What is a lacunar stroke
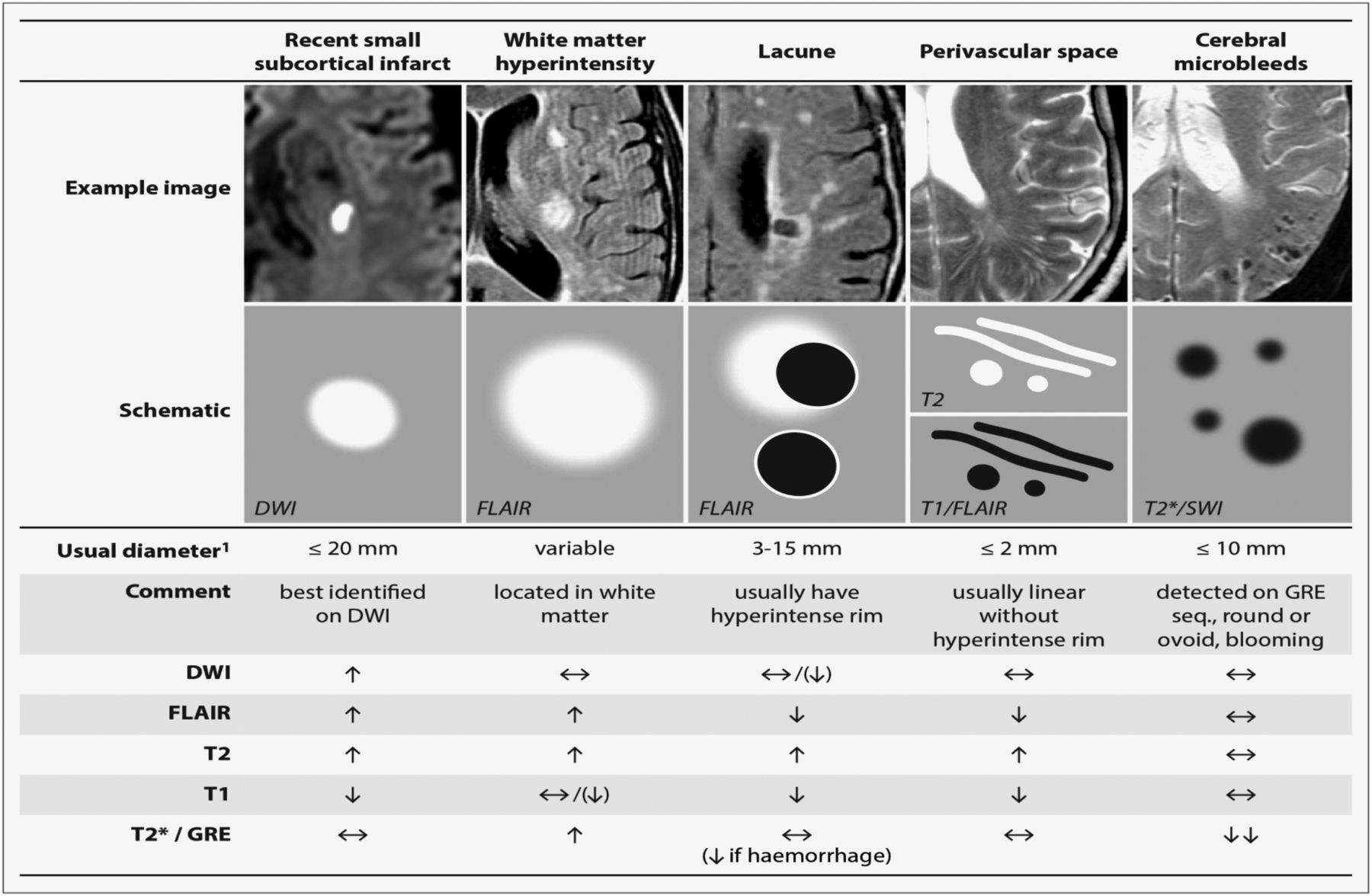
Lacunar stroke also called subcortical stroke or lacunar infarct, often arise from chronic high blood pressure, which leads to progressive narrowing and finally blockage of the small thread-like arteries within cerebral tissue of the brain. Lacunar stroke syndrome could be due to either ischemia or a small hemorrhage 1. Lacunar ischemic stroke is defined as a stroke that is attributable to a recent small infarct <1.5 (or some say 2) cm diameter in the white matter, basal ganglia, pons or brainstem, and is consistent with a lacunar clinical syndrome 2. It is commonly attributed to an abnormality in a single small deep perforating (or lenticulostriate) artery. On MRI, an acute lacunar infarct is shown as hyperintense on diffusion-weighted imaging (DWI), hypointense on an apparent diffusion coefficient map, hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR), hypointense on T1 and hypoattenuated on CT (see Figure 1). It can be rounded, ovoid or tubular 3. Generally, the Oxfordshire Community Stroke Project (OCSP) classification, which uses only clinical features to diagnose the stroke subtype, can predict correctly the size and location of a recent brain infarct on imaging in 75–80% of patients with stroke 4. However, up to 20% of acute lacunar infarcts can present with cortical symptoms, and conversely cortical infarcts can present with lacunar syndromes.9 One explanation is that lacunar infarcts closer to the cortex are more likely to cause cortical symptoms 5. Therefore, in studies where stroke diagnosis relied mainly on the clinical presentations, this ‘mismatch’ may have added ‘noise’. Thus, in epidemiology, mechanistic studies or clinical trials, it is important to verify stroke lesions using sensitive imaging wherever possible.
