Kimura disease
Kimura disease also known as eosinophilic hyperplastic lymphogranuloma, is a rare benign inflammatory disease of unknown cause that characteristically manifests as painless enlargement of cervical lymph nodes (painless lymphadenopathy) and salivary glands 1. Kimura disease was first reported in China by Kim in 1937 as eosinophilic hyperplastic lymphogranuloma 2. Until 1948, Kimura disease was widely recognized in Japan and systematically described by Professor Kimura as ‘unusual granulations combined with hyperplastic changes in lymphoid tissue’ 3. Kimura disease is a chronic disease and its etiology has not been fully elucidated to date 4. An infectious agent is presumed to be the cause of an immunological response, however no specific pathogen has as yet been identified 5. Patients suffering from Kimura disease mainly present with solitary or multiple painless masses in the maxillofacial and other regions, which often recur after treatment 6. Clinically, Kimura disease is always accompanied by enlarged regional lymph nodes, eosinophilia and markedly elevated serum immunoglobulin E (IgE) levels 7.
Kimura disease typically affects males (80%) between 20 and 40 years of age (80% of cases) 8 and is most frequently seen in East and Southeast Asia, with a small number of cases reported in Europe and the Middle East 9. Although more rare, pediatric populations can develop Kimura disease, and cases have been reported in persons aged as young as 15 months 9. Kimura disease has been most commonly diagnosed in middle-aged individuals in the Far East, for example China and Japan 10. Sporadic cases are seen in other geographic regions, however these are uncommon. Thus far, only 200 cases have been reported worldwide.
Controversy has existed in the literature regarding whether Kimura disease and angiolymphoid hyperplasia with eosinophilia (ALHE) are the same entity 4. Some authors believe that Kimura disease represents a chronic, deeper form of angiolymphoid hyperplasia with eosinophilia (ALHE); however, most papers distinguish the two on the basis of clinical and histopathologic characteristics 11. Angiolymphoid hyperplasia with eosinophilia (ALHE) appears to represent an arteriovenous malformation with secondary inflammation. Kimura disease may represent a primary inflammatory process with secondary vascular proliferation 12. Reports have described both diseases presenting simultaneously 13.
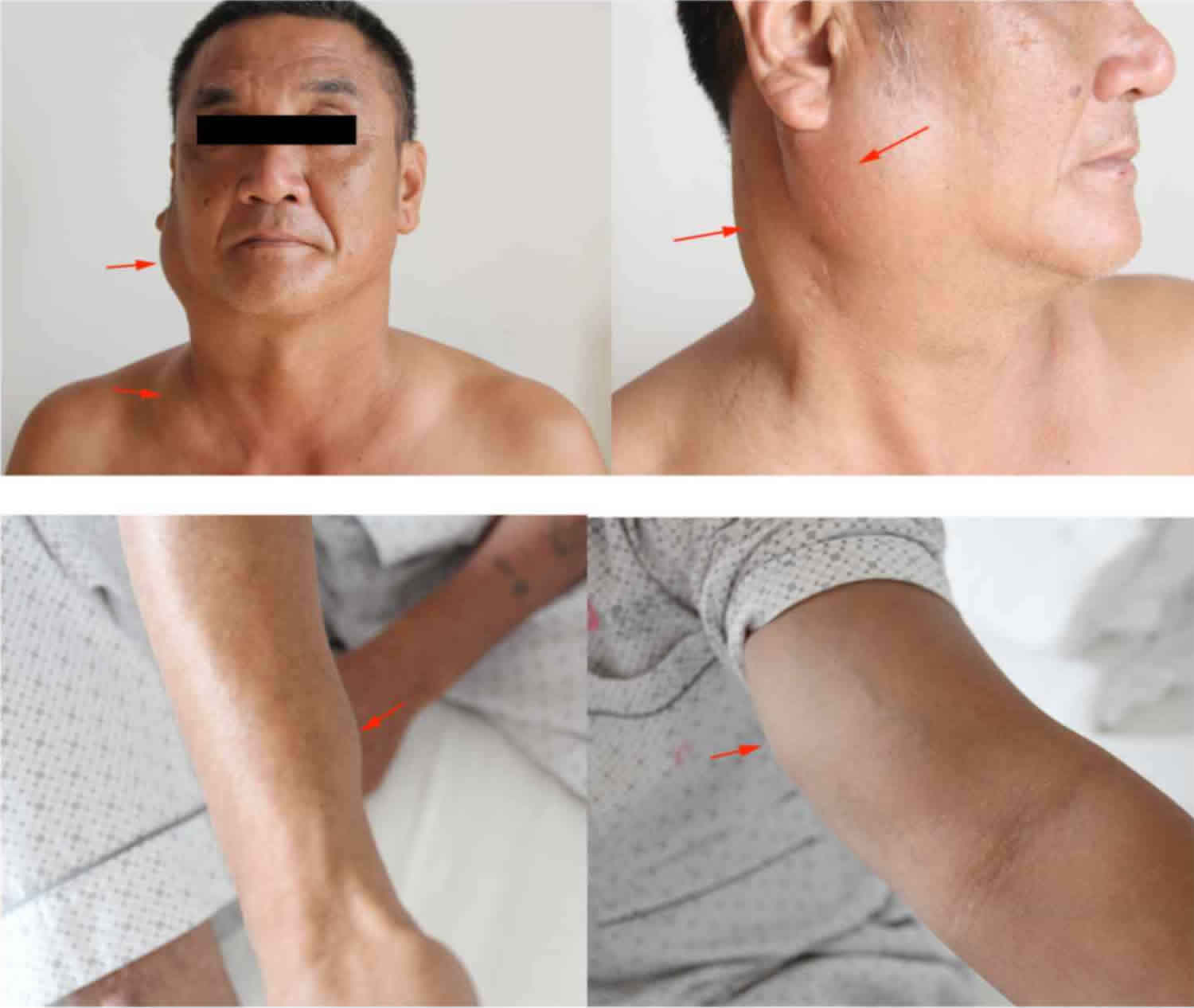
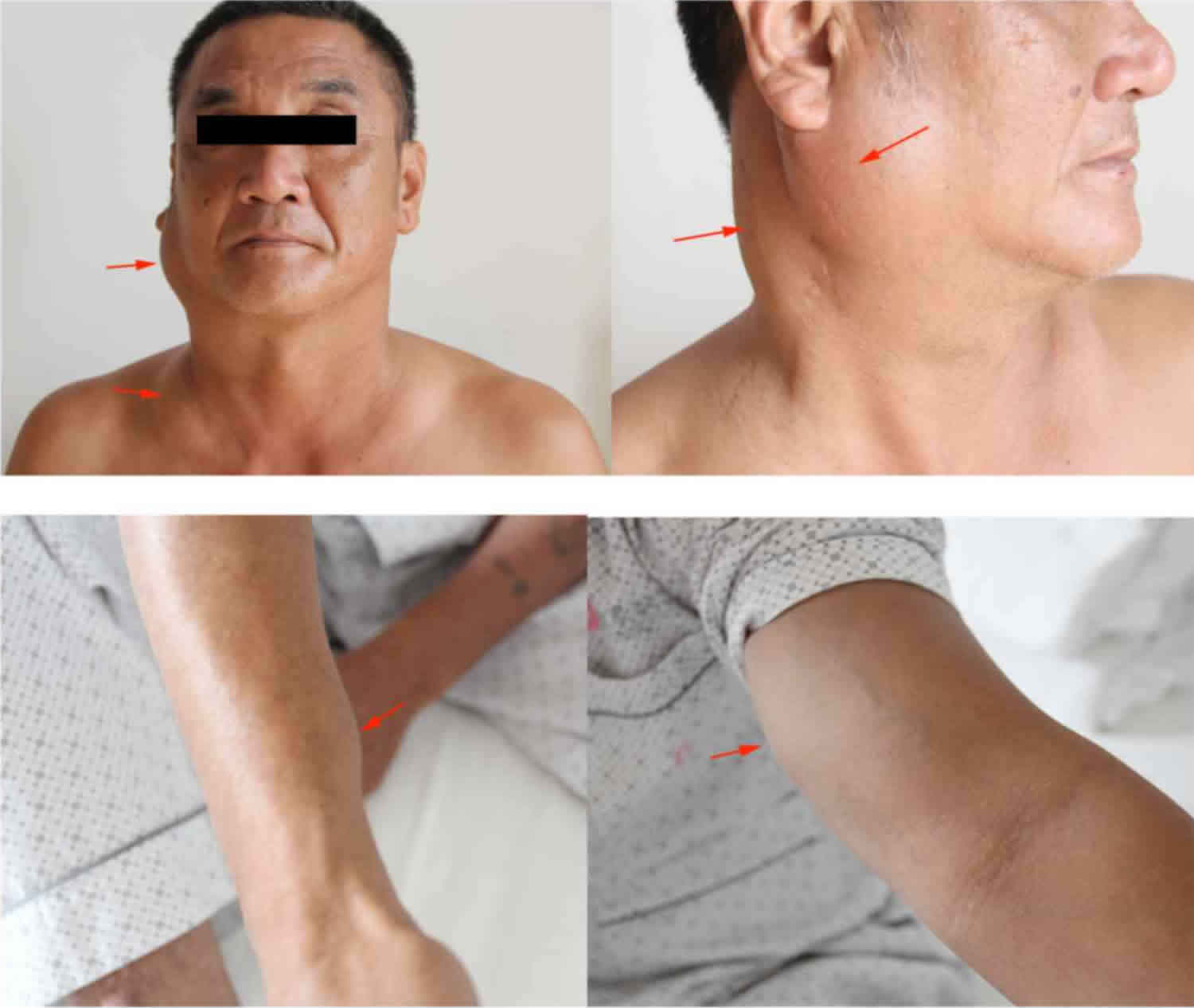
Figure 1. Kimura disease
Footnote: The patient who presented with painless masses (arrows) in the region of the right parotid gland and neck, the left supraclavicular fossa, the right forearm near the wrist joint and the left medial upper arm.
