What is immunofixation
Immunofixation consists of an electrophoresis phase and a fixation phase 1. In immunofixation electrophoresis, the serum is applied to an agarose gel, and the negatively charged serum proteins move toward the cathode under the influence of an electric current 2. The speed of movement is dictated by their charge. During the fixation phase, antiserum containing anti-IgG, anti-IgA, anti-IgM, anti-light chain kappa, or anti-light chain lambda is inoculated with the serum proteins 2. If a monoclonal protein is present, a precipitant will form at this phase. Finally, the sample is washed to remove the proteins that do not precipitate and then stained, destained, and dried. Immunofixation electrophoresis is a method used to identify abnormal bands seen on serum, urine, or cerebrospinal fluid (CSF) protein electrophoresis, in order to determine which type of antibody (immunoglobulin) is present.
Immunofixation can either reveal a normal pattern or identify a monoclonal protein or a polyclonal immunoglobulin pattern. Immunofixation permits the detection and typing of monoclonal antibodies or immunoglobulins in serum or urine. Serum or urine immunofixation negative for a monoclonal protein or a polyclonal pattern is considered to be normal. CSF immunofixation that does not reveal oligoclonal bands is also considered normal. A polyclonal immunoglobulin pattern in the serum or urine immunofixation is considered to be nonspecific.
Immunofixation electrophoresis identifies the type of immunoglobulin protein(s) present in monoclonal bands on a protein electrophoresis pattern; typically immunofixation determines the presence of a heavy chain (IgG, IgM or IgA) and a light chain (kappa or lambda).
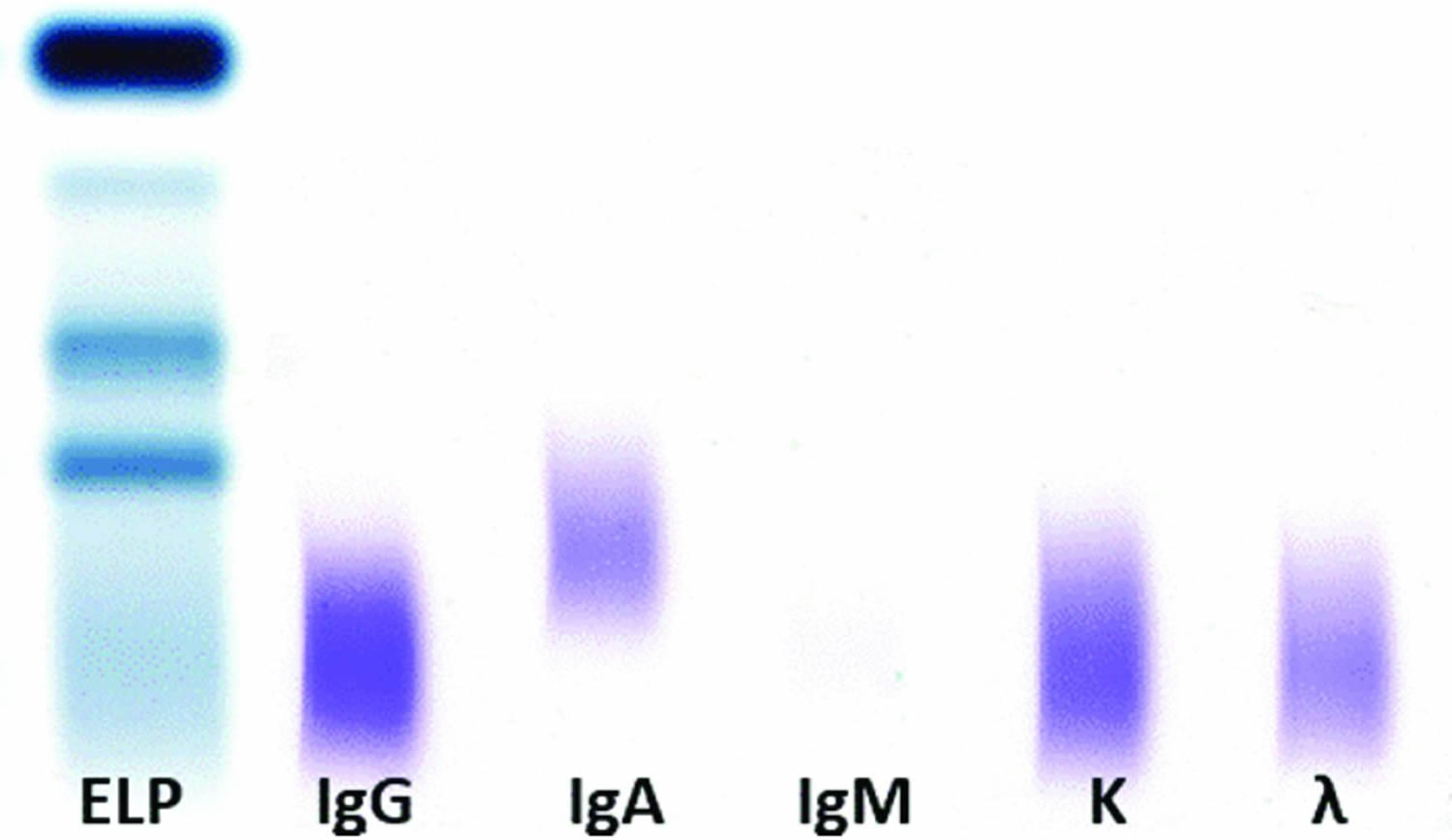
A normal result includes a darker immunoglobulin G (IgG) lane, a lighter immunoglobulin A (IgA), an absent immunoglobulin M (IgM), and a denser kappa compared to lambda lane, with ratio of 2:1. In a normal result, the lanes are broad and there is a gradual and smooth reduction in the color density toward the edges of the lane with no narrow dense band with sharp borders identified within the lane.
In some cases, all the lanes are homogeneously darkened to the same degree. This pattern represents the presence of polyclonal immunoglobulin. Again, the lanes are broad and the transition to the lane borders is smooth. In this case, the IgM lane, which is normally absent, is broad with smooth borders.
