What is hyperparathyroidism
Hyperparathyroidism is an excess of parathyroid hormone (PTH) in the bloodstream due to overactivity of one or more of the body’s four parathyroid glands. The parathyroid glands are about the size of a grain of rice and are located in your neck.
The parathyroid glands produce parathyroid hormone (PTH), which helps maintain an appropriate balance of calcium in the bloodstream and in tissues that depend on calcium for proper functioning.
Two types of hyperparathyroidism exist:
- In primary hyperparathyroidism, an enlargement of one or more of the parathyroid glands causes overproduction of the hormone, resulting in high levels of calcium in the blood (hypercalcemia), which can cause a variety of health problems. Surgery is the most common treatment for primary hyperparathyroidism.
- Secondary hyperparathyroidism occurs as a result of another disease that initially causes low levels of calcium in the body and over time, increased parathyroid hormone levels occur.
- Tertiary hyperparathyroidism usually occurs after a successful kidney transplant fails to normalize the production of parathyroid hormone (PTH) 1. Persistently increased serum levels of PTH occur in up to 30% of patients after renal transplantation 2. People with tertiary hyperparathyroidism are almost always under the care of kidney specialists.
Hyperparathyroidism symptoms
Hyperparathyroidism is often diagnosed before signs or symptoms of the disorder are apparent. When symptoms do occur, they’re the result of damage or dysfunction in other organs or tissues due to high calcium levels circulating in the blood and urine or too little calcium in bones.
Symptoms may be so mild and nonspecific that they don’t seem at all related to parathyroid function, or they may be severe.
If you do have symptoms, they can be wide ranging and include:
- depression
- tiredness
- feeling thirsty and peeing a lot
- feeling sick and losing your appetite
- muscle weakness
- constipation
- tummy pain
- loss of concentration
- mild confusion
Left untreated, high blood calcium levels may cause:
- vomiting
- drowsiness
- dehydration
- confusion
- muscle spasms
- bone pain or tenderness
- joint pain
- irregular heartbeat
- high blood pressure (hypertension)
It can also cause a number of other possible complications, including:
- osteoporosis and bone fractures
- kidney stones and blockage, and kidney damage or failure
- peptic ulcers
- pancreatitis (inflammation of the pancreas)
In very severe cases of hyperparathyroidism, high calcium levels can lead to rapid kidney failure, loss of consciousness, coma, or serious life-threatening heart rhythm abnormalities.
But hyperparathyroidism is usually diagnosed at an early stage, and these complications are extremely rare.
Hyperparathyroidism complications
Complications of hyperparathyroidism are primarily related to the long-term effect of too little calcium in your bones and too much calcium circulating in your bloodstream. Common complications include:
- Osteoporosis. The loss of calcium often results in weak, brittle bones that fracture easily (osteoporosis).
- Kidney stones. The excess of calcium in your blood may lead to excess calcium in your urine, which can cause small, hard deposits of calcium and other substances to form in your kidneys. A kidney stone usually causes significant pain as it passes through the urinary tract.
- Cardiovascular disease. Although the exact cause-and-effect link is unclear, high calcium levels are associated with cardiovascular conditions, such as high blood pressure (hypertension) and certain types of heart disease.
- Neonatal hypoparathyroidism. Severe, untreated hyperparathyroidism in pregnant women may cause dangerously low levels of calcium in newborns.
What are the parathyroid glands
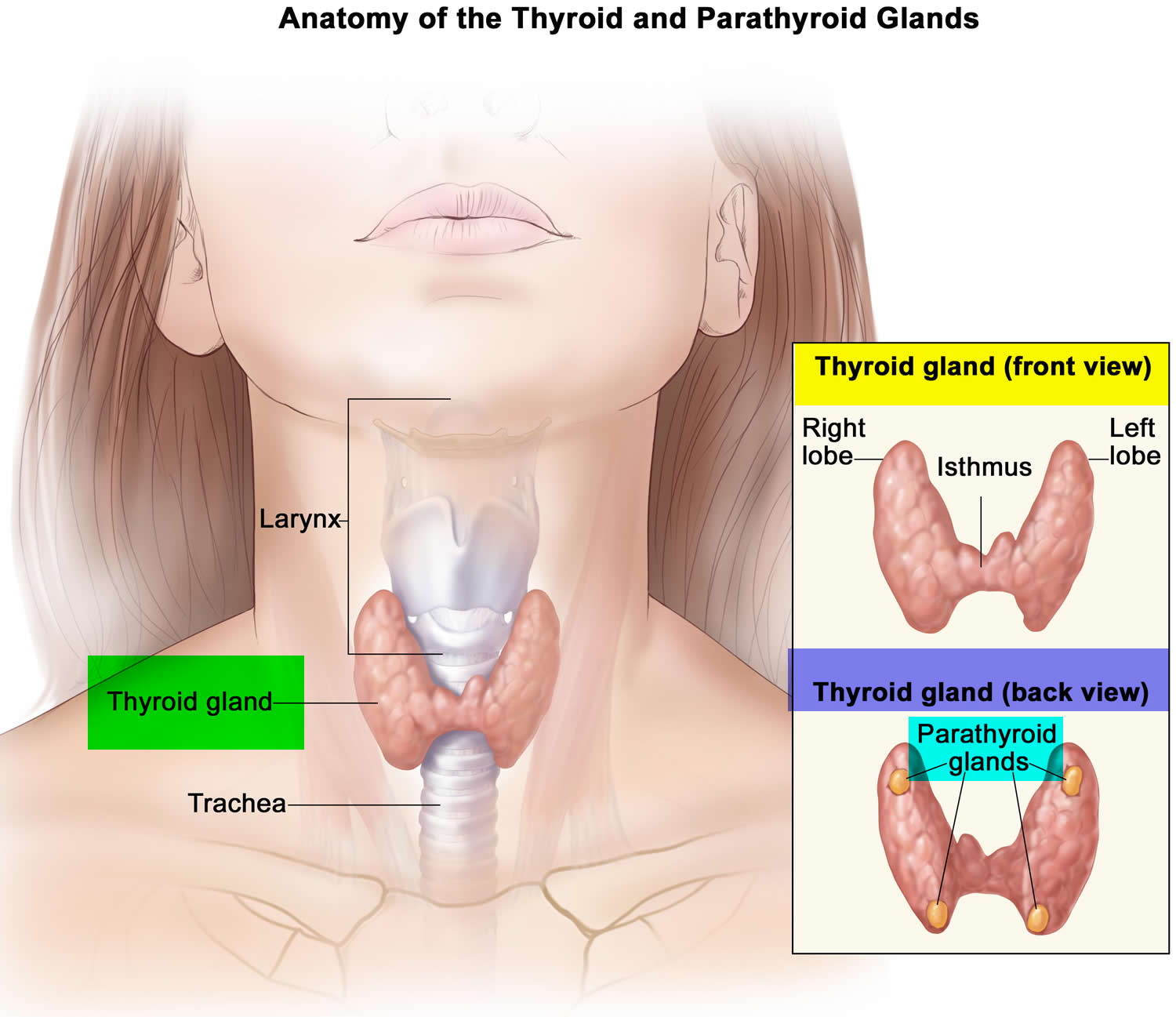
The parathyroid glands are ovoid glands, usually four in number, partially embedded in the posterior (behind) surface of the thyroid. Each parathyroid gland has a mass of about 40 mg and is about 3 to 8 mm long and 2 to 5 mm wide. Usually, one superior and one inferior parathyroid gland are attached to each lateral thyroid lobe, for a total of four. The parathyroid glands are separated from the thyroid follicles by a thin fibrous capsule and adipose tissue. Often, the parathyroid glands occur in other locations ranging from as high as the hyoid bone to as low as the aortic arch, and about 5% of people have more than four parathyroids.
Microscopically, the parathyroid glands contain two kinds of epithelial cells. The more numerous cells, called chief cells or principal cells, produce parathyroid hormone (PTH), also called parathormone. The function of the other kind of cell, called an oxyphil cell, is not known in a normal parathyroid gland. Oxyphil cells do not appear until after puberty, and then their numbers increase with age. However, its presence clearly helps to identify the parathyroid gland histologically due to its unique staining characteristics. Furthermore, in a cancer of the parathyroid glands, oxyphil cells secrete excess parathyroid hormone (PTH).
