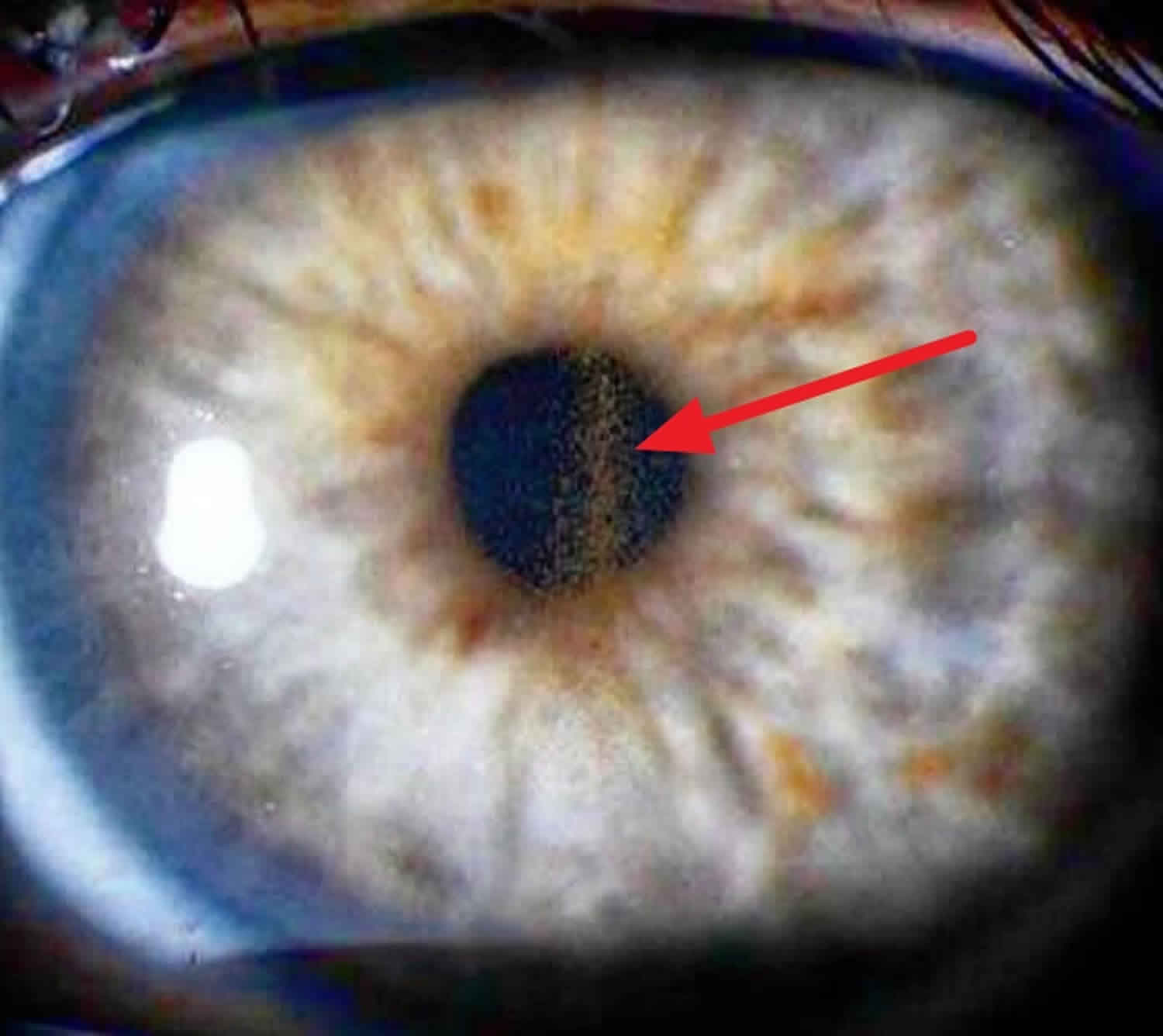
Krukenberg spindle
Krukenberg’s spindle is the name given to melanin pigment pattern on the inner surface of the cornea formed by pigmented iris cells that are shed during the mechanical rubbing of posterior pigment layer of the iris with the zonular fibrils that are deposited as a result of the currents of the aqueous humor, forming a vertical line on the posterior central cornea in contrast to other melanin pigment depositions that tend to be more circular or diffuse in distribution.