[Source 1 ]Kimura disease causes
The cause and pathophysiology of Kimura disease remains unknown. It has been confirmed that there is no correlation with tuberculosis, bacterial, fungal or viral infections, poisoning or syphilis 14. It has been hypothesized that an infection or toxin may trigger an autoimmune phenomenon or lead to a type 1 (immunoglobulin E [IgE]–mediated) hypersensitivity reaction 15. Googe et al 16 reported increased IgE levels in Kimura disease patients with renal involvement, which supports the theory that Kimura disease is an immune response; furthermore, clinical application of steroid therapy appears to be effective, further supporting this theory.
Some evidence has suggested a predominance of T helper type 2 (Th2) cells and and type 1 CD8+ T cells (Tc1) in patients with Kimura disease 17. Other studies demonstrated that the release of cytokines in patients with Kimura disease can increase the permeability of the glomerular basement membrane, causing proteinuria, which may ultimately cause renal damage 18. Chim et al 19 considered a clonal T-cell lymphoproliferative disorder as the possible cause of Kimura disease; however, this result was from a single case study, and the etiological analysis remains speculative.
Additional studies have shown elevated granulocyte-macrophage stimulating-factor (GM-CSF), tumor necrosis factor-α (TNF-α), soluble interleukin (IL)–2 receptor (sIL-2R), IL-4, IL-5, IL-10, and IL-13 20. Another study indicated that the activation of the IL-21/pERK1/2 pathway is a component of Kimura disease immunopathogenesis and that pERK1/2 could be a potential prognostic indicator of Kimura disease 21. These findings may help lay the groundwork for elucidating the underlying pathophysiology of Kimura disease.
Kimura disease symptoms
Kimura disease typically presents as a painless subcutanous mass or masses (enlargement of the lymph nodes) in the head and neck region particularly near the angle of the mandible and post-auricular groups, with occasional pruritus of the overlying skin. Salivary glands (particularly the parotid and submandibular glands) and lymph nodes of the axilla, groin, popliteal fossa, medial epicondyle 22 and elsewhere may also be involved 23. Involvement of adjacent soft tissues is uncommon, although direct extension into the pinna of the ear has been described. There have been case reports of eosinophillic panniculitis in patients with Kimura disease 24.
Renal disease, nephrotic syndrome in particular, is present in up to 20% of patients with Kimura disease 25. An estimated 12-16% of patients with Kimura disease exhibit proteinuria upon examination, of which 59-78% have nephrotic syndrome 26. Less commonly, several reports in the literature have linked Kimura disease with a hypercoagulable state in patients without associated nephrotic syndrome 27. Visible ischemia of the extremities may be present as a result of the hypercoagulable complication of Kimura disease, and related chronic diseases such as Raynaud phenomenon and acute limb ischemia have been reported when left untreated 28.
These changes are usually associated with eosinophilia in the peripheral blood and in tissues, and marked increase in serum levels of immunoglobulin E (IgE) 29.
Physical examination
Patients with Kimura disease typically present with nontender subcutaneous nodules and masses in the head and neck, especially in the parotid and submandibular regions. These lesions are often associated with lymphadenopathy. Less frequently, the orbit (including the eyelids, conjunctiva, and lacrimal glands) 30, paranasal sinuses, epiglottis, tympanic membrane, parotid gland, parapharyngeal space, palate, axilla, groin, and breast 31 may be involved 32. Although Kimura disease mainly affects the head and neck, involvement of the extremities and inguinal lymph nodes has been reported 33. In addition, a presentation of Kimura disease as a pulmonary hilar mass has been described 34.