Attached to the chief cells of these parathyroid glands is a newly discovered molecule called the Calcium Sensing Receptor (CaSR). This Calcium Sensing Receptor (CaSR) responds to very small changes in the blood level of calcium to turn the parathyroid glands on and off when needed. The Calcium Sensing Receptor (CaSR) is working as the thermostat. If the body is not getting enough calcium from the diet, for example, the Calcium Sensing Receptor (CaSR) senses a need to get calcium from somewhere. The immediate response is for the parathyroid glands to make and secrete more of their active hormone – parathyroid hormone (PTH). This sets off a chain of events that get more calcium into the bloodstream. First, parathyroid hormone (PTH) goes to the calcium bank – namely your bones, where 98% of the body’s store of calcium is warehoused. This is good for the blood calcium and for the cells that need calcium, but not good for the bones themselves. The second line of defense against a need for more calcium is the kidneys. Here parathyroid hormone (PTH) does two things. First, it limits the kidneys from excreting too much calcium, thereby keeping the blood levels up. Next, it works on the kidneys to produce another hormone called calcitriol or “active vitamin D.”
Figure 1. Parathyroid gland location (hidden behind the thyroid gland)
Figure 2. Thyroid gland location
Parathyroid gland function
Parathyroid hormone (PTH) is secreted by the parathyroid glands, which regulates blood calcium levels (Ca 2+).
Furthermore, the parathyroid hormone (PTH) is also the major regulator of the levels of magnesium (Mg 2+), and phosphate (HPO4 2−) ions in the blood. The specific action of parathyroid hormone (PTH) is to increase the number and activity of osteoclasts. The result is elevated bone resorption, which releases ionic calcium (Ca 2+) and phosphates (HPO4 2−) into the blood.
Parathyroid hormone (PTH) also acts on the kidneys. First, it slows the rate at which Ca 2+ and magnesium (Mg 2+) are lost from blood into the urine. Second, it increases loss of phosphate (HPO4 2−) from blood into the urine. Because more phosphate (HPO4 2−) is lost in the urine than is gained from the bones, PTH decreases blood phosphate (HPO4 2−) level and increases blood Ca2+ and magnesium (Mg 2+) levels.
A third effect of PTH on the kidneys is to promote formation of the hormone calcitriol, the active form of vitamin D. Calcitriol, also known as 1,25-dihydroxyvitamin D3, increases the rate of calcium (Ca 2+), phosphate (HPO4 2−) and magnesium (Mg 2+) absorption from the gastrointestinal tract into the blood.
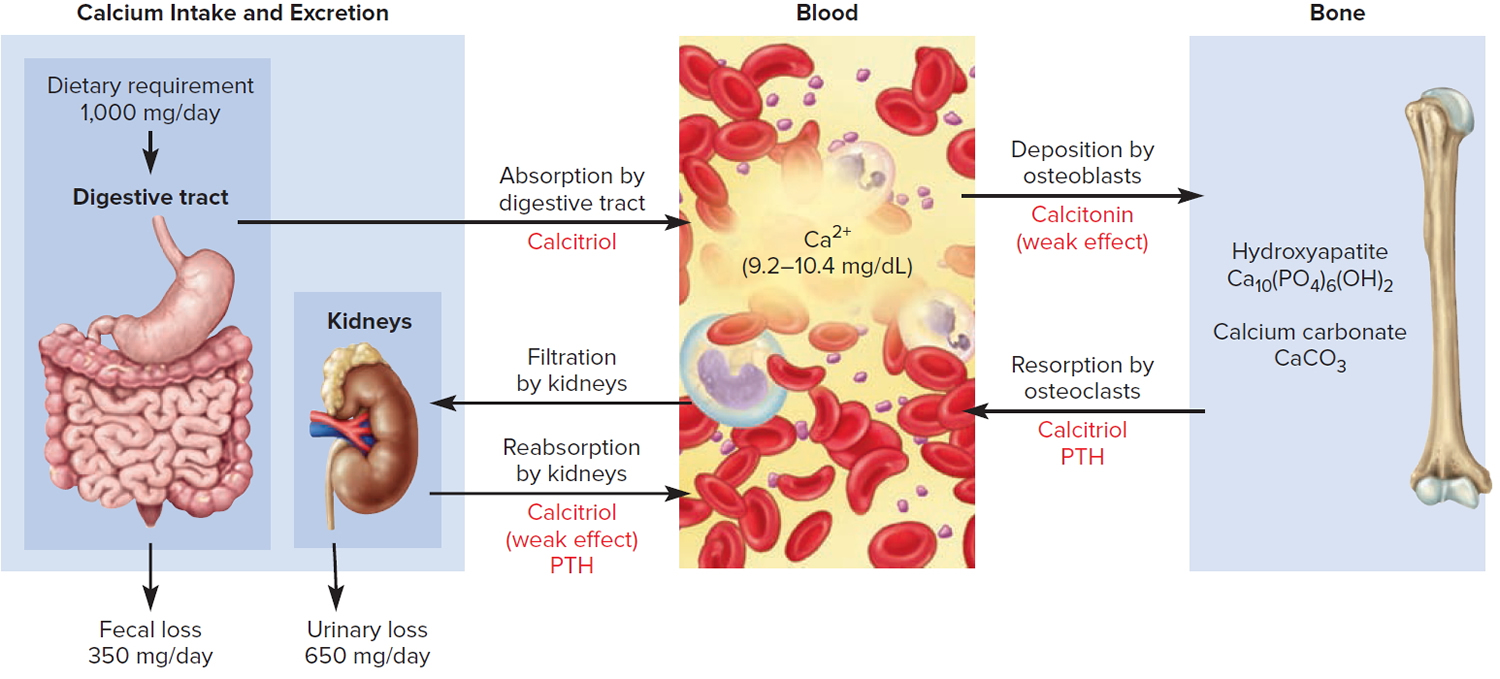
Figure 3. Hormonal control of calcium balance
Note: The central panel represents the blood reservoir of calcium and shows its normal (safe) range. Calcitriol and Parathyroid Hormone (PTH) regulate calcium exchanges between the blood and the small intestine and kidneys (left). Calcitonin, calcitriol, and Parathyroid Hormone (PTH) regulate calcium exchanges between blood and bone (right).
What is parathyroid hormone
Parathyroid hormone (PTH) is a hormone made by the parathyroid gland that helps your body maintain stable levels of calcium in your blood. Also called parathormone and parathyrin, the parathyroid hormone (PTH) helps your body store and use calcium. It is part of a feedback loop that includes calcium, parathyroid hormone, vitamin D, and to some extent, phosphorus (phosphate) and magnesium. Conditions and diseases that disrupt this feedback loop can cause inappropriate elevations or decreases in calcium and parathyroid hormone levels and lead to symptoms of hypercalcemia or hypocalcemia. A higher-than-normal amount of parathyroid hormone (hyperparathyroidism) causes high levels of calcium in the blood and may be a sign of disease. A condition characterized by underactivity of the parathyroid glands and reduced production of parathyroid hormone (hypoparathyroidism), symptoms may include tingling in the fingers and toes, muscle aches and spasms, fatigue, dry skin and brittle nails, headaches, anxiety, and depression.
- As calcium levels begin to increase in the blood, parathyroid hormone normally decreases.
- A decrease in the ionized calcium in the blood is the stimulus for the parathyroid hormone secretion.
- Magnesium also influences the parathyroid hormone level. Hypermagnesemia suppress the parathyroid hormone secretion, although not like calcium.
Parathyroid hormone is produced by four button-sized parathyroid glands that are located in the neck behind the thyroid gland. Normally, these glands secrete parathyroid hormone into the bloodstream in response to low blood calcium levels. The hormone works in three ways to help raise blood calcium levels back to normal:
- Parathyroid hormone promotes the release of calcium from bones into the bloodstream.
- Parathyroid hormone stimulates the kidneys to convert vitamin D from the inactive to the active form, which in turn increases the absorption of calcium from food in the intestines.
- Parathyroid hormone acts on the kidneys to suppress the excretion of calcium in the urine while encouraging excretion of phosphorus.
Parathyroid hormone itself is composed of 84 amino acids sometimes called parathyroid hormone 1-84. Intact and fragmented hormone is present in and secreted by the parathyroid gland. The intact hormone represents a smaller fraction, but its portion is increased when calcium levels are low and decreased when calcium levels are high. The “intact” and “whole or bioactive” parathyroid hormone assays have different reference ranges, but typically their results yield similar interpretations. The effects of fragmentation and disease are still being studied, and there are instances when the two assays may yield a different interpretation.
