What is hidradenitis suppurativa
Hidradenitis suppurativa also known as acne inversa or Verneuil’s disease, is a chronic (long-term) painful inflammatory disease of the apocrine sweat glands in your armpits, groin, under the breasts and/or genitals and anal regions of the body, although other sites may be involved, that causes deep scarring and impacts quality of life 1, 2, 3. People who have hidradenitis suppurativa develop painful red bumps or sores in places where they grow hair and where the skin rubs together, such as the armpits, groin, buttocks and breasts 4. Hidradenitis suppurativa is characterized by persistent or recurrent boil-like lumps and abscesses (swollen area within body tissue containing a pocket of pus) that culminate in a purulent discharge, unnels under the skin (sinuses), and scarring. Hidradenitis suppurativa can persist for many years and worsen over time, with serious effects on your daily life and emotional well-being. Hidradenitis suppurativa can cause a significant psychological impact, and many patients suffer from anxiety, depression, and impairment of body image 5.
Hidradenitis suppurativa is primarily considered a severe form of acne (an acne-like follicular occlusion disease) where apocrine glands (sweat glands) only become involved in the context of peri-follicular inflammation – it is not a primary disease of apocrine glands (sweat glands).
In hidradenitis suppurativa, the sweat glands (apocrine glands) become clogged due to a variety of reasons, leading to enlargement (dilatation) of the ducts behind them. This dilatation predisposes the area to inflammation and infection by a variety of bacteria. Those with hidradenitis suppurativa typically have multiple large, recurrent boil-like nodules (red bumps) and abscesses on the buttocks, breasts, groin, and armpits. The bumps gradually get larger and drain pus. After multiple bouts of this cycle of plugging, enlargement, drainage and difficult-to-heal open wounds (sinuses), there may be scarring.
Hidradenitis suppurativa tends to start after puberty and generally occurs in young adulthood to middle adulthood, most common between 18 to 39 years of age 6. Onset before puberty is uncommon, but does correlate with a greater severity later in life and a positive family history 7. Females and blacks are more than twice as likely to be affected 3. Additional risk factors include family history, smoking, and obesity 3. Hidradenitis suppurativa is associated with several comorbidities, including diabetes mellitus and Crohn disease 3.
The prevalence of hidradenitis suppurativa in the United States is approximately 0.1%, with increasing incidence over the past 10 years 8, 6.
Potential complications of hidradenitis suppurativa include keloid scars, fistulas, lymphedema, squamous cell carcinoma (SCC), anemia of chronic disease, amyloid of the skin and/or kidney with the development of nephrotic syndrome, scarring, and limb contractures 9. Cancer, in particular squamous cell carcinoma (SCC) may rarely occur 10. Patients may die. The typical patient is one with long term, severe disease who develops a very painful nodule or lesion, often in the perianal or perineal area.
At the present time, there is no cure for hidradenitis suppurativa, but you can work with your doctor to treat existing lesions and prevent new ones.
Clinical management of hidradenitis suppurativa is often complex and includes medical and surgical treatments, which are often combined, especially in moderate-to-severe disease 11. The reduction of symptoms and inflammation activity as well as prevention of formation of chronic hidradenitis suppurativa lesions and scarring represent key therapeutic goals 1.
Hidradenitis suppurativa treatment options:
- Weight loss
- Smoking cessation
- Topical or oral antibiotics
- Intralesional steroids
- Low-dose prednisone
- 1064 nm nd:YAG Laser
- Laser hair removal
- Adalimumab
- Diabetic agents (e.g. metformin)
- Surgery
In current hidradenitis suppurativa treatment guidelines, an individualized patient-oriented approach, based on the individual subjective impact and objective disease severity, is recommended 12. Treatment includes wearing loose-fitting clothes, losing weight if overweight, and smoking cessation 3. Topical clindamycin alone can be effective for patients with mild disease. Patients with moderate disease can be treated with oral antibiotics, such as tetracyclines, in addition to topical clindamycin. Adalimumab, a tumor necrosis factor alpha inhibitor, is effective for patients with moderate to severe hidradenitis suppurativa. Surgical procedures are often necessary for definitive treatment and include local procedures, such as punch debridement and unroofing/deroofing. Wide excision is indicated for patients with severe, extensive disease and scarring 3.
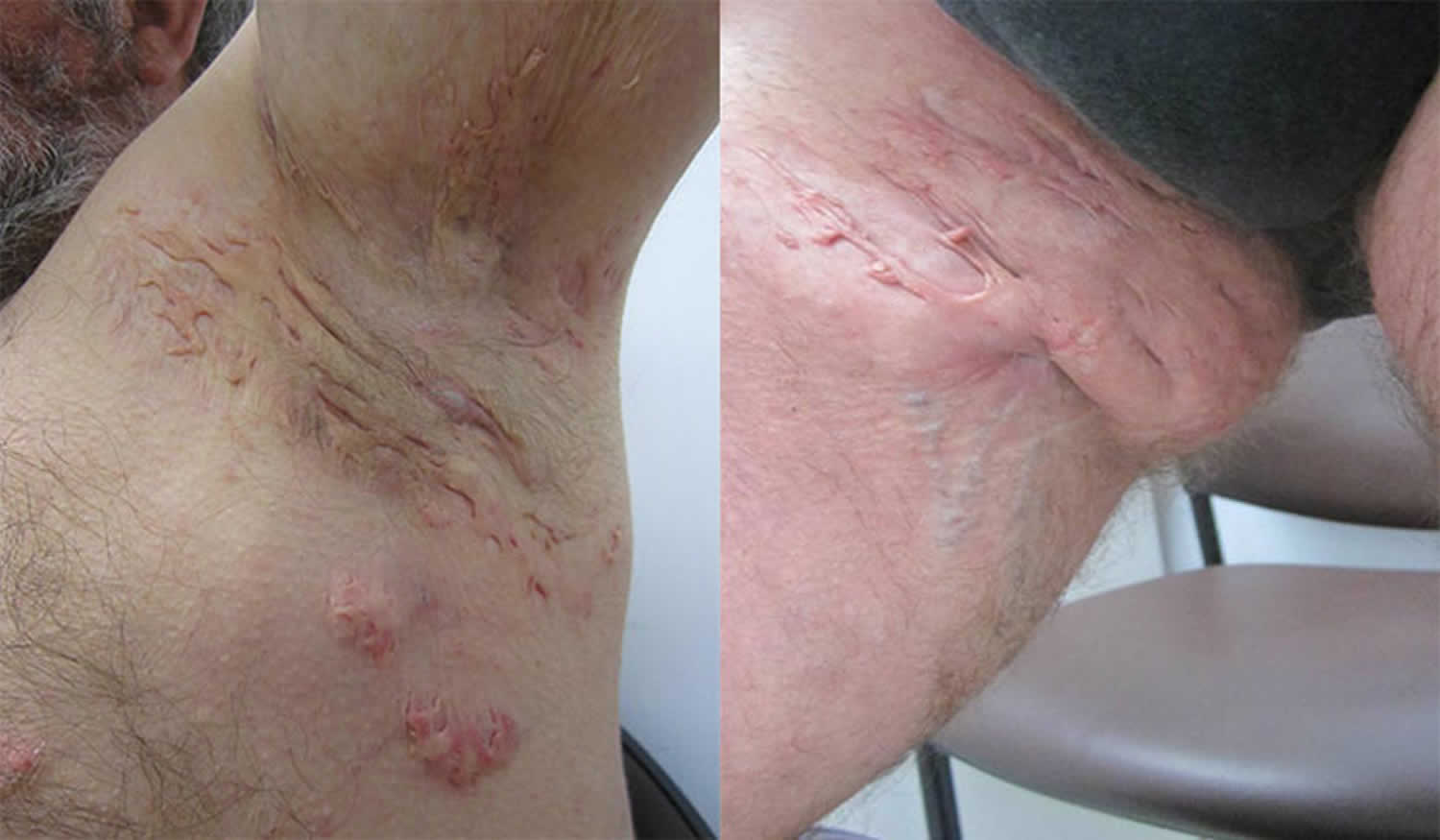
Figure 1. Hidradenitis suppurativa armpit
Figure 2. Hidradenitis suppurativa groin
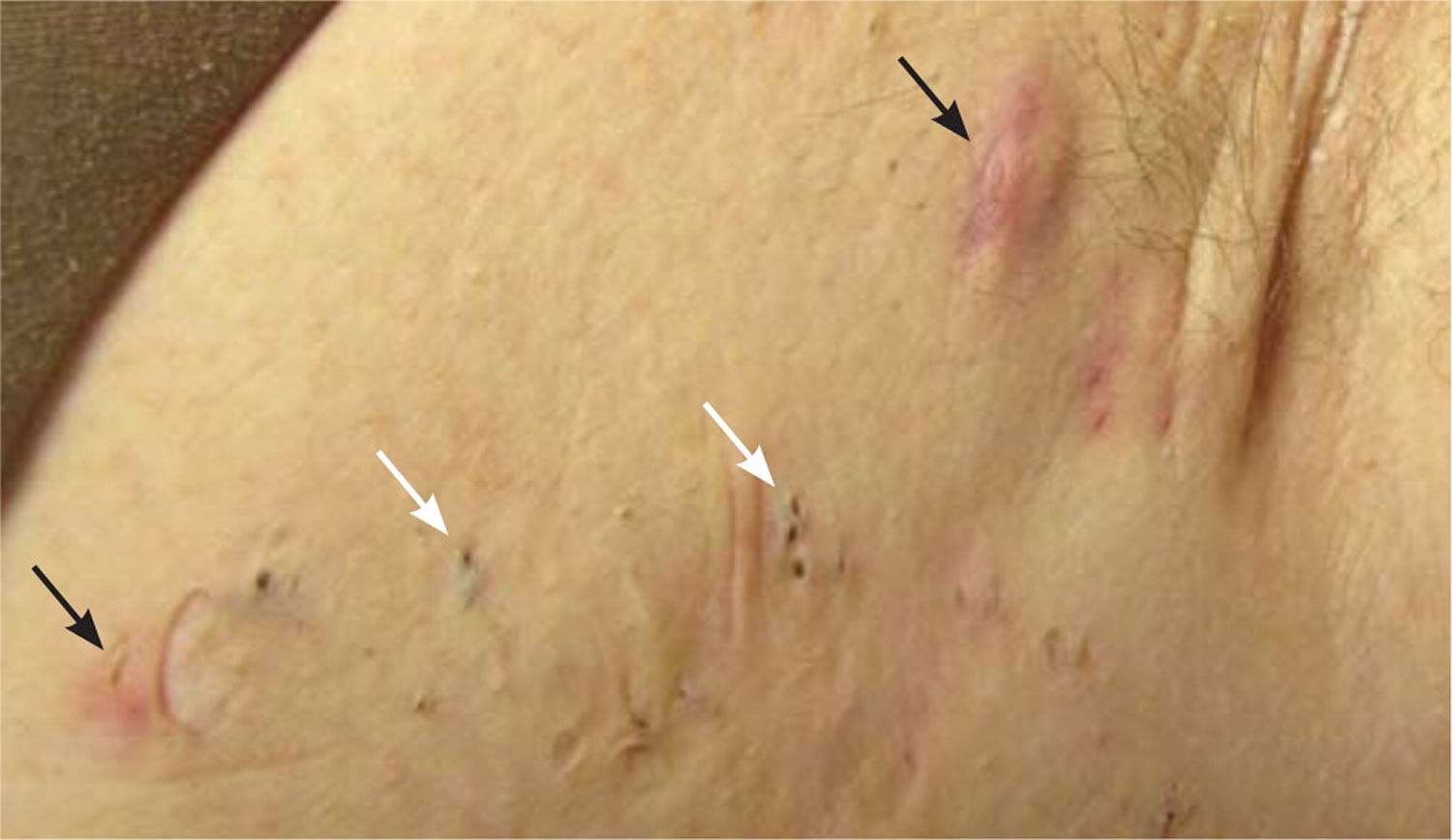
[Source 13 ]Figure 3. Axillary hidradenitis suppurativa
Footnotes: Axillary hidradenitis suppurativa showing inflammatory nodules (black arrows), multiheaded open comedones (white arrows), and scarring.
[Source 3 ]Figure 4. Hidradenitis suppurativa
Footnotes: Typical appearance of an open double “tomb stone” comedone in a patient with hidradenitis suppurativa. Multiheaded comedones are an end-stage result of inflammatory destruction of the folliculopilosebaceous unit.
[Source 3 ]Early diagnosis of hidradenitis suppurativa is key to getting effective treatment. See your doctor or a skin specialist (dermatologist) if your condition:
- Is painful
- Makes it difficult to move
- Doesn’t improve in a few weeks
- Returns within weeks of treatment
- Appears in several locations
- Flares often
Hidradenitis suppurativa is not just a boil, and many people with this condition also have related conditions. People with hidradenitis suppurativa benefit from a health care team with medical and surgical dermatologists at the core. Other specialists are involved as needed.
Is hidradenitis suppurativa contagious?
No. Hidradenitis suppurativa is non-contagious and you cannot spread hidradenitis suppurativa to another person through contact.
What are apocrine sweat glands?
Sweating prevents overheating of your body, because sweat cools your skin as it evaporates. Only mammals have sweat glands (sudoriferous glands). Humans have more than 2.5 million sweat glands distributed over the entire skin surface, except on the nipples and parts of the external genitalia. Humans normally produce about 500 ml of sweat per day, but this amount can increase to 12 liters (over 3 gallons) on hot days during vigorous exercise. Hair interferes with the evaporation of sweat and the ability to cool the body, so the need for increased temperature regulation through sweating led to a reduction of hairiness in humans.
There are two types of sweat glands, both of which increase their secretion in response to stress as well as to heat: eccrine and apocrine glands.
Eccrine glands (“secreting”) are by far the more numerous type (Figure 3). They are most abundant on the palms, soles, and forehead. Each is a coiled version of a simple tubular gland. The coiled, secretory base lies in the deep dermis and hypodermis, and the duct runs superficially to open at the skin surface through a funnel-shaped pore. (Although most pores on the skin surface are sweat pores, the “pores” seen on the face are openings of hair follicles.)
Apocrine glands are mostly confined to the armpits, anal, and genital areas. They are larger than eccrine glands, and their ducts open into hair follicles. Apocrine glands produce a special kind of sweat consisting of fatty substances and proteins, in addition to the components of true sweat. For this reason, apocrine sweat is viscous and sometimes has a milky or yellow color. This product is odorless when first secreted, but as its organic molecules are decomposed by bacteria on the skin, it takes on a musky smell. This is the source of body odor.
Apocrine glands start to function at puberty under the influence of androgens (a group of sex hormones that help start puberty and play a role in reproductive health and body development). Testosterone is the most common androgen. Apocrine glands activity is increased by sexual foreplay, and they enlarge and recede with the phases of a woman’s menstrual cycle. The secretions from the apocrine glands were identified as true human pheromones (chemical signals that convey information to a member of the same species) in the late 1990s when it was shown that they are responsible for the synchrony of the menstrual cycle that occurs in females who live together.
Apocrine glands are involved with sexual signaling and appear to function in attractiveness and mate selection. The genes that encode for the immune system, the major histocompatibility complex (MHC), also influence secretions from apocrine glands. Each person has a unique set of these genes. In experiments, the body odor scents that women selected as “sexy” or “attractive” came from men who had immune system genes most different from their own. Mates with complementary immune system genes provide their offspring with greater disease protection and decreased likelihood of recessive disorders.
Figure 5. Skin and apocrine sweat glands
Who’s at risk of hidradenitis suppurativa?
Hidradenitis suppurativa usually starts soon after puberty and continues into adult life. Hidradenitis suppurativa is most active between the ages of 20 and 40 years. Hidradenitis suppurativa is three times more common in females than in males and and in women, it can resolve at menopause. Hidradenitis suppurativa is also common in African Americans. Hair removal from shaving or using depilatories, deodorants, and irritation from anything rubbing against the affected area can worsen the condition. Hidradenitis suppurativa is often associated with smoking, obesity, and increased hormones. Additionally, it often runs in families.
Risk factors for developing hidradenitis suppurativa include 14, 15, 16, 17, 18, 19, 20, 21, 12, 22, 23:
- Other family members with hidradenitis suppurativa
- Obesity and insulin resistance (metabolic syndrome)
- Cigarette smoking
- Follicular occlusion disorders: acne conglobata, dissecting cellulitis, pilonidal sinus
- Other skin disorders: psoriasis, acne, hirsutism
- Inflammatory bowel disease (Crohn’s disease)
- Comorbidities: hypertension, diabetes mellitus, dyslipidaemia, thyroid disorders, spondyloarthritis, polycystic ovary syndrome (PCOS), adverse cardiovascular outcomes
- Drugs: lithium, sirolimus, biologics
- Rare autoinflammatory syndromes associated with abnormalities of PSTPIP1 gene.
- PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum and acne),
- PASH syndrome (pyoderma gangrenosum, acne, suppurative hidradenitis)
- PAPASH syndrome (pyogenic arthritis, pyoderma gangrenosum, acne, suppurative hidradenitis).
Hidradenitis suppurativa stages
The severity and extent of hidradenitis suppurativa should be recorded at assessment and when determining the impact of a treatment. Two scoring systems are in common use: Hurley Stages and Sartorius Hidradenitis Suppurativa Score. However, a standardized and internationally accepted score is still missing, resulting in the utilization of different classification tools in clinical studies for the evaluation of treatments 1.