Kimura disease complications
Rarely, large nodules or tumors from Kimura disease have ulcerated. After treatment, recurrence also has been reported. Abbas et al 35 reported one case of postsurgical facial disfigurement effectively treated with photodynamic therapy.
On two known occasions, brain embolisms have occurred due to Kimura disease–induced thrombi 36.
Kimura disease diagnosis
There are currently no uniform diagnostic criteria for Kimura disease. The following characteristics should raise suspicion of Kimura disease 37:
- Young male, with head and/or neck painless mass;
- Local enlarged lymph nodes;
- Llong history;
- Parts of body other than the head and neck displaying multiple painless masses, accompanied by pruritus of the overlying skin;
- Increased blood eosinophil count and serum IgE level (a high reference value is needed to make a correct diagnosis); and
- CT and MRI showing a wide range of lesions and multiple enlarged lymph nodes.
Based on these clinical manifestations and blood test results, the diagnosis of Kimura disease may be preliminarily considered, but the final diagnosis relies on pathological examination. The histopathological characteristics of Kimura disease include follicular hyperplasia, eosinophilic infiltrates and proliferation of postcapillary venules 38.
Kimura disease patients almost always have marked peripheral eosinophilia and elevated serum IgE levels. Some studies report that blood eosinophil counts are closely associated with the size of the lesion, i.e., the bigger the lesion, the higher the eosinophil count 39. Serum eosinophil cationic protein levels parallel the course of the disease 40. Blood urea nitrogen, creatinine, and urinary protein levels should be obtained to exclude concomitant renal dysfunction (especially nephrotic syndrome).
Physical examination along with ultrasound, CT and MRI may help determine the characteristics, boundaries and blood supply of the mass, as well as the presence of lymph node involvement. On ultrasound, the mass may exhibit heterogeneous or homogeneous echogenicity 41, occasionally displaying increased vascularity. On CT scans, the lesions are strongly enhanced, reflecting their vascular nature; lymphadenopathy is reported among the typical findings. On MRI, the lesion exhibits intermediate to high signal intensity on T1-weighted images and hyperintense signals on T2-weighted images 42. Therefore, imaging may be a useful way of demonstrating the morphology and extent of the lesion, as well as its association with adjacent structures 10.
Histologically Kimura disease is characterized by:
- proliferation of lymphoid follicles
- cellular infiltrates
- eosinophils (majority), sometimes progressing to eosinophilic abscesses
- also plasma cells lymphocytes, and mast cells
- vascular proliferation of post-capillary venules
- fibrosis
Kimura disease differential diagnosis
Clinically, the differential diagnosis of Kimura disease may include angiolymphoid hyperplasia with eosinophilia (ALHE), Hodgkin’s lymphoma, angioimmunoblastic T-cell lymphoma, Langerhans cell histiocytosis, florid follicular hyperplasia, Castleman’s disease, dermatopathic lymphadenopathy, sinonasal eosinophilic angiocentric fibrosis, drug reactions and parasitic infections. In the literature, the most common misdiagnosis is ALHE 38; however, patients with ALHE have normal IgE levels and no kidney damage 43.
Kimura disease treatment
A standard treatment for Kimura disease has not yet been established. The goal of treatment currently is to maintain appearance and functionality, while preventing recurrence and long-term complications, including nephritis and myocarditis.
Observation is acceptable if the Kimura disease lesions are neither symptomatic nor disfiguring 44.
Although surgery is the most widely used treatment method and it can help reach a definite diagnosis 39, other options include radiotherapy, systemic corticosteroids, cytotoxic agents, cyclosporin and pentoxifylline 45.
Oral corticosteroids are usually recommended in cases of symptomatic nephrotic syndrome 18 and, in order to prevent relapse and reduce the long-term side effects of steroid therapy, postoperative radiation therapy may be used (low-dose local irradiation, ~25–30 Gy) 46. Reportedly, the recurrence rate appears to be lower if two treatment modalities are combined 47.
Oral corticosteroids are commonly used; however, the disease frequently recurs after cessation of therapy. Intralesional corticosteroids may be effective for localized disease 48.
Oral corticosteroids in combination with cetirizine may prove to be an effective alternative treatment to surgery to reduce related nodular masses. In one patient, continued daily cetirizine prevented recurrence 4 months after tapering steroids 49.
