. No additional benefit appears to exist with further delay in operative management 82. Although some have advocated that excision should be performed early to prevent irreversible loss of motion, a comparison between 18 patients with elbow ankylosis and 27 patients with partial restriction of motion demonstrated comparable return of motion 83. Especially for heterotopic ossification at specific anatomic sites (such as tendinous heterotopic ossification or intraperitoneal heterotopic ossification), the benefits of removal must be weighed against the morbidity of the surgical procedure itself. As previously alluded to, surgical management in fibrodysplasia ossificans progressiva is generally not recommended because this may lead to additional spread of heterotopic lesions.
Even though complete excision is not always practical or possible, incomplete resection of the heterotopic ossification is associated with recurrence 20. Interposition of soft tissue is not of clear benefit 81. It is important to note that, unlike neoplastic processes, heterotopic ossification does not always respect natural anatomic barriers and may encase major neurovascular structures. This is perhaps responsible for the high reported rates of neurovascular injury after operative intervention in these patients.
Heterotopic ossification prognosis
Complications of heterotopic ossification present itself through decreased function and mobility, peripheral nerve entrapment, and pressure ulcers. Up to 70% of cases involving hip arthroplasty are asymptomatic. Ankylosis, vascular compression, and lymphedema can also be complications manifested in heterotopic ossification 84. Prognosis is generally good after surgery. Mean time from injury to surgery is 3.6 years. Once the surgery is performed, studies have shown that average range of motion (ROM) in the hip can improve from 24.3°(flexion and extension) to 98.5°(flexion and extension) 85. After surgery, improvement was maintained in follow up 6 months after surgery. Complications from surgical resection of heterotopic ossification, such as infection, severe hematoma, and DVT 85.
- Meyers C, Lisiecki J, Miller S, et al. Heterotopic Ossification: A Comprehensive Review. JBMR Plus. 2019;3(4):e10172. Published 2019 Feb 27. doi:10.1002/jbm4.10172 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6478587[↩]
- Sun E, Hanyu-Deutmeyer AA. Heterotopic Ossification. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519029[↩][↩][↩][↩]
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J. Nucl. Med. 2002 Mar;43(3):346-53.[↩][↩][↩][↩][↩]
- Dorfman HD, Czerniak B. Bone tumors: Maryland Heights, MO: Mosby; 1998.[↩]
- Rosenberg AE. Pseudosarcomas of soft tissue. Arch Pathol Lab Med. 2008;132(4):579–86. [↩]
- Nuovo MA, Norman A, Chumas J, Ackerman LV. Myositis ossificans with atypical clinical, radiographic, or pathologic findings: a review of 23 cases. Skeletal Radiol. 1992;21 (2):87–101[↩]
- Bedi A, Zbeda RM, Bueno VF, Downie B, Dolan M, Kelly BT. The incidence of heterotopic ossification after hip arthroscopy. Am J Sports Med. 2012;40 (4):854–63.[↩]
- Hong CC, Nashi N, Hey HW, Chee YH, Murphy D. Clinically relevant heterotopic ossification after elbow fracture surgery: a risk factors study. Orthop Traumatol Surg Res. 2015;101 (2):209–13[↩]
- Sandeep KN, Suresh G, Gopisankar B, Abhishek N, Sujiv A. Does excision of heterotopic ossification of the elbow result in satisfactory patient‐rated outcomes? Malays Orthop J. 2017;11(1):35–40[↩]