The Hurley staging system describes hidradenitis suppurativa into three distinct clinical stages 24 and due to its simplicity is the most widely used classification system for hidradenitis suppurativa in routine clinical practice. The Hurley staging classifies hidradenitis suppurativa into three stages, mainly based on the presence of sinus tracts and scarring, allows medical professionals a fast and simple clinical based evaluation. However, the Hurley staging system is not applicable for monitoring of hidradenitis suppurativa, as it represents a static and non-quantitative tool and inflammation activity is not captured 25. A revised Hurley staging system, that takes account of inflammation activity and subcategorizes Hurley stages 1 and 2 into mild, moderate and severe disease, has been described recently; however, clinical application is limited 26.
Hidradenitis suppurativa stage 1
Solitary or multiple, isolated abscess formation without scarring and sinus tracts.
Hidradenitis suppurativa stage 2
Recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation.
Hidradenitis suppurativa stage 3
Diffuse or broad involvement, with multiple interconnected sinus tracts and abscesses.
Severe hidradenitis (Hurley Stage 3) has been associated with:
- Male gender
- Armpit and perianal involvement
- Obesity
- Smoking
- Disease duration.
Sartorius Hidradenitis Suppurativa Score
The Sartorius Hidradenitis Suppurativa Score is made by counting involved regions, nodules and sinus tracts 27:
- Anatomic region involved (axilla, groin, genital, gluteal, or other inflammatory region left and/or right): 3 points per region involved
- Number and scores of lesions (abscesses, nodules, fistulas, scars): 2 points for each nodule, 4 points for each fistula, 1 point for each scar, 1 point each for “other”
- Longest distance between 2 relevant lesions (ie, nodules and fistulas, in each region, or size if only 1 lesion): Less than 5 cm, 2 points; less than 10 cm, 4 points; more than 10 cm, 8 points
- Lesions clearly separated by normal skin in each region: If yes, 0 points; if no, 6 points.
Although the Sartorius Hidradenitis Suppurativa Score is a dynamic system, suitable for treatment monitoring, due to its complexity its use is often time-consuming and especially in severe cases difficult to apply 27.
Hidradenitis suppurativa physician global assessment (HS-PGA) score
The hidradenitis suppurativa physician global assessment (HS-PGA) score is a frequently used tool to assess disease severity. HS is classified into six severity grades by counting the numbers of inflammatory nodules, abscesses and sinus tracts 28. As a dynamic scoring tool hidradenitis suppurativa physician global assessment (HS-PGA) can be used for treatment evaluation, although especially in cases of extensive disease with multiple lesions the clinical correlation is limited 29.
Hidradenitis suppurativa clinical response (HiSCR)
The Hidradenitis Suppurativa Clinical Response (HiSCR) is defined as a ≥ 50% reduction in inflammatory lesion count (abscesses + inflammatory nodules), and no increase in abscesses or draining fistulas when compared with baseline 30. The Hidradenitis Suppurativa Clinical Response (HiSCR) is commonly used as a primary endpoint for treatment evaluation in recent studies and has been shown to be more responsive than hidradenitis suppurativa physician global assessment (HS-PGA) 31. It has been recently used to assess the effectiveness of treatment with biologics 31. According to the psoriasis area and severity index (PASI), HiSCR75- and HiSCR90- rates are further developed outcome parameters, assessed in current studies 32.
Severity assessment of hidradenitis suppurativa (SAHS) score
The severity assessment of hidradenitis suppurativa (SAHS) score was developed by Hessam et al. 33 and is another clinical based scoring tool, that considers the number of affected body regions, fistulas and other inflammatory lesions as well as hidradenitis suppurativa-related pain and the number of new or flared boils in the last four weeks. The severity assessment of hidradenitis suppurativa (SAHS) score enables a dynamic evaluation of hidradenitis suppurativa severity and can also be used for treatment evaluation in clinical practice and studies 34.
Cardiff Dermatology Life Quality Index (DLQI)
The Cardiff Dermatology Life Quality Index (DLQI) or DLQI questionnaire is often used to assess the degree of pain, the number of flares, and the impact on daily life in hidradenitis suppurativa.
International hidradenitis suppurativa severity score system (IHS4)
The international hidradenitis suppurativa severity score system (IHS4) represents a dynamic and easy-to-use scoring tool, and assesses disease severity by counting of inflammatory HS lesions (nodules 1 point, abscesses 2 points, sinus tracts 4 points) 35. A total score of 3 or less signifies mild, 4–10 signifies moderate, and 11 or higher signifies severe disease 35. Recently, a modified and dichotomous IHS4-55 score has been developed as a potential parameter for the measurement of treatment outcomes 36.
Hidradenitis Suppurativa Burden of Disease (HSBOD)
A Hidradenitis Suppurativa Burden of Disease (HSBOD) tool has been described but is not fully validated 37.
Hidradenitis suppurativa symptoms
Hidradenitis can affect a single or multiple areas in the armpits, neck, under the breasts, and inner thighs. Anogenital involvement most commonly affects the groin, mons pubis, vulva (in females), sides of the scrotum (in males), perineum, buttocks and perianal folds.
Some people with hidradenitis suppurativa experience only mild symptoms. The course of the disease is highly variable. Excess weight and being a smoker are associated with worse symptoms, but even people who are thin and don’t smoke can experience severe disease.
Signs of hidradenitis suppurativa include:
- Open double-headed comedones (also known as blackheads). Blackheads appear in small pitted areas of skin, often appearing in pairs.
- Painful firm papules and nodules (lumps). Hidradenitis suppurativa usually starts with a single, painful lump under the skin that persists for weeks or months. More bumps may form later, usually in areas where you have more sweat and oil glands or where the skin rubs together, such as the armpits, groin, buttocks and breasts.
- Pustules, fluctuant pseudocysts, and abscesses
- Leaking bumps or sores. Some bumps or sores get bigger, break open and drain pus with an odor.
- Tunnels draining sinuses linking inflammatory lesions. Over time, tunnels might form under the skin, connecting the lumps. These wounds heal very slowly, if at all, and drain blood and pus.
- Hypertrophic and atrophic scars.
Many patients with hidradenitis suppurativa also suffer from other skin disorders, including acne, hirsutism and psoriasis.
The severity and extent of hidradenitis suppurativa is recorded at assessment and when determining the impact of a treatment.
Table 1. Clinical phenotypes of hidradenitis suppurativa
| Phenotype | Clinical features |
|---|---|
| Regular hidradenitis suppurativa | Most common form Recurrent inflammatory lesions in typical locations for at least 6 months |
| Frictional furuncle | Regular hidradenitis suppurativa plus multiple deep nodules and abscesses on sites exposed to enhanced friction (abdomen, thighs, buttocks) |
| Scarring folliculitis | Regular hidradenitis suppurativa plus pustules, cysts, superficial nodules, depressed cribriform scarring, and double-headed comedones Significant scarring |
| Conglobata | Cyst formation and acne conglobata lesions on the back and face |
| Syndromic | hidradenitis suppurativa in conjunction with syndromic features (PAPA, PASH, PAPASH) |
Hidradenitis suppurativa causes
The cause of hidradenitis suppurativa is unknown. It is thought that there is an exaggerated inflammatory response to the presence of bacteria trapped in obstructed hair follicles in the skin. Hidradenitis suppurativa isn’t exactly an “autoimmune disease,” but it is caused by over-activity of the immune system, which causes inflammation. Inflammation in the skin causes the mix of redness, swelling, itching, pain, sores, and drainage. People with hidradenitis suppurativa also have systemic inflammation that can cause joint pain or fatigue.
In some cases there does seem to be a genetic component. Bacteria seem to play some role, which is why antibiotics may be used in treatment. However, bacteria are not the primary cause of hidradenitis suppurativa. Other factors such as obesity, smoking and stress also play a role.
Contributing factors include:
- Friction from clothing and body folds
- Aberrant immune response to commensal bacteria
- Abnormal cutaneous or follicular microbiome
- Follicular occlusion
- Release of pro-inflammatory cytokines
- Inflammation causing rupture of the follicular wall and destroying apocrine glands and ducts
- Secondary bacterial infection
- Certain drugs.
Follicular Occlusion
Hidradenitis suppurativa was originally thought to be caused by a defect of the sweat gland, but this view has changed and it is now widely accepted to be a problem with the hair follicle. Build-up of keratin (a type of protein) within the hair follicle eventually plugs the hair follicle, causing further build up, and eventually the hair follicle ruptures and releases this material into the surrounding skin resulting in an intense inflammatory response 38.
Bacteria
Sometimes bacteria can be isolated from hidradenitis suppurativa lesions, while other times cultures from hidradenitis suppurativa lesions are sterile (no bacteria found) 39. Those affected by hidradenitis suppurativa frequently experience the characteristic symptoms of an infection, such as pain, tenderness, warmth, and purulent drainage. It may be reasonable to assume that since symptoms tend to improve after antibiotics, that hidradenitis suppurativa is caused primarily by bacteria, however this is not the case.
Biofilms
Recently, hidradenitis suppurativa has been described as a biofilm disease. When numerous bacteria secrete a sticky material known as a polysaccharide matrix and bind to a surface they now exist in what is known as a biofilm. When bacteria exist in a biofilm they become highly resistant to antibiotics. A recent study looking at biopsy samples (pieces of skin) from patients with hidradenitis suppurativa, demonstrated that biofilms were associated with chronic hidradenitis suppurativa 40. Although, biofilms likely don’t form primarily, this may be the reason that hidradenitis suppurativa become very resistant to medical management as it becomes more severe.
Aberrant Immunity
Several markers of inflammation exist in the blood and skin and can be used to assess the inflammatory state of the body. Abnormal levels of these markers have been implicated in the development of several diseases and similarly found to be abnormal in hidradenitis suppurativa. Additional research is needed to determine the implications of these abnormal levels, but these findings have led to the use of biologic medications, such as adalimumab and infliximab for the treatment of hidradenitis suppurativa.
Diet
Nutrition is an understudied and notoriously difficult topic to research in medicine. The majority of evidence is based on individual experience with few published articles in the literature, but the impact of diet on hidradenitis suppurativa infiltrates through social media and online message boards with reports of improvement that ranges from minimal or no effect to complete resolution. In one survey study involving 47 patients who followed dairy free diets, no patients experienced disease progression and 83% improved to various degrees 41. In another study, 12 subjects underwent surgical excision followed by diet modification with no wheat or brewers yeast consumption with immediate disease stabilization during a 12 month period of follow up 42.
Additional research is needed in order to allow for comprehensive diet counseling. Some patients have reported improvement after exclusion of nightshades, simple carbohydrates, and dairy, but the impact of diet modification appears to be highly individual and should be discussed with your physician or nutritionist. Dieticians can provide individualized meal plans to make sure that you are getting all of the required nutrients.
Other Factors
Several other factors have been implicated in the development of hidradenitis suppurativa. Smoking is more common among people with hidradenitis suppurativa than among the general population, and the components of smoke are known to promote hyperkeratosis (causing follicular occlusion) and also increases inflammation potentially worsening hidradenitis suppurativa 43. Mechanical factors caused by skin to skin contact and obesity may also contribute to the overall inflammatory state of hidradenitis suppurativa and the development or progression of hidradenitis suppurativa.
Lastly, hormones are potentially implicated in hidradenitis suppurativa. It is known that more women are affected by hidradenitis suppurativa than men and therefore a hormonal component has been hypothesized. Androgens, a type of hormone, can increase proliferation of the keratinocytes (skin cells), however the hormonal profile of patients with hidradenitis suppurativa is usually found to be normal 43.
With numerous factors implicated in the development of hidradenitis suppurativa, it is clear that additional research is needed and the trigger for one patient may not be consistent across the entire population.
Hidradenitis suppurativa pathophysiology
The pathologic process of hidradenitis suppurativa begins when a defective hair follicle becomes occluded and ruptures, spilling its contents, including keratin and bacteria, into the surrounding dermis 44. A chemotactic inflammatory response by surrounding neutrophils and lymphocytes can lead to abscess formation and subsequent destruction of the pilosebaceous unit and other adjacent structures 44. Other possible contributors to pathology include abnormal antimicrobial peptides, abnormal secretion of apocrine glands, abnormal invaginations of the epidermis leading to tract formation, and deficient numbers of sebaceous glands 45.
Immunological abnormalities have also been observed. The inflammatory cascade in hidradenitis suppurativa involves tumor necrosis factor (TNF)-α, interleukin (IL)-17 and interleukin-1β 46. Recognizing hidradenitis suppurativa as an autoinflammatory disease, and not a result of infection, has important clinical implications including the need to address comorbidities such as visceral adiposity, insulin resistance and hormonal factors that can activate or exacerbate the underlying inflammatory mechanisms. About one-third of patients have a family history of hidradenitis suppurativa, and at least 23 potentially pathogenic gene sequence variants have been identified 47. The microbiome in hidradenitis suppurativa differs significantly from healthy controls in both affected and non-affected skin 48. Bacterial infection, when it does occur, is likely to be secondary to skin damage and underlying inflammation rather than a primary contributor to pathogenesis. Aspirate from an unruptured lesion typically yields a sterile culture. However, bacterial infection and colonization during the process can secondarily worsen hidradenitis suppurativa 49.
Hidradenitis suppurativa prevention
It is difficult to prevent hidradenitis suppurativa or flare-ups caused by the disease. However, obesity and smoking are related to this condition. Quitting smoking and healthy eating and exercise may help to prevent the likelihood of hidradenitis suppurativa and limit flare-ups in those that have it. Try to lose weight if you are overweight. Also work to stay healthy by exercising and getting enough sleep.
Quit smoking
The link between tobacco smoking and hidradenitis suppurativa has been suggested in several studies. In a retrospective study, 92.2% of hidradenitis suppurativa patients were smokers and clinical remission was more often reported among non-smoking patients 50. Moreover, Sartorius et al. 51 found lower disease severities, evaluated by the Sartorius Hidradenitis Suppurativa Score, in non-smokers compared to active smokers. At a molecular level, components of cigarette smoke have been demonstrated to further promote inflammation in hidradenitis suppurativa via inhibition of the already compromised Notch signaling, induction of proinflammatory cytokine expression and causing infundibular epithelia hyperplasia and hypercornification 52, 53. Although clear evidence of tobacco as a trigger of hidradenitis suppurativa has not been found yet, patients should be encouraged to quit smoking 12.
Weight reduction
Overweight and obesity are considered as frequent comorbidities of hidradenitis suppurativa. In two case–control studies an increment of the likelihood of hidradenitis suppurativa with every body mass index (BMI) unit increase was reported 54. In another retrospective study 55, the point prevalence of hidradenitis suppurativa was much higher in an obese study population compared to the general population. This may be explained by changes of the skin microbiome and increased friction of skin folds in obese patients 56, 57. However, evidence for improvement of hidradenitis suppurativa after body weight reduction is limited. A retrospective study reported a significant reduction of inflammatory activity in hidradenitis suppurativa patients undergoing bariatric surgery 55, 58. Moreover, obese hidradenitis suppurativa patients reported lower remission rates than non-obese hidradenitis suppurativa patients 50.
Obese patients should be motivated to reduce their body weight by initiating physical activity and dietary changes 59. In cases of severe obesity, bariatric surgery (weight-loss surgery) may be an option 60.
Consider altering your diet. Hidradenitis symptoms might be worsened by diets that include dairy, red meat and foods with a high glycemic index. If your diet includes these foods, talk with a dietitian about the benefits of eliminating them.
Hidradenitis suppurativa complications
Persistent and severe hidradenitis suppurativa can cause complications, including:
- Infection. The affected area is susceptible to secondary infection, but the presence of pus is common in hidradenitis suppurativa and doesn’t necessarily mean infection.
- Scars and skin changes. The wounds may heal but leave ropelike scars or pitted skin.
- Restricted movement. Sores and scar tissue can cause limited or painful movement, especially when the disease affects the armpits or thighs.
- Skin cancer. Squamous cell carcinoma (SCC) has been reported with long-term hidradenitis suppurativa, particularly in people whose condition involves the perianal area.
- Swelling in the arms, legs or genitals (lymphedema). The most common sites for hidradenitis suppurativa also contain many lymph nodes. Scar tissue can interfere with the lymph drainage system, which can result in swelling in the arms, legs or genitals.
- Pyogenic granuloma. Pyogenic granulomas are small, raised, and red bumps on the skin. The bumps have a smooth surface and may be moist. They bleed easily because of the high number of blood vessels at the site. It is a benign (noncancerous) growth.
- Granuloma is a histological term for a chronic inflammatory pattern characterized by the localized aggregation of histiocytes with or without other inflammatory cells, with or without necrosis, with or without vasculitis, with or without calcification, with or without foreign bodies. A granuloma may be due to infection, chronic inflammatory disease, or reaction to foreign material.
- Anemia of chronic disease.
- Psychological effects and social isolation. The location, drainage and odor of the sores can cause embarrassment and reluctance to go out in public, leading to anxiety or depression.
Hidradenitis suppurativa diagnosis
Hidradenitis suppurativa can be mistaken for an infection, an ingrown hair or other conditions. Many people live with undiagnosed hidradenitis suppurativa for years before receiving a correct diagnosis.
Your doctor will base a diagnosis on your signs and symptoms, skin appearance and medical history. You might be referred to a doctor who specializes in skin conditions (dermatologist), as hidradenitis suppurativa can be difficult to diagnose and requires specialized care.
No laboratory test is available to diagnose hidradenitis suppurativa. But if pus or drainage is present, your doctor might take a sample for testing in a lab.
Skin biopsy can help differentiate hidradenitis suppurativa from other diagnoses, such as cutaneous Crohn disease in those with perianal involvement.