Leflunomide in combination with oral prednisone may be an option for treating patients with or without renal involvement who are unresponsive to corticosteroids alone. The subject of one study remained disease free at the 12-month follow up after treatment with leflunomide and methylprednisone 50. Leflunomide may have an antiproliferative effect on eosinophils.
Cyclosporine has been reported to induce remission in patients with Kimura disease 51. A dose of 5 mg/kg/d was effective, but the lesions may recur upon cessation of therapy 52.
Intravenous immunoglobulin (IVIg) was used in one patient as a steroid-sparing agent, and he remained disease free more than 6 years after follow-up 53.
Oral pentoxifylline has been reported to be effective in one patient with Kimura disease; however, the lesions relapsed after discontinuation of therapy 54.
All trans-retinoic acid in combination with prednisone has resulted in remission of Kimura disease in one patient, and he remained disease free 12 months after discontinuation of all therapy 55.
Imatinib may be an effective treatment for Kimura disease, based on advances in research for therapy in hypereosinophilic syndrome, but further investigation is necessary 56.
Photodynamic therapy has been used successfully in one patient who experienced recurrence of disease after initial surgical management 35.
Radiotherapy has occasionally been used to treat recurrent or persistent Kimura disease lesions. A report by Hareyama et al 57 described the use of radiotherapy at dosages of 26-30 Gy; local control was achieved in 74% of lesions. Another study demonstrated that radiotherapy (20-45 Gy) was more effective than local excision and steroid treatment, with local response rates of 64.3% versus 22.2%, respectively. No adverse effects were observed during a mean follow-up period of 65 months 58. New technology such as three-dimensional printing is being explored for preventing collateral damage during radiotherapy bolus delivery to radiosensitive areas such as the head and neck 59. However, considering the benign nature of Kimura disease, radiation should be reserved for recurrent or disfiguring lesions.
Recently, anti-IgE therapy has been introduced 60, and the size of lesion and peripheral blood eosinophil count of Kimura disease patients were reported to decrease following anti-IgE therapy.
The largest retrospective meta-analysis of Kimura disease treatment to date (n=639) concluded that surgical resection with low-dose postoperative radiotherapy resulted in the lowest recurrence rate of Kimura disease. Furthermore, it suggested that corticosteroid therapy should be a second-line option for treatment, owing to the potential adverse effects of long-term corticosteroid use and the high rates of recurrence when used alone 61. The same conclusions were made in another study comparing recurrence rates in 46 patients who underwent steroid therapy, surgical excision, radiotherapy, or surgical excision with radiotherapy 48.
Kimura disease prognosis
The course of Kimura disease is chronic, with lesions frequently persisting or recurring in the original location despite treatment, with a recurrence rate of up to 25% 62, posing a major challenge to the physician and patient 63. Kimura disease can lead to disfigurement secondary to the growth of untreated lesions, particularly given the predilection for the head and neck. Additionally, recurrence after treatment is well described. Smoking habits and a history of systemic disease also have been associated with poor prognosis of the disease 64. To date, malignant transformation of Kimura disease has not been described in the literature.
