What is iontophoresis
Iontophoresis is a procedure in which an electrical current is passed through skin soaked in tap water or normal saline (0.9%), allowing ionized (charged) particles to cross the normal skin barrier. Iontophoresis is a method of non-invasive transdermal drug delivery based on the transfer of charged molecules using a low-intensity electric current 1. Iontophoresis has been used to reduce sweating and enhances the delivery of drugs and macromolecules into and through the skin. Iontophoresis is safe, effective and inexpensive.
Battery-powered direct current (DC) and mains-powered alternating current (AC) iontophoresis devices are available. Individual machines vary, and the instructions provided by the manufacturer should be followed carefully.
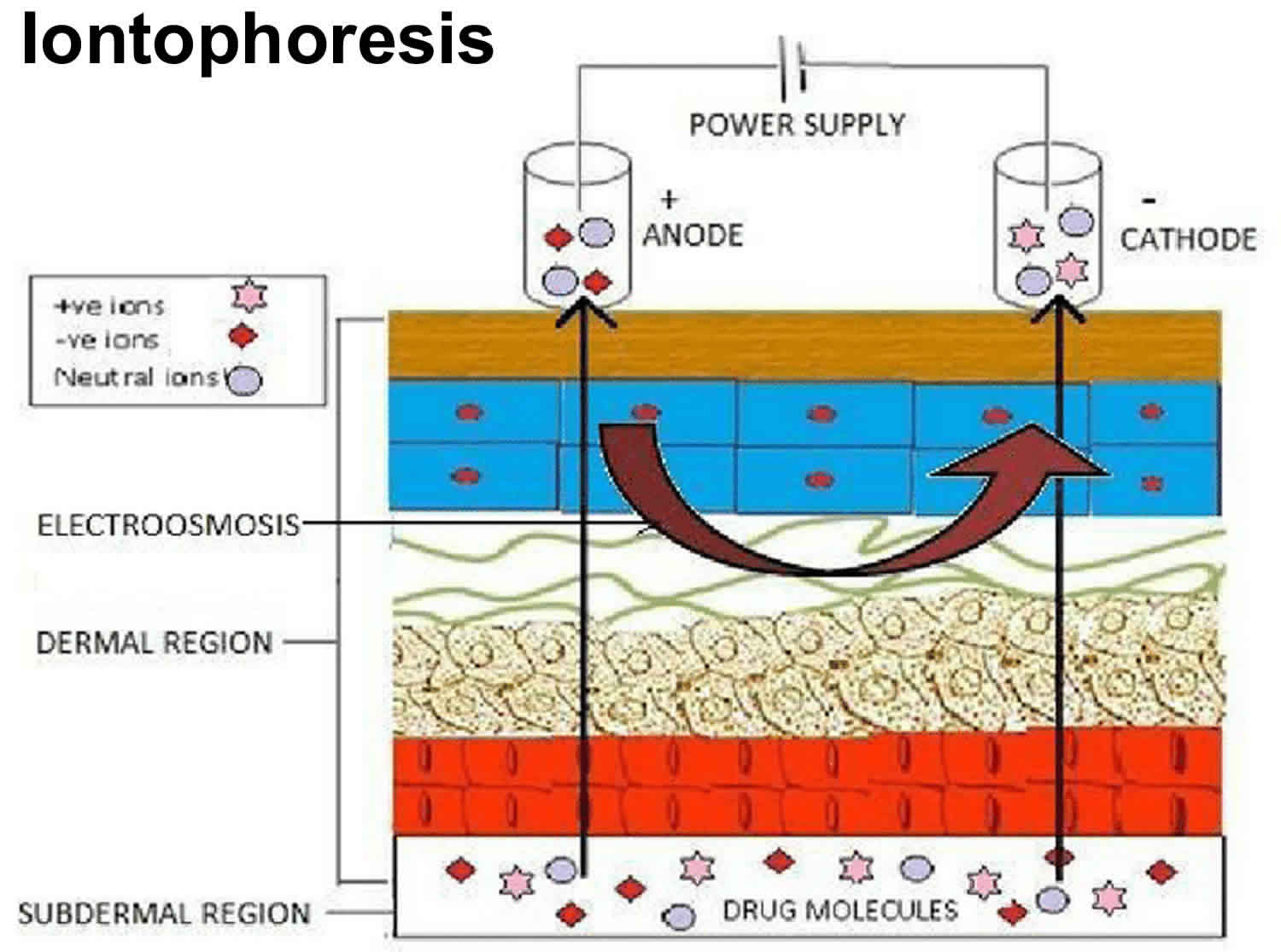
Two mechanisms are involved in iontophoretic transport. Electromigration (also referred to as electrorepulsion) is the movement of ions across a membrane (i.e. the skin) under the direct influence of an electric field. Negatively charged drugs are therefore repelled into the skin under the cathode, whereas the transfer of positively charged drugs occurs under the anode (Figure 1). The second mechanism is called electro-osmosis, which can be schematized as the volume flow induced by the current flow. As the isoelectric point (pI) of human skin is around 4–4.5, which is below its pH in physiological conditions, the skin will be charged negatively. Application of an electric field across the skin will therefore favour the movement of cations. Therefore, volume flow will be directed in the anode-to-cathode direction, facilitating the transport of positively charged drugs (Figure 1). Electro-osmosis also allows the diffusion of neutral molecules with anodal iontophoresis. The respective part of the transfer explained by electromigration or electro-osmosis depends mostly on the physicochemical properties of the molecules and the polarity of the applied current.
