Hypokalemia
Hypokalemia is a metabolic imbalance characterized by serum potassium level less than 3.6 milliequivalent/L (mEq/L) or less than 3.6 millimoles/L (mmol/L) 1, 2, 3. Severe potassium deficiency or hypokalemia is most common in hospitalized patients, affecting up to 21% of hospitalized patients, usually because of the use of water pills (e.g., loop diuretics and thiazide diuretics) and other medications that cause your body to excrete too much potassium, but it is rare among healthy people with normal kidney function 4, 5. Because your kidneys work to maintain normal blood levels of potassium by flushing out excess amounts through urine. Potassium can also be lost through stool and sweat. Hypokalemia is also seen in people with inflammatory bowel diseases (Crohn’s disease, ulcerative colitis) that may cause diarrhea and malabsorption of nutrients.
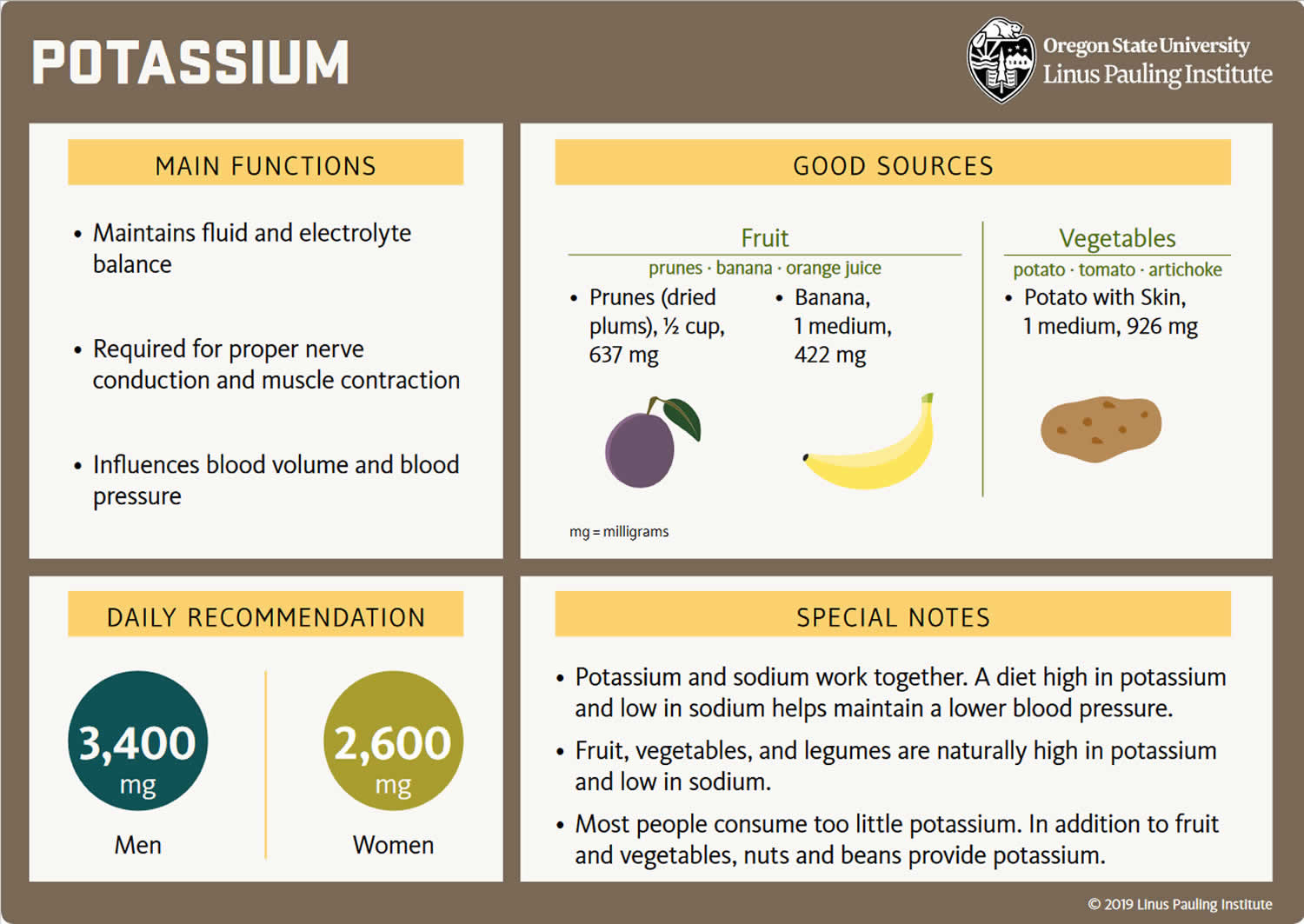
The adequate intake (intake at this level is assumed to ensure nutritional adequacy) for potassium for women is 2,600 mg/day and 3,400 mg/day for men from food is needed because of normal daily losses 6, 7. The World Health Organization (WHO) recommends a potassium intake of at least 3,510 mg per day for adults for optimal cardiovascular health 8, 9, 10. High dietary potassium intake has been found to lower blood pressure in patients with high blood pressure (hypertension), although it may precipitate hyperkalemia (a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L) if your kidney function is impaired 9, 11, 10, 12. An increased dietary potassium intake is associated with a lower risk of stroke 13. Potassium-rich foods are common to a healthy diet (see Table 3) 8, 14, 15. A low-potassium diet is generally recommended for patients with advanced chronic kidney disease 8, 14, 15.
Your body needs potassium for the contraction of muscles (including the heart), and for the functioning of many complicated proteins (enzymes). Potassium is found primarily in the skeletal muscle and bone, and participates with sodium to contribute to the normal flow of body fluids between the cells in the body. Potassium is predominantly intracellular (within a cell) where it is the most abundant cation (positive charged ion) and involved in cell regulation and several cellular processes. The fraction of potassium in the extracellular fluid is small. Therefore, plasma or serum potassium levels are not a reliable indicator of total body potassium stores. The normal concentration of potassium in the body is maintained through a combination of adjustments in acute cellular shifts between the extracellular and intracellular fluid compartments, regulated by your kidneys through the excretion of urine and, to a lesser extent, gastrointestinal losses. When your kidneys are functioning normally, the amount of potassium in your diet is sufficient for use by your body and the excess is usually excreted through urine and sweat. Body chemicals and hormones such as aldosterone also regulate potassium balance. Secretion of the hormone insulin, which is normally stimulated by food, prevents a temporary diet-induced hypokalemia by increasing cell absorption of potassium. When hypokalemia occurs, there is an imbalance resulting from a dysfunction in this normal process, or the rapid loss of urine or sweat without replacement of sufficient potassium.
Potassium deficiency or hypokalemia is rarely caused by low dietary potassium intake alone because potassium is found in so many foods (see Table 3) 6, 7, 16. However, insufficient dietary potassium in patients at risk of hypokalemia can precipitate hypokalemia 17. In rare cases, habitual consumption of large amounts of black licorice has resulted in hypokalemia 18, 19. Licorice contains a compound called glycyrrhizic acid that has similar physiologic effects to those of aldosterone, a hormone that increases urinary excretion of potassium.
Too little potassium in your blood or hypokalemia may be a sign of 20:
- Use of prescription diuretics (water pills)
- Fluid loss from diarrhea, vomiting, or heavy sweating
- Using too many laxatives
- Adrenal gland disorders, including Cushing’s syndrome and aldosteronism
- Kidney disease
- Alcohol use disorder
- Eating a lot of real licorice, which comes from licorice plants. Most licorice products sold in the U.S. don’t contain any real licorice. Check the package ingredient label to be sure.
- A diet too low in potassium (not common). Bananas, apricots, green leafy vegetables, avocados and many other foods are good sources of potassium that are part of a healthy diet.
Potassium deficiency or hypokalemia is most commonly a result of excessive loss of potassium from prolonged vomiting or diarrhea, use of some diuretics, laxative abuse, eating clay (a type of pica), heavy sweating, dialysis, using certain medications, some forms of kidney disease, inflammatory bowel disease (Crohn’s disease or ulcerative colitis)or metabolic disturbances. Hypokalemia can also be caused by refeeding syndrome (the metabolic response to initial refeeding after a starvation period) because of potassium’s movement into cells 1, 21, 22, 23, 24.
Magnesium deficiency also known as hypomagnesemia can also contribute to hypokalemia by increasing urinary potassium losses 25, 26, 27. Magnesium deficiency can also increase the risk of cardiac arrhythmias by decreasing intracellular potassium concentrations. More than 50% of individuals with clinically significant hypokalemia might have magnesium deficiency 27. In people with hypomagnesemia and hypokalemia, both should be treated concurrently 22.
In general, hypokalemia is associated with diagnoses of cardiac disease, kidney failure, malnutrition, and shock 28. Hypothermia (occurs when your body loses heat faster than it can produce heat and your body temperature falls below 95°F (35°C)) and increased blood cell production (for example, leukemia) are additional risk factors for developing hypokalemia 28. There are subsets of patients that are susceptible to the development of hypokalemia. For instance, psychiatric patients are at risk for hypokalemia due to their drug therapy 28. Hypokalemia is also prevalent in hospitalized patients, in particular, children, those who have a fever and those who are critically ill 28. Additionally, in developing countries, an increased risk of death is observed in children when severe hypokalemia is associated with diarrhea and severe malnutrition 28.
The symptoms of hypokalemia are related to alterations in membrane potential and cellular metabolism 17. Mild hypokalemia (serum potassium level is 3 to 3.4 mmol/L) is characterized by fatigue, muscle weakness and cramps, not feeling well, tiredness, and intestinal paralysis, which may lead to bloating, constipation, and abdominal pain 1, 7. Moderate to severe hypokalemia (serum potassium level less than about 2.5 mmol/L) can cause increased urination (polyuria or large volume of dilute urine); decreased brain function (encephalopathy) in patients with kidney disease; high blood sugar levels or glucose intolerance; muscle paralysis; difficulty breathing; and cardiac arrhythmias (irregular heartbeats), especially in individuals with underlying heart disease 25, 1, 2. Severe hypokalemia (serum potassium level less than about 2.5 mmol/L) may result in muscular paralysis or abnormal heart rhythms (cardiac arrhythmias) that can be life threatening 17, 25.
Getting too little potassium can increase blood pressure (hypertension), deplete calcium in bones, and increase the risk of kidney stones formation 7, 6. In the absence of treatment, hypokalemia can have serious complications and even be fatal.
Treatment of low potassium or hypokalemia is directed at the underlying cause and may include potassium supplements. Don’t start taking potassium supplements without talking to your doctor first. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your low potassium level.
What is Potassium?
Potassium (K+) is a mineral that is vital to cell metabolism. Potassium is a major intracellular cation (positively charged ion) and a type of electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance 29, 30, 31. Potassium is present in all body tissues and is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients 32, 33. Potassium helps transport nutrients into cells and removes waste products out of cells. Potassium is essential for the proper functioning of the heart, kidneys, muscles, nerves, and digestive system. Potassium is also important in muscle function, helping to transmit messages between nerves and muscles 32.
Potassium (K+) is a positively charged ion (cation), which is present throughout your body in both intracellular and extracellular fluids. The majority of body potassium, > 90%, are intracellular. It moves freely from intracellular fluid (ICF) to extracellular fluid (ECF) and vice versa when adenosine triphosphate (ATP) increases the permeability of the cell membrane. Potassium (K+) is mainly replaced inside or outside the cells by another cation, sodium (Na+). The movement of potassium into or out of the cells is linked to certain body hormones and also to certain physiological states. Standard laboratory tests measure extracellular fluid (ECF) potassium. Potassium enters the body rapidly during food ingestion. Insulin is produced when a meal is eaten; this causes the temporary movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). Over the ensuing hours, the kidneys excrete the ingested potassium and homeostasis is returned. In the critically ill patient, suffering from high potassium level or hyperkalemia, this mechanism can be manipulated beneficially by administering high concentration (50%) intravenous glucose. Insulin can be added to the glucose, but glucose alone will stimulate insulin production and cause movement of potassium from extracellular fluid (ECF) to intracellular fluid (ICF). The stimulation of alpha receptors causes increased movement of potassium from intracellular fluid (ICF) to extracellular fluid (ECF). A noradrenaline infusion can elevate serum potassium levels. An adrenaline infusion, or elevated adrenaline levels, can lower serum potassium levels. Metabolic acidosis causes a rise in extracellular potassium levels (hyperkalemia). In this situation, excess of hydrogen ions (H+) are exchanged for intracellular potassium ions, probably as a result of the cellular response to a falling blood pH. Metabolic alkalosis causes the opposite effect, with potassium moving into the cells 34.