- Li, Xuesheng & Wang, Jing & Li, Hongbo & Zhang, Ming. (2018). Misdiagnosed recurrent multiple Kimura’s disease: A case report and review of the literature. Molecular and Clinical Oncology. 10. 10.3892/mco.2018.1793[↩][↩]
- Kim HT: Eosinophilic hyperplastic lymphogranuloma, comparison with Mikulicz’s disease. Chin Med J. 23:6991937[↩]
- Kimura T and Yoshimura S: Unusual granuloma combined with hyperplastic changes in lymphatic tissues. Trans Soc Path Jpn. 13:179–180. 1948.[↩]
- Kimura Disease. https://emedicine.medscape.com/article/1098777-overview[↩][↩]
- Briggs PL. Kimura disease is not angiolymphoid hyperplasia with eosinophilia: clinical and pathological correlation with literature review and definition of diagnostic criteria (Portuguese). An. Bras. Dermatol. 2006;81 (2): 167-173. https://www.scielo.br/pdf/abd/v81n2/en_v81n02a09.pdf[↩]
- Abuel-Haija M, Hurford MT. Kimura disease. Arch Pathol Lab Med. 2007 Apr;131(4):650-1. https://meridian.allenpress.com/aplm/article/131/4/650/64574/Kimura-Disease[↩]
- Kuo TT, Shih LY and Chan HL: Kimura’s disease. Involvement of regional lymph nodes and distinction from angiolymphoid hyperplasia with eosinophilia. Am J Surg Pathol. 12:843–854. 1988.[↩]
- Ahuja A, Ying M, Mok JS, Anil CM. Gray scale and power Doppler sonography in cases of Kimura disease. AJNR Am J Neuroradiol. 2001 Mar;22(3):513-7. http://www.ajnr.org/content/22/3/513.long[↩]
- AlGhamdi FE, Al-Khatib TA, Marzouki HZ, AlGarni MA. Kimura disease: No age or ethnicity limit. Saudi Med J. 2016 Mar. 37 (3):315-9.[↩][↩]
- Park SW, Kim HJ, Sung KJ, Lee JH and Park IS: Kimura disease: CT and MR imaging findings. AJNR Am J Neuroradiol. 33:784–788. 2012.[↩][↩]
- García Carretero R, Romero Brugera M, Rebollo-Aparicio N, Vazquez-Gomez O. Eosinophilia and multiple lymphadenopathy: Kimura disease, a rare, but benign condition. BMJ Case Rep. 2016 Aug 31. 2016[↩]
- Tariq N, Sadiq S, Si K. Angiolymphoid Hyperplasia with Eosinophillia – A Rare Entity. Internat J Pathol. 2016. 14(1):1-5.[↩]
- Reddy PK, Prasad AL, Sumathy TK, Shivaswamy KN, Ranganathan C. An Overlap of Angiolymphoid Hyperplasia with Eosinophilia and Kimura’s Disease: Successful Treatment of Skin Lesions with Cryotherapy. Indian J Dermatol. 2015 Mar-Apr. 60 (2):216.[↩]
- Asadi AK: Angiolymphoid hyperplasia with eosinophilia. Dermatol Online J. 8:102002. [↩]
- Kimura Y, Pawankar R, Aoki M, Niimi Y and Kawana S: Mast cells and T cells in Kimura’s disease express increased levels of interleukin-4, interleukin-5, eotaxin and RANTES. Clin Exp Allergy. 32:1787–1793. 2002.[↩]
- Googe PB, Harris NL and Mihm MC Jr: Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: Two distinct histopathological entities. J Cutan Pathol. 14:263–271. 1987.[↩]
- Ohta N, Fukase S, Suzuki Y, Ito T, Yoshitake H, Aoyagi M. Increase of Th2 and Tc1 cells in patients with Kimura’s disease. Auris Nasus Larynx. 2011 Feb. 38(1):77-82.[↩]
- Uysal IO, Eryilmaz MA, Salk I and Abasiyanik F: Kimura disease in the parotid gland. J Craniofac Surg. 22:337–338. 2011.[↩][↩]
- Chim CS, Fung A, Shek TW, Liang R, Ho WK and Kwong YL: Analysis of clonality in Kimura’s disease. Am J Surg Pathol. 26:1083–1086. 2002.[↩]
- Hosoki K, Hirayama M, Kephart GM, et al. Elevated numbers of cells producing interleukin-5 and interleukin-10 in a boy with Kimura disease. Int Arch Allergy Immunol. 2012. 158 Suppl 1:70-4.[↩]
- Chen QL, Li CX, Shao B, Gong ZC, Liu H, Ling B, et al. Expression of the interleukin-21 and phosphorylated extracellular signal regulated kinase 1/2 in Kimura disease. J Clin Pathol. 2017 Jan 20.[↩]