For palmar hyperhidrosis, each hand is placed in a tray of water that contains the electrodes. It is recommended that petroleum jelly is applied to the skin at the water-line, and to any small cuts or wounds to prevent discomfort and localized inflammation. The device is switched on so that current passes through the water between the electrodes. The initial current should be very low, and then adjusted according to tolerance.
Initially treatment is undertaken for 20–30 minutes every 1–3 days until the desired effect is achieved, and then reduced to once per week to maintain the result. It is up to the patient to find what treatment regime works best for them long term to maintain results.
Figure 1. Iontophoresis
Footnote: Schematic representation of iontophoretic transport. Positively charged drugs (+ve) migrate under the anode, whereas negatively charges drugs (-ve) migrate under the cathode. The black arrows represent anodal and cathodal electromigration. The red arrow represents electro-osmosis.
Iontophoresis as drug delivery module
The potential of iontophoresis has been exploited for the transdermal delivery of many drugs with poor penetration properties e.g., high molecular weight electrolytes such as proteins, peptides and oligonucleotides which are normally difficult to administer except through parenteral route. It also offers a great potential for the delivery of charged peptides used as drugs. Although iontophoresis has been able to achieve significant increase in the transdermal absorption of many drugs, it has not been able to show significant permeation of larger peptides like insulin 2.
An iontophoretic drug delivery system has these basic components:
- An energy source of electronic current, which usually consists of a battery and controlled electronics;
- An active reservoir, which contains the ionic therapeutic agent; and
- An indifferent or return reservoir system, which contains an electrolyte and serves to complete the electric circuit.
- Also a control system, to monitor the overall process.
When the active and indifferent reservoir systems are placed on the skin, the current source causes electronic current to flow to the active reservoir where the electronic current is transformed into ionic current. The ionic current flows through the active reservoir, through the skin, beneath the skin towards the indifferent reservoir, and back through the skin into the indifferent reservoir. At the indifferent reservoir, it is transformed back into electronic current, completing the circuit at the opposite pole of the current source 3.
Factors affecting iontophoresis transport system
Human skin is not all the same. There are numerous differences among patient groups as well as between various regions of the body, age and ethnicity. Various factors have been shown to affect the results of iontophoresis. The following factors have to be considered 4 because they may improve the delivery from the device and drug release kinetics. One of these factors, regional blood flow (dermal blood supply), determines the systemic and underlying tissue solute absorption. Blood supply, however, does not appear to affect the drug penetration fluxes through the epidermis during iontophoretic delivery. Cross and Roberts showed that solute in the upper layer of the skin following iontophoresis was comparable in anaesthetized rats and sacrificed rats. It can thus be presumed that the blood did not affect the penetration through the epidermis since the latter has no blood supply 5. Condition of skin also affects the penetrating properties of permeant. Roberts et al., studied the in-vivo passive diffusion of methyl salicylate using skin from different areas of the human body and observed the following rank order: abdomen > forearm > instep > heel > planter, for all subjects.
Drug concentration is another factor influencing iontophoretic transfer 1. However, while in some cases there is an almost linear relationship between concentration and flux, the flux often reaches a plateau as the concentration increases, which means that above a given concentration there is a saturation in the iontophoretic transport 6. The quantity of charge, the skin area and the drug concentration are experimental variables that can be adjusted to modify the electrophoretic transfer.
