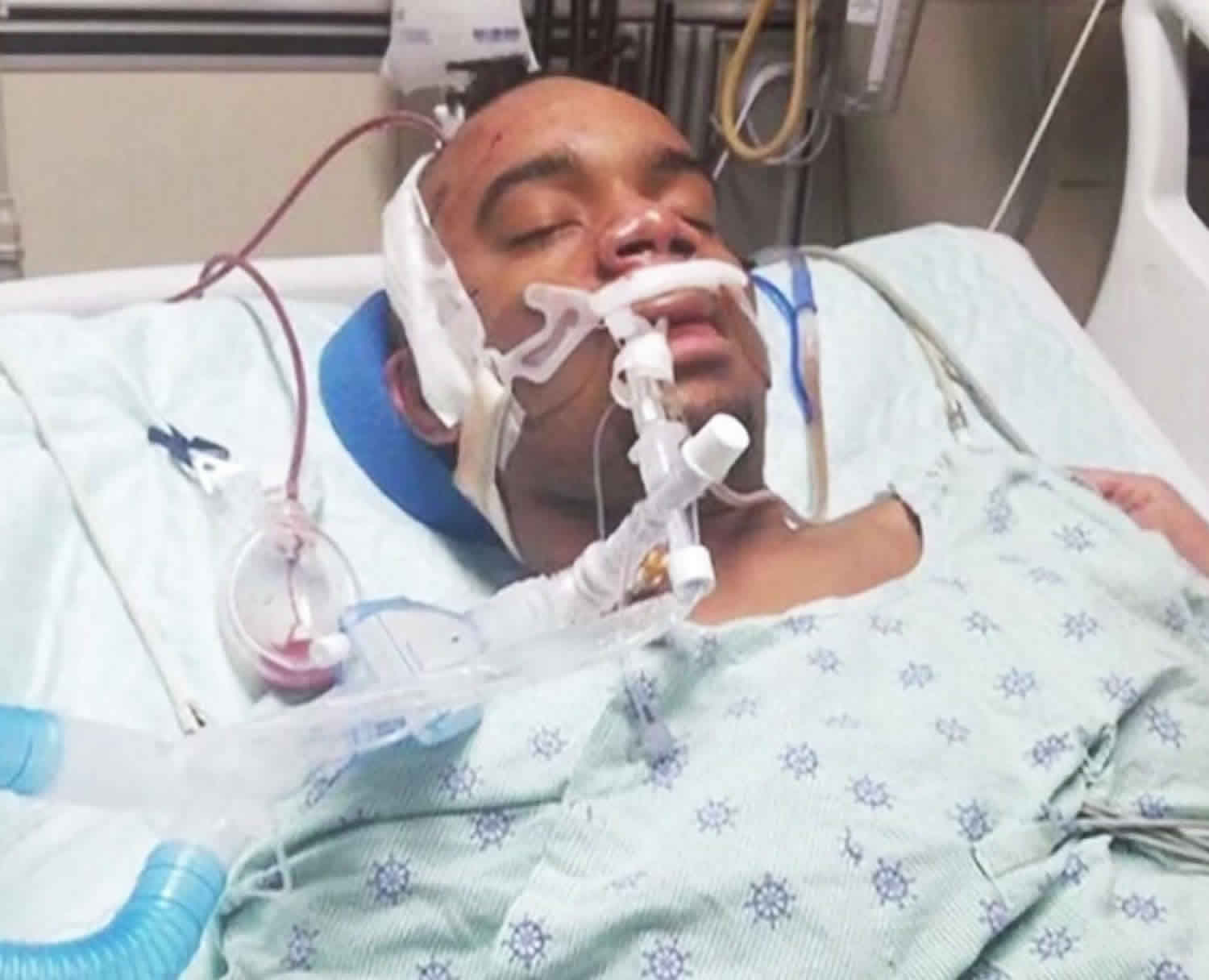
Induced coma
Induced coma also known as a medically-induced coma or medical coma, is a drug-induced state of profound brain inactivation and unconsciousness used to treat refractory intracranial hypertension and to manage treatment-resistant epilepsy (refractory status epilepticus) 1. The state of coma is achieved by continually monitoring the patient’s brain activity with an electroencephalogram (EEG) and manually titrating the anesthetic infusion rate to maintain a specified level of burst suppression, an EEG marker of profound brain inactivation in which bursts of electrical activity alternate with periods of quiescence or suppression. The medical coma is often required for several days.
