Hydroxychloroquine
Hydroxychloroquine also known as hydroxychloroquine sulfate, Plaquenil or Plaquenil Sulfate, is a derivative of chloroquine, was developed in 1946. Both chloroquine and hydroxychloroquine chemically belongs to the 4-aminoquinolones class 1. Hydroxychloroquine is a medicine used to prevent and treat acute attacks of malaria, a disease caused by parasites that enter your body through the bite of a mosquito. However, hydroxychloroquine is not used to treat severe or complicated malaria. Malaria is common in areas such as Africa, South America, and Southern Asia. Hydroxychloroquine is not effective against all strains of malaria or against malaria in areas where the infection has been resistant to a similar drug called chloroquine. Hydroxychloroquine is also used to treat autoimmune diseases, such as discoid lupus erythematosus (DLE, a chronic inflammatory condition of the skin) or systemic lupus erythematosus (SLE, a chronic inflammatory condition of the body) and rheumatoid arthritis, in patients whose symptoms have not improved with other treatments. Hydroxychloroquine is not approved for treating lupus or rheumatoid arthritis in anyone younger than 18 years old. Hydroxychloroquine has also been used as therapy of porphyria cutanea tarda where it seems to act by increasing excretion of porphyrins 2.
Hydroxychloroquine may cause blurred vision and may impair your reactions. Check with your doctor immediately if blurred vision, difficulty with reading, or any other change in vision occurs during or after treatment. Your doctor may want your eyes be checked by an ophthalmologist (eye doctor). Avoid driving or hazardous activity until you know how this medicine will affect you.
Hydroxychloroquine comes as a tablet to take by mouth. If you are an adult and taking hydroxychloroquine to prevent malaria, one dose is usually taken once a week on exactly the same day of each week. You will begin treatment 1 to 2 weeks before you travel to an area where malaria is common and then continue during your time in the area and for 4 weeks after you return. If you are an adult and taking hydroxychloroquine to treat malaria, the first dose is usually taken right away, followed by another dose 6 to 8 hours later and then additional doses on each of the next 2 days. For prevention or treatment of malaria in infants and children, the amount of hydroxychloroquine is based on the child’s weight. Your doctor will calculate this amount and tell you how much hydroxychloroquine your child should receive.
If you are taking hydroxychloroquine to treat lupus erythematosus (DLE or SLE), it is usually taken once or twice a day. If you are taking hydroxychloroquine to treat rheumatoid arthritis, it is usually taken once or twice a day.
Swallow the tablets whole; do not split, chew, or crush them.
Hydroxychloroquine tablets can be taken with a glass of milk or a meal to decrease nausea. If you are taking antacids, take them 4 hours before or 4 hours after hydroxychloroquine. If you are taking ampicillin, take it at least 2 hours before or 2 hours after hydroxychloroquine. Avoid taking an antacid or Kaopectate (kaolin-pectin) within 4 hours before or 4 hours after you take hydroxychloroquine.
Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take hydroxychloroquine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
If you are taking hydroxychloroquine for symptoms of rheumatoid arthritis, your symptoms should improve within 6 months. If your rheumatoid arthritis symptoms do not improve, or if they worsen, stop taking the drug and call your doctor. Once you and your doctor are sure the drug works for you, do not stop taking hydroxychloroquine without talking to your doctor. Symptoms of rheumatoid arthritis will return if you stop taking hydroxychloroquine.
Your doctor may order certain lab tests and electrocardiograms (ECG, a test to monitor your heart rate and rhythm) to check your response to hydroxychloroquine.
Hydroxychloroquine common side effects include headaches, dizziness, gastrointestinal upset and rash. Hydroxychloroquine can cause serious vision problems. Retinopathy is a serious side effect of hydroxychloroquine and regular ophthalmologic monitoring is recommended for patients on long term therapy.
If you experience any changes in vision, stop taking hydroxychloroquine and call your doctor immediately. If you are taking hydroxychloroquine for a long period of time, your doctor will recommend frequent eye exams. It is very important that you keep these appointments.
Do not let anyone else take your medication. Ask your pharmacist any questions you have about refilling your prescription.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.
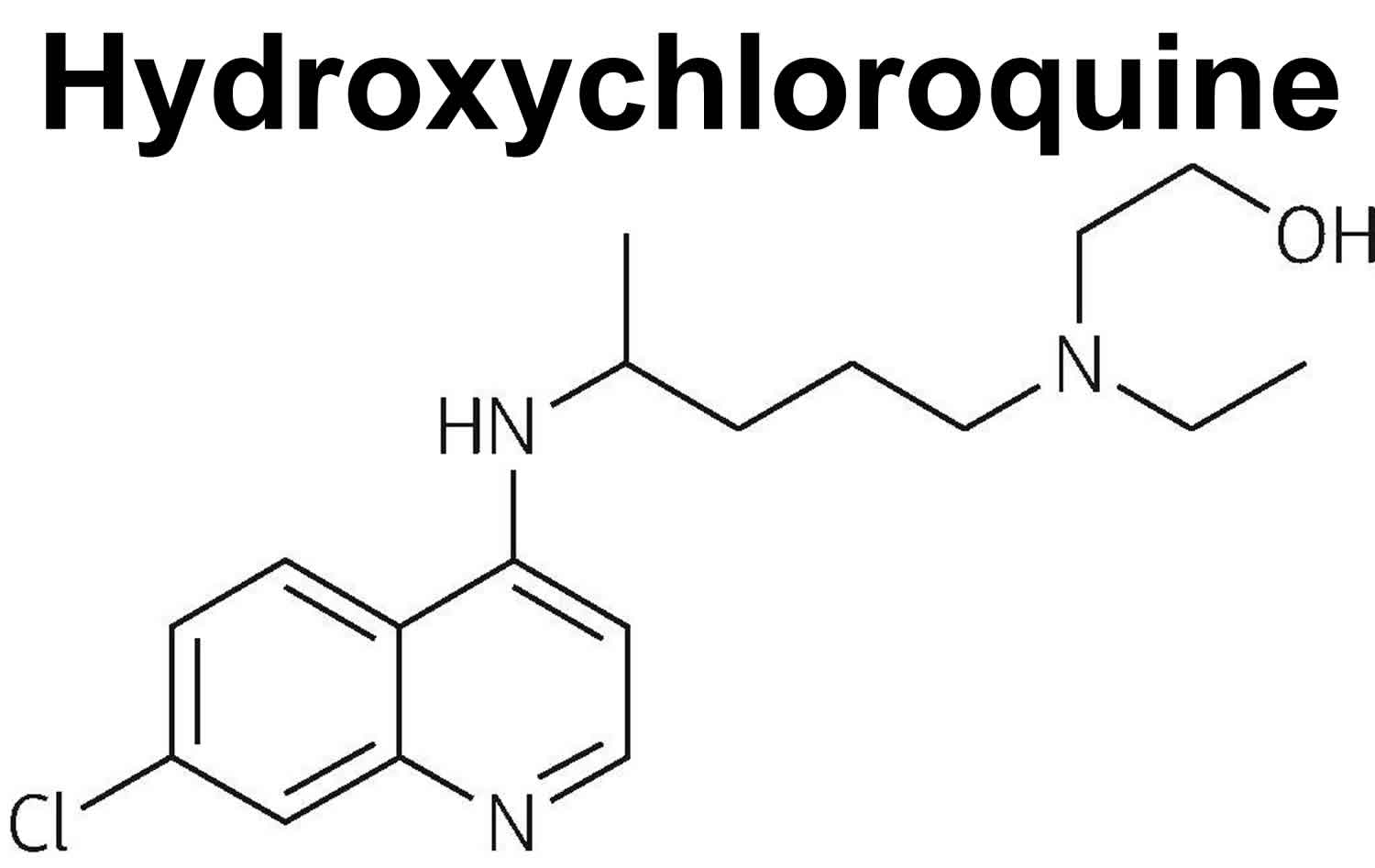
Figure 1. Chemical structure of hydroxychloroquine
Hydroxychloroquine can cause dangerous effects on your heart, especially if you also use certain other medicines including the antibiotic azithromycin (Z-Pak). Seek emergency medical attention if you have fast or pounding heartbeats and sudden dizziness (like you might pass out).