The so-called intact parathyroid hormone is the most frequently ordered parathyroid hormone test, which typically measures the whole molecule (PTH 1-84) and the larger fragments (predominantly PTH 1-74). Intact parathyroid hormone is used to help diagnose the cause of a low or high calcium level and to help distinguish between parathyroid-related and non-parathyroid-related causes. Intact parathyroid hormone may also be used to monitor the effectiveness of treatment when an individual has a parathyroid-related condition. Parathyroid hormone is routinely monitored for people with chronic kidney disease or who are on dialysis.
A calcium test is almost always ordered along with a parathyroid hormone test. It is not just the level in the blood that is important but the balance between calcium and parathyroid hormone and the response of the parathyroid glands to changing levels of calcium. Usually, health practitioners are concerned about either severe imbalances in calcium regulation that may require medical intervention or persistent imbalances that indicate an underlying problem.
Parathyroid hormone levels can be used to monitor people who have conditions or diseases that cause chronic calcium imbalances or to monitor those who have had surgery or another treatment for a parathyroid tumor.
Once released into the blood stream, parathyroid hormone has a very short life span; levels fall by half in less than 5 minutes due to uptake and cleavage in the liver and kidneys. The fragments are referred to as C-terminal fragments and are variably sized, missing anywhere from 6 amino acids to more than half the N-terminal portion of the molecule. C-terminal fragments have a longer half-life, exist in much higher concentrations, and are eventually cleared by the kidneys. Although it was originally thought that the C-terminal fragments were inactive, it now appears that certain fragments may have biologic activities that are able to oppose those of intact parathyroid hormone.
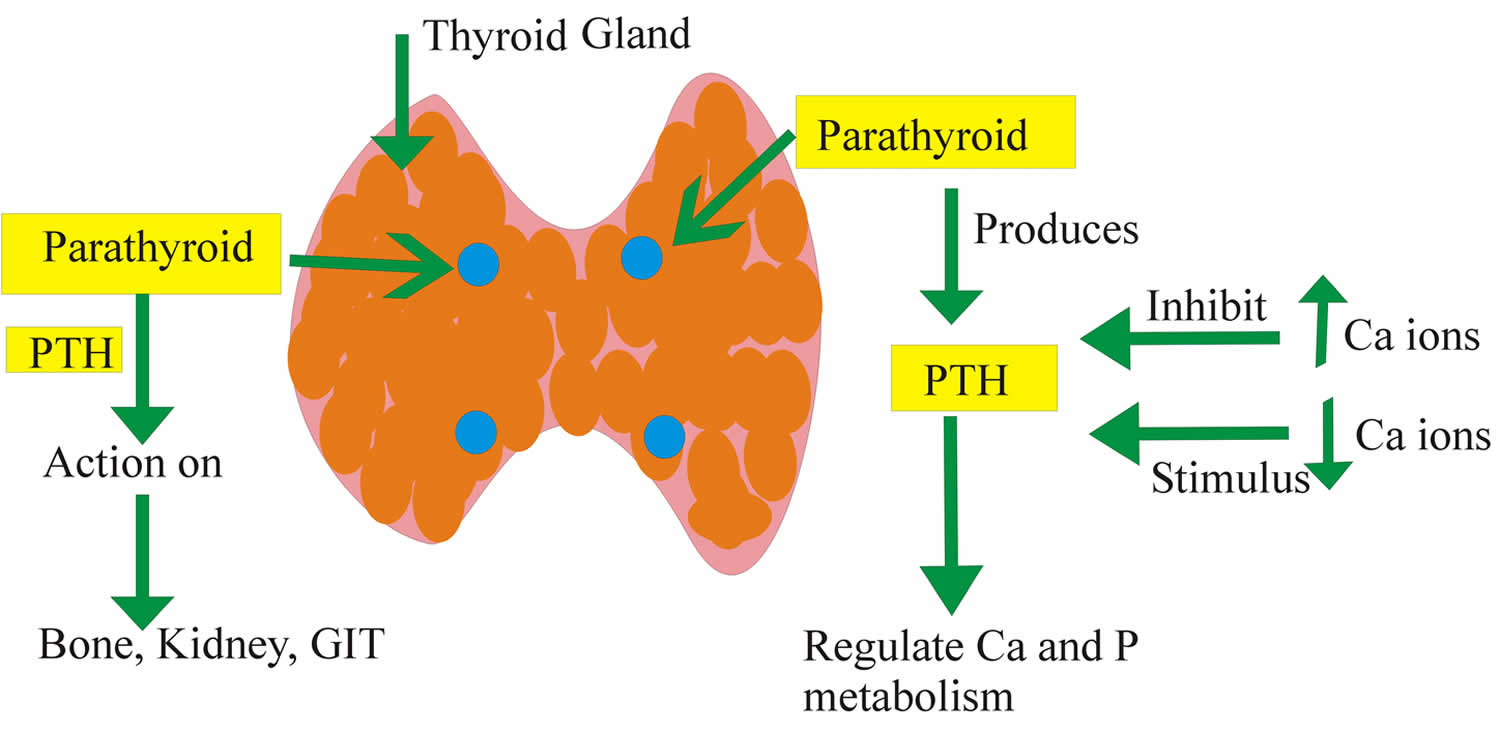
Figure 4. Parathyroid hormone function
Abbreviations: Ca = Calcium; P = Phosphate or Phosphorus; PTH = parathyroid hormone; GIT = gastrointestinal tract
Increased parathyroid hormone level is seen in:
- Primary hyperparathyroidism.
- Failure of the body to respond to parathyroid hormone (pseudohypoparathyroidism or secondary hyperparathyroidism).
- Vitamin D deficiency (hereditary) and rickets.
- Vitamin D disorders, including not enough sunlight in older adults and problems absorbing, breaking down, and using vitamin D in the body
- Zollinger Ellison syndrome.
- Non-parathyroid hormone producing tumors give rise to paraneoplastic syndrome. They produce parathyroid hormone like protein which acts like parathyroid hormone.
- Chronic renal failure.
- Hypocalcemia. Lack of calcium, which may be due to not eating enough calcium, not absorbing calcium in the gut, or losing too much calcium in your urine
- Malabsorption.
- Drugs that may increase parathyroid hormone levels include phosphates, anticonvulsants, steroids, isoniazid, lithium, and rifampin.
- Disorders that increase phosphate or phosphorous levels in the blood, such as long-term (chronic) kidney disease
- Pregnancy or breastfeeding (uncommon)
- Tumors in the parathyroid gland, called adenomas
Decreased parathyroid hormone level is seen in:
- Parathyroid glands do not produce enough parathyroid hormone (hypoparathyroidism)
- Non-Parathyroid hypercalcemia.
- Secondary hypoparathyroidism. Accidental removal of parathyroid glands during thyroid surgery.
- Sarcoidosis and tuberculosis
- Metastatic bone tumors.
- Excess vitamin D intake.
- Milk-alkali syndrome.
- Excess calcium over a long period of time usually from excess calcium supplements or certain antacids, that contain calcium carbonate or sodium bicarbonate (baking soda)
- DiGeorge syndrome is a primary immunodeficiency disease where baby may also be born without parathyroid glands.
- Autoimmune destruction of the parathyroid gland
- Cancers that start in another part of the body (such as the breast, lungs, or colon) and spread to the bone.
- Low levels of magnesium in the blood.
- Radiation to the parathyroid glands.
Calcium – Parathyroid hormone Relationship
- If calcium levels are low and parathyroid hormone levels high, then the parathyroid glands are responding appropriately, producing appropriate amounts of parathyroid hormone. Depending on the degree of hypocalcemia, a health practitioner may investigate a low calcium level further by measuring vitamin D, phosphorus, and magnesium levels.
- If calcium levels are low and parathyroid hormone levels are normal or low, then parathyroid hormone is not responding properly and the person tested probably has hypoparathyroidism. Hypoparathyroidism is a failure of the parathyroid glands to produce sufficient parathyroid hormone. It may be due to a variety of conditions and may be persistent, progressive, or transient. Causes include an autoimmune disorder, parathyroid damage or removal during surgery, a genetic condition, or a severe illness. Those affected will generally have low parathyroid hormone levels, low calcium levels, and high phosphorus levels.
- If calcium levels are high and parathyroid hormone levels are high, then the parathyroid glands are producing inappropriately high amounts of parathyroid hormone. A health practitioner may order X-rays or other imaging studies to help determine the cause and evaluate the severity of hyperparathyroidism. Hyperparathyroidism is a group of conditions characterized by an overproduction of parathyroid hormone by the parathyroid glands that is separated into primary, secondary, and tertiary hyperparathyroidism.