About 55% of the glucose and oxygen utilization by the brain is meant for its electrical activity and the rest for all other activities like metabolism. This is recognized by something such as an electroencephalogram (EEG), which measures electrical activity in the brain. When barbiturates are given to a brain injured patients for induced coma, they act by reducing the electrical activity of the brain, which in theory reduces the metabolic and oxygen demand. Once there is improvement in the patient’s general condition, the barbiturates are withdrawn gradually and the patient regains consciousness.
Following a traumatic brain injury, an anesthetic drug such as a barbiturate or propofol, is administered continuously to provide brain protection by decreasing the cerebral metabolism and blood flow, and thereby, intracranial hypertension 2. In the treatment of status epilepticus the anesthetic is administered to directly inhibit activity in the seizure foci 3. For treating both refractory intracranial hypertension and status epilepticus, the state of medical coma is achieved by continually monitoring the patient’s brain activity with the electroencephalogram (EEG) and titrating the anesthetic drug infusion rate to maintain a specified level of burst suppression. Burst suppression is an EEG pattern characterized by intervals of electrical bursts that alternate with isoelectric or quiescent intervals termed suppressions 4 and is an EEG marker of profound brain inactivation. In most cases, once burst suppression is achieved, it can be controlled by decreasing or increasing the infusion rate of the anesthetic to decrease or increase the suppression level.
No guidelines have been set to define what level of burst suppression should be achieved to maintain a medically induced coma 3. A common practice is for the intensive care unit team to agree upon a target level of burst suppression, monitor continually the EEG and adjust manually the infusion rate of the anesthetic to maintain the target level. In most cases, the medical coma is required for at least 24 hours and frequently longer. Delivering medically induced coma therapy for refractory status epilepticus patients is challenging because it requires frequent patient monitoring, subjective interpretation of the EEG, and manual titration of IV anesthetic drugs by busy intensive care staff for prolonged periods often lasting 24–48 hours 5. Moreover, subspecialists who are trained to interpret EEG and administer anesthetic drugs are often unavailable, leaving the tasks to non-experts. As a result, many have questioned the quality of induced-coma provided to refractory status epilepticus patients and have searched for ways to improve the therapy 6.
Reasons for medically induced coma
Induced coma is intended to reduce the metabolic demand of the brain cells and is used to protect the brain during major neurosurgery. Medically induced coma is currently used in clinical settings as treatment for patients with high risk of brain injury either from physical trauma, drug overdose or disease such as intracranial hypertension and refractory status epilepticus 7. Refractory status epilepticus is a life threatening condition with a mortality rate of up to 40% 8. Refractory status epilepticus is defined as generalized or focal continuous seizures that fail to respond to first and second line pharmacological treatment 9 and lasting more than 30 minutes 10. International guidelines advocate treating refractory status epilepticus with medically induced coma achieved with a continuous infusion of intravenous anesthetic drugs, such as midazolam, propofol, and/or barbiturates 11. Medically induced coma with a continuous infusion of intravenous anesthetic drugs aims to suppress brain activity in order for normal physiology to resume and abort seizures 12. It requires a careful balance between maintaining sufficient brain inactivation and minimizing the risks of intravenous anesthetic drug exposure 9. As brain inactivation cannot be measured easily, clinicians typically use a distinctive pattern on the electroencephalogram (EEG) called burst suppression as a surrogate to guide titration of intravenous anesthetic drugs. The burst suppression pattern consists of alternating periods of high (‘bursts’) and low (‘suppressions’) voltage. Although anesthetics may have differential effects on excitatory and inhibitory neuronal activity 13 the overall effect of burst suppression is a profound global reduction in overall neuronal activity, including seizure activity 14.
Barbiturates reduce the metabolic rate of brain tissue, as well as the cerebral blood flow. With these reductions, the blood vessels in the brain narrow, decreasing the amount of volume occupied by the brain, and hence the intra-cranial pressure. The hope is that, with the swelling relieved, the pressure decreases and some or all brain damage may be averted. Several studies have supported this theory by showing reduced mortality when treating refractory intracranial hypertension with a barbiturate coma 15.