Taking hydroxychloroquine long-term or at high doses may cause irreversible damage to the retina of your eye that could progress to permanent vision problems.
Stop taking hydroxychloroquine and call your doctor at once if you have blurred vision, trouble focusing, distorted vision, blind spots, trouble reading, changes in your color vision, increased sensitivity to light.
Hydroxychloroquine has been studied for the treatment and prevention of coronavirus disease 2019 (COVID-19).
On March 28, 2020, the Food and Drug Administration (FDA) granted an Emergency Use Authorization (EUA) for use of oral formulations of hydroxychloroquine and chloroquine to treat adults and adolescents who weigh at least 110 pounds (50 kg) and who are hospitalized with COVID-19, but who are unable to participate in a clinical study 3. However, FDA canceled this on June 15, 2020 because clinical studies showed that hydroxychloroquine is unlikely to be effective for treatment of COVID-19 in these patients and some serious side effects, such as irregular heartbeat, were reported 4.
The COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of chloroquine or hydroxychloroquine and/or azithromycin for the treatment of COVID-19 in hospitalized patients and in nonhospitalized patients 5. The FDA and the National Institutes of Health state that hydroxychloroquine should ONLY be taken for the treatment of COVID-19 under the direction of a doctor in a clinical study. Do not buy this medication online without a prescription. If you experience irregular heartbeats, dizziness, or fainting while taking hydroxychloroquine, call your local emergency services number for emergency medical treatment. If you have other side effects, be sure to tell your doctor.
Is hydroxychloroquine effective for COVID-19?
Coronavirus disease 2019 (COVID-19) is caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus type 2), the cause of the global pandemic of respiratory illness. In laboratory tests and cell culture systems, both hydroxychloroquine and chloroquine have been shown to have a spectrum of antiviral activity that is believed to be due to interference with viral binding to glycoprotein cell receptors or inhibition of endosomal pH regulation, which inhibits fusion between SARS-CoV-2 and the host cell membrane 6. Hydroxychloroquine can prevent the SARS-CoV and SARS-CoV-2 viruses from attaching to and entering cells. Chloroquine inhibits glycosylation of the cellular angiotensin-converting enzyme 2 (ACE 2) receptor, which may interfere with the binding of SARS-CoV to the cell receptor 7. In vitro studies (test tube studies) have suggested that both chloroquine and hydroxychloroquine may block the transport of SARS-CoV-2 from early endosomes to endolysosomes, possibly preventing the release of the viral genome 7. Both chloroquine and hydroxychloroquine also have immunomodulatory effects, which have been hypothesized to be another potential mechanism of action for the treatment of COVID-19.
Azithromycin has antiviral and anti-inflammatory properties. When used in combination with hydroxychloroquine, it has been shown to have a synergistic effect on SARS-CoV-2 in vitro and in molecular modeling studies 8, 9. However, despite demonstrating antiviral activity in some in vitro systems, neither hydroxychloroquine plus azithromycin nor hydroxychloroquine alone reduced upper or lower respiratory tract viral loads or demonstrated clinical efficacy in a rhesus macaque model 10.
In face of the growing burden of severe illness posed by COVID-19, chloroquine and hydroxychloroquine were proposed as possibly effective in preventing or ameliorating the course and prevent mortality 11, 12, 13. However, multiple human clinical studies have provided data that hydroxychloroquine (Plaquenil) does not provide a medical benefit for hospitalized patients with COVID-19 14, 15, 16.
Use of hydroxychloroquine is controversial, and has been politicized in the U.S. by various groups. Mixed studies have reported both a positive and negative effect, and data may not be robust or reliable: it can include data from study reviews, nonrandomized groups, retrospective research, observational data or from a statistically small sample size of patients. Research for COVID-19 is often quick to be published in non-peer reviewed, preprint online services due to the urgency of the pandemic. However, in general, preprint data should not be used to guide clinical practice. In addition, some hydroxychloroquine studies have been retracted due to lack of confidence in the data, including a Lancet study 17 and one from the New England Journal of Medicine 18.
Hydroxychloroquine, chloroquine, and azithromycin are not approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 5. Furthermore, in June 15, 2020, the FDA revoked the emergency use authorization (EUA) of oral hydroxychloroquine and chloroquine phosphate for the treatment of COVID-19 4. An emergency use authorization (EUA) can allow quicker access to critical medical products when there are no approved alternative options.
- Based on an evaluation of the scientific data to date, the FDA concluded that chloroquine and hydroxychloroquine are not likely to be effective in the treatment of COVID-19 for the authorized uses in the EUA.
