Human liver regeneration
The liver is a unique organ with an abundant regenerative capacity. Human liver is the only solid organ that uses regenerative mechanisms to ensure that the liver-to-bodyweight ratio is always at 100% of what is required for body homeostasis 1. Other solid organs such as your lungs, kidneys and pancreas adjust to tissue loss but do not return to 100% of normal. Even when almost two-thirds of the liver is surgically removed, the remaining liver can rapidly restore its original size. In rodents, the remaining liver after 70% hepatectomy or 30% partial liver graft returns to a size of 100% in 7–10 days after the operation, with a peak in DNA synthesis in the first 3 days. In humans, restoration of the liver mass is complete around 3 months following right or left hepatectomy, with a peak in DNA synthesis at days 7–10 after the operation 2. In living donor liver transplantation, Haga et al. 3 reported that the remaining left lobe of donors and left lobe graft of recipients regenerate more rapidly than the remaining right lobe of donors and right lobe graft of recipients. Moreover, liver regeneration rate is higher in the recipient’s left lobe graft than in the donor’s remaining left lobe 3. They also showed that the recipient liver volume increased rapidly in the first 2 months, exceeding the standard liver volume, and then gradually decreased to 90% of the standard liver volume 3. Mature hepatocytes (liver cells) are the source of new liver cells, not stem cells; however, stem cells play an important role in some cases of liver regeneration 4. Using genetic fate mapping and a high-throughput imaging system of individual hepatocytes, Miyaoka et al. 5 reported that the volume of hepatectomy affected the mode of liver regeneration; i.e., while the remnant liver following 30% partial hepatectomy regenerated solely by hypertrophy without cell division, hypertrophy and subsequent proliferation almost equally contributed to regeneration after 70% partial hepatectomy. Moreover, although most hepatocytes entered the cell cycle after 70% partial hepatectomy, only about half of the hepatocytes underwent cell division during liver regeneration, leading to an increase in hepatocyte ploidy 5. These results suggest that liver regeneration is a flexible and variable process that depends on the condition of the remaining liver, graft, and metabolic environment of the host. Partial hepatectomy also known as Minimally Invasive Liver Surgery, is surgery to remove a portion of the liver. Only people with good liver function who are healthy enough for surgery and who have a single liver tumor that has not grown into blood vessels can have this operation. Table 1 summarizes the main molecular factors in liver regeneration.
Human liver regenerative capacity is significantly impaired during chronic liver diseases due to the accumulation of senescent hepatocytes 6. In this case, liver mass restoration is performed by hepatic progenitor cells 7. Hepatic progenitor cells are located in the canals of Hering and have a bipotential nature; in other words, they can differentiate into both hepatocytes and biliary epithelial cells, the choice of which is determined by the activation of certain genes 8. The canals of Hering connect the hepatocyte canalicular system and the biliary tree, and such a location of hepatic progenitor cells is consistent with their bipotential features 9. Transplantation of current cells leads to liver regeneration enhancement via hepatic progenitor cell proliferation and differentiation, which can be applicable for the treatment of certain liver diseases 10. CK19, EpCAM and CD133 are markers common to both hepatic progenitor cells and biliary epithelial cells. Trop2 (Tacstd2) is a transmembrane molecule that is present on the hepatic progenitor cell surface and absent on biliary epithelial cells; therefore, it can play a role as a specific marker, similar to Foxl1 11.
The origin of hepatic progenitor cells is still being researched. Many scientists think that hepatic progenitor cells arise from mature differentiated biliary epithelial cells due to the presence of similar markers and cell localization. The expression of hepatocyte markers, such as albumin, AFP and HNF4α, appears earlier than hepatic progenitor cell expansion. Newly formed hepatic progenitor cells have various markers on their surface, including the biliary epithelial cell markers HNF1b and CK19, which are maintained until the hepatic progenitor cells differentiate into mature hepatocytes 12. Hepatocytes and biliary epithelial cells are formed from common cells, called hepatoblasts, during the second trimester of embryonic development. Consequently, the possibility of hepatocyte to biliary epithelial cell transdifferentiation and vice versa is genetically feasible and might be programmed to form a facultative pool of progenitors 13.
Hepatic progenitor cell compartment activation in the human liver is called ductular reaction because of the role of ductular epithelium activation. In the niche, hepatic progenitor cells are surrounded by epithelial and nonparenchymal cells, immune cells, and the components of the ECM, which transport activating signals 14. As long as hepatic progenitor cells drive the regeneration of massive or chronic damage facilitated by immune cells, inflammatory cytokines, such as TNF-α, lymphotoxin-β, interferon-γ and IL-6, will play a crucial role in hepatic progenitor cell activation. TNF-like weak inducer of apoptosis (TWEAK) is a TNF superfamily member and the main inducer of hepatic progenitor cell activation 15. Macrophages and NK cells are primary sources of TWEAK ligands. The interaction with target cells is realized by FGF-inducible 14 receptors. The TWEAK/ FGF-inducible 14 interaction leads to ductular reaction initiation via activation of the NF-κB signaling pathway 16. Hepatic progenitor cell regulation is also performed by free oxygen radicals, which act as second messengers, realizing the balance between self-renewal and the differentiation of current cells. Low reactive oxygen species levels promote hepatic progenitor cell proliferation via extracellular-signal-regulated kinase 1/2, Jun 1/2, Wnt and NF-κB signaling 17.
Hepatic progenitor cell differentiation into hepatocytes and biliary epithelial cells is regulated by a variety of signaling pathways. FGF9, the HGF-с-MET signaling pathway 18 and oncostatin M activate AKT and STAT3, which are required for hepatic progenitor cell differentiation into hepatocytes, whereas HNF-6, HNF-1β and NOTCH signaling lead to biliary epithelial cell development 19. All-trans retinoic acid is a significant active metabolite of vitamin A that is involved in hepatic progenitor cell differentiation by increasing miR-200a expression, which regulates cell autophagy 20. Ma et al 21 demonstrated the regulatory function of autophagy in hepatic progenitor cell differentiation into hepatocytes via activation of the Wnt/β-catenin signaling pathway. Autophagy can also regulate hepatic progenitor cell differentiation into biliary epithelial cells since it inhibits the Notch1 signaling pathway, which is required for the development of biliary duct cells. Therefore, autophagy is decreased during the early stages of liver regeneration 22.
Recently, a new pool of multipotential biliary progenitor cells, which can differentiate into hepatocytes, biliary epithelial cells and the islets of Langerhans cells, was identified in peribiliary glands, which are epithelial invaginations of extrahepatic and large intrahepatic biliary ducts 8. This pool was named biliary tree stem/progenitor cells (BTSCs). Biliary tree stem/progenitor cells express stem cell markers such as Sox17, Pdx1, Sox9, EpCAM, Sall4 and Lgr5 on their surface. Biliary tree stem/progenitor cells are primarily involved in biliary epithelium regeneration in chronic diseases such as primary sclerosing cholangitis, cholangiocarcinoma, nonanastomotic strictures and biliary atresia 23.
Table 1. Main factors driving liver regeneration
| Factor of regeneration | Influence on liver regeneration |
| TNF-α | Induction of CDK-1 |
| IL-6 | Activation of the JAK/STAT, MAPK, and PI3K/AKT signaling pathways |
| Hh signaling pathway | ECM remodulation; induction of progenitor cell and liver epithelial cell expansion; induction of glutaminolysis; inhibition of hepatocyte, biliary epithelial cell, Ito cell and progenitor cell apoptosis |
| ALR | lfALR: Enhancement of the hepatocyte response to IL-6 and STAT3 phosphorylation induction. MAPK signaling pathway activation; NK cells inhibition; increase in IL-6, TNFα and iNOS production by Kupffer cells, sfALR: Inhibition of proapoptotic stimuli |
| NRF2 | Regulation of M phase entry, hepatocyte proliferation, maintenance of newly formed hepatocytes in a differentiated state |
| Growth factors (HGF, TGF-α, EGF, HB-EGF) | Stimulation of DNA synthesis and cell proliferation via Ras-MAPK and PI3K/AKT signaling pathway activation |
| Bile acids | Activation of CDK2, cell cycle, regulation of termination phase and terminate liver size, decrease in the inflammatory cytokine production, enhancement of bile acid excretion and HCO3ˉ, Clˉ secretion, control of BA polarity |
| Wnt-β-catenin | Hepatocyte proliferation induction |
| Notch signaling pathway | Modulation of hepatic progenitor cells differentiation toward biliary epithelial cells, regulation of hepatocyte proliferation, mitotic rhythms, cyclin E1, A2 and B1 |
| IL-1 | DNA synthesis inhibitor |
| SOCSs | c-MET and JAK-STAT signaling pathway inhibition |
| TGF-β1, activin A, BMPs | Induction of apoptosis to correct excessive liver mass |
| HNF4 | Regulation of hepatocyte differentiation, initiation of the termination phase, antagonism YAP and TGF-β/SMAD3, prevention of excessive connective tissue synthesis, inhibition of hepatic progenitor cells proliferation and migration |
| Hippo/YAP signaling pathway | Terminal liver size control |
Abbreviations: TNF-α = Tumor necrosis factor-α; IL-6 = Interleukin-6; CDK-1 = Cyclin-dependent kinase 1; JAK/STAT = Janus kinase/signal transducer and activator of transcription; Hh = Hedgehog; ECM = Extracellular matrix; MAPK = Mitogen-activated protein kinase; ALR = Augmenter of liver regeneration; lfALR = Long-form ALR; sfALR = Short-form ALR; iNOS = Inducible nitric oxide synthase; NRF2 = Nuclear factor erythroid 2-related factor 2; BA = Bile acids; EGF = Epidermal growth factor; HGF = Hepatocyte growth factor; HPC = Hepatic progenitor cells; BEC = Biliary epithelial cells; MET = Methionine; BMP = Bone morphogenetic proteins; HNF4 = Hepatocyte nuclear factor 4 alpha; YAP = Yes-associated protein.