Potassium (K+), along with other electrolytes such as sodium (Na+), chloride (Cl–), and bicarbonate (HCO3–), helps regulate the amount of fluid in your body and maintains a stable acid-base balance. Potassium is present in all body fluids, but most potassium is found within the cells (intracellularly). Only a small amount is present in fluids outside the cells and in the liquid part of the blood (called serum or plasma).
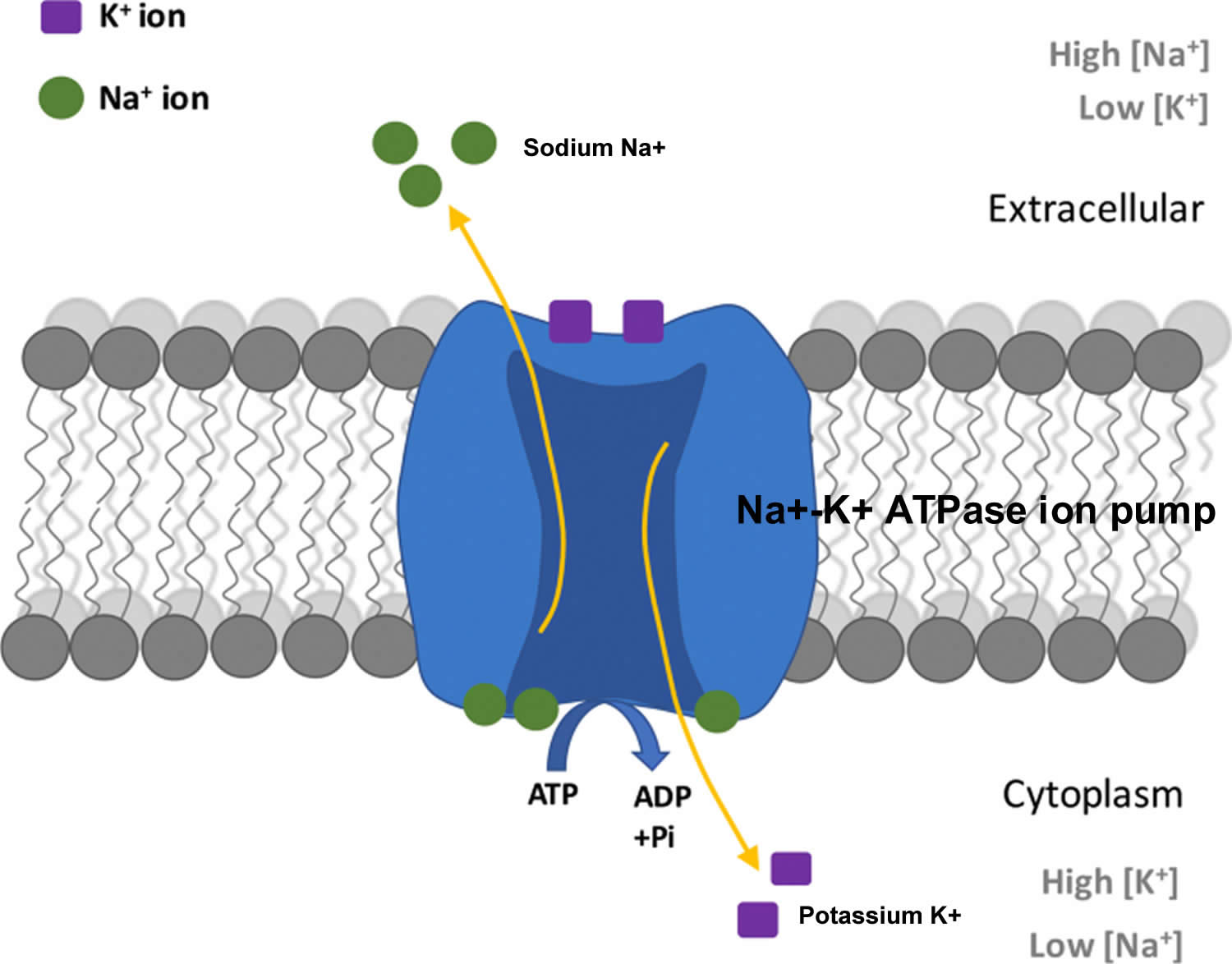
The total amount of potassium (K+) in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 millimole [mmol] = 1 milliequivalent [mEq] = 39.1 mg of potassium) 1. Most potassium are found within the cells (intracellularly) and a small amount is in extracellular fluid. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps) 35. When activated, the sodium-potassium ATPase pump (Na+-K+ ATPase ion pumps) exchanges 2 extracellular potassium (K+) ions for 3 intracellular sodium (Na+) ions, influencing membrane potential based on physiological excitation or inhibition. These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) are partially responsible, along with the sodium-potassium-chloride (Na+-K+-2Cl) co-transporter and sodium-calcium (Ca) exchanger, for maintaining the potential difference across the resting cell membrane as well. In addition to maintaining cellular tonicity, this gradient is required for proper nerve transmission, muscle contraction, and kidney function 32, 36, 37.
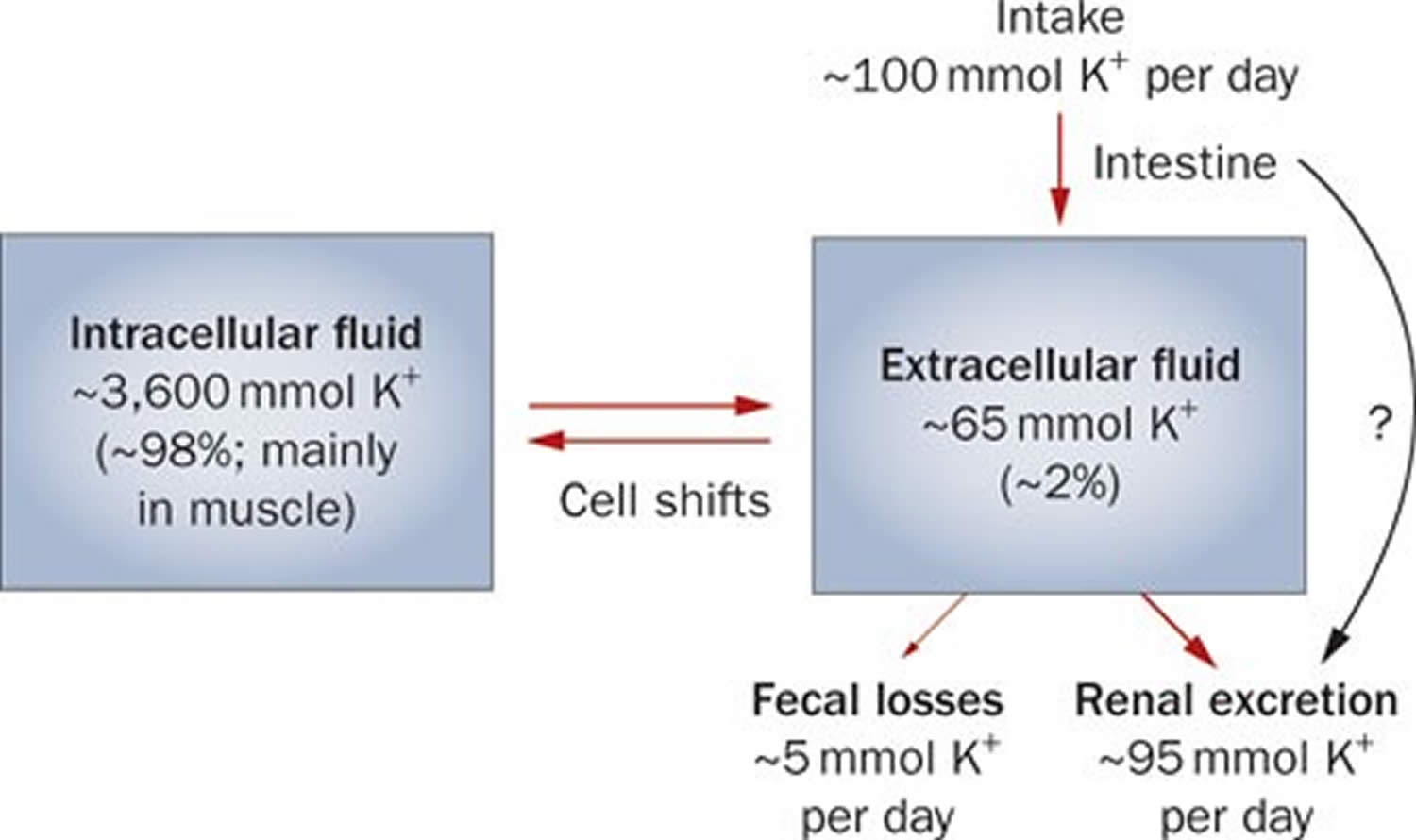
Potassium (K+) homeostasis depends on external balance (dietary intake [typically 100 mmol per day] versus excretion [95% via the kidney; 5% via the colon]) and internal balance (the distribution of potassium (K+) between intracellular and extracellular fluid compartments). The uneven distribution of potassium (K+) across cell membranes means that a mere 1% shift in its distribution can cause a 50% change in plasma potassium (K+) concentration 31. Hormonal mechanisms involving insulin, beta-adrenergic agonists and aldosterone modulate potassium (K+) distribution by promoting rapid transfer of potassium (K+) across the plasma membrane 38. Your body uses what potassium (K+) it requires and your kidneys eliminate the rest in the urine. Your body tries to keep the blood potassium level within a very narrow range. Levels are mainly controlled by aldosterone, a hormone produced by the adrenal glands in the kidneys. Extrarenal potassium (K+) losses from the body are usually small, but can be marked in individuals with chronic diarrhea, severe burns or prolonged sweating 31, 38. Under normal circumstances, the kidney’s distal nephron secretes potassium (K+) and determines final urinary excretion. In patients with low potassium levels or hypokalemia (plasma K+ concentration <3.5 mmol/l), after the exclusion of extrarenal causes, alterations in sodium ion (Na+) delivery to the distal nephron, aldosterone status, or a specific inherited or acquired defect in distal nephron function (each of which affects distal nephron K+ secretion), should be considered 31.
Figure 1. Potassium physiology
Because most potassium (K+) ions are found within the cells (a major intracellular cation), it is widely distributed in foods once derived from living tissues. Potassium concentration is higher in fruits and vegetables than in cereals and meat. You get most of the potassium you need from the foods that you eat and most people have an adequate intake of potassium. Recommended adequate intakes for potassium were set by the Food and Nutrition Board of the Institute of Medicine at 4700 mg/day 33. However it should be noted that the Food and Nutrition Board of the Institute of Medicine Recommended adequate intakes (AIs) for potassium at 4700 mg/day for adults is substantially higher than the World Health Organization’s (WHO) guidelines, which recommend 3150 mg/day for adults 39. The National Health and Nutrition Examination Survey (NHANES) data indicates that 99.2% of potassium in the US diet is naturally occurring, with the remaining 0.8% coming from fortified foods 40. These naturally occurring potassium sources include milk and other non-alcoholic beverages, as well as potatoes and fruit, which rank highest as sources of potassium intake among American adults 41. In addition, Western dietary practices with higher consumption of cereal, low nutrient density processed foods and lower consumption of fruits and vegetables has led to a diet lower in potassium and higher in sodium in recent decades 33. Salting foods and discarding the liquid induces sodium (Na+) for potassium (K+) exchange and reduces the potassium content of foods. Few Americans meet the recommended intakes; the average intake is 2591 ± 9 mg/day 40. This large gap between potassium intakes and recommended intakes led to potassium being called a shortfall nutrient in the Dietary Guidelines for Americans 42.
Actual potassium requirements would vary with an individual’s genetics, blood pressure (BP) status, and sodium intake 32. Blood pressure is currently the primary criterion for determining potassium requirements, with African Americans being more vulnerable to high blood pressure (hypertension) and more responsive to potassium supplementation than whites; individuals with high blood pressure (hypertension) are more responsive to increasing potassium intakes than individuals with normal blood pressure, and potassium having a greater benefit for those consuming a high salt diet 43. Other benefits of increasing potassium consumption may include improved blood sugar (glucose) control, glucose intolerance and insulin resistance becoming a concern for individuals with high blood pressure (hypertension) prescribed potassium wasting diuretics (water pills) 44. These differences support personalized nutrition approaches. Understanding movement of potassium within the body may help to improve these health outcomes.