Other parameters, such as the physicochemical properties of the molecule, cannot easily be influenced by the operator. The size, the partition ratio and, of course, the charge of the molecule are of primary importance 7. In general, small and hydrophilic molecules are transported at a faster rate than larger, lipophilic molecules 7. Nonetheless, this rule is not exclusive, because larger molecules, such as peptides, can be administered iontophoretically 8. In the same way, iontophoresis of rather lipophilic drugs is possible (e.g. treprostinil log octanol/water partition coefficient is 4) 9.
The pH of the solution is of primary importance, because it will determine the ionization of the compound 1. Indeed, according to the Henderson–Hasselbalch equation for weak acids, the ionic fraction increases with increasing pH of the solution. On the contrary, undissociated weak base increases with increasing pH. Therefore, one can calculate the percentage of ionization if the pKa of the compound (or its pI when there are several weakly acidic or basic groups) and the pH of the solution are known. Practically, the pH must be carefully set and controlled to ensure that the drug is ionized, while preserving skin integrity.
Figure 1. Factors affecting iontophoresis transport system
[Source 10 ]Iontophoresis treatment
The main use of iontophoresis is to treat focal areas of excessive sweating (hyperhidrosis), particularly on the palms or soles. Tap water iontophoresis is less effective in the axilla (armpit).
Limited robust data is available; however some studies suggested that up to 80-85% of patients with palmar hyperhidrosis notice subjective improvement in their symptoms within 2–4 weeks. One study showed that 33% of sole sites, and 37.5% of axillae sites had improved after 14 days, which increased to 78%, and 75% respectively over 20 days. Delivering antiperspirants or botulinum toxin A via iontophoresis has not been shown to be superior to tap water alone.
Iontophoresis has also been successfully used to deliver drugs to the skin in order to:
- Reduce sweating further using botulinum toxin A.
- Anesthetize an area of skin with lidocaine.
- Treat fungal infection of nail plate (onychomycosis).
- Eradicate infection due to resistant micro-organisms using silver ions
- Treat bursitis or tendonitis with anti-inflammatory drugs.
Iontophoresis for hyperhidrosis
The mechanism of action of iontophoresis in reducing sweating is not completely understood. Sweat forms in response to an electrical gradient produced by sympathetic nerve activity on the cells of the sweat gland. There are several theories as to how a change in electrical gradient reduces sweat production.
- Ions produced by iontophoresis may physically block the sweat ducts in the stratum corneum.
- The external electrical current may disrupt normal sympathetic nerve transmission.
- The pH drops in the sweat gland due to an accumulation of hydrogen ions.
Iontophoresis for hyperhidrosis is usually carried out with ordinary tap water, however, an electrolyte solution or drugs (eg an anticholinergic agent such as glycopyrronium bromide) can be added if the water alone is not effective.
Iontophoresis physical therapy
Iontophoresis of acetylcholine and sodium nitroprusside, when combined with laser Doppler, have been used as markers of microvascular endothelium-dependent and -independent vasodilatation, respectively 11. Several decades before being used as reactivity tests, iontophoresis had known therapeutic applications, particularly in physical therapy and dermatology 12. Indeed, tap water has been used in the treatment of palmar-plantar and axillary hyperhidrosis 13. In the same way, the current itself may enhance wound healing 14, as exploited in electrotherapy. In this section, however, we will only focus on iontophoretically delivered drugs approved by regulatory agencies or undergoing clinical development. Iontophoresis may be used as a route of administration for systemic drugs or as local therapy. Indeed, cutaneous iontophoretic delivery is faster than the usual topical routes and allows the administration of high concentrations in the target tissue while limiting systemic toxicity.
Iontophoresis medication
Systemic drugs
Fentanyl
Iontophoretic delivery of fentanyl, an opioid analgesic, through a patient-controlled transdermal system (Ionsys®; Janssen, Beerse, Belgium) was approved in 2006 in Europe for the management of acute moderate to severe postoperative pain. Unlike lidocaine, fentanyl is not used topically but as a systemic drug, and iontophoresis allows on-demand administration 15. In a large prospective, randomized, unblinded, controlled trial comparing iontophoresis of fentanyl with conventional patient-controlled analgesia with morphine, the investigators did not show any difference in efficacy 16, which was confirmed by analysis of pooled data from three trials 17. However, although not statistically different, withdrawals because of inadequate analgesia were fewer in the intravenous patient-controlled analgesia morphine group than in the fentanyl iontophoresis group (10.3 and 15.2%, respectively) 16.