[Source 24 ]Figure 1. Location of the human liver
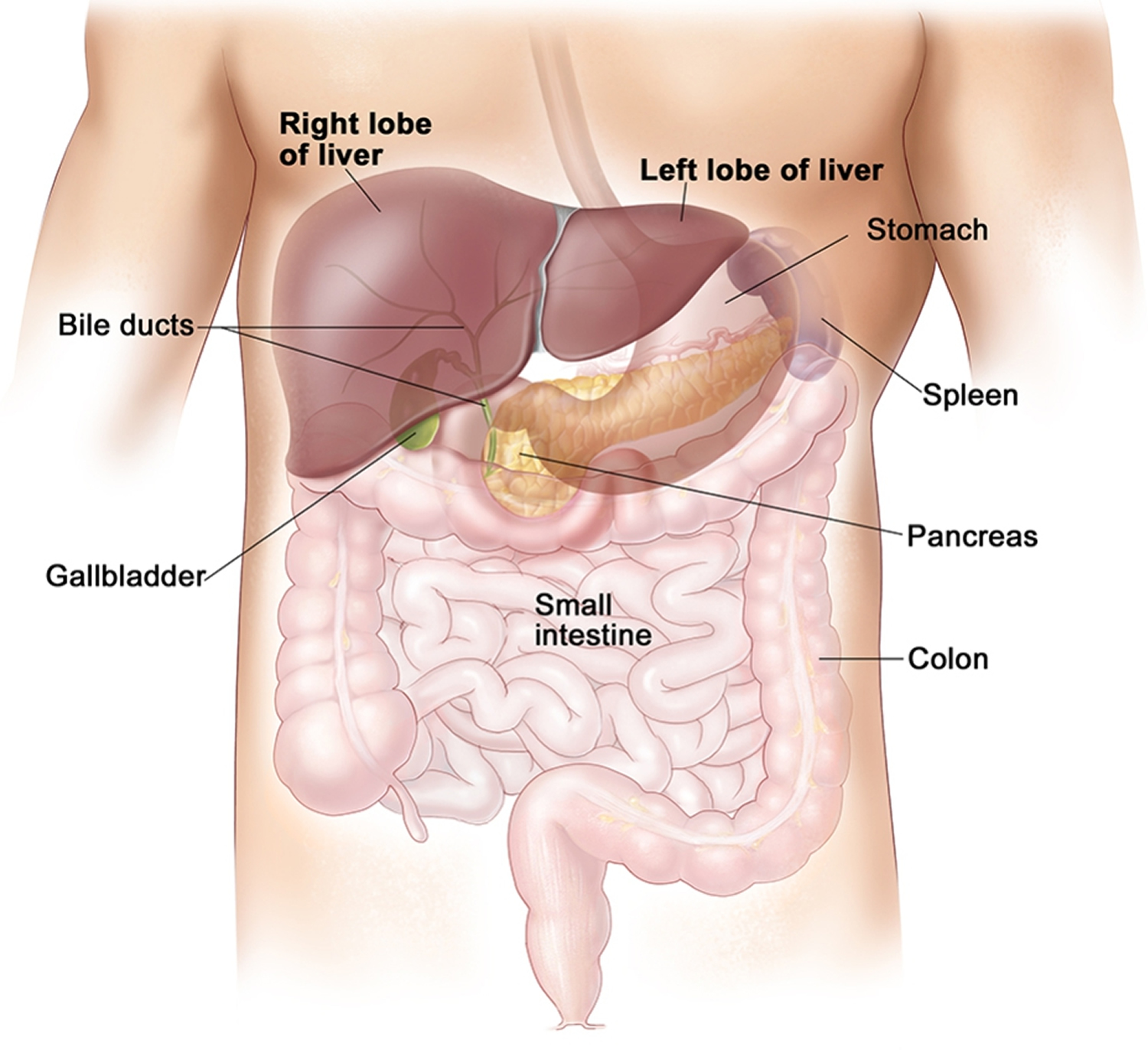
Figure 2. Liver anatomy
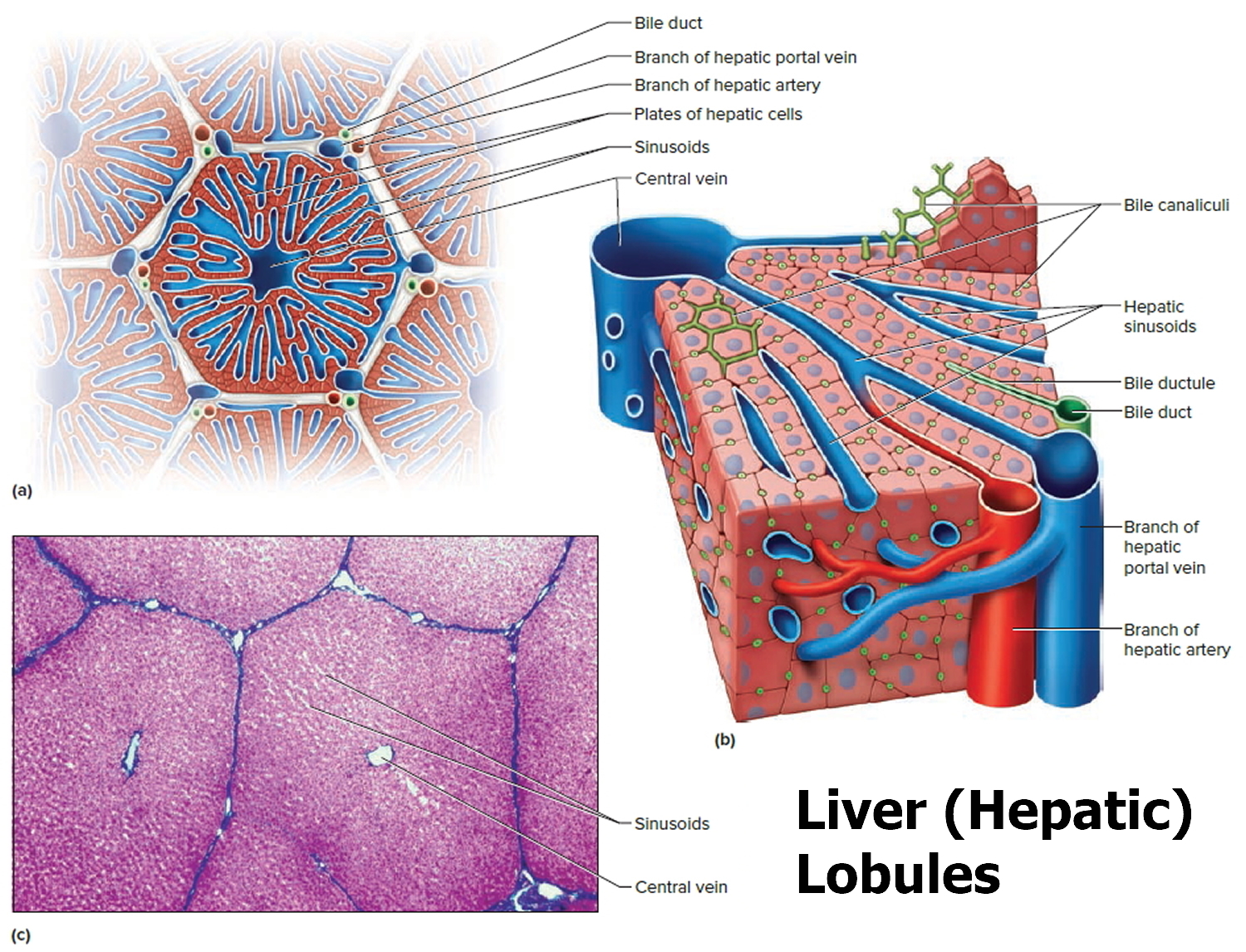
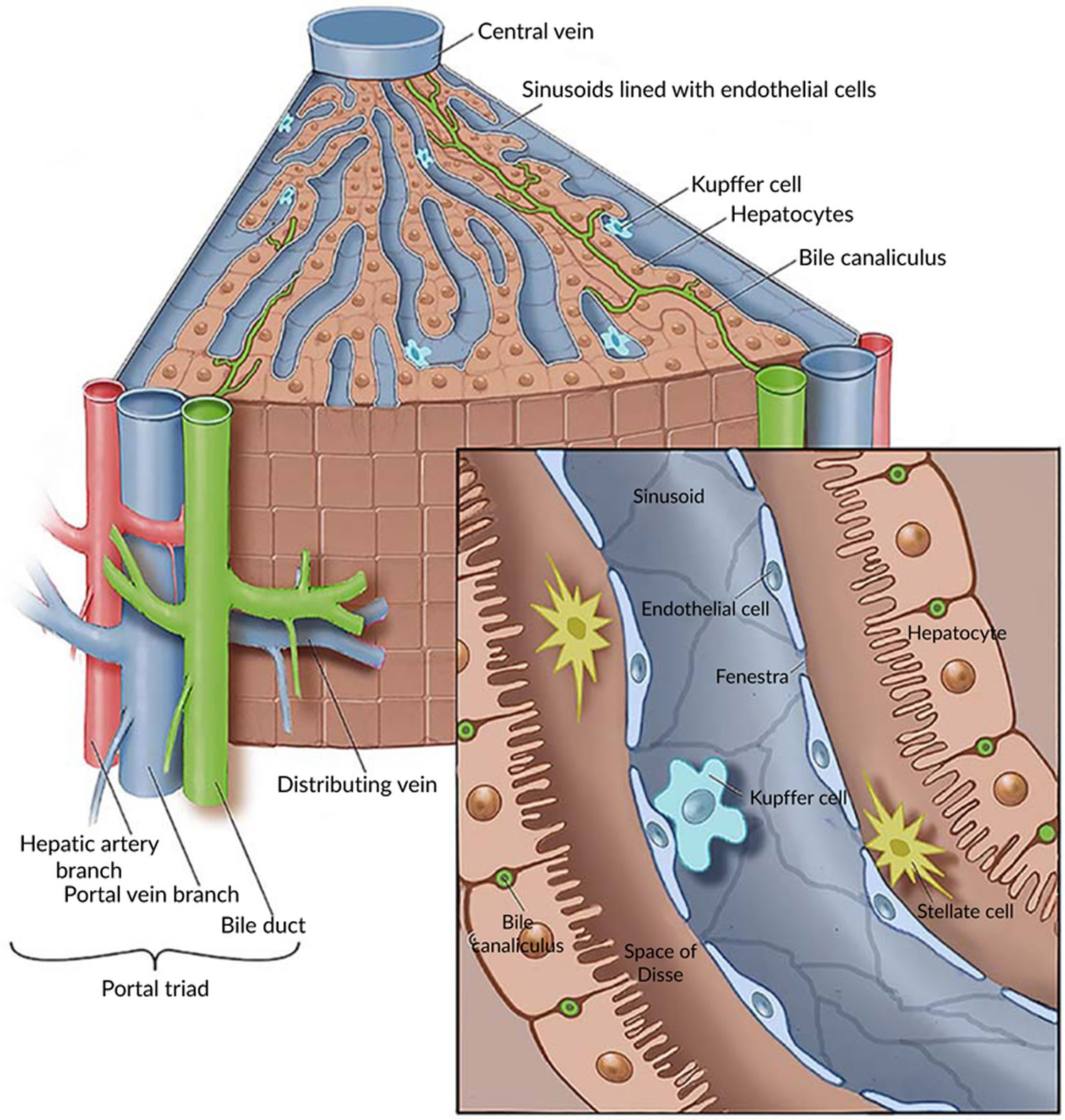
Figure 3. Liver lobule
Footnote: (a) Cross section of a hepatic lobule. (b) Enlarged longitudinal section of a hepatic lobule. (c) Light micrograph of hepatic lobules in cross section.
Figure 4. Human liver microscopic anatomy
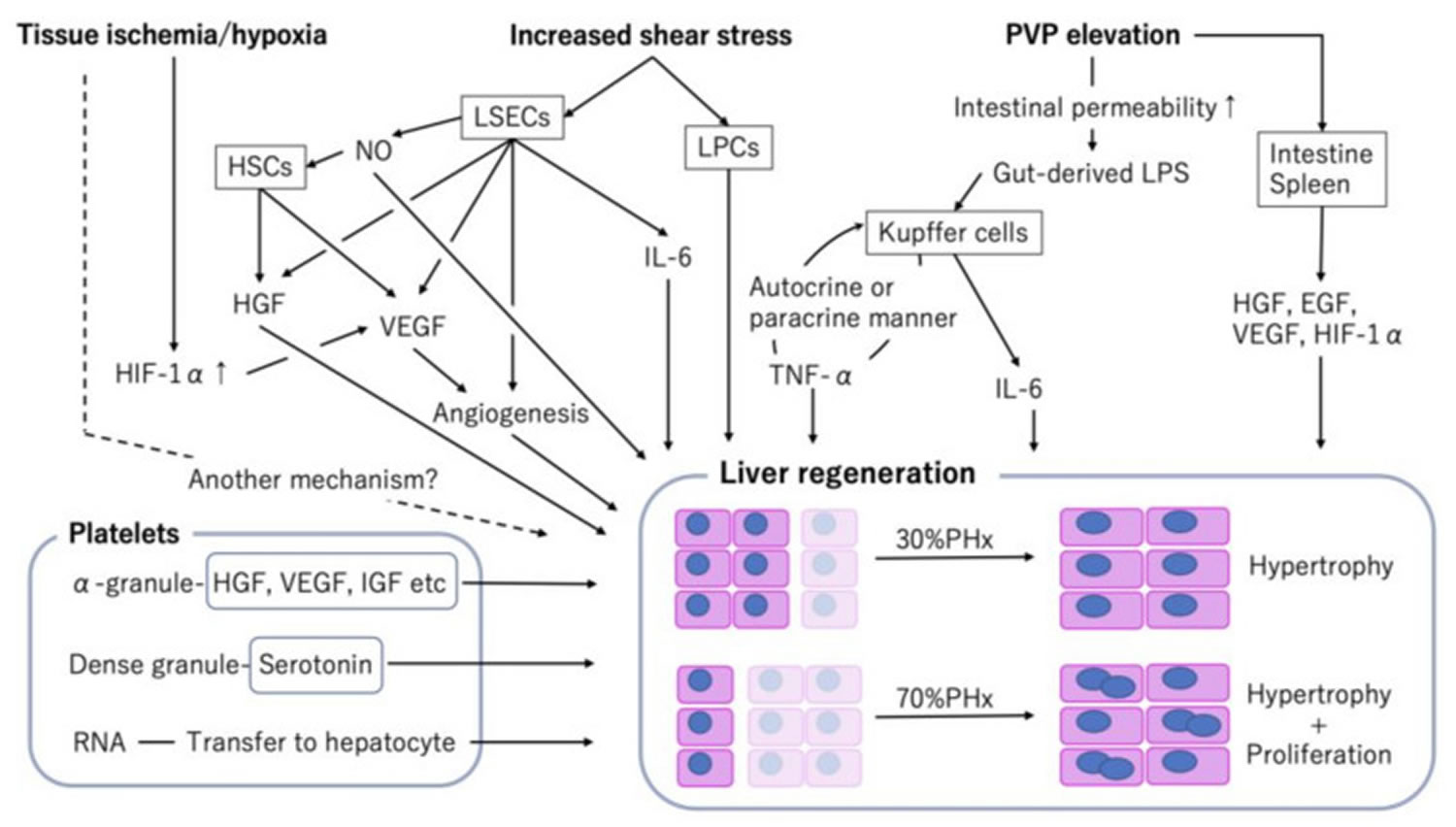
Figure 5. Factors associated with liver regeneration
Abbreviations: PHx = partial hepatectomy; PLTx = partial liver transplantation; EGF = epidermal growth factor; HGF = hepatocyte growth factor; HIF-1α = hypoxia inducible factor 1-alpha; HSCs = hepatic stellate cells; IGF = insulin-like growth factor; IL = interleukin; LSECs = liver sinusoidal endothelial cells; LPCs = liver progenitor cells; LPS = lipopolysaccharide; NO = nitric oxide; PVP = portal vein pressure; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
[Source 25 ]Types of liver regeneration
Until recently, it was believed that the liver mass after partial hepatectomy or injury recovers via hepatocyte proliferation for 1–2 cell cycles; however, recent studies have shown that different stimuli define the type of liver regeneration that occurs 26. There are two known types of liver regeneration 24:
- The first is called Typical liver regeneration that is conducted through the hypertrophy and/or hyperplasia of hepatocytes and biliary epithelial cells. Typical regeneration is specific to a healthy liver that was exposed to resection or an acute liver injury;
- The second is called Progenitor-Dependent liver regeneration that requires the reprogramming of specific hepatic cells, whose activation depends on the volume of the residual liver mass. Progenitor-dependent regeneration is specific to chronic liver diseases and massive acute liver injuries 27. Therefore, a 2/3 hepatectomy leads to the immediate hypertrophy of hepatocytes and further hyperplasia, whereas a 1/3 hepatectomy only triggers cell hypertrophy. Various chronic diseases and massive injuries initiate the activation of hepatic progenitor cells, which are responsible for liver regeneration 26. Consequently, typical liver regeneration is driven by mature hepatocytes and biliary epithelial cells, whereas the alternative regeneration method is performed by hepatic progenitor cells 28.
