What is kernicterus
Kernicterus is a rare but irreversible brain damage that can result from high levels of bilirubin in a baby’s blood being deposited in baby’s brain tissue. Kernicterus is usually seen only in infants with untreated jaundice, and it very rarely occurs in adults. Kernicterus is the pathological finding of deep-yellow staining of neurons and neuronal necrosis of the basal ganglia and brainstem nuclei 1. The incidence of kernicterus in the USA is estimated to be 1 in 40,000 births, with about 1 in 650 to 1000 neonates born >35 weeks’ postmenstrual age experiencing transient hyperbilirubinemia (>25 mg/dL) 2. Bilirubin has a neurotoxic effect on brain regions including the globus pallidus and subthalamic nuclei, which result in motor-related sequelae ranging from lack of coordination to severe movement disorders such as dyskinetic cerebral palsy. Clinical manifestations of kernicterus vary in form and severity depending on degree of prematurity and stage of regional brain development, degree, and duration of hyperbilirubinemia, as well as other perinatal risk factors such as sepsis, hypoalbuminemia, and genetic predispositions. Regional brain development and vulnerability determine periods during which moderate hyperbilirubinemia could result in auditory versus motor-predominant symptoms of bilirubin-induced neurologic dysfunction. These manifestations are distinct form those associated with exposure to high levels of neonatal total serum bilirubin that are known to cause severe neonatal motor symptoms and long-term sequelae such as dyskinetic cerebral palsy.
Kernicterus isn’t common because babies usually are treated before jaundice becomes severe. If untreated, kernicterus can cause:
- Athetoid cerebral palsy. Babies with this condition have uncontrollable movements in the arms, legs, face and other body parts.
- Hearing loss
- Vision problems
- Dental problems
- Intellectual disabilities
Classic clinical symptoms of kernicterus included lethargy, high-pitched crying, seizures, eye weakness (ophthalmoplegia), and truncal arching (opisthotonus) 3. Involvement of the central nervous system (CNS) suggested that bilirubin was crossing the blood–brain barrier (BBB) and damaging neural structures related to movement.
In newborn babies with very high levels of bilirubin in the blood (hyperbilirubinemia), the bilirubin can cross the thin layer of tissue that separates the brain and blood (the blood-brain barrier).
The bilirubin can damage the brain and spinal cord, which can be life threatening. Brain damage caused by high levels of bilirubin is also called bilirubin encephalopathy.
Total serum bilirubin is composed of conjugated and unconjugated bilirubin, but in neonates, and particularly those preterm, the unconjugated form dominates due to low activity of the enzyme uridine 5′-diphospho-glucuronosyltransferase (UDPGT), or glucuronyltransferase 3. As a water-insoluble compound, unconjugated bilirubin travels in the blood bound to proteins such as albumin. The level of albumin is low in the neonate and has a high degree of physiological variation, but increases with gestational age-at-birth and birth weight 4. When the binding affinity of bilirubin–albumin is decreased, as occurs in medically unstable neonates, or the carrying proteins become saturated, bilirubin exists in its unbound (“unbound bilirubin”) or “free” toxic form and is able to cross the blood-brain barrier 5. Albumin-bound bilirubin cannot reach the brain unless the blood-brain barrier becomes disrupted.
Your baby may be at risk of developing kernicterus if:
- they have a very high level of bilirubin in their blood
- the level of bilirubin in their blood is rising rapidly
- they don’t receive any treatment
Initial symptoms of kernicterus in babies include:
- decreased awareness of the world around them – for example, they may not react when you clap your hands in front of their face
- their muscles become unusually floppy, like a rag doll
- poor feeding
As kernicterus progresses, additional symptoms can include seizures (fits) and arching of the neck or spine.
Treatment for kernicterus involves using an exchange transfusion as used in the treatment of newborn jaundice.
If significant brain damage occurs before treatment, a child can develop serious and permanent problems, such as:
- cerebral palsy – a condition that affects a child’s movement and co-ordination
- hearing loss – which can range from mild to severe
- learning difficulties
- involuntary twitching of different parts of their body
- problems maintaining normal eye movements – people affected by kernicterus have a tendency to gaze upwards or from side to side rather than straight ahead
- poor development of the teeth.
Kernicterus can cause athetoid cerebral palsy and hearing loss. Kernicterus also causes problems with vision and teeth and sometimes can cause intellectual disabilities. In the newborn, early detection and management of jaundice or conditions that lead to jaundice may help prevent kernicterus. Children affected with complications of hyperbilirubinemia can present with choreoathetoid cerebral palsy, dystonia, sensorineural hearing loss, paralysis of upward gaze, and dental enamel dysplasia 6. Low-quality evidence from 1 study with 371 infants and children with cerebral palsy found that the prevalence of kernicterus was 4.6% 7.
In the 1940s and the 1950s, severe neonatal hyperbilirubinemia and kernicterus were most often encountered with hemolytic disease of newborn, which occurs most often as a result of the incompatibilities of the Rh and ABO blood groups 6. With the advent of prenatal testing, maternal Rh°(D) immunoglobulin, phototherapy, and exchange transfusion, the incidence of severe hyperbilirubinemia drastically decreased to the point that most physicians practising today have never encountered a bilirubin-induced neurologic disorder 6. Unfortunately, severe hyperbilirubinemia continues to be the most common cause of neonatal readmission to hospital in North America, and kernicterus continues to occur in infants without risk factors or evidence of hemolytic disease of the newborn.
Advances in the care of neonatal hyperbilirubinemia have decreased the incidence of kernicterus 8. However, neonatal exposure to high levels of bilirubin continues to cause severe motor symptoms and cerebral palsy 8. Exposure to moderate levels of unconjugated bilirubin may also cause damage to the developing central nervous system, specifically the basal ganglia and cerebellum 8. Brain lesions identified using magnetic resonance imaging (MRI) following extreme hyperbilirubinemia have been linked to dyskinetic cerebral palsy. Newer imaging techniques, such as diffusion tensor imaging or single-photon emission computed tomography, allow quantification of more subtle white matter injury following presumed exposure to unbound bilirubin, and may explain more subtle movement disorders.
In Canada, a recent 2-year Canadian Paediatric Surveillance Program study of severe hyperbilirubinemia reported 258 cases of term infants, 60 days of age or younger, with either exchange transfusion or an unconjugated bilirubin level of 425 μmol/L or greater 6. Most of these infants (72%) were readmitted to hospital at a median age of 5 days. More important, 81% of infants were exclusively breast-fed, and 11% of confirmed cases had a documented 10% to 15% weight loss. Of those with available data, only 36% had a cause identified; the most common cause was ABO blood group incompatibility and glucose-6–phosphate dehydrogenase (G6PD) deficiency 9. Regardless of the rarity of kernicterus even with bilirubin levels of 425 μmol/L, readmission creates potentially unnecessary distress and disruption for these families and can be prevented 6.
Neuroanatomy of bilirubin-associated motor impairment
Original autopsy studies of kernicterus identified yellow staining and necrosis of the basal ganglia, specifically in the globus pallidus, indicative of “unbound bilirubin” crossing the blood–brain barrier (BBB). Other regions noted to have cellular damage after hyperbilirubinemia include the substantia nigra reticulata, subthalamic nuclei, vestibular and oculomotor nuclei, hippocampus, and cerebellar Purkinje cells. Loss of neurons, decreased myelination, and gliosis can be observed in the internal and external globus pallidus and subthalamic nuclei. These regions all have functions related to movement, balance, and posture regulation and appear to be selectively vulnerable. The globus pallidus may also serve to integrate inputs from multiple systems including somatosensory and motor pathways; thus, integration of this information may be damaged in patients with kernicterus 10. By contrast, regions such as the striatum and thalamus generally appear to be spared following hyperbilirubinemia. This specific pattern of brain injury helps distinguish bilirubin-induced neurologic dysfunction from injury following hypoxic–ischemia, in which the caudate, putamen, posterior limb of the internal capsule, and cortex are more vulnerable. One theory for the selective vulnerability of the globus pallidus and subthalamic nuclei is their relatively high resting levels of neuronal activity observed in the neonatal brain, compared to nearby regions. Johnston et al. 11 observed that regions with higher neuronal activity are more vulnerable to oxidative stressors or toxins such as bilirubin. Lesions in these regions are observed in infants with classic athetotic cerebral palsy. One hypothesis for the connection between globus pallidus injury and athetosis is that reduced activity of the globus pallidus results in decreased inhibitory input to the thalamus, resulting in its “dys-inhibition” and increased motor activity, consistent with athetosis 11.
Regional specificity of kernicterus and brain pathology resulting from mild, acute, and chronic bilirubin encephalopathies has been identified on magnetic resonance imaging (MRI). The globus pallidus, and specifically the posteromedial border, appears to be most sensitive to damage from bilirubin, possibly due to its location in the circuit of the basal ganglia 12. Regulation of motor function involves basal ganglia circuits which receive input from the motor cortex to the caudate and putamen. The internal globus pallidus contains the output neurons of the basal ganglia, which project through the motor thalamus and back to the motor cortex 13. Govaert et al. 14 observed permanent damage to the globus pallidus, evident on T2 MRI, after preterm infants had been exposed to total serum bilirubin levels below suggested thresholds. However, not all infants exposed to high levels of total serum bilirubin develop neurological symptoms or lasting globus pallidus lesions 12. Lesions in the subthalamic nuclei may be more subtle and challenging to visualize on MRI 10.