However, even with sensitive imaging like diffusion-weighted imaging (DWI), about 30% of patients with clinically definite stroke did not show any recent ischaemic change on MRI 6; when followed up for a year, the diffusion-weighted imaging (DWI)-negative patients had just as much recurrent stroke, dependency and cognitive impairment as the DWI-positive patients. Therefore, negative DWI/MRI cannot exclude stroke diagnosis. Rapid access to scanning after stroke onset can increase the chance of positive findings 7. It is also noteworthy that DWI-positive lesions can be clinically ‘silent’, for example, (1) as a second silent acute infarct in patients presenting with stroke due to another acute symptomatic infarct, or (2) in patients with acute haemorrhagic stroke, and (3) in patients with severe white matter hyperintensities who did not have any overt stroke symptoms 8.
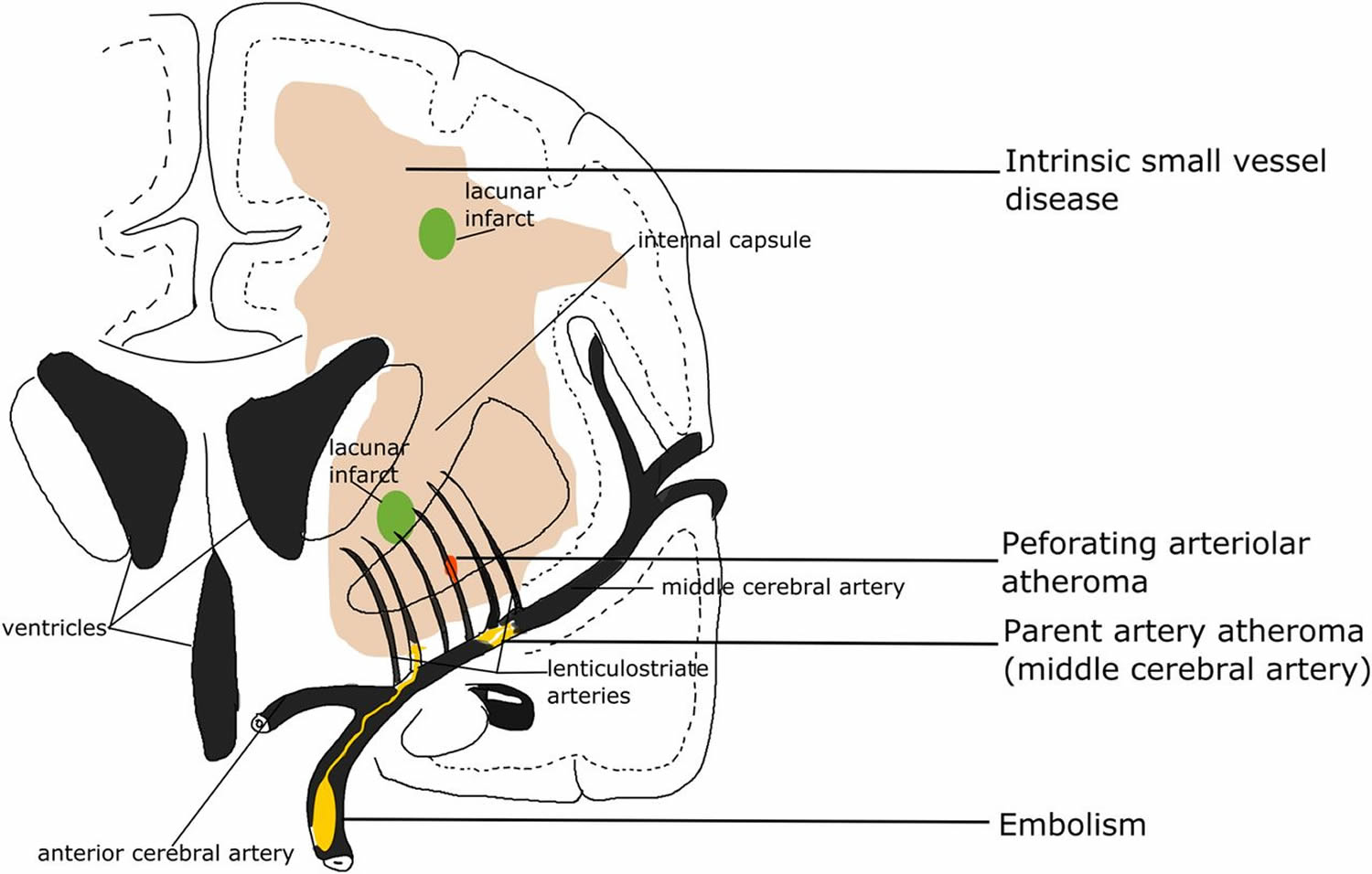
In some clinical stroke classifications such as the Trial of Org 10172 in Acute Stroke Treatment (TOAST) or the ASCO (A: atherosclerosis; S: small-vessel disease; C: cardiac pathology; O: other causes), another term ‘small vessel/artery disease’ rather than ‘lacunar stroke’ is used to represent a stroke that is supposed to be due to a small artery occlusion. However, these classifications use risk factors to decide the stroke subtype, not just the clinical presentation, so as to distinguish ‘small vessel/artery disease’ from strokes caused by large artery atherosclerosis, cardiac emboli or other unknown reasons. However, a small embolus, or atheroma in the middle cerebral artery (MCA) or perforating arterioles can all block the perforating arteriole, and any of these can cause a lacunar ischaemic stroke (see Figure 2). Therefore, it might be better to focus on the clinical presentation to assign the stroke syndrome and separately focus on the risk factors for patient management.
Lacunar strokes account for up to one-fifth of all strokes in the U.S. and are especially common among African-Americans, Hispanics and people with diabetes.