Typical liver regeneration
Partial hepatectomy causes a hemodynamic disturbance, expressed as a portal pressure escalation, which serves as a regeneration stimulus 24. Consequently, hepatocytes, biliary epithelial cells, Ito cells, Kupffer cells and sinusoid endothelial cells are proliferated. Interestingly, hepatocytes proliferate first, whereas biliary epithelial cells start to proliferate only 2-3 days after partial hepatectomy. After a 2/3 partial hepatectomy, the hepatocytes go through one cycle of DNA synthesis, which is required for the restoration of 60% of the liver mass. In the following stages, several but not all hepatocytes continue to proliferate to achieve complete liver recovery. Afterward, apoptotic activity increases with the purpose of correcting an excessive regenerative response 13.
Phases of typical liver regeneration
The beginning of each phase is initiated by a certain molecule set released in response to organ damage 29. The earliest regeneration drivers are portal pressure changes and an increasing level of urokinase plasminogen activator (uPA) 30.
Priming phase
During the first phase of regeneration, hepatocytes, driven by various cytokines, simultaneously enter the G1 phase of the cell cycle 27.
The increasing blood pressure in the hepatic sinusoids is conditioned by the incompatibility between the volume of the liver and the volume of inflowing venous blood 31, which results in a turbulent flow and mechanically stimulates sinusoid endothelial cells to secrete large amounts of urokinase plasminogen activator (uPA). uPA promotes plasminogen-plasmin transformation, leading to matrix metalloproteinase (MMP) activation and fibrinogen degradation. Plasmin and MMPs are involved in extracellular matrix (ECM) remodeling, resulting in the release of growth factors, such as hepatocyte growth factor (HGF) 30.
Two proinflammatory cytokines are the main mediators of the first phase: Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6); these cytokines are secreted primarily by liver macrophages under the influence of bacterial lipopolysaccharide and the C3a and C5a components of the complement system 32. IL-6 drives the acute phase response and initiates cytoprotection and the proliferation of hepatocytes via the IL-6-IL-6R interaction and the activation of coreceptor glycoprotein 130 (gp130), which activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT), Mitogen-activated protein kinase (MAPK) and PI3K/AKT signaling pathways 33. Although gp130 is present on the surface of most cells, IL-6R is primarily located on hepatocytes. However, there are also soluble IL-6Rs that initiate the trans-signaling pathway within cells lacking IL-6R and enhance the regenerative response of hepatocytes 34. Fazel Modares et al 35 elicited the crucial role of the trans-signaling pathway in liver regeneration after partial hepatectomy because hepatocyte IL-6R activation alone was not sufficient to initiate cell proliferation. TNF-α has two main functions: It activates the NF-κβ signaling pathway through direct interaction with TNF-R1 on Kupffer cell surfaces and through the indirect induction of inhibitory KB kinase; it also stimulates hepatocyte c-Jun N-terminal kinase (JNK). JNK phosphorylates the c-Jun transcription factor in the nucleus to induce cyclin-dependent kinase 1 transcription, which activates hepatocyte proliferation 30.
The augmenter of liver regeneration (ALR) protein, which has three isoforms (15, 21 and 23 kDa) and is expressed primarily in the liver, testes, kidneys and brain, plays a crucial role in liver regeneration. Each isoform of ALR has a different location within the cell and thus plays a different role 36. For example, mitochondrial long-form ALR translocates proteins and initiates MitoNEEt release, which leads to cell proliferation. Long-form ALR expression increases in cases of pathology and reduces liver damage, protects against oxidative stress and endoplasmic reticulum stress by decreasing Ca++ levels, and has an antimetastatic effect on hepatocellular carcinoma. Cytoplasmic short-form ALR enhances the hepatocyte response to IL-6 by inducing the phosphorylation of STAT3; it also has an antimetastatic effect on hepatoma by inhibiting the migrative and invasive capacity of cells 37. After partial hepatectomy, the ALR concentration increases immediately and activates MAPK signaling; enhances IL-6, TNF-α and inducible nitricoxide synthase production by Kupffer cells; and inhibits NK cell activity. Short-form ALR protects hepatocytes by inhibiting apoptosis stimuli 38.
Proliferative phase
During the second phase of liver regeneration, the G1/M phase transition occurs, which is driven by two groups of mitogens: Complete mitogens, including hepatocyte growth factor (HGF), TGF-α, epidermal growth factor (EGF), and HB-EGF; and the stimulation of DNA synthesis and cell proliferation via Ras-MAPK and PI3K/AKT signaling activation and auxiliary mitogens, including bile acids, vascular endothelial growth factor (VEGF), noradrenalin, insulin-like growth factors (IGFs), estrogen and serotonin 34.
Hepatocyte growth factor (HGF) is produced by mesenchymal liver cells and interacts with the methionine (MET) receptor, leading to PI3K and MAPK signaling protein phosphorylation followed by PI3K/AKT and extracellular-signal-regulated kinase 1/2 signaling activation. This process results in the proliferation, migration, and differentiation of liver cells and antiapoptotic effects 39. Epidermal growth factor receptor (EGFR)-transmembrane receptors with tyrosine kinase activity interact with EGF, TGFa, amphiregulin, epigen, and HB-EGF, leading to MAPK, PI3K/AKT–mammalian target of rapamycin (mTOR) and STAT signaling activation, which drives hepatocyte proliferation 40. Natarajan et al 41 identified impaired liver regenerative capacity and delayed cyclin D1 expression in mice lacking EGFR.
Nuclear factor erythroid 2-related factor 2 (NRF2) transcription factors, which regulate a wide range of genes including antioxidant proteins and detoxifying enzymes, are activated in response to increased reactive oxygen species levels. The expression of this molecule increases in the earliest stages of liver regeneration as a result of cellular damage 42. Zou et al 43 discovered the important role of Nrf2 in the regulation of cell cycle progression in mice. Nfr2 is a transcriptional suppressor of Cyclin A2 and a regulator of the Wee1/Cdc2/Cyclin B1 pathway, which controls the beginning of the M phase 43. Nfr2 also regulates hepatocyte proliferation by modulating the insulin/IGF-1 and Notch1 signaling activities and facilitates the capability of hepatocyte nuclear factor 4 alpha (HNF4α) to keep newly formed hepatocytes in a differentiated state 44.
Bile acids are the main end products of cholesterol metabolism and are synthesized exclusively in the liver, where they function as signaling molecules that activate membrane G-protein-coupled bile acid receptor 1 (or TGR5) and nuclear farnesoid X receptor (FXR) 45. After the loss of liver mass due to partial hepatectomy, the bile acids concentration increases during the first minute, which leads to FXR activation, resulting in inhibited bile acid synthesis and induction of the FOXM1B gene 46. FOXM1B is a transcription factor that regulates DNA synthesis and mitosis via cyclin-dependent kinase 2 (CDK2) activation, which is required for the G1/S transition and CDK1 activation and is responsible for the S/M transition 47. FXR activation also appears in enterocytes and leads to the induction of fibroblast growth factor (FGF)15/FGF19 expression. The Fgfr4/β-Klotho receptor, which is located on the hepatocyte surface, inhibits BA synthesis and activates the cell cycle via FOXM1B induction when activated 48. Fgfr4/β-Klotho activation also regulates the termination of liver regeneration and terminal organ size. Kong et al 49 showed that mice with enhanced Fgf15 expression have the most active Hippo signaling pathway, which induces cellular senescence and suppresses transcriptional activation. TGR5, which is located on Kupffer cells, SEC and BEC surfaces, leads to cAMP induction and nuclear factor kappa B (NF-κB)-signaling inhibition 50. As a result, decreased proinflammatory cytokine synthesis occurs in Kupffer cellss and bone marrow macrophages via the protein kinase B-dependent activation of the mTOR 51. TGR5 protects the liver from BA overload by increasing its excretion with urine; it also enhances the secretion of HCO3ˉ and Clˉ and controls BA polarity because inordinately hydrophobic molecules can damage the regenerating liver 52.
Wnt ligands are glycoproteins secreted by nonparenchymal liver cells, mostly Kupffer cellss and sinusoid endothelial cells, and are crucial molecules of liver regeneration 53. Wnt ligands lead to the integration of Axin into the cytoplasmic membrane through interaction with the Frizzled receptor and the coreceptors LRP5/6, resulting in impaired function of the β-catenin degradation complex. Therefore, Wnt ligands lead to β-catenin accumulation, followed by its translocation to the nucleus and interaction with members of the transcriptional T cell factor family, resulting in target gene transcription, for example, of cyclin D1, leading to hepatocyte proliferation 54. Preziosi et al 55 identified the constitutional secretion of Wnt2 and Wnt9b by central vein endotheliocytes and the essential role of these molecules in the basal activation of β-catenin and metabolic zonation of hepatocytes. partial hepatectomy leads to increased Wnt2 and Wnt4 expressions within all zones of the hepatic acinus and the additional secretion of Wnt9b and Wnt5b within the pericentral zone during the first 12 h. This leads to a 7–8-fold increase in cyclin D1 expression within the periportal and intermediate zones and 20-and 100-fold increases in glutamine synthetase expression within the intermediate zone and the pericentral zone, respectively. The role of increased glutamine synthetase expression remains unknown but is thought to be an enhancer of pericentral detoxification since the other 2/3 of hepatocytes restore organ mass 55.
The Hedgehog (Hh) signaling pathway is a morphogenic pathway that regulates embryonic development and is implicated in homeostasis maintenance 56. Among vertebrates, this pathway is activated within a special organelle, the primary cilium, via the interaction of Hh ligands Sonic hedgehog, Indian hedgehog and Desert hedgehog and the Ptched receptor 57. After that, phosphatidylinositol 4-phosphate 58, sumoylated molecules and cholesterol 59 form a complex with smoothened (Smo), which leads to its activation. Activated Smo dislocates to the apex of the PC and activates Glis (including Gli1, Gli2 and Gli3), which then translate to the nucleus and regulate gene transcription 60. The said pathway is canonical, but there are also different types of noncanonical Hh signaling pathways; for example, the Smo-free activation of Glis or the Hh pathway arises beyond the primary cilium 61. Ochoa et al 62 identified a meaningful role of Hh signaling in liver regeneration. partial hepatectomy leads to Hip inhibition, thus activating the Hh pathway via an increase in the Indian hedgehog level in the replicative period and an increase in the Sonic hedgehog level in the postreplicative period. Platelet-derived growth factor, TGF-β, and EGF are secreted in response to liver damage induced by JNK-dependent Hh ligand synthesis 63. Hh signaling activation occurs within hepatocytes, Ito cells 64 and biliary epithelial cells 65, leading to ECM remodeling, progenitor cell expansion and liver epithelial cell proliferation 60. Additionally, Hh signaling controls Yes-associated protein 1 (YAP) of activated Ito cells 66. The Hh-YAP signaling pathway induces the glutaminolysis required for Ito cell activation to regulate liver regeneration 67. Furthermore, Hh signaling facilitates cell survival via inhibiting hepatocytes, biliary epithelial cells, Ito cells and progenitor cell apoptosis 68.
Notch signaling is an important pathway in embryonic development, homeostasis maintenance, and liver regeneration 69. Mammals have 4 types of receptors for this pathway (Notch1, Notch2, Notch3, and Notch4); Notch1 and Notch2 are located primarily on biliary epithelial cells and HPCs whereas Notch3 and Notch4 are expressed by the mesenchymal compartment of the liver and are poorly represented on epithelial liver cells. JAG-1 and DLL-4 are ligands of Notch signaling that are expressed in the liver 70. The main role of this pathway in liver development is the JAG1-NOTCH2 interaction, which results in the differentiation of hepatoblasts to biliary epithelial cells and the development of the intrahepatic biliary tree 71. Lu et al 72 showed that the role of the Notch–RBPJ interaction is to drive hepatic progenitor cell differentiation to biliary epithelial cells via Yap inactivation after partial hepatectomy in mice. The direction of hepatic progenitor cell differentiation is defined by the balance of NOTCH signaling and Wnt ligands 73. Ortica et al 74 pointed out the important role of Notch3 in HPC differentiation to hepatocytes. Zhang et al 75 elicited the regulatory role of Notch signaling in hepatocyte proliferation via the NICD/Akt/Hif-1α pathway after partial hepatectomy, whereas its inhibition leads to delayed S phase entry, impaired S phase and M phase progression, and the loss of the hepatocyte mitotic rhythm due to cyclin E1, A2 and B1 dysregulation. Yang et al 76 demonstrated the involvement of Notch signaling in the regeneration of 8 types of liver cells, which is performed by the activity of 9 different pathways and regulates cellular proliferation, apoptosis, the cell cycle, etc.
Termination phase
When the needed Liver mass: Body mass ratio is achieved, cellular proliferation stops due to inhibitory molecules that control the rapidity and direction of liver regeneration. Among the inhibitors of cell proliferation, IL-1, which is synthesized by nonparenchymal liver cells, inhibits the DNA synthesis induced by HGF, EGF and TGF-α. IL-6 is multifunctional and plays a role as both a liver regeneration inducer and inhibitor; its effect depends on the time and dose of the molecule. The IL-6-dependent inhibition of proliferation is likely to occur by increasing p21 expression 77. The JAK/STAT signaling pathway is inhibited by 8 members of the SOCS family of proteins; hereafter, only SOCS1 and SOCS3 contain the extended SH2 and kinase inhibitory region. SOCS1 directly binds and inhibits JAK, whereas SOCS3 binds to cytokine receptors, forms a complex with JAK and inhibits the STAT3 signaling pathway. SOCS3 is the main suppressor of the signaling pathway activated by IL-6; it inhibits the phosphorylation of coreceptor gp130, JAK and STAT3. SOCS1 negatively regulates the hepatocyte proliferation induced by HGF via c-MET signaling inhibition and likely regulates the TNF-α levels because it interacts with toll-interleukin 1 receptor domain-containing adaptor protein, which drives the synthesis of a current mediator 78.