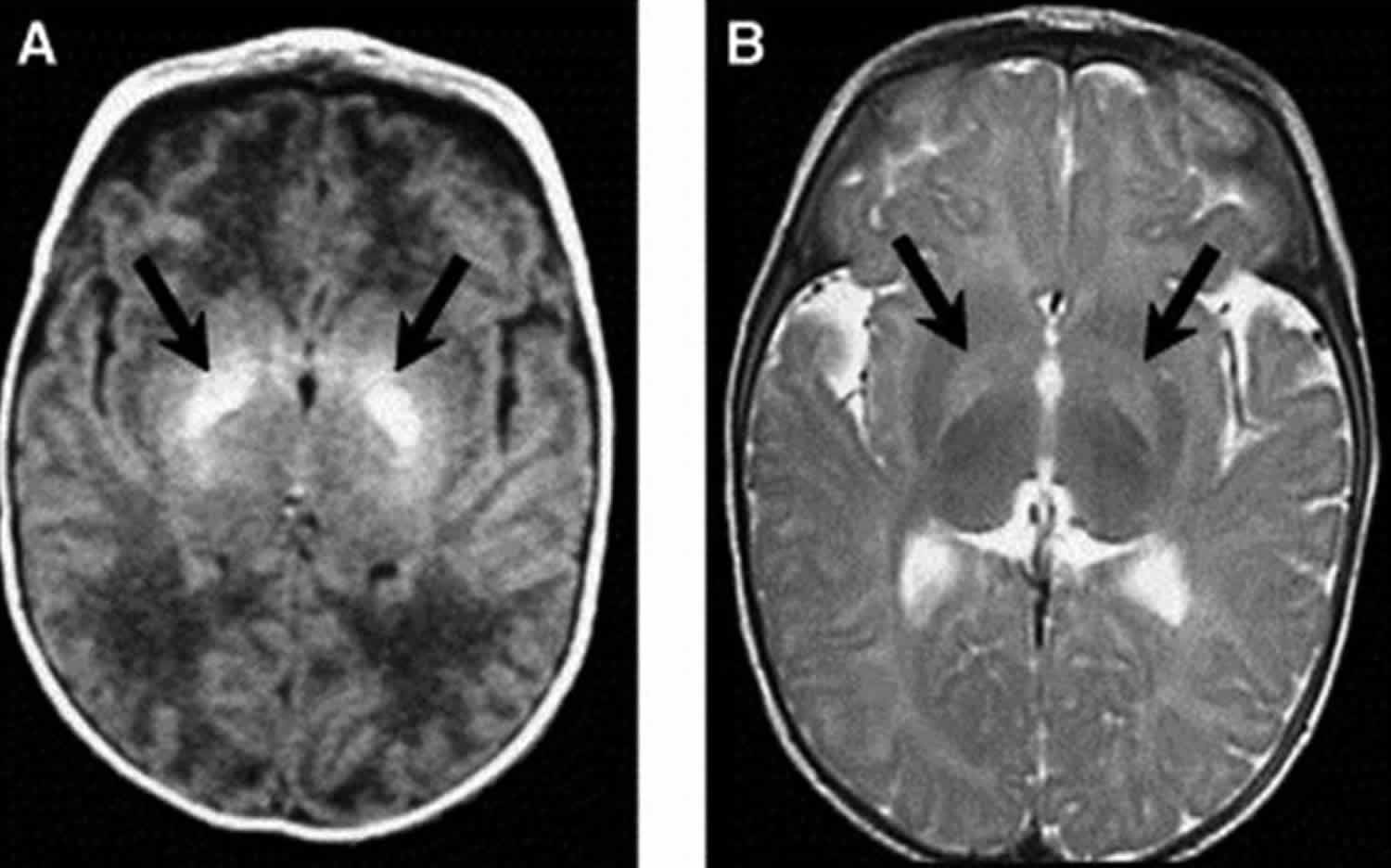
There have been few studies on longitudinal microstructural changes in white and gray matter of the brain following hyperbilirubinemia in relation to motor disorders. Gkoltsiu et al. 15 described inconsistent patterns between early imaging abnormalities following (i) bilirubin exposure and (ii) motor outcome 15. Among children in this cohort who developed cerebral palsy, all demonstrated classic signs of kernicterus on MRI, i.e., high signal intensity in the globus pallidus on T2 images. Early images, however, were not reliable in their correlation with outcome. Shapiro et al. 16 have found similar MRI patterns demonstrating acute and long-term changes on MRI, specifically bilateral hyperintense lesions in the globus pallidus, corresponding to dystonic kernicterus (Figure 1). One explanation for the challenge of using early scans is the change of brain tissue following injury, and the resulting changes in appearance on MRI 17. For example, signs of kernicterus on T1 are only visible in the short term following hyperbilirubinemia, but T2 hyperintensities are longer-lasting. Signal hyperintensities on T1 images are characteristic of the acute stages of bilirubin-induced astroglial necrosis, whereas the more permanent signals on T2 may reflect the long-term neuronal loss, demyelination, or gliosis 13. Symmetric involvement of the globus pallidus on T1 has been found to be related to severity of hyperbilirubinemia, and severity of outcome may be predicted by the characteristic switch from hyperintensities visible on T1 to T2 18. Normalization of T1 imaging is common in chronic bilirubin encephalopathy.
Advanced methods have begun to be used to study the brains of infants with kernicterus. Okumura et al. 19 used single-photon emission computed tomography (SPECT) in three children (aged four to six years) born preterm with athetoid cerebral palsy following kernicterus. All three demonstrated no brain abnormalities on ultrasound during the neonatal period. Though all had identifiable hyperintensities on MRI in the globus pallidus at five to nine months of age, abnormalities were mild in two of the infants, and in one of these two, abnormalities disappeared on follow-up MRI. SPECT was able to identify decreased blood flow to the basal ganglia region in all three patients, suggesting that single-photon emission computed tomography (SPECT) may be more useful than MRI in identifying brain abnormalities in older children after neonatal kernicterus 19.
Figure 1. Kernicterus brain
Footnote: Axial magnetic resonance imaging (MRI) of bilateral hyperintense lesions in the globus pallidus in axial projections (arrows). (A) T1-weighted axial image of a six-day-old, 37-week gestation boy with peak total bilirubin of 34.6 mg/dL. At age seven years, this child was highly intelligent, but moderately to severely disabled with dystonic, athetoid kernicteric cerebral palsy; he ambulates with a walker. (B) Axial T2-weighted MRI of a two-year-old who has classic dystonic kernicterus. Note the increased intensity of the globus pallidus bilaterally (shown with arrows and dotted line on right side only). There were no abnormalities noted in brainstem or cerebellum.
[Source 8 ]Your baby will usually be examined for signs of jaundice within 72 hours of being born, during the newborn physical examination.
If your baby develops signs of jaundice after this time, see to your doctor as soon as possible for advice.
While jaundice isn’t usually a cause for concern, it’s important to determine whether your baby needs treatment.
How do you know if your baby has jaundice?
When a baby has jaundice, a yellowish color usually first appears on his face. It then may spread to his chest, belly, arms, legs and white parts of his eyes. The best way to see jaundice is in good light, like in daylight or under fluorescent lights. Jaundice can be harder to see in babies with darker skin.
See your baby’s health care provider right away if your baby:
- Looks very yellow, orange or greenish-yellow
- Is hard to wake up or won’t sleep at all
- Has trouble breastfeeding or sucking from a bottle
- Is very fussy
- Has too few wet or dirty diapers
Call your local emergency number for an ambulance or take your baby to the hospital if he/she:
- Won’t stop crying or has a high-pitched cry
- Arches backward
- Has a stiff, limp or floppy body
- Has strange eye movements
These may be warning signs of dangerously high levels of bilirubin that need quick treatment to prevent kernicterus. This is a kind of brain damage caused by high bilirubin levels. Kernicterus isn’t common because babies usually are treated before jaundice becomes severe. If untreated, kernicterus can cause:
- Athetoid cerebral palsy. Babies with this condition have uncontrollable movements in the arms, legs, face and other body parts.
- Hearing loss
- Vision problems
- Dental problems
- Intellectual disabilities
Kernicterus signs and symptoms
Kernicterus is reserved for a diagnosis with a combination of clinical features and MRI findings. The classic diagnosis includes a tetrad of features: (1) dystonia and/or athetosis; (2) hearing impairment or deafness; (3) lack of upward gaze; and (4) dental enamel dysplasia, along with abnormal MRI findings in the globus pallidus and subthalamic nuclei. Other less common accompanying symptoms may include hypotonia or sensorimotor abnormalities, and oculomotor abnormalities may vary and include strabismus or other misalignment of the eyes 20.
Use of the term kernicterus, however, is debated as it has traditionally been used to describe infants exposed to very high total serum bilirubin levels (>20 mg/dL) who develop immediate clinical and neuroradiological signs of the disease. Infants exposed to lower total serum bilirubin levels, not severe enough to cause kernicterus, may have mild damage to the basal ganglia and cerebellum that may manifest as mild hypotonia, lack of coordination, or generalized clumsiness – not severe enough to be classified as a specific movement disorder. Bilirubin-induced neurologic dysfunction (BIND), a term introduced to include a wider spectrum of infants with clinical symptoms associated with moderate hyperbilirubinemia, has been associated with decreased sucking behavior, lethargy, hypotonia, or stupor 21. Abnormal tone in the limbs is often observed during acute injury or as post-icteric sequelae; symptoms include alternating hypotonia and hypertonia, along with opisthotonus and retrocollis. Another classic sign is the “setting sun sign,” a term to describe the downward gaze of the eyes due to damage to the nuclei controlling upward gaze, which may be associated with a mask-like facies. This acute phase will likely correspond to hyperintensity in the globus pallidus on T1 images. After the first week of acute phase of injury, hypertonia will sometimes but not always predominate.
