What is leukocytosis
Leukocytosis is an increase in the number of white blood cells to more than 11,000 cells per mm³ (11.0 × 109 per L) of blood in nonpregnant adults 1. Leukocytosis in the range of approximately 50,000 to 100,000 per mm³ (50.0 to 100.0 × 109 per L) is sometimes referred to as a leukemoid reaction. This level of elevation can occur in some severe infections, such as Clostridium difficile infection, sepsis, organ rejection, or in patients with solid tumors 2. Leukocytosis greater than 100,000 per mm³ is almost always caused by leukemias or myeloproliferative disorders 3. Leukocytes are white blood cells that exist in the blood, the lymphatic system, and tissues and are an important part of your body’s defense system. Leukocytes help protect you against infections and they have a role in inflammation, allergic responses, and protection against cancer. The white blood cell (WBC) count totals the number of leukocytes in a person’s sample of blood. It is one test among several that is included in a complete blood count (CBC), which is often used in the general evaluation of a person’s health.
If you have had your spleen removed, you may have a persistent mild to moderate increase in white blood cell (WBC) count.
Intense exercise or severe emotional or physical stress can cause leukocytosis, but the white blood cell (WBC) count is not used to evaluate these conditions. Pregnancy in the final month and labor may also be associated with leukocytosis.
In the U.S. population, white blood cell (WBC) counts are related to one’s age, sex, ethnicity, and smoking status. It is not uncommon for the elderly to fail to develop leukocytosis as a response to infection.
There are many drugs that cause both increased and decreased white blood cell (WBC) counts.
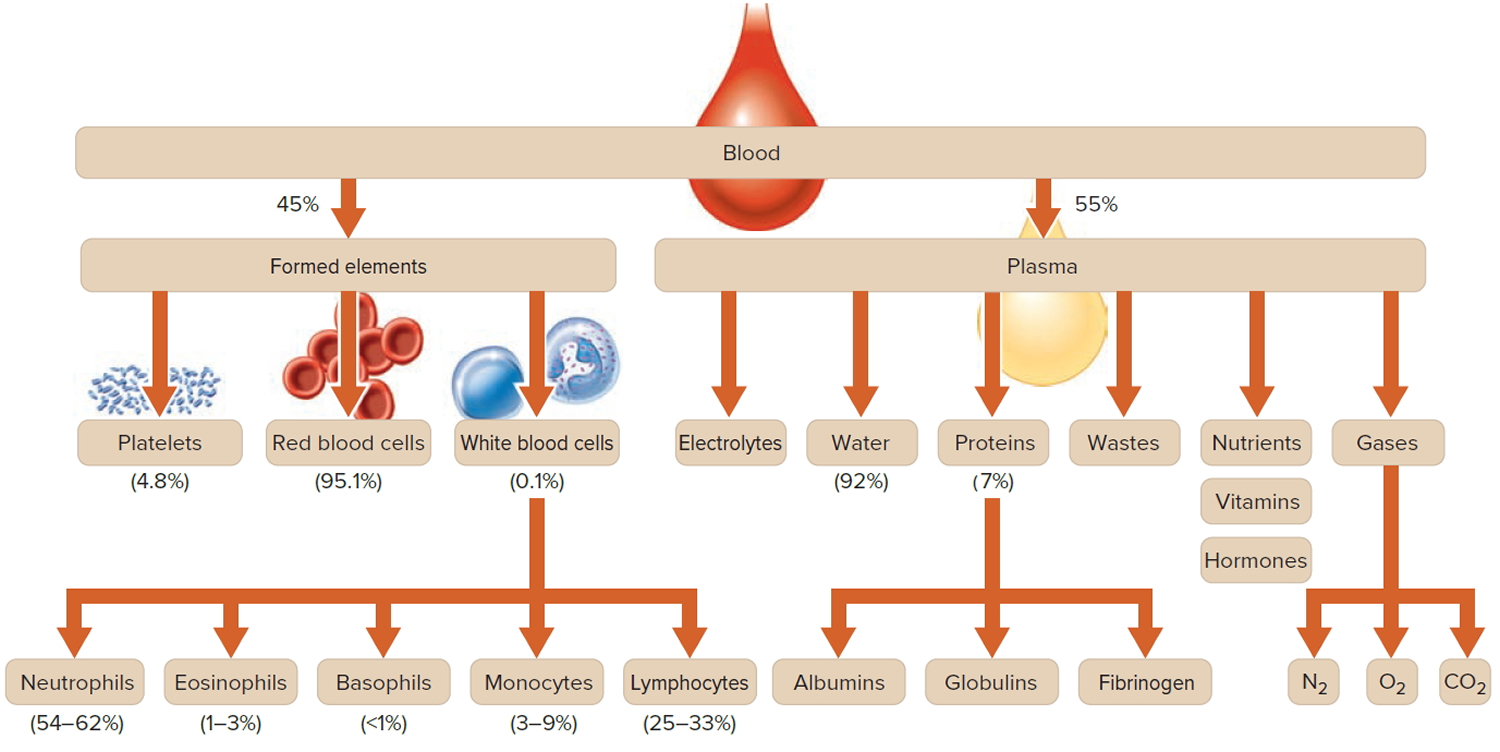
Blood is made up of a few different types of cells suspended in fluid called plasma. In addition to leukocytes (white blood cells), there are red blood cells and platelets. All of these blood cells are produced in your bone marrow and subsequently released into the blood to circulate. There are five types of leukocytes, and each has a different function.