Some TGF-β family members function as inhibitors of proliferation. In particular, TGF-β1 plays a special role in binding to receptor types 1 and 2 and inducing cell apoptosis to correct an excessive liver mass. Outside of the liver, TGF-β1 is synthesized in platelets and the spleen. The spleen might be involved in the termination phase for it inhibits HGF and its c-MET receptor expression. In this regard, splenectomy leads to increased hepatocyte proliferation in the first 48 hour after partial hepatectomy. Other members of the TGF-β family are involved in the termination phase of liver regeneration, including activin A-hepatocyte proliferation inhibitors and bone morphogenetic proteins (BMPs) 34. BMP9 is expressed exclusively by liver tissues and in part by hepatocytes. BMP9 regulates a variety of biological functions such as glucose and lipid metabolism, angiogenesis, oncogenesis, and fibrogenesis, and it affects liver regeneration after acute injuries 79. Addante et al 80 reported a regulatory function of BMP9 over hepatic progenitor cells that is affected by anaplastic lymphoma kinase 2 type I receptor activation, resulting in SMAD 1, 5, and 8 induction, hepatic progenitor cell apoptosis stimulation, and a reduction in hepatic progenitor cells. Apart from its negative influence on liver regeneration, BMP9 also has profibrogenic activity and promotes hepatocellular carcinoma (HCC) proliferation and invasion. Additionally, BMP9 enhances the expression of TLR4 on the SEC surface, leading to inflammatory cell recruitment. Therapy with anti-BMP-9/ anaplastic lymphoma kinase 1 can potentially enhance hepatocyte proliferation among patients with chronic liver diseases and decrease the probability of fibrosis and HCC development 81.
HNF4-α regulates hepatocyte differentiation and, according to Huck et al 82, promotes the termination of liver regeneration. The expression of the current molecule significantly decreased during the priming phase and increased during the following phases, which is necessary for termination and hepatocyte function recovery after partial hepatectomy 82. HNF4-α is a YAP and TGF-β/SMAD3 antagonist; therefore, decreased expression of this molecule stimulates promitogenic functions and activates connective tissue growth factor. Increased HNF4-α expression during the subsequent phases of regeneration prevents the excessive synthesis of connective tissue and therefore fibrosis 83. Hnf4-α also leads to the inhibition of HPC proliferation and migration in rats 84.
Integrin-linked kinase is a suppressor of hepatocyte proliferation that is located under the cytoplasmic membrane and is associated with a3/b1 integrins of the ECM. Interruption of this connection results in hepatostat imbalance and excessive liver mass. Focal adhesion kinase is also associated with a3/b1 integrin and promotes hepatocyte proliferation 85.
The Hippo signaling pathway is a crucial regulator of the terminal organ size within mammals. The key component of the mammalian Hippo pathway is a kinase cascade in which the Ste20-like kinases 1/2 phosphorylate and activate large tumor suppressor 1/2, its adapter protein Mps one binder 1, and the transcriptional coactivators Yap and Taz 86. Phosphorylated Yap and Taz emerge from the nucleus, where they are bound to transcription factors that control the proliferation and differentiation of cells, such as TEAD family members 87. The Hippo/Yap signaling pathway is likely an integrator of a large number of alternative growth factor signaling pathways and regulates liver size by balancing negative and positive regulatory signals 85. The Hippo signaling pathway does not have any specific receptors and is regulated by molecules that control cellular polarity and morphology, intercellular adhesion and other processes. The activity of this pathway is modulated in response to mechanical deformation and intercellular adhesion defects and cell adhesion to the intercellular matrix. Consequently, Hippo signaling senses cellular and tissue integrity 88. Intranuclear Yap is located in periportal hepatocytes and biliary epithelial cells, whereas pericentral hepatocytes contain few current molecules, which is exactly the opposite of the constitutive Wnt ligand content; therefore, current pathways inhibit one another 89.
Progenitor-dependent liver regeneration
The mechanisms described above are specific for healthy livers and occur among living liver donors. However, in most cases, liver resection occurs within patients with impaired liver function, and subsequent regeneration proceeds in a nonstandard way, which can lead to hepatic failure and death 90. Hepatocytes are the main cells driving typical liver regeneration, whereas alternative liver regeneration is performed by hepatic progenitor cells (HPCs) 91. The process of progenitor-dependent liver regeneration is shown in Figure 6 below.
Acute liver failure caused by intoxication, viral hepatitis A, B or E, autoimmune liver disease, etc., is often followed by widespread necrotic and apoptotic zones, and adequate liver regeneration becomes impossible 92. During acute liver failure, the main regenerative role is given to hepatic progenitor cells, as indicated by the increased level of alpha-fetoprotein (AFP). Therefore, a high AFP level is correlated with a positive prognosis after acetaminophen-induced liver damage 13. The immune system regulates liver regeneration via necrotic cell phagocytosis and controls inflammatory reactions in response to injury. The number of proliferative macrophages in the liver significantly increases after organ damage, and monocytes are recruited from the bloodstream and differentiate into macrophages in response to increasing the colony-stimulating factor 1 levels. Colony-stimulating factor 1 injection promotes liver regeneration after partial hepatectomy; conversely, a low level of the current factor is correlated with a negative patient prognosis 28.
Liver steatosis is associated with an impaired regenerative function, in which GADD34 plays an important role since its increased expression promotes liver regeneration within mice. Ischemia-reperfusion injury often complicates the posttransplantation period and impairs typical liver regeneration. The current complication is followed by increased receptor for advanced glycation end product levels, which might be a therapeutic target. Thus, receptor for advanced glycation end product inhibitor injection leads to a reduction in organ damage and the induction of liver regeneration. The excessive synthesis of extracellular matrix components by activated hepatic stellate cells (Ito cells) inhibits hepatocyte proliferation, and if macrophage matrix metalloproteinases do not promote connective tissue restitution, the angioarchitecture of hepatic lobules is impaired, resulting in cirrhosis 92. In the liver, damage due to cirrhosis and hepatitis B or C often reveals hepatocytes with biliary epithelial cell markers, such as epithelial cell adhesion molecule (EpCAM), on their surface. The presence of these intermediate hepatobiliary cells is thought to be explained by their origin from biliary compartment progenitors 8.
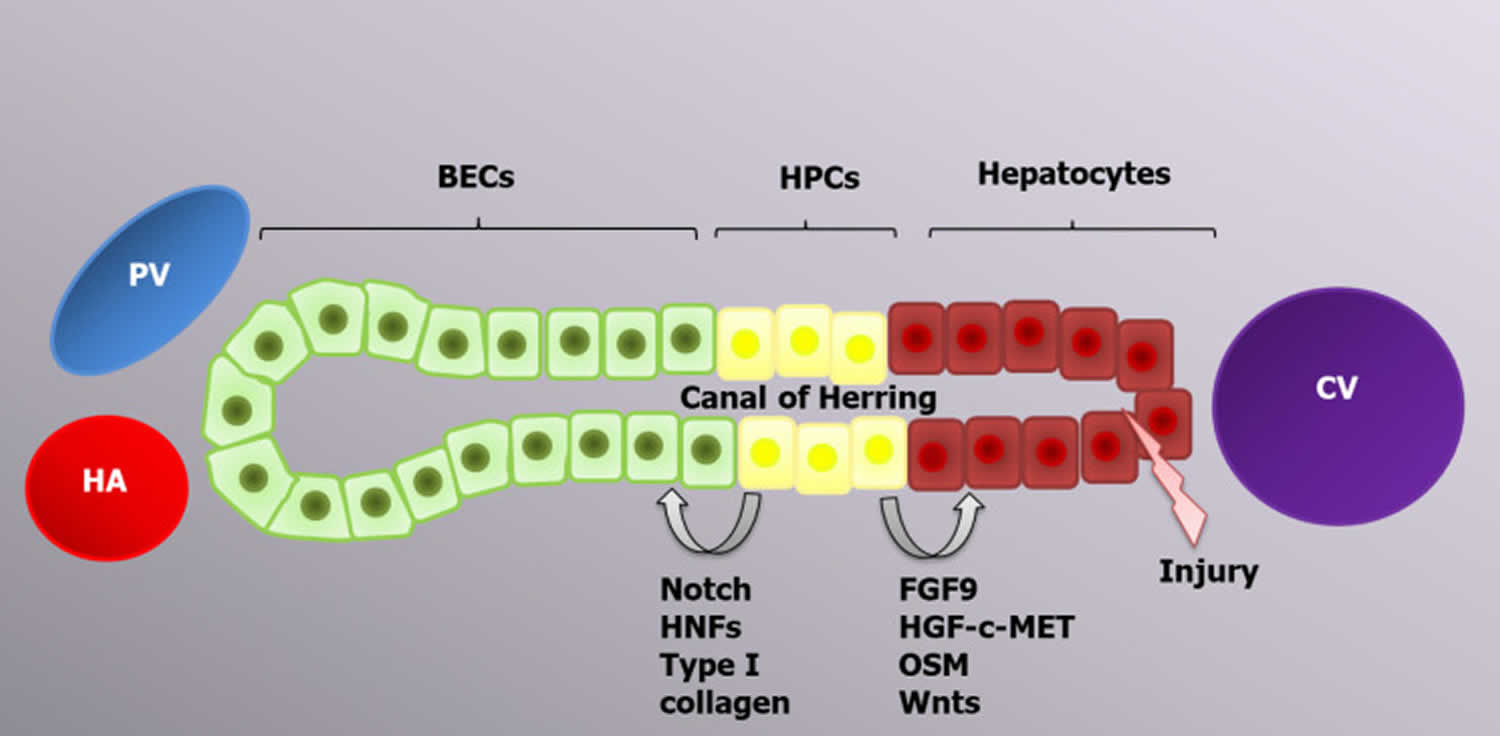
Figure 6. Progenitor-dependent liver regeneration
Footnote: In case of excessive acute injury or chronic liver diseases, hepatic progenitor cell activation occurs in response to different inflammatory cytokines, including tumor necrosis factor-like weak inducer of apoptosis. Depending on the type of stimulus, hepatic progenitor cells can differentiate into biliary epithelial cells or hepatocytes to restore the liver mass.
Abbreviations: PV = Portal vein; HA = Hepatic artery; CV = Central vein; BECs = Biliary epithelial cells; HPCs = Hepatic progenitor cells; HNFs = Hepatocyte nuclear factors.
[Source 24 ]Biomarkers for liver regeneration
Colony stimulating factor 1 (CSF1) was measured in serum samples from 55 patients after hepatic resection and 78 patients with acetaminophen overdose acute liver failure 93. Serum CSF1 levels increased in patients with hepatic resection in proportion to the resected liver volume, and a low serum CSF1 level was associated with worse prognosis (King’s College criteria) and increased mortality/transplantation in patients with acetaminophen overdose acute liver failure. Serum CSF1 level was a better predictor than HMGB1 released by necrotic cells and proposed as a prognostic marker for acute liver failure 94. Furthermore, as subcutaneous CSF1-Fc administration promoted the macrophage accumulation and liver regeneration in mice with acetaminophen acute liver injury 94, CSF1 could be not only a prognostic marker, but also a therapeutic target for liver regeneration in acute liver injury. Recombinant human macrophage-CSF was apparently well tolerated in humans 95.
The microRNAs mediate both activation and inhibition of gene expression at the posttranscriptional or translational levels, and affect biological pathways including cellular differentiation, proliferation, apoptosis, and tissue remodeling 96. Recently, microRNAs have been demonstrated to regulate cell proliferation during liver regeneration, and to serve as putative therapeutic targets for liver regeneration 97. Patients with spontaneous recovery from acute liver failure showed significantly higher serum levels of microRNA-122, microRNA-21, and microRNA-221, compared to non-recovered patient cohort 98. The elevated microRNAs serum levels were accompanied with down-regulation of growth inhibitory targets in the liver tissue, such as heme oxygenase-1, programmed cell death 4, and the cyclin-dependent kinase inhibitors p21, p27, and p57, as well as increased cyclin D1 expression and hepatocyte proliferation. Thus, microRNA-122, microRNA-21, and microRNA-221 involved in liver regeneration might also serve as a biomarker to predict acute liver failure 99.
Above all, most simple and available marker suggesting liver regeneration is alpha-fetoprotein (AFP). AFP is also known as a hepatic progenitor-associated marker besides a representative marker of hepatocellular carcinoma 100. In a prospective study of 206 patients, the AFP ratio (AFP concentration at day 3 of admission divided by that at day 1) ≥1 predicted the survival of acute liver failure patients 101. Rising AFP levels over the first 3 hospital stay days might indicate a better survival rate.
What helps the liver regenerate?
Liver regeneration is a highly orchestrated process influenced by various factors, with many aspects of liver regeneration still not understood 24 and the mode of liver regeneration is influenced by the amount of liver resection 5. Liver regeneration is a complex process regulated by the interaction between growth factors and cytokines secreted near the site of injury or transferred to the liver by the blood. This strictly orchestrated process is divided into 3 phases: Priming, proliferation, and termination 102. The sum of all signals that sense the physiologically necessary liver mass is called the “hepatostat”, which can initiate and terminate liver regeneration 103. This phenomenon reflects the correlation between the needs of organisms and the organ mass that is required for homeostasis 104.