Several classification systems have been proposed to describe the variety of motor symptoms that may follow severe neonatal hyperbilirubinemia. Shapiro 21 has proposed a classification for bilirubin-induced neurologic dysfunction (BIND) based on location of the injury, severity of symptoms, and timing of the peak of the total serum bilirubin levels. For infants with kernicterus, characterization is based on the type of symptoms, specifically as “auditory predominant” or “motor predominant.” Generally, motor-predominant forms of kernicterus are due to lesions in the external and internal globus pallidus as well as in the subthalamic nuclei. Injury to the Purkinje cells of the cerebellum, and small lesions in the brainstem within the auditory, vestibular, and oculomotor nuclei, also are likely to be present, though current imaging techniques are limited and unable to visualize this small-scale damage. Classic motor symptoms caused by bilirubin-induced neurologic dysfunction are identified as the athetotic or dyskinetic form of cerebral palsy and are related to lesions in the globus pallidus and subthalamic nuclei most prominently as well as the cerebellum and brainstem. Specific symptoms may also be involved due to damage to the vestibular nuclei, the interstitial nucleus of Cajal (responsible for upward gaze), and Dieter’s nucleus (involved in truncal tone) 10. Movement disorders associated with dyskinetic cerebral palsy are distinct from those of spastic cerebral palsy, which represents the majority of cases of cerebral palsy, and involves symptoms of weakness, spasticity, short muscle tendon length, and impaired selective motor control characterized by flexor and extensor synergies. Spastic cerebral palsy is associated with white matter injury to the corticospinal tract.
Kernicterus symptoms
Initial symptoms of kernicterus in babies include:
- decreased awareness of the world around them – for example, they may not react when you clap your hands in front of their face
- their muscles become unusually floppy, like a rag doll
- poor feeding
As kernicterus progresses, additional symptoms can include seizures (fits) and arching of the neck or spine.
Mild kernicterus may manifest with motor symptoms including dystonia with or without athetosis and mild gross motor delays such as late developmental milestones such as age of initiation of walking. These infants generally can ambulate well on their own later in childhood and can speak with some clarity.
Moderate kernicterus may be accompanied by a moderate degree of hyperkinetic dystonia, also classified as athetoid cerebral palsy. These children may have more difficulty ambulating without assistance due to the choreoathetoid movements. They may also have delayed speech but can benefit from therapy to improve quality of speech.
Severe “motor predominant” kernicterus is characterized by severe dystonia/athetosis preventing controlled voluntary movements including ambulation, speech, and self-feeding. This may also be accompanied by severe hypertonia and muscle cramping. Though some children may experience improvement in disability with time, classification of severity generally remains consistent over time.
Kernicterus prognosis
Long-term outcomes of infants with kernicterus vary widely based on severity as well as a variety of risk factors described previously. Increased incidence of dyskinetic cerebral palsy occurs following high bilirubin exposure in full-term infants or low–moderate levels of bilirubin exposure in preterm infants 22. Cerebral palsy was most recently defined by an international committee as “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain 23. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, perception, cognition, communication, behavior, by epilepsy, and by secondary musculoskeletal problems” 24.
The subtype of cerebral palsy attributed to bilirubin neurotoxicity is best classified as dyskinetic cerebral palsy to encompass the dystonic and athetotic movements that occur 22. The definition and distinction between athetosis and dystonia has been heavily debated. Athetosis has been used to describe children with dyskinesia with involuntary movements as well as lack of postural control 22. Przekop et al. 22 suggests that “choreoathetosis” may be a better term to use to describe the athetotic movements observed in children, to distinguish it from athetosis in adults characterized by slow writhing, often in the fingers or toes. Athetosis in infants is characterized by “slow continuous writhing movements that prevent maintenance of a stable posture” 23, as defined by the 2008 Taskforce on Childhood Movement Disorders, whereas dystonia is more defined by hypertonic postures 23. Shapiro 21 has observed that infants with cerebral palsy related to kernicterus rarely present initially with fixed postures or contractures. In addition, cognitive function is generally preserved due to limited injury to cortical and subcortical white matter pathways.
Activities of everyday life are often compromised in children with moderate–severe cerebral palsy. Speech is challenging, particularly for children with dyskinetic cerebral palsy that also includes deafness or hearing impairment. Swallowing and eating can also be challenging due to spasms of pharyngeal muscles and involuntary movements of the tongue. Aspiration is a risk and children with severe cerebral palsy may need gastrostomy tubes to supplement their caloric intake 22.
Kernicterus bilirubin level
There is no reliable strategy to identify all infants who will develop serious hyperbilirubinemia, nor any one bilirubin level that predicts the development of neurologic damage, the Canadian Paediatric Society 1 has recently published new guidelines for the detection, management, and prevention of hyperbilirubinemia in term and late preterm newborn infants.
Acute bilirubin encephalopathy – a clinical syndrome, in the presence of severe hyperbilirubinemia, of lethargy, hypotonia and poor suck, which may progress to hypertonia (with opisthotonos and retrocollis) with a high-pitched cry and fever, and eventually to seizures and coma.
Chronic bilirubin encephalopathy – the clinical sequelae of acute encephalopathy with athetoid cerebral palsy with or without seizures, developmental delay, hearing deficit, oculomotor disturbances, dental dysplasia and mental deficiency 25.
Severe hyperbilirubinemia – a total serum bilirubin concentration greater than 340 µmol/L at any time during the first 28 days of life.
Critical hyperbilirubinemia – a total serum bilirubin concentration greater than 425 µmol/L during the first 28 days of life.
Figure 2. Total serum bilirubin concentration in newborns
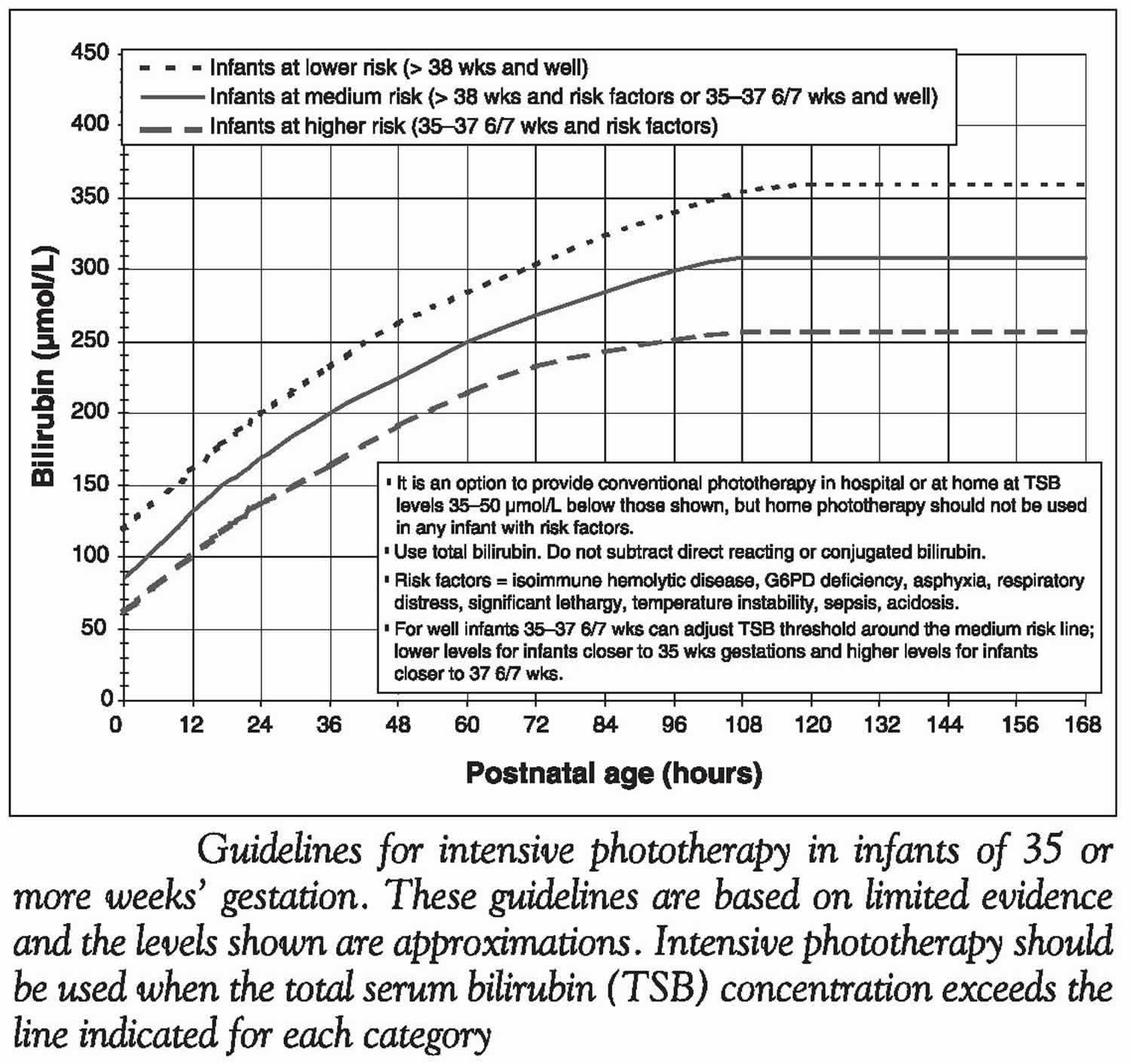
[Source 1 ]Figure 3. Guidelines for intensive phototherapy based on infants age and total serum bilirubin concentration