Krukenberg spindle runs the course of being mild pigment dispersion syndrome to a condition known as pigmentary glaucoma where the loose pigment in the eye can plug up the drainage system leading to high intra-ocular pressure and optic nerve damage. Pigmentary glaucoma is a type of secondary open-angle glaucoma characterized by heavy homogenous pigmentation of the trabecular meshwork, iris transillumination defects, and pigment along the corneal endothelium (Krukenberg spindle). Individuals with these same findings who do not demonstrate optic nerve damage and/or visual field loss are classified as having pigment dispersion syndrome, even if the intraocular pressure (IOP) is elevated.
Krukenberg spindles have been described in conditions other than pigment dispersion syndrome and pigmentary glaucoma, including uveitis and trauma 1. Trauma, previous ocular surgery, and pseudoexfoliation can also produce heavy trabecular pigmentation. Exfoliation syndrome presents in older age group and clinical signs include peri-pupillary transillumination defects, exfoliative material on the anterior lens capsule, and more uneven pigment distribution in the angle. However, it is important to remember that the exfoliation syndrome is more common in pigment dispersion syndrome/pigmentary glaucoma than in the general population. Some patients may have both, an entity described as “overlap syndrome”. Sulcus intraocular lens placement and iris melanoma can also produce a pigmentary glaucoma.
Treatment options for pigmentary glaucoma are similar to primary open-angle glaucoma and include medical therapy, laser trabeculoplasty, and incisional surgery with either trabeculectomy or glaucoma drainage implant. The efficacy of laser iridotomy in the prevention of pigment dispersion syndrome and subsequent pigmentary glaucoma is not firmly established.
Figure 1. Krukenberg spindle is a spindle-shaped, vertical deposit of chocolate-brown colored pigment in the cornea (arrow)
Krukenberg spindle pigment dispersion syndrome
Pigment dispersion syndrome and pigmentary glaucoma represent a spectrum of the same disease characterized by excessive pigment liberation throughout the anterior segment of the eye 1. The classic triad consists of dense trabecular meshwork pigmentation, mid-peripheral iris transillumination defects, and pigment deposition on the posterior surface of the central cornea. Pigment accumulation in the trabecular meshwork reduces aqueous outflow facility and may result in elevation of intraocular pressure (IOP), as seen in pigment dispersion syndrome, or in optic nerve damage associated with visual field loss, as seen in pigmentary glaucoma. Pigmentary glaucoma and pigment dispersion syndrome occur when pigment is released from the iris pigment epithelium due to rubbing of the posterior iris against the anterior lens zonules. The disease is more prevalent in males, and typically presents in the 3rd-4th decade of life.
The prevalence of pigment dispersion syndrome and pigmentary glaucoma in the general population is poorly defined. A screening of New York City employees reported that 2.5% had at least one slit lamp finding consistent with pigment dispersion syndrome 2, while a retrospective review of charts from a glaucoma practice demonstrated that roughly 1 in 25 patients (4%) was followed for either pigment dispersion syndrome or pigmentary glaucoma 3. In Olmstead County, Minnesota, the annual incidence of diagnosed pigment dispersion syndrome and pigmentary glaucoma was 4.8/100,000 and 1.4/100,000, respectively 4. True incidences are likely substantially higher, as many people with pigment dispersion syndrome and pigmentary glaucoma may have had undiagnosed disease.
Krukenberg spindle causes
The underlying mechanism responsible for pigment dispersion syndrome and pigmentary glaucoma is the presence of a concave iris contour which causes rubbing of the posterior iris surface against the anterior lens zonules bundles during physiological pupil movement, leading to disruption of the iris pigment epithelial cell membrane and release of pigment granules 5. Pigment granules can produce temporary elevation of intraocular pressure (IOP) by overwhelming the trabecular meshwork and reducing outflow 6. Over time, pathological changes in the trabecular endothelial cells and collagen beams can lead to increased resistance to aqueous outflow with chronic elevation of IOP and secondary glaucoma 5. Patients with pigment dispersion syndrome or pigmentary glaucoma have a 15-fold higher concentration of aqueous pigment granules in their anterior chamber compared to normal controls 7.