Therapeutic methods for insufficient liver regeneration treatment are lacking, although many studies have focused on the efficiency of various molecules in promoting liver regeneration 24. Further studies in this field can help determine how to prevent liver failure after surgical interventions and acute and chronic injuries via improving liver regeneration. Shi et al 105 determined that baicalin can stimulate liver regeneration after acetaminophen-induced acute liver injury in mice via inducing hepatocyte proliferating cell nuclear antigen, increasing cyclin D1 expression and Nrf2 cytosolic accumulation, and enhancing IL-18 Levels, leading to the upregulation of hepatocyte proliferation. So et al 106 showed the promotion of liver regeneration after the inhibition of EGFR or MEK/extracellular signal-regulated kinase (ERK) and the genetic suppression of the EGFR-ERK-SOX9 axis via inducing HPC-to-hepatocyte differentiation in zebrafish. The research of Xiang et al 107 noted the therapeutic effect of IL-22Fc in inducing liver regeneration in acute-on-chronic liver failure patients due to the shift from anti-regenerative IFN-γ/STAT1 to the pro-regenerative IL-6/STAT3 pathway. Li et al 108 reported that aldose reductase is a new potential therapeutic target for enhancing normal and fatty liver regeneration after surgery and ischemia-reperfusion injury because the knockout of aldose reductase leads to enhanced oxisome proliferator activated receptor-α and oxisome proliferator activated receptor-γ expression, thus improving energy metabolism in the liver. The research of Loforese et al 109 revealed that the inhibition of MST1 and MST2 with si-RNA resulted in improved hepatocyte proliferation in aged mice after partial hepatectomy; therefore, Ste20-like kinases 1/2 may be a potential therapeutic target. Many other molecules and molecular pathways have been shown to enhance liver regeneration in experimental models. Further studies would help implicate the potential therapy in the clinic and improve the survival of patients with different liver diseases in the near future.
Mesenchymal stem cells (MSCs) have a self-renewal capacity and are derived from the bone marrow, adipose tissue, umbilical cord, etc. They are the subject of focus in regenerative medicine and serve as a potential therapy for different liver diseases 110, 111. Mesenchymal stem cells were shown to improve liver regeneration in patients with cirrhosis by elevating anti-apoptotic factors, such as HGF and IGF-1, and angiogenic and mitogenic factors. In acute liver failure animal models, mesenchymal stem cells have been shown to promote liver regeneration mostly by suppressing the oxidative stress and inflammation via reducing TNF-α, IFN-γ and IL-4 Levels and stimulating liver regeneration with various released factors such as PGE2 and delta-like 4 112, 113. Mesenchymal stem cells can also stimulate liver regeneration after partial hepatectomy by upregulating hepatic cell proliferation and downregulating fat accumulation and hepatocyte growth factor (HGF), IL-6, IL-10 and TNF-α serum levels 114.
Table 2. Possible targets and roles in liver regeneration for acute severe injury
| Application to severe liver injury | ||||||
| Role in liver regeneration | Targets | Animal study | Human study (evidence in liver regeneration) | Prevention of acute liver failure following small-for-size syndrome | Ongoing acute liver failure or extensive hepatocyte injury | References |
| Promotion | PVE, ALPPS, two-stage hepatectomy | Yes | Yes (Clinically standard technique) | Yes | N/A | 115 |
| Modulation of portal flow | Yes | Yes (Retospective study, beneficial) | Yes | N/A | 116 | |
| Pentoxifylline | Yes | Yes (Randomized controlled clinical trial, beneficial) | Yes | N/A | 117 | |
| Omega-3 fatty acid | Yes | Yes (Randomized controlled clinical trial, beneficial) | Yes | N/A | 118 | |
| Platelet, serotonin | Yes | Yes (Prospective observational study, beneficial) | Yes | N/A | 119 | |
| G-CSF | Yes | Yes (Randomized controlled clinical trial, beneficial) | N/A | Yes | 120 | |
| FXR agonist, FGF19 analogue | Yes | No | Yes | N/A | 121 | |
| RAGE inhibitor | Yes | No | Yes | N/A | 122 | |
| TGFβR1 inhibitor | Yes | No | N/A | Yes | 123 | |
| mTORC1 inhibitor (Autophagy inducer) | No | No | N/A | N/A | 124 | |
| Inhibition | Steatosis | Yes | Yes (Equivocal due to inaccuracy of diagnosis) | 125 | ||
| Fibrosis | Yes | Yes (Equivocal due to inaccuracy of diagnosis) | Risk factors | 126 | ||
| Aged liver | Yes | Yes (Retrospective study, injurious) | 127 | |||
| Cholangitis | Yes | Yes (Retrospective study, injurious) | 128 | |||
| Support (Alternation) | Bioartificial liver | Yes | Yes (Randomized controlled clinical trial, no beneficial) | N/A | Yes | 129 |
| Cell transplantation | Yes | Yes (Case series, limited beneficial) | N/A | Yes | 130 | |
| Organ engineering | Yes | No | N/A | Yes | 131 | |
Abbreviations: ALF = acute liver failure, ALPPS = associating liver partition and portal vein ligation for staged hepatectomy; BAL = bioartificial liver; FGF = fibroblast growth factor; FXR = farnesoid X nuclear receptor; G-CSF = granulocyte colony-stimulating factor; mTORC1 = mammalian target of rapamycin complex 1; N/A = not available; PVE = portal vein embolization; RAGE = receptor for advanced glycation end products; RCT = randomized controlled clinical trial; SFSS = small-for-size syndrome; TGFβR1 = transforming growth factor beta receptor 1
[Source 99 ]Pentoxifylline
The effects of pentoxifylline, which enhances production of interleukin (IL)-6 while inhibiting the TNFα, were evaluated in 101 non-cirrhotic patients undergoing major hepatectomy 117. Continuous intravenous administration of pentoxifylline, starting 12 hours before and ending 72 hours after the surgery, resulted in significantly higher regeneration volume for small liver (remnant liver to body weight ratio ≤ 1.2%) and stronger induction of interleukin-6 (IL-6) mRNA levels. This study suggested the beneficial effects of pentoxifylline on liver regeneration in small remnant livers, mediated by IL-6 production 99. Perioperative treatment with pentoxifylline might enhance liver regeneration following major hepatic resection.
Omega-3 fatty acids
Omega-3 fatty acids nutrition for 7 days after the surgery was reported to mitigate liver injury, reduce infection morbidities, and shorten the post-hospital stay in patients receiving liver transplantation 132. Another clinical study 118 showed that liver regeneration calculated with volume factors was significantly increased in patients receiving omega-3 fatty acids enriched lipid emulsion for 3 days (2 days before surgery and postoperative day 0) compared with controls after hepatic resection. Experimentally, polyunsaturated fatty acids (PUFAs) administration enhanced expression of the liver kinase B1-adenosine monophosphate-activated protein kinase (LKB1-AMPK) signaling pathway after 70% partial hepatectomy in rats, leading to improved integrity of tight junctions and hepatocellular function 133. LKB1 was necessary in the phosphorylation of Akt downstream targets, including FoxO3a and glycogen synthase kinase 3β (GSK3β) 134. As AMPK activation stimulates the transport from nucleus to cytoplasm of Hu antigen R (HuR), which is an RNA-binding protein that increases the half-life of target mRNA involved in cell cycle progression 135, the deletion of AMPKα1 was reported to delay liver regeneration 136. Clinical and experimental studies suggest that omega-3 fatty acids might promote liver regeneration through the LKB1 and AMPK activation even despite a short term administration.
Resveratrol
Studies suggest that the bioactive polyphenolic compound resveratrol, found naturally in certain foods such as red grapes and peanuts, may be able to ameliorate liver damage 137. However, the effects and efficacy of long-term treatment with resveratrol remain unclear. In an experiment involving C56BL/6 mice, scientists use acetaminophen (acetaminophen; 400 mg/kg/day for 15 days) overdose model to induce liver. Three days after the intoxication was stopped, they observed biochemical, histological and ultrastructural alterations in the livers of these mice. The acetaminophen-treated animals were then given resveratrol (10 mg/kg/day) for 60 days. Blood and tissue were analyzed at days 7, 30 and 60. The data showed that long-term resveratrol treatment (60 days) ameliorates the liver injury caused by acetaminophen intoxication, restoring histological features, ultrastructural organization and serum biochemical parameters (albumin, alanine aminotransferase) 137. Ck18- and F4/80-positive cells (indicators of hepatocyte recovery) were reestablished and the number of α-SMA positive cells was normalized after long-term resveratrol treatment 137. Additionally, downregulation of the drug transporter BCRP (Breast Cancer Resistance Protein) was observed. Electron microscopy revealed that treatment with resveratrol was effective in restoring the shape and size of hepatic microvilli and normalizing both the number and viability of mitochondria. Taken together, these results indicate that long-term treatment with resveratrol is effective in alleviating liver injury caused by acetaminophen administration.
Platelets and serotonin
A recent study 119 evaluated the effects of intra-platelet serotonin on liver regeneration and oncologic outcome in 96 patients. This study demonstrated that patients with preoperative high intra-platelet serotonin levels exhibited a significant reduction in liver dysfunction after hepatic resection for malignant tumors. On the other hand, these patients suffered from a high incidence of postoperative tumor recurrence 119. In addition, patients receiving perioperative selective serotonin reuptake inhibitor (SSRI) treatment, which effectively reduced the intra-platelet serotonin levels, displayed a substantial increase in postoperative morbidities and had no tumor recurrence within 12 months after the surgery. This study suggested the potential of serotonin in both liver regeneration and tumor growth. Induction of thrombocytosis drives liver regeneration, while inhibition of platelet aggregation or reduction of platelet number impairs liver regeneration in mice after partial hepatectomy 138. It has been commonly conceived that release of platelet granule contents including serotonin and other growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1) may enhance liver regeneration. A recent study demonstrated that pro- and anti-regenerative proteins in the granules of platelet were selectively released upon activation and were involved in liver regeneration 139. However, the role of platelet in liver regeneration has not been fully elucidated. It is also controversial whether serotonin may directly exert mitogenic activity on hepatocytes or indirectly influences liver regeneration through platelet activation. It has been suggested that the role of serotonin on tumor growth is concentration-dependent; while high serotonin dose stimulates tumor growth, low doses of serotonin can inhibit tumor growth by decreasing blood supply 140. Although it is unclear which factors contribute to liver regeneration, the increase of platelet and serotonin levels might promote liver regeneration. Of note, mitogenic activity of serotonin should be carefully considered in patients with carcinoma 119.
Granulocyte colony-stimulating factor (G-CSF)
A randomized controlled clinical trial 141 evaluated the impact of granulocyte colony-stimulating factor (G-CSF) on liver regeneration in 47 consecutive patients with acute-on-chronic liver failure. Twelve doses of subcutaneous G-CSF, starting within 48 hours of admission, significantly improved patient survival in addition to Child-Pugh score, Model for End-Stage Liver Disease (MELD), and Sequential Organ Failure Assessment (SOFA), which all relate to severity (acuity) of the liver disease 141. As a matter of course, neutrophil counts were higher in G-CSF treated patient cohort. A recent experimental study in mice with acetaminophen-induced acute liver injury demonstrated that neutrophil infiltration was involved not only in injury amplification at the early phase, but also during liver-tissue repair at the later phase 120. The administration of matrix metalloproteinases (MMP) inhibitor in those mice successfully ameliorated hepatic damage and mitigated local inflammation along with reduction of neutrophil migration into the liver. However, matrix metalloproteinases (MMP) inhibitor impaired liver tissue repair and function restoration at later time points. Neutrophils seem to contribute to tissue regeneration through the various mechanisms, such as MMP delivery 142. By regulating neutrophil function, G-CSF is a promising new therapeutic agent for hepatocellular regeneration after severe liver injury.
Farnesoid X nuclear receptor (FXR) agonist and FGF19 analogue
Although there is no evidence of Farnesoid X nuclear receptor (FXR) agonist or fibroblast growth factor-19 (FGF19) analogue to affect acute liver failure in humans, these are attractive therapeutic targets for liver regeneration 99. Patients without biliary drainage had significantly higher serum bile acids levels and regenerated liver volumes than patients with biliary drainage after major hepatectomy 143. Bile acids drive liver growth by activating their receptor FXR, and are involved in the maintenance of normal liver size through the regulation of fibroblast growth factor (FGF)15 in mice and FGF19 in humans. FGF15/19 binding to FGF receptor (FGFR) 4 on hepatocytes negatively regulates bile acids synthesis with suppression of CYP7A1 144. Mice with humanized livers, without normal FGF15/19 regulation, demonstrate increased bile acids levels, ultimately leading to hepatocyte proliferation and enlarged liver size 145. Inhibition of FXR or FGFR4 signaling was reported to impair hepatocyte proliferation due to reduction of the downstream forkhead box protein M1 (FoxM1) and signal transducer and activator of transcription 3 (STAT3) after PHx in mice 146. In marked contrast, activating FXR enhances hepatocyte proliferation. Oral administration of obeticholic acid, a potent FXR activator, promoted liver regeneration after portal vein embolization in a rabbit model 121. FGF19 conjugated to apolipoprotein A-I to increase half-life of FGF19 in circulation, successfully reduced liver steatosis and improved the liver regeneration after partial hepatectomy in obese mice 147. Thus, FXR and FGF19 analogue have been reported to promote liver regeneration in animal models. The therapeutic effects of FXR agonist and FGF19 analogue in humans have been studied for nonalcoholic steatohepatitis, primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) 148. It might be worth investigating whether FXR agonist or FGF19 analogue impacts on liver regeneration in humans, with obeticholic acid being a good candidate to support liver enlargement after portal vein embolization.