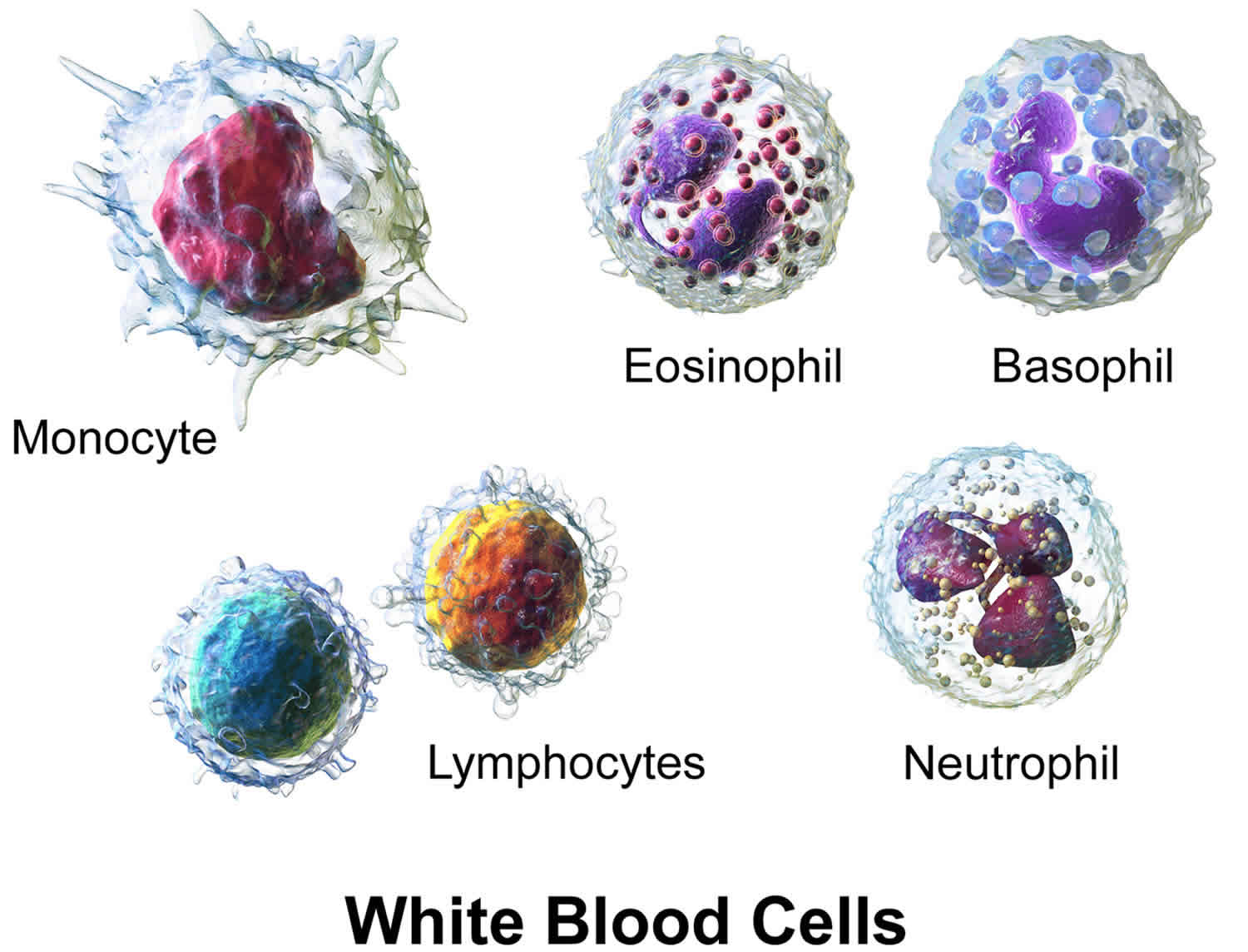
Three types of leukocytes are referred to as “granulocytes” because of the granules present in their cytoplasm. These granules release chemicals and other substances as part of the immune response. Granulocytes include neutrophils, which normally make up the largest number of circulating leukocytes, eosinophils, and basophils. The other two types of leukocytes are monocytes and lymphocytes. Lymphocytes are further divided into three subtypes: B lymphocytes that produce antibodies (also known as immunoglobulins), T lymphocytes, and natural killer cells (NK cells).
When there is an infection or an inflammatory process somewhere in your body, the bone marrow produces more leukocytes, releasing them into the blood, and through a complex process, they move to the site of infection or inflammation. As the condition resolves, the production of leukocytes by the bone marrow subsides and the number of leukocytes drops to normal levels again.
In addition to infections and inflammation, there are a number of conditions that can affect the production of leukocytes by the bone marrow or the survival of leukocytes in the blood, such as cancer or an immune disorder, resulting in either increased or decreased numbers of leukocytes in the blood. The white blood cell count, along with the other components of the complete blood count (CBC), alerts a health practitioner to possible health issues. Results are often interpreted in conjunction with additional tests such as a white