Potassium is absorbed via passive diffusion, primarily in the small intestine 35. About 90% of ingested potassium is absorbed and used to maintain its normal intracellular and extracellular concentrations 21. There is around 50 mEq/kg of potassium (K+) in the body such that total body potassium (K+) in a 70-kg person is 3,500 mEq. Around 98% of potassium (K+) is found mainly within cells, and about 2% of the bodies’ potassium (K+) is in the extracellular fluid. The normal concentration of potassium (K+) in the extracellular fluid is 3.5–5.3 mEq/L. Large deviations from these values are not compatible with life.
Approximately 90% of the daily potassium (K+) intake is excreted in the urine, whereas a smaller percentage (10%) is excreted by the gastrointestinal tract in the stool and a very small amount is lost in sweat 45, 38, 46. Therefore, within the body, the kidney is the major organ responsible for potassium (K+) homeostasis. The kidneys control potassium excretion in response to changes in dietary intakes, and potassium excretion increases rapidly in healthy people after potassium consumption, unless body stores are depleted 32. The kidney facilitates potassium (K+) homeostasis by adjusting renal potassium (K+) excretion over several hours in response to a potassium load. Initial changes in extracellular potassium (K+) concentration are buffered by movement of potassium (K+) into or out of skeletal muscle cells. Internal potassium (K+) balance is a term used to refer to regulation of potassium (K+) distribution between the intracellular and extracellular space. Insulin, catecholamines, and, to a lesser extent, aldosterone are critical factors responsible for maintaining the normal internal distribution of potassium (K+) 38, 46.
The kidneys can adapt to variable potassium intakes in healthy individuals even in the setting of high dietary intake, but a minimum of 5 mmol (about 195 mg) potassium is excreted daily in urine 1. To demonstrate this, studies have shown potassium (K+) levels are kept within the normal range even when there are increases to ~15 g daily of dietary potassium (K+) intake sustained for 20 days 47, 48. Recent findings have identified the presence of an enteric potassium (K+) sensing mechanism that initiates the renal secretory process upon K+ entry into the gastrointestinal tract 46. The distal convoluted tubule has been identified as a site critical for potassium (K+) homeostasis, where it acts as a potassium (K+) sensor capable of initiating potassium (K+) excretion independent of mineralocorticoid activity 46. Combined with other obligatory losses, potassium balance cannot be achieved with intakes less than about 400–800 mg/day 38, 46.
Assessing potassium status is not routinely done in clinical practice, and it is difficult to do because most potassium in the body is inside cells 6. Although blood potassium levels can provide some indication of potassium status, they often correlate poorly with tissue potassium stores 1, 49, 50. Other methods to measure potassium status include collecting balance data (measuring net potassium retention and loss); measuring the total amount of potassium or the total amount of exchangeable potassium in the body; and conducting tissue analyses (e.g., muscle biopsies), but all have limitations 49.
Normal serum concentrations of potassium range from about 3.6 to 5.0 mmol/L and are regulated by a variety of mechanisms 1, 22. Diarrhea, vomiting, kidney disease, use of certain medications, and other conditions that alter potassium excretion or cause transcellular potassium shifts can cause low potassium level also called hypokalemia (serum potassium levels below 3.6 mmol/L) or high potassium level also called hyperkalemia (serum potassium levels above 5.0 mmol/L) 22. Otherwise, in healthy individuals with normal kidney function, abnormally low or high blood levels of potassium are rare.
Because the blood concentration of potassium is so small, minor changes can have significant consequences. If potassium levels are too low (serum potassium levels below 3.6 mmol/L) or too high (serum potassium levels above 5.0 mmol/L), there can be serious health consequences; a person may be at risk for developing shock, respiratory failure, or heart rhythm disturbances. An abnormal potassium level can alter the function of the nerves and muscles; for example, the heart muscle may lose its ability to contract.
Your body needs potassium to:
- Build proteins
- Break down and use carbohydrates
- Build muscle
- Maintain normal body growth
- Control the electrical activity of the heart
- Control the acid-base balance
Reduced potassium consumption has been associated with hypertension and cardiovascular diseases, and appropriate consumption levels could be protective against these conditions 51. A recent meta-analysis including 11 cohort studies reported an inverse association between potassium intake and risk of stroke 52. Additionally, two meta-analyses of trials comparing increased potassium to lower potassium intake found that increased potassium intake lowers blood pressure 53, 54. These results were further supported by a systematic review without a meta-analysis, which concluded that increased potassium intake results in decreased blood pressure in adults 55. Thus, a public health intervention aimed at increasing potassium intake from food could be a cost-effective strategy to reduce the burden of cardiovascular morbidity and mortality. Moreover, increasing potassium consumption from food in the population is safe; in individuals without renal impairment caused by medical conditions or drug therapy, the body is able to efficiently adapt and excrete excess potassium via the urine when consumption 56.
The American Heart Association recommended potassium intake for an average adult is 4,700 milligrams (mg) per day. Most of us aren’t getting nearly that much. On average, adult males eat almost 3,200 mg/day, and adult females eat about 2,400 mg/day 57. Remember that potassium is only part of an overall heart-healthy eating pattern. Other dietary factors that may affect blood pressure include amount and type of dietary fat; cholesterol; protein, sugar and fiber; calcium and magnesium, and of course, sodium.
For example, the DASH (Dietary Approaches to Stop Hypertension) diet study found that a diet rich in fruits, vegetables, fat-free or low-fat (1 percent) milk and milk products, whole-grain foods, fish, poultry, beans, seeds and unsalted nuts reduced blood pressure compared to a typical American diet. The DASH eating plan also had less sodium; sweets, added sugars and sugar-containing beverages; saturated and trans fats; and red meats than the typical American diet.
People with kidney problems, especially those on dialysis, should not eat too many potassium-rich foods. Your health care provider will recommend a special diet.
What does potassium do?
Potassium (K+) is the principal positively charged ion (cation) in the fluid inside of cells, while sodium (Na+) is the principal cation in the extracellular fluid. Potassium (K+) concentrations are about 30 times higher inside than outside cells, while sodium (Na+) concentrations are more than 10 times lower inside than outside cells 7. The concentration differences of these charged particles causes a difference in electric potential between the inside and outside of cells, known as the membrane potential. A cell’s membrane potential is maintained by ion pumps in the cell membrane, especially the Sodium-Potassium ATPase pumps (Na+-K+ ATPase ion pumps). These sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) use ATP (energy) to pump sodium (Na+) of the cell and potassium (K+) into the cell, leading to a potassium (K+) gradient across the cell membrane [potassium (K+) in > potassium (K+) out], which is partially responsible for maintaining the cell membrane potential (Figure 2). The sodium-potassium ATPase pumps (Na+-K+ ATPase ion pumps) activity has been estimated to account for 20%-40% of the resting energy consumption in a typical adult 7. The large proportion of energy dedicated to maintaining sodium/potassium concentration gradients emphasizes the importance of this function in sustaining life 7. The cell membrane potential created by potassium and sodium ions allows the cell generate an action potential–a “spike” of electrical discharge. The ability of cells to produce electrical discharge is critical for body functions such as nerve impulse transmission, muscle contraction, and heart function 58, 59, 60.
Potassium is also an essential mineral needed to regulate water balance, blood pressure and levels of acidity 61. The more potassium you eat, the more sodium you pass out of the body through urine. Increased potassium intake has no adverse effect on blood lipid concentration, catecholamine concentrations, or renal function in apparently healthy adults without impaired renal handling of potassium 57. The largest benefit was detected when sodium intake was more than 4 g/day, which is the intake of most populations globally 62, so increased potassium intake should benefit most people in most countries. However, the authors also found a statistically significant decrease in blood pressure with increased potassium when sodium intake was 2-4 g/day. Therefore, increased potassium can continue to be beneficial in terms of blood pressure even as individuals and populations decrease their sodium intake. Studies examining both nutrients simultaneously support this concept, showing an increased benefit with simultaneous reduction in sodium and increase in potassium compared with changes in one nutrient individually 63, 64.
Potassium also helps relax blood vessel walls, which helps lower blood pressure 57.
World Health Organization recommends an increase in potassium intake from food to reduce blood pressure and risk of cardiovascular disease, stroke and coronary heart disease in adults. World Health Organization suggests a potassium intake of at least 90 mmol/day (3510 mg/day) for adults (conditional recommendation) 56.
Potassium also acts as a cofactor for some enzymes activity. For example, the activation of Na+/K+-ATPase requires the presence of sodium and potassium. The presence of potassium is also required for the activity of pyruvate kinase, an important enzyme in carbohydrate metabolism 65.
Figure 2. Sodium-Potassium ATPase pump
What are normal potassium levels?
Normal serum potassium values are between 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L) 29, 66, 67, 12. However, there can be slight variation between laboratories and for this reason, it is important to look for the specific reference interval listed on your test report. Potassium levels outside this range, 3.5 to 5.0 millimoles/L (mmol/L) or 3.5 to 5.0 milliequivalent/L (mEq/L), are not compatible with life with increased rates of death from several causes 68, 69.
Your health care provider may order a potassium blood test as part of your regular checkup or to monitor an existing condition, such as diabetes, kidney disease, or adrenal gland disorders. You may also need this test if you take medicines that could affect your potassium levels or if you have symptoms of having too much or too little potassium.
Interpretation of a potassium test requires carefully considering the result, the laboratory reference range, and your health situation. Because potassium is frequently measured with other electrolytes, levels may be evaluated together. For a blood test, the report should list the amount of potassium measured in either milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L). The test report will also show a reference range, which the laboratory considers an expected range for potassium levels.
How much potassium do I need?
The amount of potassium you need each day depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg). Table 1 lists the current Adequate Intakes (AIs) for potassium for healthy individuals. Intake recommendations for potassium and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine 33. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and sex, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
When the Food and Nutrition Board evaluated the available data in 2005, it found the data insufficient to derive an Estimated Average Requirement (average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) for potassium, so the board established Adequate Intake (intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance) for all ages based on potassium intakes in healthy populations 70. In 2019, a National Academies of Sciences, Engineering, and Medicine committee updated the Dietary Reference Intake (DRI) for potassium and sodium 33. The committee found the data insufficient to derive an Estimated Average Requirement (EAR) for potassium. Therefore, they established Adequate Intakes (AIs) for all ages based on the highest median potassium intakes in healthy children and adults, and on estimates of potassium intakes from breast milk and complementary foods in infats. The National Academies of Sciences, Engineering, and Medicine committee also used an expanded Dietary Reference Intake (DRI) model to include a recommended intake level for a nutrient to reduce the risk of chronic disease, what they termed the chronic disease risk reduction intake 70. In 2019, a National Academies of Sciences, Engineering, and Medicine committee updated the Dietary Reference Intake (DRI) for potassium and sodium 33. According to the model, a chronic disease risk reduction intake might be set for a nutrient like potassium when there is a causal relationship between a certain level of intake and a reduced risk of chronic disease based on evidence of at least moderate strength. However, the committee found the evidence to be insufficient to derive a chronic disease risk reduction intake for potassium 33.
Table 1. Average daily recommended intake for Potassium
| Birth to 6 months | 400 mg |
|---|---|
| Infants 7–12 months | 860 mg |
| Children 1–3 years | 2,000 mg |
| Children 4–8 years | 2,300 mg |
| Children 9–13 years (boys) | 2,500 mg |
| Children 9–13 years (girls) | 2,300 mg |
| Teens 14–18 years (boys) | 3,000 mg |
| Teens 14–18 years (girls) | 2,300 mg |
| Adults 19+ years (men) | 3,400 mg |
| Adults 19+ years (women) | 2,600 mg |
| Pregnant teens | 2,600 mg |
| Pregnant women | 2,900 mg |
| Breastfeeding teens | 2,500 mg |
| Breastfeeding women | 2,800 mg |
Footnote: *Adequate Intakes (AIs) do not apply to individuals with impaired potassium excretion because of medical conditions (e.g., kidney disease) or the use of medications that impair potassium excretion.
[Source 6 ]Hypokalemia causes
Low potassium (hypokalemia) has many causes. Hypokalemia is always a symptom of another disorder, rather than a disease that occurs by itself. The most common cause is excessive loss of potassium through the urine (kaliuresis) due to prescription water or fluid pills (diuretics). Water pills or diuretics medications are often prescribed for people who have high blood pressure or heart disease.