Receptor for advanced glycation end products (RAGE)
The levels of soluble receptor for advanced glycation end products (sRAGE) and RAGE ligands including high mobility group box-1 (HMGB1) were significantly higher in 60 patients with acetaminophen-related acute liver failure as compared with 30 normal controls 122. In those 60 patients with acetaminophen-related acute liver failure, soluble receptor for advanced glycation end products (sRAGE) levels were significantly higher in 30 patients who underwent liver transplantation and/or died as compared with 30 spontaneous survivors. In mouse studies, blockage of RAGE signaling using sRAGE reduced hepatocyte apoptosis and increased liver regeneration in both hepatic ischemia and partial hepatectomy models 122. Receptor for advanced glycation end products (RAGE) in liver remnants was up-regulated in mice with 85% partial hepatectomy as compared with 70% partial hepatectomy and sRAGE therapy increased the survival of 85% partial hepatectomy mice from 30% to 90% 149. While soluble receptor for advanced glycation end products (sRAGE) suppresses ligand-induced stimulation of RAGE serving as a decoy receptor, which binds ligands of RAGE competitively, sRAGE seems to be associated with severity of liver injury in humans. Another experimental study using mice demonstrated that angiotensin II receptor antagonist, losartan mitigated ischemia-reperfusion injury through peroxisome proliferator-activated receptor γ (PPAR-γ) activation, which inhibited RAGE signaling 150. Further studies are required to make the RAGE signaling clinically relevant in liver regeneration.
Transforming growth factor beta receptor 1 (TGFβR1) inhibitor
This study 123 demonstrated that administration of transforming growth factor beta receptor 1 (TGFβR1) inhibitor after 12 hours of acetaminophen-induced acute liver injury mitigated senescence induction, increased hepatocyte proliferation, and reduced jaundice in mice. As TGFβR1 inhibitor has been already evaluated for hepatocellular carcinoma in humans 151, its application as a novel therapeutic strategy to stimulate liver regeneration and reduce hepatocyte senescence in acute severe injury may be possible in the future.
What foods help regenerate the liver?
After your liver transplant, it is especially important to eat a healthy, well-balanced diet to help you recover and keep your liver healthy 152. A dietitian or nutritionist can help you create a healthy eating plan that meets your nutrition and diet needs. In general, your diet after liver transplant should be low in salt, cholesterol, fat and sugar. To prevent damaging your liver, it’s important to avoid alcohol and cigarettes. Do not drink alcoholic beverages or use alcohol in cooking.
Your dietitian will also provide you with several healthy food options and ideas to use in your nutrition plan. Your dietitian’s recommendations may include:
- Eating at least five servings of fruits and vegetables each day
- Avoiding grapefruit and grapefruit juice because of their effect on a group of immunosuppression medications
- Having enough fiber in your daily diet
- Choosing whole-grain foods over processed ones
- Drinking low-fat or fat-free dairy products, which is important to maintain optimal calcium and phosphorus levels
Your dietitian may also recommend:
- Eating lean meats, poultry and fish
- Following food safety guidelines
- Staying hydrated by drinking adequate water and other fluids each day
You should avoid consuming the following:
- water from lakes and rivers
- unpasteurized milk products
- raw or undercooked
- eggs
- meats, particularly pork and poultry
- fish and other seafood.
- Michalopoulos, G.K., Bhushan, B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 18, 40–55 (2021). https://doi.org/10.1038/s41575-020-0342-4[↩]
- Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. J. Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016[↩]
- Haga J., Shimazu M., Wakabayashi G., Tanabe M., Kawachi S., Fuchimoto Y., Hoshino K., Morikawa Y., Kitajima M., Kitagawa Y. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transplant. 2008;14:1718–1724. doi: 10.1002/lt.21622[↩][↩][↩]
- Mao SA, Glorioso JM, Nyberg SL. Liver regeneration. Transl Res. 2014 Apr;163(4):352-62. doi: 10.1016/j.trsl.2014.01.005[↩]
- Miyaoka Y., Ebato K., Kato H., Arakawa S., Shimizu S., Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr. Biol. 2012;22:1166–1175. doi: 10.1016/j.cub.2012.05.016[↩][↩][↩]
- Van Haele M, Roskams T. Hepatic Progenitor Cells: An Update. Gastroenterol Clin North Am. 2017 Jun;46(2):409-420. doi: 10.1016/j.gtc.2017.01.011[↩]
- Raven A, Forbes SJ. Hepatic progenitors in liver regeneration. J Hepatol. 2018;69:1394–1395.[↩]
- Itoh T. Stem/progenitor cells in liver regeneration. Hepatology. 2016 Aug;64(2):663-8. doi: 10.1002/hep.28661[↩][↩][↩]
- Zhu C, Coombe DR, Zheng MH, Yeoh GC, Li L. Liver progenitor cell interactions with the extracellular matrix. J Tissue Eng Regen Med. 2013;7:757–766.[↩]
- Alwahsh SM, Rashidi H, Hay DC. Liver cell therapy: is this the end of the beginning? Cell Mol Life Sci. 2018;75:1307–1324.[↩]
- Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574.[↩]
- Michalopoulos GK, Khan Z. Liver Stem Cells: Experimental Findings and Implications for Human Liver Disease. Gastroenterology. 2015;149:876–882.[↩]
- Kholodenko IV, Yarygin KN. Cellular Mechanisms of Liver Regeneration and Cell-Based Therapies of Liver Diseases. Biomed Res Int. 2017;2017:8910821. doi: 10.1155/2017/8910821[↩][↩][↩]
- Best J, Manka P, Syn WK, Dollé L, van Grunsven LA, Canbay A. Role of liver progenitors in liver regeneration. Hepatobiliary Surg Nutr. 2015;4:48–58.[↩]
- Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. 2011;149:231–239.[↩]
- Dwyer BJ, Olynyk JK, Ramm GA, Tirnitz-Parker JE. TWEAK and LTβ Signaling during Chronic Liver Disease. Front Immunol. 2014;5:39.[↩]
- di Bello G, Vendemiale G, Bellanti F. Redox cell signaling and hepatic progenitor cells. Eur J Cell Biol. 2018;97:546–556.[↩]
- Liu W, Wang Y, Sun Y, Wu Y, Ma Q, Shi Y, He R, Zhang T, Ma Y, Zuo W, Wu Z. Clonal expansion of hepatic progenitor cells and differentiation into hepatocyte-like cells. Dev Growth Differ. 2019;61:203–211.[↩]
- Mao Y, Tang S, Yang L, Li K. Inhibition of the Notch Signaling Pathway Reduces the Differentiation of Hepatic Progenitor Cells into Cholangiocytes in Biliary Atresia. Cell Physiol Biochem. 2018;49:1074–1082.[↩]
- Hu C, Liang X, Fang S, Xu L, Gong M, Wang Y, Bi Y, Hong S, He Y. ATRA induces the differentiation of hepatic progenitor cells by upregulating microRNA-200a. In Vitro Cell Dev Biol Anim. 2019;55:713–722.[↩]
- Ma Z, Li F, Chen L, Gu T, Zhang Q, Qu Y, Xu M, Cai X, Lu L. Autophagy promotes hepatic differentiation of hepatic progenitor cells by regulating the Wnt/β-catenin signaling pathway. J Mol Histol. 2019;50:75–90.[↩]
- Zeng J, Jing Y, Shi R, Pan X, Lai F, Liu W, Li R, Gao L, Hou X, Wu M, Wei L. Autophagy regulates biliary differentiation of hepatic progenitor cells through Notch1 signaling pathway. Cell Cycle. 2016;15:1602–1610.[↩]
- Overi D, Carpino G, Cardinale V, Franchitto A, Safarikia S, Onori P, Alvaro D, Gaudio E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int J Mol Sci. 2018 Sep 25;19(10):2917. doi: 10.3390/ijms19102917[↩]
- Kiseleva, Y. V., Antonyan, S. Z., Zharikova, T. S., Tupikin, K. A., Kalinin, D. V., & Zharikov, Y. O. (2021). Molecular pathways of liver regeneration: A comprehensive review. World journal of hepatology, 13(3), 270–290. https://doi.org/10.4254/wjh.v13.i3.270[↩][↩][↩][↩][↩][↩]
- Yagi, S., Hirata, M., Miyachi, Y., & Uemoto, S. (2020). Liver Regeneration after Hepatectomy and Partial Liver Transplantation. International journal of molecular sciences, 21(21), 8414. https://doi.org/10.3390/ijms21218414[↩]
- Gilgenkrantz H, Collin de l’Hortet A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am J Pathol. 2018 Jun;188(6):1316-1327. doi: 10.1016/j.ajpath.2018.03.008[↩][↩]
- López-Luque J, Fabregat I. Revisiting the liver: from development to regeneration – what we ought to know! Int J Dev Biol. 2018;62(6-7-8):441-451. doi: 10.1387/ijdb.170264JL[↩][↩]
- Wirth KM, Kizy S, Steer CJ. Liver Regeneration in the Acute Liver Failure Patient. Clin Liver Dis. 2018 May;22(2):269-287. doi: 10.1016/j.cld.2018.01.004[↩][↩]
- Bhat M, Pasini E, Baciu C, Angeli M, Humar A, Macparland S, Feld J, McGilvray I. The basis of liver regeneration: A systems biology approach. Ann Hepatol. 2019;18:422–428.[↩]
- Abu Rmilah, A., Zhou, W., Nelson, E., Lin, L., Amiot, B., & Nyberg, S. L. (2019). Understanding the marvels behind liver regeneration. Wiley interdisciplinary reviews. Developmental biology, 8(3), e340. https://doi.org/10.1002/wdev.340[↩][↩][↩]
- Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13.[↩]
- Min JS, DeAngelis RA, Reis ES, Gupta S, Maurya MR, Evans C, Das A, Burant C, Lambris JD, Subramaniam S. Systems Analysis of the Complement-Induced Priming Phase of Liver Regeneration. J Immunol. 2016;197:2500–2508.[↩]
- Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016 Jun;64(6):1403-15. doi: 10.1016/j.jhep.2016.02.004[↩]
- Tao, Y., Wang, M., Chen, E., & Tang, H. (2017). Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediators of inflammation, 2017, 4256352. https://doi.org/10.1155/2017/4256352[↩][↩][↩]
- Fazel Modares N, Polz R, Haghighi F, Lamertz L, Behnke K, Zhuang Y, Kordes C, Häussinger D, Sorg UR, Pfeffer K, Floss DM, Moll JM, Piekorz RP, Ahmadian MR, Lang PA, Scheller J. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology. 2019 Dec;70(6):2075-2091. doi: 10.1002/hep.30774[↩]
- Gupta P, Venugopal SK. Augmenter of liver regeneration: A key protein in liver regeneration and pathophysiology. Hepatol Res. 2018;48:587–596.[↩]
- Ibrahim S, Weiss TS. Augmenter of liver regeneration: Essential for growth and beyond. Cytokine Growth Factor Rev. 2019;45:65–80.[↩]
- Nalesnik MA, Gandhi CR, Starzl TE. Augmenter of liver regeneration: A fundamental life protein. Hepatology. 2017;66:266–270.[↩]
- Fajardo-Puerta AB, Mato Prado M, Frampton AE, Jiao LR. Gene of the month: HGF. J Clin Pathol. 2016;69:575–579.[↩]
- Berasain C, Avila MA. The EGFR signalling system in the liver: from hepatoprotection to hepatocarcinogenesis. J Gastroenterol. 2014;49:9–23.[↩]
- Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci . 2007;104:17081–17086.[↩]
- Morales-González Á, Bautista M, Madrigal-Santillán E, Posadas-Mondragón A, Anguiano-Robledo L, Madrigal-Bujaidar E, Álvarez-González I, Fregoso-Aguilar T, Gayosso-Islas E, Sánchez-Moreno C, Morales-González JA. Nrf2 modulates cell proliferation and antioxidants defenses during liver regeneration induced by partial hepatectomy. Int J Clin Exp Pathol. 2017;10:7801–7811.[↩]
- Zou Y, Hu M, Lee J, Nambiar SM, Garcia V, Bao Q, Chan JY, Dai G. Nrf2 is essential for timely M phase entry of replicating hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2015;308:G262–G268.[↩][↩]
- Zou Y, Lee J, Nambiar SM, Hu M, Rui W, Bao Q, Chan JY, Dai G. Nrf2 is involved in maintaining hepatocyte identity during liver regeneration. PLoS One. 2014;9:e107423[↩]
- Liu HX, Keane R, Sheng L, Wan YJ. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502–1510.[↩]
- van de Laarschot LF, Jansen PL, Schaap FG, Olde Damink SW. The role of bile salts in liver regeneration. Hepatol Int. 2016;10:733–740.[↩]
- de Haan L, van der Lely SJ, Warps AK, Hofsink Q, Olthof PB, de Keijzer MJ, Lionarons DA, Mendes-Dias L, Bruinsma BG, Uygun K, Jaeschke H, Farrell GC, Teoh N, van Golen RF, Li T, Heger M. Post-hepatectomy liver regeneration in the context of bile acid homeostasis and the gut-liver signaling axis. J Clin Transl Res. 2018;4:1–46.[↩]