blood cell differential and a blood smear review. A differential may inform the health practitioner as to which type of leukocyte may be low or high, and a blood smear can reveal the presence of abnormal and/or immature populations of leukocyte.
If results indicate a problem, a wide variety of other tests maybe performed in order to help determine the cause. A health practitioner will typically consider an individual’s signs and symptoms, medical history, and results of a physical examination to decide what other tests may be necessary. For example, as needed, a bone marrow biopsy will be performed to evaluate the bone marrow status.
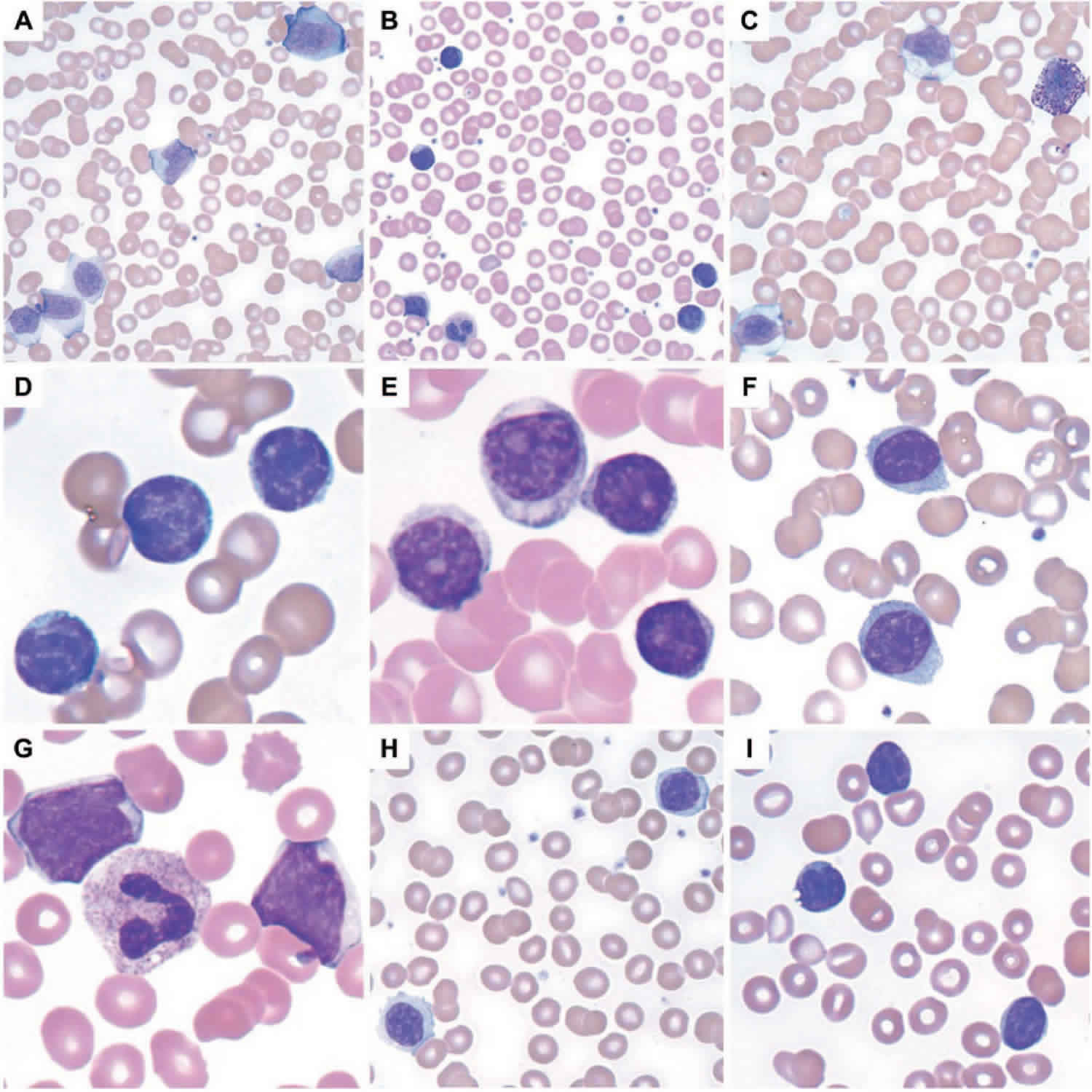
Figure 1. Leukocytes (white blood cells)
Types of leukocytes
All leukocytes have nuclei, which distinguishes them from the other blood cells, the anucleated red blood cells and platelets (Figure 1). Leukocytes differ in size, the nature of their cytoplasm, the shape of the nucleus, and their staining characteristics, and they are named for these distinctions. Two pairs of broadest categories classify them either by structure (granulocytes or agranulocytes) or by cell division lineage (myeloid cells or lymphoid cells) (see Figure 3). These broadest categories can be further divided into the five main types: neutrophils, eosinophils, basophils, lymphocytes, and monocytes 4. These types are distinguished by their physical and functional characteristics. Monocytes and neutrophils are phagocytic. Further subtypes can be classified; for example, among lymphocytes, there are B cells, T cells, and NK (natural killer) cells.