However, technical issues concerning the device led to a suspension of marketing authorization by the European Medicines Agency in January 2009.
Treatment of migraine
Among the drugs in the pipeline which could lead to new marketing applications soon, agonists of the 5-HT1 family receptors used as anti-migraine agents (i.e. triptans) have raised interest. Indeed, subcutaneous (s.c.) administration of sumatriptan leads to a rapid but transient effect, whereas oral or nasal administration suffers from poor bioavailability. The pharmacokinetics of iontophoretically delivered sumatriptan (4 mA for 1 h followed by 2 mA for 3 h) in healthy subjects showed comparable concentration over time areas under the curve to the s.c. route 18, but the maximal concentration was about 30% of that by the s.c. route, whereas time to maximum plasma concentration was 5.6- to 8.3-fold that of the s.c. route 19. Given that it avoids patient exposure to a rapid increase and high plasma concentrations of sumatriptan in comparison to s.c. administration, iontophoresis may reduce typical triptan-related adverse events (i.e. chest tightness, chest heaviness, paresthesias and sedation/fatigue/malaise) 20. A new drug application for an iontophoretic device containing sumatriptan (Zecuity®) has been approved by the US Food and Drug Administration (FDA) in January 2013 1.
Other iontophoretically delivered 5-HT1 agonists, such as zolmitriptan or almotriptan, have been studied recently; to date, only preclinical data are available and suggest that these molecules are appropriate candidates for iontophoresis 21.
Other potential applications
Iontophoresis has been suggested as a route of administration for nicotine in smoking cessation 22.
Recent investigations in animals highlighted the potential of iontophoresis of ranitidine 23 or phenobarbital 24 in paediatric patients.
Finally, administration of proteins has raised interest in the past few years. Iontophoresis of insulin has been extensively studied since the mid-1980s, but a low penetration rate precluded its consideration for therapeutic use 25. Recent improvements in penetration enhancement techniques and in formulation (i.e. nanovesicles) are encouraging 26. Iontophoretic delivery of other proteins, such as calcitonin, luteinizing hormone-releasing hormone or vasopressin, has been also investigated in the late 1990s 25, but no clinical applications have emerged yet from these experiments.
Local treatment
Lidocaine/epinephrine
Lidocaine combined with epinephrine was approved by the FDA as a local anaesthetic in 2004 (Lidosite®). Combination with epinephrine aims at decreasing skin blood flow, thus reducing skin clearance of the drug and consequently increasing the dermal concentration 27 and prolonging the anaesthetic action of lidocaine in a dose-dependent manner 28. The current density of Lidosite® is 0.35 mA cm−2, and it is applied for 10 min with a patch electrode pH of 4.5, at which both lidocaine and epinephrine are positively charged 29.
In a randomized, open-label, crossover study conducted in children undergoing repeated procedures requiring peripheral intraveous access, pain relief after 10 min of lidocaine/epinephrine iontophoresis (Iontocaine®) was similar to that found after 60 min of local anaesthesia with lidocaine/prilocaine cream 30. Two of 22 children discontinued iontophoresis before the complete dose was delivered because of intolerable tingling, itching and discomfort from the procedure 30. In a randomized, double-blind study comparing iontophoresis of lidocaine/epinephrine with that of placebo, erythema at the site of iontophoresis was observed in about 50% of adults and in 60% of children, whatever the group. In most cases, it was mild, but still detectable after 24 h in about 6.5% of the adults 31. Additional adverse events included mild edema, itching and urticaria 31.
The pharmacokinetics of lidocaine iontophoresis have been assessed in animals and in humans. In a study using microdialysis in animal models, iontophoresis of lidocaine at 0.15 mA/cm² resulted in a 40-fold increase in dermal lidocaine concentrations in comparison to passive drug delivery 32. In children, lidocaine plasma concentrations were below the lower limit of detection (<5 ng/ml; or below 10 ng/ml for one observation after three applications) 33.