The British Association of Dermatologists guidelines state that a triad of “typical lesions, in characteristic locations, with a recurrent pattern” is required for diagnosis 61:
- specifically, two typical lesions (defined as inflamed nodules or abscesses) in typical locations (armpits, submammary, abdominal fold, groin, genital, neck, or perianal areas) over six months or a history of at least five such lesions over a lifetime.
Hidradenitis suppurativa differential diagnoses
Differential diagnoses for hidradenitis suppurativa can include the following conditions.
- Staphylococcal skin infections, including abscesses, carbuncles, and furuncles.
- Cysts, like Bartholin cyst or epidermoid cyst.
- Cutaneous Crohn disease.
- Anogenital Crohn disease.
- Acne conglobata: Severe nodular acne on the back, chest, face and neck; predominantly affects men.
- Granuloma inguinale: Genital ulcerations and subcutaneous granulomas.
- Lymphogranuloma venerum: Unilateral groin adenopathy; proctocolitis in men who have sex with men.
- Pilonidal cyst: Sacrococcygeal cyst or abscess; occurs more often in men.
Hidradenitis suppurativa treatment
There is no cure for hidradenitis suppurativa and treatments aren’t one-size-fits-all 62. The overall goals of treatment is to treat existing lesions to minimize pain and drainage, decreasing the frequency of recurrence, and preventing disease progression. Since limited studies compare treatment regimens, most treatment algorithms are based on expert opinion and consensus 44.
The type of treatment you receive depends on how severe your condition is. Treatment with medications, surgery or both can help control symptoms and prevent complications of hidradenitis suppurativa. Expect to have regular follow-up visits with your dermatologist. Some people might need the comprehensive care provided by a multidisciplinary health care team.
For mild cases, apply a warm compress to the affected area. Wash the area with anti-bacterial soap. Non-steroidal anti-inflammatory drugs (NSAIDs) can help reduce swelling and relieve pain.
More severe cases may require antibiotics. These can be topical (applied to your skin) or oral (taken by mouth). Antibiotics help prevent or treat infection. For some people, hidradenitis suppurativa gets worse over time. Scarring can occur in the affected area. These people may need surgery to remove the sores and scars. People who have surgery to remove hidradenitis suppurativa may get it again.
Talk with your doctor about the risks and benefits of the treatment options and how to develop an approach tailored to you.
Hidradenitis suppurativa management principles are outlined below:
In early uncomplicated HS diseases, topical antibiotics are the first-line treatment. Topical clindamycin has been the most effective 63. Intralesional corticosteroids can reduce local inflammation, and partial de-roofing (punch debridement) of individual lesions can facilitate healing.
Treatment for Hurley Stage 2 and resistant Hurley Stage 1 involves oral antibiotics. Antibiotics in the tetracycline family have been the most effective 64. If treatment failure persists, combination therapy with oral clindamycin plus rifampin is recommended 65. Anti-androgenic hormonal therapy can also be helpful, including cyproterone acetate, oral contraceptives, spironolactone, and finasteride 66. Oral retinoids have shown mixed responsiveness. While isotretinoin is most effective in acne, acitretin appears more effective in hidradenitis suppurativa 67, 68. Systemic steroids are effective for some individuals.
For Hurley stage 3 and resistant lower stages, tumor necrosis factor-alpha inhibitors are indicated. Adalimumab is the only FDA-approved medication to treat hidradenitis suppurativa 69, 70.
Surgery is often needed for Hurley stage 3 and involves a wide excision to include the lesions, tracts, and scars of an entire affected area. A combination of medical treatment and surgical excision is often the preferred approach. Other therapeutic options may include localized laser and pulsed light therapy, which help to disrupt the inflammatory process 71, 72, 73.
Pain management is also critical. The pain of hidradenitis suppurativa is both inflammatory and non-inflammatory. Sources of pain can include scarring (causing tensile pain), keloids, abscesses, open ulcerations, sinus tracts, frictional pain, lymphedema, anal fissures, and arthritis. Depending on disease severity and type of pain, topical agents (lidocaine and anti-inflammatories), systemic nonsteroidal anti-inflammatories, acetaminophen, atypical anticonvulsants, including gabapentin or pregabalin, and serotonin-norepinephrine reuptake inhibitors may be beneficial. Duloxetine is especially helpful if there is comorbid depression 74, 75.
Regardless of the stage of the HS disease, treatment should include the management of comorbidities that contribute to the development of or worsening of the disease process. Individuals who are above ideal weight or who smoke have more severe disease progression, so counseling and help with weight loss and smoking cessation are important components of treatment 53.
Treatment also involves avoidance of skin trauma. Eliminating tight and synthetic clothing, avoiding harsh products or cleaning tools (loofahs, washcloths, brushes), and avoiding adhesive dressings can be beneficial. Soft dressings with clear petroleum jelly or non-occlusive dressings can be used to prevent further irritation to draining lesions 76.
A critically important but often overlooked aspect of treatment involves the psychosocial aspect of the disease. Quality of life is diminished in individuals with this condition because of the associated pain, drainage, odor, and sensitive affected areas. Patients may become socially isolated, have employment difficulties because of missed days of work when flares occur, and may have increased sexual or relationship dysfunction. Assurance that this condition is not contagious or the result of poor hygiene can be helpful. Counseling and support groups are often beneficial additions to treatment plans 77.
Figure 6. Hidradenitis suppurativa treatment algorithm
Footnote: This treatment algorithm is used in the Henry Ford Dermatology Clinic, which provides care to more than 1,000 patients with HS. The algorithm is based on a combination of evidence-based research and provider experience.
Abbreivations: HS = hidradenitis suppurativa; BID = bis in die (twice daily); I/L = intralesional; IV = intravenously; Nd:YAG = neodymium-doped yttrium aluminum garnet; QD = quaque die (once daily)
[Source 78]General measures
Friction and moisture in the affected areas should be reduced as much as possible. Weight loss can greatly improve the condition if the patient is overweight 79. Topical clindamycin may be helpful (or benzoyl peroxide 10% wash). Bleach baths and Hibiclens may have some benefit. Patients who smoke are twice as likely to develop hidradenitis suppurativa compared with nonsmokers, and smoking is associated with decreased response to treatment 80, 81. Smokers should stop. Bacterial cultures may occasionally be done to rule out other causes or secondary infection.
Not smoking is associated with a better response to treatment 81 and a 15% weight reduction in obese patients improves disease severity 79.
- Weight loss; follow an anti-inflammatory, low-sugar, low-grain, low-dairy diet (mainly plants). Not being at a healthy weight can worsen the symptoms of hidradenitis suppurativa. Talk with your doctor or a dietitian to develop a plan. Try to find activities that don’t irritate your skin.
- Smoking cessation: this can lead to improvement within a few months
- Wearing loose-fitting clothes to prevent friction, although evidence for this is limited
- Daily unfragranced antiperspirants
- If prone to secondary infection, wash with antiseptics or take bleach baths
- Daily skin care routine. Gently wash your body with a nonsoap cleanser. It can sometimes be helpful to use an antiseptic wash such as chlorhexidine 4% or benzoyl peroxide wash when showering. First try it once a week, then increase usage up to once a day if your skin tolerates it well. Pat dry. When washing, avoid using washcloths, loofahs or other such items on affected areas, as they can irritate skin. Don’t squeeze pimples and sores. And avoid shaving or using depilatory creams.
- Apply hydrogen peroxide solution or medical grade honey to reduce malodour
- Use peeling agents such as resorcinol 15% cream to de-roof nodules
- Apply simple dressings to draining sinuses
- Analgesics, such as paracetamol (acetaminophen), for pain control
- Seek help to manage anxiety and depression.
Medications for hidradenitis suppurativa
Medical management of hidradenitis suppurativa is difficult. Treatment is required long term. Effective options are listed below.
Your doctor might prescribe one or more of the following types of medications:
- Antibiotics applied to the skin. Mild symptoms might be managed with a topical antibiotic in liquid or gel form. For more-widespread disease, your doctor might prescribe antibiotic pills, such as doxycycline (Monodox), clindamycin (Cleocin), rifampin (Rimactane) or both. People with severe disease might need to take antibiotics for months.
- Steroid injections. Triamcinolone (Aristospan, Kenalog-10) injected into the sores might help reduce swelling and inflammation.
- Hormonal therapy. Hormone pills, such as estrogen-containing combined oral contraceptives (Estrace, Prefest), might be effective for people with mild hidradenitis suppurativa.
- Biologics. These drugs, usually administered by injection, alter the immune system in a way that disrupts the disease cycle and improves symptoms and signs of disease within weeks. Several of these drugs are approved for the treatment of moderate to severe hidradenitis suppurativa. Two of them are the tumor necrosis factor (TNF) inhibitors adalimumab (Humira) and infliximab (Remicade). Many other biologics are in clinical trials for hidradenitis suppurativa.
- Retinoids. Oral retinoids might be an option for some people with acnelike (acneiform) disease. These drugs are not recommended when you’re pregnant or breastfeeding or if you intend to become pregnant.
- Pain medication. If over-the-counter pain relievers don’t help, your doctor might prescribe a stronger pain medication or refer you to a pain clinic.
Antibiotics
- Topical clindamycin, with benzoyl peroxide to reduce bacterial resistance
- Short course of oral antibiotics for acute staphylococcal abscesses, eg flucloxacillin
- Oral antibiotics can be very helpful. A current study assessed the duration of antibiotic treatments in HS patients and showed that in the majority of oral antibiotic courses the duration of treatment was less than 12 weeks 82. Prolonged courses (minimum 3 months) of tetracycline, metronidazole, trimethoprim + sulphamethoxazole, fluoroquinolones, ertapenem or dapsone for their anti-inflammatory action.
- Doxycycline 100 mg oral twice daily ongoing can be beneficial.
- Dapsone, 5-200 mg/day, may be helpful.
- 6–12 week courses of the combination of clindamycin (or doxycycline) and rifampicin for severe disease.
- Several experts recommend treatment for more severe, unstable hidradenitis suppurativa, with the combination of clindamycin 300 mg and rifampicin 300 mg both twice daily for 10 weeks. On the plus side, rifampicin acts against Clostridium difficule infections, decreasing the risk of colitis. On the down side, rifampicin can inactivate birth control pills. Also, it can turn bodily secretions orange, e.g. tears, sweat and urine.
- Other options include amoxicillin with clavulanic acid or fluoroquinolones (e.g., ciprofloxacin). Trimethoprim with sulfamethoxazole may also be beneficial. One study showed Staphylococcus epidermidis, Proteus mirabilis, and Staphylococcus aureus to be the most common isolates 83.
Recent guideline recommendations recognize tetracyclines as the first-line treatment for more widely spread hidradenitis suppurativa in Hurley stage 1 or 2 12. Established tetracycline antibiotics include tetracycline, doxycycline and minocycline 84. A prospective study comparing the efficacy of the different tetracycline antibiotics tetracycline, doxycycline and lymecycline showed a reduction of hidradenitis suppurativa in all treatment groups with greatest response in the tetracycline group. Moreover, a reduction of pain, formation of new inflammatory lesions and an improvement of quality of life was observed in all treatment groups 85.
An antibiotic combination therapy with clindamycin and rifampicin is recommended as first-line therapy for patients with Hurley stage 2 and moderate-to-severe disease and as second-line therapy for patients who do not respond on oral tetracycline treatment 12, 86. In an open-label prospective study with 56 patients, the combination of clindamycin and rifampicin showed an overall clinical response with reduction of hidradenitis suppurativa in 79.6% 87. HSS50 (50% remission) was 37% and complete remission (HSS100) was observed in 13%. Side effects were observed in 55.6% of patients with diarrhea, abdominal pain and nausea being the most commonly reported 87. This data is strengthened by several retrospective trials, which came to similar results; however, the majority of patients with initial complete remission relapsed after discontinuation of treatment 88, 89, 90. However, due to the role of rifampicin as a potent inductor of the hepatic CYP system, metabolization of clindamycin is intensified within the combination therapy 91. In one study clindamycin blood levels were decreased by around 90% within two weeks after treatment initiation, raising the question of whether the observed response rates under combination antibiotic therapy may be traced to rifampicin alone 92. More research with prospective randomized controlled trials is needed to address this topic.
For more extensive disease with severe inflammation and widespread distribution of inflammatory lesions some intensified antibiotic treatment regimens have been described; however, up to now evidence is based on case report and retrospective analyses, and these therapies have not been evaluated in prospective trials.
Join-Lambert et al. 93 described a broad-spectrum antibiotic combination therapy consisting of rifampicin, moxifloxacin and metronidazole leading to high rates of complete remissions, defined as clearance of all inflammatory lesions, especially in Hurley stage 1 or 2 patients.
Ertapenem, another broad-spectrum antibiotic, showed rapid improvement in treatment-refractory cases of hidradenitis suppurativa as a rescue therapy 94. In a retrospective pilot study with 30 patients, a 6 week course of ertapenem led to a significant and sustained improvement of disease severity, assessed with the Sartorius score 95. Through its rapid clinical improvement, ertapenem may be initiated in severe hidradenitis suppurativa as neoadjuvant therapy for bridging to surgery or other maintenance therapies 94, 96, 97.
Antiandrogens
Clinical observations in female hidradenitis suppurativa patients with premenstrual flares and cyclic alterations of inflammation activity led to the suggestion that hormonal alterations may influence the course of hidradenitis suppurativa 98. Moreover, hidradenitis suppurativa is associated with endocrine disorders such as polycystic ovary syndrome (PCOS) and metabolic syndrome 99, 100. However, the role of hormonal influences on the pathogenesis of hidradenitis suppurativa is still unclear 101. Most data regarding antiandrogenic treatment approaches for hidradenitis suppurativa are based on retrospective analyses, and case reports and prospective trials are rare.
- Long-term oral contraceptive pill; antiandrogenic progesterones drospirenone or cyproterone acetate (CPA) may be more effective than standard combined pills. These are more suitable than progesterone-only pills or devices. Successful disease control with an antiandrogenic therapy containing ethinylestradiol and cyproteronacetate has been described in a case series 102. In a double-blind controlled cross-over trial comparing two contraceptive regimens containing ethinylestradiol and norgestrel or cyproterone acetate, both treatments produced substantial improvement of disease activity; however, there was no significant difference 103. In a retrospective study, Kraft et al. 104 compared an antiandrogen treatment approach with antibiotic therapies in 66 female hidradenitis suppurativa patients and found significantly superior response rates, 55% vs. 26%, suggesting that antiandrogen therapy should be considered for all women presenting with hidradenitis suppurativa. Moreover, the authors concluded that female hidradenitis suppurativa patients should be investigated for underlying PCOS and insulin resistance 104.
- Spironolactone. Spironolactone is a potassium-sparing diuretic with antiandrogen properties due to the inhibition of mineralocorticoid receptors 105. Some women experience a premenstrual flare. For them, antiandrogen therapy (e.g., spironolactone) may be helpful. In several retrospective analyses of female hidradenitis suppurativa patients, spironolactone treatment led to a significant reduction of pain and inflammatory lesions, and improved quality of life 106, 107, 108.
- The antidiabetic agent metformin reduces insulin resistance by improving peripheral insulin sensitivity and may have some antiandrogen properties 97. In several retrospective trials and case reports, metformin showed promising clinical response rates and thus may contribute to disease control in hidradenitis suppurativa as an adjunctive treatment option 109, 110, 111.
- Finasteride has been used in children with hidradenitis suppurativa with success 112, 113. Three female patients, ages six, seven, and 15 who had failed standard therapy including isotretinoin, were treated with finasteride 1.25-10 mg/day for up to six years. Adjunctive birth control pills were given if the patient was a menstruating female. Treatment resulted in decreased frequency and severity of disease flares with no significant adverse effects.
- Response takes 6 months or longer.
Immunomodulatory treatments for severe disease
- Intralesional corticosteroids into nodules (e.g., Triamcinolone 5-10 mg/mL) can give the patient great relief. In one study utilizing triamcinolone 10 mg/mL 114, pain was reduced after 1 day and signs of inflammation after 7 days.
- Systemic corticosteroids short-term for flares. In one report 115, 13 patients were treated initially with prednisone 10 mg/day (ultimate dose varied from 5-15 mg/day). This was given in addition to existing therapy when that was not sufficient for control (e.g., acitretin, adalimumab, dapsone, clindamycin, doxycycline). All patients received calcium (1200 mg/day) and vitamin D (1000 IU/day) and monthly glucose monitoring. Overall, 11/13 patients showed a clinical response with the addition of low-dose prednisone. Side effects were minor and included hyperglycemia, sleep disturbance, and mild psychomotor agitation.
- Methotrexate, cyclosporin, and azathioprine. A few case reports highlight the benefit of cyclosporin. One patient did well on long-term cyclosporin 5 mg/kg/day, then 3 mg/kg/day 116.
- If oral and topical antibiotics plus general measures are not sufficient, a biologic agent is often added. TNF-α inhibitors adalimumab and infliximab, used in higher dose than required for psoriasis, are the most successful treatments to date. Note that paradoxically, they may sometimes induce new-onset hidradenitis suppurativa
- Other biologics are under investigation, such as the IL-1β antagonist, canakinumab
Adalimumab
Adalimumab, a fully human IgG1 monoclonal antibody specific for TNFα, is FDA-approved for moderate to severe hidradenitis suppurativa in adults and adolescent patients ≥12 years that is resistant to conventional therapies, but the dosing is weekly (for psoriasis, it is every other week) 117. Adalimumab can be administered in subcutaneous injections in two treatment regimens with 40 mg every week or 80 mg every other week after an initial loading dose 12. The therapy is usually well tolerated with injection side reactions being the most common side effects and large meta-analyses have shown non-significant safety issues compared to placebo 118
. In very rare cases malignancies (especially lymphomas) have been reported under therapy 119, 120.