The excessive excretion of potassium in the urine (kaliuresis) may also result from a deficiency of magnesium in the blood (hypomagnesemia), excessive mineralocorticoids such as aldosterone in the blood (hyperaldosteronism) which affect the electrolyte and fluid balance in the body (usually caused by endocrine diseases), kidney disorders, or from the use of high doses of penicillin.
Typically, the potassium level becomes low because too much potassium is lost from the digestive tract (gastrointestinal losses) due to prolonged diarrhea or vomiting, chronic laxative abuse, inadequate dietary intake of potassium, intestinal obstruction or infections such as fistulas in the intestines which continually drain intestinal fluids. Additionally, excessive perspiration due to hot weather or exercise can cause hypokalemia. Hypokalemia is rarely caused by consuming too little potassium because many foods (such as beans, dark leafy greens, potatoes, fish, and bananas) contain potassium.
In many adrenal disorders, such as Cushing syndrome, the adrenal glands produce too much aldosterone, a hormone that causes the kidneys to excrete large amounts of potassium.
Certain drugs (such as insulin, albuterol, and terbutaline) cause more potassium to move from blood into cells and can result in hypokalemia. However, these drugs usually cause temporary hypokalemia, unless another condition is also causing potassium to be lost.
Hypokalemia sometimes occurs with or is caused by a low level of magnesium in the blood (hypomagnesemia).
Causes of potassium loss leading to low potassium include:
- Excessive alcohol use
- Chronic kidney disease
- Diabetic ketoacidosis
- Diarrhea (causing anal irritation)
- Diuretics (water retention relievers)
- Excessive laxative use
- Excessive sweating
- Folic acid deficiency
- Prescription water or fluid pills (diuretics) use
- Primary aldosteronism
- Some antibiotic use
- Vomiting
Gastrointestinal tract losses
Gastrointestinal potassium losses are another common cause of hypokalemia, particularly among hospitalized patients 71. Abnormal gastrointestinal potassium losses occur in all of the following 72:
- Chronic diarrhea, including chronic laxative abuse and bowel diversion
- Clay (bentonite) ingestion, which binds potassium and greatly decreases absorption
- Rarely, villous adenoma of the colon, which causes massive potassium secretion
As a portion of daily potassium is excreted in the colon, lower gastrointestinal losses in the form of persistent diarrhea can also result in hypokalemia and may be accompanied by hyperchloremic acidosis 3.
Protracted vomiting or gastric suction (which removes volume and hydrochloric acid) causes renal potassium losses due to metabolic alkalosis and stimulation of aldosterone due to volume depletion; aldosterone and metabolic alkalosis both cause the kidneys to excrete potassium 72.
Kidney potassium losses
Various disorders can increase kidney potassium excretion.
Diuretic use is a common cause of renally mediated hypokalemia 73. When given in the same dosage, chlorthalidone is more likely to induce hypokalemia than hydrochlorothiazide, which is more often implicated because of its widespread use 74, 75. Diuretic-induced hypokalemia is dose-dependent and tends to be mild (3 to 3.5 mEq per L [3 to 3.5 mmol per L]), although it can be more severe when accompanied by other causes (e.g., gastrointestinal losses) 76.
Excess mineralocorticoid (ie, aldosterone) effect can directly increase potassium secretion by the distal nephrons and occurs in any of the following 72:
- Adrenal steroid excess that is due to Cushing syndrome, primary hyperaldosteronism, rare renin-secreting tumors, glucocorticoid-remediable aldosteronism (a rare inherited disorder involving abnormal aldosterone metabolism), and congenital adrenal hyperplasia.
- Bartter syndrome, an uncommon genetic disorder that is characterized by renal potassium and sodium wasting, excessive production of renin and aldosterone, and normotension. Bartter syndrome is caused by mutations in a loop diuretic–sensitive ion transport mechanism in the loop of Henle.
- Gitelman syndrome is an uncommon genetic disorder characterized by renal potassium and sodium wasting, excessive production of renin and aldosterone, and normotension. Gitelman syndrome is caused by loss of function mutations in a thiazide-sensitive ion transport mechanism in the distal nephron.
- Ingestion of substances such as glycyrrhizin (present in natural licorice and used in the manufacture of chewing tobacco), which inhibits the enzyme 11 beta-hydroxysteroid dehydrogenase (11β-HSDH), preventing the conversion of cortisol, which has some mineralocorticoid activity, to cortisone, which does not, resulting in high circulating concentrations of cortisol and renal potassium wasting.
- Liddle syndrome, a rare autosomal dominant disorder caused by unrestrained sodium reabsorption in the distal nephron due to one of several mutations found in genes encoding for epithelial sodium channel subunits. Inappropriately high reabsorption of sodium results in both severe hypertension and renal potassium wasting, resulting in hypokalemia.
Renal potassium wasting can also be caused by numerous congenital and acquired renal tubular diseases, such as the renal tubular acidoses and Fanconi syndrome, an unusual syndrome resulting in renal wasting of potassium, glucose, phosphate, uric acid, and amino acids 72.
Hypomagnesemia is a common correlate of hypokalemia. Much of this correlation is attributable to common causes (ie, diuretics, diarrhea), but hypomagnesemia itself may also result in increased renal potassium losses 72.
Intracellular shift
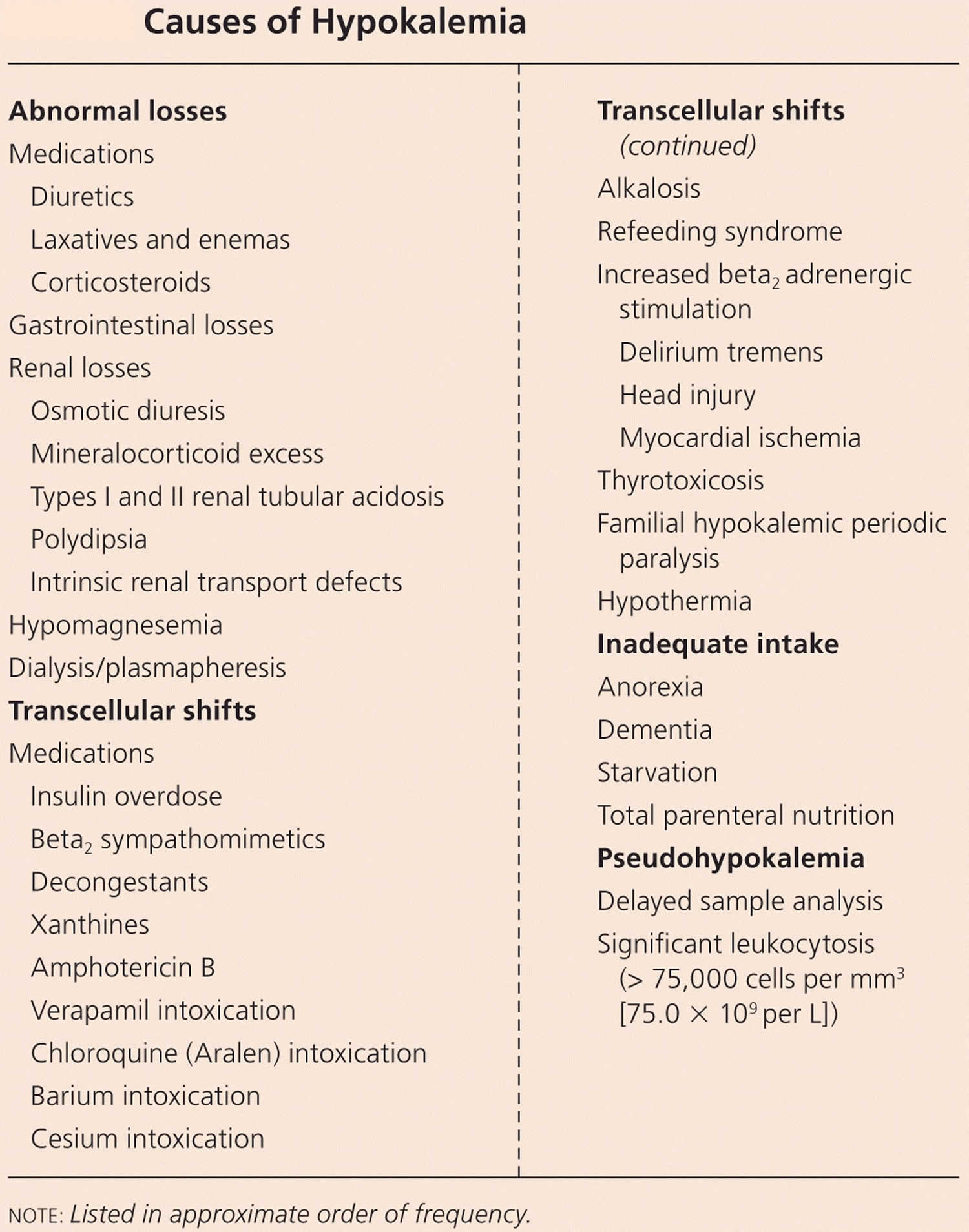
The transcellular shift of potassium into cells may also cause hypokalemia. This shift can occur in any of the following 72:
- After administration of insulin
- Familial periodic paralysis
- Glycogenesis during total parenteral nutrition or enteral hyperalimentation (stimulating insulin release)
- Stimulation of the sympathetic nervous system, particularly with beta 2-agonists (eg, albuterol, terbutaline), which may increase cellular potassium uptake
- Thyrotoxicosis (occasionally) due to excessive beta-sympathetic stimulation (hypokalemic thyrotoxic periodic paralysis)
Familial periodic paralysis is a rare autosomal dominant disorder characterized by transient episodes of profound hypokalemia thought to be due to sudden abnormal shifts of potassium into cells. Episodes frequently involve varying degrees of paralysis. They are typically precipitated by a large carbohydrate meal or strenuous exercise.
Drug interactions
Several classes of medications are known to induce low serum potassium or hypokalemia see Table 2 below.
Diuretics are by far the most commonly used drugs that cause hypokalemia. Potassium-wasting diuretics that block sodium reabsorption proximal to the distal nephron include 72, 77:
- Loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®)
- Osmotic diuretics
- Thiazide diuretics, such as chlorothiazide (Diuril®) and metolazone (Zaroxolyn®)
Experts recommend monitoring potassium status in people taking these medications, and initiating potassium supplementation if warranted 77.
By inducing diarrhea, laxatives, especially when abused, can cause hypokalemia. Secret diuretic or laxative use or both is a frequent cause of persistent hypokalemia, particularly among patients preoccupied with weight loss and among health care practitioners with access to prescription drugs 72.
Other drugs that can cause hypokalemia include 72:
- Aminoglycosides
- Anti-fungal agents (amphotericin-B, fluconazole)
- Antipseudomonal penicillins (eg, carbenicillin)
- Cisplatin. Cisplatin can damage the renal tubular epithelium and lead to severe potassium loss.
- Corticoids. Corticoids cause sodium retention that leads to a compensatory increase in urinary potassium excretion 7.
- Penicillin in high doses. Penicillins formulated as sodium salts also stimulate potassium excretion 7.
- Theophylline (both acute and chronic intoxication)
Outdated tetracycline antibiotics have been linked to electrolyte disturbances 7.
Table 2. Medications associated with Hypokalemia (low serum potassium)
| Medication Family | Specific Medications |
|---|---|
| Aminoglycosides | amikacin (Amikin), gentamicin (Garamycin), kanamycin (Kantrex), tobramycin (Nebcyn), streptomycin |
| Antibiotics | Penicillins: penicillin G sodium (Pfizerpen), mezlocillin (Mezlin), carbenicillin (Geocillin), ticarcillin (Ticar) Tetracyclines (when outdated) |
| Anti-cancer agent | cisplatin (Platinol-AQ) |
| Anti-fungal agents | amphotericin B (Abelcet, Amphotec, AmBisome, Amphocin, Fungizone), fluconazole (Diflucan) |
| Beta-adrenergic agonists | albuterol (Salbutamol, Ventolin), bitolterol (Tornalate), metaproterenol (Alupent) |
| Diuretics | Loop diuretics: bumetanide (Bumex), ethacrynic acid (Edecrin), furosemide (Lasix), torsemide (Demadex) Thiazide diuretics: Acetazolamide, thiazides, chlorthalidone (Hygroton), indapamide (Lozol), metolazone (Zaroxolyn), chlorothiazide (Diuril) |
| Mineralocorticoids | fludrocortisone (Florinef), hydrocortisone (Cortef), cortisone (Cortone), prednisone (Deltasone) Substances with mineralocorticoid effects: licorice, carbenoxolone, gossypol |
| Other | methylxanthines (e.g., theophylline), sodium polystyrene sulfonate, sodium phosphates, caffeine |
Groups at Risk of Hypokalemia
Potassium deficiency can occur with intakes that are below the Adequate Intake (AI) [intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA)] but above the amount required to prevent hypokalemia. The following groups are more likely than others to have poor potassium status.