- In addition, the risk for serious side effects with hydroxychloroquine and chloroquine phosphate are a concern. This includes the possibility of adverse cardiovascular (heart) events such as an abnormal heart rhythm which could be fatal.
- Additional worldwide studies are still ongoing to assess the use of these agents for the treatment or prevention or COVID-19, including early-stage outpatient and use with supplements such as zinc or vitamin D or with azithromycin. However, the FDA states hydroxychloroquine should not be used outside of clinical trials in the U.S.
The World Health Organization (WHO) and the U.S. National Institutes of Health (NIH) have also stopped studies evaluating hydroxychloroquine for the treatment of COVID-19 due to a lack of benefit. Current NIH and US treatment guidelines do not recommend use of hydroxychloroquine and chloroquine phosphate for COVID-19 treatment outside of clinical studies.
Although earlier studies suggested that hydroxychloroquine could inhibit the SARs-CoV-2 virus and was more potent than chloroquine, recent studies do not support the use of hydroxychloroquine or chloroquine phosphate. The FDA stated on June 15, 2020 that the suggested dosing regimens for chloroquine and hydroxychloroquine are unlikely to kill or inhibit the virus that causes COVID-19 4.
Do studies show hydroxychloroquine is not effective for COVID-19?
Yes, multiple studies provide data that hydroxychloroquine is ineffective in the treatment of SARS-CoV-2, the virus that causes COVID-19 disease.
Hospitalized patients
In a large, randomized, controlled, open-label study evaluating a number of potential treatments for patients hospitalized with COVID-19 in the United Kingdom, the RECOVERY Trial from the University of Oxford 19. The RECOVERY study is being conducted by researchers at the University of Oxford in the UK (the hydroxychloroquine arm is now halted) 20. Hydroxychloroquine did not decrease 28-day mortality when compared to the usual standard of care 21. Patients who were randomized to receive hydroxychloroquine had a longer median hospital stay than those who received the standard of care. In addition, among patients who were not on invasive mechanical ventilation at the time of randomization, those who received hydroxychloroquine were more likely to subsequently require intubation or die during hospitalization than those who received the standard of care 21.
- In the RECOVERY Trial, investigators reported that there was no beneficial effect or reduction of death in hospitalized patients with COVID-19 receiving hydroxychloroquine 20.
- In this study, 1561 patients received hydroxychloroquine and were compared to 3155 patients receiving standard care only. No difference was found in the primary endpoint, which was the incidence of death at 28 days (26.8% hydroxychloroquine vs. 25% usual care).
- In addition, hydroxychloroquine treatment was associated with an increased length of stay in the hospital and increased need for invasive mechanical ventilation.
- Based on this data, investigators stopped enrollment in the RECOVERY hydroxychloroquine arm on June 5th, 2020 20.
The results from several additional large randomized controlled trials have been published; these trials have failed to show a benefit for hydroxychloroquine with or without azithromycin or azithromycin alone in hospitalized adults with COVID-19. In the Solidarity trial 22, an international randomized controlled platform trial that enrolled hospitalized patients with COVID-19, the hydroxychloroquine arm was halted for futility. There was no difference in in-hospital mortality between patients in the hydroxychloroquine arm and those in the control arm 22. Similarly, PETAL 23, a randomized, placebo-controlled, blinded study, was stopped early for futility. In this study, there was no difference in the median scores on the COVID Outcomes Scale between patients who received hydroxychloroquine and those who received placebo 23. Data from two additional randomized studies of hospitalized patients with COVID-19 did not support using hydroxychloroquine plus azithromycin over hydroxychloroquine alone 24, 25. In RECOVERY, azithromycin alone (without hydroxychloroquine) did not improve survival or other clinical outcomes when compared to the usual standard of care 19.
In addition to these randomized trials, data from large retrospective observational studies do not consistently show evidence of a benefit for hydroxychloroquine with or without azithromycin in hospitalized patients with COVID-19 26, 27, 28.
In a multicenter, randomized, open-label, controlled trial published in July 2020 by Cavalcanti and colleagues in the New England Journal of Medicine 29, hydroxychloroquine use was studied in patients who were hospitalized with mild-to-moderate COVID-19.
- Patients received hydroxychloroquine (400 mg twice daily for 7 days), hydroxychloroquine with azithromycin (hydroxychloroquine 400 mg twice daily + azithromycin 500 mg once daily for 7 days), or standard care only.
- The clinical status of these patients at day 15 was not improved as compared with the patients receiving only standard care.
- In addition, researchers noted that prolonged QT intervals (which may lead to abnormal heart rates and death) and elevated liver enzymes were higher in patients receiving hydroxychloroquine, either with or without azithromycin.
A retrospective, observational study conducted from March to early May of 2020 28 did report a positive effect with hydroxychloroquine on hospitalized patient mortality, used alone and with azithromycin when compared to no treatment. The study authors note a limitation to their analysis was the retrospective, non-randomized, non-blinded study design 28.
- Researchers looked at 2,541 patients, with a median total hospitalization time of 6 days.
- Mortality, by treatment, was 20.1% for hydroxychloroquine + azithromycin, 13.5% for hydroxychloroquine alone, 22.4% for azithromycin alone, and 26.4% for neither drug. The primary cause of death was respiratory failure in 88% of patients.
- Adjunct therapy with corticosteroids (methylprednisolone and/or prednisone) and anti-IL-6 tocilizumab was provided in 68% and 4.5% of patients, respectively.
- Factors such as greater glucocorticoid use in the hydroxychloroquine groups and the nonrandomized study design suggested this data may be flawed and that prospective, randomized controlled studies were needed to validate these results.
Given the lack of a benefit seen in the randomized clinical trials, the COVID-19 Treatment Guidelines Panel (the Panel) recommends against using hydroxychloroquine or chloroquine and/or azithromycin to treat COVID-19 in hospitalized patients 30.
Nonhospitalized patients
Several randomized trials have not shown a clinical benefit for hydroxychloroquine in nonhospitalized patients with early, asymptomatic, or mild COVID-19 31, 32. In an open-label trial, Mitja et al. 32 randomized 307 nonhospitalized people who were recently confirmed to have COVID-19 to receive hydroxychloroquine or no antiviral treatment. Patients in the hydroxychloroquine arm received hydroxychloroquine 800 mg on Day 1 followed by 400 mg daily for an additional 6 days. The authors reported no difference in the mean reduction in SARS-CoV-2 RNA at Day 3 or the time to clinical improvement between the two arms 32. In another trial 33, treating patients who had asymptomatic or mild COVID-19 with hydroxychloroquine with or without azithromycin did not result in greater rates of virologic clearance (as measured by a negative polymerase chain reaction [PCR] result on Day 6).