- Alvarez-Sola G, Uriarte I, Latasa MU, Jimenez M, Barcena-Varela M, Santamaría E, Urtasun R, Rodriguez-Ortigosa C, Prieto J, Berraondo P, Fernandez-Barrena MG, Berasain C, Avila MA. Bile acids, FGF15/19 and liver regeneration: From mechanisms to clinical applications. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1326–1334.[↩]
- Kong B, Sun R, Huang M, Chow MD, Zhong XB, Xie W, Lee YH, Guo GL. Fibroblast Growth Factor 15-Dependent and Bile Acid-Independent Promotion of Liver Regeneration in Mice. Hepatology. 2018;68:1961–1976.[↩]
- Péan N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L, Schoonjans K, Rainteau D, Tordjmann T. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–1460.[↩]
- Keitel V, Häussinger D. Role of TGR5 (GPBAR1) in Liver Disease. Semin Liver Dis. 2018;38:333–339.[↩]
- Merlen G, Ursic-Bedoya J, Jourdainne V, Kahale N, Glenisson M, Doignon I, Rainteau D, Tordjmann T. Bile acids and their receptors during liver regeneration: “Dangerous protectors”. Mol Aspects Med. 2017;56:25–33.[↩]
- Valizadeh A, Majidinia M, Samadi-Kafil H, Yousefi M, Yousefi B. The roles of signaling pathways in liver repair and regeneration. J Cell Physiol. 2019 Feb 15. doi: 10.1002/jcp.28336[↩]
- Russell JO, Monga SP. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol. 2018;13:351–378.[↩]
- Preziosi M, Okabe H, Poddar M, Singh S, Monga SP. Endothelial Wnts regulate β-catenin signaling in murine liver zonation and regeneration: A sequel to the Wnt-Wnt situation. Hepatol Commun. 2018;2:845–860.[↩][↩]
- Chapouly C, Guimbal S, Hollier PL, Renault MA. Role of Hedgehog Signaling in Vasculature Development, Differentiation, and Maintenance. Int J Mol Sci. 2019 Jun 24;20(12):3076. doi: 10.3390/ijms20123076[↩]
- Qi X, Schmiege P, Coutavas E, Wang J, Li X. Structures of human Patched and its complex with native palmitoylated sonic hedgehog. Nature. 2018;560:128–132.[↩]
- Jiang K, Liu Y, Fan J, Zhang J, Li XA, Evers BM, Zhu H, Jia J. PI(4)P Promotes Phosphorylation and Conformational Change of Smoothened through Interaction with Its C-terminal Tail. PLoS Biol. 2016;14:e1002375[↩]
- Hu A, Song BL. The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling. Curr Opin Cell Biol. 2019;61:31–38.[↩]
- Gao L, Zhang Z, Zhang P, Yu M, Yang T. Role of canonical Hedgehog signaling pathway in liver. Int J Biol Sci. 2018;14:1636–1644.[↩][↩]
- Ho Wei L, Arastoo M, Georgiou I, Manning DR, Riobo-Del Galdo NA. Activation of the Gi protein-RHOA axis by non-canonical Hedgehog signaling is independent of primary cilia. PLoS One. 2018;13:e0203170[↩]
- Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–1723.[↩]
- Machado MV, Diehl AM. Hedgehog signalling in liver pathophysiology. J Hepatol. 2018;68:550–562.[↩]
- Langiewicz M, Schlegel A, Saponara E, Linecker M, Borger P, Graf R, Humar B, Clavien PA. Hedgehog pathway mediates early acceleration of liver regeneration induced by a novel two-staged hepatectomy in mice. J Hepatol. 2017;66:560–570.[↩]
- Jalan-Sakrikar N, De Assuncao TM, Lu J, Almada LL, Lomberk G, Fernandez-Zapico ME, Urrutia R, Huebert RC. Hedgehog Signaling Overcomes an EZH2-Dependent Epigenetic Barrier to Promote Cholangiocyte Expansion. PLoS One. 2016;11:e0168266[↩]
- Swiderska-Syn M, Xie G, Michelotti GA, Jewell ML, Premont RT, Syn WK, Diehl AM. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–244.[↩]
- Du K, Hyun J, Premont RT, Choi SS, Michelotti GA, Swiderska-Syn M, Dalton GD, Thelen E, Rizi BS, Jung Y, Diehl AM. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology 2018; 154: 1465-1479. :e13.[↩]
- Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–373.[↩]
- Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017;97:1235–1294.[↩]
- Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol. 2013;37:447–454.[↩]
- Adams JM, Jafar-Nejad H. The Roles of Notch Signaling in Liver Development and Disease. Biomolecules. 2019 Oct 14;9(10):608. doi: 10.3390/biom9100608[↩]
- Lu J, Zhou Y, Hu T, Zhang H, Shen M, Cheng P, Dai W, Wang F, Chen K, Zhang Y, Wang C, Li J, Zheng Y, Yang J, Zhu R, Wang J, Lu W, Xia Y, De Assuncao TM, Jalan-Sakrikar N, Huebert RC, Bin Zhou, Guo C. Notch Signaling Coordinates Progenitor Cell-Mediated Biliary Regeneration Following Partial Hepatectomy. Sci Rep. 2016;6:22754[↩]
- Morell CM, Strazzabosco M. Notch signaling and new therapeutic options in liver disease. J Hepatol. 2014;60:885–890.[↩]
- Ortica S, Tarantino N, Aulner N, Israël A, Gupta-Rossi N. The 4 Notch receptors play distinct and antagonistic roles in the proliferation and hepatocytic differentiation of liver progenitors. FASEB J. 2014;28:603–614.[↩]
- Zhang F, Zhang J, Li X, Li B, Tao K, Yue S. Notch signaling pathway regulates cell cycle in proliferating hepatocytes involved in liver regeneration. J Gastroenterol Hepatol. 2018;33:1538–1547.[↩]
- Yang X, He C, Zhu L, Zhao W, Li S, Xia C, Xu C. Comparative Analysis of Regulatory Role of Notch Signaling Pathway in 8 Types Liver Cell During Liver Regeneration. Biochem Genet. 2019;57:1–19.[↩]
- Liu M, Chen P. Proliferation‑inhibiting pathways in liver regeneration (Review). Mol Med Rep. 2017 Jul;16(1):23-35. doi: 10.3892/mmr.2017.6613[↩]
- Khan MGM, Ghosh A, Variya B, Santharam MA, Kandhi R, Ramanathan S, Ilangumaran S. Hepatocyte growth control by SOCS1 and SOCS3. Cytokine. 2019;121:154733.[↩]
- Pascale RM, Feo F, Calvisi DF. The complex role of bone morphogenetic protein 9 in liver damage and regeneration: New evidence from in vivo and in vitro studies. Liver Int. 2018;38:1547–1549.[↩]
- Addante A, Roncero C, Almalé L, Lazcanoiturburu N, García-Álvaro M, Fernández M, Sanz J, Hammad S, Nwosu ZC, Lee SJ, Fabregat I, Dooley S, Ten Dijke P, Herrera B, Sánchez A. Bone morphogenetic protein 9 as a key regulator of liver progenitor cells in DDC-induced cholestatic liver injury. Liver Int. 2018;38:1664–1675.[↩]
- Marí M, Morales A. Bone morphogenetic protein-9/activin-like kinase 1 axis a new target for hepatic regeneration and fibrosis treatment in liver injury. Hepatobiliary Surg Nutr. 2017;6:414–416.[↩]
- Huck I, Gunewardena S, Espanol-Suner R, Willenbring H, Apte U. Hepatocyte Nuclear Factor 4 Alpha Activation Is Essential for Termination of Liver Regeneration in Mice. Hepatology. 2019;70:666–681.[↩][↩]
- Zhou J, Sun X, Yang L, Wang L, Ran G, Wang J, Cao Q, Wu L, Bryant A, Ling C, Pi L. Hepatocyte nuclear factor 4α negatively regulates connective tissue growth factor during liver regeneration. FASEB J. 2020;34:4970–4983.[↩]
- Wang P, Cong M, Liu T, Xu H, Wang L, Sun G, Yang A, Zhang D, Huang J, Sun Y, Zhao W, Ma H, Jia J, You H. Inhibitory effects of HNF4α on migration/maltransformation of hepatic progenitors: HNF4α-overexpressing hepatic progenitors for liver repopulation. Stem Cell Res Ther. 2017;8:183.[↩]
- Michalopoulos GK. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384–1392.[↩][↩]
- Konishi T, Schuster RM, Lentsch AB. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2018;314:G471–G482.[↩]
- Lu L, Finegold MJ, Johnson RL. Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp Mol Med. 2018;50:e423.[↩]
- Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20:211–226.[↩]
- Patel SH, Camargo FD, Yimlamai D. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology. 2017;152:533–545.[↩]
- Forbes SJ, Newsome PN. Liver regeneration – mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473–485.[↩]
- Shang H, Wang Z, Song Y. Liver progenitor cells-mediated liver regeneration in liver cirrhosis. Hepatol Int. 2016;10:440–447.[↩]
- Forbes SJ, Newsome PN. Liver regeneration – mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016 Aug;13(8):473-85. doi: 10.1038/nrgastro.2016.97[↩][↩]
- Stutchfield BM, Antoine DJ, Mackinnon AC, et al. CSF1 Restores Innate Immunity After Liver Injury in Mice and Serum Levels Indicate Outcomes of Patients With Acute Liver Failure. Gastroenterology. 2015;149:1896–1909 e1814[↩]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. RETRACTED: Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012 May;56(5):1070-1079. doi: 10.1016/j.jhep.2011.12.019. Epub 2012 Jan 17. Retraction in: J Hepatol. 2020 Nov;73(5):1297.[↩][↩]
- VandePol CJ, Garnick MB. Clinical applications of recombinant macrophage-colony stimulating factor (rhM-CSF). Biotechnol Ther. 1991;2(3-4):231-9.[↩]
- Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016 Oct 13;17(10):1712. doi: 10.3390/ijms17101712[↩]
- Lv T, Kong L, Jiang L, Wu H, Wen T, Shi Y, Yang J. Dicer1 facilitates liver regeneration in a manner dependent on the inhibitory effect of miR-21 on Pten and Rhob expression. Life Sci. 2019 Sep 1;232:116656. doi: 10.1016/j.lfs.2019.116656[↩]
- John K, Hadem J, Krech T, Wahl K, Manns MP, Dooley S, Batkai S, Thum T, Schulze-Osthoff K, Bantel H. MicroRNAs play a role in spontaneous recovery from acute liver failure. Hepatology. 2014 Oct;60(4):1346-55. doi: 10.1002/hep.27250[↩]
- Kojima, H., Nakamura, K., & Kupiec-Weglinski, J. W. (2020). Therapeutic targets for liver regeneration after acute severe injury: a preclinical overview. Expert opinion on therapeutic targets, 24(1), 13–24. https://doi.org/10.1080/14728222.2020.1712361[↩][↩][↩][↩]
- Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015 Aug;17(8):971-983. doi: 10.1038/ncb3203[↩]
- Schiødt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, Lee WM. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl. 2006 Dec;12(12):1776-81. doi: 10.1002/lt.20886[↩]
- Tao Y, Wang M, Chen E, Tang H. Liver Regeneration: Analysis of the Main Relevant Signaling Molecules. Mediators Inflamm. 2017;2017:4256352[↩]
- Manco R, Leclercq IA, Clerbaux LA. Liver Regeneration: Different Sub-Populations of Parenchymal Cells at Play Choreographed by an Injury-Specific Microenvironment. Int J Mol Sci. 2018 Dec 18;19(12):4115. doi: 10.3390/ijms19124115[↩]
- Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013 Jan;3(1):485-513. doi: 10.1002/cphy.c120014[↩]
- Shi L, Zhang S, Huang Z, Hu F, Zhang T, Wei M, Bai Q, Lu B, Ji L. Baicalin promotes liver regeneration after acetaminophen-induced liver injury by inducing NLRP3 inflammasome activation. Free Radic Biol Med. 2020 Nov 20;160:163-177. doi: 10.1016/j.freeradbiomed.2020.05.012[↩]
- So J, Kim M, Lee SH, Ko S, Lee DA, Park H, Azuma M, Parsons MJ, Prober D, Shin D. Attenuating the Epidermal Growth Factor Receptor-Extracellular Signal-Regulated Kinase-Sex-Determining Region Y-Box 9 Axis Promotes Liver Progenitor Cell-Mediated Liver Regeneration in Zebrafish. Hepatology. 2021 Apr;73(4):1494-1508. doi: 10.1002/hep.31437[↩]
- Xiang X, Feng D, Hwang S, Ren T, Wang X, Trojnar E, Matyas C, Mo R, Shang D, He Y, Seo W, Shah VH, Pacher P, Xie Q, Gao B. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol. 2020 Apr;72(4):736-745. doi: 10.1016/j.jhep.2019.11.013[↩]
- Li CX, Wang HW, Jiang WJ, Li GC, Zhang YD, Luo CH, Li XC. The Inhibition of Aldose Reductase Accelerates Liver Regeneration through Regulating Energy Metabolism. Oxid Med Cell Longev. 2020 Feb 27;2020:3076131. doi: 10.1155/2020/3076131[↩]
- Loforese G, Malinka T, Keogh A, Baier F, Simillion C, Montani M, Halazonetis TD, Candinas D, Stroka D. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol Med. 2017;9:46–60.[↩]
- Hu, C., Zhao, L., Zhang, L., Bao, Q., & Li, L. (2020). Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem cell research & therapy, 11(1), 377. https://doi.org/10.1186/s13287-020-01895-1[↩]
- Varderidou-Minasian, S., & Lorenowicz, M. J. (2020). Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics, 10(13), 5979–5997. https://doi.org/10.7150/thno.40122[↩]