When a narrow band with sharp borders can be identified, it implies the presence of a monoclonal protein. In most cases in which a monoclonal protein is present, it shows as a narrow band at the IgG lane in combination with a monoclonal band at the kappa or lambda lane. IgA or IgM bands are less common. Finally, isolated light chain bands, either kappa or lambda, represent more commonly pure kappa or lambda light chain gammopathies or, less commonly, immunoglobulin D (IgD) or immunoglobulin E (IgE) gammopathies. Given the dismal impact on prognosis in the latter scenario 3, an additional assay for the identification of IgD or IgE must be performed. If the result is negative, it is safe to conclude that a pure kappa or lambda light chain is present 4.
Finally, when several bands with sharp borders are seen after immunofixation in cerebrospinal fluid (CSF) but not in serum, the result is reported as positive for oligoclonal bands.
The identification of a monoclonal immunoglobulin is helpful in the diagnosis of the following conditions 5:
- Monoclonal gammopathy of undetermined significance (MGUS) 6
- Multiple myeloma 7
- Waldenstrom macroglobulinemia 8
- Amyloidosis 9
The identification of oligoclonal bands in CSF is helpful in the diagnosis of multiple sclerosis 10.
In addition, immunofixation can be used to monitor therapy in plasma cell dyscrasias (ie, multiple myeloma and Waldenstrom macroglobulinemia). If the monoclonal protein level decreases or is undetectable after chemotherapy, it might indicate a response to treatment. On the contrary, a persistent monoclonal protein despite treatment is a sign of refractory disease 4.
The presence of oligoclonal bands in CSF but not in the serum, given their intrathecal production, is helpful in the diagnosis of multiple sclerosis 2. Other inflammatory conditions of the CNS can present with oligoclonal bands in CSF resulting from intrathecal production of antibodies 11. HIV-related encephalitis, neurosyphilis, Lyme meningoencephalitis, neurosarcoidosis, acute disseminated encephalomyelitis (ADEM), systemic lupus erythematosus (SLE), Sjögren syndrome, subacute sclerosing panencephalitis, CNS malignancy, neuromyelitis optica, and transverse myelitis can all reveal oligoclonal banding in CSF immunofixation, precluding the use of immunofixation for the detection of oligoclonal bands as a sole diagnostic technique in multiple sclerosis.
Figure 1. Serum immunofixation
Immunofixation test applications
Purpose of the immunofixation test
- To identify the monoclonal gammopathy.
- To monitor the treatment with monoclonal gammopathy.
- To find the light chains / heavy chain of immunoglobulin in the urine.