- Forsberg JA, Pepek JM, Wagner S, et al. Heterotopic ossification in high‐energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91 (5):1084–91[↩]
- Teasell RW, Mehta S, Aubut JL, et al. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord. 2010;48 (7):512–21[↩]
- Engber WD, Reynen P. Post‐burn heterotopic ossification at the elbow. Iowa Orthop J. 1994;14:38–41.[↩]
- Daniels CM, Pavey GJ, Arthur J, Noller M, Forsberg JA, Potter BK. Has the proportion of combat‐related amputations that develop heterotopic ossification increased? J Orthop Trauma. 2018;32 (6):283–7[↩]
- Hurvitz EA, Mandac BR, Davidoff G, Johnson JH, Nelson VS. Risk factors for heterotopic ossification in children and adolescents with severe traumatic brain injury. Arch Phys Med Rehabil. 1992 May;73(5):459-62[↩]
- Chen HC, Yang JY, Chuang SS, Huang CY, Yang SY. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009 Sep;35(6):857-62.[↩]
- Yoon BH, Park IK, Sung YB. Ankylosing Neurogenic Myositis Ossificans of the Hip: A Case Series and Review of Literature. Hip Pelvis. 2018 Jun;30(2):86-91[↩][↩][↩]
- Rossier AB, Bussat P, Infante F, Zender R, Courvoisier B, Muhelm G, Donath A, Vasey H, Taillard W, Lagier R, Gabbiani G, Baud CA, Pouezat JA, Very JM, Hachen HJ. Current facts of para-osteo-arthropathy (POA). Paraplegia. 1973 May;11(1):38-78.[↩]
- Ruo Redda MG, De Colle C, Bianco L, Ruggieri A, Nassisi D, Rossi A, Gino E, Airaldi C. Heterotopic ossifications: role of radiotherapy as prophylactic treatment. Radiol Med. 2018 Jun;123(6):463-468.[↩][↩]
- Al-Jarallah K, Al-Saeed O, Shehab D, Dashti K, Sheikh M. Ossification of ligamentum flavum in Middle East Arabs: a hospital-based study. Med Princ Pract. 2012;21(6):529-33[↩]
- Winkler S, Wagner F, Weber M, et al. Current therapeutic strategies of heterotopic ossification––a survey amongst orthopaedic and trauma departments in Germany. BMC Musculoskelet Disord. 2015;16:313[↩][↩][↩]
- Ritter MA, Sieber JM. Prophylactic indomethacin for the prevention of heterotopic bone formation following total hip arthroplasty. Clin Orthop Relat Res. 1985(196):217–25.[↩]
- Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double‐blind randomized placebo‐controlled trial. J Bone Joint Surg Am. 2015;97(24):2032–7.[↩]
- Sagi HC, Jordan CJ, Barei DP, Serrano‐Riera R, Steverson B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma. 2014;28 (7):377–83[↩]
- Vavken P, Dorotka R. Economic evaluation of NSAID and radiation to prevent heterotopic ossification after hip surgery. Arch Orthop Trauma Surg. 2011;131(9):1309–15[↩]
- Joice M, Vasileiadis GI, Amanatullah DF. Non-steroidal anti-inflammatory drugs for heterotopic ossification prophylaxis after total hip arthroplasty: a systematic review and meta-analysis. Bone Joint J. 2018 Jul;100-B(7):915-922[↩]
- Aubut JA, Mehta S, Cullen N, Teasell RW., ERABI Group. Scire Research Team. A comparison of heterotopic ossification treatment within the traumatic brain and spinal cord injured population: An evidence based systematic review. NeuroRehabilitation. 2011;28(2):151-60[↩][↩]
- Aubut JA, Mehta S, Cullen N, Teasell RW., ERABI Group. Scire Research Team. A comparison of heterotopic ossification treatment within the traumatic brain and spinal cord injured population: An evidence based systematic review. NeuroRehabilitation. 2011;28(2):151-60.[↩][↩]