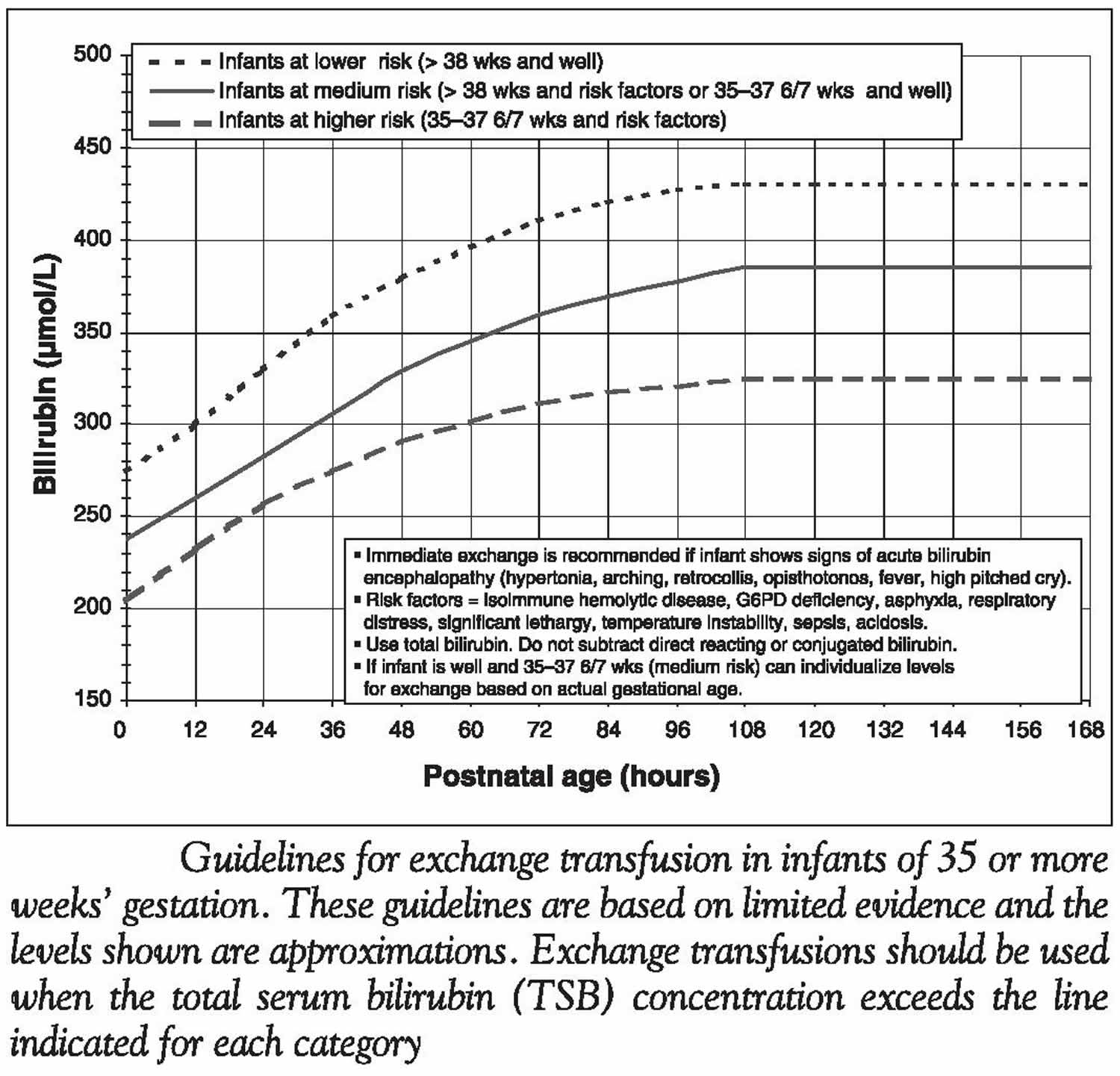
[Source 1 ]Figure 4. Guidelines for exchange transfusion based on infants age and total serum bilirubin concentration
[Source 1 ]The prevention, detection and management of jaundice in otherwise healthy term and late preterm newborn infants remain a challenge, partly because jaundice is so common and kernicterus is so rare in comparison 26. It is estimated that 60% of term newborns develop jaundice and 2% reach a total serum bilirubin concentration greater than 340 µmol/L 27. Acute encephalopathy does not occur in full-term infants whose peak total serum bilirubin concentration remains below 340 µmol/L and is very rare unless the peak total serum bilirubin concentration exceeds 425 µmol/L 1. Above this level, the risk for toxicity progressively increases 28. More than three-quarters of the infants in the United States’ kernicterus registry (between 1992 and 2002) had a total serum bilirubin concentration of 515 µmol/L or greater, and two-thirds had a concentration exceeding 600 µmol/L 29. Even with concentrations greater than 500 µmol/L, there are still some infants who will escape encephalopathy. All of the reasons for the variable susceptibility of infants are not known; however, dehydration, hyperosmolarity, respiratory distress, hydrops, prematurity, acidosis, hypoalbuminemia, hypoxia and seizures are said to increase the risk of acute encephalopathy in the presence of severe hyperbilirubinemia 30, although reliable evidence to confirm these associations is lacking 31. In addition, some infants with severe hyperbilirubinemia are found to have sepsis, but both sepsis and hyperbilirubinemia are common in the neonatal period, and sepsis appears to be uncommon in the well-appearing infant with severe hyperbilirubinemia.
Milder degrees of hyperbilirubinemia not leading to a clinical presentation of acute encephalopathy may also be neurotoxic and cause less severe long-term complications. This remains controversial; however, if there are bilirubin concentrations at which subtle cerebral injury can occur, the thresholds are unknown 32. The collaborative perinatal project, examining 54,795 live births in the United States, was unable to find any consistent association between peak total serum bilirubin concentrations below critical levels and IQ or other adverse outcomes 31. Therefore, prevention of acute encephalopathy remains the justification for the prevention, detection and treatment of severe hyperbilirubinemia 33.
The incidence of acute encephalopathy is uncertain, but it continues to occur. The Canadian Paediatric Surveillance Program recently reported 258 full-term infants over a two-year period (2002 to 2004) who either required exchange transfusion or had critical hyperbilirubinemia (excluding infants with rhesus isoimmunization) 34. Twenty per cent of these infants had at least one abnormal neurological sign at presentation, and 5% had documented hearing loss or significant neurological sequelae at discharge. During this period, the live birth rate in Canada was approximately 330,000 per year, leading to a calculated minimal incidence of this degree of severity of hyperbilirubinemia of approximately four in 10,000 live births. If we assume that the entire 20% of infants with neurological findings at presentation had acute bilirubin encephalopathy, the incidence of this complication would be one in 10,000 live births, an incidence similar to that of phenylketonuria. The incidence of chronic encephalopathy is also uncertain, but it has been estimated to be approximately one in 100,000 35. This situation occurs despite the fact that a large number of infants already receive intensive preventive therapy 30. The Canadian Paediatric Surveillance Program report 34 noted that 13 of the infants continued to have important neurological abnormalities at final discharge, suggesting a chronic bilirubin encephalopathy incidence of one in 50,000, similar to the frequency reported from a Danish study36.
Acute bilirubin encephalopathy was first recognized in infants with rhesus hemolytic disease; this cause is now largely avoidable and, consequently, has become rare. Reports 37 indicate that acute bilirubin encephalopathy continues to occur in otherwise healthy infants with, and occasionally without, identifiable risk factors. Prevention of this rare but serious disease requires appropriate clinical assessment, interpretation of total serum bilirubin concentration and treatment, which must include all systems involved in the provision of health care and community support.
Can severe hyperbilirubinemia be accurately predicted?
Timed total serum bilirubin measurements
Carefully timed total serum bilirubin measurements can be used to predict the chances of developing severe hyperbilirubinemia. A study 38 in a North American multi-ethnic population of appropriate weight for gestational age term and late preterm infants (35 weeks or greater) who did not have a positive direct Coombs test demonstrated that a timed measurement of total serum bilirubin concentration at discharge (between 18 hours and three days of age) could predict a later total serum bilirubin measurement greater than the 95th percentile within stated confidence limits (the 95th percentile was approximately 300 µmol/L after 96 hours of age). When the total serum bilirubin concentration was below the 40th percentile at the time of measurement, there were no cases of subsequent total serum bilirubin concentration greater than the 95th percentile. When the total serum bilirubin concentration was between the 40th and the 75th percentiles, only 2.2% of infants developed a total serum bilirubin concentration greater than the 95th percentile. Finally, when the total serum bilirubin concentration was above the 75th percentile, 12.9% of infants subsequently exceeded the 95th percentile 38. Routine total serum bilirubin estimation at 6 hours of life can also be used in term and late preterm infants to predict a total serum bilirubin concentration greater than 238 µmol/L in infants with a birth weight of 2 kg to 2.5 kg, and a total serum bilirubin concentration greater than 289 µmol/L in infants with a birth weight greater than 2.5 kg 32. Combining a timed total serum bilirubin measurement at younger than 48 hours with a clinical risk score improved the prediction of a subsequent total serum bilirubin concentration greater than 342 µmol/L 39; this improvement was almost entirely due to the effect of including gestational age. Thus, a total serum bilirubin concentration between the 75th and the 94th percentiles was associated with a 12% risk of subsequent total serum bilirubin concentration greater than 342 µmol/L in the infant of 36 weeks’ gestation, and with approximately a 3% risk in the infant of 40 weeks’ gestation 40.
