What is intraocular pressure
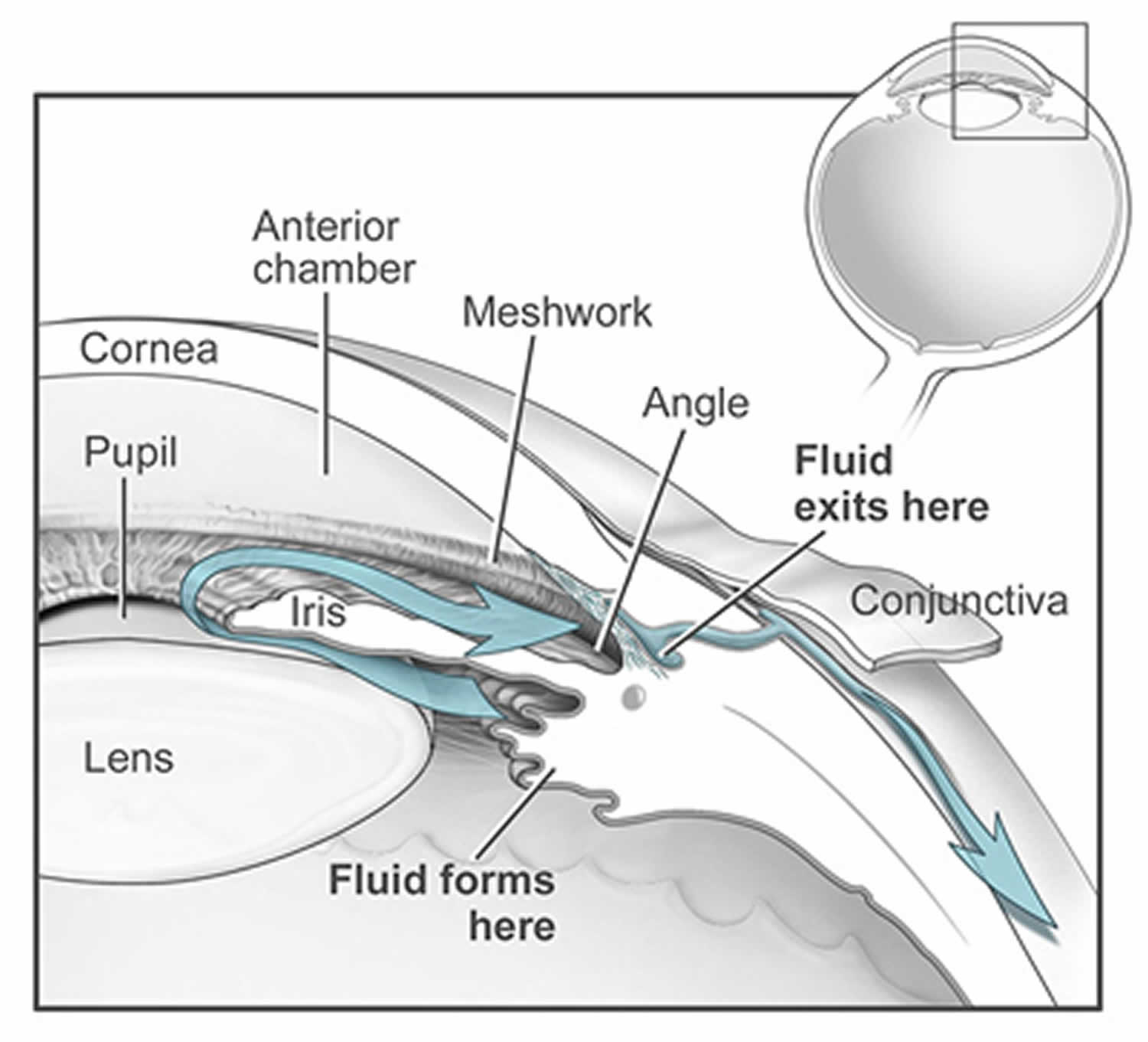
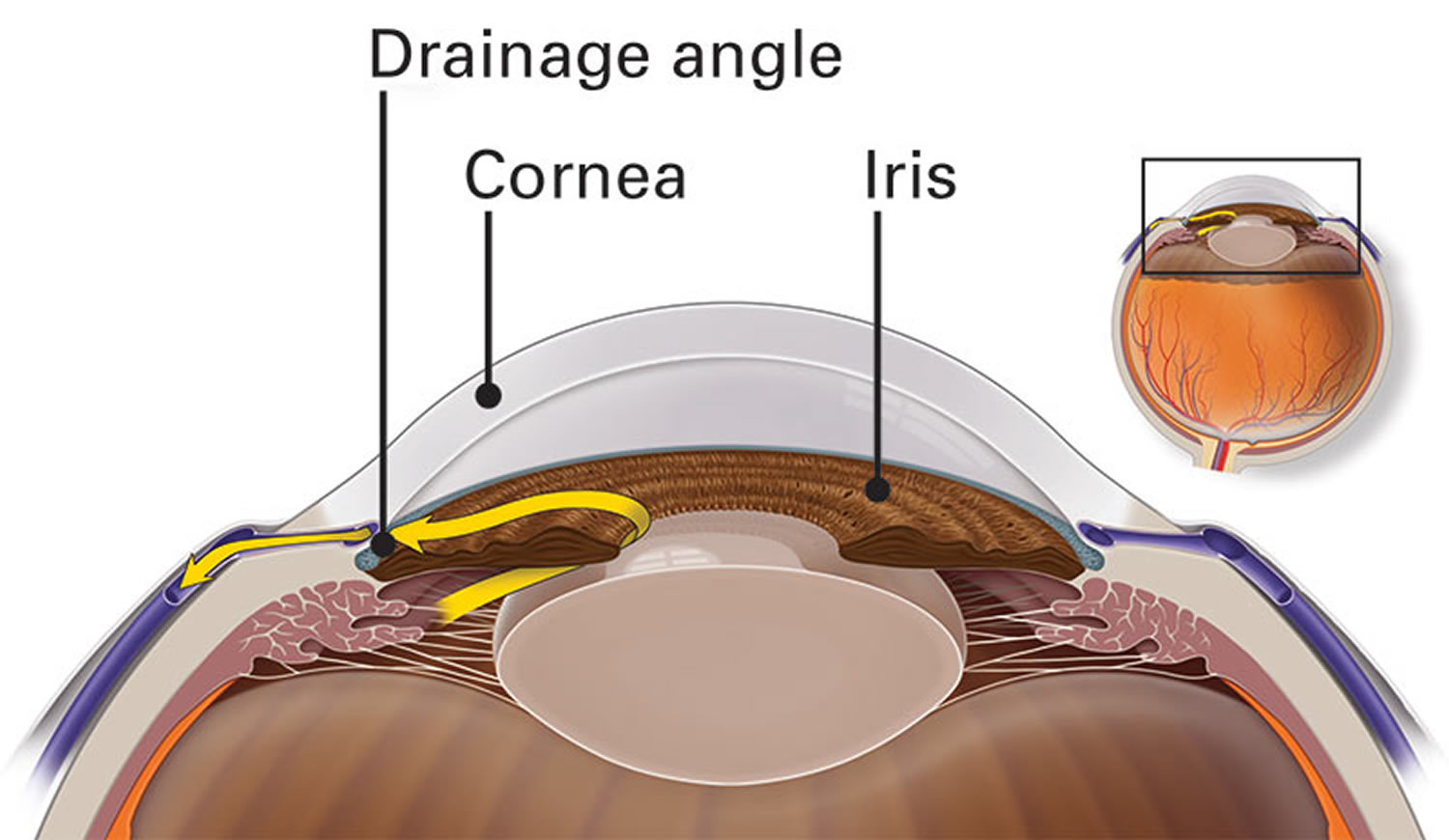
The space in your eye that is behind the cornea and in front of the iris is called the anterior chamber. The anterior chamber is filled with a water-like fluid called the aqueous humor, which nourishes the cornea and the lens, providing oxygen and vital nutrients. The aqueous humor also provides the necessary pressure to help maintain the shape of your eye. To maintain a constant eye pressure, aqueous humor also drains from the eye in an area called the drainage angle. Doctors call this intraocular pressure or IOP. As you will read, maintaining the right amount of pressure within the eye is very important to protecting your vision. Measuring the intraocular pressure (IOP) is one of the ways your eye doctor tests for glaucoma.