Controversy exists, however, over the benefits of using barbiturates to control intracranial hypertension. Some studies have shown that barbiturate-induced coma can reduce intracranial hypertension but does not necessarily prevent brain damage. Furthermore, the reduction in intracranial hypertension may not be sustained. Some randomized trials have failed to demonstrate any survival or morbidity benefit of induced coma in diverse conditions such as neurosurgical operations, head trauma 16, intracranial aneurysm rupture, intracranial hemorrhage, ischemic stroke, and status epilepticus. If the patient survives, cognitive impairment may also follow recovery from the coma 17.
There is considerable amount of controversy on the use of continuous infusion of intravenous antiepileptics for treating refractory status epilepticus. Controversy exists as to the most efficacious agent to use, preference of high monotherapy over combination antiepileptics, therapeutic dose ranges, duration of coma, depth of coma, thresholds for treating abnormal rhythms on EEG, and how continuous should the electroencephalography monitoring be 18. A systematic review of the literature by Claasen and colleagues 19 showed that outcomes of patients in status epilepticus are poor. Additionally, there is no difference in outcome on choice of antiepileptic or titration goal 19. However, they did find that pentobarbital coma is associated with less short-term treatment failure, breakthrough seizures, and the need to switch to another antiepileptic 19. However, the use of pentobarbital coma has significant morbidity and mortality 20.
Medically induced coma side effects
A study highlights the high morbidity of patients with refractory status epilepticus treated with pentobarbital coma 21. A total of seven patients had an infection from various sources. Increased propensity to infection may be related to the immunosuppressant effects of pentobarbital coma 22. Barbiturates cause immunosuppression by reduction of phagocytic activity of leukocytes, decreasing activation of peripheral lymphocytes, and depression of chemotactic migration of white blood cells 23. Immunosuppression increases the risk of IV line-associated infections and ventilator-associated pneumonia. Pentobarbital also reduces gastric motility putting a patient at potential risk for transmural translocation of intestinal bacteria and sepsis from intestinal organisms 22. Altered gastric motility also makes absorption of orally-available antiepileptics less reliable and compromises nutritional status. The study authors also observed hemodynamic instability and myocardial dysfunctions (i.e. heart failure and prolongation of QTc) resulting in hypotension and volume overload 24. Patients in pentobarbital coma have longer mechanical ventilation times and require aggressive venous thrombosis prophylaxis due to prolong immobility. Indeed, two patients in their study developed DVTs and one with a pulmonary embolus 21. It has been suggested that since barbiturates redistributes in tissue in a nonlinear fashion after prolonged use, accumulation occurs leading to prolonged recovery 23. Pentobarbital coma also is known to either contribute directly to the development of a peripheral polyneuropathy or its development may be a manifestation of prolonged critical illness 25. Overall, seven of their patients had had clinical signs of polyneuropathy and myopathy with one having an electromyography (EMG) / nerve conduction study (NCS) verifying the clinical suspicion.
Lactic acidosis is also encountered in prolonged pentobarbital comas 26. This may be secondary to the propylene glycol base (40% by volume) used with pentobarbital 24. Propylene glycol is predominantly metabolized in the liver to lactate, acetate, and pyruvate with the remainder excreted unchanged through the kidney 27. A total of seven patients in their series had prolonged lactic acidosis (median 9 days). Overall, one patient had a propylene glycol level of 58 mg/dl with no associated renal failure 27. It has been suggested that propylene glycol decreases renal clearance by saturating the proximal tubule 28. A propylene glycol level was available only in one patient, but four other patients required hemodialysis for acute renal failure with metabolic abnormalities or volume overload.
Generalized cerebral atrophy was seen in 33% of their patients. The progression of cerebral atrophy in patients with refractory status epilepticus is unclear. It may be a reflection of the etiology causing the refractory status epilepticus or the status epilepticus itself with its subsequent treatment and critical illnesses. Cross-sectional studies of this phenomenon are complicated by the possibility of cortical damage from the initial ictal insult, nonlesional MRI, possibility of cerebral atrophy from prolonged critical illness and associated multisystem/metabolic abnormalities, and effects of prolonged high dose multi-antiepileptic therapy 29. It has been proposed that prolonged sedation with anesthetics disrupts the ascending reticular activating system allowing for decoupling from the posterior parietal cortex, medial temporal lobe, and prefrontal cortex 30. This prolonged decoupling may lead to excitotoxicity and ultimately apoptosis 30. This could be one hypothesis to explain the cerebral atrophy. This hypothesis further highlights the urgency of aggressive intervention in patients who present with status epilepticus.