A randomized, double-blind, placebo-controlled trial from Skipper and colleagues published in the Annals of Internal Medicine in July 2020 31 was conducted in 423 outpatients (not in the hospital) with early COVID-19.
- Patients received oral hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 more days) or a placebo (inactive treatment).
- Researchers found that over a 14 day period a change in symptom severity and the percent of patients with ongoing symptoms did not differ significantly between groups, signaling no effect from the hydroxychloroquine treatment.
- However, side effects were significantly greater in the group receiving hydroxychloroquine compared to placebo (43% hydroxychloroquine versus 22% placebo). Rates of hospitalizations and deaths did not differ significantly.
An open-label, prospective, randomized trial compared oral azithromycin 500 mg once daily for 3 days plus standard of care to standard of care alone in nonhospitalized, high-risk, older adults who had laboratory-confirmed or suspected COVID-19 34. No differences were observed between the arms in the primary endpoints of time to first self-reported recovery and hospitalization or death due to COVID-19 34. These findings remained consistent in an analysis that was restricted to participants with positive SARS-CoV-2 PCR results. The study was ultimately halted due to futility 34. Similarly, in a preliminary report from ATOMIC-2, adding oral azithromycin 500 mg once daily to standard of care for 14 days did not reduce the risk of hospitalization or death among 292 participants with mild to moderate COVID-19 35.
While ongoing clinical trials are still evaluating the use of chloroquine, hydroxychloroquine, and azithromycin in outpatients, the existing data suggest that it is unlikely that clinical benefits will be identified for these agents. The COVID-19 Treatment Guidelines Panel (the Panel) recommends against the use of chloroquine or hydroxychloroquine and/or azithromycin for the treatment of COVID-19 in nonhospitalized patients 30.
Hydroxychloroquine study for prevention after exposure to COVID-19
A randomized, double-blind, placebo-controlled study published online in the New England Journal of Medicine in June 2020 36 looked at prevention of COVID-19 after exposure to the virus (post-exposure prophylaxis or PEP).
- Researchers evaluated over 800 people in the U.S. and Canada who had been exposed to COVID-19. The primary outcome was the incidence of either laboratory-confirmed COVID-19 or illness compatible with the virus within 14 days.
- Hydroxychloroquine was given as 800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days. Patients started treatment within 4 days after exposure, defined as being in close contact with a COVID-19 patient for more than 10 minutes without protection.
- Results showed that hydroxychloroquine did not prevent COVID-19 when compared to a placebo (used as post-exposure prophylaxis). The incidence of COVID-19 did not differ significantly between those who took hydroxychloroquine (11.8%) and those who took placebo (14.3%).
- Side effects were more common in the hydroxychloroquine group (40.1% compared to 16.8% with placebo), but were not reported as serious. Common adverse events included nausea, loose stools, and stomach pain.
- Limitations in this study 36 were many, and included inability to confirm self-reported COVID-19 exposure, adherence to study drug, starting drug up to 4 days after reported exposure to the virus, lack of survey completion, and enrollment of a lower-risk population.
Hydroxychloroquine special precautions before taking hydroxychloroquine
You should NOT use hydroxychloroquine if you are allergic to hydroxychloroquine, chloroquine, primaquine, quinine, or any other drugs.
High doses or long-term use of hydroxychloroquine may cause irreversible damage to your retina (the membrane layer inside your eye that helps produce vision). This could progress to permanent vision problems. The risk of retinal damage is higher in people with pre-existing eye problems, kidney disease, or people who also take tamoxifen.
To make sure hydroxychloroquine is safe for you, tell your doctor if you have ever had:
- vision changes or damage to your retina caused by an anti-malaria medication
- heart disease, heart rhythm disorder (such as long QT syndrome)
- diabetes
- a stomach disorder
- an allergy to quinine
- liver or kidney disease
- psoriasis
- alcoholism
- porphyria (a genetic enzyme disorder that causes symptoms affecting the skin or nervous system)
- a genetic enzyme deficiency called glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Using hydroxychloroquine alone or with other medicines (eg, azithromycin) may increase your risk of heart rhythm problems (eg, QT prolongation, ventricular fibrillation, ventricular tachycardia).
Tell your doctor if you have or have ever had liver disease, heart disease, a prolonged QT interval (a rare heart problem that may cause irregular heartbeat, fainting, or sudden death), an irregular heartbeat, a low level of magnesium or potassium in your blood, psoriasis, porphyria or other blood disorders, G6PD deficiency (an inherited blood disease), dermatitis (skin inflammations), seizures, vision problems, diabetes, kidney problems, or if you drink large amounts of alcohol.
Tell your doctor if you are pregnant or plan to become pregnant. Malaria is more likely to cause serious illness or death in a pregnant woman. Having malaria during pregnancy may also increase the risk of miscarriage, stillbirth, premature delivery, and low birth weight.
It is not known whether hydroxychloroquine will harm an unborn baby. If you are pregnant, ask your doctor about the risks of traveling to areas where malaria is common such as Africa, South America, and Southern Asia.
It may not be safe to breastfeed while using this medicine. Ask your doctor about any risk.
Hydroxychloroquine is not approved for treating lupus or rheumatoid arthritis in anyone younger than 18 years old.
Tell your doctor and pharmacist what prescription and nonprescription drugs, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention acetaminophen (Tylenol, others); azithromycin (Zithromax); cimetidine (Tagamet); cyclosporine (Gengraf, Neoral, Sandimmune); digoxin (Lanoxin), insulin and oral medication for diabetes; medications for seizures such as carbamazepine (Carbatrol, Epitol, Equetro, Tegretol, Teril), phenytoin (Dilantin, Phenytek), or valproic acid (Depakene); certain medications for irregular heartbeat such as amiodarone (Pacerone); methotrexate (Trexall, Xatmep); moxifloxacin (Avelox); praziquantel (Biltricide); and tamoxifen (Nolvadex). Your doctor may need to change the doses of your medications or monitor you carefully for side effects. Many other medications may also interact with hydroxychloroquine, so be sure to tell your doctor about all the medications you are taking, even those that do not appear on this list.