Normally, five types of leukocytes are in circulating blood. For example, leukocytes with granular cytoplasm are called granulocytes, whereas those without cytoplasmic granules are called agranulocytes (see Figure. 3).
A typical granulocyte is about twice the size of a red blood cell. Members of this group include neutrophils, eosinophils, and basophils. Granulocytes develop in red bone marrow as do red blood cells, but they have short life spans, averaging about 12 hours.
Neutrophils have fine cytoplasmic granules that appear light purple in neutral stain. The nucleus of an older neutrophil is lobed and consists of two to five sections (segments, so these cells are sometimes called segs) connected by thin strands of chromatin (Figure 4). Younger neutrophils are also called bands because their nuclei are C-shaped. Neutrophils account for 54% to 62% of the leukocytes in a typical blood sample from an adult.
Eosinophils contain coarse, uniformly sized cytoplasmic granules that appear deep red in acid stain (Figure 4). The nucleus usually has only two lobes (termed
bilobed). Eosinophils make up 1% to 3% of the total number of circulating leukocytes.
Basophils are similar to eosinophils in size and in the shape of their nuclei, but they have fewer, more irregularly shaped cytoplasmic granules that appear deep
blue in basic stain (Figure 4). Basophils usually account for less than 1% of the circulating leukocytes.