Much of our current knowledge of lacunar strokes is due to Fisher’s prior cadaveric dissection of postmortem stroke patients 9. Fisher found that most symptomatic lacunar strokes are due to the occlusion of penetrating arteries of 200-800 μm in diameter, whereas those with smaller-diameter penetrating artery infarcts tended to be asymptomatic 9. The term ‘lacune’ was used by Fisher to describe a small fluid cavity in the brain which he thought was a healed lacunar infarct. Therefore, in cerebral small vessel disease research, it is very common that terms like ‘lacunar infarction’, ‘lacunar stroke’ and ‘silent brain infarct’ were used to refer to the CSF-filled cavities on brain MRI or autopsy 10. In fact, lacunes are not always ‘ischemic’. They can also be the residual lesion of a small hemorrhage 11. Also, it is common that many non-cavitated lacunar ischaemic strokes were not counted as ‘lacunar infarcts’. Therefore, in order to avoid more confusion, the term ‘lacune of presumed vascular origin’ was proposed to replace ‘lacune’ and the term ‘lacunar infarct’ should NOT be used to describe ‘lacunes’ any more.
Lacunar stroke may be defined as small subcortical infarcts (< 15 mm in diameter) in the territory of the deep penetrating arteries; these lesions may present with specific lacunar syndromes or they may be asymptomatic 12. Unfortunately, the 5 classic lacunar syndromes established by Fisher and their radiologic appearances are not specific for lacunes. Lacunes occur most frequently in the basal ganglia and in the internal capsule, thalamus, corona radiata, and pons.
In the United States and other Western nations, lacunar stroke account for 15-25% of all ischemic strokes 13. However, differences in the reported incidence rates of lacunar strokes between US and European studies may be due in part to different definitions used in the studies. In two community-based studies in the United States, the annual incidence rates of lacunar strokes were 13.4 and 19.5 cases per 100,000 population. However, two European community-based studies found higher annual incidence rates (31.7 and 53 cases per 100,000 population).
A study by Rukn et al 14 indicated that among ischemic strokes in Middle Eastern and North African countries, lacunar strokes are the second most common type (24.1%).
The incidence of lacunar strokes increases with age (mean age of first lacunar stroke, 65 years), and men may be affected more than women. Some studies have also found higher frequencies of lacunar strokes in black persons, Mexican Americans, and Hong Kong Chinese 15.
Figure 1. Lacunar stroke MRI
Footnote: STRIVE, STandards for Reporting and Imaging of Small Vessel Disease: example findings (upper), schematic representation (middle) and a summary of imaging characteristics (lower) of MRI features for changes related to small vessel disease 3.
Abbreviations: DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; SWI = susceptibility-weighted imaging; GRE = gradient-recalled echo.
[Source 16 ]Figure 2. Lacunar stroke
Footnote: Four possible mechanisms that cause a lacunar infarct (from bottom to top): (A) an embolus from the big arteries or cardiac sources goes up to MCA (middle cerebral artery) and ends up entering and occluding lenticulostriate arteries, resulting in a lacunar lesion in basal ganglia; (B) if the atheroma in the parent artery (ie, MCA) is positioned at the opening of its penetrating branches, it could lead to an acute occlusion of one or several penetrating arteries, hence causing a lacunar infarct; (C) a lacunar infarct could also be due to atheroma in the perforating artery if an acute occlusion happens; (D) intrinsic small vessel disease may lead to diffused disrupted blood–brain barrier. If this happens at an arteriolar level, plasma fluid components would enter and deposit in the vessel wall, resulting in narrowing of the arteriolar lumen, vessel wall thickening and eventually a secondary luminal occlusion and traditional infarct. MCA, middle cerebral arteries.
[Source 16 ]What causes a lacunar stroke?
Lacunar strokes are caused by occlusion of a single penetrating artery. The deep penetrating arteries are small, nonbranching end arteries (usually smaller than 500 μm in diameter), which arise directly from much larger arteries (eg, the middle cerebral artery, anterior choroidal artery, anterior cerebral artery, posterior cerebral artery, posterior communicating artery, cerebellar arteries, basilar artery). Their small size and proximal position predispose them to the development of microatheroma and lipohyalinosis 17.
Four possible main causes for lacunar ischemic stroke have been proposed (see Figure 2): atheroma of parent arteries usually middle cerebral artery (MCA) or perforating arterioles, embolism from the heart or carotid arteries, and intrinsic small vessel disease (lipohyalinosis or fibrinoid necrosis) 16. Atheroma in middle cerebral artery (MCA) appears to cause <20% of lacunar ischaemic stroke. In the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, only 11% (38/347) of all patients with stroke were lacunar type 18, which is surprising if middle cerebral artery (MCA) stenosis is supposed to be a common cause of lacunar stroke. A recent study also did not find any association between lacunar stroke and middle cerebral artery (MCA) stenosis 19. A systematic review of Asian studies showed that parent artery atherosclerosis accounted for 20% of single lacunar infarcts in anterior circulation territory; however, these hospital-based studies were rather small (n=71–118) and some were even retrospective 20. Larger and tubular lacunar infarcts might be more likely to be caused by proximal artery diseases 21. However, the results of both our study and the Secondary Prevention of Small Subcortical Stokes Trial (SPS3) suggest that it is not possible to identify the cause of a particular recent lacunar ischaemic stroke based on its size, shape or location 22.
Initially, lipohyalinosis was thought to be the predominant small-vessel pathology of lacunar stroke; however, microatheroma now is thought to be the most common mechanism of arterial occlusion (or stenosis). Occasionally, atheroma in the parent artery blocks the orifice of the penetrating artery (luminal atheroma), or atheroma involves the origin of the penetrating artery (junctional atheroma).
A hemodynamic (hypoperfusion) mechanism is suggested when there is a stenosis (and not occlusion) of the penetrating artery. When no evidence of small-vessel disease is found on histologic examination, an embolic cause is assumed, either artery-to-artery embolism or cardioembolism. In one series, 25% of patients with clinical, radiologically defined lacunar stroke had a potential cardiac cause for their strokes 23.