If you are taking antacids, take them 4 hours before or 4 hours after hydroxychloroquine. If you are taking ampicillin, take it at least 2 hours before or 2 hours after hydroxychloroquine.
Tell your doctor if you have ever had vision changes while taking hydroxychloroquine, chloroquine (Aralen), or primaquine.
Children
Appropriate studies performed to date have not demonstrated pediatric-specific problems that would limit the usefulness of hydroxychloroquine to prevent and treat malaria in children. However, use is not recommended in children weighing less than 31 kilograms (kg). Safety and efficacy of hydroxychloroquine to treat lupus and arthritis have not been established in children.
Geriatric
Appropriate studies performed to date have not demonstrated geriatric-specific problems that would limit the usefulness of hydroxychloroquine in the elderly. However, elderly patients are more likely to have age-related kidney, liver, or heart problems, which may require caution and an adjustment in the dose for patients receiving this medicine.
Hydroxychloroquine drug interactions
Hydroxychloroquine can cause a serious heart problem. Your risk may be higher if you also use certain other medicines for infections, asthma, heart problems, high blood pressure, depression, mental illness, cancer, malaria, or HIV.
The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using hydroxychloroquine with any of the following medicines is not recommended. Your doctor may decide not to treat you with hydroxychloroquine or change some of the other medicines you take:
- Aurothioglucose
- Bepridil
- Cisapride
- Dronedarone
- Mesoridazine
- Pimozide
- Piperaquine
- Saquinavir
- Sparfloxacin
- Terfenadine
- Thioridazine
- Ziprasidone
Using hydroxychloroquine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Acarbose
- Albiglutide
- Alfuzosin
- Alogliptin
- Amiodarone
- Amisulpride
- Amitriptyline
- Anagrelide
- Apomorphine
- Aripiprazole
- Aripiprazole Lauroxil
- Arsenic Trioxide
- Asenapine
- Astemizole
- Atazanavir
- Auranofin
- Azithromycin
- Bedaquiline
- Buprenorphine
- Buserelin
- Canagliflozin
- Carbamazepine
- Ceritinib
- Chloroquine
- Chlorpromazine
- Chlorpropamide
- Cimetidine
- Ciprofloxacin
- Citalopram
- Clarithromycin
- Clofazimine
- Clomipramine
- Clozapine
- Crizotinib
- Cyclobenzaprine
- Cyclosporine
- Dabrafenib
- Dapagliflozin
- Dasatinib
- Degarelix
- Delamanid
- Desipramine
- Deslorelin
- Deutetrabenazine
- Disopyramide
- Dofetilide
- Dolasetron
- Domperidone
- Donepezil
- Doxepin
- Droperidol
- Dulaglutide
- Ebastine
- Efavirenz
- Empagliflozin
- Encorafenib
- Entrectinib
- Eribulin
- Erythromycin
- Escitalopram
- Exenatide
- Famotidine
- Felbamate
- Fingolimod
- Flecainide
- Fluconazole
- Fluoxetine
- Formoterol
- Foscarnet
- Fosphenytoin
- Fostemsavir
- Galantamine
- Gatifloxacin
- Gemifloxacin
- Glasdegib
- Glimepiride
- Glipizide
- Glyburide
- Gonadorelin
- Goserelin
- Granisetron
- Halofantrine
- Haloperidol
- Histrelin
- Hydroquinidine
- Hydroxyzine
- Ibutilide
- Iloperidone
- Imipramine
- Inotuzumab Ozogamicin
- Insulin
- Insulin Aspart, Recombinant
- Insulin Bovine
- Insulin Degludec
- Insulin Detemir
- Insulin Glulisine
- Insulin Lispro, Recombinant
- Itraconazole
- Ivabradine
- Ivosidenib
- Ketoconazole
- Lapatinib
- Lefamulin
- Lenvatinib
- Leuprolide
- Levofloxacin
- Linagliptin
- Liraglutide
- Lixisenatide
- Lofexidine
- Lumefantrine
- Macimorelin
- Mefloquine
- Metformin
- Methadone
- Methotrimeprazine
- Metronidazole
- Mifepristone
- Miglitol
- Mirtazapine
- Mizolastine
- Moricizine
- Moxifloxacin
- Nafarelin
- Nateglinide
- Nelfinavir
- Nilotinib
- Norfloxacin
- Octreotide
- Ofloxacin
- Olanzapine
- Ondansetron
- Osilodrostat
- Osimertinib
- Ozanimod
- Paliperidone
- Panobinostat
- Papaverine
- Paroxetine
- Pasireotide
- Pazopanib
- Pentamidine
- Perphenazine
- Pimavanserin
- Pioglitazone
- Pipamperone
- Pitolisant
- Ponesimod
- Posaconazole
- Pramlintide
- Probucol
- Procainamide
- Prochlorperazine
- Promethazine
- Propafenone
- Protriptyline
- Quetiapine
- Quinidine
- Quinine
- Ranolazine
- Remdesivir
- Repaglinide
- Ribociclib
- Rilpivirine
- Risperidone
- Ritonavir
- Rosiglitazone
- Saxagliptin
- Selpercatinib
- Semaglutide
- Sertindole
- Sertraline
- Sevoflurane
- Siponimod
- Sitagliptin
- Sodium Phosphate
- Sodium Phosphate, Dibasic
- Sodium Phosphate, Monobasic
- Solifenacin
- Sorafenib
- Sotalol
- Sulpiride
- Sultopride
- Sunitinib
- Tacrolimus
- Tamoxifen
- Telaprevir
- Telavancin
- Telithromycin
- Tetrabenazine
- Tolazamide
- Tolbutamide
- Tolterodine
- Toremifene
- Trazodone
- Triclabendazole
- Trimipramine
- Triptorelin
- Vandetanib
- Vardenafil
- Vemurafenib
- Venlafaxine
- Vilanterol
- Vildagliptin
- Vinflunine
- Voclosporin
- Voriconazole
- Vorinostat
- Zotepine
- Zuclopenthixol
Using hydroxychloroquine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Ampicillin
- Digoxin
This list is not complete. other drugs may interact with hydroxychloroquine, including prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible drug interactions are listed here.
Other interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of hydroxychloroquine. Make sure you tell your doctor if you have any other medical problems, especially:
- Allergy to 4-aminoquinoline compounds (eg, chloroquine). Hydroxychloroquine should not be used in patients with this condition.