Intraocular pressures (IOP) are typically less than 20 mm Hg. However, that pressure measurement can be falsely elevated by a thicker, and potentially more rigid cornea. Crucially, intraocular pressure is not fixed, but varies considerably during the 24-hour cycle and between one visit and another 1. Indeed, a single intraocular pressures (IOP) measurement during so-called office hours is a poor surrogate of the entire intraocular pressure profile of a patient with glaucoma 2. Consequently, a single intraocular pressure (IOP) measurement during so-called office hours is insufficient to characterize the real intraocular pressure pathology of a patient with glaucoma. Raised intraocular pressure (IOP) has been well recognized as a major risk factor for glaucoma, and intraocular pressure reduction is an effective treatment to prevent or slow glaucoma progression. The Ocular Hypertension Treatment Study found that study patients with elevated intraocular pressures (IOP) greater than 24 mm Hg and thicker corneas, were dramatically lower risk of developing glaucoma than those patient with elevated pressures and normal or thin corneas.
Figure 1. Increased intraocular pressure
Figure 2. High intraocular pressure (glaucoma)
Glaucoma suspects are adults demonstrating findings consistent with an increased risk for glaucoma development in at least one eye 3. Such findings include an enlarged cup (taking into account optic disc size) or asymmetric cup–disc ratios, notching or narrowing of the neuroretinal rim, visual field changes commensurate with glaucoma, or intraocular pressure readings above the statistically accepted upper limit (over 21 mmHg) 3. The last of these is particularly important since the possibility of glaucoma development is significantly correlated with an elevated intraocular pressure whereas glaucoma may progress faster in patients with higher levels of intraocular pressure 4. Glaucoma suspects actually outnumber patients with established glaucoma in the average ophthalmologist’s practice and comprise the majority of patients receiving anti-glaucomatous medications in the USA, implying that adequate intraocular pressure monitoring strategies are required for their management 5.
Importantly, short- and long-term intraocular pressure fluctuation influenced by a variety of physiological factors including blood pressure and heart rate, breathing, accommodation, eyelid blink, pupillary size, eye movement, central venous pressure (Valsalva maneuver), ingestion of water and osmolarity, sleep, postural changes, physical activity and hormonal factors all need to be borne in mind [30]. Moreover, temperature-related and seasonal variations in intraocular pressure have also been reported 6. A single office intraocular pressure measurement of a glaucoma suspect only provides a snapshot in the continuum of intraocular pressure change. However, since the intraocular pressure is considered the primary cause of glaucoma development, the decision to treat glaucoma suspects rests heavily on the intraocular pressure level documented during follow-up. Thus, an apparently low level of recorded intraocular pressure may prove misleading and may mask the real risk of glaucoma development. On the other hand, an erroneously high level of intraocular pressure may lead to unnecessary administration of medication with potentially toxic effects on a long-term basis 7. Therefore, to obtain a true picture of intraocular pressure levels for a glaucoma suspect, several intraocular pressure readings are required 8. While a diurnal intraocular pressure curve may suffice in most cases, a complete 24-hour or 48-hours curve may be needed in selected cases to unmask periods of exposure to higher levels of intraocular pressure during the night 8.
The current methodology for recording diurnal fluctuations of intraocular pressure involves repeated measurements by applying conventional applanation tonometry with the patient in a sitting or horizontal posture (“phasing”) 5. Consequently, they are unable to detect dynamic variations of intraocular pressure in response to a wide range of daily activities (such as Valsalva manoeuvres or touching and rubbing the eyes) or by truly measuring sleeping pressure 9. Efforts to monitor intraocular pressure conventionally during the day or the night may disrupt daily activities and normal sleep patterns. More importantly, isolated intraocular pressure measurements may not be sufficient to truly identify continuing intraocular pressure changes.