People with inflammatory bowel diseases
Potassium is secreted within the colon, and this process is normally balanced by absorption 78. However, in inflammatory bowel disease (including Crohn’s disease and ulcerative colitis), potassium secretion increases, which can lead to poor potassium status. Inflammatory bowel diseases are also characterized by chronic diarrhea, which can further increase potassium excretion 79.
People who use certain medications, including diuretics and laxatives
Certain diuretics (e.g., thiazide diuretics, loop diuretics) that are commonly used to treat high blood pressure increase urinary potassium excretion and can cause hypokalemia 22, 3. Potassium- sparing diuretics, however, do not increase potassium excretion and can actually cause hyperkalemia. Large doses of laxatives and repeated use of enemas can also cause hypokalemia because they increase losses of potassium in stool 6.
People with pica
Pica is the persistent eating of non-nutritive substances, such as clay. When consumed, clay binds potassium in the gastrointestinal tract, which can increase potassium excretion and lead to hypokalemia 21, 23, 24. Cessation of pica combined with potassium supplementation can restore potassium status and resolve symptoms of potassium deficiency.
Hypokalemia pathophysiology
Hypokalemia can occur via the following pathogenetic mechanisms 80:
- Decreased potassium intake
- Increased potassium loss
- Shift from the extracellular to the intracellular space (transcellular shifts)
Hypokalemia can occur as a result of decreased potassium intake, transcellular shifts (a shift from the extracellular to the intracellular space) or increased potassium loss (skin, gastrointestinal and renal losses) 28. Decreased potassium intake, in isolation, rarely results in hypokalemia due to the ability of the kidneys to effectively minimize potassium excretion 6, 7, 16. However, reduced potassium intake can be a contributor to hypokalemia in the presence of other causes, such as malnutrition or diuretic therapy 28. Cellular uptake of potassium is promoted by alkalosis (an abnormal pathophysiological condition characterized by the buildup of excess base or alkali in the body), insulin, beta-adrenergic stimulation, aldosterone and xanthines, such as caffeine 28. Most cases of hypokalemia result from gastrointestinal or kidney losses. Kidney potassium losses are associated with increased mineralocorticoid-receptor stimulation such as occurs with primary hyperreninism and primary aldosteronism 28. Increased delivery of sodium and/or non-absorbable ions (diuretic therapy, magnesium deficiency, genetic syndromes) to the distal nephron can also result in renal potassium wasting. Gastrointestinal potassium losses are a common cause of hypokalemia with severe or chronic diarrhea being the most common extrarenal cause of hypokalemia 81, 82, 83.
Increased potassium loss
The most common mechanisms leading to increased renal potassium losses include the following 80:
- Enhanced sodium delivery to the collecting duct, as with diuretics
- Mineralocorticoid excess, as with primary or secondary hyperaldosteronism
- Increased urine flow, as with an osmotic diuresis
Gastrointestinal losses, from diarrhea, vomiting, or nasogastric suctioning, also are common causes of hypokalemia. Vomiting leads to hypokalemia via a complex pathogenesis. Gastric fluid itself contains little potassium, approximately 10 mEq/L. However, vomiting produces volume depletion and metabolic alkalosis, which are accompanied by increased renal potassium excretion.
Volume depletion leads to secondary hyperaldosteronism, which in turn leads to enhanced cortical collecting tubule secretion of potassium in response to enhanced sodium reabsorption. Metabolic alkalosis also increases collecting tubule potassium secretion due to the decreased availability of hydrogen ions for secretion in response to sodium reabsorption.
Shift from the extracellular to the intracellular space
Hypokalemia caused by a shift from extracellular to intracellular space often accompanies increased excretion, leading to a potentiation of the hypokalemic effect of excessive loss 80. Intracellular shifts of potassium often are episodic and frequently are self-limited, as, for example, with acute insulin therapy for hyperglycemia 80.
Hypokalemia prevention
Most people can prevent potassium deficiency by eating a healthy, balanced diet. If you are at increased risk, for example if you are taking diuretics, talk to your doctor about your potassium levels. Routine potassium replacement is not necessary in most patients receiving diuretics. However, serum potassium should be monitored during diuretic use when risk of hypokalemia or of its complications is high. Risk is high in 72:
- Patients with decreased left ventricular function
- Patients taking digoxin
- Patients with diabetes (in whom insulin concentrations can fluctuate)
- Patients with asthma who are taking beta 2-agonists
Triamterene 100 mg orally once a day or spironolactone 25 mg orally 4 times a day does not increase potassium excretion and may be useful in patients who become hypokalemic but must use diuretics. When hypokalemia develops, potassium supplementation, usually with oral potassium chloride, is indicated.
Hypokalemia may also be minimized by dietary restriction of salt (sodium) since high rates of sodium excretion promote urinary potassium losses. People who participate in vigorous sports or exercise in warm weather should be sure to replace potassium that is lost through excessive sweating. This can be accomplished through dietary planning.
Foods high in Potassium
Potassium is found in many foods. You can get recommended amounts of potassium by eating a variety of foods, including the following 84:
- Fruits, such as dried apricots, prunes, raisins, orange juice, and bananas
- Vegetables, such as acorn squash, potatoes, spinach, tomatoes, and broccoli
- Lentils, kidney beans, soybeans, and nuts
- Milk and yogurt
- Meats, poultry, and fish
Potassium is found in a wide variety of plant and animal foods and in beverages. Many fruits and vegetables are excellent sources, as are some legumes (e.g., soybeans) and potatoes. Meats, poultry, fish, milk, yogurt, and nuts also contain potassium 21. Among starchy foods, whole-wheat flour and brown rice are much higher in potassium than their refined counterparts, white wheat flour and white rice 85. Selected food sources of potassium are listed in Table 2. People with kidney problems, especially those on dialysis, should not eat too many potassium-rich foods. Your health care provider will recommend a special diet low in potassium.
Milk, coffee, tea, other nonalcoholic beverages, and potatoes are the top sources of potassium in the diets of American adults 86. Among children in the United States, milk, fruit juice, potatoes, and fruit are the top sources 87.
It is estimated that the body absorbs about 85%–90% of dietary potassium 32. The forms of potassium in fruits and vegetables include potassium phosphate, sulfate, citrate, and others, but not potassium chloride 88.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing potassium ordered by nutrient content (https://www.nal.usda.gov/sites/www.nal.usda.gov/files/potassium.pdf). The 2015–2020 Dietary Guidelines for Americans provides a a list of foods containing potassium 89.
[Source 2 ]Table 3. Food Sources of Potassium
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Apricots, dried, ½ cup | 755 | 16 |
| Lentils, cooked, 1 cup | 731 | 16 |
| Squash, acorn, mashed, 1 cup | 644 | 14 |
| Prunes, dried, ½ cup | 635 | 14 |
| Raisins, ½ cup | 618 | 13 |
| Potato, baked, flesh only, 1 medium | 610 | 13 |
| Kidney beans, canned, 1 cup | 607 | 13 |
| Orange juice, 1 cup | 496 | 11 |
| Soybeans, mature seeds, boiled, ½ cup | 443 | 9 |
| Banana, 1 medium | 422 | 9 |
| Milk, 1%, 1 cup | 366 | 8 |
| Spinach, raw, 2 cups | 334 | 7 |
| Chicken breast, boneless, grilled, 3 ounces | 332 | 7 |
| Yogurt, fruit variety, nonfat, 6 ounces | 330 | 7 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 326 | 7 |
| Beef, top sirloin, grilled, 3 ounces | 315 | 7 |
| Molasses, 1 tablespoon | 308 | 7 |
| Tomato, raw, 1 medium | 292 | 6 |
| Soymilk, 1 cup | 287 | 6 |
| Yogurt, Greek, plain, nonfat, 6 ounces | 240 | 5 |
| Broccoli, cooked, chopped, ½ cup | 229 | 5 |
| Cantaloupe, cubed, ½ cup | 214 | 5 |
| Turkey breast, roasted, 3 ounces | 212 | 5 |
| Asparagus, cooked, ½ cup | 202 | 4 |
| Apple, with skin, 1 medium | 195 | 4 |
| Cashew nuts, 1 ounce | 187 | 4 |
| Rice, brown, medium-grain, cooked, 1 cup | 154 | 3 |
| Tuna, light, canned in water, drained, 3 ounces | 153 | 3 |
| Coffee, brewed, 1 cup | 116 | 2 |
| Lettuce, iceberg, shredded, 1 cup | 102 | 2 |
| Peanut butter, 1 tablespoon | 90 | 2 |
| Tea, black, brewed, 1 cup | 88 | 2 |
| Flaxseed, whole, 1 tablespoon | 84 | 2 |
| Bread, whole-wheat, 1 slice | 81 | 2 |
| Egg, 1 large | 69 | 1 |
| Rice, white, medium-grain, cooked, 1 cup | 54 | 1 |
| Bread, white, 1 slice | 37 | 1 |
| Cheese, mozzarella, part skim, 1½ ounces | 36 | 1 |
| Oil (olive, corn, canola, or soybean), 1 tablespoon | 0 | 0 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs (Daily Values) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value (DV) for potassium is 4,700 mg for adults and children age 4 years and older 90. FDA requires the new food labels to list potassium content. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 85 ]Hypokalemia signs and symptoms
Most often, a slight decrease in the potassium level in blood or mild hypokalemia (serum potassium 3 to 3.5 mEq/L [3 to 3.5 mmol/L]) usually causes no symptoms or asymptomatic. In most cases, low potassium is found by a blood test that is done because of an illness, or because you are taking diuretics (water pills). Hypokalemia affects up to 21% of hospitalized patients, usually because of the use of diuretics and other medications 4, 5, 91. It is rare for low potassium to cause isolated symptoms such as muscle cramps if you are feeling well in other respects.
Low potassium symptoms may include 20:
- Muscle weakness
- Weak or twitching muscles
- Nausea and vomiting
- Fatigue
- Muscle cramps
- Constipation
- Feeling of skipped heart beats or palpitations
- Tingling or numbness
Serum potassium less than 3 mEq/L (less than 3 mmol/L) generally causes muscle weakness and may lead to muscular paralysis and possibly respiratory failure. Other muscular dysfunction includes cramping, muscle twitches (fasciculations), paralysis of the bowel (paralytic ileus), hypoventilation, low blood pressure (hypotension), tetany (involuntary contraction of muscles), and rhabdomyolysis. Severe hypokalemia may also lead to disruption of skeletal muscle cells, particularly during exercise. The normal physical response to exercise requires the local release of potassium from muscle. In potassium depleted muscle, the lack of potassium prevents adequate widening of blood vessels, resulting in decreased muscle blood flow, cramps and the destruction of skeletal muscle. Other symptoms may include loss of appetite, nausea and vomiting, confusion, distention of the abdomen and a decrease in mental activity.
Persistent hypokalemia may also impair the ability of the kidneys to concentrate urine, resulting in excessive urination (polyuria) with secondary excessive thirst (polydipsia).
A large drop in potassium level (serum potassium level less than about 2.5 mmol/L) may lead to abnormal heart rhythms (arrhythmias), especially in people with underlying heart disease that can be fatal and requires urgent medical attention 17, 25. This can cause you to feel lightheaded or faint. Abnormal heart rhythms (arrhythmias) are the most worrisome complication of very low potassium levels, particularly in people with underlying heart disease. A very low potassium level can even cause your heart to stop.
Chronic low potassium levels (chronic hypokalemia) is associated with high blood pressure (hypertension), kidney stone formation, increased bone turnover, urinary calcium excretion, and salt sensitivity (meaning that changes in sodium intakes affect blood pressure to a greater than normal extent).