Evidence for embolism as a common cause for lacunar ischaemic stroke is limited. Presence of cardioembolic sources was found significantly less often in lacunar than in non-lacunar ischaemic stroke.19 ,20 Few if any associations were found between ipsilateral carotid stenosis and lacunar ischaemic stroke or other features of CSVD.21 ,22 In primate models, <6% of emboli injected into carotid arteries entered the lenticulostriate arteries, while the majority entered the cortical arteries.23 Lacunar ischaemic strokes in the basal ganglia were marginally more often associated with embolism than those in the centrum semiovale (11% vs 3%, respectively), but the overall rate of known embolic sources in symptomatic lacunar ischaemic stroke was very low (11%).18
Intrinsic small vessel pathologies remain the most common cause of lacunar ischemic stroke, although the underlying mechanism is unclear. Fisher attributed the lipohyalinosis in small arteries to hypertension. However, the diagnosis and treatment of hypertension were less good when Fisher was working in the 1950s and 1960s and he may have seen some particularly severe cases of hypertension. Now, epidemiology data show that hypertension is equally common in non-lacunar as in lacunar ischaemic stroke 24; and many patients with lacunar stroke are normotensive. Similarly, other traditional risk factors like diabetes mellitus, hypercholesterolaemia and smoking were as frequent in lacunar stroke as in other ischemic strokes 25. Risk factor profiles of lacunar stroke seemed different in China, but it might be too early to say so. The Beijing stroke registry (n=1184) showed a higher proportion of hypertension in lacunar (acute stroke symptoms+subcortical lesion <2 cm diameter on acute CT/MRI) than in non-lacunar stroke after adjusting for age and gender 26. Some other studies had similar findings, but the stroke diagnosis varied: in some studies, the differentiation between lacunar stroke and ‘large artery atherosclerosis’ stroke relied only on lesion size, and clinical classification included risk factors 27. Additionally, most studies were hospital-based. Hence, population scale data on lacunar stroke are lacking. It is important to distinguish lacunar stroke from other subtypes because of the mechanism, hence prevention and treatment might differ. More data and careful separation of lacunar stroke from other subtypes are required in future studies.
Risk factors for lacunar stroke
The cause of lacunar infarction is occlusion of a single small penetrating artery. This occlusion may be due to microatheroma and lipohyalinosis, which are associated with hypertension, smoking, and diabetes, or may result from microembolism from the heart or carotid arteries 13.
Study results initially indicated that almost all patients with lacunar stroke have hypertension. However, later studies found hypertension in only 44-75% of patients. In the setting of chronic hypertension, the penetrating arteries, which usually are not affected by atherosclerosis, may develop microatheroma and lipohyalinosis.
Diabetes mellitus is well recognized as a risk factor for development of small-vessel disease throughout the body, including in the penetrating arteries, and smoking is also an established risk factor for lacunar stroke.
Traditionally, embolism (either cardioembolism or artery-to-artery embolism) was considered a rare mechanism of lacunar stroke, but a potential embolic cause is not uncommon when lacunar stroke are defined clinicoradiologically. A potential embolic cause may be a coincidental finding only.
Atrial fibrillation and ipsilateral carotid stenosis have a stronger association with nonlacunar infarcts 28.
The data are less clear regarding a strong association between other risk factors and lacunar stroke, including alcohol consumption, elevated cholesterol, and history of previous stroke.
Lacunar stroke symptoms
Lacunar stroke symptoms may occur suddenly or may evolve in either a fluctuating (eg, the capsular warning syndrome) or a progressive manner 29.
Fisher’s studies 9 led to development of his 5 classic lacunar syndromes, of which each has a symptom complex that is relatively specific to it:
- Pure motor stroke or hemiparesis,
- Ataxic hemiparesis,
- Dysarthria/clumsy hand,
- Pure sensory stroke, and
- Mixed sensorimotor stroke.
Occasionally, cortical infarcts and intracranial hemorrhages can mimic a lacunar syndrome 30. Cortical symptoms (eg, aphasia, neglect) and visual field defects are absent.
Pure motor stroke or hemiparesis
Pure motor stroke or hemiparesis is the most common (33-50%) lacunar syndrome; the lacunar infarct stroke is usually in the posterior limb of the internal capsule, which carries the descending corticospinal and corticobulbar tracts, or the basis pontis.
This syndrome consists of hemiparesis or hemiplegia that typically affects the face, arm, and leg equally. However, the face or leg can be involved to a lesser extent than other regions, and occasionally, only arm or leg weakness is noted by patients. Transient sensory symptoms (but not signs) may be present. Dysarthria and dysphagia may also be present.
Hemiparesis or hemiplegia is noted, with hyperreflexia and Babinski sign; no involvement of any other system is observed.
Ataxic hemiparesis
Ataxic hemiparesis is the second most frequent lacunar syndrome. The most frequent sites of infarction are the posterior limb of the internal capsule, basis pontis, and corona radiata.
This syndrome features a combination of cerebellar and motor symptoms, including weakness and clumsiness, on the ipsilateral side of the body. The leg is usually more affected than the arm; hence, it is known also as homolateral ataxia and crural paresis. The onset of symptoms is often over hours or days.
A combination of pyramidal signs (eg, hemiparesis, hyperreflexia, Babinski sign) and cerebellar ataxia on the same side of the body. The lower extremities are typically more involved than the upper extremities. Nystagmus may be present.
Dysarthria or clumsy hand
Although now considered to be a variant of ataxic hemiparesis, this disorder is usually still classified as a separate lacunar syndrome. The lesion is in the pons.
The main symptoms are dysarthria and clumsiness (ie, weakness) of the hand, which are often most prominent when the patient is writing.
Unilateral lower facial weakness with dysarthric speech is noted. On protrusion, the tongue may deviate to the side of facial weakness. A mild, ipsilateral hemiparesis is usually noted, but the arm is ataxic. Ipsilateral hyperreflexia and Babinski sign may be observed.