- If calcium levels are high and parathyroid hormone levels are low, then the parathyroid glands are responding properly, but a health practitioner is likely to perform further investigations to check for non-parathyroid-related reasons for the elevated calcium, such as rare mutations in calcium receptors or tumors that secrete a peptide that has parathyroid hormone-like activity and increases calcium concentration, which in turn decreases parathyroid hormone.
Table 1. Summarizes results of Calcium – Parathyroid hormone Relationship
| Calcium | Parathyroid hormone | Interpretation |
| Normal | Normal | Calcium regulation system functioning OK |
| Low | High | Parathyroid hormone is responding correctly; may run other tests to check for other causes of hypocalcemia |
| Low | Normal or Low | Parathyroid hormone not responding correctly; probably have hypoparathyroidism |
| High | High | Parathyroid gland producing too much parathyroid hormone; may do imaging studies to check for hyperparathyroidism |
| High | Low | Parathyroid hormone is responding correctly; may run other tests to check for non-parathyroid-related causes of elevated calcium |
| Normal | High | Mild hyperparathyroidism |
Can I have an abnormal parathyroid hormone level without having symptoms?
Yes, if your calcium level changes slowly, you may not have any noticeable symptoms. In this case, the imbalance will most likely be detected by finding an abnormal calcium level during a regular health check, then checking your parathyroid hormone level.
Primary hyperparathyroidism
Primary hyperparathyroidism is a disorder of the parathyroid glands, also called parathyroids. “Primary” means this disorder originates in the parathyroid glands. In primary hyperparathyroidism, one or more of the parathyroid glands are overactive. As a result, the gland releases too much parathyroid hormone (PTH). The disorder includes the problems that occur in the rest of the body as a result of too much parathyroid hormone (PTH)—for example, loss of calcium from bones.
In the United States, about 100,000 people develop primary hyperparathyroidism each year 3. The disorder is diagnosed most often in people between age 50 and 60, and women are affected about three times as often as men 4.
Key facts
- Primary hyperparathyroidism is a disorder of the parathyroid glands, in which one or more of the parathyroid glands are overactive. As a result, the gland releases too much parathyroid hormone (PTH).
- High PTH levels trigger the bones to release increased calcium into the blood, causing blood calcium levels to rise above normal. The loss of calcium from bones may weaken the bones. In response to high blood calcium levels, the kidneys excrete more calcium in the urine, which can lead to kidney stones.
Most people with primary hyperparathyroidism have no symptoms. When symptoms appear, they are often mild and nonspecific, such as muscle weakness, fatigue, increased need for sleep, feelings of depression, or aches and pains in bones and joints. - People with more severe primary hyperparathyroidism may have symptoms such as loss of appetite, nausea, vomiting, constipation, confusion or impaired thinking and memory, and increased thirst and urination.
- Health care providers diagnose primary hyperparathyroidism when a person has high blood calcium and PTH levels.
- Surgery to remove the overactive parathyroid gland or glands is the only definitive treatment for the disorder. When performed by experienced endocrine surgeons, surgery cures primary hyperparathyroidism in more than 95 percent of operations. Some people who have mild primary hyperparathyroidism may not need immediate or even any surgery and can be safely monitored. People with primary hyperparathyroidism due to familial hypocalciuric hypercalcemia should not have surgery.
Primary hyperparathyroidism causes
In about 80 percent of people with primary hyperparathyroidism, a benign or noncancerous tumor called an adenoma has formed in one of the parathyroid glands 4. The tumor causes the parathyroid gland to become overactive. In most other cases, the excess hormone comes from two or more overactive parathyroid glands, a condition called multiple tumors or hyperplasia. Rarely, primary hyperparathyroidism is caused by cancer of a parathyroid gland.
Primary hyperparathyroidism occurs because of some problem with one or more of the four parathyroid glands:
- A noncancerous growth (adenoma) on a gland is the most common cause. In 4 out of 5 cases, primary hyperparathyroidism is caused by a non-cancerous tumour called an adenoma on one of the parathyroid glands.
- Enlargement (hyperplasia) of two or more parathyroid glands accounts for most other cases. Less commonly, it can occur if 2 or more parathyroid glands become enlarged (hyperplasia).
- A cancerous (malignant) tumor is a rare cause of primary hyperparathyroidism.
Women are twice as likely to develop primary hyperparathyroidism than men. Most women who develop it are 50 to 60 years of age.
Primary hyperparathyroidism usually occurs randomly, but some people inherit a gene that causes the disorder.
Familial causes of hyperparathyroidism 5
- Multiple endocrine neoplasia Type 1
- Multiple endocrine neoplasia Type 2
- Familial hyperparathyroidism
- Hyperparathyroidism-jaw tumor syndrome
In most cases, health care providers don’t know why adenoma or multiple tumors occur in the parathyroid glands. Most people with primary hyperparathyroidism have no family history of the disorder, but some cases can be linked to an inherited problem. For example, familial multiple endocrine neoplasia type 1 is a rare, inherited syndrome that causes multiple tumors in the parathyroid glands as well as in the pancreas and the pituitary gland. Another rare genetic disorder, familial hypocalciuric hypercalcemia, causes a kind of hyperparathyroidism that is atypical, in part because it does not respond to standard parathyroid surgery.
Risk factors for primary hyperparathyroidism
You may be at an increased risk of primary hyperparathyroidism if you:
- Are a woman who has gone through menopause
- Have had prolonged, severe calcium or vitamin D deficiency
- Have a rare, inherited disorder, such as multiple endocrine neoplasia, type 1, which usually affects multiple glands
- Have had radiation treatment for cancer that has exposed your neck to radiation
- Have taken lithium, a drug most often used to treat bipolar disorder
Primary hyperparathyroidism symptoms
Most people with primary hyperparathyroidism have no symptoms.
When hyperparathyroidism symptoms appear, they are often mild and nonspecific, such as:
- muscle weakness
- fatigue and an increased need for sleep
- feelings of depression
- aches and pains in bones and joints
People with more severe disease may have:
- loss of appetite
- nausea
- vomiting
- constipation
- confusion or impaired thinking and memory
- increased thirst and urination
These symptoms are mainly due to the high blood calcium levels that result from excessive PTH.
Primary hyperparathyroidism diagnosis
Medical professionals diagnose primary hyperparathyroidism when a person has high blood calcium and PTH levels. High blood calcium is usually the first sign that leads health care providers to suspect parathyroid gland overactivity. Other diseases can cause high blood calcium levels, but only in primary hyperparathyroidism is the elevated calcium the result of too much PTH.
Routine blood tests that screen for a wide range of conditions, including high blood calcium levels, are helping health care providers diagnose primary hyperparathyroidism in people who have mild forms of the disorder and are symptom-free. For a blood test, blood is drawn at a health care provider’s office or commercial facility and sent to a lab for analysis.
What tests may be done to check for possible complications?
Once the diagnosis of primary hyperparathyroidism is established, other tests may be done to assess complications:
- Bone mineral density test. Dual energy x-ray absorptiometry, sometimes called a DXA or DEXA scan, uses low-dose x rays to measure bone density. During the test, a person lies on a padded table while a technician moves the scanner over the person’s body. DXA scans are performed in a health care provider’s office, outpatient center, or hospital by a specially trained technician and may be interpreted by a metabolic bone disease expert or radiologist—a doctor who specializes in medical imaging—or other specialists; anesthesia is not needed. The test can help assess bone loss and risk of fractures.
- Ultrasound. Ultrasound uses a device, called a transducer, that bounces safe, painless sound waves off organs to create an image of their structure. The procedure is performed in a health care provider’s office, outpatient center, or hospital by a specially trained technician, and the images are interpreted by a radiologist; anesthesia is not needed. The images can show the presence of kidney stones.
- Computerized tomography (CT) scan. CT scans use a combination of x rays and computer technology to create three-dimensional (3-D) images. A CT scan may include the injection of a special dye, called contrast medium. CT scans require the person to lie on a table that slides into a tunnel-shaped device where the x rays are taken. The procedure is performed in an outpatient center or hospital by an x-ray technician, and the images are interpreted by a radiologist; anesthesia is not needed. CT scans can show the presence of kidney stones.