Pigment release requires irido-zonular contact and pupillary movement. Risk factors for irido-zonular contact based on ocular anatomy. Irido-zonular contact has been demonstrated to increase with blinking in eyes with pigment dispersion syndrome or pigmentary glaucoma 8. Blinking has been hypothesized to burp fluid from the posterior chamber to the anterior chamber in these eyes, resulting in a higher pressure in the anterior chamber as compared to the posterior chamber 9. The resulting pressure gradient results in a posterior-bowing (concave) iris with greater than normal iridolenticular contact (also referred to as reverse pupillary block) which has been shown to be reduced with suppression of blinking 10. The similar sounding Inverse Pupillary Block refers to blocking of pupil by crystalline lens with large anteroposterior diameter in Microspherophakia.
Greater iridolenticular contact also occurs with accommodation, in which the anterior lens surface moves anteriorly with contraction of the ciliary ring 11. Increase in iris concavity secondary to accommodation has also been reported in myopic eyes without pigment dispersion syndrome and normal eyes. This suggests that irides in eyes with pigment dispersion syndrome and pigmentary glaucoma may have inherent susceptibility to pigment liberation and factors other than iris shape and size may be at play 12. Pupillary movement produced by pharmacologic dilation has been observed to produce pigment release and increased IOP in some patients with pigmentary glaucoma or pigment dispersion syndrome 6. Likewise, physiological changes in pupil size resulting from lighting changes or accommodation, may also produce pigment release in individuals with iridozonular contact 13. In some patients with pigmentary glaucoma or pigment dispersion syndrome, significant pigment release accompanied by IOP elevation has been observed after strenuous exercise 14. However, systematic observation of IOP in patients with pigment dispersion syndrome or pigmentary glaucoma suggests that most patients do not have pigment release or IOP elevation after exercise 7. Pigment release producing elevated IOP and glaucoma has also been observed with sulcus placement of certain intraocular lens designs after cataract surgery 15. The terms pigment dispersion syndrome and pigmentary glaucoma are not applied to this secondary form of glaucoma, despite some underlying mechanistic similarities.
Risk factors for Krukenberg spindle pigment dispersion syndrome
- Male gender. Pigmentary glaucoma has a strong male predominance, with all case series showing a male to female ratio of between 2:1 and 5:1. Much less of a male predominance is noted for pigment dispersion syndrome, with case series describing male to female ratios between 1:1 and 2:1 4.
- Age. Male patients with pigmentary glaucoma and pigment dispersion syndrome most often present in their 30s, whereas female patients typically present roughly a decade later in life 16. Cases of pigment dispersion syndrome have been identified in patients as young as 12-15 years of age 17. Disease may be more frequent in middle age when the lens has enlarged and the iris is flexible enough to form a concave position 18.
- Myopia. The most common refractive error noted in eyes with pigment dispersion syndrome and pigmentary glaucoma is moderate myopia, with mean spherical equivalents typically in the range of -3 to -4 D. A broad range of refractive errors is typically found, though hyperopia is relatively rare, usually accounting for only 5-10% of patients in most case series 19.
- Race. Both pigment dispersion syndrome and pigmentary glaucoma occur infrequently (<5% of patients identified in case series) in persons of African ancestry 3. However, the actual prevalence may be higher than reported as persons of African ancestry have thick brown irides making detection of iris transillumination defects more difficult 20.
- Concave iris and posterior iris insertion. Patients with pigment dispersion syndrome and pigmentary glaucoma have greater iridolenticular contact than individuals without the disease. Increased iridolenticular contact results from a combination of a concave iris and a more posterior iris insertion, both of which are more common in patients with pigment dispersion syndrome or pigmentary glaucoma 11.
- Flat corneas. Patients with pigment dispersion syndrome and pigmentary glaucoma have significantly flatter corneas than control subjects of similar age and refractive error 21. A flat cornea might be more likely to result in burping of aqueous humor from the posterior chamber to the anterior chamber with blinking, resulting in increased iridozonular contact 21.