- Blood or bone marrow problems
- Diabetes
- Eye or vision problems
- Muscle problems
- Nerve problems
- Porphyria (blood disorder)
- Psoriasis (skin disease)
- Stomach or bowel problems–Use with caution. May make these conditions worse.
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency. May cause hemolytic anemia in patients with this condition.
- Heart disease (eg, heart attack, heart failure)
- Heart rhythm problems (eg, bradycardia, ventricular dysrhythmia), or history of heart rhythm problems
- Hypokalemia (low potassium in the blood), uncorrected low potassium
- Hypomagnesemia (low magnesium in the blood), uncorrected. Use with caution. May prolong the QT interval.
- Kidney disease
- Liver disease. Use with caution. The effects may be increased because of the slower removal of hydroxychloroquine from the body.
Hydroxychloroquine uses
Hydroxychloroquine is a derivative of chloroquine that has both antimalarial and antiinflammatory activities. Hydroxychloroquine sulfate is approved to treat and prevent malaria infection in areas or regions where it is known that other medicines (eg, chloroquine) may not work. Hydroxychloroquine is now most often used as an antirheumatologic agent in systemic lupus erythematosis and rheumatoid arthritis. Hydroxychloroquine has also been used as therapy of porphyria cutanea tarda where it seems to act by increasing excretion of porphyrins 2. Take this medicine only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered.
Swallow the tablet whole. Do not crush, break, or chew it. Take hydroxychloroquine with a meal or a glass of milk to lessen stomach upset, unless otherwise directed by your doctor.
- To treat lupus or arthritis, hydroxychloroquine is usually taken daily.
- To prevent malaria: Hydroxychloroquine is usually taken once per week on the same day each week. Start taking the medicine 2 weeks before entering an area where malaria is common. Keep taking the medicine during your stay and for at least 4 weeks after you leave the area.
- To treat malaria: Hydroxychloroquine is usually given as one high dose followed by smaller doses during the next 2 days in a row.
Keep using this medicine for the full treatment time, even if you feel better after the first few doses. Your infection may not clear up if you stop using the medicine too soon.
Call your doctor as soon as possible if you have been exposed to malaria, or if you have fever or other symptoms of illness during or after a stay in an area where malaria is common.
Use protective clothing, insect repellents, and mosquito netting around your bed to further prevent mosquito bites that could cause malaria.
No medication is 100% effective in treating or preventing all types of malaria. Talk with your doctor if you have fever, vomiting, or diarrhea during your treatment.
While using hydroxychloroquine, you may need frequent medical tests and vision exams.
Hydroxychloroquine dose
The dose of hydroxychloroquine will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of hydroxychloroquine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
Adult dose for malaria prevention
For the prophylaxis of malaria in geographic areas where chloroquine resistance is not reported.
For oral dosage form (tablets): 400 mg orally once a week on the same day of each week starting 2 weeks before traveling to an area where malaria occurs, and continued for 4 weeks after leaving the area.
Comments:
- An alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria
- Prophylaxis should start 1 to 2 weeks prior to exposure or travel to malarious areas and should continue for 4 weeks after leaving the endemic area.
- Hydroxychloroquine should be administered on the same day of each week.
- If malaria develops while using this drug for chemoprophylaxis, it should not be used as part of the treatment regimen.
Children dose for malaria prevention
For the prophylaxis of malaria in geographic areas where chloroquine resistance is not reported.
For oral dosage form (tablets):
- Children weighing 31 kilograms (kg) or more: Dose is based on body weight and must be determined by your doctor. At first, 6.5 mg per kilogram (kg) of body weight, not to exceed 400 mg, once weekly on the same day of the week starting 2 weeks before traveling to an area where malaria occurs, and continued for 4 weeks after leaving the area.
- Children weighing less than 31 kg: Use is not recommended.
Comments:
- An alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria
- Prophylaxis should start 1 to 2 weeks prior to exposure or travel to malarious areas and should continue for 4 weeks after leaving the endemic area.
- Hydroxychloroquine should be administered on the same day of each week.
- If malaria develops while using this drug for chemoprophylaxis, it should not be used as part of the treatment regimen.
Adult dose for treatment of malaria
For the treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax.
For treatment of malaria: At first, 800 milligrams (mg) (4 tablets) taken as a single dose. Then, 400 mg taken 6 hours, 24 hours, and 48 hours after the first dose.
Comments:
- Recommended for uncomplicated malaria (Plasmodium falciparum or species not identified) in regions with chloroquine sensitivity
- Recommended for uncomplicated malaria (Plasmodium malariae, Plasmodium knowlesi, Plasmodium vivax [unless chloroquine-resistant Plasmodium vivax suspected], or Plasmodium ovale) in all regions; if treating Plasmodium vivax or Plasmodium ovale infections, concomitant treatment with primaquine (after quantitative testing to rule out glucose-6-phosphate dehydrogenase [G6PD] deficiency) is recommended.
- Recommended for uncomplicated malaria treatment for pregnant women in regions with chloroquine sensitivity
- Concomitant therapy with an 8-aminoquinoline compound is necessary for radical cure of Plasmodium vivax and Plasmodium ovale infections.
Children dose for treatment of malaria
For the treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax.
For treatment of malaria:
- Children weighing 31 kilograms (kg) or more: Dose is based on body weight and must be determined by your doctor. At first, 13 mg per kg of body weight taken as a single dose. Then, 6.5 mg per kg of body weight taken 6 hours, 24 hours, and 48 hours after the first dose. However, dose is usually not more than 800 mg for the first dose and not more than 400 mg for the next doses.
- Children weighing less than 31 kg: Use is not recommended.
Comments:
- Recommended for uncomplicated malaria (Plasmodium falciparum or species not identified) in regions with chloroquine sensitivity
- Recommended for uncomplicated malaria (Plasmodium malariae, Plasmodium knowlesi, Plasmodium vivax [unless chloroquine-resistant Plasmodium vivax suspected], or Plasmodium ovale) in all regions; if treating Plasmodium vivax or Plasmodium ovale infections, concomitant treatment with primaquine (after quantitative testing to rule out glucose-6-phosphate dehydrogenase [G6PD] deficiency) is recommended.
- Recommended for uncomplicated malaria treatment for pregnant women in regions with chloroquine sensitivity
- Concomitant therapy with an 8-aminoquinoline compound is necessary for radical cure of Plasmodium vivax and Plasmodium ovale infections.
Adult dose for Systemic Lupus Erythematosus
For the treatment of chronic discoid lupus erythematosus (DLE) and systemic lupus erythematosus (SLE).