In one randomized double blind controlled trial, adalimumab weekly was superior to both adalimumab every other week and placebo 121.
Other biologic agents
In recent years, adalimumab biosimilars have become a frequently used alternative to the adalimumab originator agent and currently six adalimumab biosimilars are approved by the FDA and European Medicines Agency 122.
Infliximab is a chimeric mouse/human IgG1 monoclonal antibody with high affinity to soluble and transmembrane bound TNF⍺. A recent metanalysis calculated a pooled response rate for infliximab of 83% in patients with moderate-to-severe hidradenitis suppurativa and described a low toxicity with a rate of 2.9% for severe adverse events 123. In a phase 2 placebo-controlled crossover study, 57% of patients under infliximab treatment reached a reduction of the Hidradenitis Suppurativa Severity index (HSSI) of >50% compared to 5% in the placebo group. Additionally, improvements in pain intensity and quality of life were observed with concomitant reduction in clinical markers of inflammation 124. In another prospective trial, sustained treatment responses were observed in hidradenitis suppurativa patients after a single course of infliximab 125. Overall, infliximab is a promising treatment option for hidradenitis suppurativa; however, larger prospective randomized trials are needed to investigate its efficacy compared to other biologic therapies 123.
Etanercept, a fusion protein consisting of the extracellular ligand-binding domain of the 75 kDa TNF⍺ receptor and the Fc portion of human IgG1, inhibits TNF⍺ signaling through competitive binding of its ligand 126. In a small prospective placebo-controlled phase 2 trial, etanercept showed no significant efficacy compared to placebo 127.
In comparing all the biologic agents, one review put the percent responders at 89% infliximab, 79% adalimumab, and 56% for etanercept 128. Other TNF⍺ inhibitors like golimumab and certolizumab pegol showed inconstant response rates, and evidence is limited on several case reports and small retrospective studies 129, 130, 131. Ustekinumab has also been used in a few patients.
Infliximab seems to be most effective but in one study 22% experienced serious adverse events, the most common being infusion reactions, and one patient died of pneumococcal sepsis. In one study 132 of 11 patients with severe hidradenitis suppurativa unresponsive to more than three prior therapies, infliximab 5 mg/kg every four weeks was given. Nine of the 11 patients remain well-controlled on this regimen.
Canakinumab is a human IgGk monoclonal antibody targeting IL-1B. It was beneficial in two patients with severe hidradenitis suppurativa 133.
Interleukin-17
In recent years, interleukin 17 (IL-17) has emerged as a major player in various autoimmune and inflammatory skin disorders, and its proinflammatory isoforms IL-17A, IL-17C and IL-17F are presumed as key cytokines in hidradenitis suppurativa 134, 135. IL-17A and IL-17F are mainly produced by T-helper 17 cells (TH17) and play a major role in control of many fungal and bacterial infections, while IL-17C is released by epithelial cells like keratinocytes, and promotes further inflammation by mediation of the production of other inflammatory molecules and further enhancing the inflammation cascade through stimulation of IL-17A and IL-17F production in TH17-cells as a feed forward loop 136.
The first evidence for the role of IL-17 in hidradenitis suppurativa was reported by Schlapbach et al. 137, who found a 30-fold increased expression of IL-17A in hidradenitis suppurativa lesions compared to healthy skin. Another study confirmed these findings and identified a dysregulated cytokine milieu in perilesional skin, suggesting that subclinical inflammation may be present in hidradenitis suppurativa skin prior to the formation of active lesions 138. Moreover, Navrazhina et al. 139 found significantly increased expressions of the proinflammatory IL-17 isoforms A, C and F in lesional, perilesional and even unaffected skin of hidradenitis suppurativa patients compared to healthy individuals. Given these accumulating findings pointing toward a key role of IL-17 signaling in the pathogenesis of hidradenitis suppurativa, targeting the IL-17 pathway seems like a promising treatment approach. However, as hidradenitis suppurativa can be associated with inflammatory bowel disease, it should be kept in mind that paradoxical exacerbations of pre-existing inflammatory bowel disease have been reported under anti-IL17 treatment 100, 140.
Secukinumab is a recombinant human monoclonal IgG1κ antibody that selectively targets IL-17A and blocks its interaction with the IL-17 receptor 141. In an open-label pilot study with nine patients, 78% reached HiSCR after 24 weeks of treatment with secukinumab 300 mg every 4 weeks 142. Another open-label trial with 20 enrolled patients tested two dose levels of secukinumab (300 mg every 2 or 4 weeks after an initial loading dose) and observed pooled hidradenitis suppurativa clinical response (HiSCR) rates of 70%. Interestingly, clinical responses were also observed in patients with failure to prior anti-TNF⍺ treatment 143.
Recently, the first results of two randomized, placebo-controlled, multicenter phase 3 trials 144 evaluating the efficacy of secukinumab in two dose regimens (every 2 weeks or every 4 weeks) have been presented. After 16 weeks, the primary endpoint was reached in both studies for the every 2 weeks regimen and in one for the every 4 weeks regimen, demonstrating the superiority of secukinumab over placebo in patients with moderate-to-severe hidradenitis suppurativa (HiSCR rates: 45% vs. 33.7% (study 1) and 42.3% vs. 31.2% (study 2) for secukinumab 300 mg every 2 weeks, and 41.8% vs. 33.7% (study 1) and 46.1% vs. 31.2% (study 2) for secukinumab every 4 weeks). In both treatment groups secukinumab significantly reduced inflammatory lesions and pain, and improved patients’ quality of life 144, 145.
Bimekizumab is another humanized IgG1κ monoclonal antibody, targeting IL-17A and IL-17F. In a recent double-blind, placebo-controlled, phase 2 clinical trial 146 90 patients were randomized to receive bimekizumab (640 mg at week 0, 320 mg every 2 weeks), adalimumab (160 mg at week 0, 80 mg at week 2 and 40 mg every week for weeks 4–10) or placebo. Hidradenitis suppurativa clinical response (HiSCR) was reached in 57.3% of the bimekizumab group compared to 26.1% in the placebo arm. Moreover, 46% of the patients under bimekizumab achieved HiSCR75 and 32% achieved HiSCR90, compared to 10% and 0% in the placebo group, respectively 32. These promising results led to the initiation of three placebo-controlled phase 3 studies, which are currently ongoing – NCT04901195 147, NCT04242498 148 and NCT04242446 149.
Nanobodies represent a novel innovative class of antibody-derived targeted therapies. Consisting of one or more domains based on the small antigen binding variable regions of heavy chain antibodies, they are much smaller compared to conventional monoclonal antibodies, facilitating their tissue penetration 150. Moreover, several nanobodies can be linked to obtain multi-specific molecules 151. Sonelokimab is a trivalent nanobody containing three domains with specificity for IL-17A, IL-17F and human serum albumin, and has already showed clinical efficacy in the treatment of plaque psoriasis in a recent phase 2 study 152. An ongoing randomized placebo-controlled trial evaluates the efficacy of sonelokimab in patients with moderate-to-severe hidradenitis suppurativa (ClinicalTrials.gov NCT05322473) 153.
Izokibep, another small, molecular, antibody-mimetic IL-17A inhibitor has recently shown significant clinical responses in patients with psoriatic arthritis and is currently in a placebo-controlled phase 2 trial in hidradenitis suppurativa 154.
JAK/STAT Inhibitors
The Janus kinase and signal transducers and activators of transcription (JAK/STAT) pathway represents a rapid membrane-to-nucleus cell signaling module, which regulates the expression of various critical mediators of cancer and inflammation 155. Inhibitors of the JAK/STAT pathway are a potential approach for future treatment of hidradenitis suppurativa, as they enable the simultaneous modulation of expression of different cytokines that are involved in the complex inflammatory process of hidradenitis suppurativa 156.
The efficacy of the JAK1 inhibitor INCB054707 was evaluated in two phase 2 studies (ClinicalTrials.gov NCT03569371, NCT03607487) in patients with moderate-to-severe hidradenitis suppurativa 157. Janus Kinase 1 Inhibitor INCB054707 was well tolerated and clinical improvement of disease was observed; however, the effect was only significant in the high-dose group with 90 mg (HiSCR 88% vs. 57% placebo) 157.
Upadacitinib is a second-generation JAK inhibitor with selectivity for JAK1. In a retrospective cohort study 75% and 100% of patients treated with upadacitinib reached hidradenitis suppurativa clinical response (HiSCR) after 4 and 12 weeks of treatment, respectively. HiSCR75 rates were 30% and 95% after 4 weeks and 12 weeks 158. These promising observations led to the initiation of a phase II randomized controlled trial with results unpublished (ClinicalTrials.gov NCT04430855) [182].
A phase 2 trial for topical treatment of early-stage hidradenitis suppurativa lesions with the JAK1/JAK2 inhibitor ruxolitinib is currently ongoing (ClinicalTrials.gov NCT04414514) 159.
Other medical treatments
- Metformin in patients with insulin resistance. Metformin 160 and more recently with glucagon-like peptide-1 agonist liraglutide 161 have shown benefit in difficult to control hidradenitis suppurativa. In overweight or obese patients with recalcitrant disease, these agents may be beneficial.
- Acitretin (unsuitable for females of childbearing potential). Acitretin has been evaluated for the treatment of moderate-to-severe hidradenitis suppurativa in a small prospective trial with 17 patients and showed an overall response rate of 47%; however, another 47% of patients dropped out due to lack of efficacy or adverse events 162. Acitretin in a retrospective study of 12 patients, acitretin showed promising results with all treated patients achieving clinical remission and reduction of pain 68. The average dose was 0.67 mg/kg/day. Nine patients achieved marked or complete remission after one course. The improvement generally started within two months and further improvement was achieved within the first six months of therapy. Another recent retrospective cohort study reported significant clinical responses under acitretin treatment and identified the follicular hidradenitis suppurativa phenotype, a history of follicular plugging diseases and a family history of hidradenitis suppurativa as potential predictive markers for treatment response 163.
- Isotretinoin has only shown limited efficacy for treatment of hidradenitis suppurativa and several retrospective studies reported treatment response rates between 16.1% and 35.9% 164, 165, 166. Clinical exacerbations and occurrence of new flares of hidradenitis suppurativa have been reported after initiation of isotretinoin in various cases, which could be explained by the further reduction of the size and action of sebaceous glands due to isotretinoin therapy 167, 168.
- Colchicine and minocycline. This prospective study or case series (n = 20) indicates that the combination of the anti-inflammatory actions of colchicine and minocycline is effective in disease control in hidradenitis suppurativa 169.
- Zinc salts (zinc gluconate) show anti-inflammatory effects in hidradenitis suppurativa, probably through inhibition of chemotaxis of neutrophil granulocytes, modulation of cytokine expression and anti-androgen properties 170. In hidradenitis suppurativa, a high-dose therapy with 90 mg zinc gluconate per day in gradual dose escalation can be considered as a maintenance therapy for limited disease 12. In several small studies, zinc gluconate showed promising results in mild-to-moderate hidradenitis suppurativa 171, 172, 173. As zinc competes with copper in gastrointestinal resorption, long-term use of high doses of zinc may cause hypocupremia and anemia; thus, routine monitoring of copper levels and hemoglobin is recommended 174. Gastrointestinal discomfort is a frequent reported side effect.
- Anakinra (IL-1 receptor antagonist) showed benefit in a study of five patients 175. Another study randomized 20 patients to receive either anakinra or placebo for 12 weeks. The disease activity score was decreased at the end of treatment in 20% (2 of 10) of the placebo arm compared with 67% (6 of 9) of the anakinra arm 176. Several case reports described inconstant clinical responses with partial improvements and failure of anakinra treatment 177, 178, 179, 180, 181.
- Bermekimab (also known as MABp1) is a recombinant monoclonal antibody that neutralizes IL-1⍺. In a prospective randomized controlled phase 2 trial, patients with moderate-to-severe hidradenitis suppurativa, that were not eligible for or failed a prior anti-TNF⍺ treatment, showed significant clinical responses with hidradenitis suppurativa clinical response (HiSCR) rates of 60% compared to 10% in the placebo group after 12 weeks 182. Patients who were initially randomized to the placebo group were allowed to continue in an open-label extension study and 75% showed clinical response (HiSCR) after 12 weeks of bermekimab treatment 183. In another phase 2 study with 42 patients with moderate-to-severe hidradenitis suppurativa who were naïve to or had failed prior anti-TNF⍺ therapy, significant clinical responses were observed in both groups with HiSCR rates of 63% in the TNF⍺-failure group and 61% in the TNF⍺-naïve group, respectively, qualifying bermekimab as potential alternative treatment option for non-responders to anti-TNF⍺ 184. A randomized placebo and active-comparator-controlled phase IIa/b trial evaluating the efficacy of bermekimab compared to placebo and adalimumab is currently ongoing (ClinicalTrials.gov NCT04988308) 185.
- Medical management of anxiety and depression
- Nd:YAG laser hair removal. For axillary involvement, laser hair removal can be quite helpful. In addition, the 1064 nm nd:YAG laser has been used ongoing with benefit in uncontrolled studies 186.
Hidradenitis suppurativa surgery
If medical therapy is not successful and the patient desires a “cure,” a surgical approach may be considered 187. This usually consists of excision of all diseased tissue in the area. Limited excisions usually result in recurrence adjacent to the scar. In one study, limited excision of diseased tissue had a 43% recurrence rate whereas radical excision had only a 27% recurrence rate 188. With appropriate patient selection, patient satisfaction with surgery is high 189.
There are both localized and extensive surgical interventions. Although there is no consensus on the best approach, procedures are carried out based on disease severity and location, with the overall goal of removing lesional tissue and sparing healthy skin to optimize outcomes.
- Incision and drainage of acute abscesses. In acute cases with abscess formation, incision and drainage can be considered for acute pain relief, although this is only a symptom treatment with nearly 100% recurrence rates 190. As deroofing can be performed approximately in the same amount of time, coming with higher recurrence-free rates, experts recommend deroofing over incision and drainage 11.
- Deroofing. Deroofing describes a superficial removal of the skin covering an inflammatory nodule or a solitary sinus tract, exposing the partially epithelialized basis of the lesion with subsequently curettage of the gelatinous granulation tissue 191. The advantages of this procedure are its simplicity, cost-effectiveness and that it can be performed under local anesthesia, qualifying this method for treatment of inflammatory nodules, abscesses and solitary sinus tracts 192. In a small study with 44 patients undergoing deroofing of axillary or inguinal hidradenitis suppurativa lesions, 87% were recurrence-free in the follow-up interval of five years 193.
- Skin-tissue-sparing excision with electrosurgical peeling (STEEP). The skin-tissue-sparing excision with electrosurgical peeling (STEEP) method represents a similar surgical approach and was first described by Blok et al. 194. Here, successive tangential excisions are performed with an electrosurgical wire loop until the epithelialized bottom of the lesion is exposed, whilst saving as much healthy tissue as possible. Although this surgical technique is associated with a short time to wound healing and a low risk of wound contraction, no long-term outcomes are reported and evidence is limited on small case series 195.
- Excision. Excision describes the complete removal of the affected tissue and represents a more invasive surgical approach in hidradenitis suppurativa. Depending on the extent of resection experts differentiate between limited, wide and radical excisions; however, there are no generally accepted definitions and distinctions between surgical interventions are blurred 192. Van Rappard et al. 196 defined limited or localized excisions as the complete excision of the affected tissue, beyond the borders of activity, leaving clear margins. Limited excisions represent a low-invasive surgical approach, appropriate for the treatment of recurrent inflammatory nodules or abscesses and solitary sinus tracts in Hurley stage 1 or 2, and can be performed under local anesthesia in an outpatient setting 196. Wide excisions are understood as the surgical removal of the affected tissue including the surrounding subcutaneous fat and perilesional skin with an additional resection margin 197. In severe cases, radical excisions with the complete removal of the entire hair bearing area including the subcutaneous fat to the underlying fascia of an affected body region can be performed 12. Nesmith et al. 198 suggested an additional superficial lymphadenectomy for a further reduction of the risk of recurrence. Although there are no accepted definitions for surgical techniques and recurrence in hidradenitis suppurativa, it is generally considered that more extensive resections are associated with a lower risk of recurrence 199.
- In cases of extensive disease with multiple or confluent sinus tracts, preoperative MRI- or ultrasound-based imaging methods or an intraoperative use of dye mapping methods, such as methyl violet or iodine starch, can contribute to an improved visualization of the surgical site 200.
- Laser ablation of nodules, abscesses and sinuses. Various laser and light-based therapies have gained increasing attention as a possible treatment modality for hidradenitis suppurativa 201.
- Carbon dioxide (CO2) laser acts at a wavelength of 10,600 nm and can be used for tissue ablation in different modalities, namely vaporization and excision. CO2-laser vaporization represents a tissue-sparing treatment option, that aims on a focal destruction of chronic or inflammatory hidradenitis suppurativa lesions, and was first described by Dalrymple et al. for the treatment of sinus tracts 202. In two case series, Lapins et al. 203, 204 reported low local recurrence rates and satisfactory postoperative outcomes after a stepwise horizontal CO2-laser evaporation of sinus tracts in Hurley stage 2 patients. Another retrospective study showed similar outcomes, but described local recurrence rates of 29% 205. A retrospective study evaluated the outcomes of 185 treated sites of 61 hidradenitis suppurativa patients after CO2-laser excision or marsupialization with secondary intention healing, and demonstrated fast healing rates and a local recurrence rate of 1% 206. Summarizing, CO2-laser interventions are tissue-sparing treatment options for hidradenitis suppurativa lesions with fast postoperative wound healing and low complication rates. As they can be performed under local anesthesia and wounds are generally allowed to heal by secondary intention, these treatments seem suitable for an outpatient setting 201.
- Neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers act as non-ablative lasers at a wavelength of 1064 nm and enable a selective photothermolysis of hair follicles 207. Their efficacy has been demonstrated in several randomized controlled studies, leading to significant clinical improvements and prevention of new inflammatory lesions in the treated body areas of hidradenitis suppurativa patients 208, 209. In a histopathologic study, an initially increased perifollicular inflammation, observed after 1 week of treatment, was followed by a fibrotic tissue transformation after 4–8 weeks of treatment 210. Axillary and inguinal areas have been shown to be more responsive to Nd:YAG-laser treatment compared to inframammary and gluteal regions 209. Moreover, combination treatments consisting of Nd:YAG-laser-mediated hair follicle destruction and CO2-laser ablation of hidradenitis suppurativa lesions have shown superior clinical responses 211.
Many approaches exist to manage hidradenitis suppurativa, including local destruction, incision and drainage, standard unroofing, and wide excision techniques 212. Local destruction is used to ablate hidradenitis suppurativa lesions and may be carried out with electrosurgery, cryotherapy, or laser removal. Incision and drainage may be used for decompression in acute episodes of unbearable pain. However, incision and drainage does not assist in the long-term resolution of disease, as the inflamed tissue remains and infection is almost certain to recur 213. Unroofing techniques are effective for both small and large lesional units and are carried out by opening the surface of all connected abscesses and tracts within an hidradenitis suppurativa lesion. The contents are removed by curettage, often leaving the site open to heal by secondary intention 214. Unroofing techniques are preferred for Hurley stage I/II, whereas skin-tissue-saving excision with electrosurgical peeling (STEEP) is preferred for Hurley stage II/III. Skin-tissue-saving excision with electrosurgical peeling (STEEP) similarly removes diseased, fibrotic tissue via electrosurgical loop while sparing healthy skin to decrease sequelae following the surgical procedure 215. Lesions that cannot be unroofed may be excised, which includes the removal of the entire diseased area up to the margins of normal-appearing subcutaneous tissue 212.
The best surgical approach for long-term outcomes is controversial and is dependent on the patient’s disease severity and location of the lesions. A recent systematic review and meta-analysis assessed for recurrence rates with varying methods of surgical management. The lowest rates of recurrence occurred following wide excision therapy when compared to local excision and deroofing procedures. This study also indicated that recurrence rates were lowest with skin grafts and skin flaps compared to primary closure, although this was limited by retrospective analysis and lack of randomization of closures 216. Wide excision followed by secondary intention healing was also shown to be functional and aesthetically acceptable to patients 217. Bias may exist in the discrepancy between recurrence rates and type of closure owing to the fact that primary closure is possible only with smaller wound sizes compared to larger excisions that simply cannot be closed by primary intention. Although this has not been formally assessed, the size of the wound may play a role.
The use of lasers in hidradenitis suppurativa management has gained recent popularity. The 1064 nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser appears to be an effective, novel modality in hidradenitis suppurativa management. After a series of treatments, two trials reported decreases in hidradenitis suppurativa-associated inflammation, scarring, and fibrosis, indicating successful, selective photothermolysis to manage the disease 218. It is best used in recalcitrant Hurley stage 1 and 2 patients.
Another type of laser used in the treatment of hidradenitis suppurativa is the carbon dioxide (CO2) laser, which is used to excise hidradenitis suppurativa lesions and ablate pathologic tissues. CO2 laser has been used since the late 1980s for hidradenitis suppurativa, although it is now increasing in popularity. Laser excision followed by marsupialization has been shown to be effective for the management of persistent or late-stage cases of hidradenitis suppurativa, with overall patient satisfaction in post-operative quality of life and pain measures 219. Additionally, a retrospective study (n = 58) of CO 2 laser evaporation techniques reported a 29% recurrence rate of disease within 12.2 months of the procedure (noted around the borders of treated regions), while 95% of patients reported some or great improvement in disease status 220. A reported complication of CO2 excision is scar contracture, restricted range of motion, and delayed wound healing. Nicholson et al. determined that fractional CO 2 therapy could be a helpful adjunct in these cases 221.
Psychological support
Due to its chronic recurrent course with painful lesions and purulent discharge, hidradenitis suppurativa has a pronounced impact on the patient’s life and professional support may be required. Esmann et al. 222 described an increased risk of social isolation due to shame and fear of stigmatization. Quality of life can be severely affected by hidradenitis suppurativa and hidradenitis suppurativa has been observed as one of the most distressing conditions in dermatology 223, 224. Hidradenitis suppurativa is associated with an increased risk for distinct psychiatric disorders including depression, anxiety disorders and substance-related abuse 225. Moreover, a meta-analysis found a more than two-fold increased risk for suicide in hidradenitis suppurativa patients 225. For this reason, physicians treating hidradenitis suppurativa patients should perform a screening for psychiatric comorbidities and initiate psychiatric referral if necessary.
Hidradenitis suppurativa prognosis
The way hidradenitis suppurativa develops in individual people is not very well understood and there is no cure for this condition but is generally controllable with one or more of the above treatments. Hidradenitis suppurativa prognosis is variable and not all cases progress to the most severe form of the disease. However, the prognosis worsens if there is a delay in diagnosis and treatment during the early stages of the disease and if comorbid conditions of smoking and obesity (if present) are not addressed and improved 226.
Treatment and control of the inflammation is important to minimize HS skin disease from progressing and scarring.
Weight gain and smoking worsen hidradenitis suppurativa and should be avoided. Hidradenitis suppurativa is generally most active during teenage years and young adulthood. It often, but not always, improves as the person gets older.
Hidradenitis suppurativa complications include both physical and psychological conditions 227. Physically, recurrence of lesions leading to abscesses, tracts, and scarring can cause chronic pain, limb contractures, and impaired mobility. Lymphatic obstruction can lead to peripheral lymphedema. Long-term effects of chronic inflammation can also occur, including anemia, hyperproteinemia, and amyloidosis, as well as axial and peripheral arthropathy. In rare cases, a superimposed infection can lead to systemic illness of variable severity. Squamous cell carcinoma can occur in the setting of hidradenitis suppurativa, sometimes occurring up to 30 years after diagnosis. There is an associated increase in buccal and hepatocellular cancer based on observational data.
Hidradenitis suppurativa can have a psychological impact as well. Combining chronic pain with drainage, odor, and deformity of skin appearance can lead to depression, social isolation, decreased relationship satisfaction, sexual dysfunction, reduced work productivity, and even suicide in extreme cases 227.
- Ocker L, Abu Rached N, Seifert C, Scheel C, Bechara FG. Current Medical and Surgical Treatment of Hidradenitis Suppurativa-A Comprehensive Review. J Clin Med. 2022 Dec 6;11(23):7240. doi: 10.3390/jcm11237240[↩][↩][↩]
- Smith MK, Nicholson CL, Parks-Miller A, Hamzavi IH. Hidradenitis suppurativa: an update on connecting the tracts. F1000Research. 2017;6:1272. doi:10.12688/f1000research.11337.1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5538037[↩]
- Hidradenitis Suppurativa: Rapid Evidence Review. Am Fam Physician. 2019;100(9):562-569. https://www.aafp.org/pubs/afp/issues/2019/1101/p562.html[↩][↩][↩][↩][↩][↩][↩][↩]
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009 Apr;60(4):539-61; quiz 562-3. doi: 10.1016/j.jaad.2008.11.911[↩]
- Hidradenitis suppurativa. https://dermnetnz.org/topics/hidradenitis-suppurativa[↩][↩]
- Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and Age-Adjusted Population Analysis of Prevalence Estimates for Hidradenitis Suppurativa in the United States. JAMA Dermatol. 2017 Aug 1;153(8):760-764. doi: 10.1001/jamadermatol.2017.0201[↩][↩]
- Deckers IE, van der Zee HH, Boer J, Prens EP. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J Am Acad Dermatol. 2015 Mar;72(3):485-8. doi: 10.1016/j.jaad.2014.11.017[↩]
- Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017 Jul;77(1):118-122. doi: 10.1016/j.jaad.2017.02.005[↩]
- JEADV 2016;230;174[↩]
- Maclean GM, Coleman DJ. Three fatal cases of squamous cell carcinoma arising in chronic perineal hidradenitis suppurativa. Ann R Coll Surg Engl. 2007 Oct;89(7):709-12. doi: 10.1308/003588407X209392[↩]
- Alikhan A., Sayed C., Alavi A., Alhusayen R., Brassard A., Burkhart C., Crowell K., Eisen D.B., Gottlieb A.B., Hamzavi I., et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: A Publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, Intralesional, and Systemic Medical Management. J. Am. Acad. Dermatol. 2019;81:91–101. doi: 10.1016/j.jaad.2019.02.068[↩][↩]
- Zouboulis C.C., Desai N., Emtestam L., Hunger R.E., Ioannides D., Juhász I., Lapins J., Matusiak L., Prens E.P., Revuz J., et al. European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa. J. Eur. Acad. Dermatol. Venereol. 2015;29:619–644. doi: 10.1111/jdv.12966[↩][↩][↩][↩][↩][↩][↩][↩]
- Zarchi K, Dufour DN, Jemec GBE. Successful Treatment of Severe Hidradenitis Suppurativa With Anakinra. JAMA Dermatol. 2013;149(10):1192–1194. doi:10.1001/jamadermatol.2013.5377[↩]
- Shlyankevich J., Chen A.J., Kim G.E., Kimball A.B. Hidradenitis Suppurativa Is a Systemic Disease with Substantial Comorbidity Burden: A Chart-Verified Case-Control Analysis. J. Am. Acad. Dermatol. 2014;71:1144–1150. doi: 10.1016/j.jaad.2014.09.012[↩]
- Deckers I.E., Benhadou F., Koldijk M.J., Del Marmol V., Horváth B., Boer J., van der Zee H.H., Prens E.P. Inflammatory Bowel Disease Is Associated with Hidradenitis Suppurativa: Results from a Multicenter Cross-Sectional Study. J. Am. Acad. Dermatol. 2017;76:49–53. doi: 10.1016/j.jaad.2016.08.031[↩]
- geberg A., Gislason G.H., Hansen P.R. Risk of Major Adverse Cardiovascular Events and All-Cause Mortality in Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2016;152:429–434. doi: 10.1001/jamadermatol.2015.6264[↩]
- Patel K.R., Lee H.H., Rastogi S., Vakharia P.P., Hua T., Chhiba K., Singam V., Silverberg J.I. Association between Hidradenitis Suppurativa, Depression, Anxiety, and Suicidality: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2020;83:737–744. doi: 10.1016/j.jaad.2019.11.068[↩]
- Richette P., Molto A., Viguier M., Dawidowicz K., Hayem G., Nassif A., Wendling D., Aubin F., Lioté F., Bachelez H. Hidradenitis Suppurativa Associated with Spondyloarthritis—Results from a Multicenter National Prospective Study. J. Rheumatol. 2014;41:490–494. doi: 10.3899/jrheum.130977[↩]
- Sabat R., Chanwangpong A., Schneider-Burrus S., Metternich D., Kokolakis G., Kurek A., Philipp S., Uribe D., Wolk K., Sterry W. Increased Prevalence of Metabolic Syndrome in Patients with Acne Inversa. PLoS ONE. 2012;7:e31810. doi: 10.1371/journal.pone.0031810[↩]
- Garg A., Malviya N., Strunk A., Wright S., Alavi A., Alhusayen R., Alikhan A., Daveluy S.D., Delorme I., Goldfarb N., et al. Comorbidity Screening in Hidradenitis Suppurativa: Evidence-Based Recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J. Am. Acad. Dermatol. 2022;86:1092–1101. doi: 10.1016/j.jaad.2021.01.059[↩]
- Timila Touhouche A., Chaput B., Marie Rouquet R., Montastier E., Caron P., Gall Y., Aquilina C., Boulinguez S., Claude Marguery M., Giordano-Labadie F., et al. Integrated Multidisciplinary Approach to Hidradenitis Suppurativa in Clinical Practice. Int. J. Womens Dermatol. 2020;6:164–168. doi: 10.1016/j.ijwd.2020.02.006[↩]
- Seyed Jafari S.M., Hunger R.E., Schlapbach C. Hidradenitis Suppurativa: Current Understanding of Pathogenic Mechanisms and Suggestion for Treatment Algorithm. Front. Med. 2020;7:68. doi: 10.3389/fmed.2020.00068[↩]
- Sabat R., Jemec G.B.E., Matusiak Ł., Kimball A.B., Prens E., Wolk K. Hidradenitis Suppurativa. Nat. Rev. Dis. Primer. 2020;6:18. doi: 10.1038/s41572-020-0149-1[↩]
- Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigh R, Roenigh H, eds. Dermatologic surgery. New York: Marcel Dekker; 1989:729-739.[↩]
- Napolitano M., Megna M., Timoshchuk E.A., Patruno C., Balato N., Fabbrocini G., Monfrecola G. Hidradenitis Suppurativa: From Pathogenesis to Diagnosis and Treatment. Clin. Cosmet. Investig. Dermatol. 2017;10:105–115. doi: 10.2147/CCID.S111019[↩]
- Rondags A., van Straalen K.R., van Hasselt J.R., Janse I.C., Ardon C.B., Vossen A.R., Prens E.P., van der Zee H.H., Horváth B. Correlation of the Refined Hurley Classification for Hidradenitis Suppurativa with Patient-Reported Quality of Life and Objective Disease Severity Assessment. Br. J. Dermatol. 2019;180:1214–1220. doi: 10.1111/bjd.17508[↩]
- Sartorius, K., Emtestam, L., Jemec, G.B.E. and Lapins, J. (2009), Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. British Journal of Dermatology, 161: 831-839. https://doi.org/10.1111/j.1365-2133.2009.09198.x[↩][↩]
- Kimball A.B., Kerdel F., Adams D., Mrowietz U., Gelfand J.M., Gniadecki R., Prens E.P., Schlessinger J., Zouboulis C.C., van der Zee H.H., et al. Adalimumab for the Treatment of Moderate to Severe Hidradenitis Suppurativa: A Parallel Randomized Trial. Ann. Intern. Med. 2012;157:846–855. doi: 10.7326/0003-4819-157-12-201212180-00004[↩]
- Monfrecola G., Megna M. Hidradenitis Suppurativa. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2017. Classification and Severity Scales; pp. 39–45.[↩]