To address these issues, a variety of intraocular or extraocular devices with integrated pressure sensors have been developed and proposed for continuous intraocular pressure monitoring 10. The obvious advantage of these devices apart from the possibility of continuous intraocular pressure monitoring is that they do not interrupt physiologic rhythms, thus providing better information on the “natural history” of intraocular pressure changes in the individual patient. Such designs include radiotelemetry from a device applanating the inferior sclera 11, various concepts of contact lens embedded piezoelectric sensors 12, tracking secondary speckle pattern trajectories produced by the reflection of an illuminating laser beam from the iris or the sclera 13 and various proposals for surgically implanted sensors with capabilities for external signal recording through telemetry. The first implantable device proposed by Collins in 1967 included a gas bubble encapsulated in a thin and flexible film 9. Since then several other designs have been proposed such as an elastic band placed around the globe equator 14, a subchoroidal implant 15 or a variety of sensor-integrated intraocular lens designs 16. Although the level of agreement of all these devices with standard Goldmann applanation tonometry varies 9, they may be of value in earlier glaucoma diagnosis and in the identification of true intraocular pressure characteristics and better delineation of the risk for glaucoma progression by revealing hidden peaks of intraocular pressure in glaucoma suspects.
Although the theoretical value of obtaining more information on the continuous changes of intraocular pressure may be important, the optimal practical way to take advantage of this increased amount of information is currently not known 9. Continuous intraocular pressure monitoring may reveal higher intraocular pressure peaks than isolated measurements, but the mean intraocular pressure should be roughly the same for both strategies and currently there is no consensus on whether the mean or peak intraocular pressure is the most relevant determinant factor for glaucoma diagnosis 4. Moreover, associated factors such as the long-term role of intraocular pressure nyctohemeral fluctuations and the significance of the nocturnal intraocular pressure rise in glaucoma progression has not so far been sufficiently investigated 9, although a previous study has reported that the best time to catch the peak intraocular pressure in glaucoma suspects is in the period from early morning until mid-afternoon, and the best time to record the minimum intraocular pressure would be from the late evening until past midnight 5. Moreover, the common practice of hospitalizing patients to perform phasing of the intraocular pressure may underestimate the intraocular pressure, since evidence has shown that intraocular pressure recordings are consistently lower during hospitalization than after discharge from the hospital 17.
Since the ideal strategy for glaucoma detection should be tailor-made for each case, the goal of intraocular pressure monitoring should be to create a more comprehensive, individualized pressure profile for each glaucoma suspect, identifying his or her individual patterns of intraocular pressure peaks and troughs, and focusing on these time points for the early detection of unacceptable intraocular pressure characteristics, necessitating timely intervention. Taking into account that glaucomatous optic nerve damage results from a combination of pathogenetic factors, measurements could be expanded to additional physiological parameters, such as the continuous recording of ocular perfusion and breathing patterns, which may play an even more critical role than intraocular pressure in glaucoma development and progression 18. Concomitant ophthalmic conditions predisposing to different forms of glaucoma should also be taken into consideration. The concept of obtaining better evidence on diurnal or 24-h intraocular pressure characteristics (peak, mean and fluctuation) in glaucoma suspects may be particularly important in patients with narrow or occludable angles, with a view to detecting earlier subclinical repeated episodes of asymptomatic acute intraocular pressure elevation 19. Furthermore, ophthalmic conditions predisposing to the development of secondary open-angle glaucoma with potentially non-linear and fast progression, such as exfoliation syndrome or pigment dispersion syndrome, should be taken into account in any intraocular pressure monitoring strategy, while clinicians need to have a lower threshold of concern for glaucoma suspects with exfoliation syndrome and pigment dispersion syndrome, obtaining more pressure measurements in these patients [92]. In any case, our management strategy with glaucoma suspects must aim at detecting the thin line that separates the indication for observation from the indication for treatment. It is not an easy task and certainly requires gathering as much useful intraocular pressure information as possible. A key role for clinicians is to accurately translate diurnal intraocular pressure characteristics into risk for glaucoma progression. For this purpose, the development and targeted use of future technologies enabling reliable and continuous intraocular pressure monitoring may be of great diagnostic value.