- Urine collection. A 24-hour urine collection may be done to measure selected chemicals, such as calcium and creatinine, which is a waste product healthy kidneys remove. The person collects urine over a 24-hour period, and the urine is sent to a laboratory for analysis. The urine collection may provide information on kidney damage, the risk of kidney stone formation, and the risk of familial hypocalciuric hypercalcemia.
- 25-hydroxy-vitamin D blood test. This test is recommended because vitamin D deficiency is common in people with primary hyperparathyroidism.
Imaging tests before surgery
If your doctor recommends surgery, he or she will likely use one of these imaging tests to locate the parathyroid gland or glands that are causing problems:
- Sestamibi parathyroid scan. Sestamibi is a radioactive compound that is absorbed by overactive parathyroid glands and can be detected by a scanner that detects radioactivity. The normal thyroid gland also absorbs sestamibi. To eliminate uptake in the thyroid obscuring the uptake in a parathyroid adenoma, radioactive iodine, which is only taken up by the thyroid, also is given and the thyroid image is digitally subtracted. Computerized tomography (CT) scanning may be combined with the sestamibi scan to improve detection of an abnormality.
- Ultrasound. Ultrasound uses sound waves to create images of your parathyroid glands and surrounding tissue. A small device held against your skin (transducer) emits high-pitched sound waves and records the sound wave echoes as they reflect off internal structures. A computer converts the echoes into images on a monitor.
Primary hyperparathyroidism treatment
Watchful waiting
Some people who have mild primary hyperparathyroidism may not need immediate or even any surgery and can be safely monitored. People may wish to talk with their health care provider about long-term monitoring if they:
- are symptom-free
- have only slightly elevated blood calcium levels
- have normal kidneys and bone density
Your doctor may recommend no treatment and regular monitoring if:
- Your calcium levels are only slightly elevated
- Your kidneys are functioning normally, and you have no kidney stones
- Your bone density is normal or only slightly below normal
- You have no other symptoms that may improve with treatment
If you choose this watch-and-wait approach, you’ll likely need periodically scheduled tests to monitor your blood-calcium levels and bone density.
Long-term monitoring should include periodic clinical evaluations, annual serum calcium measurements, annual serum creatinine measurements to check kidney function, and bone density measurements every 1 to 2 years.
Vitamin D deficiency should be corrected if present. Patients who are monitored need not restrict calcium in their diets.
Lifestyle and home remedies
If you and your doctor have chosen to monitor, rather than treat, your primary hyperparathyroidism, the following suggestions can help prevent complications:
- Monitor how much calcium and vitamin D you get in your diet. Restricting dietary calcium intake is not advised for people with hyperparathyroidism. The Institute of Medicine recommends 1,000 milligrams (mg) of calcium a day for adults ages 19 to 50 and men ages 51 to 70. That calcium recommendation increases to 1,200 mg a day for women age 51 and older and men age 71 and older. The Institute of Medicine also recommends 600 international units (IUs) of vitamin D a day for people ages 1 to 70 and 800 IUs a day for adults age 71 and older. Talk to your doctor about dietary guidelines that are appropriate for you.
- Drink plenty of fluids. Drink enough fluids, mostly water, to produce nearly clear urine to lessen the risk of kidney stones.
- Exercise regularly. Regular exercise, including strength training, helps maintain strong bones. Talk to your doctor about what type of exercise program is best for you.
- Don’t smoke. Smoking may increase bone loss as well as increase your risk of a number of serious health problems. Talk to your doctor about the best ways to quit.
- Avoid calcium-raising drugs. Certain medications, including some diuretics such as thiazides and lithium, can raise calcium levels. If you take such drugs, ask your doctor whether another medication may be appropriate for you.
Either immobilization—the inability to move due to illness or injury—or gastrointestinal illness with vomiting or diarrhea that leads to dehydration can cause blood calcium levels to rise further in someone with primary hyperparathyroidism. People with primary hyperparathyroidism should seek medical attention if they find themselves immobilized or dehydrated due to vomiting or diarrhea.
Hyperparathyroidism surgery
Surgery to remove the overactive parathyroid gland or glands is the only definitive treatment for primary hyperparathyroidism and provides a cure in about 95 percent of all cases, particularly if the patient has a very high blood calcium level or has had a fracture or a kidney stone. In patients without any symptoms, guidelines are used to identify who might benefit from parathyroid surgery 6.
When performed by experienced endocrine surgeons, surgery cures primary hyperparathyroidism in more than 95 percent of operations 4.
Surgeons often use imaging tests before surgery to locate the overactive gland to be removed. The most commonly used tests are sestamibi and ultrasound scans. In a sestamibi scan, the patient receives an injection of a small amount of radioactive dye that is absorbed by overactive parathyroid glands. The overactive glands can then be viewed using a special camera.
If all four glands are affected, a surgeon will likely remove only three glands and perhaps a portion of the fourth — leaving some functioning parathyroid tissue.
Surgery may be done as an outpatient procedure, allowing you to go home the same day. In such cases, the surgery can be done through very small incisions in the neck, and you receive only local anesthetics.
Surgeons use two main strategies to remove the overactive parathyroid gland or glands:
- Minimally invasive parathyroidectomy. This type of surgery, which can be done on an outpatient basis, may be used when only one of the parathyroid glands is likely to be overactive. Guided by a tumor-imaging test, the surgeon makes a small incision in the neck to remove the gland. The small incision means that patients typically have less pain and a quicker recovery than with more invasive surgery. Local or general anesthesia may be used for this type of surgery.
- Standard neck exploration. This type of surgery involves a larger incision that allows the surgeon to access and examine all four parathyroid glands and remove the overactive ones. This type of surgery is more extensive and typically requires a hospital stay of 1 to 2 days. Surgeons use this approach if they plan to inspect more than one gland. General anesthesia is used for this type of surgery.
Almost all people with primary hyperparathyroidism who have symptoms can benefit from surgery. Experts believe that those without symptoms but who meet guidelines for surgery will also benefit from surgery. Surgery can lead to improved bone density and fewer fractures and can reduce the chance of forming kidney stones. Other potential benefits are being studied by researchers.
Complications from surgery aren’t common. Risks include:
- Damage to nerves controlling the vocal cords
- Long-term low calcium levels requiring the use of calcium and vitamin D supplements
Surgery for primary hyperparathyroidism has a complication rate of 1–3 percent when performed by experienced endocrine surgeons 7. Rarely, patients undergoing surgery experience damage to the nerves controlling the vocal cords, which can affect speech. A small number of patients lose all their healthy parathyroid tissue and thus develop chronic low calcium levels, requiring lifelong treatment with calcium and some form of vitamin D. This complication is called hypoparathyroidism. The complication rate is slightly higher for operations on multiple tumors than for a single adenoma because more extensive surgery is needed.
People with primary hyperparathyroidism due to familial hypocalciuric hypercalcemia should not have surgery.
Medications
Medications to treat hyperparathyroidism include the following:
- Calcimimetics. A calcimimetic is a drug that mimics calcium circulating in the blood. The drug may trick the parathyroid glands into releasing less parathyroid hormone. Calcimimetic drug is sold as cinacalcet (Sensipar). The U.S. Food and Drug Administration approved cinacalcet to treat secondary hyperparathyroidism caused by chronic kidney disease caused by dialysis—a blood-filtering treatment for kidney failure and primary hyperparathyroidism caused by parathyroid cancer. Some doctors may prescribe it for the management of hypercalcemia associated with primary hyperparathyroidism, particularly if surgery hasn’t successfully cured the disorder or a person isn’t a good candidate for surgery. The most commonly reported side effects of cinacalcet are joint and muscle pain, diarrhea, nausea, and respiratory infection.
- Hormone replacement therapy. For women who have gone through menopause and have signs of osteoporosis, hormone replacement therapy (selective estrogen receptor modulators) may help bones retain calcium. This treatment doesn’t address the underlying problems with the parathyroid glands. Prolonged use of hormone replacement therapy can increase the risk of blood clots and breast cancer. Work with your doctor to evaluate the risks and benefits to help you decide what’s best for you. Some common side effects of hormone replacement therapy include breast pain and tenderness, dizziness, and headache.
- Bisphosphonates. Bisphosphonates also prevent the loss of calcium from bones and may lessen osteoporosis caused by hyperparathyroidism. Some side effects associated with bisphosphonates include low blood pressure, fever and vomiting.
Eating, Diet, and Nutrition
Eating, diet, and nutrition have not been shown to play a role in causing or preventing primary hyperparathyroidism.