Hypokalemia complications
Someone with severe hypokalemia can experience:
- Decreased brain function
- High blood sugar levels (hyperglycemia)
- Muscle paralysis
- Difficulty breathing (dyspnea)
- irregular heartbeat (cardiac arrhythmias). Abnormal heart rhythms (arrhythmias) are the most worrisome complication of very low potassium levels, particularly in people with underlying heart disease.
- In severe hypokalemia cases, life-threatening paralysis may develop, such as with hypokalemic periodic paralysis.
- Chronic low potassium levels (chronic hypokalemia) is associated with high blood pressure (hypertension) and kidney stone formation.
Severe hypokalemia (serum potassium level less than about 2.5 mmol/L) may result in muscular paralysis or abnormal heart rhythms (cardiac arrhythmias) that can be life threatening 17, 25.
Talk to your doctor about what your blood test results mean. You may need to change a medication that’s affecting your potassium level, or you may need to treat another medical condition that’s causing your low potassium level.
Hypokalemia diagnosis
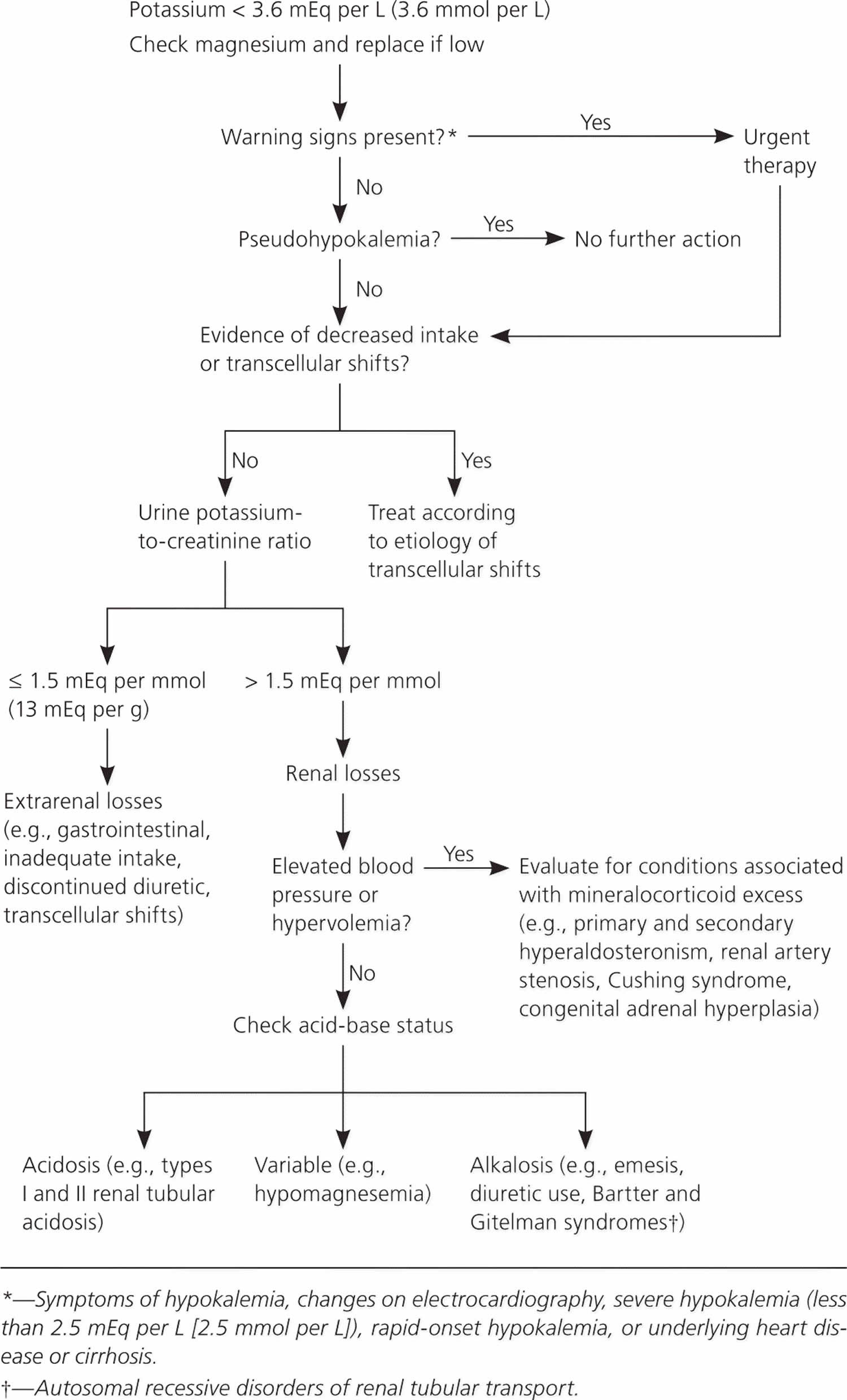
Hypokalemia is often asymptomatic. The diagnosis of hypokalemia (serum potassium < 3.5 mEq/L [< 3.5 mmol/L]) may be found during routine serum electrolyte measurement. Doctors then try to identify what is causing the potassium level to decrease (see Figure 3) 92, 93. The cause may be clear based on your symptoms (such as vomiting) or use of drugs or other substances. If the cause is not clear, further investigation is warranted and doctors measure how much potassium is excreted in urine to determine whether excess excretion is the cause. Because low potassium levels can cause abnormal heart rhythms, doctors usually do electrocardiography (ECG) to check for abnormal heart rhythms.
Evaluation begins with a search for warning signs or symptoms warranting urgent treatment 22. These include weakness or palpitations, changes on electrocardiography (ECG), severe hypokalemia (less than 2.5 mEq per L [2.5 mmol per L]), rapid-onset hypokalemia, or underlying heart disease or cirrhosis 92, 94. Most cases of hypokalemia-induced rhythm disturbances occur in individuals with underlying heart disease 73. Early identification of potassium transcellular shifts is important because management may differ. Identification and treatment of concurrent hypomagnesemia are also important because magnesium depletion impedes potassium repletion and can exacerbate hypokalemia-induced rhythm disturbances 95, 96.
It should be suspected in patients with typical changes on an ECG or who have muscular symptoms and risk factors and confirmed by blood testing.
Figure 3. Hypokalemia diagnostic algorithm
[Source 22 ]History and physical examination
A focused history includes evaluation for possible gastrointestinal losses, review of medications, and assessment for underlying cardiac comorbidities 22. A history of paralysis, hyperthyroidism, or use of insulin or beta agonists suggests possible transcellular shifts leading to redistributive hypokalemia. The physical examination should focus on identifying cardiac arrhythmias and neurologic manifestations, which range from generalized weakness to ascending paralysis.
Electrocardiography (ECG)
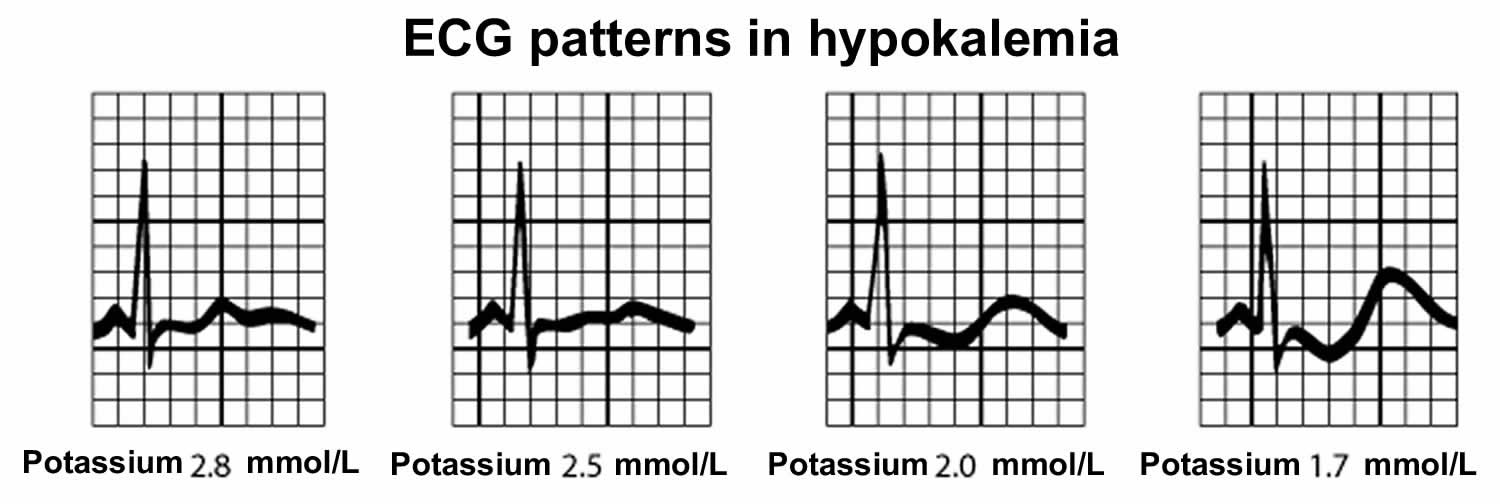
Electrocardiography (ECG) should be done on patients with hypokalemia. Cardiac effects of hypokalemia are usually minimal until serum potassium concentrations are less than 3 mEq/L (< 3 mmol/L). Hypokalemia causes sagging of the ST segment, depression of the T wave (decreased T-wave amplitude), and elevation of the U wave (see Figure 4) 72. With marked hypokalemia can lead to PR-interval prolongation, ST-interval depression, the T wave becomes progressively smaller or T-wave inversions and the U wave becomes increasingly larger 72. Sometimes, a flat or positive T wave merges with a positive U wave, which may be confused with QT prolongation (see figure ECG patterns in hypokalemia). Hypokalemia may cause sinus bradycardia, premature ventricular beats and premature atrial contractions, ventricular tachycardia or fibrillation and supraventricular tachyarrhythmias, torsade de pointes and 2nd- or 3rd-degree atrioventricular block 97, 72. Such arrhythmias become more severe with increasingly severe hypokalemia; eventually, ventricular fibrillation may occur 72. Although the risk of ECG changes and arrhythmias increases as serum potassium concentration decreases, these findings are not reliable because some patients with severe hypokalemia do not have ECG changes 98. Patients with significant preexisting heart disease and patients receiving digoxin are at risk of cardiac conduction abnormalities as a result of even mild hypokalemia.
Figure 4. ECG patterns in hypokalemia
[Source 72 ]Laboratory and urine tests
Potassium deficiency or hypokalemia diagnosis should be confirmed with a repeat serum potassium measurement. Other laboratory tests include serum glucose and magnesium levels, urine electrolyte and creatinine levels, and acid-base balance. After acidosis and other causes of intracellular potassium shift (increased beta-adrenergic effect, hyperinsulinemia) have been eliminated, 24-hour urinary potassium and serum magnesium concentrations are measured.
The most accurate method for evaluating urinary potassium excretion is a 24-hour timed urine potassium collection; normal kidneys excrete no more than 15 to 30 mEq per L (15 to 30 mmol per L) of potassium per day in response to hypokalemia. In hypokalemia, potassium secretion is normally less than 15 mEq/L (< 15 mmol/L) 72. Extrarenal gastrointestinal potassium loss or decreased potassium ingestion is suspected in chronic unexplained hypokalemia when renal potassium secretion is less than 15 mEq/L (< 15 mmol/L). Secretion of more than 15 mEq/L (> 15 mmol/L) suggests a renal cause for potassium loss 72. A more practical approach is calculation of the urine potassium-to-creatinine ratio from a spot urine specimen; a ratio greater than 1.5 mEq per mmol (13 mEq per g) of creatinine is indicative of renal potassium wasting 99, 28.
Unexplained hypokalemia with increased renal potassium secretion and hypertension suggests an aldosterone-secreting tumor or Liddle syndrome 72. Unexplained hypokalemia with increased renal potassium loss and normal blood pressure suggests Bartter syndrome or Gitelman syndrome, but hypomagnesemia, surreptitious vomiting, and diuretic abuse are more common and should also be considered 72.
If no cause is identified with the initial workup, assessment of thyroid and adrenal function should be considered.
Hypokalemia treatment
Treatment of low potassium is directed at the underlying cause and may include potassium supplements. If your condition is mild, your doctor will likely prescribe oral potassium pills. If your condition is severe, you may need to get potassium through a vein (IV). Treatment urgency depends on the severity of your hypokalemia, the existence of comorbid conditions and the rate of decline of serum potassium levels 28. Rapid correction is possible with oral potassium; the fastest results are likely best achieved by combining oral (e.g., 20 to 40 mmol) and intravenous administration 100.