Current clinical investigations
Iontophoretic delivery of terbinafine has been proposed in the treatment of onychomycosis 34. Preliminary data in humans compared terbinafine patches with (100 μA/cm², the active electrode polarity being positive) or without current during 4 weeks (6–8 h overnight, every day, 5 days a week). Clinical improvement was observed in the iontophoresis group compared with control subjects from the second follow-up visit 35. At the end of follow-up, mycological improvement (i.e. decrease in fungal elements) was also observed in patients from the iontophoresis group compared with control subjects 35. The investigators hypothesized that deeper penetration of the drug into the nail bed under the influence of the electric field was responsible for these encouraging results 35. Despite the prolonged treatment with elevated intensity, iontophoresis of terbinafine was well tolerated. Besides a tingling sensation, local irritation was reported by only two of 20 patients at the first application of the patch, and none of them stopped the therapy 35. Nonetheless, iontophoresis was performed over a small area (1 cm²) on the toenail, which is less sensitive than plain skin.
Iontophoresis of antiviral agents was proposed in the treatment of herpes labialis as early as the mid-1980s. Indeed, the efficacy of topical cream is limited by low penetration of the drug into the basal epidermis. A single 10 min iontophoretic application of 5% acyclovir cream was superior to placebo in the time to healing 36. Further research using a modified, self-administered, iontophoretic device (SoloVir®) was conducted in 2007, but the results have not been published (clinicaltrials.gov identifier: NCT00469300), and the development of SoloVir® was stopped 37.
Among other local therapies being investigated, a study comparing the efficacy and safety of iontophoretically administered azelaic acid twice weekly to topical cream twice daily in women with melasma is ongoing (clinicaltrials.gov identifier: NCT00848458).
Finally, iontophoresis of corticosteroids has been extensively studied, especially since the marketing of new devices (e.g. EyeGate® delivery system) which enable drug delivery to both the anterior and posterior segments of the human eye. Recent controlled studies suggest that dexamethasone administered through ophthalmic iontophoresis may be an effective treatment of dry eye 38, but this is beyond the scope of this review. On the skin, however, a pilot study reports the potential benefit of dexamethasone iontophoresis for temporomandibular joint involvement in juvenile idiopathic arthritis 39. In contrast, dexamethasone iontophoresis was not effective in the treatment of mild to moderate carpal tunnel syndrome 40.
Besides tap water iontophoresis, which has been used for years in hyperhidrosis, Clostridium botulinum toxin type A (BTX-A) was successfully administered iontophoretically (100 IU diluted in 3 ml of saline; 1.1–2.2 mA/cm² for 5 min) to two patients with severe palmar hyperhidrosis 41. A small (n = 8), double-blind, randomized, placebo-controlled study performed by the same group showed reduced palmar sweating in the BTX-A group at 14 days post-treatment 42. A preliminary randomized study comparing iontophoretically administered vs. intradermal injections of botulinum toxin treatment in patients with palmar hyperhidrosis recently showed that injections are more effective but more painful for the administration of BTX-A 43. A larger trial is currently ongoing (clinicaltrials.gov identifier: NCT01262339) and will provide further data.
Iontophoresis contraindications
Iontophoresis should not be used by:
- Patients who are epileptic or have a history of seizures
- Patients with heart conditions or a pacemaker
- Patients with metal implants
- Pregnant woman
Patients should delay treatment if they have recent wounds, skin grafts, or scars in the area requiring treatment, as iontophoresis may be painful and the treatment less effective.
Iontophoresis side effects
Iontophoresis is generally a safe procedure. It is important to avoid direct contact with the electrodes during treatment, as it may cause a mild electric shock.
A feeling of pins and needles or burning sensation is experienced by most people. Adverse effects may include:
- Redness of treated skin
- Small blisters(vesicles) or pompholyx
- Dry and cracked skin or dermatitis
Although these side effects from iontophoresis are expected to resolve within a few days, emollients/moisturisers should be applied several times daily to reduce symptoms. Topical corticosteroids can be applied.
If used, anticholinergic drugs such as glycopyrronium may be absorbed into the body and produce systemic side effects such as dizziness, dry eyes and dry mouth.