Vitamin D
Experts suggest correcting vitamin D deficiency in people with primary hyperparathyroidism to achieve a serum level of 25-hydroxy-vitamin D greater than 20 nanograms per deciliter (50 nanomoles per liter). Research is ongoing to determine optimal doses and regimens of vitamin D supplementation for people with primary hyperparathyroidism.
For the healthy public, the Institute of Medicine (IOM) guidelines for vitamin D intake are:
- people ages 1 to 70 years may require 600 International Units (IUs)
- people age 71 and older may require as much as 800 IUs
The Institute of Medicine also recommends that no more than 4,000 IUs of vitamin D be taken per day.
Calcium
People with primary hyperparathyroidism without symptoms who are being monitored do not need to restrict calcium in their diet. People with low calcium levels due to loss of all parathyroid tissue from surgery will need to take calcium supplements for the rest of their life.
Secondary hyperparathyroidism
Secondary hyperparathyroidism is the result of another condition that lowers calcium levels in your body and over time, increased parathyroid hormone (PTH) levels occur. Therefore, your parathyroid glands overwork to compensate for the loss of calcium. Factors that may contribute to secondary hyperparathyroidism include:
- Severe calcium deficiency. Your body may not get enough calcium from your diet, often because your digestive system doesn’t absorb the calcium from it.
- Severe vitamin D deficiency. Vitamin D helps maintain appropriate levels of calcium in the blood, and it helps your digestive system absorb calcium from your food. Your body produces vitamin D when your skin is exposed to sunlight, and you consume some vitamin D in food. If you don’t get enough vitamin D, then calcium levels may drop.
- Chronic kidney failure. Your kidneys convert vitamin D into a form that your body can use. If your kidneys function poorly, usable vitamin D may decline and calcium levels drop. Chronic kidney failure is the most common cause of secondary hyperparathyroidism.
Secondary hyperparathyroidism, a common, serious, and progressive complication of chronic kidney disease (CKD), is characterized by high serum PTH, parathyroid gland hyperplasia, and disturbances in mineral metabolism, mainly hypocalcemia and hyperphosphatemia 8. These mineral disturbances mainly cause renal osteodystrophy, progressive vascular calcification, and in turn, cardiovascular disease and death, especially in patients receiving hemodialysis 9. Together, this constellation of comorbidities is known as chronic kidney disease–mineral and bone disorder 10. In the US, the estimated prevalence of secondary hyperparathyroidism in patients with chronic kidney failure ranges from 2 to nearly 5 million individuals, with 30%–50% of end-stage renal disease (ESRD) patients affected by secondary hyperparathyroidism 11.
Secondary hyperparathyroidism symptoms
The signs and symptoms of hyperparathyroidism include:
- Fragile bones that easily fracture (osteoporosis)
- An increase in bone fractures or breaks
- Kidney stones
- Excessive urination
- Abdominal pain
- Tiring easily or weakness
- Depression or forgetfulness
- Bone and joint pain
- Frequent complaints of illness with no apparent cause
- Nausea, vomiting or loss of appetite
- Feeling weak or tired most of the time
- General aches and pains
- Frequent heart burn (the high calcium level in your blood can cause your stomach to make too much acid)
- Confusion and memory loss
- Excessive urination
- High blood pressure. High blood pressure occurs more commonly in people with hyperparathyroidism and may need to be treated whether or not specific treatment is recommended for the hyperparathyroidism.
Signs and symptoms of kidney failure. You may have some of the following symptoms:
- nausea
- little or no urination
- headaches
- drowsiness
- trouble sleeping
- loss of appetite
- weight loss
- weakness
- fatigue, or feeling tired
- generalized itching or numbness
- weight loss
- muscle cramps (especially in the legs)
- high blood pressure
- edema—swelling, usually in the legs, feet, or ankles and less often in the hands or face
- anemia (a low blood count)
- trouble sleeping
- darkened skin
- dry skin
- trouble concentrating
- vomiting
Secondary hyperparathyroidism treatment
Current treatment for secondary hyperparathyroidism should follow three steps:
- Reduction of phosphorus uptake by dietary restriction or the use of phosphate binders;
- Control of parathyroid hormone (PTH) with the use of vitamin D metabolites; and
- Use of calcimimetics, currently agents that allosterically modify the calcium-sensing receptor (CaSR) to enhance activation in the presence of circulating levels of calcium, thus reducing parathyroid hormone (Table 2) 12.
Parathyroidectomy is usually a treatment strategy of last resort, after pharmacotherapy has failed 13. The goal of treatment is to maintain serum calcium, serum phosphorus, and PTH within accepted targeted ranges 14. Because of the limitations associated with the standard-of-care treatment for secondary hyperparathyroidism, PTH targets are not met for many patients 15.
Table 2. Treatment options for secondary hyperparathyroidism
| Treatment | Dose formulation | Initial dose | Most common side effects |
|---|---|---|---|
| Phosphate binders | |||
| Calcium-containing | |||
| Calcium acetate (prescription for end-stage renal disease) | 667 mg capsules | 667–1,334 mg | Nausea, vomiting, hypercalcemia |
| Calcium carbonate (OTC) | 250 to 1,000 mg tablets | 500–1,000 mg | Nausea, vomiting, diarrhea, dyspepsia, abdominal pain, flatulence, constipation |
| Non-calcium-containing | |||
| Sevelamer hydrochloride/carbonate | 800 mg tablets, 2.4 g packets powder for oral suspension | 800–1,600 mg TID with meals | Headache, diarrhea, stomach upset |
| Lanthanum carbonate (for ESRD) | 250, 500, 750, and 1,000 mg chewable tablets | 1,500 mg daily | Diarrhea, nausea, abdominal pain, vomiting |
| Vitamin D analogs | |||
| Calcitriol | Oral (0.25 µg capsules) | 0.25 µg daily | Hypercalcemia, headache, abdominal pain, nausea, rash, urinary tract infection |
| Injectable (1 µg/mL) | 1–2 µg three times weekly or every other day | Weakness, headache, somnolence, nausea, vomiting, dry mouth, constipation, muscle pain, bone pain, metallic taste, anorexia, abdominal pain, epigastric discomfort | |
| Doxercalciferol | Oral (0.5, 1.0, and 2.5 µg capsules) | 1 µg daily (predialysis) 10 µg three times weekly at dialysis | Edema, headache, malaise, dyspepsia, nausea, vomiting, dizziness, dyspnea (similar for both dosing forms) |
| Injectable 4 µg/2 mL | 4 µg three times weekly at the end of dialysis or every other day | ||
| Paricalcitol | Oral (1, 2, and 4 µg capsules), injectable | 1–2 µg daily (PTH ≤500 pg/mL) or 2–4 µg every other day (PTH >500 pg/mL) | Diarrhea, hypertension, dizziness, vomiting |
| Calcimimetic | |||
| Cinacalcet | 30, 60, and 90 mg tablets | 30 mg once daily | Nausea, vomiting, diarrhea |
Abbreviations: AE = adverse event; ESRD = end-stage renal disease; OTC = over the counter; PTH = parathyroid hormone; TID = three times per day.
[Source 8 ]Table 3. Stages of chronic kidney disease
| Stage | Definition | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage and normal or elevated GFR | ≥90 |
| 2 | Kidney damage and mild reduction in GFR | 60–89 |
| 3 | Moderate reduction in GFR | 30–59 |
| 4 | Severe reduction in GFR | 15–29 |
| 5 | Kidney failure | <15 (or dialysis) |
Abbreviation: GFR = glomerular filtration rate.
Low-phosphorus diet and phosphate binders
Current guidelines suggest maintaining serum calcium and phosphorous with the normal range via balanced diet and/or phosphate binders administration 14. The use of vegetarian products as well as protein restriction is commonly suggested to limit phosphate intake 14. Cooking methods such as boiling are also proposed to limit (20%–70%) the amount of phosphate contained in different foods 15. A recent meta-analysis suggested a significant reduction of phosphate levels of about 0.8 mg/dL 16.