- Monoclonal gammopathy of undetermined significance (MGUS)
- Waldenstrom’s macroglobulinemia.
- Multiple myeloma (used more than 100 years).
- Amyloidosis.
Protein electrophoresis is used to identify the presence of abnormal proteins, to identify the absence of normal proteins, and to determine when different groups of proteins are present in unusually high or low amounts in blood or other body fluids.
Proteins do many things in the body, including the transport of nutrients, removal of toxins, control of metabolic processes, and defense against invaders.
Protein electrophoresis separates proteins based on their size and electrical charge. This forms a characteristic pattern of bands of different widths and intensities on a test media and reflects the mixture of proteins present in the body fluid evaluated. The pattern is divided into five fractions, called albumin, alpha 1, alpha 2, beta, and gamma. In some cases, the beta fraction is further divided into beta 1 and beta 2.
Immunofixation electrophoresis can be used as needed to further identify abnormal bands, in order to determine which type of antibody (immunoglobulin) is present. Alterations to the usual appearance of the patterns formed can help in the diagnosis of disease. The presence of an abnormality on a protein electrophorectic pattern is seldom diagnostic in itself. Instead, it provides a clue. Follow-up testing is then usually performed, based on that clue, to try to identify the nature of the underlying disease.
Follow-up tests may include, for example, albumin, immunoelectrophoresis, serum free light chains, quantitative immunoglobuins, alpha-1 antitrypsin or cryoglobulins.
A 24-hour concentrated urine sample is appropriate for urine immunofixation. This sample is often difficult to collect, and suboptimal collection might alter the result. A concomitant measurement of the creatinine in the 24-hour urine sample for correction of sampling errors has been proposed 12.
Detection of a paraprotein by immunofixation is possible in lymphomas 13.
Primary screening for plasma cell dyscrasias
No studies have addressed the role of serum or urine immunofixation in screening of the general population for multiple myeloma and related disorders. Therefore, such an approach cannot be currently recommended 10..
Surveillance among patients with monoclonal gammopathy of undetermined significance (MGUS) or smoldering (asymptomatic) multiple myeloma
Immunofixation of the urine and serum should be included in a battery of tests after 2-3 months following diagnosis of smoldering myeloma, then every 4-6 months for 1 year, and, finally, every 6-12 months if the results are stable. Surveillance for multiple myeloma in patients with monoclonal gammopathy of undetermined significance (MGUS) and favorable prognostic factors (ie, low levels of monoclonal protein and IgG type) should include monitoring at 6 months and then every 2-3 years thereafter. For patients with monoclonal gammopathy of undetermined significance (MGUS) and high risk for progression to multiple myeloma, surveillance should be performed at 6 months and then yearly thereafter 14.
Diagnosis of plasma cell dyscrasias
Immunofixation is clearly indicated upon clinical or laboratory evidence of a plasma cell dyscrasia for the diagnosis of multiple myeloma, Waldenstrom macroglobulinemia, or amyloid light-chain amyloidosis 2. Typically, the monoclonal protein is discovered, and workup then ensues. These conditions should be promptly diagnosed and treated. In particular, when serum and urine protein electrophoresis results are negative, immunofixation is indicated as a more sensitive test if the clinical suspicion remains high. In addition, when protein electrophoresis assays are positive for a monoclonal protein, serum and urine immunofixation are indicated for the appropriate identification of the immunoglobulin and the corresponding light chain. However, most patients with a monoclonal protein will be finally diagnosed with monoclonal gammopathy of undetermined significance (MGUS) rather than any of the malignant plasma cell dyscrasias for which treatment is recommended.
A negative urine or serum immunofixation result does not always rule out a plasma cell dyscrasia, as a nonsecretory or oligosecretory multiple myeloma can present with a negative immunofixation finding in both the urine and the serum. A serum free light chain ratio is indicated in the case of a negative immunofixation result when the suspicion for multiple myeloma is still high 15.
Follow-up of multiple myeloma, Waldenstrom macroglobulinemia, and amyloid light-chain amyloidosis
Immunofixation of urine and serum samples should be performed in all patients with multiple myeloma, Waldenstrom macroglobulinemia, and amyloid light-chain amyloidosis every 3 months after the completion of treatment in order to evaluate for response or to document a relapse 2.