- Shafer DM, Bay C, Caruso DM, Foster KN. The use of eidronate disodium in the prevention of heterotopic ossification in burn patients. Burns. 2008;34 (3):355–60.[↩]
- Shimono K, Tung WE, Macolino C, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor‐gamma agonists. Nat Med. 2011;17 (4):454–60[↩]
- Popovic M, Agarwal A, Zhang L, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta‐analysis of published data. Radiother Oncol. 2014;113 (1):10–7[↩][↩]
- Liu JZ, Frisch NB, Barden RM, Rosenberg AG, Silverton CD, Galante JO. Heterotopic ossification prophylaxis after total hip arthroplasty: randomized trial of 400 vs 700 cGy. J Arthroplasty. 2017;32 (4):1328–34[↩]
- Milakovic M, Popovic M, Raman S, Tsao M, Lam H, Chow E. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta‐analysis of randomized controlled trials. Radiother Oncol. 2015;116(1):4–9.[↩][↩]
- Mishra MV, Austin L, Parvizi J, Ramsey M, Showalter TN. Safety and efficacy of radiation therapy as secondary prophylaxis for heterotopic ossification of non‐hip joints. J Med Imaging Radiat Oncol. 2011;55(3):333–6.[↩]
- Museler AC, Grasmucke D, Jansen O, et al. In‐hospital outcomes following single‐dose radiation therapy in the treatment of heterotopic ossification of the hip following spinal cord injury—an analysis of 444 cases. Spinal Cord. 2017;55 (3):244–6.[↩]
- Ploumis A, Belbasis L, Ntzani E, Tsekeris P, Xenakis T. Radiotherapy for prevention of heterotopic ossification of the elbow: a systematic review of the literature. J Shoulder Elbow Surg. 2013;22 (11):1580–8.[↩]
- Hamid N, Ashraf N, Bosse MJ, et al. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am. 2010;92 (11):2032–8[↩]
- Sheybani A, TenNapel MJ, Lack WD, et al. Risk of radiation‐induced malignancy with heterotopic ossification prophylaxis: a case‐control analysis. Int J Radiat Oncol Biol Phys. 2014;89 (3):584–9[↩]
- Mazonakis M, Berris T, Lyraraki E, Damilakis J. Cancer risk estimates from radiation therapy for heterotopic ossification prophylaxis after total hip arthroplasty. Med Phys. 2013;40 (10):101702.[↩]
- Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80.[↩][↩][↩]
- The medical management of FOP: current treatment considerations. In: Kaplan FS, editor; , Shore EM, editor; , Pignolo RJ, editor. , editors. The International Consortium on FOP. Philadelphia: The Center for Research in FOP and Related Disorders, The University of Pennsylvania, School of Medicine; 2011. p. 1–100.[↩]
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J. Nucl. Med. 2002 Mar;43(3):346-53[↩]
- Citak M, Suero EM, Backhaus M, Aach M, Godry H, Meindl R, Schildhauer TA. Risk factors for heterotopic ossification in patients with spinal cord injury: a case-control study of 264 patients. Spine. 2012 Nov 01;37(23):1953-7[↩]
- Mary Jiayi T, Linda P, Michael P, Hans K, Markku N, Richard J, Bo Angela W, May T, Elizabeth B, Edward C. Potential discrepancy between plain films and CT scans in Brooker classification of heterotopic ossification. Br J Radiol. 2017 Dec;90(1080):20170263[↩][↩]
- Hoch B, Montag A. Reactive bone lesions mimicking neoplasms. Semin Diagn Pathol. 2011; 28 (1):102–12.[↩]
- Conner GA, Duffy M. Myositis ossificans: a case report of multiple recurrences following third molar extractions and review of the literature. J Oral Maxillofac Surg. 2009;67 (4):920–6.[↩]
- Botzoris VG, Argyropoulou MI, Voulgari PV, Zikou AK, Drosos AA. Heterotopic ossification in systemic sclerosis. Scand J Rheumatol. 2009;38 (4):317–9.[↩]