- Choi JA, Lee GK, Kong KY, Hong SH, Suh JS, Ahn JM, Lee YJ, Cho KH, Park JG, Choi JY, Kang HS. Imaging findings of Kimura’s disease in the soft tissue of the upper extremity. AJR Am J Roentgenol. 2005 Jan;184(1):193-9. doi: 10.2214/ajr.184.1.01840193[↩]
- Takahashi S, Ueda J, Furukawa T, Tsuda M, Nishimura M, Orita H, Tsujimura T, Araki Y. Kimura disease: CT and MR findings. AJNR Am J Neuroradiol. 1996 Feb;17(2):382-5.[↩]
- Maleki, D., Sayyah, A., Rahimi-Rad, M.H. et al. Kimura’s disease with eosinophilic panniculitis – treated with cyclosporine: a case report. All Asth Clin Immun 6, 5 (2010). https://doi.org/10.1186/1710-1492-6-5[↩]
- Rajpoot DK, Pahl M, Clark J. Nephrotic syndrome associated with Kimura disease. Pediatr Nephrol. 2000 Jun. 14(6):486-8.[↩]
- Gong Y, Gu JY, Labh S, Shi YL. Kimura disease accompanied with nephrotic syndrome in a 45-year-old male. Diagn Pathol. 2015 Apr 28. 10:43.[↩]
- Jennifer Lee and Yeon-Sik Hong. Kimura Disease complicated with bowel infarction and multiple arterial thromboses in the extremities. J Clin Rheumatol. Jan 2014. 20:38-41.[↩]
- Heo W, Jun HJ, Kang DK, Min HK, Hwang YH, Kim JY, et al. Acute Limb Ischemia and Coronary Artery Disease in a Case of Kimura’s Disease. Korean J Thorac Cardiovasc Surg. 2017 Apr. 50 (2):114-118.[↩]
- Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. Lippincott Williams & Wilkins. (2008) ISBN:0781775965[↩]
- Yoganathan P, Meyer DR, Farber MG. Bilateral lacrimal gland involvement with Kimura disease in an African American male. Arch Ophthalmol. 2004 Jun. 122(6):917-9.[↩]
- Goncalves AC, Moritz RB, Aldred VL, Monteiro ML. Bilateral extraocular muscles enlargement from Kimura’s disease of the orbit. Indian J Ophthalmol. 2016 Jul. 64 (7):538-40.[↩]
- Bonfils P, Moya-Plana A, Badoual C, Nadéri S, Malinvaud D, Laccourreye O. Intraparotid Kimura disease. Eur Ann Otorhinolaryngol Head Neck Dis. 2013 Apr. 130(2):87-9.[↩]
- Yadla M, Sriramnaveen P, Sivakumar V, Sandeep Reddy Y, Sridhar AV, Krishna Kishore C. Epitrochlear mass in a patient on maintenance hemodialysis-Kimura disease. Hemodial Int. 2012 Jan 26.[↩]
- Li D, Li YJ, Zhan FH, Dang CJ. The false-positive finding of left pulmonary Kimura disease on 18F-FDG PET/CT. Clin Nucl Med. 2013 Jul. 38(7):569-72.[↩]
- Abbas S, Jerjes W, Upile T, Vincent A, Hopper C. Treatment of Kimura disease with photodynamic therapy: a case study. Photodiagnosis Photodyn Ther. 2012 Mar. 9(1):83-6.[↩][↩]
- Tanaka Y, Ueno Y, Shimada Y, Yamashiro K, Tanaka R, Urabe T, et al. Paradoxical brain embolism associated with Kimura disease mimics watershed infarction. J Stroke Cerebrovasc Dis. 2015 Feb. 24 (2):e55-7.[↩]
- Li, X., Wang, J., Li, H., & Zhang, M. (2019). Misdiagnosed recurrent multiple Kimura’s disease: A case report and review of the literature. Molecular and Clinical Oncology, 10, 352-356. https://doi.org/10.3892/mco.2018.1793[↩]
- Chen H, Thompson LD, Aguilera NS and Abbondanzo SL: Kimura disease: A clinicopathologic study of 21 cases. Am J Surg Pathol. 28:505–513. 2004.[↩][↩]
- Sun QF, Xu DZ, Pan SH, Ding JG, Xue ZQ, Miao CS, Cao GJ and Jin DJ: Kimura disease: Review of the literature. Intern Med J. 38:668–672. 2008.[↩][↩]
- Ohta N, Okazaki S, Fukase S, Akatsuka N, Aoyagi M, Yamakawa M. Serum concentrations of eosinophil cationic protein and eosinophils of patients with Kimura’s disease. Allergol Int. 2007 Mar. 56(1):45-9.[↩]
- Ragu R, Eng JY and Azlina AR: Kimura’s disease of the parotid: A complete clinical-radiological-pathology report. Med J Malaysia. 69:199–201. 2014.[↩]
- Takahashi S, Ueda J, Furukawa T, Tsuda M, Nishimura M, Orita H, Tsujimura T and Araki Y: Kimura disease: CT and MR findings. AJNR Am J Neuroradiol. 17:382–385. 1996.[↩]