Therefore, the best available method for predicting severe hyperbilirubinemia appears to be the use of a timed total serum bilirubin measurement analyzed in the context of the infant’s gestational age. Infants of less than 38 weeks’ gestation whose total serum bilirubin concentration is greater than the 75th percentile have a greater than 10% risk of developing severe hyperbilirubinemia; similarly, infants of 39 to 40 weeks’ gestation whose total serum bilirubin concentration is above the 95th percentile have a greater than 10% risk 1.
Umbilical cord blood total serum bilirubin
A total serum bilirubin concentration greater than 30 µmol/L in umbilical cord blood 41 is statistically correlated with a peak neonatal total serum bilirubin concentration greater than 300 µmol/L, but the positive predictive value is only 4.8% for the term infant, rising to 10.9% in the late preterm infant, and the specificity is very poor.
Universal hemoglobin assessment
Although bilirubin is derived from the breakdown of hemoglobin, routine umbilical cord blood hemoglobin or hematocrit measurement does not aid in the prediction of severe hyperbilirubinemia 42.
Blood group and Coombs testing
ABO isoimmunization is a common cause of severe hyperbilirubinemia. Babies whose mothers are blood group O have an odds ratio (OR) of 2.9 for severe hyperbilirubinemia (because most infants with jaundice due to ABO isoimmunization are blood group A or B infants born to a mother with group O blood) 43. Odds ratio (OR) is a measure of association between an exposure and an outcome. Odds ratio (OR)>1 means exposure associated with higher odds of outcome. The need for phototherapy is increased in ABO-incompatible infants who are direct antiglobulin test (direct Coombs test)-positive compared with those who are direct antiglobulin test (direct Coombs test)-negative 40. Universal testing for incompatibility with blood grouping, and for isoimmunization using the direct antiglobulin test (direct Coombs test), on cord blood does not improve clinical outcomes compared with testing only infants whose mothers are group O 44. Testing all babies whose mothers are group O does not improve outcomes compared with testing only those with clinical jaundice 45. Therefore, it is reasonable to perform a direct antiglobulin test (direct Coombs test) in clinically jaundiced infants of mothers who are group O and in infants with an elevated risk of needing therapy (i.e., in the high-intermediate zone [see Figure 2]). The results will determine whether they are low risk or high risk, and may therefore affect the threshold at which therapy would be indicated (Figure 3).
The usual antenatal screen for a panel of red cell antibodies occasionally identifies additional mothers who will deliver infants at increased risk of hemolysis. The significance of the various antibodies differs; in such infants, analysis of blood group and a direct antiglobulin test (direct Coombs test) is usually required, closer follow-up and earlier therapy may be needed, and a consultation with a paediatric hematologist or neonatologist is suggested.
Kernicterus causes
Jaundice is caused by too much bilirubin in the blood. This is known as hyperbilirubinaemia. Jaundice usually occurs in newborns because theirs livers are not fully developed.
Jaundice is common in newborn babies because babies have a high level of red blood cells in their blood, which are broken down and replaced frequently.
The liver in newborn babies is also not yet fully developed, so it’s less effective at processing the bilirubin and removing it from the blood.
This means the level of bilirubin in babies can be about twice as high as in adults.
Bilirubin is a yellow substance produced when red blood cells, which carry oxygen around the body, are broken down.
The bilirubin travels in the bloodstream to the liver. The liver changes the form of the bilirubin so it can be passed out of the body in poo.
However, if there’s too much bilirubin in the blood or the liver can’t get rid of it, excess bilirubin causes jaundice.
By the time a baby is around two weeks old, they’re producing less bilirubin and their liver is more effective at removing it from the body. This means the jaundice often corrects itself by this point without causing any harm.
Breastfeeding
Breastfeeding your baby can increase their chances of developing jaundice. However, there’s no need to stop breastfeeding your baby if they have jaundice as the symptoms normally pass in a few weeks.
The benefits of breastfeeding outweigh any potential risks associated with the condition.
If your baby needs to be treated for jaundice, he or she may need extra fluids and more frequent feeds during treatment.
The reason why breastfed babies are more likely to develop jaundice is unclear, although a number of theories have been suggested. For example, it may be that breast milk contains certain substances that reduce the ability of the liver to process bilirubin.
Newborn jaundice thought to be linked to breastfeeding is sometimes called breast milk jaundice.
Underlying health conditions
In some cases, jaundice may be the result of another health problem. This is sometimes called pathological jaundice.
Some other medical conditions that make newborn jaundice worse are:
- Blood group incompatibility – baby’s blood type does not match with his or her mother’s blood type
- Rhesus factor disease – a condition that can occur if the mother has rhesus-negative blood and the baby has rhesus-positive blood
- Baby is born with too many red blood cells (polycythemia). Some babies have too many red blood cells. This is more common in some twins and babies who are small for gestational age. This means a baby who is smaller than normal based on the number of weeks he’s been in the womb.
- Baby has an infection in his or her blood (sepsis)
- Baby has an infection e.g. a urinary tract infection
- Baby has bruises from birth. A bruise happens when blood leaks out of a blood vessel. Sometimes babies get bruises during labor and birth. When large bruises heal, bilirubin levels may rise.
- Internal bleeding. This is bleeding inside the body.
- Baby swallowed blood during birth
- Baby’s mother has diabetes
- Baby has an underactive thyroid gland (hypothyroidism) – where the thyroid gland doesn’t produce enough hormones
- Baby has a blockage or problem in his/her bile ducts and gallbladder – these create and transport bile, a fluid used to help digest fatty foods
- A problem with your baby’s liver. Your baby’s liver may not work well if he has an infection, like hepatitis, or a disease, like cystic fibrosis, that affects the liver.
- Crigler-Najjar syndrome – an inherited condition that affects the enzyme responsible for processing bilirubin
- Baby has an inherited enzyme deficiency known as glucose 6 phosphate dehydrogenase (G6PD) could also lead to jaundice or kernicterus. This condition is when your body doesn’t have enough G6PD, an enzyme that helps your red blood cells work the right way. If you have a family history of G6PD, it’s important to let your doctor or pediatrician know and your baby’s jaundice symptoms are closely monitored. Newborns with glucose-6-phosphate dehydrogenase deficiency (G6PD) have an increased incidence of severe hyperbilirubinemia. Testing for G6PD deficiency in babies whose ethnic group or family history suggest an increased risk of G6PD deficiency is advised (e.g., Mediterranean , Middle Eastern, African 46 or Southeast Asian origin). Although G6PD deficiency is an X-linked disease, female heterozygotes can have more than 50% of their red cells deficient in the enzyme because of random inactivation of the X chromosome. Females with greater proportions of their red cells affected have an increased risk of severe neonatal hyperbilirubinemia 47; therefore, testing of both girls and boys who are at risk is advised 48. G6PD deficiency increases the likelihood of requiring exchange transfusion in infants with severe hyperbilirubinemia; therefore, a test for G6PD deficiency should be considered in all infants with severe hyperbilirubinemia. It should also be recognized that in the presence of hemolysis, G6PD levels can be overestimated and this may obscure the diagnosis 49. Females in particular can have misleading results on the common screening tests 50. G6PD-deficient newborns may require intervention at a lower total serum bilirubin concentration because they are more likely to progress to severe hyperbilirubinemia 51. Unfortunately, in many centers, it currently takes several days for a G6PD deficiency screening test result to become available. Improving the turnaround time for this test would improve care of the newborn. Because G6PD deficiency is a disease with lifelong implications, testing infants at risk is still of value.
Some babies are more likely than others to have jaundice. These include:
- Premature babies. A premature baby is one who is born too early, before 37 weeks of pregnancy. A premature baby is more likely than others to have jaundice because his liver may not be fully developed.
- Breastfed babies, especially babies who aren’t breastfeeding well. If you’re breastfeeding, feed your baby when he’s hungry. For most newborns, this is once every 2 to 3 hours (about eight to 12 times each day). Feeding this often helps keep your baby’s bilirubin level down. If you’re having trouble breastfeeding, ask your baby’s provider, a nurse or a lactation consultant for help. A lactation consultant is a person with special training in helping women breastfeed.
- Babies with East Asian or Mediterranean ethnic backgrounds. Ethnic background means the part of the world or the ethnic group your ancestors come from. An ethnic group is a group of people, often from the same country, who share language or culture. Ancestors are family members who lived long ago, even before your grandparents.
Risk factors for severe hyperbilirubinemia
About 60% of term and 80% of preterm infants have clinical jaundice in the first week after birth but only 2% to 16% of them develop severe hyperbilirubinemia (total serum bilirubin > 25mg/dl), which is an emergency because it may cause neonatal bilirubin encephalopathy (kernicterus), which can result in death or irreversible brain damage in survivor 52. Some babies are more likely to have severe jaundice and higher bilirubin levels than others. Babies with any of the following risk factors need close monitoring and early jaundice management:
Preterm Babies
- Babies born before 37 weeks, or 8.5 months, of pregnancy might have jaundice because their liver is not fully developed. The young liver might not be able to get rid of so much bilirubin.
Babies with Darker Skin Color
- Jaundice may be missed or not recognized in a baby with darker skin color. Checking the gums and inner lips may detect jaundice. If there is any doubt, a bilirubin test should be done.
East Asian or Mediterranean Descent
- A baby born to an East Asian or Mediterranean family is at a higher risk of becoming jaundiced. Also, some families inherit conditions (such as G6PDdeficiency), and their babies are more likely to get jaundice.
Feeding Difficulties
- A baby who is not eating, wetting, or stooling well in the first few days of life is more likely to get jaundice.
Sibling with Jaundice
- A baby with a sister or brother that had jaundice is more likely to develop jaundice.
Bruising
- A baby with bruises at birth is more likely to get jaundice. A bruise forms when blood leaks out of a blood vessel and causes the skin to look black and blue. The healing of large bruises can cause high levels of bilirubin and your baby might get jaundice.