- Kimball A.B., Jemec G.B.E., Yang M., Kageleiry A., Signorovitch J.E., Okun M.M., Gu Y., Wang K., Mulani P., Sundaram M. Assessing the Validity, Responsiveness and Meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as the Clinical Endpoint for Hidradenitis Suppurativa Treatment. Br. J. Dermatol. 2014;171:1434–1442. doi: 10.1111/bjd.13270[↩]
- Kimball A.B., Sobell J.M., Zouboulis C.C., Gu Y., Williams D.A., Sundaram M., Teixeira H.D., Jemec G.B.E. HiSCR (Hidradenitis Suppurativa Clinical Response): A Novel Clinical Endpoint to Evaluate Therapeutic Outcomes in Patients with Hidradenitis Suppurativa from the Placebo-Controlled Portion of a Phase 2 Adalimumab Study. J. Eur. Acad. Dermatol. Venereol. JEADV. 2016;30:989–994. doi: 10.1111/jdv.13216[↩][↩]
- Glatt S., Jemec G.B.E., Forman S., Sayed C., Schmieder G., Weisman J., Rolleri R., Seegobin S., Baeten D., Ionescu L., et al. Efficacy and Safety of Bimekizumab in Moderate to Severe Hidradenitis Suppurativa: A Phase 2, Double-Blind, Placebo-Controlled Randomized Clinical Trial. JAMA Dermatol. 2021;157:1279–1288. doi: 10.1001/jamadermatol.2021.2905[↩][↩]
- Hessam S, Scholl L, Sand M, Schmitz L, Reitenbach S, Bechara FG. A Novel Severity Assessment Scoring System for Hidradenitis Suppurativa. JAMA Dermatol. 2018 Mar 1;154(3):330-335. doi: 10.1001/jamadermatol.2017.5890[↩]
- Kokolakis G., Sabat R. Distinguishing Mild, Moderate, and Severe Hidradenitis Suppurativa. JAMA Dermatol. 2018;154:971–972. doi: 10.1001/jamadermatol.2018.1599[↩]
- Zouboulis C.C., Tzellos T., Kyrgidis A., Jemec G.B.E., Bechara F.G., Giamarellos-Bourboulis E.J., Ingram J.R., Kanni T., Karagiannidis I., Martorell A., et al. Development and Validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a Novel Dynamic Scoring System to Assess HS Severity. Br. J. Dermatol. 2017;177:1401–1409. doi: 10.1111/bjd.15748[↩][↩]
- Tzellos T., van Straalen K.R., Kyrgidis A., Alavi A., Goldfarb N., Gulliver W., Jemec G.B.E., Lowes M.A., Marzano A.V., Prens E.P., et al. Development and Validation of IHS4-55, an IHS4 Dichotomous Outcome to Assess Treatment Effect for Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2022:1–7. doi: 10.1111/jdv.18632[↩]
- Pinard J, Vleugels RA, Joyce C, Merola JF, Patel M. Hidradenitis suppurativa burden of disease tool: Pilot testing of a disease-specific quality of life questionnaire. J Am Acad Dermatol. 2018 Jan;78(1):215-217.e2. https://www.jaad.org/article/S0190-9622(17)32263-6/fulltext[↩]
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34(6):994-999.[↩]
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24(10):727-731.[↩]
- Ring HC, Bay L, Nilsson M, et al. Bacterial Biofilm in Chronic Lesions of Hidradenitis Suppurativa. Br J Dermatol. 2016.[↩]
- Danby FW. Diet in the prevention of hidradenitis suppurativa (acne inversa). J Am Acad Dermatol. 2015;73(5 Suppl 1):S52-54.[↩]
- Cannistra C, Finocchi V, Trivisonno A, Tambasco D. New perspectives in the treatment of hidradenitis suppurativa: surgery and brewer’s yeast-exclusion diet. Surgery. 2013;154(5):1126-1130.[↩]
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: An update. J Am Acad Dermatol. 2015;73(5 Suppl 1):S8-11.[↩][↩]
- Ballard K, Shuman VL. Hidradenitis Suppurativa. [Updated 2022 Jul 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534867[↩][↩][↩]
- van der Zee HH, Laman JD, Boer J, Prens EP. Hidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp Dermatol. 2012 Oct;21(10):735-9. doi: 10.1111/j.1600-0625.2012.01552.x[↩]
- Vekic, D.A., Frew, J.W., Woods, J. and Cains, G.D. (2016), Adopting the orphan: The importance of recognising hidradenitis suppurativa as a systemic auto-inflammatory disease. Australasian Journal of Dermatology, 57: 69-70. https://doi.org/10.1111/ajd.12287[↩]
- Frew, J.W., Vekic, D.A., Woods, J. and Cains, G.D. (2017), A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol, 177: 987-998. https://doi.org/10.1111/bjd.15441[↩]
- Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Riis PT, Larsen N, Andersen LO, Nielsen HV, Miller IM, Bjarnsholt T, Fuursted K, Jemec GB. The Follicular Skin Microbiome in Patients With Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017 Sep 1;153(9):897-905. doi: 10.1001/jamadermatol.2017.0904[↩]
- Woodruff CM, Charlie AM, Leslie KS. Hidradenitis Suppurativa: A Guide for the Practicing Physician. Mayo Clin Proc. 2015 Dec;90(12):1679-93. doi: 10.1016/j.mayocp.2015.08.020[↩]
- Kromann C.B., Deckers I.E., Esmann S., Boer J., Prens E.P., Jemec G.B.E. Risk Factors, Clinical Course and Long-Term Prognosis in Hidradenitis Suppurativa: A Cross-Sectional Study. Br. J. Dermatol. 2014;171:819–824. doi: 10.1111/bjd.13090[↩][↩]
- Sartorius K., Emtestam L., Jemec G.B.E., Lapins J. Objective Scoring of Hidradenitis Suppurativa Reflecting the Role of Tobacco Smoking and Obesity. Br. J. Dermatol. 2009;161:831–839. doi: 10.1111/j.1365-2133.2009.09198.x[↩]
- Melnik B.C., John S.M., Chen W., Plewig G. T Helper 17 Cell/Regulatory T-Cell Imbalance in Hidradenitis Suppurativa/Acne Inversa: The Link to Hair Follicle Dissection, Obesity, Smoking and Autoimmune Comorbidities. Br. J. Dermatol. 2018;179:260–272. doi: 10.1111/bjd.16561[↩]
- Bukvić Mokos Z, Miše J, Balić A, Marinović B. Understanding the Relationship Between Smoking and Hidradenitis Suppurativa. Acta Dermatovenerol Croat. 2020 Jul;28(1):9-13.[↩][↩]
- Revuz J.E., Canoui-Poitrine F., Wolkenstein P., Viallette C., Gabison G., Pouget F., Poli F., Faye O., Roujeau J.C., Bonnelye G., et al. Prevalence and Factors Associated with Hidradenitis Suppurativa: Results from Two Case-Control Studies. J. Am. Acad. Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020[↩]
- Kromann C.B., Ibler K.S., Kristiansen V.B., Jemec G.B.E. The Influence of Body Weight on the Prevalence and Severity of Hidradenitis Suppurativa. Acta Derm. Venereol. 2014;94:553–557. doi: 10.2340/00015555-1800[↩][↩]
- Brandwein M., Katz I., Katz A., Kohen R. Beyond the Gut: Skin Microbiome Compositional Changes Are Associated with BMI. Hum. Microbiome J. 2019;13:100063. doi: 10.1016/j.humic.2019.100063[↩]
- Darlenski R., Mihaylova V., Handjieva-Darlenska T. The Link Between Obesity and the Skin. Front. Nutr. 2022;9:855573. doi: 10.3389/fnut.2022.855573[↩]
- Gallagher C., Kirthi S., Burke T., O’Shea D., Tobin A.-M. Remission of Hidradenitis Suppurativa after Bariatric Surgery. JAAD Case Rep. 2017;3:436–437. doi: 10.1016/j.jdcr.2017.06.008[↩]
- Khan A., Chang M.W. The Role of Nutrition in Acne Vulgaris and Hidradenitis Suppurativa. Clin. Dermatol. 2022;40:114–121. doi: 10.1016/j.clindermatol.2022.04.001[↩]
- Canard C., Cives A.D., Gaubil-Kaladjian I., Bertin E., Viguier M. Impact of Bariatric Surgery on Hidradenitis Suppurativa. Acta Derm. Venereol. 2021;101:adv00471. doi: 10.2340/00015555-3830[↩]
- Hasan S B, Harris C, Collier F. Hidradenitis suppurativa BMJ 2022;379:e068383 https://doi.org/10.1136/bmj-2021-068383[↩]
- HS Treatments. https://www.hs-foundation.org/hs-treatments[↩]
- Clemmensen, O.J. (1983), Topical Treatment of Hidradenitis Suppurativa with Clindamycin. International Journal of Dermatology, 22: 325-328. https://doi.org/10.1111/j.1365-4362.1983.tb02150.x[↩]
- Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998 Dec;39(6):971-4. doi: 10.1016/s0190-9622(98)70272-5[↩]
- van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219(2):143-7. doi: 10.1159/000228337[↩]
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007 Jul-Aug;11(4):125-31. doi: 10.2310/7750.2007.00019[↩]
- Soria A, Canoui-Poitrine F, Wolkenstein P, Poli F, Gabison G, Pouget F, Viallette C, Revuz J. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218(2):134-5. doi: 10.1159/000182261[↩]
- Boer, J. and Nazary, M. (2011), Long-term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer?. British Journal of Dermatology, 164: 170-175. https://doi.org/10.1111/j.1365-2133.2010.10071.x[↩][↩]
- Hayashi N, Hayama K, Takahashi K, Kurokawa I, Okazaki M, Kashiwagi T, Iwashita E, Terui T. Real-world safety and effectiveness of adalimumab in patients with hidradenitis suppurativa: 12-week interim analysis of post-marketing surveillance in Japan. J Dermatol. 2022 Apr;49(4):411-421. doi: 10.1111/1346-8138.16297[↩]
- Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, Armstrong AW, Kerdel F, Gold MH, Forman SB, Korman NJ, Giamarellos-Bourboulis EJ, Crowley JJ, Lynde C, Reguiai Z, Prens EP, Alwawi E, Mostafa NM, Pinsky B, Sundaram M, Gu Y, Carlson DM, Jemec GB. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016 Aug 4;375(5):422-34. doi: 10.1056/NEJMoa1504370[↩]
- Tricarico PM, Zupin L, Ottaviani G, Rupel K, Celsi F, Genovese G, Boniotto M, Crovella S, Marzano AV. Photobiomodulation as potential novel third line tool for non-invasive treatment of hidradenitis suppurativa. G Ital Dermatol Venereol. 2020 Feb;155(1):88-98. doi: 10.23736/S0392-0488.19.06247-3[↩]
- Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016 Sep;17(3):343-351. doi: 10.1007/s11154-016-9328-5[↩]
- Hazen PG, Hazen BP. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010 Feb;36(2):208-13. doi: 10.1111/j.1524-4725.2009.01427.x[↩]
- Scheinfeld N. Treatment of hidradenitis supprurativa associated pain with nonsteroidal anti-inflammatory drugs, acetaminophen, celecoxib, gabapentin, pegabalin, duloxetine, and venlafaxine. Dermatol Online J. 2013 Nov 15;19(11):20616. https://doi.org/10.5070/D31911020616[↩]
- Fernandez JM, Thompson AM, Borgstrom M, Orenstein LAV, Hsiao JL, Shi VY. Pain management modalities for hidradenitis suppurativa: a patient survey. J Dermatolog Treat. 2022 May;33(3):1742-1745. doi: 10.1080/09546634.2020.1822501[↩]
- Antia C, Alavi A, Alikhan A. Topical management and wound care approaches for hidradenitis suppurativa. Semin Cutan Med Surg. 2017 Jun;36(2):58-61. doi: 10.12788/j.sder.2017.020[↩]
- Matusiak, Ł. (2020), Profound consequences of hidradenitis suppurativa: a review. Br. J. Dermatol., 183: e171-e177. https://doi.org/10.1111/bjd.16603[↩]
- Smith MK, Nicholson CL, Parks-Miller A, Hamzavi IH. Hidradenitis suppurativa: an update on connecting the tracts. F1000Research. 2017;6:1272. doi:10.12688/f1000research.11337.1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5538037/[↩]
- Kromann CB, Ibler KS, Kristiansen VB, Jemec GB. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol. 2014 Sep;94(5):553-7. doi: 10.2340/00015555-1800[↩][↩]
- Garg, A., Papagermanos, V., Midura, M. and Strunk, A. (2018), Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A.. Br J Dermatol, 178: 709-714. https://doi.org/10.1111/bjd.15939[↩]
- Denny G, Anadkat MJ. The effect of smoking and age on the response to first-line therapy of hidradenitis suppurativa: An institutional retrospective cohort study. J Am Acad Dermatol. 2017 Jan;76(1):54-59. doi: 10.1016/j.jaad.2016.07.041[↩][↩]
- Kitts S., Govea R., Maczuga S., Kirby J. Long-Term Antibiotic Use for the Treatment of Hidradenitis Suppurativa Consistent with Guideline Recommendations. Clin. Exp. Dermatol. 2021;46:582–583. doi: 10.1111/ced.14512[↩]
- Acta Derm Venereol 2014; 94: 699–702[↩]
- Marasca C., Tranchini P., Marino V., Annunziata M.C., Napolitano M., Fattore D., Fabbrocini G. The Pharmacology of Antibiotic Therapy in Hidradenitis Suppurativa. Expert Rev. Clin. Pharmacol. 2020;13:521–530. doi: 10.1080/17512433.2020.1762571[↩]
- Jørgensen A.-H.R., Yao Y., Thomsen S.F., Ring H.C. Treatment of Hidradenitis Suppurativa with Tetracycline, Doxycycline, or Lymecycline: A Prospective Study. Int. J. Dermatol. 2021;60:785–791. doi: 10.1111/ijd.15459[↩]
- Gulliver W., Zouboulis C.C., Prens E., Jemec G.B.E., Tzellos T. Evidence-Based Approach to the Treatment of Hidradenitis Suppurativa/Acne Inversa, Based on the European Guidelines for Hidradenitis Suppurativa. Rev. Endocr. Metab. Disord. 2016;17:343–351. doi: 10.1007/s11154-016-9328-5[↩]
- Yao Y., Jørgensen A.-H.R., Ring H.C., Thomsen S.F. Effectiveness of Clindamycin and Rifampicin Combination Therapy in Hidradenitis Suppurativa: A 6-Month Prospective Study. Br. J. Dermatol. 2021;184:552–553. doi: 10.1111/bjd.19578[↩][↩]
- Gener G., Canoui-Poitrine F., Revuz J.E., Faye O., Poli F., Gabison G., Pouget F., Viallette C., Wolkenstein P., Bastuji-Garin S. Combination Therapy with Clindamycin and Rifampicin for Hidradenitis Suppurativa: A Series of 116 Consecutive Patients. Dermatol. Basel Switz. 2009;219:148–154. doi: 10.1159/000228334[↩]
- van der Zee H.H., Boer J., Prens E.P., Jemec G.B.E. The Effect of Combined Treatment with Oral Clindamycin and Oral Rifampicin in Patients with Hidradenitis Suppurativa. Dermatol. Basel Switz. 2009;219:143–147. doi: 10.1159/000228337[↩]
- Mendonça C.O., Griffiths C.E.M. Clindamycin and Rifampicin Combination Therapy for Hidradenitis Suppurativa. Br. J. Dermatol. 2006;154:977–978. doi: 10.1111/j.1365-2133.2006.07155.x[↩]
- Wynalda M.A., Hutzler J.M., Koets M.D., Podoll T., Wienkers L.C. In Vitro Metabolism of Clindamycin in Human Liver and Intestinal Microsomes. Drug Metab. Dispos. Biol. Fate Chem. 2003;31:878–887. doi: 10.1124/dmd.31.7.878[↩]
- Albrecht J., Barbaric J., Nast A. Rifampicin Alone May Be Enough: Is It Time to Abandon the Classic Oral Clindamycin–Rifampicin Combination for Hidradenitis Suppurativa? Br. J. Dermatol. 2019;180:949–950. doi: 10.1111/bjd.17422[↩]
- Join-Lambert O., Coignard H., Jais J.-P., Guet-Revillet H., Poirée S., Fraitag S., Jullien V., Ribadeau-Dumas F., Thèze J., Le Guern A.-S., et al. Efficacy of Rifampin-Moxifloxacin-Metronidazole Combination Therapy in Hidradenitis Suppurativa. Dermatol. Basel Switz. 2011;222:49–58. doi: 10.1159/000321716[↩]
- Chahine A.A., Nahhas A.F., Braunberger T.L., Rambhatla P.V., Hamzavi I.H. Ertapenem Rescue Therapy in Hidradenitis Suppurativa. JAAD Case Rep. 2018;4:482–483. doi: 10.1016/j.jdcr.2017.12.010[↩][↩]
- Join-Lambert O., Coignard-Biehler H., Jais J.-P., Delage M., Guet-Revillet H., Poirée S., Duchatelet S., Jullien V., Hovnanian A., Lortholary O., et al. Efficacy of Ertapenem in Severe Hidradenitis Suppurativa: A Pilot Study in a Cohort of 30 Consecutive Patients. J. Antimicrob. Chemother. 2016;71:513–520. doi: 10.1093/jac/dkv361[↩]
- Braunberger T.L., Nartker N.T., Nicholson C.L., Nahhas A.F., Parks-Miller A., Hanna Z., Jayaprakash R., Ramesh M.S., Rambhatla P.V., Hamzavi I.H. Ertapenem—A Potent Treatment for Clinical and Quality of Life Improvement in Patients with Hidradenitis Suppurativa. Int. J. Dermatol. 2018;57:1088–1093. doi: 10.1111/ijd.14036[↩]
- Ghanian S., Yamanaka-Takaichi M., Naik H.B., Alavi A. Medical Management of Hidradenitis Suppurativa with Non-Biologic Therapy: What’s New? Am. J. Clin. Dermatol. 2022;23:167–176. doi: 10.1007/s40257-021-00667-8[↩][↩]