- Family history. Direct examination of a small set of family members of pigment dispersion syndrome patients showed that disease was present in 2/19 (12%) 22. A second examination of family members reported signs of pigment dispersion syndrome in 36% of subjects’ parents and 50% of siblings, but no children, suggesting a possible autosomal dominant inheritance pattern with incomplete penetrance 23. Families with pigmentary glaucoma have also been described across multiple generations 24. Roughly 50% of family members in the described families had pigment dispersion syndrome or pigmentary glaucoma, reinforcing the idea of an autosomal dominant inheritance pattern.
Risk factors for stage of disease or disease progression include:
- Intraocular pressure. A retrospective study from Olmstead County Minnesota found IOP > 21 to be the only risk factor for progression from pigment dispersion syndrome to pigmentary glaucoma. Age, refractive error and family history were not associated with conversion to pigmentary glaucoma 4.
- Degree of iridolenticular contact in patients with asymmetric disease. The more affected eye was noted to have more iris-lens contact than the less affected eye. Features associated with greater iridolenticular contact (greater iris concavity, more posterior iris insertion) were also more common in patients with pigment dispersion syndrome or pigmentary glaucoma 25.
- Greater trabecular meshwork pigmentation. In eyes with bilateral pigment dispersion syndrome, worse disease is typically found in the eye with more severe trabecular meshwork pigmentation 3.
Krukenberg spindle signs and symptoms
Visual symptoms are unusual except in patients with visual field loss. Some patients may describe episodes of haloes and blurry vision resulting from intermittent IOP elevation. Patients with such symptoms should be asked if they are brought about by exercise or dark exposure, which have been previously described in patients with pigment dispersion syndrome or pigmentary glaucoma.
Krukenberg spindle complications
Rise in intraocular pressure after iridotomy is greater in pigment dispersion syndrome and pigmentary glaucoma patients than in patients with occludable angles. To avoid this, lower energy levels should be used, alpha adrenergic agonists should be administered before and after the laser treatment, and argon laser should be used, as it is less disruptive than a YAG laser in terms of pigment liberation and inflammation 26.
Krukenberg spindle diagnosis
Pigment dispersion syndrome is diagnosed clinically based on the presence of iris transillumination defects in the mid-peripheral iris, pigment on the corneal endothelium (Krukenberg spindle, vertically oriented due to convection currents), and heavy pigmentation of the trabecular meshwork. The presence of all three of these findings in the absence of another cause (i.e. history of trauma or posterior chamber IOL) suggests definite disease. No formal criteria have been set forth in defining whether disease exists in eyes with 1 or 2 of the above findings. Disease is likely present when 2 of the above 3 findings are present, particularly if other exam findings consistent with pigment dispersion syndrome or pigmentary glaucoma (i.e. elevated IOP, zonular pigment, posterior capsular pigment) are present. Pigmentary glaucoma is present when the criteria for pigment dispersion syndrome are accompanied by optic nerve cupping and/or visual field loss 27. Pigment dispersion syndrome can occur with normal or elevated IOP.
Patients should be asked about a history of previous trauma, surgery, or eye disease. The presence of family members with glaucoma (and the type of glaucoma) should be queried.
Physical examination
Careful slit lamp examination is critical to identification of pigment dispersion syndrome. Findings are typically bilateral, but can be markedly asymmetric on occasion.
- The posterior surface of the central cornea should be carefully examined for the presence of pigment. Pigment is often arranged in a the shape of a Krukenberg spindle, narrow or rounded oval of brown pigment, usually 0.5 to 2.5 mm wide, and 2-6 mm in length. Pigment is typically densest at the center, thinning out at the edges in the shape of a spindle 28. Krukenberg spindles are present in roughly 90% of patients with pigment dispersion syndrome or pigmentary glaucoma. Whether a dense Krukenberg spindle or very fine granules of pigment are present, visual acuity is not reported to be affected. Corneas of patients with pigment dispersion syndrome or pigmentary glaucoma have not been demonstrated to be thicker or to have decreased endothelial cell counts 29.
- The anterior chamber should be examined for the presence of pigment granules and depth. Examination should be performed prior to and after pupillary dilation.