Intraocular pressure measurement
Eye pressure is a very important measurement for ophthalmologists (eye doctors) to use when evaluating your eye health.
Types of intraocular pressure measurement:
- Applanation Tonometry
- Pneumatonometry
- Rebound Tonometry
- Air-Puff Tonometry
- Other Forms of Tonometry
Applanation Tonometry
There are many different ways to measure eye pressure. One such method is a painless procedure, called “applanation tonometry.” The term applanate means to flatten. In most ophthalmologist’s offices, eye pressure is measured using “Goldmann applanation tonometry,” and this is considered a “gold standard” eye pressure measurement.
In this test, the eyes are anesthetized with numbing drops. In addition, a small amount of non-toxic dye is placed in the eye. Your ophthalmologist will instruct you to position your head into a device called the slit lamp. Then, a small tip gently touches the surface of the eye and the eye pressure is measured. The eye pressure is measured based on the force required to gently flatten a fixed area of the cornea.
This test can be affected by various conditions, such as when there is too much or too little dye present in the eye, or depending on the thickness or thinness of the cornea. For example, we know that in thin corneas, which can occur naturally or be induced because a patient has had laser correction eye surgery, the eye pressure measured with this device can be artificially low. In addition, naturally thin corneas are an independent risk factor for glaucoma, so measurement of corneal thickness is part of a comprehensive eye exam and should be performed at your initial and/or subsequent visits.
Pneumatonometry
Alternative measurement methods include pneumatonometry, which is particularly useful in cases of scarred corneas, but can also be used as part of routine practice. In this case, numbing drops are also used but no dye is instilled. The pneumatonometer will also print out a tracing so that the quality of the measurement can be assessed. Pneumatonometry is thought to be less influenced by corneal thickness.
Rebound Tonometry
Rebound tonometry is yet another form of eye pressure measurement. A small plastic-tipped probe bounces gently against the cornea. Numbing drops are not required for this measurement, and there are devices, such as iCare, that can be used at home. It is a portable device and easy to use with children or patients who cannot comply with the more traditional eye pressure measurement techniques. Another device that is portable and easy to use is the Tonopen, a form of electronic indentation tonometry. However, in contrast to iCare, numbing drops are required in order to use the Tonopen.
Air-Puff Tonometry
Non-contact tonometry (or air-puff tonometry) is a form of eye pressure measurement that uses a rapid air pulse to applanate (flatten) the cornea. Although there is some controversy about its accuracy, modern devices have been shown to correlate with Goldmann tonometry.
Other Forms of Tonometry
In addition to the methods of eye pressure measurement mentioned above, there are other forms that you may encounter and also new methods that are being developed. It is important to remember that when you have your eye pressure checked once to 4 or 6 times per year, we are only capturing your eye pressure with a very small “snapshot.” It is known that eye pressure varies over the course of the day, and that these eye pressure fluctuations may be harmful, especially for glaucoma patients. Thus, novel methods have been developed to measure eye pressure over 24 hours, for example, a contact lens that contains an eye pressure sensor. As of yet these devices are not a part of routine care, but stay tuned for future innovation in this important area of glaucoma care.
What is normal intraocular pressure
Intraocular pressures (IOP) is measured in millimeters of mercury, like the way a thermometer measures temperature using mercury. Normal eye pressure is usually considered to be between 10 and 20 millimeters of mercury (mmHg). Having eye pressure that’s too low or too high can damage your vision.
Elevated Intraocular pressures with no other symptoms is ocular hypertension. Some people can have higher eye pressure with no damage. Other people may lose vision even if the pressure is in the normal range.