However, a study by Lahuerta et al 16 indicated that, with the exception of persons with light-chain–only disease, urine immunofixation is not needed in combination with serum immunofixation to define complete response in multiple myeloma. Among 161 patients in whom both serum and urine demonstrated positive results for monoclonal protein and who were, posttreatment, negative for serum immunofixation, just 3 (1.9%) displayed positive urine immunofixation.
Diagnosis of multiple sclerosis
Immunofixation in CSF for the detection of oligoclonal bands is indicated every time there is suspicion for multiple sclerosis. However, the presence of oligoclonal bands does not necessarily confirm the diagnosis, as other conditions can present with oligoclonal bands in the CSF.
Immunofixation analysis
Normal
- No monoclonal band is identified.
The monoclonal band is seen in:
- Multiple myelomas show in 99 % of the patients in serum and urine.
- Waldenstrom macroglobulinemia always shows serum IgM type monoclonal band.
- Monoclonal light chains also called κ or Bence Jones protein, seen in the urine of Multiple myeloma cases in 75 % of the patients.
- Waldenstrom macroglobulinemia also shows light chains in the urine in 75 % of the patients.
- In Amyloidosis can see the light chain or heavy chain in the urine.
Polyclonal bands of Ig are seen in:
- Chronic infections.
- Autoimmune diseases.
- Chronic liver diseases.
- Amyloidosis.
Monoclonal Ig is seen in urine:
- Multiple myelomas.
- Waldenstrom macroglobulinemia.
- Cawley LP, Minard BJ, Tourtellotte WW, et al. Immunofixation electrophoretic techniques applied to identification of proteins in serum and cerebrospinal fluid. Clin Chem. 1976. 22:1262-8.[↩]
- Immunofixation. https://emedicine.medscape.com/article/2086976-overview[↩][↩][↩][↩][↩]
- Morris C, Drake M, Apperley J, et al. Efficacy and outcome of autologous transplantation in rare myelomas. Haematologica. 2010. 95:2126-33.[↩]
- Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel. Blood. 2011. 117:4701-5.[↩][↩]
- Guido Tricot. Multiple Myeloma. Ronald Hoffman, Leslie E. Silberstein, Sanford J. Shattil, Bruce Furie, Philip McGlave. Hematology: Basic Principles and Practice. 5th. Philadelphia, PA, USA: Elsevier Churchill Livingstone; 2008. chapter 87.[↩]
- Fanning SR, Hussein MA. Monoclonal gammopathies of undetermined significance. Medscape Drugs & Diseases. 2018 Sep 7.[↩]
- Beaumont-Epinette MP, Moreau C, Besnard S, Latute F, Collet N, Sebillot M, et al. Heavy/light chain specific immunoglobulin ratios provides no additional information than serum proteins electrophoresis and immunofixation for the diagnosis and the follow-up of intact immunoglobulin multiple myeloma patients. Pathol Biol (Paris). 2015 Aug 26.[↩]
- Waldenstrom Macroglobulinemia. https://emedicine.medscape.com/article/207097-overview[↩]
- Urbancek S, Gascon P, Schwartz RA, Jacobson DR, Buxbaum JN, Bogdan CA. Immunoglobulin-related amyloidosis. Medscape Drugs & Diseases. 2017 Dec 28.[↩]
- Multiple Sclerosis. https://emedicine.medscape.com/article/1146199-overview[↩][↩]
- Cavuoti D, Baskin L, Jialal I. Detection of oligoclonal bands in cerebrospinal fluid by immunofixation electrophoresis. Am J Clin Pathol. 1998. 109:585-8.[↩]
- Kaplan JS, Horowitz GL. Twenty-four-hour Bence-Jones protein determinations: can we ensure accuracy?. Arch Pathol Lab Med. 2011. 135:1048-51[↩]
- Bird J, Behrens J, Westin J, et al. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol. 2009. 147:22-42.[↩]
- Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010. 24:1121-7.[↩]
- Sasson SC, McGill K, Wienholt L, Carr A, Brown DA, Kelleher AD, et al. Comparison of the Freelite serum free light chain (SFLC) assay with serum and urine electrophoresis/immunofixation and the N Latex FLC assay. Pathology. 2015 Oct. 47 (6):564-9.[↩]
- Lahuerta JJ, Jimenez-Ubieto A, Paiva B, et al. Role of urine immunofixation in the complete response assessment of MM patients other than light-chain-only disease. Blood. 2019 Jun 20. 133 (25):2664-8.[↩]