- 200 milligrams (mg) once a day or 400 mg taken once a day or in two divided doses.
Comments:
- Doses above 400 mg/day are not recommended.
- Higher incidence of retinopathy reported when this maintenance dose is exceeded.
Adult dose for Rheumatoid Arthritis
For the treatment of acute and chronic rheumatoid arthritis.
- At first, 400 to 600 milligrams (mg) taken as a single dose or in two divided doses per day. Then, 200 mg once a day or 400 mg taken as a single dose or in two divided doses per day. Your doctor may adjust your dose if needed.
Comments:
- The action of this drug is cumulative and may require weeks to months to achieve the maximum therapeutic effect.
- When a good response is obtained, the initial dose may be reduced by 50% and continued at a maintenance dose.
- Higher incidence of retinopathy reported when this maintenance dose is exceeded; 600 mg or 6.5 mg/kg, whichever is lower, should not be exceed per day.
- Corticosteroids and salicylates may be used with this drug, and they can generally be decreased gradually in dosage or eliminated after a maintenance dose of this drug has been achieved.
What should I do if I forget a dose?
If you miss a dose of this medicine, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular dosing schedule. Do not double doses.
Hydroxychloroquine side effects
Hydroxychloroquine may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- headache
- dizziness
- loss of appetite
- nausea
- diarrhea
- stomach pain
- vomiting
- rash
If you experience any of the following symptoms, call your doctor immediately:
- difficulty reading or seeing (words, letters, or parts of objects missing)
- sensitivity to light
- blurred vision
- changes in vision
- seeing light flashes or streaks
- difficulty hearing
- ringing in ears
- muscle weakness
- unusual bleeding or bruising
- bleaching or loss of hair
- mood or mental changes
- irregular heartbeat
- drowsiness
- convulsions
- decreased consciousness or loss of consciousness
- thinking about harming or killing yourself
Hydroxychloroquine overdose
In case of hydroxychloroquine overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call your local emergency services number.
Symptoms of hydroxychloroquine overdose may include:
- headache
- drowsiness
- visual disturbances
- convulsions
- irregular heartbeat.
- Browning D.J. Springer; New York, NY: 2014. Pharmacology of Chloroquine and Hydroxychloroquine, Hydroxychloroquine and Chloroquine Retinopathy; pp. 35–63.[↩]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Hydroxychloroquine. [Updated 2021 Apr 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548738[↩][↩]
- https://www.fda.gov/media/136534/download[↩]
- https://www.fda.gov/media/138945/download[↩][↩][↩]
- Chloroquine or Hydroxychloroquine and/or Azithromycin. Last Updated: July 8, 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/chloroquine-or-hydroxychloroquine-and-or-azithromycin[↩][↩]
- Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., Shi, Z., Hu, Z., Zhong, W., & Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research, 30(3), 269–271. https://doi.org/10.1038/s41422-020-0282-0[↩]
- Vincent, M. J., Bergeron, E., Benjannet, S., Erickson, B. R., Rollin, P. E., Ksiazek, T. G., Seidah, N. G., & Nichol, S. T. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology journal, 2, 69. https://doi.org/10.1186/1743-422X-2-69[↩][↩]
- Fantini, J., Chahinian, H., & Yahi, N. (2020). Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. International journal of antimicrobial agents, 56(2), 106020. https://doi.org/10.1016/j.ijantimicag.2020.106020[↩]
- Andreani, J., Le Bideau, M., Duflot, I., Jardot, P., Rolland, C., Boxberger, M., Wurtz, N., Rolain, J. M., Colson, P., La Scola, B., & Raoult, D. (2020). In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microbial pathogenesis, 145, 104228. https://doi.org/10.1016/j.micpath.2020.104228[↩]
- Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R, Naninck T, Pizzorno A, Lemaitre J, Gonçalves A, Kahlaoui N, Terrier O, Fang RHT, Enouf V, Dereuddre-Bosquet N, Brisebarre A, Touret F, Chapon C, Hoen B, Lina B, Calatrava MR, van der Werf S, de Lamballerie X, Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020 Sep;585(7826):584-587. doi: 10.1038/s41586-020-2558-4[↩]
- Infante, M., Ricordi, C., Alejandro, R., Caprio, M., & Fabbri, A. (2021). Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert review of anti-infective therapy, 19(1), 5–16. https://doi.org/10.1080/14787210.2020.1799785[↩]
- Frie K., Gbinigie K. The Centre for Evidence-Based Medicine; 2020. Chloroquine and Hydroxychloroquine: Current Evidence for Their Effectiveness in Treating COVID-19.[↩]
- Spinelli F.R., Ceccarelli F., Di Franco M., Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann. Rheum. Dis. 2020;79:666–667.[↩]
- Singh, B., Ryan, H., Kredo, T., Chaplin, M., & Fletcher, T. (2021). Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. The Cochrane database of systematic reviews, 2(2), CD013587. https://doi.org/10.1002/14651858.CD013587.pub2[↩]
- Joshi, G., Thakur, S., Mayank, & Poduri, R. (2021). Exploring insights of hydroxychloroquine, a controversial drug in Covid-19: An update. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 151, 112106. https://doi.org/10.1016/j.fct.2021.112106[↩]
- Abd-Elsalam, S., Esmail, E. S., Khalaf, M., Abdo, E. F., Medhat, M. A., Abd El Ghafar, M. S., Ahmed, O. A., Soliman, S., Serangawy, G. N., & Alboraie, M. (2020). Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study. The American journal of tropical medicine and hygiene, 103(4), 1635–1639. https://doi.org/10.4269/ajtmh.20-0873[↩]
- Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Published:May 22, 2020 https://doi.org/10.1016/S0140-6736(20)31180-6[↩]
- Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med 2020; 382:e102 DOI: 10.1056/NEJMoa2007621 https://www.nejm.org/doi/full/10.1056/NEJMoa2007621[↩]