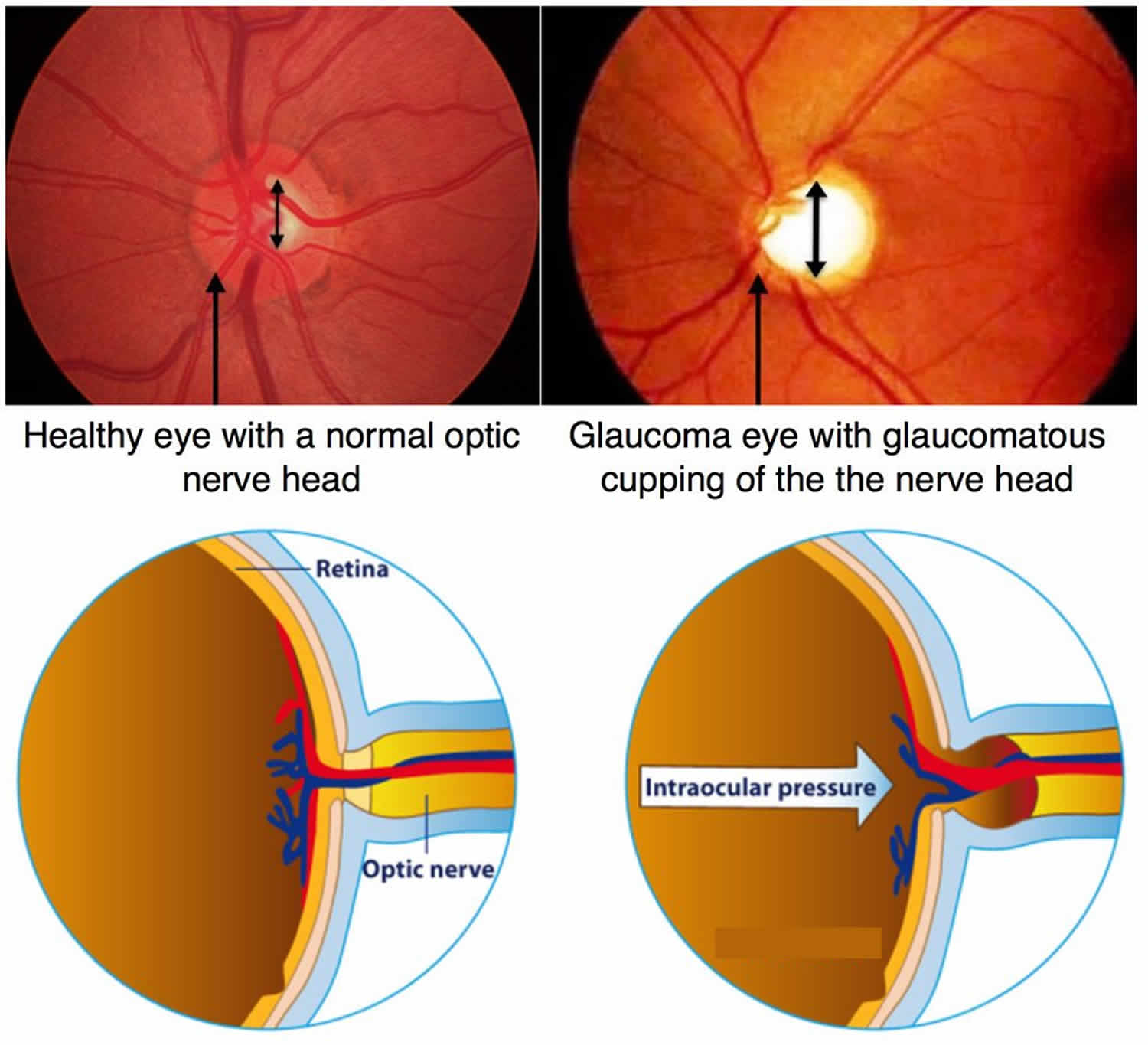
When someone has glaucoma, eye pressure damages the optic nerve. This damage permanently reduces vision. If glaucoma is not treated, it can lead to total blindness.