Blood Type
Women with an O blood type or Rh negative blood factor might have babies with higher bilirubin levels. A mother with Rh incompatibility should be given Rhogam.
Risk factors for severe hyperbilirubinemia include the following 1:
- Hemolysis due to ABO or other incompatibility,
- Cephalhematoma or substantial bruising,
- Excessive weight loss (≥ 10%), particularly in breastfeeding infants,
- East Asian ancestry,
- Gestational age of 35 to 36 weeks,
- History of a sibling receiving phototherapy, and
- Jaundice observed in the first 24 hours.
Any infant who requires resuscitation at birth or treatment for sepsis is also at increased risk.
Several risk factors have been identified for the development of severe hyperbilirubinemia in the newborn (Table 1). These risk factors are all common and the attributable risk of each is therefore very low. They are of limited use in directing surveillance, investigation or therapy by themselves, but can be useful in combination with timed total serum bilirubin analysis. It should also be noted that although a large number of studies have demonstrated an increased risk of severe hyperbilirubinemia with breastfeeding, one study 53 found that exclusive breastfeeding was associated with a lower incidence of hyperbilirubinemia. This may represent cultural differences in the approach to breastfeeding and the support mechanisms in place.
Table 1. Risk factors for the development of severe hyperbilirubinemia
| Risk Factor | Approximate odds ratio in comparison with the rest of the population |
| Visible jaundice at younger than 24 h | Unclear |
| Visible jaundice before discharge at any age | Unclear |
| Shorter gestation (less than 38 weeks) | For 36 weeks, 1.9 to 7.7 |
| Previous sibling with severe hyperbilirubinemia | 4.8 |
| Visible bruising | 2.6 |
| Cephalhematoma | 3.6 |
| Male sex | 1.3 to 1.7 |
| Maternal age older than 25 years of age | 2.6 |
| Asian or European background | 5.2 or 1.2, respectively |
| Dehydration | Depends on severity |
| Exclusive and partial breastfeeding | Very variable in the literature |
Who should have their bilirubin concentration measured, when and how?
Previous recommendations were to measure total serum bilirubin concentration in all infants with clinical jaundice at any time in the first four days of life, and to measure total serum bilirubin concentration in those who are not clinically jaundiced but have increased risk factors. Because of the high occurrence of the risk factors, this recommendation requires total serum bilirubin measurement in a large majority of infants (exceptions include females of certain ethnic groups who are fully formula fed and more than 37 weeks’ gestation). Despite these recommendations, infants continue to present with severe hyperbilirubinemia during or after their initial hospitalization. Recent data from the Canadian Paediatric Surveillance Program 34 demonstrated that 185 of 289 infants with critical hyperbilirubinemia presented after hospital discharge. There is an opportunity to perform universal screening for either total serum bilirubin or transcutaneous bilirubin (TcB) before the period of highest risk 35 and to use this to determine the risk profile and individualize follow-up. Furthermore, clinical assessment of jaundice is inadequate for diagnosing hyperbilirubinemia. Jaundice is not evident on clinical examination when the total serum bilirubin concentration is less than 68 µmol/L, and only 50% of babies with a total serum bilirubin concentration greater than 128 µmol/L appear jaundiced. One study 50 showed a difference of up to 100 µmol/L between visual and laboratory estimates of bilirubin concentration. In one study 54, all infants with a total serum bilirubin concentration greater than 204 µmol/L were identified as being jaundiced; in another study 55, 19% of infants with total serum bilirubin concentrations this high were not considered to be jaundiced by neonatologists. Although there have been no prospective, controlled trials to evaluate the effectiveness or cost-benefit relationship of universal screening, it appears to be a reasonable strategy, and an observational study 56 has reported it to be effective.
The peak total serum bilirubin concentration usually occurs between three and five days of life, at which time the majority of babies have already been discharged from hospital. At the usual age of discharge, total serum bilirubin concentrations that are in a high-risk zone on the nomograms cannot be reliably detected by visual inspection, especially in infants with darker skin colours. To predict the occurrence of severe hyperbilirubinemia, it is therefore recommended that either total serum bilirubin or transcutaneous bilirubin (TcB) concentration be measured in all infants between 24 hours and 72 hours of life; if the infant does not require immediate treatment, the results should be plotted on the predictive nomogram to determine the risk of progression to severe hyperbilirubinemia. The total serum bilirubin or transcutaneous bilirubin (TcB) concentration and the predictive zone should be recorded, a copy should be given to the family at the time of discharge, and follow-up arrangements should be made for infants who are at higher risk (Table 2).
Table 2. Response to results of bilirubin screening
| Zone | Greater than 37 weeks’ gestation and DAT-negative | 35 to 37 6/7 weeks’ gestation or DAT-positive | 35 to 37 6/7 weeks’ gestation and DAT-positive |
| High | Further testing or treatment required* | Further testing or treatment required* | Phototherapy required |
| High-intermediate | Routine care | Follow-up within 24 h to 48 | Further testing or treatment required* |
| Low-intermediate | Routine care | Routine care | Further testing or treatment required* |
| Low | Routine care | Routine care | Routine care |
Footnote: DAT = Direct Antiglobulin Test (direct Coombs test)
[Source 1 ]If the total serum bilirubin concentration had not been measured earlier because of clinical jaundice, a total serum bilirubin measurement should be obtained at the same time as the metabolic screening test to avoid an increase in the number of painful procedures and to minimize costs; alternatively, a transcutaneous bilirubin (TcB) measurement should be obtained either at discharge or before 72 hours of life. The prediction of severe hyperbilirubinemia is more accurate if the gestational age at birth is included in the prediction model 40.
Some of the most severely affected infants require therapy to be started before the time of the metabolic screen to prevent severe hyperbilirubinemia and its complications. Sudden increases in total serum bilirubin concentration may also occasionally occur after the first two to three days 57. This may occur particularly in association with excessive postnatal weight loss. Therefore, the institution of a program of universal screening complements, but does not replace, careful ongoing assessment of newborn infants beginning from the first hours of life and continuing through the first weeks. Systems to ensure follow-up within the recommended intervals after hospital discharge must be in place so that an infant who develops severe hyperbilirubinemia can be identified and treated promptly. This requires, for example, that an infant discharged from hospital in the first 24 hours of life be reviewed within 24 hours, any day of the week, by an individual with the training to recognize neonatal hyperbilirubinemia, obtain measurement of total serum bilirubin or transcutaneous bilirubin without delay and refer the infant to a treatment facility if required. This individual may be from any medical or nursing discipline.
In addition to universal measurement, all newborns should be clinically assessed for jaundice repeatedly within the first 24 hours, and again, at a minimum, 24 hours to 48 hours later. This should be performed by an individual competent in the assessment of the newborn who can, if necessary, immediately obtain a total serum bilirubin or transcutaneous bilirubin measurement and arrange treatment for the infant, whether in hospital or after discharge.
Measurement of bilirubin
It is possible to measure bilirubin concentration using capillary or venous blood samples or transcutaneously. There is no systematic difference between the results of capillary or venous samples 58. Capillary sampling is the method used most often in Canada and in most studies, including those of Bhutani et al 38. There are several limitations to transcutaneous bilirubin measurements 59: they become unreliable after initiation of phototherapy 60, and they may be unreliable with changes in skin colour and thickness 61. However, the results are more accurate at lower levels of bilirubin, and therefore, use of transcutaneous bilirubin as a screening device is reasonable 56. The available devices differ in accuracy; safe use of the device mandates knowledge of the accuracy of the particular device. The 95% confidence intervals for total serum bilirubin concentration based on the transcutaneous bilirubin measurement range from approximately 37 µmol/L to 78 µmol/L 62. For example, if the 95% confidence interval is 37 µmol/L, then a transcutaneous bilirubin concentration greater than 37 µmol/L below the treatment threshold on Figure 3 should be safe (i.e., if the threshold at 24 hour is 170 µmol/L, then a transcutaneous bilirubin of less than 133 µmol/L should be safe).
Measurement of free bilirubin
Displacement of bilirubin from albumin-binding sites by certain toxic medications and additives has caused numerous cases of kernicterus in the past, mostly in the neonatal intensive care unit population 63. It is believed to be free bilirubin (ie, not bound to albumin) that crosses the blood-brain barrier and causes neuronal damage 64. The clinical value of measurement of free bilirubin is currently uncertain and it is not readily available 65.
Measurement of conjugated bilirubin
Although early neonatal jaundice is generally due to unconjugated hyperbilirubinemia, in some situations the conjugated fraction may be elevated, such as in rhesus erythroblastosis, liver disease and cholestasis 66. In infants placed on phototherapy, measurement of the conjugated fraction should be considered. However, previous reports 33 on the epidemiology of bilirubin toxicity use the total serum bilirubin concentration as the standard, which remains the deciding value for phototherapy and other therapies. The conjugated bilirubin fraction should be estimated in an infant with persistent jaundice (longer than two weeks) and/or hepatosplenomegaly 67. A total conjugated bilirubin concentration greater than 18 µmol/L or greater than 20% of the total serum bilirubin concentration warrants further investigation 68.
Recommendations
- Either total serum bilirubin or transcutaneous bilirubin concentration should be measured in all infants during the first 72 hours of life. If not required earlier because of clinical jaundice, a total serum bilirubin measurement should be obtained at the same time as the metabolic screening test; alternatively, a transcutaneous bilirubin (TcB) measurement should be obtained either at discharge or, if not yet discharged, at 72 hours of life.
- If the total serum bilirubin concentration does not require immediate intervention, the results should be plotted on the predictive nomogram. The result of the total serum bilirubin measurement, the time at which it was obtained and the zone should be recorded, and a copy should be given to the parents. Follow-up of the infant should be individualized according to the risk assessment.