- Recovery Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605-612. https://doi.org/10.1016/S0140-6736(21)00149-5[↩][↩]
- https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf[↩][↩][↩]
- RECOVERY Collaborative Group, Horby, P., Mafham, M., Linsell, L., Bell, J. L., Staplin, N., Emberson, J. R., Wiselka, M., Ustianowski, A., Elmahi, E., Prudon, B., Whitehouse, T., Felton, T., Williams, J., Faccenda, J., Underwood, J., Baillie, J. K., Chappell, L. C., Faust, S. N., Jaki, T., … Landray, M. J. (2020). Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. The New England journal of medicine, 383(21), 2030–2040. https://doi.org/10.1056/NEJMoa2022926[↩][↩]
- WHO Solidarity Trial Consortium, Pan, H., Peto, R., Henao-Restrepo, A. M., Preziosi, M. P., Sathiyamoorthy, V., Abdool Karim, Q., Alejandria, M. M., Hernández García, C., Kieny, M. P., Malekzadeh, R., Murthy, S., Reddy, K. S., Roses Periago, M., Abi Hanna, P., Ader, F., Al-Bader, A. M., Alhasawi, A., Allum, E., Alotaibi, A., … Swaminathan, S. (2021). Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. The New England journal of medicine, 384(6), 497–511. https://doi.org/10.1056/NEJMoa2023184[↩][↩]
- Self WH, Semler MW, Leither LM, et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. 2020;324(21):2165–2176. doi:10.1001/jama.2020.22240 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7653542[↩][↩]
- Furtado, R., Berwanger, O., Fonseca, H. A., Corrêa, T. D., Ferraz, L. R., Lapa, M. G., Zampieri, F. G., Veiga, V. C., Azevedo, L., Rosa, R. G., Lopes, R. D., Avezum, A., Manoel, A., Piza, F., Martins, P. A., Lisboa, T. C., Pereira, A. J., Olivato, G. B., Dantas, V., Milan, E. P., … COALITION COVID-19 Brazil II Investigators (2020). Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet (London, England), 396(10256), 959–967. https://doi.org/10.1016/S0140-6736(20)31862-6[↩]
- Cavalcanti, A. B., Zampieri, F. G., Rosa, R. G., Azevedo, L., Veiga, V. C., Avezum, A., Damiani, L. P., Marcadenti, A., Kawano-Dourado, L., Lisboa, T., Junqueira, D., de Barros E Silva, P., Tramujas, L., Abreu-Silva, E. O., Laranjeira, L. N., Soares, A. T., Echenique, L. S., Pereira, A. J., Freitas, F., Gebara, O., … Coalition Covid-19 Brazil I Investigators (2020). Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. The New England journal of medicine, 383(21), 2041–2052. https://doi.org/10.1056/NEJMoa2019014[↩]
- Rosenberg, E. S., Dufort, E. M., Udo, T., Wilberschied, L. A., Kumar, J., Tesoriero, J., Weinberg, P., Kirkwood, J., Muse, A., DeHovitz, J., Blog, D. S., Hutton, B., Holtgrave, D. R., & Zucker, H. A. (2020). Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA, 323(24), 2493–2502. https://doi.org/10.1001/jama.2020.8630[↩]
- Geleris, J., Sun, Y., Platt, J., Zucker, J., Baldwin, M., Hripcsak, G., Labella, A., Manson, D. K., Kubin, C., Barr, R. G., Sobieszczyk, M. E., & Schluger, N. W. (2020). Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. The New England journal of medicine, 382(25), 2411–2418. https://doi.org/10.1056/NEJMoa2012410[↩]
- Arshad, S., Kilgore, P., Chaudhry, Z. S., Jacobsen, G., Wang, D. D., Huitsing, K., Brar, I., Alangaden, G. J., Ramesh, M. S., McKinnon, J. E., O’Neill, W., Zervos, M., & Henry Ford COVID-19 Task Force (2020). Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases, 97, 396–403. https://doi.org/10.1016/j.ijid.2020.06.099[↩][↩][↩]
- Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020; 383:2041-2052 DOI: 10.1056/NEJMoa2019014 https://www.nejm.org/doi/full/10.1056/NEJMoa2019014[↩]
- Chloroquine or Hydroxychloroquine and/or Azithromycin. Last Updated: July 8, 2021 https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/chloroquine-or-hydroxychloroquine-and-or-azithromycin[↩][↩]
- Skipper, C. P., Pastick, K. A., Engen, N. W., Bangdiwala, A. S., Abassi, M., Lofgren, S. M., Williams, D. A., Okafor, E. C., Pullen, M. F., Nicol, M. R., Nascene, A. A., Hullsiek, K. H., Cheng, M. P., Luke, D., Lother, S. A., MacKenzie, L. J., Drobot, G., Kelly, L. E., Schwartz, I. S., Zarychanski, R., … Boulware, D. R. (2020). Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19 : A Randomized Trial. Annals of internal medicine, 173(8), 623–631. https://doi.org/10.7326/M20-4207[↩][↩]
- Mitjà, O., Corbacho-Monné, M., Ubals, M., Tebe, C., Peñafiel, J., Tobias, A., Ballana, E., Alemany, A., Riera-Martí, N., Pérez, C. A., Suñer, C., Laporte, P., Admella, P., Mitjà, J., Clua, M., Bertran, L., Sarquella, M., Gavilán, S., Ara, J., Argimon, J. M., … BCN PEP-CoV-2 RESEARCH GROUP (2020). Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaa1009. Advance online publication. https://doi.org/10.1093/cid/ciaa1009[↩][↩][↩]
- Omrani AS, Pathan SA, Thomas SA, et al. Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe COVID-19. EClinicalMedicine. 2020. Published online 2020 Nov 20. doi: 10.1016/j.eclinm.2020.100645 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7678437[↩]
- PRINCIPLE Trial Collaborative Group (2021). Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet (London, England), 397(10279), 1063–1074. https://doi.org/10.1016/S0140-6736(21)00461-X[↩][↩][↩]
- A randomised clinical trial of azithromycin versus standard care in ambulatory COVID-19 – the ATOMIC2 trial. Timothy SC Hinks, Lucy Cureton, Ruth Knight, Ariel Wang, Jennifer L Cane, Vicki S Barber, Joanna Black, Susan J Dutton, James Melhorn, Maisha Jabeen, Phil Moss, Rajendar Garlapati, Tanya Baron, Graham Johnson, Fleur Cantle, David Clarke, Samer Elkhodair, Jonathan Underwood, Daniel Lasserson, Ian D Pavord, Sophie Morgan, Duncan Richards. medRxiv 2021.04.21.21255807; doi: https://doi.org/10.1101/2021.04.21.21255807[↩]
- Boulware D, Pullen M, Bangdiwala A, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med 2020; 383:517-525 DOI: 10.1056/NEJMoa2016638 https://www.nejm.org/doi/full/10.1056/nejmoa2016638[↩][↩]