The leukocytes of the agranulocyte group include monocytes and lymphocytes. Monocytes generally arise from red bone marrow. Lymphocytes are formed in the organs of the lymphatic system, as well as in the red bone marrow.
Monocytes, the largest blood cells, are two to three times greater in diameter than red blood cells. Their nuclei are round, kidney-shaped, oval, or lobed. They usually make up 3% to 9% of the leukocytes in a blood sample and live for several weeks or even months.
Lymphocytes are only slightly larger than red blood cells. A typical lymphocyte has a large, round nucleus surrounded by a thin rim of cytoplasm. These cells account for 25% to 33% of circulating leukocytes. Lymphocytes may live for years.
Blood composition
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
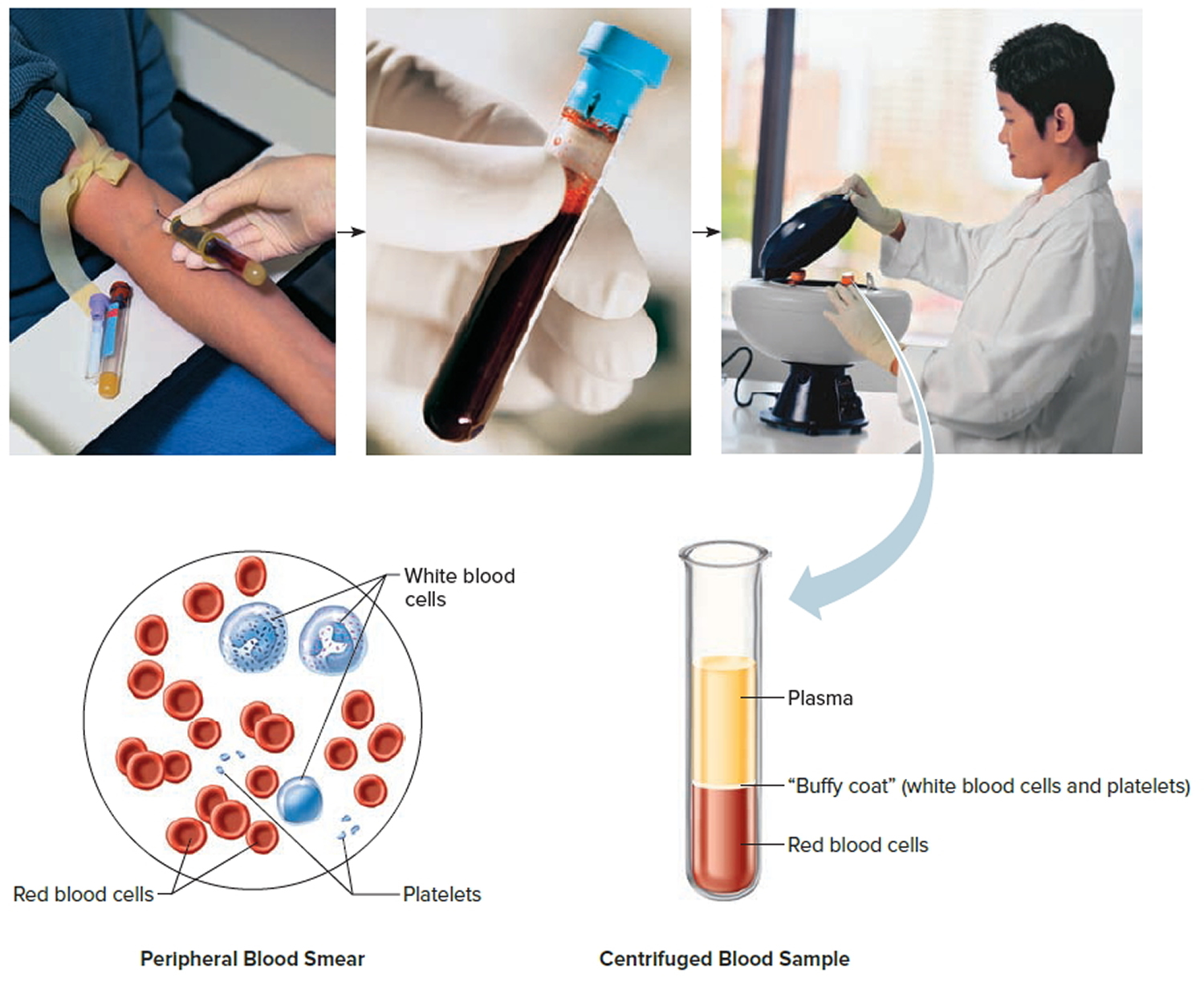
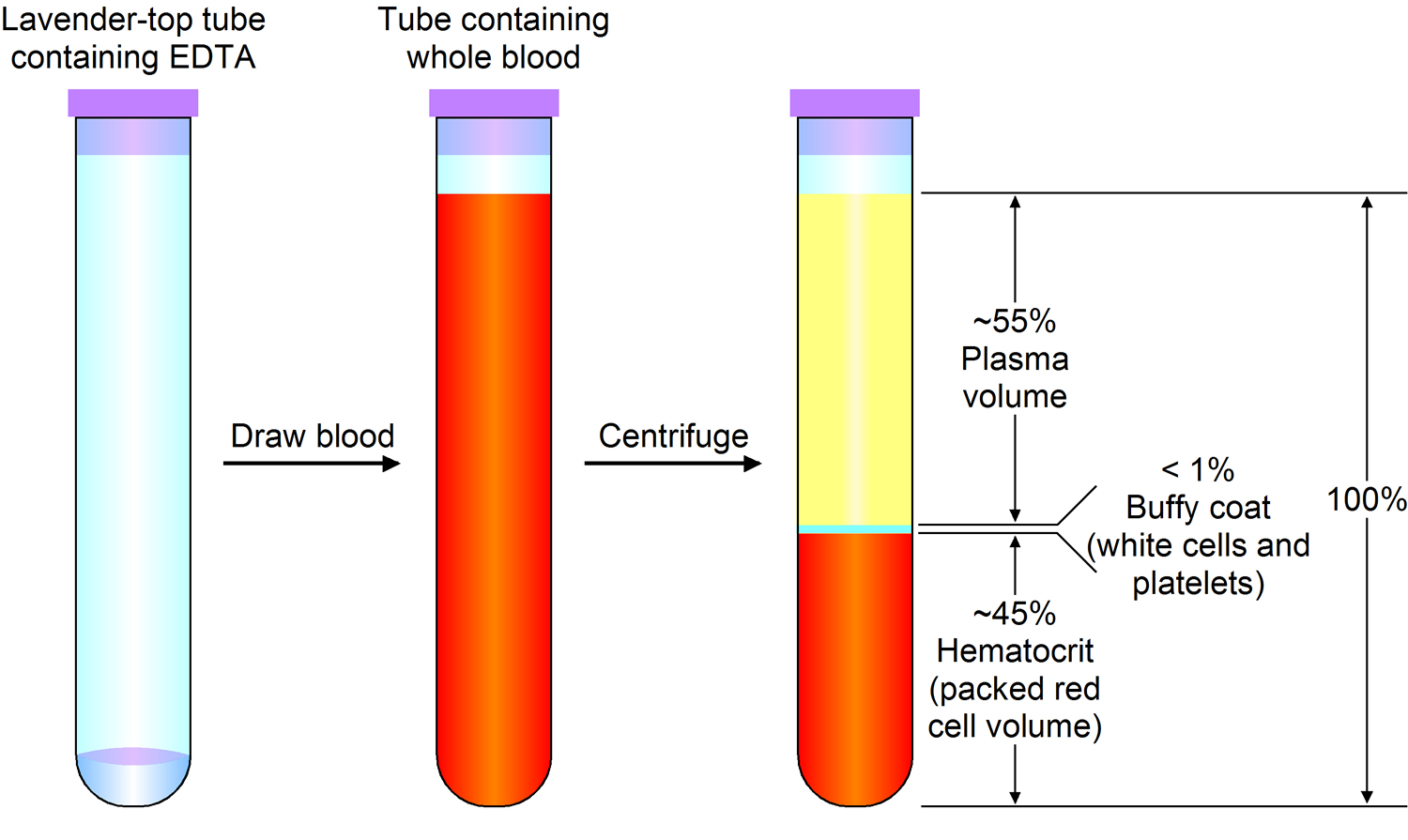
Note: Blood consists of a liquid portion called plasma and a solid portion (the formed elements) that includes red blood cells, white blood cells, and platelets. When blood components are separated by centrifugation, the white blood cells and platelets form a thin layer, called the “buffy coat,” between the plasma and the red blood cells, which accounts for about 1% of the total blood volume. Blood cells and platelets can be seen under a light microscope when a blood sample is smeared onto a glass slide.