- The iris should be examined with retroillumination to look for iris transillumination defects. Transillumination defects are most common in the mid-peripheral iris, where contact between iris pigment epithelium and anterior lens zonules is maximal. Transillumination defects appear in a spoke-like configuration, and are most common or most prominent inferiorly or inferonasally 9. Roughly 90% of pigment dispersion syndrome/pigmentary glaucoma patients demonstrate iris transillumination defects in at least 1 eye 30, though transillumination defects may be absent in patients with thick, dark irides 20. In asymmetric cases, frank iris heterochromia with increased iris pigmentation in the eye with greater pigmentation can be noted in the more affected eye 31. The eye with greater pigment loss can also have a larger pupil resulting in clinical anisocoria, possibly secondary to iris dilator hypertrophy 32.
- The lens should be examined for the presence of pigment on the anterior surface, along the zonules, and along the posterior surface. Zonular pigment is best noted after pupillary dilation with the patient gazing upwards to bring the inferior zonules into view 33. Rarely, pigment can migrate posteriorly, where it can be found trapped between the posterior lens capsule and anterior hyaloid 34.
- Gonioscopy should be completed prior to dilation to evaluate the extent of trabecular pigmentation. The angle is typically widely open and the trabecular meshwork typically shows dense, homogenous pigmentation 31. Pigment deposition may be noted on Schwalbe’s line.
- Intraocular pressure should be measured. In a community based retrospective study, IOP at time of diagnosis for a population of patients with pigmentary glaucoma and pigment dispersion syndrome was 29 mm Hg and 24 mm Hg, respectively 4. Other studies confirm that patients presenting with pigmentary glaucoma typically have higher pressures 16.
Mydriatic provocative testing has limited utility in diagnosing or predicting the course of pigment dispersion syndrome or pigmentary glaucoma. In one case series, roughly 1/3 of patients demonstrated extensive anterior chamber pigment after phenylephrine administration, and only a fraction of these (20%) had an associated IOP rise 13.
Diagnostic procedures
Patients with suspected pigment dispersion syndrome or pigmentary glaucoma should undergo gonioscopy to document the extent of trabecular pigmentation. In older patients, the only sign of pigment dispersion syndrome may be the “pigment reversal sign,” where the trabecular meshwork is found to be darker in the superior quadrant when compared with the inferior quadrant. This finding helps to differentiate patients with “burned out” pigmentary glaucoma from other types of glaucomas. Iris concavity and the extent of iridolenticular contact can also be examined using ultrasound biomicroscopy (UBM) or anterior segment optical coherence tomography (AS-OCT). However, neither test is necessary for diagnosis.
Krukenberg spindle treatment
Treatment for pigmentary glaucoma and pigment dispersion syndrome is similar to the treatment of primary open angle glaucoma, though laser iridotomy may be considered as a prophylactic treatment. Men and persons of African descent often present with advanced disease, and may require more aggressive therapy 35. In one case series, patients with pigment dispersion syndrome or pigmentary glaucoma were more likely to require surgery than a control group with primary open-angle glaucoma.
Medical therapy
Pilocarpine has been demonstrated to reduce iris concavity and has been shown to block the exercise-induced elevation of IOP found in some patients 36. However, pilocarpine treatment can induce additional myopia and accommodative spasms. Peripheral retina should be carefully examined before the initiation of miotics since lattice degeneration is present in up to 20% of these eyes, and the incidence of retinal detachment in patients with pigment dispersion syndrome and pigmentary glaucoma is higher than in general population 9. Therefore, treatment with pilocarpine has largely been replaced by newer medical agents including topical prostaglandins, beta-blockers, carbonic anhydrase inhibitors, and alpha-adrenergic agonists. Prostaglandin analogues may be preferred over aqueous suppressants as treatment with aqueous suppressants slows down the clearance of pigment from the trabecular meshwork.
Medical follow up
Patients treated medically should be followed up periodically every 3-6 months to ensure adequate control of intraocular pressure and to confirm that the glaucoma has not progressed through examination, visual field testing, and/or imaging of the optic nerve head and nerve fiber layer.