In spite of these data, phosphate binders have long been perceived as a cornerstone in the treatment of secondary hyperparathyroidism 17. Indeed, due to poor treatment adherence, diet is often insufficient to reach a desirable control of serum phosphate levels, and a wide range of phosphate binders are now available 18. Although effective, aluminum-based phosphate binders have been replaced by calcium and iron-based and polymer-based phosphate binders due to their toxicity 18. Current guidelines suggest to limit aluminum-based phosphate binders in case of severe hyperphosphatemia and for a short period of time 14. When compared to placebo, all available compounds have been shown to lower serum phosphate to similar extent 15. However, differences among drugs may exist. Calcium-free phosphate binders are associated with lower serum calcium, and differences in PTH control with various drugs have been reported 16. Preliminary data also suggest that phosphate restriction and calcium-free phosphate binders may reduce FGF-23 18. Although the clinical relevance of different biochemical profiles still needs to be elucidated, some lines of evidence suggest that calcium-based phosphate binders may accelerate vascular calcification deposition and progression when compared to calcium-free phosphate binders 19. Calcium supplements can represent a substantial portion of an individual’s daily calcium intake and contribute to deposition of calcium crystals in soft tissues 19 in individuals with normal renal function 20 as well as renal function impairment 19. Also, evidence supports the notion that calcium-free phosphate binders are associated with a better survival when compared to calcium-based phosphate binders 21, although future efforts are still required to shed light on this vexing question. Similarly, future studies should investigate the impact of combination therapies for chronic kidney disease-mineral bone disorder on calcium and phosphate balance and their effect on outcome in chronic kidney disease patients 22.
Vitamin D and vitamin D derivatives
In patients with chronic kidney disease stages 3–5 (Table 3 above), both nutritional (cholecalciferol, ergocalciferol, calcifediol) and vitamin D receptor activators (VDRAs) such as calcitriol and its analogs can improve abnormal mineral homeostasis 14. Although the optimal target of 25(OH)D remains uncertain, serum levels >30 ng/mL are accepted as a normal threshold 23. Clinical data support a mild effect of nutritional vitamin D against nonsevere SHPT in chronic kidney disease stages 3–5 24. International guidelines suggest the correction of nutritional vitamin D deficiency as a first-line therapy to counteract the onset and progression of chronic kidney disease-mineral and bone disorder in predialysis patients 25. However, due to the inconsistency of current evidence on the best approach to correct 25(OH)D deficiency, the KDIGO, NICE, and ERBP guidelines are unable to provide any therapeutic suggestion on how to replenish low 25(OH) D levels. Doses originally recommended by KDOQI guidelines were often insufficient to achieve the expected 25(OH)D levels 26. Higher doses of cholecalciferol and ergocalciferol were thus suggested in 2012 27, showing the superiority of cholecalciferol compared to ergocalciferol in replenishing 25(OH)D deficiency 28. Wetmore et al. 29 have recently randomized 44 nondialysis chronic kidney disease patients to receive cholecalciferol 50,000 IU/wk versus ergocalciferol 50,000 IU/wk for 12 weeks, without suspending concomitant active therapy. Although cholecalciferol was more effective than ergocalciferol at raising serum 25(OH)D levels (45±16.5 ng/mL and 30.7 versus 15.3 ng/mL), changes in serum PTH or active vitamin D were similar between the groups, and a similar decline of 25(OH)D levels between the groups was observed after cessation of therapy 29. Recent data support an innovative efficacy of a modified-release formulation of calcifediol 30, probably favored by its capability to raise 25(OH)D levels slowly enough for limiting an abrupt activation of vitamin D catabolism 31. This novel compound was effective in reducing iPTH levels in 44 predialysis chronic kidney disease patients in a dose-dependent manner compared to placebo 30. Independently from the type and dose of nutritional vitamin D adopted, 25(OH)D levels should not exceed 100 ng/mL, and particular caution should be paid while supplementing patients at high risk of 1-α-hydroxylase activity, as kidney transplant patients and those affected by sarcoidosis or B-cell lymphoma 24. Vitamin D receptor activator was shown to be effective in counteracting secondary hyperparathyroidism in nondialysis chronic kidney disease patients 32. Selective vitamin D receptor activators such as paricalcitol and doxercalciferol were developed to provide vitamin D receptor activation with a lower risk of positive Ca and P balance 33. In a 12-week, placebo-controlled, randomized study in patients receiving dialysis, paricalcitol effectively reduced PTH levels, with 6.7% of assessments indicative of hypercalcemia (serum calcium >10.5 mg/dL) versus 3.3% with placebo 34. Doxercalciferol has also been shown to be effective in reducing PTH in hemodialysis patients, with a modest but clinically notable incidence of hypercalcemia in 15% of assessments during treatment 35, while more recently a minimal increase in Ca and P levels was reported among patients receiving paricalcitol 36 vitamin D receptor activators are largely used to treat secondary hyperparathyroidism in dialysis patients. Improved survival among end-stage-renal disease patients receiving vitamin D receptor activator was inconsistently reported in observational studies 37. Similarly, observational data showed heterogeneous results about the superiority of paricalcitol against nonselective vitamin D receptor activator in improving survival 38. Although selective vitamin D receptor activators have been purported to limit calcitriol administration in chronic kidney disease patients at higher cardiovascular risk 39, it must be noted that the impact of different vitamin D receptor activator on hard end points has never been tested in head-to-head randomized clinical trials. Due to the widespread genomic effect elicited by vitamin D receptor activation, vitamin D is receiving growing interest for other targets than secondary hyperparathyroidism such as proteinuria 40, left ventricular hypertrophy (LVH) 41 and anemia 42. Thus, vitamin D represents a culprit of medical therapy for chronic kidney disease-mineral and bone disorder. However, further studies are advocated to shed light on the many unresolved issues of this multifaceted therapy 43.
Parathyroidectomy
Advances in the pharmacological treatment of secondary hyperparathyroidism often obviate parathyroidectomy; however, some researchers have reported that parathyroidectomy may be more cost-effective than cinacalcet in some patients with end-stage renal disease and uncontrolled secondary hyperparathyroidism 44. Successful parathyroidectomy can yield a dramatic reduction in PTH level and clinical symptoms 45. Dialysis patients undergoing parathyroidectomy have an increased risk of cardiopulmonary complications and mortality compared to patients not on dialysis. The risks of parathyroidectomy in dialysis patients are likely similar to other commonly performed procedures for dialysis patients. However, a retrospective review of dialysis patients with severe and unresponsive secondary hyperparathyroidism indicated that parathyroidectomy did not improve cardiovascular outcomes compared with standard medical treatment 46. In some instances, secondary hyperparathyroidism may continue after parathyroidectomy because of incomplete resection or because of ongoing PTH secretion from autotransplanted parathyroid tissue 13.
Tertiary hyperparathyroidism
Tertiary hyperparathyroidism usually occurs after a successful kidney transplant fails to normalize the production of parathyroid hormone (PTH) 1. Persistently increased serum levels of PTH occur in up to 30% of patients after renal transplantation 2. Parathyroidectomy is currently the only curative treatment. Indications for parathyroidectomy in patients with tertiary hyperparathyroidism include severe hypercalcemia, persistent hypercalcemia more than three months to one year after transplantation, decreased bone mineral density, or symptoms of hyperparathyroidism, including pruritus, nephrocalcinosis, and pathologic bone fracture 47. The diagnosis of tertiary hyperparathyroidism is controversial, and has been defined classically by a post-transplant increase in serum PTH with corresponding hypercalcemia 1. However, as scientists learn more about the underlying pathophysiology of post-transplant parathyroid function, many patients after successful renal transplantation have increased serum PTH with either a normal or increased serum calcium concentration. Additionally, when left untreated, increased levels of PTH even with normocalcemia have adverse effects on bone health and may worsen osteopenia and fracture rates 48.
Recently, calcimimetics, such as cinacalcet, have received attention with respect to treating tertiary hyperparathyroidism in the hope that parathyroidectomy can be delayed or even avoided. Several single-center, prospective studies have treated a small number of renal transplant patients with persistent post-transplant hyperparathyroidism with cinacalcet and have reported promising results with the normalization of calcium 49. These studies, however, were of short duration and did not examine the effect of cinacalcet on bone markers. Therefore, tertiary hyperparathyroidism is not an approved indication currently for patients to receive cinacalcet 50. Cinacalcet is being utilized increasingly in the tertiary hyperparathyroidism population, however, this does not seem to affect rates of cure or recurrence at parathyroidectomy 51.
If cinacalcet cannot be continued for financial reasons or intolerance to the drug, then subtotal parathyroidectomy should be considered to treat tertiary hyperparathyroidism 1. Since involution of hyperplastic parathyroid glands and a resultant decline in PTH concentration occur over a year, many kidney transplant physicians prefer to wait for at least a year after kidney transplantation before proceeding to parathyroidectomy surgery, provided there is no graft dysfunction related to hypercalcemia. Also, treatment of osteoporosis with bisphosphonates and denosumab may improve serum calcium levels 52.