In another study involving 31 patients 20, 11 had no side effects related to starting continuous infusions of pentobarbital (Table 1). Overall, ventilator-associated pneumonia (32%) and hypotension requiring pressors (32%) were the most frequently encountered complications during continuous infusions of pentobarbital treatment, followed by urinary tract infection (13%), deep venous thrombosis (10%) and ileus (10%). After initiation of continuous infusions of pentobarbital, the percentage of patients requiring vasopressors increased from 13 to 32% (Figure 1). In all cases hypotension responded to fluid administration and vasopressors, and did not require a switch in continuous infusions of anesthetic drugs: 15% of the patients (n = 7) had other side effects such as neuropathy (n = 1), brain edema and bleeding following brain biopsy (n = 1), and sepsis which started before continuous infusions of pentobarbital (n = 3). One patient died of post-anoxic encephalopathy after a bradycardic arrest the day following continuous infusions of pentobarbital initiation, which occurred in the setting of several episodes of bradycardia requiring atropine administration prior to starting continuous infusions of pentobarbital. Another patient with N-methyl-D-aspartate receptor encephalitis developed propylene-glycol intoxication with severe acidosis, which resolved after cessation of continuous infusions of pentobarbital. All patients in the study were mechanically ventilated prior to starting continuous infusions of pentobarbital.
Table 1. Side effects that developed in 31 patients during treatment with pentobarbital
| Side effects | Number of patients (%) |
|---|---|
| None | 11 (35) |
| Ventilator-associated pneumonia | 10 (32) |
| Hypotension | 10 (32) |
| Urinary tract infection | 4 (13) |
| Deep venous thrombosis | 3 (10) |
| Ileus | 3 (10) |
| Other | 7 (23) |
A study by Sutter et al 31, showed the use of IV anesthetic drugs in status epilepticus was associated with an increased relative risk of death independent of possible confounders and without significant changes in the risk by different grades of status epilepticus severity and different status epilepticus etiologies. Regarding different IV anesthetic drugs, the association with death was strongest with midazolam followed by barbiturates or propofol in their study—results that are underscored by a study of patients with mainly convulsive status epilepticus 32. In a multicenter study, coma induction in status epilepticus patients was associated with death in the univariable analysis but lost significance in the multivariable model 33. However, the proportion of patients with coma induction was small (25%, 39/154), possibly impeding the statistical power, and the proportion of survivors in the coma induction group was lower (54%) than in the group without (65%).
Several hazards may contribute to poor outcome, including cardiotoxicity with phenobarbital and pentobarbital, severe hypotension from thiopental 34 or hepatotoxicity, metabolic acidosis, rhabdomyolysis, and cardiac failure seen in the propofol infusion syndrome 35. Furthermore, infections affect one-third of patients in some studies 36. In a randomized trial regarding effectiveness of midazolam, propofol, and pentobarbital in refractory status epilepticus, mortality was still 48% independent of drug or treatment intensity, and with a higher proportion of severe hypotension in patients on pentobarbital 37, a frequent side effect in status epilepticus patients treated with IV anesthetic drugs 38. A higher proportion of infections and the use of vasopressors indicating severe hypotension were identified as possible mediators for poor outcome in their cohort.
The risks regarding the use of IV anesthetic drugs in status epilepticus in their study raise great concern. There is growing uncertainty regarding the benefits of these drugs in status epilepticus vs their potential harm. Should patients be treated with rapid intubation and high-dose IV anesthetic drugs, or managed less urgently, even when delayed seizure control might incur neuronal damage? This decision is complex 39. Simplified “one-fits-all” management algorithms are not tenable, as morbidity and mortality vary considerably among different status epilepticus etiologies. The question whether status epilepticus in general damages the brain remains unresolved. While animal models support neuronal damage, there are insufficient data in humans 40. Most clinical studies emphasize that status epilepticus etiology remains the main determinant of outcome 41. Hence the main therapeutic goals should include developing strategies tailored to status epilepticus etiology and worst seizure type at onset, and consequently avoiding therapeutic harm from adverse effects. New generations of broad-spectrum anti-epileptic drugs with fewer adverse effects were not associated with increased risk of death in their cohort and are promising alternatives that may reduce the use of IV anesthetic drugs (e.g., topiramate or lacosamide) 42, 43.. In a study of refractory status epilepticus, seizures were terminated in 71% of patients within 72 hours and in 9% within 24 hours after administration of topiramate 42. However, status epilepticus termination could not be linked to topiramate, as most patients received additional nonanesthetic antiepileptic drugs and IV anesthetic drugs. While the effect of topiramate on outcome without IV anesthetic drugs remains unclear, the independent association between lacosamide and favorable short-term outcome in patients with refractory status epilepticus was detected with no differences in the use of IV anesthetic drugs in patients with and without lacosamide 43.