Cutaneous adverse events
Iontophoresis induces a sensation of tingling or itching, depending on the density of the applied current. Besides these uncomfortable but harmless effects, skin irritation is the most common local adverse effect of cutaneous iontophoresis. It occurs at both the anode and the cathode. Erythema is the most frequently described adverse effect, with a variable frequency according to the iontophoresis protocols 31. It was shown to occur consistently after 3 h iontophoresis at 250 μA/cm², was rated as very slight (grade 1) and lasted up to 3 h after removal of iontophoresis 44. Using the same protocol, edema was observed in half of the patients, and both erythema and oedema were enhanced by non-occlusive pretreatment with a surfactant 44. Skin irritation spontaneously and rapidly resolves, does not lead to permanent skin damage and does not disturbs the barrier function of the skin 44.
Although rare, burns have been observed, mainly due to operator error and the incorrect choice of electrodes/formulation composition. Indeed, the electrochemistry at bare metal or graphite electrodes involves electrolysis of water, which induces changes in the pH of the skin by generating H+ and OH− at the positive and negative electrodes, respectively 45. Variations in pH beyond the buffer capacities of the skin may lead to burns. High current density or prolonged application, as well as positioning of the electrodes over skin defects (which decrease skin resistance) increase the risk of burns. Burns are generally more serious under the cathode, due to involvement of OH− and rise in pH 12. Indeed, an alkaline phase erodes the epidermis and reduces skin resistance, making skin erosion worse 45. Therefore, an appropriate choice of buffer concentration in the formulation is needed to reduce the risk of burns. A better solution is to use Ag–AgCl rather than bare metal or carbon active electrodes, because they function at a lower potential and do not operate by water electrolysis.
Other cutaneous adverse effects have occasionally been described, such as galvanic urticaria 46. However, this was observed at high current intensities up to 24 mA, in the treatment of hyperhidrosis 47.
Several simple recommendations can decrease the risk of skin injury, such as avoiding pressure on the electrodes (not taping, binding or compressing either electrode) and ensuring that the electrode is uniformly wetted [68]. Indeed, most of the commercially available electrodes are made of small sponges in contact with the skin. After dampening the sponge with the drug solution, it conducts the current. Therefore, heterogeneity in sponge dampening locally increases current density and may lead to skin injury. In the same way, the adhesive seal should adhere uniformly to the skin to avoid leaks 48. Moreover, cleansing the skin with alcohol and avoiding skin defects and contact between metal components and the skin are also recommended 48. Finally, current intensity should be <0.5 mA/cm² 49.
Material defects
Material defects are the main cause of skin injuries, such as burns, usually resulting from a contact between metal components and the skin. For example, a partial-thickness burn has been reported in a paediatric patient, attributed to contact between the skin and a defect in the coating of the wires connecting the controller to the electrode patch 31.
Another consequence of material defects can be overdose, which is potentially harmful when drugs are delivered for a systemic action and have a narrow therapeutic index. The most striking example in the past few years was the iontophoretic delivery of fentanyl (Ionsys®); after receiving marketing authorization for the whole European Union in 2006, corrosion of a component within the system was found in one batch. Although no case of fentanyl overdose was reported, this defect could have resulted in fentanyl release without activation by the patient. This could have exposed patients to fentanyl overdose (the maximal theoretical dose was 3.2 mg), with a risk of severe respiratory depression 50. Ionsys® has not been marketed in Europe since October 2008, and the marketing authorization holder did not apply for renewal of authorization 51. Ionsys® has recently been acquired by another pharmaceutical company (Incline Therapeutics Inc., Redwood City CA, USA) currently developing new features into the system in order to obtain regulatory approval in the next few years.
- Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol. 2013;77(1):63-71. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3895348/[↩][↩][↩][↩]
- Iontophoresis – an approach for controlled drug delivery: a review. Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Curr Drug Deliv. 2007 Jan; 4(1):1-10. http://www.eurekaselect.com/58408/article[↩]
- Bronaugh R, Maibach H. Percutaneous Absorption: Mechanisms – Methodology – Drug Delivery. Marcel Dekker; New York and Basel: 1989. pp. 150–163.[↩]
- Banga AK, Chien YW. Iontophoretic delivery of drugs: Fundamentals, developments and biomedical applications. J Control Release. 1988;7:1–24. http://dx.doi.org/10.1016/0168-3659(88)90075-2[↩]
- Singh I, Prasanthi S. Percutaneous penetration enhancement in transdermal drug delivery. Asian J Pharm. 2010:92–101.[↩]
- Iontophoretic drug delivery. Kalia YN, Naik A, Garrison J, Guy RH. Adv Drug Deliv Rev. 2004 Mar 27; 56(5):619-58.[↩]
- Iontophoresis – an approach for controlled drug delivery: a review. Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Curr Drug Deliv. 2007 Jan; 4(1):1-10.[↩][↩]
- Non-invasive iontophoretic delivery of peptides and proteins across the skin. Gratieri T, Kalaria D, Kalia YN. Expert Opin Drug Deliv. 2011 May; 8(5):645-63.[↩]
- Cathodal iontophoresis of treprostinil induces a sustained increase in cutaneous blood flux in healthy volunteers. Blaise S, Roustit M, Hellmann M, Millet C, Cracowski JL. J Clin Pharmacol. 2013 Jan; 53(1):58-66.[↩]
- Dhote V, Bhatnagar P, Mishra PK, Mahajan SC, Mishra DK. Iontophoresis: a potential emergence of a transdermal drug delivery system. Sci Pharm. 2011;80(1):1-28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3293348/[↩]
- Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19:47–64.[↩]
- Costello CT, Jeske AH. Iontophoresis: applications in transdermal medication delivery. Phys Ther. 1995;75:554–563.[↩][↩]
- Eisenach JH, Atkinson JL, Fealey RD. Hyperhidrosis: evolving therapies for a well-established phenomenon. Mayo Clin Proc. 2005;80:657–666.[↩]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460.[↩]
- Chelly JE, Grass J, Houseman TW, Minkowitz H, Pue A. The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: a multicenter, placebo-controlled trial. Anesth Analg. 2004;98:427–433. table of contents.[↩]
- Viscusi ER, Reynolds L, Chung F, Atkinson LE, Khanna S. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA. 2004;291:1333–1341.[↩][↩]
- Viscusi ER, Siccardi M, Damaraju CV, Hewitt DJ, Kershaw P. The safety and efficacy of fentanyl iontophoretic transdermal system compared with morphine intravenous patient-controlled analgesia for postoperative pain management: an analysis of pooled data from three randomized, active-controlled clinical studies. Anesth Analg. 2007;105:1428–1436. table of contents.[↩]
- Siegel SJ, O’Neill C, Dube LM, Kaldeway P, Morris R, Jackson D, Sebree T. A unique iontophoretic patch for optimal transdermal delivery of sumatriptan. Pharm Res. 2007;24:1919–1926.[↩]
- Pierce M, Marbury T, O’Neill C, Siegel S, Du W, Sebree T. Zelrix: a novel transdermal formulation of sumatriptan. Headache. 2009;49:817–825.[↩]
- Rapoport AM, Freitag F, Pearlman SH. Innovative delivery systems for migraine: the clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929–940.[↩]
- Calatayud-Pascual MA, Balaguer-Fernandez C, Serna-Jimenez CE, Del Rio-Sancho S, Femenia-Font A, Merino V, Lopez-Castellano A. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharm. 2011;416:189–194.[↩]
- Escobar-Chavez JJ, Merino V, Lopez-Cervantes M, Rodriguez-Cruz IM, Quintanar-Guerrero D, Ganem-Quintanar A. The use of iontophoresis in the administration of nicotine and new non-nicotine drugs through the skin for smoking cessation. Curr Drug Discov Technol. 2009;6:171–185.[↩]
- Djabri A, Guy RH, Delgado-Charro MB. Transdermal iontophoresis of ranitidine: an opportunity in paediatric drug therapy. Int J Pharm. 2012;435:27–32.[↩]
- Djabri A, Guy RH, Delgado-Charro MB. Passive and iontophoretic transdermal delivery of phenobarbital: implications in paediatric therapy. Int J Pharm. 2012;435:76–82.[↩]
- Gratieri T, Kalaria D, Kalia YN. Non-invasive iontophoretic delivery of peptides and proteins across the skin. Expert Opin Drug Deliv. 2011;8:645–663.[↩][↩]