- Any infant discharged before 24 hours of life should be reviewed within 24 hours by an individual with experience in the care of the newborn who has access to testing and treatment facilities.
- There should be a systematic approach to the risk assessment of all infants before discharge and institution of follow-up care if the infant develops jaundice.
- All newborns who are visibly jaundiced in the first 24 hours of life should have their bilirubin level determined.
- Transcutaneous bilirubinometry is an acceptable method, either as a routine procedure or in infants with visible jaundice. The result should be summed with the 95% confidence interval of the device to estimate the maximum probable total serum bilirubin concentration.
- Total serum bilirubin concentration may be estimated on either a capillary or a venous blood sample.
- Infants with severe or prolonged hyperbilirubinemia should be further investigated, including measurement of the conjugated component of bilirubin.
How should severe hyperbilirubinemia be treated?
- Infants with a total serum bilirubin concentration above the thresholds shown on Figure 4 should have immediate intensive phototherapy, and should be referred for further investigation and preparation for exchange transfusion.
- An infant with clinical signs of acute bilirubin encephalopathy should have an immediate exchange transfusion.
Phototherapy
An infant who presents with severe hyperbilirubinemia, or who progresses to severe hyperbilirubinemia despite initial treatment, should receive immediate intensive phototherapy. The bilirubin concentration should be checked within 2 hours to 6 hours of initiation of treatment to confirm response. Consideration of further therapy should commence and preparations for exchange transfusion may be indicated. Supplemental fluids are indicated, and IV immunoglobulin (IVIG) should be given if not already commenced for the infant with isoimmunization.
Exchange transfusion
If phototherapy fails to control the rising bilirubin concentrations, exchange transfusion is indicated to lower total serum bilirubin concentrations. For healthy term newborns without risk factors, exchange transfusion should be considered when the total serum bilirubin concentration is between 375 µmol/L and 425 µmol/L (despite adequate intensive phototherapy). Because blood collected after an exchange transfusion is of no value for investigating many of the rarer causes of severe hyperbilirubinemia, these investigations should be considered before performing the exchange transfusion. Appropriate amounts of blood should be taken and stored for tests such as those for red cell fragility, enzyme deficiency (G6PD or pyruvate kinase deficiency) and metabolic disorders, as well as for hemoglobin electrophoresis and chromosome analysis. Preparation of blood for exchange transfusion may take several hours, during which time intensive phototherapy, supplemental fluids and IV immunoglobulin (IVIG) (in case of isoimmunization) should be used. If an infant whose total serum bilirubin concentration is already above the exchange transfusion line presents for medical care, then repeat measurement of the total serum bilirubin concentration just before performance of the exchange is reasonable, as long as therapy is not thereby delayed. In this way, some exchange transfusions, with their attendant risks, may be avoided. Exchange transfusion is a procedure with substantial morbidity that should only be performed in centres with the appropriate expertise under supervision of an experienced neonatologist. An infant with clinical signs of acute bilirubin encephalopathy should have an immediate exchange transfusion.
Jaundice in newborns diagnosis
Your baby will be checked for jaundice within 72 hours of being born during the newborn physical examination.
However, you should keep an eye out for signs of the condition after you return home as it can sometimes take up to a week to appear.
When you’re at home with your baby, you should look out for yellowing of their skin or the whites of their eyes. Gently pressing your fingers on the tip of their nose or on their forehead can make it easier for you to spot any yellowing.
You should also check your baby’s urine and poo. Your baby may have jaundice if their urine is yellow (a newborn baby’s urine should be colorless) or their poo is pale.
You should speak to your midwife or healthcare provider as soon as possible if you think your baby may have jaundice. Tests will need to be carried out to determine whether any treatment is necessary.
Visual examination
A visual examination of your baby will be carried out to look for signs of jaundice. Your baby needs to be undressed during this so their skin can be looked at under good – preferably natural – light.
Other things that may also be checked include:
- the whites of your baby’s eyes
- your baby’s gums
- the color of your baby’s urine or poo
Bilirubin test
If it’s thought your baby has jaundice, the level of bilirubin in their blood will need to be tested. This can be done using:
- a small device called a bilirubinometer, which beams light on to your baby’s skin – it calculates the level of bilirubin by analyzing how the light reflects off or is absorbed by the skin
- a blood test of a sample of blood taken by pricking your baby’s heel with a needle – the level of bilirubin in the liquid part of the blood (the serum) is then measured
In most cases, a bilirubinometer is used to check for jaundice in babies. Blood tests are usually only necessary if your baby developed jaundice within 24 hours of birth or the reading is particularly high.
The level of bilirubin detected in your baby’s blood is used to decide whether any treatment is necessary.
Further blood tests
Further blood tests may need to be carried out if your baby’s jaundice lasts longer than two weeks or treatment is needed. The blood is analyzed to determine:
- the baby’s blood group – this is to see if it’s incompatible with the mother’s
- whether any antibodies (infection-fighting proteins) are attached to the baby’s red blood cells
- the number of cells in the baby’s blood
- whether there’s any infection
- whether there’s an enzyme deficiency
These tests help determine whether there’s another underlying cause for the raised levels of bilirubin.
Kernicterus treatment
Treatment for kernicterus involves using an exchange transfusion as used in the treatment of newborn jaundice.
Exchange transfusion is the standard method of therapy for immediate treatment of severe hyperbilirubinemia and prevention of kernicterus. Although the frequency of neonatal exchange transfusion has declined markedly in the past two decades, this procedure is still performed in many countries, especially in those with a high incidence of neonatal hyperbilirubinemia.
Exchange transfusion is effective and considered to be safe procedure ; however, it is not without risks. Complications have been reported and mortality rates vary from 0.5 to 3.3%. therefore,the current recommendation for performing exchange transfusion are based on balance between the risks of encephalopathy and complications related to the procedure .
Most of these complications are transient, such as severe thrombocytopenia, apnea, hypocalcemia , bradycardia, and hyperkalemia and recovery is expected along with appropriate care and follow up. But serious complications and even death can occurs due to cardiovascular collapse during exchange , necrotizing enterocolitis, bacterial sepsis, and pulmonary hemorrhage that can be avoided by careful cardio-pulmonary and oxygen saturation monitoring.
There is currently no specific treatment for dyskinetic cerebral palsy, but supportive therapy can be instituted to improve symptoms. Dystonia can be managed with a combination of physical, occupational, and speech therapies. The hypertonia generally disappears with sleep, and botulinum toxin (Botox) can be used to relax muscle tone. Orthopedic procedures, including tendon-lengthening as typically used to treat patients with spastic cerebral palsy, are not indicated for dyskinetic cerebral palsy, and medication and Botox injections are preferred 10. For severe forms of dystonia, baclofen pumps may be useful in reducing muscle tone and severity of muscle spasms.
Due to hypertonia and hyperkinetic activity, infants with dyskinetic cerebral palsy may have higher caloric requirements, complicated by frequent challenges in swallowing or self-feeding. Decreased or abnormal motility of gastrointestinal muscles can complicate digestion, creating further challenges with adequate nutrition.
Based on success of deep-brain stimulation (DBS) in other movement disorders such as Parkinson disease, deep-brain stimulation (DBS) has been suggested as a possible therapy for dyskinetic cerebral palsy. Stimulators placed in the globus pallidus internus may help control dystonia, but a limited number of trials has demonstrated only small improvements in motor symptoms. Results following deep-brain stimulation (DBS) in patients with dystonia have been most effective in certain subgroups, such as individuals with genetic-based dystonia caused by a DYT1 mutation, but less effective in individuals with secondary dystonia 69. Kim et al. 70 demonstrated improvements in dystonia in adults with cerebral palsy, but little improvement in overall disability measures.
- Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr Child Health 2007;12(Suppl B):1B-12B https://www.cps.ca/en/documents/position/hyperbilirubinemia-newborn[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kernicterus in the 21st century: frequently asked questions. Bhutani VK, Johnson L. J Perinatol. 2009 Feb; 29 Suppl 1():S20-4. https://www.ncbi.nlm.nih.gov/pubmed/19177056/[↩]
- Bilirubin toxicity in the developing nervous system. Shapiro SM. Pediatr Neurol. 2003 Nov; 29(5):410-21.[↩][↩]
- Criteria for exchange transfusion in jaundiced newborns. Ahlfors CE. Pediatrics. 1994 Mar; 93(3):488-94.[↩]
- Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Oh W, Stevenson DK, Tyson JE, Morris BH, Ahlfors CE, Bender GJ, Wong RJ, Perritt R, Vohr BR, Van Meurs KP, Vreman HJ, Das A, Phelps DL, O’Shea TM, Higgins RD, NICHD Neonatal Research Network Bethesda MD. Acta Paediatr. 2010 May; 99(5):673-678.[↩]
- Shaw E, Grenier D. Prevention of kernicterus: new guidelines and the critical role of family physicians. Can Fam Physician. 2008;54(4):575-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2294096/[↩][↩][↩][↩][↩]
- Ipek, B., Ecevit, C., Ipek, I., Kocabas, O., Kavakli, T., Ozturk, A., The evaluation of 371 cases with cerebral palsy between January 1984 and December 2004, Journal of Neurological Sciences, 24, 270–279, 2007[↩]
- Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin Fetal Neonatal Med. 2014;20(1):20-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4388741/[↩][↩][↩][↩]
- Sgro M, Campbell DM, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ. 2006;175(6):587–90.[↩]
- Shapiro SM. Kernicterus. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the jaundiced neonate. New York: McGraw-Hill Medical; 2012.[↩][↩][↩][↩]
- Johnston MV, Hoon AH. Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–91.[↩][↩]
- Yilmaz Y, Alper G, Kilicoglu G, Celik L, Karadeniz L, Yilmaz-Degirmenci S. Magnetic resonance imaging findings in patients with severe neonatal indirect hyperbilirubinemia. J Child Neurol. 2001;16:452–5.[↩][↩]
- Nolte J. The human brain: an introduction to its functional anatomy. 6th. Philadelphia, PA: Mosby/Elsevier; 2009.[↩][↩]
- Govaert P, Lequin M, Swarte R, et al. Changes in globus pallidus with (pre)term kernicterus. Pediatrics. 2003;112(6 Pt 1):1256–63.[↩]
- Gkoltsiou K, Tzoufi M, Counsell S, Rutherford M, Cowan F. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev. 2008;84:829–38.[↩][↩]
- Shapiro SM, Bhutani VK, Johnson L. Hyperbilirubinemia and kernicterus. Clin Perinatol. 2006;33:387–410.[↩]
- Falcão AS, Silva RFM, Fernandes A, Brito MA, Brites D. Influence of hypoxia and ischemia preconditioning on bilirubin damage to astrocytes. Brain Res. 2007;1149:191–9[↩]
- Mao J, Fu J, Chen L, Wang X, Xue X. Changes of globus pallidus in the newborn infants with severe hyperbilirubinemia. Chin J Pediatr. 2007;45:24–9.[↩]
- Single photon emission computed tomography and serial MRI in preterm infants with kernicterus. Okumura A, Hayakawa F, Maruyama K, Kubota T, Kato K, Watanabe K. Brain Dev. 2006 Jul; 28(6):348-52.[↩][↩]
- Birth-related syndromes of athetosis and kernicterus. Przekop A, Sanger TD. Handb Clin Neurol. 2011; 100():387-95.[↩]
- Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND) J Perinatol. 2005;25:54–9.[↩][↩][↩]
- Przekop A, Sanger TD. Birth-related syndromes of athetosis and kernicterus. Handb Clin Neurol. 2011;100:387–95.[↩][↩][↩][↩][↩]
- Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25:1538–49.[↩][↩][↩]
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14.[↩]
- Hansen TW. Mechanisms of bilirubin toxicity: Clinical implications. Clin Perinatol 2002;29:765-778,viii.[↩]
- American Academy of Pediatrics, Subcommittee on Neonatal Hyperbilirubinemia. Neonatal jaundice and kernicterus. Pediatrics 2001;108:763-5.[↩]
- Stevenson DK, Fanaroff AA, Maisels MJ, et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 2001;108:31-9.[↩]
- Harris MC, Bernbaum JC, Polin JR, Zimmerman R, Polin RA. Developmental follow-up of breastfed term and near-term infants with marked hyperbilirubinemia. Pediatrics 2001;107:1075-80.[↩]
- Bhutani VK, Johnson LH, Maisels JM, et al. Kernicterus: Epidemiological strategies for its prevention through systems-based approaches. J Perinatol 2004;24:650-62.[↩]
- American Academy of Pediatrics, Subcommittee on Neonatal Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316. (Erratum in 2004;114:1138).[↩][↩]
- Ip S, Glicken S, Kulig J, et al. Management of Neonatal Hyperbilirubinemia. Evidence Report/Technology Assessment No. 65 (Prepared by Tufts-New England Medical Center Evidence-based Practice Center under Contract No. 290-97-0019). AHRQ Publication No. 03-E011. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality, 2003.[↩][↩]
- Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics 2004;113:775-80.[↩][↩]
- Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 2004;114:e130-53.[↩][↩]
- Sgro M, Campbell D, Shah V. Incidence and causes of severe neonatal hyperbilirubinemia in Canada. CMAJ 2006;175:587-90.[↩][↩][↩]
- Centers for Disease Control and Prevention (CDC). Kernicterus in full term infants – United States, 1994-1998. MMWR Morb Mortal Wkly Rep 2001;50:491-4.[↩][↩]
- Ebbesen F. Recurrence of kernicterus in term and near-term infants in Denmark. Acta Paediatr 2000;89:1213-7.[↩]
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001;344:581-90.[↩]
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6-14.[↩][↩][↩]
- Newman TB, Liljestrand P, Escobar GJ. Combining clinical risk factors with serum bilirubin levels to predict hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med 2005;159:113-9.[↩]
- Sarici SU, Yurdakok M, Serdar MA, et al. An early (sixth-hour) serum bilirubin measurement is useful in predicting the development of significant hyperbilirubinemia and severe ABO hemolytic disease in a selective high-risk population of newborns with ABO incompatibility. Pediatrics 2002;109:e53.[↩][↩][↩]
- Carbonell X, Botet F, Figueras J, Riu-Godo A. Prediction of hyperbilirubinaemia in the healthy term newborn. Acta Paediatr 2001;90:166-70.[↩]
- Madlon-Kay DJ. The clinical significance of ABO blood group incompatibility. Arch Fam Med 1993;2:285-7.[↩]
- Ozolek JA, Watchko JF, Mimouni F. Prevalence and lack of clinical significance of blood group incompatibility in mothers with blood type A or B. J Pediatr 1994;125:87-91.[↩]
- Dinesh D. Review of positive direct antiglobulin tests found on cord blood sampling. J Paediatr Child Health 2005;41:504-7.[↩]
- Madan A, Huntsinger K, Burgos A, Benitz WE. Readmission for newborn jaundice: The value of the Coombs’ test in predicting the need for phototherapy. Clin Pediatr (Phila) 2004;43:63-8.[↩]
- Kaplan M, Herschel M, Hammerman C, Karrison T, Hoyer JD, Stevenson DK. Studies in hemolysis in glucose-6-phosphate dehydrogenase-deficient African American neonates. Clin Chim Acta 2006;365:177-82.[↩]
- Meloni T, Forteleoni G, Dore A, Cutillo S. Neonatal hyperbilirubinaemia in heterozygous glucose-6-phosphate dehydrogenase deficient females. Br J Haematol 1983;53:241-6.[↩]
- Kaplan M, Beutler E, Vreman HJ, et al. Neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Pediatrics 1999;104(1 Pt 1):68-74.[↩]
- Gourley GR, Kreamer B, Arend R. The effect of diet on feces and jaundice during the first 3 weeks of life. Gastroenterology 1992;103:660-7.[↩]
- Riskin A, Kugelman A, Abend-Weinger M, Green M, Hemo M, Bader D. In the eye of the beholder: How accurate is clinical estimation of jaundice in newborns? Acta Paediatr 2003;92:574-6. Erratum in 2005;94:1168[↩][↩]
- Bhutani VK, Gourley GR, Adler S, Kreamer B, Dalin C, Johnson LH. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics 2000;106:e17.[↩]
- Complications of Exchange Transfusion in Neonates (COET). https://clinicaltrials.gov/ct2/show/NCT03195049[↩]
- Seidman DS, Ergaz Z, Paz I, et al. Predicting the risk of jaundice in full-term healthy newborns: A prospective population-based study. J Perinatol 1999;19(8 Pt 1):564-7.[↩]
- Madlon-Kay DJ. Recognition of the presence and severity of newborn jaundice by parents, nurses, physicians, and icterometer. Pediatrics 1997;100:E3.[↩]
- Riskin A, Abend-Weinger M, Bader D. How accurate are neonatologists in identifying clinical jaundice in newborns? Clin Pediatr (Phila) 2003;42:153-8.[↩]
- Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics 2006;117:e855-62.[↩][↩]
- Watchko JF. Vigintiphobia revisited. Pediatrics 2005;115:1747-53.[↩]
- Amato M, Huppi P, Markus D. Assessment of neonatal jaundice in low birth weight infants comparing transcutaneous, capillary and arterial bilirubin levels. Eur J Pediatr 1990;150:59-61.[↩]
- Rubaltelli FF, Gourley GR, Loskamp N, et al. Transcutaneous bilirubin measurement: A multicentre evaluation of a new device. Pediatrics 2001;107:1264-71.[↩]
- Cremer RJ, Perryman PW, Richards DH. Influence of light on the hyperbilirubinaemia of infants. Lancet 1958;1:1094-7.[↩]
- Rubaltelli FF, Carli M. The effect of light on cutaneous bilirubin. Biol Neonate 1971;18:457-62.[↩]
- Maisels MJ, Ostrea EM Jr, Touch S, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics 2004;113:1628-35.[↩]
- Hansen TW. Recent advances in the pharmacotherapy for hyperbilirubinaemia in the neonate. Expert Opin Pharmacother 2003;4:1939-48.[↩]
- Ahlfors CE. Unbound bilirubin associated with kernicterus: A historical approach. J Pediatr 2000;137:540-4.[↩]
- McDonagh AF, Maisels MJ. Bilirubin unbound: Deja vu all over again? Pediatrics 2006;117:523-5.[↩]
- Venigalla S, Gourley GR. Neonatal cholestasis. Semin Perinatol 2004;28:348-55.[↩]
- Butler DA, MacMillan JP. Relationship of breast feeding and weight loss to jaundice in the newborn period: Review of the literature and results of a study. Cleve Clin Q 1983;50:263-8.[↩]
- Keffler S, Kelly DA, Powell JE, Green A. Population screening for neonatal liver disease: A feasibility study. J Pediatr Gastroenterol Nutr 1998;27:306-11.[↩]
- Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–9.[↩]
- Kim AR, Chang JW, Chang WS, Park ES, Cho SR. Two-year outcomes of deep brain stimulation in adults with cerebral palsy. Ann Rehabil Med. 2014;38:209.[↩]