Surgery
Given that pigment dispersion syndrome or pigmentary glaucoma results from a pressure differential across the iris (from the anterior to posterior chambers), it has been suggested that the underlying mechanism of disease might be eliminated by treatment with laser iridotomy. Indeed, reports have demonstrated that laser iridotomy can eliminate iris concavity and reduce iridolenticular contact in eyes with pigment dispersion syndrome 37. However, some eyes may retain a concave iris configuration even after laser treatment 38 and this intervention may not always prevent exercise-induced pigment release and IOP elevation 39. Limited data is available regarding whether laser iridotomy is effective in controlling IOP in patients with pigment dispersion syndrome or pigmentary glaucoma. A small randomized controlled trial of 21 patients demonstrated a lower rate of IOP elevation over 2 years of follow up in eyes treated with laser as compared to eyes not receiving laser (52% vs. 5%) 40, while a retrospective study of 60 patients did not suggest any benefit for laser iridotomy with regards to the future IOP course 41. Given the heavy trabecular pigmentation in pigment dispersion syndrome and pigmentary glaucoma, argon laser trabeculoplasty may be an effective treatment option 42. However, long-term control of IOP is unlikely, and 1/3 of eyes or more may require trabeculectomy 42. Younger patients are more likely to have long-term IOP lowering after argon laser trabeculoplasty 42. The effects of Selective Laser Trabeculoplasty as a treatment in pigment dispersion syndrome and pigmentary glaucoma has not been well studied. With either argon laser trabeculoplasty or selective laser trabeculoplasty, lower energy settings should be used to avoid release of pigment and IOP spikes.
Patients demonstrating disease progression despite treatment with medicines and/or trabeculoplasty should be considered for trabeculectomy or other incisional surgery. Long term results of trabeculectomy for pigmentary glaucoma have not been reported. The use of newer surgical modalities in the treatment of pigmentary glaucoma has not been well described.
Surgical follow up
Follow-up after laser iridotomy is similar to the follow-up for iridotomy performed for angle closure. Follow-up after argon laser trabeculoplasty and selective laser trabeculoplasty is similar for the follow-up schedule used for argon laser trabeculoplasty or selective laser trabeculoplasty with primary open angle glaucoma.
Krukenberg spindle prognosis
Blindness from pigmentary glaucoma is rare. In a community based study of 113 pigment dispersion syndrome and pigmentary glaucoma patients followed for a median of 6 years, 1 patient went unilaterally blind while a second went bilaterally blind 4. In the same study, 10% of pigment dispersion syndrome patients progressed to pigmentary glaucoma at 5 years, while 15% progressed at 10 years, though 23% of patients were noted to have pigmentary glaucoma at diagnosis 4. Forty-four percent of patients with pigmentary glaucoma had worsening of visual fields over a mean follow-up period of 6 years. Similar rates of blindness were found in a group of patients followed in a glaucoma clinic, but higher rates of progression from pigment dispersion syndrome to pigmentary glaucoma were observed (35% over a median follow-up at 15 years) and roughly 40% of pigmentary glaucoma patients were observed to have worsening of optic nerve damage 43. No risk factor for progression has been identified other than elevated intraocular pressure 4. In some cases, pigmentary glaucoma may regress over time. Both trabecular meshwork pigmentation and iris transillumination defects have been observed to normalize over time 44. Even elevated IOP has been observed to normalize, suggesting return of normal trabecular meshwork function 45. Additionally, older patients with diagnosis of normal tension glaucoma have been identified with iris transillumination defects and dense trabecular meshwork pigmentation suggesting they may have had pigmentary glaucoma with subsequent normalization of IOP due to cessation of pigment release 46. In such patients, presence of “pigment reversal sign” helps to distinguish between different types of glaucomas.