- Hu, C., Zhao, L., Wu, Z., & Li, L. (2020). Transplantation of mesenchymal stem cells and their derivatives effectively promotes liver regeneration to attenuate acetaminophen-induced liver injury. Stem cell research & therapy, 11(1), 88. https://doi.org/10.1186/s13287-020-01596-9[↩]
- Zhang, S., Yang, Y., Fan, L., Zhang, F., & Li, L. (2020). The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Annals of translational medicine, 8(8), 565. https://doi.org/10.21037/atm.2020.03.218[↩]
- Hu, C., Wu, Z., & Li, L. (2020). Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. International journal of biological sciences, 16(5), 893–903. https://doi.org/10.7150/ijbs.39725[↩]
- Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012 Mar;255(3):405-14. doi: 10.1097/SLA.0b013e31824856f5[↩]
- Lorenz L, Axnick J, Buschmann T, Henning C, Urner S, Fang S, Nurmi H, Eichhorst N, Holtmeier R, Bódis K, Hwang JH, Müssig K, Eberhard D, Stypmann J, Kuss O, Roden M, Alitalo K, Häussinger D, Lammert E. Mechanosensing by β1 integrin induces angiocrine signals for liver growth and survival. Nature. 2018 Oct;562(7725):128-132. doi: 10.1038/s41586-018-0522-3[↩]
- Petrowsky H, Breitenstein S, Slankamenac K, Vetter D, Lehmann K, Heinrich S, DeOliveira ML, Jochum W, Weishaupt D, Frauenfelder T, Graf R, Clavien PA. Effects of pentoxifylline on liver regeneration: a double-blinded, randomized, controlled trial in 101 patients undergoing major liver resection. Ann Surg. 2010 Nov;252(5):813-22. doi: 10.1097/SLA.0b013e3181fcbc5e[↩][↩]
- Ibrahim ES, Saleh SM, El Hoseeny M, El shaarawy A. Effect of omega-3 on hepatic regeneration in adult living donors undergoing hepatic resections for liver transplantation: A randomized controlled trial. J Crit Care. 2016 Feb;31(1):157-62. doi: 10.1016/j.jcrc.2015.09.022[↩][↩]
- Padickakudy R, Pereyra D, Offensperger F, Jonas P, Oehlberger L, Schwarz C, Haegele S, Assinger A, Brostjan C, Gruenberger T, Starlinger P. Bivalent role of intra-platelet serotonin in liver regeneration and tumor recurrence in humans. J Hepatol. 2017 Dec;67(6):1243-1252. doi: 10.1016/j.jhep.2017.08.009[↩][↩][↩][↩]
- Alvarenga DM, Mattos MS, Lopes ME, Marchesi SC, Araújo AM, Nakagaki BN, Santos MM, David BA, De Souza VA, Carvalho É, Sousa Pereira RV, Marques PE, Mafra K, de Castro Oliveira HM, de Miranda CDM, Diniz AB, de Oliveira THC, Teixeira MM, Rezende RM, Antunes MM, Menezes GB. Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure. Cells. 2018 Dec 6;7(12):247. doi: 10.3390/cells7120247[↩][↩]
- Olthof PB, Huisman F, Schaap FG, van Lienden KP, Bennink RJ, van Golen RF, Heger M, Verheij J, Jansen PL, Olde Damink SW, van Gulik TM. Effect of obeticholic acid on liver regeneration following portal vein embolization in an experimental model. Br J Surg. 2017 Apr;104(5):590-599. doi: 10.1002/bjs.10466[↩][↩]
- Basta G, Del Turco S, Navarra T, Lee WM; Acute Liver Failure Study Group. Circulating levels of soluble receptor for advanced glycation end products and ligands of the receptor for advanced glycation end products in patients with acute liver failure. Liver Transpl. 2015 Jun;21(6):847-54. doi: 10.1002/lt.24129[↩][↩][↩]
- Bird TG, Müller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu WY, Jamieson T, Govaere O, Campbell AD, Ferreira-Gonzalez S, Cole AM, Hay T, Simpson KJ, Clark W, Hedley A, Clarke M, Gentaz P, Nixon C, Bryce S, Kiourtis C, Sprangers J, Nibbs RJB, Van Rooijen N, Bartholin L, McGreal SR, Apte U, Barry ST, Iredale JP, Clarke AR, Serrano M, Roskams TA, Sansom OJ, Forbes SJ. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med. 2018 Aug 15;10(454):eaan1230. doi: 10.1126/scitranslmed.aan1230[↩][↩]
- Toshima T, Shirabe K, Fukuhara T, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Okano S, Maehara Y. Suppression of autophagy during liver regeneration impairs energy charge and hepatocyte senescence in mice. Hepatology. 2014 Jul;60(1):290-300. doi: 10.1002/hep.27140[↩]
- Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017 Jun 13;8:15691. doi: 10.1038/ncomms15691[↩]
- Wadhera V, Harimoto N, Lubezky N, Gomatos I, Facciuto M, Gonzalez D, Stueck A, Fiel MI, Schiano T, Facciuto ME. The impact of donor liver allograft fibrosis on patients undergoing liver transplantation. Clin Transplant. 2018 Mar;32(3):e13187. doi: 10.1111/ctr.13187[↩]
- Kubota T, Hata K, Sozu T, Ueda Y, Hirao H, Okamura Y, Tamaki I, Yoshikawa J, Kusakabe J, Tanaka H, Kageyama S, Anazawa T, Yoshizawa A, Yagi S, Yamashiki N, Okajima H, Kaido T, Uemoto S. Impact of Donor Age on Recipient Survival in Adult-to-Adult Living-donor Liver Transplantation. Ann Surg. 2018 Jun;267(6):1126-1133. doi: 10.1097/SLA.0000000000002194[↩]
- Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. The adverse effects of preoperative cholangitis on the outcome of portal vein embolization and subsequent major hepatectomies. Surgery. 2014 Nov;156(5):1190-6. doi: 10.1016/j.surg.2014.04.036[↩]
- Thompson J, Jones N, Al-Khafaji A, Malik S, Reich D, Munoz S, MacNicholas R, Hassanein T, Teperman L, Stein L, Duarte-Rojo A, Malik R, Adhami T, Asrani S, Shah N, Gaglio P, Duddempudi A, Borg B, Jalan R, Brown R, Patton H, Satoskar R, Rossi S, Parikh A, ElSharkawy A, Mantry P, Sher L, Wolf D, Hart M, Landis C, Wigg A, Habib S, McCaughan G, Colquhoun S, Henry A, Bedard P, Landeen L, Millis M, Ashley R, Frank W, Henry A, Stange J, Subramanian R; VTI-208 Study Group. Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: A multinational, prospective, controlled, randomized trial. Liver Transpl. 2018 Mar;24(3):380-393. doi: 10.1002/lt.24986[↩]
- Soltys KA, Setoyama K, Tafaleng EN, Soto Gutiérrez A, Fong J, Fukumitsu K, Nishikawa T, Nagaya M, Sada R, Haberman K, Gramignoli R, Dorko K, Tahan V, Dreyzin A, Baskin K, Crowley JJ, Quader MA, Deutsch M, Ashokkumar C, Shneider BL, Squires RH, Ranganathan S, Reyes-Mugica M, Dobrowolski SF, Mazariegos G, Elango R, Stolz DB, Strom SC, Vockley G, Roy-Chowdhury J, Cascalho M, Guha C, Sindhi R, Platt JL, Fox IJ. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017 May;66(5):987-1000. doi: 10.1016/j.jhep.2016.12.017[↩]
- Kojima H, Yasuchika K, Fukumitsu K, Ishii T, Ogiso S, Miyauchi Y, Yamaoka R, Kawai T, Katayama H, Yoshitoshi-Uebayashi EY, Kita S, Yasuda K, Sasaki N, Komori J, Uemoto S. Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. Am J Transplant. 2018 Jun;18(6):1351-1359. doi: 10.1111/ajt.14666[↩]
- Zhu, X. H., Wu, Y. F., Qiu, Y. D., Jiang, C. P., & Ding, Y. T. (2012). Liver-protecting effects of omega-3 fish oil lipid emulsion in liver transplantation. World journal of gastroenterology, 18(42), 6141–6147. https://doi.org/10.3748/wjg.v18.i42.6141[↩]
- Yan XP, Wang S, Yang Y, Qiu YD. Effects of n-3 polyunsaturated fatty acids on rat livers after partial hepatectomy via LKB1-AMPK signaling pathway. Transplant Proc. 2011 Dec;43(10):3604-12. doi: 10.1016/j.transproceed.2011.10.045[↩]
- Zhong D, Liu X, Khuri FR, Sun SY, Vertino PM, Zhou W. LKB1 is necessary for Akt-mediated phosphorylation of proapoptotic proteins. Cancer Res. 2008 Sep 15;68(18):7270-7. doi: 10.1158/0008-5472.CAN-08-1484[↩]
- Martínez-Chantar ML, Vázquez-Chantada M, Garnacho M, Latasa MU, Varela-Rey M, Dotor J, Santamaria M, Martínez-Cruz LA, Parada LA, Lu SC, Mato JM. S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology. 2006 Jul;131(1):223-32. doi: 10.1053/j.gastro.2006.04.019[↩]
- Merlen G, Gentric G, Celton-Morizur S, Foretz M, Guidotti JE, Fauveau V, Leclerc J, Viollet B, Desdouets C. AMPKα1 controls hepatocyte proliferation independently of energy balance by regulating Cyclin A2 expression. J Hepatol. 2014 Jan;60(1):152-9. doi: 10.1016/j.jhep.2013.08.025[↩]
- de Moraes ACN, de Andrade CBV, Ramos IPR, Dias ML, Batista CMP, Pimentel CF, de Carvalho JJ, Goldenberg RCDS. Resveratrol promotes liver regeneration in drug-induced liver disease in mice. Food Res Int. 2021 Apr;142:110185. doi: 10.1016/j.foodres.2021.110185[↩][↩][↩]
- Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T, Hashimoto I, Shibasaki Y, Yasue H, Ohkohchi N. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008 Sep;49(3):363-72. doi: 10.1016/j.jhep.2008.04.019[↩]
- Starlinger P, Haegele S, Offensperger F, Oehlberger L, Pereyra D, Kral JB, Schrottmaier WC, Badrnya S, Reiberger T, Ferlitsch A, Stift J, Luf F, Brostjan C, Gruenberger T, Assinger A. The profile of platelet α-granule released molecules affects postoperative liver regeneration. Hepatology. 2016 May;63(5):1675-88. doi: 10.1002/hep.28331[↩]
- Sarrouilhe D, Clarhaut J, Defamie N, Mesnil M. Serotonin and cancer: what is the link? Curr Mol Med. 2015;15(1):62-77. doi: 10.2174/1566524015666150114113411[↩]
- Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012 Mar;142(3):505-512.e1. doi: 10.1053/j.gastro.2011.11.027[↩][↩]
- Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019 Jun 17;129(7):2629-2639. doi: 10.1172/JCI124616[↩]
- Otao R, Beppu T, Isiko T, Mima K, Okabe H, Hayashi H, Masuda T, Chikamoto A, Takamori H, Baba H. External biliary drainage and liver regeneration after major hepatectomy. Br J Surg. 2012 Nov;99(11):1569-74. doi: 10.1002/bjs.8906[↩]
- Alvarez-Sola G, Uriarte I, Latasa MU, Jimenez M, Barcena-Varela M, Santamaría E, Urtasun R, Rodriguez-Ortigosa C, Prieto J, Berraondo P, Fernandez-Barrena MG, Berasain C, Avila MA. Bile acids, FGF15/19 and liver regeneration: From mechanisms to clinical applications. Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1326-1334. doi: 10.1016/j.bbadis.2017.06.025[↩]
- Naugler WE, Tarlow BD, Fedorov LM, Taylor M, Pelz C, Li B, Darnell J, Grompe M. Fibroblast Growth Factor Signaling Controls Liver Size in Mice With Humanized Livers. Gastroenterology. 2015 Sep;149(3):728-40.e15. doi: 10.1053/j.gastro.2015.05.043[↩]
- Padrissa-Altés S, Bachofner M, Bogorad RL, Pohlmeier L, Rossolini T, Böhm F, Liebisch G, Hellerbrand C, Koteliansky V, Speicher T, Werner S. Control of hepatocyte proliferation and survival by Fgf receptors is essential for liver regeneration in mice. Gut. 2015 Sep;64(9):1444-53. doi: 10.1136/gutjnl-2014-307874[↩]
- Alvarez-Sola G, Uriarte I, Latasa MU, Fernandez-Barrena MG, Urtasun R, Elizalde M, Barcena-Varela M, Jiménez M, Chang HC, Barbero R, Catalán V, Rodríguez A, Frühbeck G, Gallego-Escuredo JM, Gavaldà-Navarro A, Villarroya F, Rodriguez-Ortigosa CM, Corrales FJ, Prieto J, Berraondo P, Berasain C, Avila MA. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut. 2017 Oct;66(10):1818-1828. doi: 10.1136/gutjnl-2016-312975[↩]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015 Mar 14;385(9972):956-65. doi: 10.1016/S0140-6736(14)61933-4. Epub 2014 Nov 7. Erratum in: Lancet. 2015 Mar 14;385(9972):946. Erratum in: Lancet. 2016 Apr 16;387(10028):1618[↩]
- Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, Lu Y, Rong LL, Hofmann MA, Kislinger T, Pachydaki SI, Jenkins DG, Weinberg A, Lefkowitch J, Rogiers X, Yan SF, Schmidt AM, Emond JC. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005 Feb 7;201(3):473-84. doi: 10.1084/jem.20040934[↩]
- Koh EJ, Yoon SJ, Lee SM. Losartan protects liver against ischaemia/reperfusion injury through PPAR-γ activation and receptor for advanced glycation end-products down-regulation. Br J Pharmacol. 2013 Jul;169(6):1404-16. doi: 10.1111/bph.12229[↩]
- Faivre S, Santoro A, Kelley RK, Gane E, Costentin CE, Gueorguieva I, Smith C, Cleverly A, Lahn MM, Raymond E, Benhadji KA, Giannelli G. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 2019 Aug;39(8):1468-1477. doi: 10.1111/liv.14113[↩]
- Liver Transplantation Treatment & Management. https://emedicine.medscape.com/article/431783-treatment#d16[↩]