The goal of potassium replacement in the context of kidney or gastrointestinal losses is to immediately raise serum potassium concentration to a safe level and then replace the remaining deficit over days to weeks. A potassium-sparing diuretic should also be considered when the cause of hypokalemia involves renal potassium wasting as potassium replacement therapy alone may not be enough.
The immediate goal of treatment is the prevention of potentially life-threatening cardiac conduction disturbances and neuromuscular dysfunction by raising serum potassium to a safe level 22. Further replenishment can proceed more slowly, and attention can turn to the diagnosis and management of the underlying disorder 94. Patients with a history of congestive heart failure or heart attack (myocardial infarction) should maintain a serum potassium concentration of at least 4 mEq per L (4 mmol per L), based on expert opinion 94.
For hypokalemia associated with diuretic use, stopping the diuretic or reducing its dosage may be effective 94. Another strategy, if otherwise indicated to treat a comorbid condition, is use of an angiotensin-converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), beta blocker, or potassium-sparing diuretic because each of these drugs is associated with an elevation in serum potassium 22.
It is appropriate to increase dietary potassium in patients with low-normal and mild hypokalemia, particularly in those with a history of hypertension or heart disease 94. The effectiveness of increased dietary potassium is limited, however, because most of the potassium contained in foods is coupled with phosphate, whereas most cases of hypokalemia involve chloride depletion and respond best to supplemental potassium chloride 94, 3.
Eating foods rich in potassium can help treat and prevent low level of potassium. These foods include:
- Avocados
- Baked potato
- Bananas
- Bran
- Carrots
- Cooked lean beef
- Milk
- Oranges
- Peanut butter
- Peas and beans
- Salmon
- Seaweed
- Spinach
- Tomatoes
- Wheat germ
Many oral potassium supplements are available. Nonurgent hypokalemia is treated with 40 to 100 mmol of oral potassium per day over days to weeks. For the prevention of hypokalemia in patients with persistent losses, as with ongoing diuretic therapy or hyperaldosteronism, 20 mmol per day is usually sufficient 101. Because high single doses can cause gastrointestinal irritation and occasional bleeding, potassium deficiency are usually replaced in divided doses. Liquid potassium chloride given orally elevates concentrations within 1 to 2 hours but has a bitter taste and is tolerated particularly poorly in doses greater than 25 to 50 mEq. (> 25 to 50 mmol) 72. Wax-impregnated potassium chloride preparations are safe and better tolerated. GI bleeding may be even less common with microencapsulated potassium chloride preparations. Several of these preparations contain 8 or 10 mEq/capsule. Because a decrease in serum potassium of 1 mEq/L (1 mmol/L) correlates with about a 200- to 400-mEq (200 to 400 mmol) deficit in total body potassium stores, total deficit can be estimated and replaced over a number of days at 20 to 80 mEq (20 to 80 mmol)/day.
When hypokalemia is severe (eg, with ECG changes or severe symptoms), is unresponsive to oral therapy, or occurs in hospitalized patients who are taking digoxin or who have significant heart disease or ongoing losses, potassium must be replaced intravenously (IV) 72. Because use of intravenous (IV) potassium increases the risk of hyperkalemia (a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L) and can cause pain and vein inflammation (phlebitis), intravenous potassium should be reserved for patients with severe hypokalemia, hypokalemic ECG changes, or physical signs or symptoms of hypokalemia, or for those unable to tolerate the oral form 22.
When intravenous (IV) potassium is used, standard administration is 20 to 40 mmol of potassium in 1 L of normal saline [the concentration should not exceed 40 mEq/L (40 mmol/L)] 22. Correction typically should not exceed 20 mmol per hour, although higher rates using central venous catheters have been successful in emergency situations 100. Continuous cardiac monitoring is indicated if the rate exceeds 10 mmol per hour. In children, dosing is 0.5 to 1.0 mmol per L per kg over one hour (maximum of 40 mmol) 102. Potassium should not be given in dextrose-containing solutions because dextrose-stimulated insulin secretion can exacerbate hypokalemia 22.
In hypokalemia-induced arrhythmia, intravenous (IV) potassium chloride must be given more rapidly, usually through a central vein or using multiple peripheral veins simultaneously. Infusion of 40 mEq (40 mmol) potassium chloride/hour can be undertaken but only with continuous cardiac monitoring and hourly serum potassium determinations. Glucose solutions are avoided because elevation in the serum insulin concentrations could result in transient worsening of hypokalemia.
Even when potassium deficits are severe, it is rarely necessary to give > 100 to 120 mEq (> 100 to 120 mmol) of potassium in a 24-hour period unless potassium loss is ongoing. In potassium deficit with high serum potassium concentration, as in diabetic ketoacidosis, intravenous (IV) potassium is deferred until the serum potassium starts to fall. When hypokalemia occurs with hypomagnesemia, both the potassium and magnesium deficiencies must be corrected to stop ongoing renal potassium wasting.
Careful monitoring during treatment is essential because supplemental potassium is a common cause of hyperkalemia (a serum or plasma potassium level above the upper limits of normal, usually greater than 5.0 mEq/L to 5.5 mEq/L) in hospitalized patients 103. The risk of rebound hyperkalemia is higher when treating redistributive hypokalemia. Because serum potassium concentration drops approximately 0.3 mEq per L (0.3 mmol per L) for every 100-mEq (100-mmol) reduction in total body potassium, the approximate potassium deficit can be estimated in patients with abnormal losses and decreased intake 22. For example, a decline in serum potassium from 3.8 to 2.9 mEq per L (3.8 to 2.9 mmol per L) roughly corresponds to a 300-mEq (300-mmol) reduction in total body potassium 22. Additional potassium will be required if losses are ongoing. Concomitant hypomagnesemia should be treated concurrently.
Hypokalemia prognosis
The prognosis for patients with hypokalemia depends entirely on the underlying cause of hypokalemia 104. For example, a patient with an acute episode of hypokalemia resulting from diarrhea has an excellent prognosis. Hypokalemia due to a congenital disorder such as Bartter syndrome has a poor to nonexistent potential for resolution 104.
Hypokalemia is associated with increased mortality for patients with diabetes, chronic kidney disease, myocardial infarction, heart failure and COVID-19 pneumonia 105, 106, 107, 108.
- Preuss HG, Clouatre DL. Sodium, chloride, and potassium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:475-92.[↩][↩][↩][↩][↩][↩][↩][↩]
- Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70.https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html[↩][↩][↩]
- Gennari FJ. Hypokalemia. N Engl J Med. 1998 Aug 13;339(7):451-8. doi: 10.1056/NEJM199808133390707[↩][↩][↩][↩]
- Paice BJ, Paterson KR, Onyanga-Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J. 1986 Mar;62(725):187-91. doi: 10.1136/pgmj.62.725.187[↩][↩]
- Lippi G, Favaloro EJ, Montagnana M, Guidi GC. Prevalence of hypokalaemia: the experience of a large academic hospital. Intern Med J. 2010 Apr;40(4):315-6. doi: 10.1111/j.1445-5994.2009.02146.x[↩][↩]
- Potassium. https://ods.od.nih.gov/factsheets/Potassium-HealthProfessional[↩][↩][↩][↩][↩][↩][↩]
- Potassium. https://lpi.oregonstate.edu/mic/minerals/potassium[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Guideline: Potassium Intake for Adults and Children. Geneva: World Health Organization; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK132470[↩][↩][↩]
- Casey DE Jr, Thomas RJ, Bhalla V, Commodore-Mensah Y, Heidenreich PA, Kolte D, Muntner P, Smith SC Jr, Spertus JA, Windle JR, Wozniak GD, Ziaeian B. 2019 AHA/ACC Clinical Performance and Quality Measures for Adults With High Blood Pressure: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2019 Nov;12(11):e000057. doi: 10.1161/HCQ.0000000000000057[↩][↩]
- Filippini T, Violi F, D’Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol. 2017 Mar 1;230:127-135. doi: 10.1016/j.ijcard.2016.12.048[↩][↩]
- Staruschenko A. Beneficial Effects of High Potassium: Contribution of Renal Basolateral K+ Channels. Hypertension. 2018 Jun;71(6):1015-1022. doi: 10.1161/HYPERTENSIONAHA.118.10267[↩]
- Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018 Jul 1;4(3):180-188. doi: 10.1093/ehjcvp/pvy015[↩][↩]
- Kim MJ, Valerio C, Knobloch GK. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2023 Jan;107(1):59-70. https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html[↩]
- Cupisti A, Kovesdy CP, D’Alessandro C, Kalantar-Zadeh K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients. 2018 Feb 25;10(3):261. doi: 10.3390/nu10030261[↩][↩]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. 2013;3(1):19-62. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf[↩][↩]
- Potassium. https://www.hsph.harvard.edu/nutritionsource/potassium[↩][↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid — Base Metabolism In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease: Lippincott Williams & Wilkins; 2014:102-132.[↩][↩][↩][↩][↩]
- Mumoli N, Cei M. Licorice-induced hypokalemia. Int J Cardiol. 2008 Mar 14;124(3):e42-4. doi: 10.1016/j.ijcard.2006.11.190[↩]
- Walker BR, Edwards CR. Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol Metab Clin North Am. 1994 Jun;23(2):359-77.[↩]
- Potassium Blood Test. https://medlineplus.gov/lab-tests/potassium-blood-test[↩][↩]
- Bailey JL, Sands JM, Franch HA. Water, electrolytes, and acid-based metabolism. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:102-32.[↩][↩][↩][↩]
- Viera AJ, Wouk N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am Fam Physician. 2015 Sep 15;92(6):487-95. https://www.aafp.org/pubs/afp/issues/2015/0915/p487.html[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ukaonu C, Hill DA, Christensen F. Hypokalemic myopathy in pregnancy caused by clay ingestion. Obstet Gynecol. 2003 Nov;102(5 Pt 2):1169-71. doi: 10.1016/s0029-7844(03)00705-1[↩][↩]
- McKenna D. Myopathy, hypokalaemia and pica (geophagia) in pregnancy. Ulster Med J. 2006 May;75(2):159-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1891740[↩][↩]
- Food and Nutrition Board, Institute of Medicine. Potassium. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, D.C.: National Academies Press; 2005:186-268. https://nap.nationalacademies.org/read/10925/chapter/7[↩][↩][↩][↩][↩]
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:527-37.[↩]
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007 Oct;18(10):2649-52. doi: 10.1681/ASN.2007070792[↩][↩]
- Castro D, Sharma S. Hypokalemia. [Updated 2023 Mar 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482465[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Sur M, Mohiuddin SS. Potassium. [Updated 2022 Dec 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539791[↩][↩]
- Potassium. https://pubchem.ncbi.nlm.nih.gov/compound/potassium[↩]
- Unwin, R., Luft, F. & Shirley, D. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 7, 75–84 (2011). https://doi.org/10.1038/nrneph.2010.175[↩][↩][↩][↩]
- Stone MS, Martyn L, Weaver CM. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients. 2016 Jul 22;8(7):444. doi: 10.3390/nu8070444[↩][↩][↩][↩][↩][↩]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria M, Harrison M, Stallings VA, editors. Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019 Mar 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538102[↩][↩][↩][↩][↩][↩][↩]