- Chaudhry IH, Kazakov DV, Michal M, Mentzel T, Luzar B, Calonje E. Fibro‐osseous pseudotumor of the digit: a clinicopathological study of 17 cases. J Cutan Pathol. 2010;37 (3):323–9.[↩]
- McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34 (10):609–19.[↩][↩]
- Daniels CM, Pavey GJ, Arthur J, Noller M, Forsberg JA, Potter BK. Has the proportion of combat‐related amputations that develop heterotopic ossification increased? J Orthop Trauma. 2018;32 (6):283–7.[↩]
- Katz LD, Lindskog D, Eisen R. Neuritis ossificans of the tibial, common peroneal and lateral sural cutaneous nerves. J Bone Joint Surg Br. 2011;93 (7):992–4.[↩]
- Hashash JG, Zakhary L, Aoun EG, Refaat M. Heterotopic mesenteric ossification. Colorectal Dis. 2012;14 (1):e29–30[↩]
- Tran L, Stein N, Miller S. Fibrodysplasia ossificans progressiva: early diagnosis is critical yet challenging. J Pediatr. 2010;157 (5):860 e1[↩]
- Nakashima Y, Haga N, Kitoh H, et al. Deformity of the great toe in fibrodysplasia ossificans progressiva. J Orthop Sci. 2010;15 (6):804–9[↩]
- Shore EM. Fibrodysplasia ossificans progressiva: a human genetic disorder of extraskeletal bone formation, or how does one tissue become another? Wiley Interdiscip Rev Dev Biol. 2012;1 (1):153–65[↩][↩][↩]
- Pignolo RJ, Kaplan FS. Clinical staging of fibrodysplasia ossificans progressiva (FOP). Bone. 2018;109:111–4.[↩]
- Wentworth KL, Bigay K, Chan TV, et al. Clinical‐pathological correlations in three patients with fibrodysplasia ossificans progressiva. Bone. 2018;109:104–10.[↩]
- Wang H, Behrens EM, Pignolo RJ, Kaplan FS. ECSIT links TLR and BMP signaling in FOP connective tissue progenitor cells. Bone. 2018;109:201–9[↩]
- Barruet E, Morales BM, Lwin W, et al. The ACVR1 R206H mutation found in fibrodysplasia ossificans progressiva increases human induced pluripotent stem cell‐derived endothelial cell formation and collagen production through BMP‐mediated SMAD1/5/8 signaling. Stem Cell Res Ther. 2016;7 (1):115.[↩]
- Kaplan FS, Craver R, MacEwen GD, et al. Progressive osseous heteroplasia: a distinct developmental disorder of heterotopic ossification. Two new case reports and follow‐up of three previously reported cases. J Bone Joint Surg Am. 1994;76 (3):425–36.[↩]
- Shore EM, Ahn J, Jan de Beur S, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346(2):99–106.[↩][↩]
- Fischer JA, Egert F, Werder E, Born W. An inherited mutation associated with functional deficiency of the alpha‐subunit of the guanine nucleotide‐binding protein Gs in pseudo‐ and pseudopseudohypoparathyroidism. J Clin Endocrinol Metab. 1998;83 (3):935–8.[↩]
- Kjaersgaard-Andersen P, Pedersen P, Kristensen SS, Schmidt SA, Pedersen NW. Serum alkaline phosphatase as an indicator of heterotopic bone formation following total hip arthroplasty. Clin. Orthop. Relat. Res. 1988 Sep;(234):102-9.[↩]
- Kjaersgaard-Andersen P, Schmidt SA, Pedersen NW, Kristensen SS, Pedersen P. Erythrocyte sedimentation rate and heterotopic bone formation after cemented total hip arthroplasty. Clin. Orthop. Relat. Res. 1989 Nov;(248):189-94.[↩]
- Sherman AL, Williams J, Patrick L, Banovac K. The value of serum creatine kinase in early diagnosis of heterotopic ossification. J Spinal Cord Med. 2003 Fall;26(3):227-30.[↩]
- Tyler P, Saifuddin A. The imaging of myositis ossificans. Semin Musculoskelet Radiol. 2010;14 (2):201–16.[↩]
- Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic‐pathologic correlation. AJR Am J Roentgenol. 1991;157 (6):1243–8[↩]
- Cvitanic O, Sedlak J. Acute myositis ossificans. Skeletal Radiol. 1995;24 (2):139–41[↩]
- Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55 (8):1629–32[↩]
- Hastings H 2nd, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin. 1994;10(3):417–37[↩]
- Agrawal K, Bhattacharya A, Harisankar CN, et al. [18F]Fluoride and [18F]fluorodeoxyglucose PET/CT in myositis ossificans of the forearm. Eur J Nucl Med Mol Imaging. 2011;38 (10):1956.[↩]
- Eekhoff EMW, Botman E, Coen Netelenbos J, et al. [18F]NaF PET/CT scan as an early marker of heterotopic ossification in fibrodysplasia ossificans progressiva. Bone. 2018;109:143–6.[↩]
- Deryk S, Goethals L, Vanhove C, et al. Imaging characteristics of heterotopic mesenteric ossification on FDG PET and Tc‐99m bone SPECT. Clin Nucl Med. 2008;33(7):496–9[↩]
- Wale DJ, Wong KK, Savas H, Kandathil A, Piert M, Brown RK. Extraosseous Findings on bone scintigraphy using fusion SPECT/CT and correlative imaging. AJR Am J Roentgenol. 2015;205 (1):160–72.[↩]
- Argyropoulou MI, Kostandi E, Kosta P, et al. Heterotopic ossification of the knee joint in intensive care unit patients: early diagnosis with magnetic resonance imaging. Crit Care. 2006;10 (5):R152.[↩]
- Peterson JR, Okagbare PI, De La Rosa S, et al. Early detection of burn induced heterotopic ossification using transcutaneous Raman spectroscopy. Bone. 2013;54(1):28–34.[↩]
- Stefanidis K, Brindley P, Ramnarine R, et al. Bedside ultrasound to facilitate early diagnosis and ease of follow‐up in neurogenic heterotopic ossification: a pilot study from the intensive care unit. J Head Trauma Rehabil. 2017;32 (6):E54– E8[↩]
- Della Valle AG, Ruzo PS, Pavone V, Tolo E, Mintz DN, Salvati EA. Heterotopic ossification after total hip arthroplasty: a critical analysis of the Brooker classification and proposal of a simplified rating system. J Arthroplasty. 2002 Oct;17(7):870-5[↩][↩]
- Behery OA, Dai AZ, McLaurin TM. Posttraumatic Heterotopic Ossification of the Hip. J Orthop Trauma. 2018 Aug;32 Suppl 1:S18-S19.[↩]
- Ranganathan K, Loder S, Agarwal S, et al. Heterotopic ossification: basic‐science principles and clinical correlates. J Bone Joint Surg Am. 2015;97(13):1101–11[↩]
- Aronen JG, Garrick JG, Chronister RD, McDevitt ER. Quadriceps contusions: clinical results of immediate immobilization in 120 degrees of knee flexion. Clin J Sport Med. 2006;16 (5):383–7.[↩]
- Pavey GJ, Polfer EM, Nappo KE, Tintle SM, Forsberg JA, Potter BK. What risk factors predict recurrence of heterotopic ossification after excision in combat‐related amputations? Clin Orthop Relat Res. 2015;473 (9):2814–24.[↩][↩]
- Almangour W, Schnitzler A, Salga M, Debaud C, Denormandie P, Genet F. Recurrence of heterotopic ossification after removal in patients with traumatic brain injury: a systematic review. Ann Phys Rehabil Med. 2016;59 (4):263–9.[↩]
- Brouwer KM, Lindenhovius AL, de Witte PB, Jupiter JB, Ring D. Resection of heterotopic ossification of the elbow: a comparison of ankylosis and partial restriction. J Hand Surg Am. 2010;35(7):1115–9.[↩]
- Behery OA, Dai AZ, McLaurin TM. Posttraumatic Heterotopic Ossification of the Hip. J Orthop Trauma. 2018 Aug;32 Suppl 1:S18-S19[↩]
- Yoon BH, Park IK, Sung YB. Ankylosing Neurogenic Myositis Ossificans of the Hip: A Case Series and Review of Literature. Hip Pelvis. 2018 Jun;30(2):86-91 [↩][↩]