Pure sensory stroke
The infarct of this lacunar syndrome is usually in the thalamus. Symptoms consist of persistent or transient numbness and/or tingling on one side of the body (eg, face, arm, leg, trunk). Occasionally, patients complain of pain or burning, or of another unpleasant sensation.
Unilateral sensory loss is observed. Although the patient may complain of weakness, no weakness is found on examination.
Mixed sensorimotor stroke
The infarct in mixed sensorimotor strokes is usually in the thalamus and the adjacent posterior internal capsule (seemingly, in the carotid and vertebrobasilar territories).
With this lacunar syndrome, patients note hemiparesis or hemiplegia with ipsilateral sensory impairment.
A combination of pyramidal signs (eg, hemiparesis, hyperreflexia, Babinski sign) is noted, as is sensory loss in the absence of any cortical signs.
Lacunar strokes complications
Complications from lacunar strokes are not specific to this stroke subtype. They include the following:
- Stroke progression or recurrent stroke
- Aspiration pneumonia
- Deep vein thrombosis (DVT) and pulmonary embolism
- Urinary tract infection (UTI)
- Depression
- Shoulder-hand syndrome
- Decubitus ulcers
Lacunar stroke diagnosis
When considering the diagnosis of a lacunar stroke, other conditions should also be evaluated, including hypoglycemia, migraine headache, middle cerebral artery stroke, other stroke subtypes (large artery disease, cardioembolic, hemorrhagic), seizures (Todd paresis), and space-occupying lesions (abscess, tumor).
Routine Tests
Obtain a serum glucose level to rule out hypoglycemia.
Obtain a complete blood cell (CBC) count, as thrombocytopenia is a contraindication for thrombolysis. Coagulation studies such as prothrombin time/international normalized ratio (PT/INR) and activated partial prothrombin time (aPTT) should also be obtained, as anticoagulant use with prolonged aPTT or PT (>15 sec) or INR greater than 1.7 are contraindications to thrombolysis.
CT Scanning of the Head
Computed tomography (CT) scans are usually negative for lacunes in the acute stage; however, CT scanning is the imaging procedure of choice to rule out an intracerebral hemorrhage. It may show a large cortical stroke, an old lacune, or a space-occupying lesion.
Magnetic Resonance Studies of the Head
Magnetic resonance imaging (MRI) is more sensitive than computed tomography (CT) scanning for the identification of acute and old lacunes (particularly in the posterior fossa) 31. However, although MRI can help to identify acute hemorrhage, there is a longer acquisition time than with CT scanning.
Magnetic resonance angiography (MRA) also should be performed, because lacunes occasionally result from large-vessel disease. If further anatomic details are needed, the use of diffusion-weighted (DW) MRI may be indicated 31.
Cardiac and Arterial Studies
An electrocardiogram (ECG), a Holter monitor study, a carotid Doppler ultrasonogram, and an echocardiogram may be required to identify a potential embolic cause for the lacunar stroke 23.
Cerebral angiography is required if a severe (>70% occlusion) carotid stenosis is identified on noninvasive testing (carotid ultrasonography or magnetic resonance angiography [MRA]) and if carotid endarterectomy is contemplated.
Lacunar stroke treatment
Management of traditional risk factors is still the main approach for treating or preventing cerebral small vessel disease, despite the fact that most of these treatments have not yet shown ideal effects on long-term outcome.
The medications used in the management of lacunar stroke are not specific to this stroke subtype. Aspirin is accepted as standard antiplatelet therapy in patients with lacunar infarcts 32. The addition of clopidogrel to aspirin has been shown to reduce the risk of stroke among patients with atrial fibrillation 33 and those with acute coronary syndromes 34, but dual antiplatelet therapy has been associated with increased bleeding 35. The Secondary Prevention of Small Subcortical Strokes (SPS3) trial tested two randomized interventions, in a 2-by-2 factorial design, in patients with recent symptomatic, MRI-confirmed lacunar stroke: clopidogrel and aspirin versus aspirin alone and two target levels of systolic blood pressure. The antiplatelet component of the trial was terminated at the recommendation of the data and safety monitoring committee because of lack of efficacy combined with evidence of harm. Among patients with recent lacunar strokes, the Management of Atherothrombosis with Clopidogrel in High-risk patients (MATCH) trial showed the addition of clopidogrel to aspirin did not significantly reduce the risk of recurrent stroke and did significantly increase the risk of bleeding and death 36. Dual antiplatelet therapy was associated with a trend toward a reduction in recurrent strokes attributed to atherosclerosis but not recurrent lacunar strokes, a finding that supports the hypothesis that the role of platelets is different in different types of ischemic cerebrovascular disease 37. It has been speculated that thrombosis may have a minimal role in precipitating occlusions of small, penetrating cerebral arteries 38. In conclusion, in this clinical trial of clopidogrel and aspirin, as compared with aspirin alone, in patients with a recent lacunar stroke identified on MRI, we found that the anticipated increase in the risk of major hemorrhage with dual antiplatelet therapy was not offset by a reduction in the risk of stroke recurrence, and there was an unexpected increase in mortality 37. Also, blood pressure lowering did not show significant reduction in recurrent lacunar stroke in the Small Subcortical Strokes (SPS3) trial, although it was consistent with a modest benefit 39.
Apart from the Small Subcortical Strokes (SPS3) trial, there are very few clinical trials of antiplatelets where the results were reported by stroke subtype, and, except trials of cilostazol 40, which has weak antiplatelet effects 41, are especially scarce in Asian populations. Although some trials reported the proportion of lacunar stroke in their study population, the diagnostic criteria varied considerably and the results were not always reported by subgroup. A systematic review of randomised trials found that any single antiplatelet appeared beneficial for secondary prevention of lacunar stroke 42, but the SPS3 trial showed that long-term dual antiplatelet treatment doubled the risk of bleeding without reducing the risk of stroke recurrence in patients with recent lacunar stroke.