- Pigmentary glaucoma and Pigment Dispersion Syndrome. https://eyewiki.aao.org/Pigmentary_glaucoma_and_Pigment_Dispersion_Syndrome[↩][↩]
- Ritch R, Steinberger D, Liebmann JM. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am J Ophthalmol. 1993;115(6):707-710.[↩]
- Scheie HG, Cameron JD. Pigment dispersion syndrome: a clinical study. Br J Ophthalmol. 1981;65(4):264-269.[↩][↩][↩]
- Siddiqui Y, Ten Hulzen RD, Cameron JD, Hodge DO, Johnson DH. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135(6):794-799.[↩][↩][↩][↩][↩][↩][↩]
- Kampik A, Green WR, Quigley HA, Pierce LH. Scanning and transmission electron microscopic studies of two cases of pigment dispersion syndrome. Am J Ophthalmol. 1981;91(5):573-587.[↩][↩]
- Jewelewicz DA, Radcliffe NM, Liebmann J, Ritch R. Temporal evolution of intraocular pressure elevation after pupillary dilation in pigment dispersion syndrome.J Glaucoma. 2009;18(3):184-185.[↩][↩]
- Mardin CY, Kuchle M, Nguyen NX, Martus P, Naumann GO. Quantification of aqueous melanin granules, intraocular pressure and glaucomatous damage in primary pigment dispersion syndrome. Ophthalmology. 2000;107(3):435-440.[↩][↩]
- Liebmann JM, Tello C, Chew SJ, Cohen H, Ritch R. Prevention of blinking alters iris configuration in pigment dispersion syndrome and in normal eyes. Ophthalmology. 1995;102(3):446-455.[↩]
- Ritch R. A unification hypothesis of pigment dispersion syndrome. Trans Am Ophthalmol Soc. 1996;94:381-405; discussion 405-9.[↩][↩][↩]
- Doyle JW, Hansen JE, Smith MF, Hamed LM, McGorray S, Sherwood MB. Use of scheimpflug photography to study iris configuration in patients with pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma. 1995;4(6):398-405.[↩]
- Mora P, Sangermani C, Ghirardini S, Carta A, Ungaro N, Gandolfi S. Ultrasound biomicroscopy and iris pigment dispersion: a case–control study. Br J Ophthalmol. 2010;94(4):428-432.[↩][↩]
- Pavlin CJ, Macken P, Trope GE, Harasiewicz K, Foster FS. Accommodation and iridotomy in the pigment dispersion syndrome. Ophthalmic Surg Lasers. 1996;27(2):113-120.[↩]
- Epstein DL, Boger WP,3rd, Grant WM. Phenylephrine provocative testing in the pigmentary dispersion syndrome. Am J Ophthalmol. 1978;85(1):43-50.[↩][↩]
- Schenker HI, Luntz MH, Kels B, Podos SM. Exercise-induced increase of intraocular pressure in the pigmentary dispersion syndrome. Am J Ophthalmol. 1980;89(4):598-600[↩]
- Caplan MB, Brown RH, Love LL. Pseudophakic pigmentary glaucoma. Am J Ophthalmol. 1988;105(3):320-321.[↩]
- Gillies WE, Brooks AM. Clinical features at presentation of anterior segment pigment dispersion syndrome. Clin Experiment Ophthalmol. 2001;29(3):125-127.[↩][↩]
- Kaiser-Kupfer MI, Kupfer C, McCain L. Asymmetric pigment dispersion syndrome. Trans Am Ophthalmol Soc. 1983;81:310-324.[↩]
- Campbell DG. Pigmentary dispersion and glaucoma. A new theory. Arch Ophthalmol. 1979;97(9):1667-1672.[↩]
- Lord FD, Pathanapitoon K, Mikelberg FS. Keratometry and axial length in pigment dispersion syndrome: a descriptive case-control study. J Glaucoma. 2001;10(5):383-385.[↩]
- Roberts DK, Chaglasian MA, Meetz RE. Clinical signs of the pigment dispersion syndrome in blacks. Optom Vis Sci. 1997;74(12):993-1006.[↩][↩]
- Yip LW, Sothornwit N, Berkowitz J, Mikelberg FS. A comparison of interocular differences in patients with pigment dispersion syndrome. J Glaucoma. 2009;18(1):1-5.[↩][↩]
- Roberts DK, Meetz RE, Chaglasian MA. The inheritance of the pigment dispersion syndrome in blacks. J Glaucoma. 1999;8(4):250-256.[↩]