- Collier E.K., Price K.N., Grogan T.R., Naik H.B., Shi V.Y., Hsiao J.L. Characterizing Perimenstrual Flares of Hidradenitis Suppurativa. Int. J. Womens Dermatol. 2020;6:372–376. doi: 10.1016/j.ijwd.2020.09.002[↩]
- Garg A., Neuren E., Strunk A. Hidradenitis Suppurativa Is Associated with Polycystic Ovary Syndrome: A Population-Based Analysis in the United States. J. Investig. Dermatol. 2018;138:1288–1292. doi: 10.1016/j.jid.2018.01.009[↩]
- Shalom G., Freud T., Harman-Boehm I., Polishchuk I., Cohen A.D. Hidradenitis Suppurativa and Metabolic Syndrome: A Comparative Cross-Sectional Study of 3207 Patients. Br. J. Dermatol. 2015;173:464–470. doi: 10.1111/bjd.13777[↩][↩]
- Clark A.K., Quinonez R.L., Saric S., Sivamani R.K. Hormonal Therapies for Hidradenitis Suppurativa: Review. Dermatol. Online J. 2017;23:13030. doi: 10.5070/D32310036990[↩]
- Sawers R.S., Randall V.A., Ebling F.J. Control of Hidradenitis Suppurativa in Women Using Combined Antiandrogen (Cyproterone Acetate) and Oestrogen Therapy. Br. J. Dermatol. 1986;115:269–274. doi: 10.1111/j.1365-2133.1986.tb05741.x[↩]
- Mortimer P.S., Dawber R.P., Gales M.A., Moore R.A. A Double-Blind Controlled Cross-over Trial of Cyproterone Acetate in Females with Hidradenitis Suppurativa. Br. J. Dermatol. 1986;115:263–268. doi: 10.1111/j.1365-2133.1986.tb05740.x[↩]
- Kraft J.N., Searles G.E. Hidradenitis Suppurativa in 64 Female Patients: Retrospective Study Comparing Oral Antibiotics and Antiandrogen Therapy. J. Cutan. Med. Surg. 2007;11:125–131. doi: 10.2310/7750.2007.00019[↩][↩]
- Corvol P., Michaud A., Menard J., Freifeld M., Mahoudeau J. Antiandrogenic Effect of Spirolactones: Mechanism of Action. Endocrinology. 1975;97:52–58. doi: 10.1210/endo-97-1-52[↩]
- Golbari N.M., Porter M.L., Kimball A.B. Antiandrogen Therapy with Spironolactone for the Treatment of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2019;80:114–119. doi: 10.1016/j.jaad.2018.06.063[↩]
- Lee A., Fischer G. A Case Series of 20 Women with Hidradenitis Suppurativa Treated with Spironolactone. Australas. J. Dermatol. 2015;56:192–196. doi: 10.1111/ajd.12362[↩]
- Quinlan C., Kirby B., Hughes R. Spironolactone Therapy for Hidradenitis Suppurativa. Clin. Exp. Dermatol. 2020;45:464–465. doi: 10.1111/ced.14119[↩]
- Jennings L., Hambly R., Hughes R., Moriarty B., Kirby B. Metformin Use in Hidradenitis Suppurativa. J. Dermatol. Treat. 2020;31:261–263. doi: 10.1080/09546634.2019.1592100[↩]
- Moussa C., Wadowski L., Price H., Mirea L., O’Haver J. Metformin as Adjunctive Therapy for Pediatric Patients With Hidradenitis Suppurativa. J. Drugs Dermatol. JDD. 2020;19:1231–1234. doi: 10.36849/JDD.2020.5447[↩]
- Verdolini R., Clayton N., Smith A., Alwash N., Mannello B. Metformin for the Treatment of Hidradenitis Suppurativa: A Little Help along the Way. J. Eur. Acad. Dermatol. Venereol. JEADV. 2013;27:1101–1108. doi: 10.1111/j.1468-3083.2012.04668.x[↩]
- Randhawa HK, Hamilton J, Pope E. Finasteride for the Treatment of Hidradenitis Suppurativa in Children and Adolescents. JAMA Dermatol. 2013;149(6):732–735. doi:10.1001/jamadermatol.2013.2874[↩]
- Mota, F., Machado, S. and Selores, M. (2017), Hidradenitis Suppurativa in Children Treated with Finasteride—A Case Series. Pediatr Dermatol, 34: 578-583. https://doi.org/10.1111/pde.13216[↩]
- Riis PT, Boer J, Prens EP, Saunte DM, Deckers IE, Emtestam L, Sartorius K, Jemec GB. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): A case series. J Am Acad Dermatol. 2016 Dec;75(6):1151-1155. doi: 10.1016/j.jaad.2016.06.049[↩]
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016 Nov;75(5):1059-1062. doi: 10.1016/j.jaad.2016.06.001[↩]
- Bianchi L, Hansel K, Stingeni L. Recalcitrant severe hidradenitis suppurativa successfully treated with cyclosporine A. J Am Acad Dermatol. 2012 Dec;67(6):e278-9. doi: 10.1016/j.jaad.2012.06.011[↩]
- Markota Čagalj A., Marinović B., Bukvić Mokos Z. New and Emerging Targeted Therapies for Hidradenitis Suppurativa. Int. J. Mol. Sci. 2022;23:3753. doi: 10.3390/ijms23073753[↩]
- Lu J.-W., Huang Y.-W., Chen T.-L. Efficacy and Safety of Adalimumab in Hidradenitis Suppurativa. Medicine. 2021;100:e26190. doi: 10.1097/MD.0000000000026190[↩]
- Alballa N., Alyousef A., Alamari A., Alhumidi A.A., Zayed M.A., Zeitouni L., Alsaif F.M. Hodgkin’s Lymphoma in a Patient on Adalimumab Treatment for Psoriasis. AME Case Rep. 2018;2:49. doi: 10.21037/acr.2018.12.01[↩]
- Wong A.K., Kerkoutian S., Said J., Rashidi H., Pullarkat S.T. Risk of Lymphoma in Patients Receiving Antitumor Necrosis Factor Therapy: A Meta-Analysis of Published Randomized Controlled Studies. Clin. Rheumatol. 2012;31:631–636. doi: 10.1007/s10067-011-1895-y[↩]
- Gottlieb A, Menter A, Armstrong A, Ocampo C, Gu Y, Teixeira HD. Adalimumab Treatment in Women With Moderate-to-Severe Hidradenitis Suppurativa from the Placebo-Controlled Portion of a Phase 2, Randomized, Double-Blind Study. J Drugs Dermatol. 2016 Oct 1;15(10):1192-1196. https://jddonline.com/articles/adalimumab-treatment-in-women-with-moderate-to-severe-hidradenitis-suppurativa-from-the-placebo-cont-S1545961616P1192X[↩]
- Coghlan J., He H., Schwendeman A.S. Overview of Humira® Biosimilars: Current European Landscape and Future Implications. J. Pharm. Sci. 2021;110:1572–1582. doi: 10.1016/j.xphs.2021.02.003[↩]
- Shih T., Lee K., Grogan T., De D.R., Shi V.Y., Hsiao J.L. Infliximab in Hidradenitis Suppurativa: A Systematic Review and Meta-Analysis. Dermatol. Ther. 2022;35:e15691. doi: 10.1111/dth.15691[↩][↩]
- Grant A., Gonzalez T., Montgomery M.O., Cardenas V., Kerdel F.A. Infliximab Therapy for Patients with Moderate to Severe Hidradenitis Suppurativa: A Randomized, Double-Blind, Placebo-Controlled Crossover Trial. J. Am. Acad. Dermatol. 2010;62:205–217. doi: 10.1016/j.jaad.2009.06.050[↩]
- Mekkes J.R., Bos J.D. Long-Term Efficacy of a Single Course of Infliximab in Hidradenitis Suppurativa. Br. J. Dermatol. 2008;158:370–374. doi: 10.1111/j.1365-2133.2007.08332.x[↩]
- Jarvis B., Faulds D. Etanercept. Drugs. 1999;57:945–966. doi: 10.2165/00003495-199957060-00014[↩]
- Adams D.R., Yankura J.A., Fogelberg A.C., Anderson B.E. Treatment of Hidradenitis Suppurativa With Etanercept Injection. Arch. Dermatol. 2010;146:501–504. doi: 10.1001/archdermatol.2010.72[↩]
- Blok, J.L., van Hattem, S., Jonkman, M.F. and Horváth, B. (2013), Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. British Journal of Dermatology, 168: 243-252. https://doi.org/10.1111/bjd.12104[↩]
- Esme P., Akoglu G., Caliskan E. Rapid Response to Certolizumab Pegol in Hidradenitis Suppurativa: A Case Report. Skin Appendage Disord. 2021;7:58–61. doi: 10.1159/000511284[↩]
- Melendez-Gonzalez M.D.M., Hamad J., Sayed C. Golimumab for the Treatment of Hidradenitis Suppurativa in Patients with Previous TNF-α Treatment Failure. J. Investig. Dermatol. 2021;141:2975–2979. doi: 10.1016/j.jid.2021.04.026[↩]
- Holm J.G., Jørgensen A.-H.R., Yao Y., Thomsen S.F. Certolizumab Pegol for Hidradenitis Suppurativa: Case Report and Literature Review. Dermatol. Ther. 2020;33:e14494. doi: 10.1111/dth.14494[↩]
- B. Moriarty, Z. Jiyad, D. Creamer, Four‐weekly infliximab in the treatment of severe hidradenitis suppurativa, British Journal of Dermatology, Volume 170, Issue 4, 1 April 2014, Pages 986–987, https://doi.org/10.1111/bjd.12713[↩]
- Houriet C, Seyed Jafari SM, Thomi R, et al. Canakinumab for Severe Hidradenitis Suppurativa: Preliminary Experience in 2 Cases. JAMA Dermatol. 2017;153(11):1195–1197. doi:10.1001/jamadermatol.2017.2392[↩]
- Fletcher J.M., Moran B., Petrasca A., Smith C.M. IL-17 in Inflammatory Skin Diseases Psoriasis and Hidradenitis Suppurativa. Clin. Exp. Immunol. 2020;201:121–134. doi: 10.1111/cei.13449[↩]
- Yao Y., Thomsen S.F. The Role of Interleukin-17 in the Pathogenesis of Hidradenitis Suppurativa. Dermatol. Online J. 2017;23:1. doi: 10.5070/D3237035729[↩]
- Mills K.H.G. IL-17 and IL-17-Producing Cells in Protection versus Pathology. Nat. Rev. Immunol. 2022:1–17. doi: 10.1038/s41577-022-00746-9[↩]
- Schlapbach C., Hänni T., Yawalkar N., Hunger R.E. Expression of the IL-23/Th17 Pathway in Lesions of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2011;65:790–798. doi: 10.1016/j.jaad.2010.07.010[↩]
- Kelly G., Hughes R., McGarry T., van den Born M., Adamzik K., Fitzgerald R., Lawlor C., Tobin A.M., Sweeney C.M., Kirby B. Dysregulated Cytokine Expression in Lesional and Nonlesional Skin in Hidradenitis Suppurativa. Br. J. Dermatol. 2015;173:1431–1439. doi: 10.1111/bjd.14075[↩]
- Navrazhina K., Frew J., Krueger J. Research Letter: IL-17C Is Elevated in Lesional Tissue of Hidradenitis Suppurativa. Br. J. Dermatol. 2020;182:1045–1047. doi: 10.1111/bjd.18556[↩]
- Fauny M., Moulin D., D’Amico F., Netter P., Petitpain N., Arnone D., Jouzeau J.-Y., Loeuille D., Peyrin-Biroulet L. Paradoxical Gastrointestinal Effects of Interleukin-17 Blockers. Ann. Rheum. Dis. 2020;79:1132–1138. doi: 10.1136/annrheumdis-2020-217927[↩]
- Frieder J., Kivelevitch D., Menter A. Secukinumab: A Review of the Anti-IL-17A Biologic for the Treatment of Psoriasis. Ther. Adv. Chronic Dis. 2018;9:5–21. doi: 10.1177/2040622317738910[↩]
- Prussick L., Rothstein B., Joshipura D., Saraiya A., Turkowski Y., Abdat R., Alomran A., Zancanaro P., Kachuk C., Dumont N., et al. Open-Label, Investigator-Initiated, Single-Site Exploratory Trial Evaluating Secukinumab, an Anti-Interleukin-17A Monoclonal Antibody, for Patients with Moderate-to-Severe Hidradenitis Suppurativa. Br. J. Dermatol. 2019;181:609–611. doi: 10.1111/bjd.17822[↩]
- Casseres R.G., Prussick L., Zancanaro P., Rothstein B., Joshipura D., Saraiya A., Turkowski Y., Au S.C., Alomran A., Abdat R., et al. Secukinumab in the Treatment of Moderate to Severe Hidradenitis Suppurativa: Results of an Open-Label Trial. J. Am. Acad. Dermatol. 2020;82:1524–1526. doi: 10.1016/j.jaad.2020.02.005[↩]
- Kimball A.B. Secukinumab in Moderate-to-Severe Hidradenitis Suppurativa: Primary Endpoint Analysis from the SUNSHINE and SUNRISE Phase III Trials; Proceedings of the 31st European Academy of Dermatology and Venereology (EADV) Congress; Milan, Italy. 7–10 September 2022. https://www.emjreviews.com/dermatology/abstract/secukinumab-in-moderate-to-severe-hidradenitis-suppurativa-primary-endpoint-analysis-from-the-sunshine-and-sunrise-phase-iii-trials-j030122[↩][↩]
- EMJ Dermatol. 2022;10[1]:40-43. DOI/10.33590/emjdermatol/10182311. https://doi.org/10.33590/emjdermatol/10182311[↩]
- A Study to Test the Efficacy, Safety and Pharmacokinetics of Bimekizumab in Subjects With Moderate to Severe Hidradenitis Suppurativa. https://clinicaltrials.gov/ct2/show/NCT03248531[↩]
- A Study to Test the Long-term Treatment of Bimekizumab in Study Participants With Moderate to Severe Hidradenitis Suppurativa (BE HEARD EXT). https://clinicaltrials.gov/ct2/show/NCT04901195[↩]
- A Study to Evaluate the Efficacy and Safety of Bimekizumab in Study Participants With Moderate to Severe Hidradenitis Suppurativa (BE HEARD II). https://clinicaltrials.gov/ct2/show/NCT04242498[↩]
- A Study to Evaluate the Efficacy and Safety of Bimekizumab in Study Participants With Moderate to Severe Hidradenitis Suppurativa (BE HEARD I). https://clinicaltrials.gov/ct2/show/NCT04242446[↩]
- MoonLake. https://moonlaketx.com/science[↩]
- Svecova D., Lubell M.W., Casset-Semanaz F., Mackenzie H., Grenningloh R., Krueger J.G. A Randomized, Double-Blind, Placebo-Controlled Phase 1 Study of Multiple Ascending Doses of Subcutaneous M1095, an Anti–Interleukin 17A/F Nanobody, in Moderate-to-Severe Psoriasis. J. Am. Acad. Dermatol. 2019;81:196–203. doi: 10.1016/j.jaad.2019.03.056[↩]
- Papp K.A., Weinberg M.A., Morris A., Reich K. IL17A/F Nanobody Sonelokimab in Patients with Plaque Psoriasis: A Multicentre, Randomised, Placebo-Controlled, Phase 2b Study. Lancet. 2021;397:1564–1575. doi: 10.1016/S0140-6736(21)00440-2[↩]
- Evaluation of Sonelokimab for the Treatment of Patients With Active Moderate to Severe Hidradenitis Suppurativa. https://clinicaltrials.gov/ct2/show/NCT05322473[↩]
- Houriet C, Seyed Jafari SM, Thomi R, et al. Canakinumab for Severe Hidradenitis Suppurativa: Preliminary Experience in 2 Cases. JAMA Dermatol. 2017;153(11):1195–1197. doi:10.10 (ClinicalTrials.gov NCT05355805) ((Behrens F., Taylor P.C., Wetzel D., Brun N.C., Brandt-Juergens J., Drescher E., Dokoupilova E., Rowińska-Osuch A., Martin N.A.-K., Vlam K. de Op0258 Izokibep (Aby-035) in Patients with Active Psoriatic Arthritis—16-Week Results from a Phase 2 Study. Ann. Rheum. Dis. 2022;81:170–171. doi: 10.1136/annrheumdis-2022-eular.536[↩]
- Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1[↩]
- Zouboulis C.C., Frew J.W., Giamarellos-Bourboulis E.J., Jemec G.B.E., Marmol V., Marzano A.V., Nikolakis G., Sayed C.J., Tzellos T., Wolk K., et al. Target Molecules for Future Hidradenitis Suppurativa Treatment. Exp. Dermatol. 2021;30:8–17. doi: 10.1111/exd.14338[↩]
- Alavi A., Hamzavi I., Brown K., Santos L.L., Zhu Z., Liu H., Howell M.D., Kirby J.S. Janus Kinase 1 Inhibitor INCB054707 for Patients with Moderate-to-Severe Hidradenitis Suppurativa: Results from Two Phase II Studies. Br. J. Dermatol. 2022;186:803–813. doi: 10.1111/bjd.20969[↩][↩]
- Kozera E., Flora A., Frew J.W. Real-World Safety and Clinical Response of Janus Kinase Inhibitor Upadacitinib in the Treatment of Hidradenitis Suppurativa: A Retrospective Cohort Study. J. Am. Acad. Dermatol. 2022;87:1440–1442. doi: 10.1016/j.jaad.2022.07.047[↩]
- Topical Ruxolitinib 1.5% for Hidradenitis Suppurativa Treatment. https://clinicaltrials.gov/ct2/show/NCT04414514[↩]
- Verdolini, R., Clayton, N., Smith, A., Alwash, N. and Mannello, B. (2013), Metformin for the treatment of hidradenitis suppurativa: a little help along the way. Journal of the European Academy of Dermatology and Venereology, 27: 1101-1108. https://doi.org/10.1111/j.1468-3083.2012.04668.x[↩]
- Jennings, L., Nestor, L., Molloy, O., Hughes, R., Moriarty, B. and Kirby, B. (2017), The treatment of hidradenitis suppurativa with the glucagon-like peptide-1 agonist liraglutide. Br J Dermatol, 177: 858-859. https://doi.org/10.1111/bjd.15233[↩]
- Matusiak L., Bieniek A., Szepietowski J.C. Acitretin Treatment for Hidradenitis Suppurativa: A Prospective Series of 17 Patients. Br. J. Dermatol. 2014;171:170–174. doi: 10.1111/bjd.12884[↩]
- Sánchez-Díaz M., Díaz-Calvillo P., Rodríguez-Pozo J.Á., Arias-Santiago S., Molina-Leyva A. Effectiveness and Safety of Acitretin for the Treatment of Hidradenitis Suppurativa, Predictors of Clinical Response: A Cohort Study. Dermatology. 2022:1–8. doi: 10.1159/000526019[↩]