- Chen H, Zhu H, Zheng J, Mou D, Wan J, Zhang J, Shi T, Zhao Y, Xu H, Yang X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J Control Release. 2009;139:63–72.[↩]
- Riviere JE, Monteiro-Riviere NA, Inman AO. Determination of lidocaine concentrations in skin after transdermal iontophoresis: effects of vasoactive drugs. Pharm Res. 1992;9:211–214.[↩]
- Wakita R, Oono Y, Oogami S, Hayashi S, Umino M. The relation between epinephrine concentration and the anesthetic effect of lidocaine iontophoresis. Pain Pract. 2009;9:115–121.[↩]
- Lidosite® 2004. Full Prescribing Information. Vyteris Inc.[↩]
- Galinkin JL, Rose JB, Harris K, Watcha MF. Lidocaine iontophoresis versus eutectic mixture of local anesthetics (EMLA) for IV placement in children. Anesth Analg. 2002;94:1484–1488.[↩][↩]
- Zempsky WT, Sullivan J, Paulson DM, Hoath SB. Evaluation of a low-dose lidocaine iontophoresis system for topical anesthesia in adults and children: a randomized, controlled trial. Clin Ther. 2004;26:1110–1119.[↩][↩][↩][↩]
- Holovics HJ, Anderson CR, Levine BS, Hui HW, Lunte CE. Investigation of drug delivery by iontophoresis in a surgical wound utilizing microdialysis. Pharm Res. 2008;25:1762–1770.[↩]
- Kearns GL, Heacook J, Daly SJ, Singh H, Alander SW, Qu S. Percutaneous lidocaine administration via a new iontophoresis system in children: tolerability and absence of systemic bioavailability. Pediatrics. 2003;112:578–582[↩]
- Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, Friden PM, Murthy SN. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98:4130–4140.[↩]
- Amichai B, Nitzan B, Mosckovitz R, Shemer A. Iontophoretic delivery of terbinafine in onychomycosis: a preliminary study. Br J Dermatol. 2010;162:46–50.[↩][↩][↩][↩]
- Morrel EM, Spruance SL, Goldberg DI. Topical iontophoretic administration of acyclovir for the episodic treatment of herpes labialis: a randomized, double-blind, placebo-controlled, clinic-initiated trial. Clin Infect Dis. 2006;43:460–467.[↩]
- Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011;12:431–441.[↩]
- Patane MA, Cohen A, From S, Torkildsen G, Welch D, Ousler GW., 3rd Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5:633–643.[↩]
- Mina R, Melson P, Powell S, Rao M, Hinze C, Passo M, Graham TB, Brunner HI. Effectiveness of dexamethasone iontophoresis for temporomandibular joint involvement in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:1511–1516.[↩]
- Amirjani N, Ashworth NL, Watt MJ, Gordon T, Chan KM. Corticosteroid iontophoresis to treat carpal tunnel syndrome: a double-blind randomized controlled trial. Muscle Nerve. 2009;39:627–633.[↩]
- Kavanagh GM, Oh C, Shams K. BOTOX delivery by iontophoresis. Br J Dermatol. 2004;151:1093–1095.[↩]
- Kavanagh GM, Shams K. Botulinum toxin type A by iontophoresis for primary palmar hyperhidrosis. J Am Acad Dermatol. 2006;55:S115–117.[↩]
- Montaser-Kouhsari L, Zartab H, Fanian F, Noorian N, Sadr B, Nassiri-Kashani M, Firooz A. Comparison of intradermal injection with iontophoresis of abobotulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2012.739679.[↩]
- Li GL, Van Steeg TJ, Putter H, Van Der Spek J, Pavel S, Danhof M, Bouwstra JA. Cutaneous side-effects of transdermal iontophoresis with and without surfactant pretreatment: a single-blinded, randomized controlled trial. Br J Dermatol. 2005;153:404–412.[↩][↩][↩]
- ECRI Institute. Lesions and shocks during iontophoresis. Health Devices. 1997;26:123–125.[↩][↩]
- Meffert JJ. Galvanic urticaria. Cutis. 1999;63:327–328.[↩]
- Lacy KE, Kennedy CT. Galvanic urticaria. Clin Exp Dermatol. 2006;31:739–740.[↩]
- Warden GD. Electrical safety in iontophoresis. Rehab Manag. 2007;20:22–23.[↩][↩]
- Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis – an approach for controlled drug delivery: a review. Curr Drug Deliv. 2007;4:1–10.[↩]
- Prescrire. Fentanyl transdermal system: market withdrawal. Exposes patients to a risk of overdose. The market withdrawal, due to a manufacturing defect, is more than welcome. Prescrire Int. 2009;18:110.[↩]
- European Medicines Agency. Ionsys (fentanyl) – Non-renewal of the marketing authorisation in the European Union, 2011. https://www.ema.europa.eu/documents/public-statement/public-statement-ionsys-non-renewal-marketing-authorisation-european-union_en.pdf [↩]