- Lou I, Schneider DF, Leverson G, Foley D, Sippel R, Chen H. Parathyroidectomy is underused in patients with tertiary hyperparathyroidism after renal transplantation. Surgery. 2015;159(1):172-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4688142/[↩][↩][↩][↩]
- Evenepoel P, Claes K, Kuypers D, et al. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19(5):1281–7.[↩][↩]
- Bilezikian JP. Primary hyperparathyroidism. In: DeGroot LJ, ed.; Arnold A, section editor. Diseases of Bone and Mineral Metabolism.[↩]
- Silverberg SJ and Bilezikian JP. Primary hyperparathyroidism. In: Jameson JL and DeGroot LJ, senior eds. Endocrinology: Adult and Pediatric. 6th ed. (online version). Philadelphia: Saunders; 2010.[↩][↩][↩]
- Pokhrel B, Levine SN. Hyperparathyroidism, Primary. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441895[↩]
- Bilezikian JP, Khan A, Potts Jr JT. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Third International Workshop. Journal of Clinical Endocrinology and Metabolism. 2009;94(6):335–339.[↩]
- Udelsman R, Pasieka JL, Sturgeon C, Young JEM, and Clark OH. Surgery for asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. Journal of Clinical Endocrinology and Metabolism. 2009;94(2):366–372.[↩]
- Cozzolino M, Galassi A, Conte F, Mangano M, Di Lullo L, Bellasi A. Treatment of secondary hyperparathyroidism: the clinical utility of etelcalcetide. Ther Clin Risk Manag. 2017;13:679-689. Published 2017 Jun 1. doi:10.2147/TCRM.S108490 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5461056/[↩][↩]
- McCann LM, Beto J. Roles of calcium-sensing receptor and vitamin D receptor in the pathophysiology of secondary hyperparathyroidism. J Ren Nutr. 2010;20:141–150.[↩]
- Cozzolino M, Urena-Torres P, Vervloet MG, et al. Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant. 2014;29(10):1815–1820.[↩]
- Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm. 2007;13:397–411[↩]
- Stubbs JR, Wetmore JB. Does it matter how parathyroid hormone levels are suppressed in secondary hyperparathyroidism? Semin Dial. 2011;24:298–306.[↩]
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921.[↩][↩]
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1–S130.[↩][↩][↩][↩][↩]
- Galassi A, Cupisti A, Santoro A, Cozzolino M. Phosphate balance in ESRD: diet, dialysis and binders against the low evident masked pool. J Nephrol. 2015;28:415–429.[↩][↩][↩]
- Sekercioglu N, Angeliki Veroniki A, Thabane L, et al. Effects of different phosphate lowering strategies in patients with CKD on laboratory outcomes: a systematic review and NMA. PLoS One. 2017;12(3):e0171028.[↩][↩]
- Saliba W, El-Haddad B. Secondary hyperparathyroidism: pathophysiology and treatment. J Am Board Fam Med. 2009;22:574–581.[↩]
- Cozzolino M, Tomlinson J, Walsh L, Bellasi A. Emerging drugs for secondary hyperparathyroidism. Expert Opin Emerg Drugs. 2015;20(2):197–208.[↩][↩][↩]
- Cozzolino M, Mazzaferro S, Brandenburg V. The treatment of hyperphosphataemia in CKD: calcium-based or calcium-free phosphate binders? Nephrol Dial Transplant. 2011;26:402–407.[↩][↩][↩]
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2016;5:e003815[↩]
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis. 2016;68(5):691–702.[↩]
- di Filippo S, Carfagna F, la Milia V, et al. Assessment of intradialysis calcium mass balance by single pool variable-volume calcium kinetic model. Hemodial Int. 2017 Feb 5; Epub.[↩]
- Goldsmith DJ, Covic A, Fouque D, et al. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guidelines: a European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25(12):3823–3831.[↩]
- Morrone LF, Bolasco P, Camerini C, et al. Vitamin D in patients with chronic kidney disease: a position statement of the Working Group “Trace Elements and Mineral Metabolism” of the Italian Society of Nephrology. J Nephrol. 2016;29:305–328.[↩][↩]
- KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150.[↩]
- Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6:50–62.[↩]
- Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60(1):139–156.[↩]
- Tripkovic L, Lambert H, Hart K, et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95(6):1357–1364.[↩]
- Wetmore JB, Kimber C, Mahnken JD, Stubbs JR, Cholecalciferol V. Ergocalciferol for 25-hydroxyvitamin D (25(OH)D) repletion in chronic kidney disease: a randomised clinical trial. Br J Nutr. 2016;116(12):2074–2081.[↩][↩]
- Sprague SM, Crawford PW, Melnick JZ, et al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol. 2016;44(4):316–325.[↩][↩]
- Petkovich M, Melnick J, White J, Tabash S, Strugnell S, Bishop CW. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol. 2015;148:283–289[↩]
- Hamdy NA, Kanis JA, Beneton MN, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310(6976):358–363.[↩]
- Brown AJ. Vitamin D analogs for secondary hyperparathyroidism: what does the future hold? J Steroid Biochem Mol Biol. 2007;103(3–5):578–583.[↩]
- Martin KJ, González EA, Gellens M, Hamm LL, Abboud H, Lindberg J. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9(8):1427–1432.[↩]
- Frazao JM, Chesney RW, Coburn JW. Intermittent oral 1alpha-hydroxyvitamin D2 is effective and safe for the suppression of secondary hyperparathyroidism in haemodialysis patients. 1alphaD2 Study Group. Nephrol Dial Transplant. 1998;13(Suppl 3):68–72[↩]
- Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3–4 CKD. Clin J Am Soc Nephrol. 2014;9(9):1620–1626.[↩]
- Messa P, Cozzolino M, Brancaccio D, et al. FARO Study Group Effect of VDRA on survival in incident hemodialysis patients: results of the FARO-2 observational study. BMC Nephrol. 2015;16:11.[↩]
- Tentori F, Hunt WC, Stidley CA, et al. Medical Directors of Dialysis Clinic Inc Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70(10):1858–1865.[↩]
- Mazzaferro S, Goldsmith D, Larsson TE, Massy ZA, Cozzolino M. Vitamin D metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol. 2014;12(2):339–349.[↩]
- de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24(11):1863–1871[↩]
- Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–684.[↩]
- Riccio E, Sabbatini M, Bruzzese D, et al. Effect of paricalcitol vs calcitriol on hemoglobin levels in chronic kidney disease patients: a randomized trial. PLoS One. 2015;10(3):e0118174.[↩]
- Galassi A, Bellasi A, Auricchio S, Papagni S, Cozzolino M. Which vitamin D in CKD-MBD? The time of burning questions. Biomed Res Int. 2013;2013:864012.[↩]
- Narayan R, Perkins RM, Berbano EP, et al. Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis. 2007;49:801–813.[↩]
- Conzo G, Perna AF, Savica V, et al. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC Surg. 2013;13(Suppl 2):S4.[↩]
- Di Iorio B, Molony D, Bell C, et al. INDEPENDENT Study Investigators Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis. 2013;62(4):771–778.[↩]
- Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89(5):1227–39.[↩]
- Triponez F, Kebebew E, Dosseh D, et al. Less-than-subtotal parathyroidectomy increases the risk of persistent/recurrent hyperparathyroidism after parathyroidectomy in tertiary hyperparathyroidism after renal transplantation. Surgery. 2006;140(6):990–7. discussion 997-9.[↩]
- Serra AL, Schwarz AA, Wick FH, et al. Successful treatment of hypercalcemia with cinacalcet in renal transplant recipients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2005;20(7):1315–9.[↩]
- Food and Drug Administration. Full Prescribing Information, Sensipar (cinacalcet). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021688s017lbl.pdf[↩]
- Somnay YR, Weinlander E, Schneider DF, et al. The effect of cinacalcet on intraoperative findings in tertiary hyperparathyroidism patients undergoing parathyroidectomy. Surgery. 2014;156(6):1308–13. discussion 1313-4.[↩]
- Vangala C, Pan J, Cotton RT, Ramanathan V. Mineral and Bone Disorders After Kidney Transplantation. Front Med (Lausanne). 2018;5:211. Published 2018 Jul 31. doi:10.3389/fmed.2018.00211 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6079303/[↩]