- Boer J., van Gemert M.J. Long-Term Results of Isotretinoin in the Treatment of 68 Patients with Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 1999;40:73–76. doi: 10.1016/S0190-9622(99)70530-X[↩]
- Patel N., McKenzie S.A., Harview C.L., Truong A.K., Shi V.Y., Chen L., Grogan T.R., Bennett R.G., Hsiao J.L. Isotretinoin in the Treatment of Hidradenitis Suppurativa: A Retrospective Study. J. Dermatol. Treat. 2021;32:473–475. doi: 10.1080/09546634.2019.1670779[↩]
- Soria A., Canoui-Poitrine F., Wolkenstein P., Poli F., Gabison G., Pouget F., Viallette C., Revuz J. Absence of Efficacy of Oral Isotretinoin in Hidradenitis Suppurativa: A Retrospective Study Based on Patients’ Outcome Assessment. Dermatology. 2009;218:134–135. doi: 10.1159/000182261[↩]
- Gallagher C.G., Kirthi S.K., Cotter C.C., Revuz J.R., Tobin A.M.T. Could Isotretinoin Flare Hidradenitis Suppurativa? A Case Series. Clin. Exp. Dermatol. 2019;44:777–780. doi: 10.1111/ced.13944[↩]
- Kamp S., Fiehn A.M., Stenderup K., Rosada C., Pakkenberg B., Kemp K., Dam T.N., Jemec G.B. Hidradenitis Suppurativa: A Disease of the Absent Sebaceous Gland? Sebaceous Gland Number and Volume Are Significantly Reduced in Uninvolved Hair Follicles from Patients with Hidradenitis Suppurativa. Br. J. Dermatol. 2011;164:1017–1022. doi: 10.1111/j.1365-2133.2011.10224.x[↩]
- Armyra, K., Kouris, A., Markantoni, V., Katsambas, A. and Kontochristopoulos, G. (2017), Hidradenitis suppurativa treated with tetracycline in combination with colchicine: a prospective series of 20 patients. Int J Dermatol, 56: 346-350. https://doi.org/10.1111/ijd.13428[↩]
- Brocard A., Dréno B. Innate Immunity: A Crucial Target for Zinc in the Treatment of Inflammatory Dermatosis. J. Eur. Acad. Dermatol. Venereol. JEADV. 2011;25:1146–1152. doi: 10.1111/j.1468-3083.2010.03934.x[↩]
- Brocard A., Knol A.-C., Khammari A., Dréno B. Hidradenitis Suppurativa and Zinc: A New Therapeutic Approach. A Pilot Study. Dermatol. Basel Switz. 2007;214:325–327. doi: 10.1159/000100883[↩]
- Hessam S., Sand M., Meier N.M., Gambichler T., Scholl L., Bechara F.G. Combination of Oral Zinc Gluconate and Topical Triclosan: An Anti-Inflammatory Treatment Modality for Initial Hidradenitis Suppurativa. J. Dermatol. Sci. 2016;84:197–202. doi: 10.1016/j.jdermsci.2016.08.010[↩]
- Molinelli E., Brisigotti V., Campanati A., Sapigni C., Giacchetti A., Cota C., Offidani A. Efficacy of Oral Zinc and Nicotinamide as Maintenance Therapy for Mild/Moderate Hidradenitis Suppurativa: A Controlled Retrospective Clinical Study. J. Am. Acad. Dermatol. 2020;83:665–667. doi: 10.1016/j.jaad.2020.04.092[↩]
- Wahab A., Mushtaq K., Borak S.G., Bellam N. Zinc-Induced Copper Deficiency, Sideroblastic Anemia, and Neutropenia: A Perplexing Facet of Zinc Excess. Clin. Case Rep. 2020;8:1666–1671. doi: 10.1002/ccr3.2987[↩]
- Leslie KS, Tripathi SV, Nguyen TV, Pauli M, Rosenblum MD. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol. 2014 Feb;70(2):243-51. doi: 10.1016/j.jaad.2013.09.044[↩]
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA Dermatol. 2016;152(1):52–59. doi:10.1001/jamadermatol.2015.3903[↩]
- van der Zee H.H., Prens E.P. Failure of Anti-Interleukin-1 Therapy in Severe Hidradenitis Suppurativa: A Case Report. Dermatol. Basel Switz. 2013;226:97–100. doi: 10.1159/000343221[↩]
- André R., Marescassier H., Gabay C., Pittet B., Laffitte E. Long-Term Therapy with Anakinra in Hidradenitis Suppurativa in Three Patients. Int. J. Dermatol. 2019;58:e208–e209. doi: 10.1111/ijd.14596[↩]
- Menis D., Maroñas-Jiménez L., Delgado-Marquez A.M., Postigo-Llorente C., Vanaclocha-Sebastián F. Two Cases of Severe Hidradenitis Suppurativa with Failure of Anakinra Therapy. Br. J. Dermatol. 2015;172:810–811. doi: 10.1111/bjd.13292[↩]
- Zarchi K., Dufour D.N., Jemec G.B.E. Successful Treatment of Severe Hidradenitis Suppurativa With Anakinra. JAMA Dermatol. 2013;149:1192–1194. doi: 10.1001/jamadermatol.2013.5377[↩]
- Russo V, Alikhan A. Failure of Anakinra in a Case of Severe Hidradenitis Suppurativa. J Drugs Dermatol. 2016 Jun 1;15(6):772-4. https://jddonline.com/articles/failure-of-anakinra-in-a-case-of-severe-hidradenitis-suppurativa-S1545961616P0772X/[↩]
- Kanni T., Argyropoulou M., Spyridopoulos T., Pistiki A., Stecher M., Dinarello C.A., Simard J., Giamarellos-Bourboulis E.J. MABp1 Targeting IL-1α for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Randomized Study. J. Investig. Dermatol. 2018;138:795–801. doi: 10.1016/j.jid.2017.10.030[↩]
- Kanni T., Argyropoulou M., Dinarello C.A., Simard J., Giamarellos-Bourboulis E.J. MABp1 Targeting Interleukin-1α in Hidradenitis Suppurativa Ineligible for Adalimumab Treatment: Results of the Open-Label Extension Period. Clin. Exp. Dermatol. 2021;46:162–163. doi: 10.1111/ced.14333[↩]
- Gottlieb A., Natsis N.E., Kerdel F., Forman S., Gonzalez E., Jimenez G., Hernandez L., Kaffenberger J., Guido G., Lucas K., et al. A Phase II Open-Label Study of Bermekimab in Patients with Hidradenitis Suppurativa Shows Resolution of Inflammatory Lesions and Pain. J. Investig. Dermatol. 2020;140:1538–1545.e2. doi: 10.1016/j.jid.2019.10.024[↩]
- A Study of Bermekimab for the Treatment of Participants With Moderate to Severe Hidradenitis Suppurativa (LYRA). https://clinicaltrials.gov/ct2/show/NCT04988308[↩]
- Levoska MA, Nicholson CL, Hamzavi IH. A retrospective review of light- and laser-based management of hidradenitis suppurativa. Semin Cutan Med Surg. 2017 Jun;36(2):67-74. doi: 10.12788/j.sder.2017.023[↩]
- Kohorst JJ, Baum CL, Otley CC, Roenigk RK, Schenck LA, Pemberton JH, Dozois EJ, Tran NV, Senchenkov A, Davis MD. Surgical Management of Hidradenitis Suppurativa: Outcomes of 590 Consecutive Patients. Dermatol Surg. 2016 Sep;42(9):1030-40. doi: 10.1097/DSS.0000000000000806[↩]
- Ritz JP, Runkel N, Haier J, Buhr HJ. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13(4):164-8. doi: 10.1007/s003840050159[↩]
- Kohorst JJ, Baum CL, Otley CC, Roenigk RK, Pemberton JH, Dozois EJ, Tran NV, Davis MD. Patient Satisfaction and Quality of Life Following Surgery for Hidradenitis Suppurativa. Dermatol Surg. 2017 Jan;43(1):125-133. doi: 10.1097/DSS.0000000000000942[↩]
- Kohorst J.J., Baum C.L., Otley C.C., Roenigk R.K., Schenck L.A., Pemberton J.H., Dozois E.J., Tran N.V., Senchenkov A., Davis M.D.P. Surgical Management of Hidradenitis Suppurativa: Outcomes of 590 Consecutive Patients. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2016;42:1030–1040. doi: 10.1097/DSS.0000000000000806[↩]
- Dahmen R.A., Gkalpakiotis S., Mardesicova L., Arenberger P., Arenbergerova M. Deroofing Followed by Thorough Sinus Tract Excision: A Modified Surgical Approach for Hidradenitis Suppurativa. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG. 2019;17:698–702. doi: 10.1111/ddg.13875[↩]
- Shukla R., Karagaiah P., Patil A., Farnbach K., Ortega-Loayza A.G., Tzellos T., Szepietowski J.C., Giulini M., Schepler H., Grabbe S., et al. Surgical Treatment in Hidradenitis Suppurativa. J. Clin. Med. 2022;11:2311. doi: 10.3390/jcm11092311[↩][↩]
- van der Zee H.H., Prens E.P., Boer J. Deroofing: A Tissue-Saving Surgical Technique for the Treatment of Mild to Moderate Hidradenitis Suppurativa Lesions. J. Am. Acad. Dermatol. 2010;63:475–480. doi: 10.1016/j.jaad.2009.12.018[↩]
- Blok J.L., Spoo J.R., Leeman F.W.J., Jonkman M.F., Horváth B. Skin-Tissue-Sparing Excision with Electrosurgical Peeling (STEEP): A Surgical Treatment Option for Severe Hidradenitis Suppurativa Hurley Stage II/III. J. Eur. Acad. Dermatol. Venereol. JEADV. 2015;29:379–382. doi: 10.1111/jdv.12376[↩]
- Janse I.C., Hellinga J., Blok J.L., van den Heuvel E.R., Spoo J.R., Jonkman M.F., Terra J.B., Horváth B. Skin-Tissue-Sparing Excision with Electrosurgical Peeling: A Case Series in Hidradenitis Suppurativa. Acta Derm. Venereol. 2016;96:390–391. doi: 10.2340/00015555-2258[↩]
- van Rappard D.C., Mooij J.E., Mekkes J.R. Mild to Moderate Hidradenitis Suppurativa Treated with Local Excision and Primary Closure. J. Eur. Acad. Dermatol. Venereol. 2012;26:898–902. doi: 10.1111/j.1468-3083.2011.04203.x[↩][↩]
- Deckers I.E., Dahi Y., van der Zee H.H., Prens E.P. Hidradenitis Suppurativa Treated with Wide Excision and Second Intention Healing: A Meaningful Local Cure Rate after 253 Procedures. J. Eur. Acad. Dermatol. Venereol. 2018;32:459–462. doi: 10.1111/jdv.14770[↩]
- Nesmith R.B., Merkel K.L., Mast B.A. Radical Surgical Resection Combined With Lymphadenectomy-Directed Antimicrobial Therapy Yielding Cure of Severe Axillary Hidradenitis. Ann. Plast. Surg. 2013;70:538–541. doi: 10.1097/SAP.0b013e31828ea7cb[↩]
- Ritz J.-P., Runkel N., Haier J., Buhr H.J. Extent of Surgery and Recurrence Rate of Hidradenitis Suppurativa. Int. J. Colorectal Dis. 1998;13:164–168. doi: 10.1007/s003840050159[↩]
- Elkin K., Daveluy S., Avanaki K. Review of Imaging Technologies Used in Hidradenitis Suppurativa. Skin Res. Technol. 2020;26:3–10. doi: 10.1111/srt.12772[↩]
- Hamzavi I.H., Griffith J.L., Riyaz F., Hessam S., Bechara F.G. Laser and Light-Based Treatment Options for Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2015;73:S78–S81. doi: 10.1016/j.jaad.2015.07.050[↩][↩]
- Dalrymple J.C., Monaghan J.M. Treatment of Hidradenitis Suppurativa with the Carbon Dioxide Laser. Br. J. Surg. 1987;74:420. doi: 10.1002/bjs.1800740535[↩]
- Lapins J., Marcusson J.A., Emtestam L. Surgical Treatment of Chronic Hidradenitis Suppurativa: CO2 Laser Stripping-Secondary Intention Technique. Br. J. Dermatol. 1994;131:551–556. doi: 10.1111/j.1365-2133.1994.tb08559.x[↩]
- Lapins J., Sartorius K., Emtestam L. Scanner-Assisted Carbon Dioxide Laser Surgery: A Retrospective Follow-up Study of Patients with Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2002;47:280–285. doi: 10.1067/mjd.2002.124601[↩]
- Mikkelsen P.R., Dufour D.N., Zarchi K., Jemec G.B.E. Recurrence Rate and Patient Satisfaction of CO2 Laser Evaporation of Lesions in Patients with Hidradenitis Suppurativa: A Retrospective Study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2015;41:255–260. doi: 10.1097/DSS.0000000000000264[↩]
- Hazen P.G., Hazen B.P. Hidradenitis Suppurativa: Successful Treatment Using Carbon Dioxide Laser Excision and Marsupialization. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2010;36:208–213. doi: 10.1111/j.1524-4725.2009.01427.x[↩]
- Jfri A., Saxena A., Rouette J., Netchiporouk E., Barolet A., O’Brien E., Barolet D., Litvinov I.V. The Efficacy and Effectiveness of Non-Ablative Light-Based Devices in Hidradenitis Suppurativa: A Systematic Review and Meta-Analysis. Front. Med. 2020;7:591580. doi: 10.3389/fmed.2020.591580[↩]
- Mahmoud B.H., Tierney E., Hexsel C.L., Pui J., Ozog D.M., Hamzavi I.H. Prospective Controlled Clinical and Histopathologic Study of Hidradenitis Suppurativa Treated with the Long-Pulsed Neodymium:Yttrium-Aluminium-Garnet Laser. J. Am. Acad. Dermatol. 2010;62:637–645. doi: 10.1016/j.jaad.2009.07.048[↩]
- Tierney E., Mahmoud B.H., Hexsel C., Ozog D., Hamzavi I. Randomized Control Trial for the Treatment of Hidradenitis Suppurativa with a Neodymium-Doped Yttrium Aluminium Garnet Laser. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2009;35:1188–1198. doi: 10.1111/j.1524-4725.2009.01214.x[↩][↩]
- Xu L.Y., Wright D.R., Mahmoud B.H., Ozog D.M., Mehregan D.A., Hamzavi I.H. Histopathologic Study of Hidradenitis Suppurativa Following Long-Pulsed 1064-Nm Nd:YAG Laser Treatment. Arch. Dermatol. 2011;147:21–28. doi: 10.1001/archdermatol.2010.245[↩]
- Abdel Azim A.A., Salem R.T., Abdelghani R. Combined Fractional Carbon Dioxide Laser and Long-Pulsed Neodymium: Yttrium-Aluminium-Garnet (1064 Nm) Laser in Treatment of Hidradenitis Suppurativa; a Prospective Randomized Intra-Individual Controlled Study. Int. J. Dermatol. 2018;57:1135–1144. doi: 10.1111/ijd.14075[↩]
- Danby FW, Hazen PG, Boer J: New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S62–5. 10.1016/j.jaad.2015.07.043[↩][↩]
- Jemec GB: Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158–64. 10.1056/NEJMcp1014163[↩]
- Danby FW: Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63(3):481.e1–3. 10.1016/j.jaad.2010.01.033[↩]
- Blok JL, Spoo JR, Leeman FW, et al. : Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J Eur Acad Dermatol Venereol. 2015;29(2):379–82. 10.1111/jdv.12376[↩]
- Mehdizadeh A, Hazen PG, Bechara FG, et al. : Recurrence of hidradenitis suppurativa after surgical management: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5 Suppl 1):S70–7. 10.1016/j.jaad.2015.07.044[↩]
- Humphries LS, Kueberuwa E, Beederman M, et al. : Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: A single-center experience. J Plast Reconstr Aesthet Surg. 2016;69(4):554–66. 10.1016/j.bjps.2015.12.004[↩]
- Xu LY, Wright DR, Mahmoud BH, et al. : Histopathologic study of hidradenitis suppurativa following long-pulsed 1064-nm Nd:YAG laser treatment. Arch Dermatol. 2011;147(1):21–8. 10.1001/archdermatol.2010.245[↩]
- Hazen PG, Hazen BP: Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36(2):208–13. 10.1111/j.1524-4725.2009.01427.x[↩]
- Mikkelsen PR, Dufour DN, Zarchi K, et al. : Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: a retrospective study. Dermatol Surg. 2015;41(2):255–60. 10.1097/DSS.0000000000000264[↩]
- Nicholson CL, Hamzavi I, Ozog DM: Rapid healing of chronic ulcerations and improvement in range of motion after fractional carbon dioxide (CO 2) treatment after CO 2 excision of hidradenitis suppurativa axillary lesions: A case report. JAAD Case Rep. 2016;2(1):4–6. 10.1016/j.jdcr.2015.11.001[↩]
- Esmann S., Jemec G.B.E. Psychosocial Impact of Hidradenitis Suppurativa: A Qualitative Study. Acta Derm. Venereol. 2011;91:328–332. doi: 10.2340/00015555-1082[↩]
- Matusiak Ł., Bieniek A., Szepietowski J.C. Hidradenitis Suppurativa Markedly Decreases Quality of Life and Professional Activity. J. Am. Acad. Dermatol. 2010;62:706–708.e1. doi: 10.1016/j.jaad.2009.09.021[↩]
- Wolkenstein P., Loundou A., Barrau K., Auquier P., Revuz J. Quality of Life Impairment in Hidradenitis Suppurativa: A Study of 61 Cases. J. Am. Acad. Dermatol. 2007;56:621–623. doi: 10.1016/j.jaad.2006.08.061[↩]
- Phan K., Huo Y.R., Smith S.D. Hidradenitis Suppurativa and Psychiatric Comorbidities, Suicides and Substance Abuse: Systematic Review and Meta-Analysis. Ann. Transl. Med. 2020;8:821. doi: 10.21037/atm-20-1028[↩][↩]
- Kromann, C.B., Deckers, I.E., Esmann, S., Boer, J., Prens, E.P. and Jemec, G.B.E. (2014), Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol, 171: 819-824. https://doi.org/10.1111/bjd.13090[↩]
- Yuan JT, Naik HB. Complications of hidradenitis suppurativa. Semin Cutan Med Surg. 2017 Jun;36(2):79-85. doi: 10.12788/j.sder.2017.022[↩][↩]