- McDermott JA, Ritch R, McDermott J. Familial occurrence of pigmentary dispersion syndrome. Invest Ophthalmolol Vis Sci. 1987;28(suppl):136.[↩]
- Andersen JS, Pralea AM, DelBono EA, et al. A gene responsible for the pigment dispersion syndrome maps to chromosome 7q35-q36.Arch Ophthalmol. 1997;115(3):384-388.[↩]
- Kanadani FN, Dorairaj S, Langlieb AM, et al. Ultrasound biomicroscopy in asymmetric pigment dispersion syndrome and pigmentary glaucoma. Arch Ophthalmol. 2006;124(11):1573-1576.[↩]
- Birt CM. Intraocular pressure spike after YAG iridotomy in patients with pigment dispersion. Can J Ophthalmol. 2004;39(3):234-9.[↩]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-42.[↩]
- Evans WH, Odom RE, Wenaas EJ. Krukenberg’s spindle; A study of two hundred and two collected cases. Arch Ophthal. 1941:1023.[↩]
- Murrell WJ, Shihab Z, Lamberts DW, Avera B. The corneal endothelium and central corneal thickness in pigmentary dispersion syndrome. Arch Ophthalmol. 1986;104(6):845-846.[↩]
- Sugar HS. Pigmentary glaucoma. A 25-year review. Am J Ophthalmol. 1966;62(3):499-507.[↩]
- Lichter PR, Shaffer RN. Diagnostic and prognostic signs in pigmentary glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1970;74(5):984-998.[↩][↩]
- Feibel RM. Anisocoria in the pigmentary dispersion syndrome: further cases. J Glaucoma. 1993;2(1):37-38.[↩]
- Lichter PR. Pigmentary glaucoma–current concepts. Trans Am Acad Ophthalmol Otolaryngol. 1974;78(2):OP309-13.[↩]
- Lin DY, Volpicelli M, Singh K. Dense pigmentation of the posterior lens capsule associated with the pigment dispersion syndrome. J Glaucoma. 2003;12(6):491-493.[↩]
- Farrar SM, Shields MB, Miller KN, Stoup CM. Risk factors for the development and severity of glaucoma in the pigment dispersion syndrome. Am J Ophthalmol. 1989;108(3):223-229.[↩]
- Haynes WL, Johnson AT, Alward WL. Inhibition of exercise-induced pigment dispersion in a patient with the pigmentary dispersion syndrome. Am J Ophthalmol. 1990;109(5):601-602.[↩]
- Laemmer R, Mardin CY, Juenemann AG. Visualization of changes of the iris configuration after peripheral laser iridotomy in primary melanin dispersion syndrome using optical coherence tomography. J Glaucoma. 2008;17(7):569-570.[↩]
- Jampel HD. Lack of effect of peripheral laser iridotomy in pigment dispersion syndrome. Arch Ophthalmol. 1993;111(12):1606.[↩]
- Haynes WL, Alward WL, Tello C, Liebmann JM, Ritch R. Incomplete elimination of exercise-induced pigment dispersion by laser iridotomy in pigment dispersion syndrome. Ophthalmic Surg Lasers. 1995;26(5):484-486.[↩]
- Gandolfi SA, Vecchi M. Effect of a YAG laser iridotomy on intraocular pressure in pigment dispersion syndrome. Ophthalmology. 1996;103(10):1693-5.[↩]
- Reistad CE, Shields MB, Campbell DG, Ritch R, Wang JC, Wand M. The influence of peripheral iridotomy on the intraocular pressure course in patients with pigmentary glaucoma. J Glaucoma. 2005;14(4):255-9.[↩]
- Ritch R, Liebmann J, Robin A, et al. Argon laser trabeculoplasty in pigmentary glaucoma. Ophthalmology. 1993;100(6):909-913.[↩][↩][↩]
- Migliazzo CV, Shaffer RN, Nykin R, Magee S. Long-term analysis of pigmentary dispersion syndrome and pigmentary glaucoma. Ophthalmology. 1986;93(12):1528-1536.[↩]
- Richter CU, Richardson TM, Grant WM. Pigmentary dispersion syndrome and pigmentary glaucoma. A prospective study of the natural history. Arch Ophthalmol. 1986;104(2):211-215.[↩]
- Speakman JS. Pigmentary dispersion. Br J Ophthalmol. 1981;65(4):249-251.[↩]
- Ritch R. Nonprogressive low-tension glaucoma with pigmentary dispersion.Am J Ophthalmol. 1982;94(2):190-196.[↩]