- Buggage RR, Spraul CW, Wojno TH and Grossniklaus HE: Kimura disease of the orbit and ocular adnexa. Surv Ophthalmol. 44:79–91. 1999.[↩]
- Kimura disease. https://emedicine.medscape.com/article/1098777-treatment[↩]
- Sakamoto M, Komura A and Nishimura S: Hematoserological analysis of Kimura’s disease for optimal treatment. Otolaryngol Head Neck Surg. 132:159–160. 2005.[↩]
- Kim GE, Kim WC, Yang WI, Kim SK, Oh WY, Suh HS, Hahn JS and Park CS: Radiation treatment in patients with recurrent Kimura’s disease. Int J Radiat Oncol Biol Phys. 38:607–612. 1997.[↩]
- Hiwatashi A, Hasuo K, Shiina T, Ohga S, Hishiki Y, Fujii K and Ishitoya J: Kimura’s disease with bilateral auricular masses. AJNR Am J Neuroradiol. 20:1976–1978. 1999.[↩]
- Ye P, Ma DQ, Yu GY, Gao Y, Peng X. Comparison of the efficacy of different treatment modalities for Kimura’s disease. Int J Oral Maxillofac Surg. 2016 Sep 7.[↩][↩]
- Kuruvilla S, Kuruvilla R, Bhasi R, Lilly M. Recurrent Kimura’s disease successfully treated with steroids and cetirizine. Internat Surg J. April-June 2016. 3(2):947-9.[↩]
- Ma XR, Xin SJ, Ouyang TX, Ma YT, Chen WY, Chang ML. Successful treatment of Kimura’s disease with leflunomide and methylprednisolone: a case report. Int J Clin Exp Med. 2014. 7 (8):2219-22.[↩]
- Gupta D, Kumari R, Rajesh NG, Manobalan K, Thappa DM. Low-dose cyclosporine for rapid remission and maintenance in recurrent Kimura’s disease. Indian J Dermatol Venereol Leprol. 2017 Mar-Apr. 83 (2):262-264.[↩]
- Kaneko K, Aoki M, Hattori S, Sato M, Kawana S. Successful treatment of Kimura’s disease with cyclosporine. J Am Acad Dermatol. 1999 Nov. 41(5 Pt 2):893-4.[↩]
- Hernandez-Bautista V, Yamazaki-Nakashimada MA, Vazquez-Garcia R, Stamatelos-Albarran D, Carrasco-Daza D, Rodriguez-Lozano AL. Treatment of Kimura disease with intravenous immunoglobulin. Pediatrics. 2011 Dec. 128(6):e1633-5.[↩]
- Hongcharu W, Baldassano M, Taylor CR. Kimura’s disease with oral ulcers: response to pentoxifylline. J Am Acad Dermatol. 2000 Nov. 43(5 Pt 2):905-7.[↩]
- Boulanger E, Gachot B, Verkarre V, Valensi F, Brousse N, Hermine O. all-trans-Retinoic acid in the treatment of Kimura’s disease. Am J Hematol. 2002 Sep. 71(1):66.[↩]
- Sun QF, Xu DZ, Pan SH, et al. Kimura disease: review of the literature. Intern Med J. 2008 Aug. 38(8):668-72.[↩]
- Hareyama M, Oouchi A, Nagakura H, et al. Radiotherapy for Kimura’s disease: the optimum dosage. Int J Radiat Oncol Biol Phys. 1998 Feb 1. 40(3):647-51.[↩]
- Chang AR, Kim K, Kim HJ, Kim IH, Park CI, Jun YK. Outcomes of Kimura’s disease after radiotherapy or nonradiotherapeutic treatment modalities. Int J Radiat Oncol Biol Phys. 2006 Jul 15. 65(4):1233-9.[↩]
- Park JW, Yea JW. Three-dimensional customized bolus for intensity-modulated radiotherapy in a patient with Kimura’s disease involving the auricle. Cancer Radiother. 2016 May. 20 (3):205-9.[↩]
- Nonaka M, Sakitani E and Yoshihara T: Anti-IgE therapy to Kimura’s disease: A pilot study. Auris Nasus Larynx. 41:384–388. 2014.[↩]
- Ye P, Wei T, Yu GY, Wu LL, Peng X. Comparison of Local Recurrence Rate of Three Treatment Modalities for Kimura Disease. J Craniofac Surg. 2016 Jan. 27 (1):170-4.[↩]
- Allen PW, Ramakrishna B and MacCormac LB: The histiocytoid hemangiomas and other controversies. Pathol Annu. 27:51–87. 1992.[↩]
- Abuel-Haija M and Hurford MT: Kimura disease. Arch Pathol Lab Med. 131:650–651. 2007.[↩]
- Chen QL, Dwa S, Gong ZC, Abasi K, Ling B, Liu H, et al. Kimura’s disease: risk factors of recurrence and prognosis. Int J Clin Exp Med. 2015. 8 (11):21414-20.[↩]