Fibrinolytic agents are used to improve stroke outcome. The National Institute of Neurological Disorders and Stroke (NINDS) study on recombinant tissue-type plasminogen activator (rt-PA) showed an 11-13% absolute increase in the number of ischemic stroke patients with a favorable outcome at 3 months with tissue plasminogen activator (t-PA) 43.
A study by Matusevicius et al 44 using the SITS (Safe Implementation of Treatments in Stroke) International Stroke Thrombolysis Register found that in patients who had suffered a lacunar stroke, the likelihood of functional independency was 7.1% greater among those who were treated with intravenous (IV) thrombolysis than in patients who did not receive this therapy. However, the adjusted odds ratios for mortality at 3 months were similar between the two groups. The investigators also found that among lacunar and nonlacunar stroke patients who underwent IV thrombolysis, the risk for symptomatic intracerebral hemorrhage was lower in the former group than in the latter.
Using data from the Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial, Barow et al 45 found that among patients who had suffered a lacunar infarct, favorable functional outcomes occurred in 31 out of 53 (58.5%) persons who underwent IV thrombolysis with the recombinant tissue-type plasminogen activator (rt-PA) alteplase, compared with 24 out of 52 individuals (46.2%) who received a placebo. However, one symptomatic intracranial hemorrhage and one death occurred in the alteplase patients, with neither occurring in the placebo group. In addition, no significant difference in alteplase-related functional outcomes was found between patients with lacunar stroke and those with other stroke types.
Similarly, a retrospective Australian study, by Eggers et al 46, found a similar degree of improvement in lacunar and nonlacunar ischemic stroke patients treated with rt-PA, with functional outcomes better in both groups at 3-month follow-up than in ischemic stroke patients who did not undergo thrombolysis.
Antiplatelet agents are used for secondary stroke prevention, and if commenced within 48 hours of stroke onset, confer a small survival benefit. A literature review by Kwok et al 47 indicated that following a lacunar stroke, antiplatelet therapy has a significant benefit over placebo in the reduction of secondary stroke. The study, which included 17 trials (42,234 participants), also suggested that dual antiplatelet agent therapy has no clear benefit over monotherapy and should therefore not be used for long-term prevention of lacunar stroke.
Angiotensin-converting enzyme inhibitors are also used for secondary stroke prevention 48. Anticoagulant agents are employed for prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism.
Antihypertensive treatment produced contradictory results: it reduced white matter hyperintensity progression in some observational studies 49 but showed little or no effects in randomized controlled trials 50. Although hypertension has been reported to be highly associated with cerebral small vessel disease, other factors may be involved or be influenced by genetic factors 51, yet more evidences are required. Likewise, most lipid-lowering treatment had neutral results in preventing white matter hyperintensity, like pravastatin 52. Post hoc analysis of a 2-year follow-up study from Hong Kong showed that statins might be able to delay white matter hyperintensity progression in patients with severe baseline white matter hyperintensity 53. Statins might also have other therapeutic effects including anti-inflammatory and proendothelial activities 54. Likewise, subgroup analysis of the VITAmins TO Prevent Stroke (VITATOPS) MRI substudy shows that vitamin B supplementation may reduce white matter hyperintensity progression in patients with severe baseline cerebral small vessel disease 55.
Some patients with spasticity or joint contractures following a lacunar stroke may benefit from the injection of botulinum toxin or neurolytic agents.
Surgical intervention
Surgery (eg, gastrostomy/jejunostomy) is rarely required as a result of a lacunar stroke, but patients with severe dysphagia may require long-term tube feeding.
Consultations
A social worker should be consulted to assess personal and family resources, to inform the patient and family of available government resources, to facilitate discharge planning, and to coordinate community services.
Rehabilitation
Rehabilitation in patients following a lacunar stroke may include physical and occupational therapy, speech therapy, and recreational therapy.
If the patient who has had a lacunar stroke is functionally independent, can return safely home, and would benefit from intensive inpatient rehabilitation, transfer him/her to a rehabilitation facility.
Educate the patient and family about the common stroke symptoms. Inform them early about the importance of presentation, because tissue plasminogen activator (t-PA) (which may be indicated) can be given only within 3 hours of stroke onset 43.
Medical follow-up is necessary to assess neurologic and functional improvement, to monitor and treat risk factors, and to monitor drug compliance. Outpatient physical, occupational, and/or speech therapy may be recommended.
Discharge on aspirin and ramipril 56. If the patient remains nonambulatory and is at high risk of deep vein thrombosis, continue subcutaneous heparin.
Physical therapy
After the initial assessment of a patient who has suffered a lacunar stroke, a physical therapy program should provide passive exercises, with the major joints of the paretic limb being put through a full range of motion (ROM). As soon as patients are stable and can tolerate more active therapy, encourage them to sit up (initially in bed and later in a chair), to stand, and to transfer safely; then, they can commence ambulating with assistance and aids, as required. The physical therapist can provide splints and braces to support joints and limbs, to treat and prevent complications (eg, shoulder-hand syndrome, spasticity), and to assist the patient in walking.
Occupational therapy
When the patient who has had a lacunar stroke becomes stable, assess his/her ability to perform activities of daily living (ADLs), such as dressing and undressing, bathing, personal grooming, toileting, preparing meals, and eating. The occupational therapist can advise on equipment that may allow the patient to be more independent. If the patient is returning home, an assessment of the residence identifies potential problems and necessary modifications (eg, handrails, moving a bed to a ground level room), thereby providing confidence to the patient and family.