- Shanechi MM, Chemali JJ, Liberman M, Solt K, Brown EN. A brain-machine interface for control of medically-induced coma. PLoS Comput Biol. 2013;9(10):e1003284. doi:10.1371/journal.pcbi.1003284 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3814408[↩]
- Doyle PW, Matta BF (1999) Burst suppression or isoelectric encephalogram for cerebral protection: evidence from metabolic suppression studies. Br J Anaesth 83: 580–584.[↩]
- Hunter G, Young GB (2012) Status epilepticus: a review, with emphasis on refractory cases. Can J Neurol Sci 39: 157–169.[↩][↩]
- Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN (2012) A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci USA 109: 3095–3100.[↩]
- Parvinian B, Scully C, Wiyor H, Kumar A, Weininger S. Regulatory Considerations for Physiological Closed-Loop Controlled Medical Devices Used for Automated Critical Care: Food and Drug Administration Workshop Discussion Topics. Anesth Analg. 2017; Publish Ahead of Print. 10.1213/ANE.0000000000002329[↩]
- Ching S, Liberman MY, Chemali JJ, Westover MB, Kenny J, Solt K, et al. Real-time Closed-loop Control in a Rodent Model of Medically-induced Coma Using Burst Suppression. Anesthesiology. 2013;119.[↩]
- An J, Jonnalagadda D, Moura V, Purdon PL, Brown EN, Westover MB. Spatial variation in automated burst suppression detection in pharmacologically induced coma. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:7430–7433. doi:10.1109/EMBC.2015.7320109 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4876722[↩]
- Hocker SE, Britton JW, Mandrekar JN, Wijdicks EM, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013;70: 72–77. 10.1001/jamaneurol.2013.578[↩]
- Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011; awr215. 10.1093/brain/awr215[↩][↩]
- Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, LaRoche SM, R JJ, Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM N. C. S. S. E. G. W. Committee. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit. Care. 2012 Apr.17(1):3–23.[↩]
- An J, Jonnalagadda D, Moura V Junior, Purdon PL, Brown EN, Westover MB. Variability in pharmacologically-induced coma for treatment of refractory status epilepticus. PLoS One. 2018;13(10):e0205789. Published 2018 Oct 31. doi:10.1371/journal.pone.0205789 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6209214[↩]
- Amzica F, Kroeger D. Cellular mechanisms underlying EEG waveforms during coma. Epilepsia. 2011;52: 25–27. 10.1111/j.1528-1167.2011.03229.x[↩]
- Nita DA, Moldovan M, Sharma R, Avramescu S, Otsubo H, Hahn CD. Burst-suppression is reactive to photic stimulation in comatose children with acquired brain injury. Clin Neurophysiol. 2016;127: 2921–2930. 10.1016/j.clinph.2016.03.029[↩]
- Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50: 38–39. 10.1111/j.1528-1167.2009.02345.x[↩]
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Use of barbiturates in the control of intracranial hypertension. J Neurotrauma. 2000 Jun-Jul;17(6-7):527-30. https://doi.org/10.1089/neu.2000.17.527[↩]
- The University of Toronto head injury treatment study: a prospective, randomized comparison of pentobarbital and mannitol. Can J Neurol Sci. 1984 Nov;11(4):434-40. DOI:10.1017/s0317167100045960 https://doi.org/10.1017/S0317167100045960[↩]
- Clinical outcome and cognitive impairment in patients with severe head injuries treated with barbiturate coma. Acta Neurochir (Wien). 1992;117(3-4):153-9. DOI:10.1007/bf01400613 https://doi.org/10.1007/BF01400613[↩]
- Rossetti A., Lowenstein D. (2011) Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol 10: 922–930.[↩]
- Claassen J., Hirsch L., Emerson R., Mayer S. (2002) Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 43: 146–153.[↩][↩][↩]