Intraocular pressure monitoring strategies in glaucoma and their impact on prognosis and treatment
One of the major limiting factors in the treatment of glaucoma is the lack of robust intraocular pressure data collection capabilities. This limitation influences every step of our care for those suffering from glaucoma. At the time of diagnosis, we frequently make treatment decisions based on one or two intraocular pressure measurements that take place during clinic hours. Subsequently, assessment of treatment success or failure is often completed during the same office hours while relying on one or two measurements representing a small snapshot of the pressure experienced by the eyes in any given day. Perhaps more concerning is the fact that advancement of treatment is often decided upon in similar fashion, based on a target pressure that was set with incomplete information. It is not uncommon for patients to undergo surgery on the basis of snapshot intraocular pressure measurements that appear out of range from a pre-set goal pressure. In essence, eye doctors begin treatment of glaucoma on the basis of limited information, and therapy is maintained or altered on the basis of spot checks of equally limited data. Clearly, there is much room to enhance how eye doctorsapproach their decision-making, and efforts are underway to incorporate new algorithms for decision-making into everyday practice.
Ideally, physicians would have available an extensive intraocular pressure data set that spans a prolonged period. These data would include maximum and minimum values along with diurnal variability of pressures for many consecutive days 20. Continuous monitoring would be an ideal, and connecting the intraocular pressure profile with activities of daily living would add more in-depth information to help guide treatment decisions. For example, it would be of great value to know if a specific eye experiences pressure elevation during the nocturnal period but never during the diurnal period. Similarly, knowing if intraocular pressure elevation is related to a specific activity, playing the trumpet for example, could certainly influence patient education and treatment decisions. Unfortunately, we currently lack a method of intraocular pressure monitoring that is both continuous and reliable, and our efforts must be relegated to episodic measurements that often take place in the physician’s office, in hospital or units called sleep laboratories. These limitations have resulted in a “one size fits all” therapy approach rather than one tailored to address individual peaks, troughs and individual patterns that are influenced by both disease and activities of daily living.
Given the limited ability to measure and monitor intraocular pressure, and to obtain robust data from each individual eye, how can eye doctors alter their current practice to achieve more informed decisions for their patients? Eye doctorscould begin by making initial treatment decisions based on several intraocular pressure measurements at various time points within usual office hours 21. If diagnosis of glaucoma or ocular hypertension is made in the early morning hours, any treatment decision can be delayed until the patient returns on a subsequent day for an afternoon visit to get the most crude data set consisting of two time points and different times during the day. Alternatively, the patient may return for full diurnal plotting of pressure, by spending the day in clinic and undergoing measurements every 1–2 hours from morning through to late afternoon. The next level of vigilance would involve both diurnal and nocturnal plotting of intraocular pressure that usually involves an overnight stay for the patient in a facility that can accommodate such rigour (e.g. a formal sleep laboratory). It would be obvious to any busy clinician contemplating this information that it is not practical to implement these ideas on a regular basis given the time and expense related to running efficient practices as well as poor patient acceptance of the time required to obtain these data.
Practically speaking, there are a few practice patterns that can be implemented by most clinicians with relative ease and little expense or burden. First, unless there is an urgent need to lower intraocular pressure, treatment decisions can be delayed for one or more visits in order to obtain a clearer understanding of visit-to-visit variability as well as time of day variability in intraocular pressure. Decisions regarding efficacy of the chosen therapy as well as the need to escalate therapy can be similarly delayed for several visits to collect more robust and actionable data. In those patients who appear to have advancing disease that does not correlate with the data obtained during regular office visits, the decision can be made to first obtain a diurnal pressure curve during regular office hours. Patients going through this more rigorous data collection process would represent a small fraction of the overall patient population and would thus exert a lesser burden on any given practice. Finally, for those patients who require even more information, for example a patient with advancing disease despite what appears to be controlled pressure on one or two diurnal curve measurements, a lengthier stay in the office into evening hours or a visit to a sleep laboratory for nocturnal pressure checks might be in order. Patients with ocular hypertension often do not require the rigour of multiple checks that might be required in advancing late-stage glaucoma. Similarly, patients with labile secondary glaucomas may benefit from repeated measures on different days to better understand the intraocular pressure cycling that might be at play. By using this staged approach for the amount of rigour implemented for any given patient, physicians will be able to make decisions based on more complete data while limiting the impact of the exorbitant time required obtaining such information.