- Brooks G. Potassium additive algorithm for use in continuous renal replacement therapy. Nurs Crit Care. 2006 Nov-Dec;11(6):273-80. doi: 10.1111/j.1478-5153.2006.00185.x[↩]
- Hinderling PH. The Pharmacokinetics of Potassium in Humans Is Unusual. J Clin Pharmacol. 2016 Oct;56(10):1212-20. doi: 10.1002/jcph.713[↩][↩]
- Palmer B.F. Regulation of potassium homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813[↩]
- Unwin R.J., Luft F.C., Shirley D.G. Pathophysiology and management of hypokalemia: A clinical perspective. Nat. Rev. Nephrol. 2011;7:75–84. doi: 10.1038/nrneph.2010.175[↩]
- Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019 Nov;74(5):682-695. doi: 10.1053/j.ajkd.2019.03.427. Erratum in: Am J Kidney Dis. 2022 Nov;80(5):690.[↩][↩][↩][↩][↩]
- World Health Organisation (WHO) Guideline: Potassium Intake for Adults and Children. WHO; Geneva, Switzerland: 2012.[↩]
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257[↩][↩]
- O’Neil C.E., Keast D.R., Fulgoni V.L., Nicklas T.A. Food sources of energy and nutrients among adults in the us: Nhanes 2003–2006. Nutrients. 2012;4:2097–2120. doi: 10.3390/nu4122097[↩]
- DeSalvo K.B., Olson R., Casavale K.O. Dietary guidelines for americans. JAMA. 2016;315:457–458. doi: 10.1001/jama.2015.18396[↩]
- Weaver C.M. Potassium and health. Adv. Nutr. (Bethesda Md.) 2013;4:S368–S377. doi: 10.3945/an.112.003533[↩]
- He F.J., MacGregor G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008;133:725–735. doi: 10.1111/j.1399-3054.2007.01033.x[↩]
- Shils M.E., Shike M. Modern Nutrition in Health and Disease. Lippincott Williams & Wilkins; Baltimore, MD, USA: 2006.[↩]
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016 Dec;40(4):480-490. doi: 10.1152/advan.00121.2016[↩][↩][↩][↩][↩]
- Hené RJ, Koomans HA, Boer P, Dorhout Mees EJ. Adaptation to chronic potassium loading in normal man. Miner Electrolyte Metab. 1986;12(3):165-72.[↩]
- Rabelink TJ, Koomans HA, Hené RJ, Dorhout Mees EJ. Early and late adjustment to potassium loading in humans. Kidney Int. 1990 Nov;38(5):942-7. doi: 10.1038/ki.1990.295[↩]
- Patrick J. Assessment of body potassium stores. Kidney Int 1977;11:476-90. https://www.kidney-international.org/article/S0085-2538(15)31757-9/pdf[↩][↩]
- Chatterjee R, Yeh HC, Edelman D, Brancati F. Potassium and risk of Type 2 diabetes. Expert Rev Endocrinol Metab. 2011 Sep;6(5):665-672. doi: 10.1586/eem.11.60[↩]
- WHO. Diet, nutrition and the prevention of chronic disease. Report of a Joint WHO/FAO Expert Consultation. Geneva, World Health Organization (WHO), 2003. http://whqlibdoc.who.int/trs/WHO_TRS_916.pdf [↩]
- D’Elia L, Barba G, Cappuccio FP et al. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. Journal of the American College of Cardiology, 2011, 57(10):1210–1219. http://www.ncbi.nlm.nih.gov/pubmed/21371638[↩]
- Whelton PK, He J, Cutler JA et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. Journal of the American Medical Association, 1997, 277(20):1624–1632. http://www.ncbi.nlm.nih.gov/pubmed/9168293[↩]
- Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. Journal of Human Hypertension, 2003, 17(7):471–480. http://www.ncbi.nlm.nih.gov/pubmed/12821954[↩]
- Dietary Guidelines Advisory Committee. The report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. Washington, D.C., Department of Health and Human Services and Department of Agriculture, 2005. http://www.health.gov/dietaryguidelines/dga2005/report/default.htm[↩]
- World Health Organization. Guideline: Potassium intake for adults and children. http://apps.who.int/iris/bitstream/10665/77986/1/9789241504829_eng.pdf[↩][↩]
- American Heart Association. A Primer on Potassium. https://sodiumbreakup.heart.org/a_primer_on_potassium?utm_source=SRI&utm_medium=HeartOrg&utm_term=Website&utm_content=SodiumAndSalt&utm_campaign=SodiumBreakup[↩][↩][↩]
- Clausen T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol. 2013 Oct;142(4):327-45. doi: 10.1085/jgp.201310980[↩]
- Larsen BR, Stoica A, MacAulay N. Managing Brain Extracellular K(+) during Neuronal Activity: The Physiological Role of the Na(+)/K(+)-ATPase Subunit Isoforms. Front Physiol. 2016 Apr 22;7:141. doi: 10.3389/fphys.2016.00141[↩]
- Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, Goldhaber J, Kaplan J, Lingrel JB, Pavlovic D, Philipson K, Sipido KR, Xie ZJ. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015 Mar 15;593(6):1361-82. doi: 10.1113/jphysiol.2014.282319[↩]
- DrugBank. Potassium. http://www.drugbank.ca/drugs/DB01345[↩]
- Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol2009;38:791-813.[↩]
- Kawasaki T, Itoh K, Kawasaki M. Reduction in blood pressure with a sodium-reduced, potassium- and magnesium-enriched mineral salt in subjects with mild essential hypertension. Hypertens Res1998;21:235-43. https://www.ncbi.nlm.nih.gov/pubmed/9877516?access_num=9877516&link_type=MED&dopt=Abstract[↩]
- Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr2006;83:1289-96. http://ajcn.nutrition.org/content/83/6/1289?ijkey=52dc92efe22c9a7ff1bd4e7366b4fcecb7f45e7e&keytype2=tf_ipsecsha[↩]
- Sheng H-W. Sodium, chloride and potassium. In: Stipanuk M, ed. Biochemical and Physiological Aspects of Human Nutrition. Philadelphia: W.B. Saunders Company; 2000:686-710.[↩]
- Weiss JN, Qu Z, Shivkumar K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ Arrhythm Electrophysiol. 2017 Mar;10(3):e004667. doi: 10.1161/CIRCEP.116.004667[↩]
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 8: advanced challenges in resuscitation: section 1: life-threatening electrolyte abnormalities. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000 Aug 22;102(8 Suppl):I217-22.[↩]
- Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012 Jan 11;307(2):157-64. doi: 10.1001/jama.2011.1967[↩]
- Smyth A, Dunkler D, Gao P, Teo KK, Yusuf S, O’Donnell MJ, Mann JF, Clase CM; ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014 Dec;86(6):1205-12. doi: 10.1038/ki.2014.214[↩]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC; 2005.[↩][↩]
- Reid A, Jones G, Isles C. Hypokalaemia: common things occur commonly – a retrospective survey. JRSM Short Rep. 2012 Nov;3(11):80. doi: 10.1258/shorts.2012.011179[↩]
- Hypokalemia. https://www.msdmanuals.com/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypokalemia[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Schulman M, Narins RG. Hypokalemia and cardiovascular disease. Am J Cardiol. 1990 Mar 6;65(10):4E-9E; discussion 22E-23E. doi: 10.1016/0002-9149(90)90244-u[↩][↩]
- Greenberg A. Diuretic complications. Am J Med Sci. 2000 Jan;319(1):10-24. https://doi.org/10.1016/S0002-9629(15)40676-7[↩]
- Dhalla IA, Gomes T, Yao Z, Nagge J, Persaud N, Hellings C, Mamdani MM, Juurlink DN. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013 Mar 19;158(6):447-55. doi: 10.7326/0003-4819-158-6-201303190-00004[↩]
- Morgan DB, Davidson C. Hypokalaemia and diuretics: an analysis of publications. Br Med J. 1980 Mar 29;280(6218):905-8. doi: 10.1136/bmj.280.6218.905[↩]
- Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part II: electrolyte and acid-base disorders complicating diuretic therapy. Expert Opin Drug Saf. 2010 Mar;9(2):259-73. doi: 10.1517/14740330903499257[↩][↩]
- Barkas F, Liberopoulos E, Kei A, Elisaf M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol. 2013;26(1):23-28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959504[↩]
- Musto D, Rispo A, Testa A, Sasso F, Castiglione F. Hypokalemic myopathy in inflammatory bowel diseases. J Crohns Colitis. 2013 Sep;7(8):680. doi: 10.1016/j.crohns.2013.01.005[↩]
- Hypokalemia. https://emedicine.medscape.com/article/242008-overview#a4[↩][↩][↩][↩]
- Brown HD, Tran RH, Patka JH. Effect of Bolus Insulin Administration Followed by a Continuous Insulin Infusion on Diabetic Ketoacidosis Management. Pharmacy (Basel). 2018 Dec 7;6(4):129. doi: 10.3390/pharmacy6040129[↩]
- Shao W, Ayub S, Drutel R, Heise WC, Gerkin R. QTc Prolongation Associated With Psychiatric Medications: A Retrospective Cross-Sectional Study of Adult Inpatients. J Clin Psychopharmacol. 2019 Jan/Feb;39(1):72-77. doi: 10.1097/JCP.0000000000000992[↩]
- Cunha TDS, Heilberg IP. Bartter syndrome: causes, diagnosis, and treatment. Int J Nephrol Renovasc Dis. 2018 Nov 9;11:291-301. doi: 10.2147/IJNRD.S155397[↩]
- Potassium. https://ods.od.nih.gov/factsheets/Potassium-Consumer[↩]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28. https://ndb.nal.usda.gov/ndb/[↩][↩]
- O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients. 2012 Dec 19;4(12):2097-120. doi: 10.3390/nu4122097[↩]
- Keast DR, Fulgoni VL 3rd, Nicklas TA, O’Neil CE. Food sources of energy and nutrients among children in the United States: National Health and Nutrition Examination Survey 2003–2006. Nutrients. 2013 Jan 22;5(1):283-301. doi: 10.3390/nu5010283[↩]
- He FJ, MacGregor GA. Beneficial effects of potassium on human health. Physiol Plant. 2008 Aug;133(4):725-35. doi: 10.1111/j.1399-3054.2007.01033.x[↩]
- 2015-2020 Dietary Guidelines. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015[↩]
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels[↩]
- Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013 Mar;126(3):256-63. doi: 10.1016/j.amjmed.2012.06.037[↩]
- Weiner ID, Wingo CS. Hypokalemia–consequences, causes, and correction. J Am Soc Nephrol. 1997 Jul;8(7):1179-88. doi: 10.1681/ASN.V871179[↩][↩]
- Mount DB, Zandi-Nejad K. Disorders of potassium balance. In: Taal MW, Chertow GM, Marsden PA, Brenner BM, Rector FC, eds. Brenner and Rector’s The Kidney. Philadelphia, Pa.: Elsevier/Saunders; 2012.[↩]
- Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004 Jan 21;43(2):155-61. doi: 10.1016/j.jacc.2003.06.021[↩][↩][↩][↩][↩][↩]
- Whang R, Whang DD, Ryan MP. Refractory Potassium Repletion: A Consequence of Magnesium Deficiency. Arch Intern Med. 1992;152(1):40–45. doi:10.1001/archinte.1992.00400130066006[↩]
- Millane TA, Ward DE, Camm AJ. Is hypomagnesemia arrhythmogenic? Clin Cardiol. 1992 Feb;15(2):103-8. doi: 10.1002/clc.4960150210[↩]
- Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004 Aug;27(2):153-60. doi: 10.1016/j.jemermed.2004.04.006[↩]
- WEAVER WF, BURCHELL HB. Serum potassium and the electrocardiogram in hypokalemia. Circulation. 1960 Apr;21:505-21. doi: 10.1161/01.cir.21.4.505[↩]
- Kamel KS, Ethier JH, Richardson RM, Bear RA, Halperin ML. Urine electrolytes and osmolality: when and how to use them. Am J Nephrol. 1990;10(2):89-102. doi: 10.1159/000168062[↩]
- Kim GH, Han JS. Therapeutic approach to hypokalemia. Nephron. 2002;92 Suppl 1:28-32. doi: 10.1159/00006537[↩][↩]
- Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004 Jan 21;43(2):155-61. doi: 10.1016/j.jacc.2003.06.02[↩]
- Ingram TC, Olsson JM. In brief: hypokalemia. Pediatr Rev. 2008 Sep;29(9):e50-1. doi: 10.1542/pir.29-9-e50[↩]
- Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007 Dec;22(12):3471-7. doi: 10.1093/ndt/gfm471[↩]
- Hypokalemia. https://emedicine.medscape.com/article/242008-overview#a2[↩][↩]
- Coregliano-Ring L, Goia-Nishide K, Rangel ÉB. Hypokalemia in Diabetes Mellitus Setting. Medicina (Kaunas). 2022 Mar 16;58(3):431. doi: 10.3390/medicina58030431[↩]
- Cunningham JW, Mehra MR. Hypokalemia in heart failure: A low or a high point? Eur J Prev Cardiol. 2021 Apr 23;28(3):313-315. doi: 10.1177/2047487320914745[↩]
- Makinouchi R, Machida S, Matsui K, Shibagaki Y, Imai N. Severe hypokalemia in the emergency department: A retrospective, single-center study. Health Sci Rep. 2022 Apr 14;5(3):e594. doi: 10.1002/hsr2.594[↩]
- Chen D, Li X, Song Q, Hu C, Su F, Dai J, Ye Y, Huang J, Zhang X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw Open. 2020 Jun 1;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122[↩]