Speech therapy
A speech-language therapist can assist with speech-language problems and swallowing disorders in patients who have had a lacunar stroke. Early assessment of a patient with swallowing problems may prevent dehydration and malnutrition from inadequate intake, as well as prevent aspiration and pneumonia. In addition to the bedside assessment, cinefluoroscopy with barium swallow may be required. Treatment may require a change in food consistency, a change in positioning or compensatory swallowing technique, or placement of a feeding tube. Patients with lacunes may be dysarthric (but not dysphasic), requiring treatment to improve functional communication.
Recreational therapy
Following stroke, recreational therapy improves a patient’s independence, self-confidence, and ability to function, through participation in individual and group recreational activities that the patient previously enjoyed, as well as through participation in new ones.
The recreational therapist must assess the medical condition and physical capabilities of the patient, in addition to that individual’s interests and hobbies. Then, the therapist must help the patient to set realistic goals and to make any modifications needed to achieve them.
Recreational therapy not only allows the stroke patient to practice motor skills but also allows him/her to remain socially active. Recreational therapy includes leisure activities, such as going for a walk, fishing, and gardening, as well as involvement in family and community activities, such as playing cards or going to a restaurant or to church.
Lacunar stroke prognosis
Patient survival rates and rates of functional improvement are better for lacunar strokes than they are for other stroke subtypes 57. Between 70% and 80% of patients who have suffered a lacunar stroke are functionally independent at 1 year, compared with fewer than 50% of patients who have had a nonlacunar stroke.
The early (< 30 days) survival rate for patients who have had a lacunar stroke is approximately 96-97%. This compares to an early survival rate of 85% for patients who have suffered a nonlacunar stroke.
The late (1 year) survival rates are 87% following lacunar strokes and 65-70% following nonlacunar strokes.
The risk of recurrent lacunar stroke, no more than 10% at 1 year, is no higher (and possibly is lower) than the recurrent stroke risk noted for other stroke subtypes 58.
A study by Erdur et al 59 of in-hospital stroke recurrence over a median length of stay of 5 days found no recurrences among patients with lacunar stroke. The study involved 5106 patients with acute ischemic stroke or transient ischemic attack (TIA).
References- Mori E, Tabuchi M, Yamadori A. Lacunar syndrome due to intracerebral hemorrhage. Stroke 1985;16:454–9.
- Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. doi:10.1016/S1474-4422(13)70060-7
- Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. doi:10.1016/S1474-4422(13)70124-8
- Mead GE, Lewis SC, Wardlaw JM, et al. How well does the Oxfordshire community stroke project classification predict the site and size of the infarct on brain imaging? J Neurol Neurosurg Psychiatr 2000;68:558–62.
- Potter G, Doubal F, Jackson C, et al. Associations of clinical stroke misclassification (‘clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis 2010;29:395–402. doi:10.1159/000286342
- Makin SD, Doubal FN, Dennis MS, et al. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging: longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 2015;46:3142–8. doi:10.1161/STROKEAHA.115.010665
- Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross-sectional study. J Neurol Neurosurg Psychiatr 2011;82:540–2. doi:10.1136/jnnp.2009.190298
- Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 2009;72:1230–5. doi:10.1212/01.wnl.0000345666.83318.03
- Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968 Dec 18. 12(1):1-15.
- Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke 2011;42:359–66. doi:10.1161/STROKEAHA.110.594754
- Franke CL, van Swieten JC, van Gijn J. Residual lesions on computed tomography after intracerebral hemorrhage. Stroke 1991;22:1530–3.
- Lacunar Stroke. https://emedicine.medscape.com/article/322992-overview
- Bejot Y, Catteau A, Caillier M, Rouaud O, Durier J, Marie C, et al. Trends in incidence, risk factors, and survival in symptomatic lacunar stroke in Dijon, France, from 1989 to 2006: a population-based study. Stroke. 2008 Jul. 39(7):1945-51.
- Rukn SA, Mazya MV, Hentati F, et al. Stroke in the Middle-East and North Africa: a 2-year prospective observational study of stroke characteristics in the region-results from the Safe Implementation of Treatments in Stroke (SITS)-Middle-East and North African (MENA). Int J Stroke. 2019 Mar 12. 1747493019830331.
- Mok VC, Wong A, Lam WW, Baum LW, Ng HK, Wong L. A case-controlled study of cognitive progression in Chinese lacunar stroke patients. Clin Neurol Neurosurg. 2008 Jul. 110(7):649-56.
- Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke and Vascular Neurology 2016;1:doi: 10.1136/svn-2016-000035 https://svn.bmj.com/content/1/3/83.full
- Cerebral Small Artery Disease. Pullicino PM, Caplan LR, Hommel M, eds. Advances in Neurology. New York, NY: Raven Press; 1993. 62:
- Khan A, Kasner SE, Lynn MJ, et al. Risk factors and outcome of patients with symptomatic intracranial stenosis presenting with lacunar stroke. Stroke 2012;43:1230–3. doi:10.1161/STROKEAHA.111.641696
- Wardlaw JM, Doubal FN, Eadie E, et al. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc Dis 2011;31:12–18. doi:10.1159/000319773
- Kim JS, Yoon Y. Single subcortical infarction associated with parental arterial disease: important yet neglected sub-type of atherothrombotic stroke. Int J Stroke 2013;8:197–203. doi:10.1111/j.1747-4949.2012.00816.x
- de Jong G, Kessels F, Lodder J. Two types of lacunar infarcts: further arguments from a study on prognosis. Stroke 2002;33:2072–6.
- Asdaghi N, Pearce LA, Nakajima M, et al. Clinical correlates of infarct shape and volume in lacunar strokes: the Secondary Prevention of Small Subcortical Strokes trial. Stroke 2014;45:2952–8. doi:10.1161/STROKEAHA.114.005211
- Rojas JI, Zurru MC, Romano M, Patrucco L, Falconi M, Cristiano E. Transesophageal echocardiography findings in lacunar stroke. J Stroke Cerebrovasc Dis. 2008 May-Jun. 17(3):116-20.
- Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 2010;41:624–9. doi:10.1161/STROKEAHA.109.558809
- Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke 2005;36:891–901. doi:10.1161/01.STR.0000157949.34986.30
- Fang XH, Wang WH, Zhang XQ, et al. Incidence and survival of symptomatic lacunar infarction in a Beijing population: a 6-year prospective study. Eur J Neurol 2012;19:1114–20. doi:10.1111/j.1468-1331.2012.03709.x
- Lv P, Jin H, Liu Y, et al. Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: a cross-sectional study in China. PLoS ONE 2016;11:e0149605. doi:10.1371/journal.pone.0149605
- Inzitari D, Eliasziw M, Sharpe BL, Fox AJ, Barnett HJ. Risk factors and outcome of patients with carotid artery stenosis presenting with lacunar stroke. North American Symptomatic Carotid Endarterectomy Trial Group. Neurology. 2000 Feb 8. 54(3):660-6.
- Berberich A, Schneider C, Reiff T, Gumbinger C, Ringleb PA. Dual Antiplatelet Therapy Improves Functional Outcome in Patients With Progressive Lacunar Strokes. Stroke. 2019 Apr. 50 (4):1007-9.
- Bang OY, Joo SY, Lee PH, Joo US, Lee JH, Joo IS, et al. The course of patients with lacunar infarcts and a parent arterial lesion: similarities to large artery vs small artery disease. Arch Neurol. 2004 Apr. 61(4):514-9.
- Nitkunan A, Barrick TR, Charlton RA, Clark CA, Markus HS. Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke. 2008 Jul. 39(7):1999-2005.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276.
- The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078.
- The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. [Errata, N Engl J Med 2001;345:1506, 1716.
- Usman MHU, Notaro LA, Nagarakanti R, et al. Combination antiplatelet therapy for secondary stroke prevention: enhanced efficacy or double trouble? Am J Cardiol. 2009;103:1107–1112.
- Clopidogrel Added to Aspirin Adds No Benefit but Bleeding Risk in Patients With Recent Lacunar Stroke. Stroke. 2013;44:861–863 https://doi.org/10.1161/STROKEAHA.112.680751
- SPS3 Investigators, Benavente OR, Hart RG, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367(9):817–825. doi:10.1056/NEJMoa1204133 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4067036
- Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76:617–619.
- Group SPSS, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–15. doi:10.1016/S0140-6736(13)60852-1
- Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010;9:959–68. doi:10.1016/S1474-4422(10)70198-8
- Comerota AJ. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl 2005;6:13–19. doi:10.1016/j.atherosclerosissup.2005.09.005
- Kwok CS, Shoamanesh A, Copley HC, et al. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke 2015;46:1014–23. doi:10.1161/STROKEAHA.114.008422
- Adams HP Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1996 Sep 1. 94(5):1167-74.
- Matusevicius M, Paciaroni M, Caso V, et al. Outcome after intravenous thrombolysis in patients with acute lacunar stroke: an observational study based on SITS international registry and a meta-analysis. Int J Stroke. 2019 Apr 1. 1747493019840947.
- Barow E, Boutitie F, Cheng B, et al. Functional Outcome of Intravenous Thrombolysis in Patients With Lacunar Infarcts in the WAKE-UP Trial. JAMA Neurol. 2019 Mar 25.
- Eggers CCJ, Bocksrucker C, Seyfang L, Austrian Stroke Unit Registry Collaborators. The efficacy of thrombolysis in lacunar stroke – evidence from the Austrian Stroke Unit Registry. Eur J Neurol. 2017 Apr 27
- Kwok CS, Shoamanesh A, Copley HC, et al. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke. 2015 Apr. 46(4):1014-23.
- Brenner D, Labreuche J, Pico F, Scheltens P, Poirier O, Cambien F, et al. The renin-angiotensin-aldosterone system in cerebral small vessel disease. J Neurol. 2008 Jul. 255(7):993-1000.
- Dufouil C, de Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology 2001;56:921–6.
- Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke 2012;43:2336–42. doi:10.1161/STROKEAHA.111.648576
- Turner ST, Fornage M, Jack CR Jr., et al. Genomic susceptibility loci for brain atrophy in hypertensive sibships from the GENOA study. Hypertension 2005;45:793–8. doi:10.1161/01.HYP.0000154685.54766.2d
- ten Dam VH, van den Heuvel DM, van Buchem MA, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology 2005;64:1807–9. doi:10.1212/01.WNL.0000161844.00797.73
- Mok VC, Lam WW, Fan YH, et al. Effects of statins on the progression of cerebral white matter lesion: post hoc analysis of the ROCAS (Regression of Cerebral Artery Stenosis) study. J Neurol 2009;256:750–7. doi:10.1007/s00415-009-5008
- Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015;10:469–78. doi:10.1111/ijs.12466
- Cavalieri M, Schmidt R, Chen C, et al. B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: the VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke 2012;43:3266–70. doi:10.1161/STROKEAHA.112.665703
- Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012 Aug 30. 367(9):817-25.
- Bejot Y, Catteau A, Caillier M, Rouaud O, Durier J, Marie C, et al. Trends in incidence, risk factors, and survival in symptomatic lacunar stroke in Dijon, France, from 1989 to 2006: a population-based study. Stroke. 2008 Jul. 39(7):1945-51
- Sacco S, Marini C, Totaro R, Russo T, Cerone D, Carolei A. A population-based study of the incidence and prognosis of lacunar stroke. Neurology. 2006 May 9. 66(9):1335-8.
- Erdur H, Scheitz JF, Ebinger M, et al. In-hospital stroke recurrence and stroke after transient ischemic attack: frequency and risk factors. Stroke. 2015 Apr. 46(4):1031-7.