References- 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Quaranta L, Katsanos A, Russo A, Riva I. Surv Ophthalmol. 2013 Jan-Feb; 58(1):26-41.
- Konstas AG, Kahook MY, Araie M, et al. Diurnal and 24-h Intraocular Pressures in Glaucoma: Monitoring Strategies and Impact on Prognosis and Treatment. Adv Ther. 2018;35(11):1775–1804. doi:10.1007/s12325-018-0812-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6223998
- Glaucoma: Definitions and Classification. https://www.aao.org/disease-review/glaucoma-definitions-classification
- Konstas AGP, Katsanos A, Quaranta L, Mikropoulos DG, Tranos PG, Teus MA. Twenty-four hour efficacy of glaucoma medications. Prog Brain Res. 2015;221:297–318.
- Tajunisah I, Reddy SC, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol. 2007;245:1851–1857
- Blumenthal M, Blumenthal R, Peritz E, Best M. Seasonal variation in intraocular pressure. Am J Ophthalmol. 1970;69:608–610.
- Detorakis E, Symvoulakis E. Over-diagnosed glaucoma:possible consequences for patients and health care services. Hippokratia. 2011;15:381–382
- McMonnies CW. The importance of and potential for continuous monitoring of intraocular pressure. Clin Exp Optom. 2017;100:203–207.
- Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res. 2016;55:108–148.
- Konstas AGP, Topouzis F, Leliopoulou O, et al. 24-hour intraocular pressure control with maximum medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology. 2006;113(761–765):e1
- Cooper RL, Beale DG, Constable IJ, Grose GC. Continual monitoring of intraocular pressure: effect of central venous pressure, respiration, and eye movements on continual recordings of intraocular pressure in the rabbit, dog, and man. Br J Ophthalmol. 1979;63:799–804.
- Twa MD, Roberts CJ, Karol HJ, Mahmoud AM, Weber PA, Small RH. Evaluation of a contact lens-embedded sensor for intraocular pressure measurement. J Glaucoma. 2010;19:382–390.
- Margalit I, Beiderman Y, Skaat A, et al. New method for remote and repeatable monitoring of intraocular pressure variations. J Biomed Opt. 2014;19:027002
- Wolbarsht ML, Wortman J, Schwartz B, Cook D. A scleral buckle pressure gauge for continuous monitoring of intraocular pressure. Int Ophthalmol. 1980;3:11–17
- Rizq RN, Choi WH, Eilers D, Wright MM, Ziaie B. Intraocular pressure measurement at the choroid surface: a feasibility study with implications for implantable microsystems. Br J Ophthalmol. 2001;85:868–871
- Todani A, Behlau I, Fava MA, et al. Intraocular pressure measurement by radio wave telemetry. Invest Ophthalmol Vis Sci. 2011;20(52):9573–9580
- Hyams SW, Bergman D, Keroub C. The effect of hospitalization on intraocular pressure. Am J Ophthalmol. 1982;94:519–521
- Kanadani FN, Moreira T, Bezerra B, et al. Diurnal curve of the ocular perfusion pressure. J Curr Glaucoma Pract. 2016;10:4–6.
- Sihota R, Rao A, Gupta V, Srinivasan G, Sharma A. Progression in primary angle closure eyes. J Glaucoma. 2010;19:632–636.
- Quaranta L, Katsanos A, Russo A, Riva I. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv Ophthalmol. 2013;58:26–41
- Konstas AGP, Quaranta L, Bozkurt B, et al. 24-h efficacy of glaucoma treatment options. Adv Ther. 2016;33:481–517