- Pugin D, Foreman B, De Marchis GM, et al. Is pentobarbital safe and efficacious in the treatment of super-refractory status epilepticus: a cohort study. Crit Care. 2014;18(3):R103. Published 2014 May 21. doi:10.1186/cc13883 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4095579[↩][↩][↩]
- Newey CR, Wisco D, Nattanmai P, Sarwal A. Observed medical and surgical complications of prolonged barbiturate coma for refractory status epilepticus. Ther Adv Drug Saf. 2016;7(5):195–203. doi:10.1177/2042098616659414 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5014050[↩][↩]
- Schmutzhard E., Pfausler B. (2011) Complications of the management of status epilepticus in the intensive care unit. Epilepsia 52(Suppl 8): 39–41.[↩][↩]
- Parviainen I., Kalviainen R., Ruokonen E. (2007) Propofol and barbiturates for the anesthesia of refractory convulsive status epilepticus: pros and cons. Neurol Res 29: 667–671.[↩][↩]
- Ji T., Zubkov A., Wijdicks E., Manno E., Rabinstein A., Kotagal S. (2009) Massive tongue swelling in refractory status epilepticus treated with high-dose pentobarbital. Neurocrit Care 10: 73–75.[↩][↩]
- Hermans G., De Jonghe B., Bruyninckx F., Van den Berghe G. (2008) Clinical review: critical illness polyneuropathy and myopathy. Crit Care 12: 238.[↩]
- Miller M., Forni A., Yogaratnam D. (2008) Propylene glycol-induced lactic acidosis in a patient receiving continuous infusion pentobarbital. Ann Pharmacother 42: 1502–1506.[↩]
- Bledsoe K., Kramer A. (2008) Propylene glycol toxicity complicating use of barbiturate coma. Neurocrit Care 9: 122–124.[↩][↩]
- Zar T., Yusufzai I., Sullivan A., Graeber C. (2007) Acute kidney injury, hyperosmolality and metabolic acidosis associated with lorazepam. Nat Clin Pract Nephrol 3: 515–520.[↩]
- DeGiorgio C., Heck C., Rabinowicz A., Gott P., Smith T., Correale J. (1999) Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology 52: 746–749.[↩]
- Gunther M., Jackson J., Ely E. (2007) Loss of IQ in the ICU brain injury without the insult. Med Hypotheses 69: 1179–1182.[↩][↩]
- Sutter R, Marsch S, Fuhr P, Kaplan PW, Rüegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;82(8):656–664. doi:10.1212/WNL.0000000000000009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3945664[↩]
- Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med 2012;40:2677–2684[↩]
- Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566[↩]
- Etsten B, Li TH. Hemodynamic changes during thiopental anesthesia in humans: cardiac output, stroke volume, total peripheral resistance, and intrathoracic blood volume. J Clin Invest 1955;34:500–510[↩]
- Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med 2009;37:3024–3030[↩]
- Power KN, Flaatten H, Gilhus NE, Engelsen BA. Propofol treatment in adult refractory status epilepticus. Mortality risk and outcome. Epilepsy Res 2011;94:53–60[↩]
- Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146–153[↩]
- Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol 2005;62:1698–1702[↩]
- Ferguson M, Bianchi MT, Sutter R, et al. Calculating the risk benefit equation for aggressive treatment of non-convulsive status epilepticus. Neurocrit Care 2013;18:216–227[↩]
- Scantlebury MH, Heida JG, Hasson HJ, et al. Age-dependent consequences of status epilepticus: animal models. Epilepsia 2007;48(suppl 2):75–82[↩]
- Sokic DV, Jankovic SM, Vojvodic NM, Ristic AJ. Etiology of a short-term mortality in the group of 750 patients with 920 episodes of status epilepticus within a period of 10 years (1988-1997). Seizure 2009;18:215–219[↩]
- Hottinger A, Sutter R, Marsch S, Ruegg S. Topiramate as an adjunctive treatment in patients with refractory status epilepticus: an observational cohort study. CNS Drugs 2012;26:761–772[↩][↩]
- Sutter R, Marsch S, Rüegg S. Safety and efficacy of intravenous lacosamide for adjunctive treatment of refractory status epilepticus: a comparative cohort study. CNS Drugs 2013;27:321–329[↩][↩]