Normal Leukocyte Life Cycle and Responses
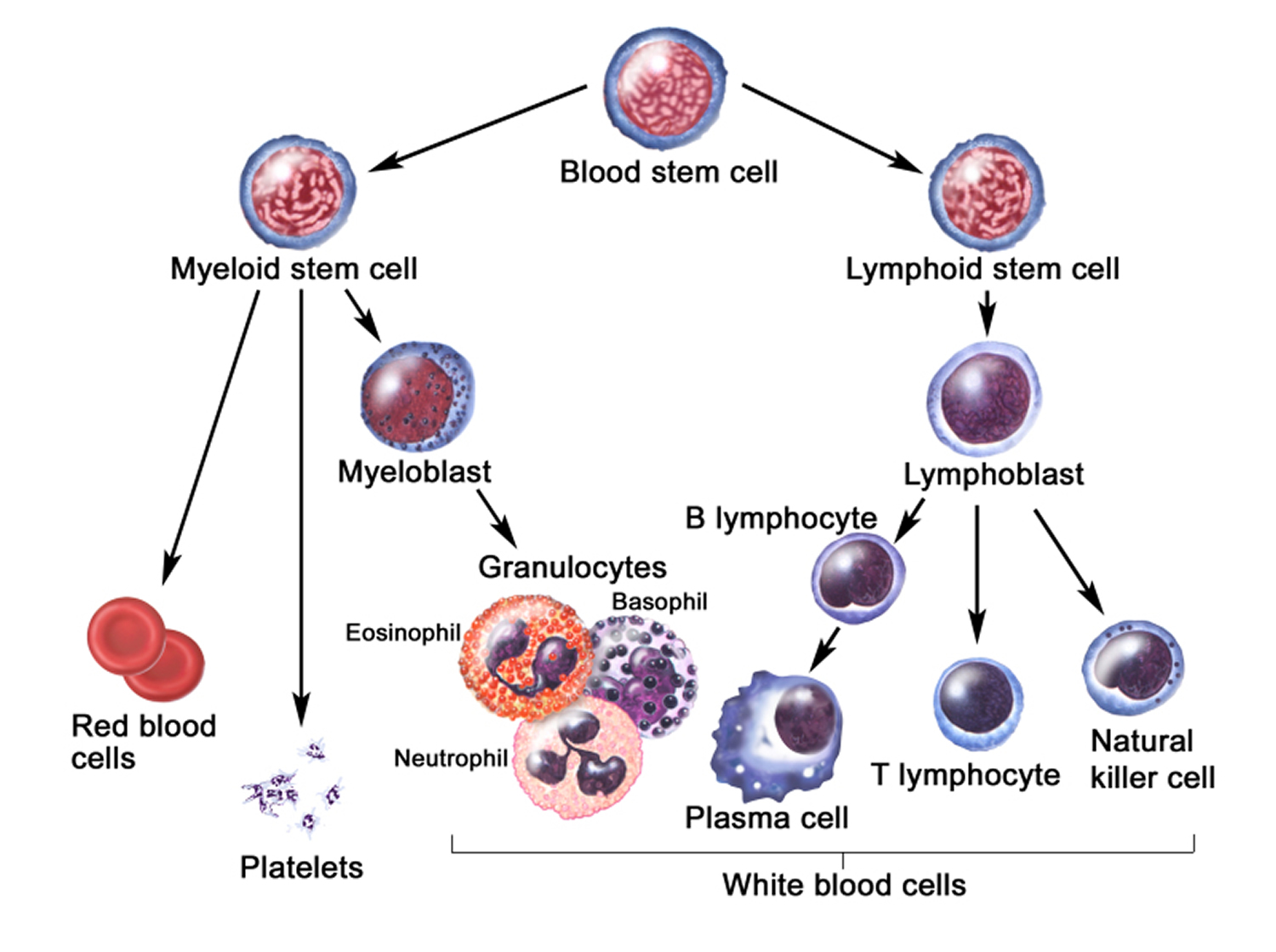
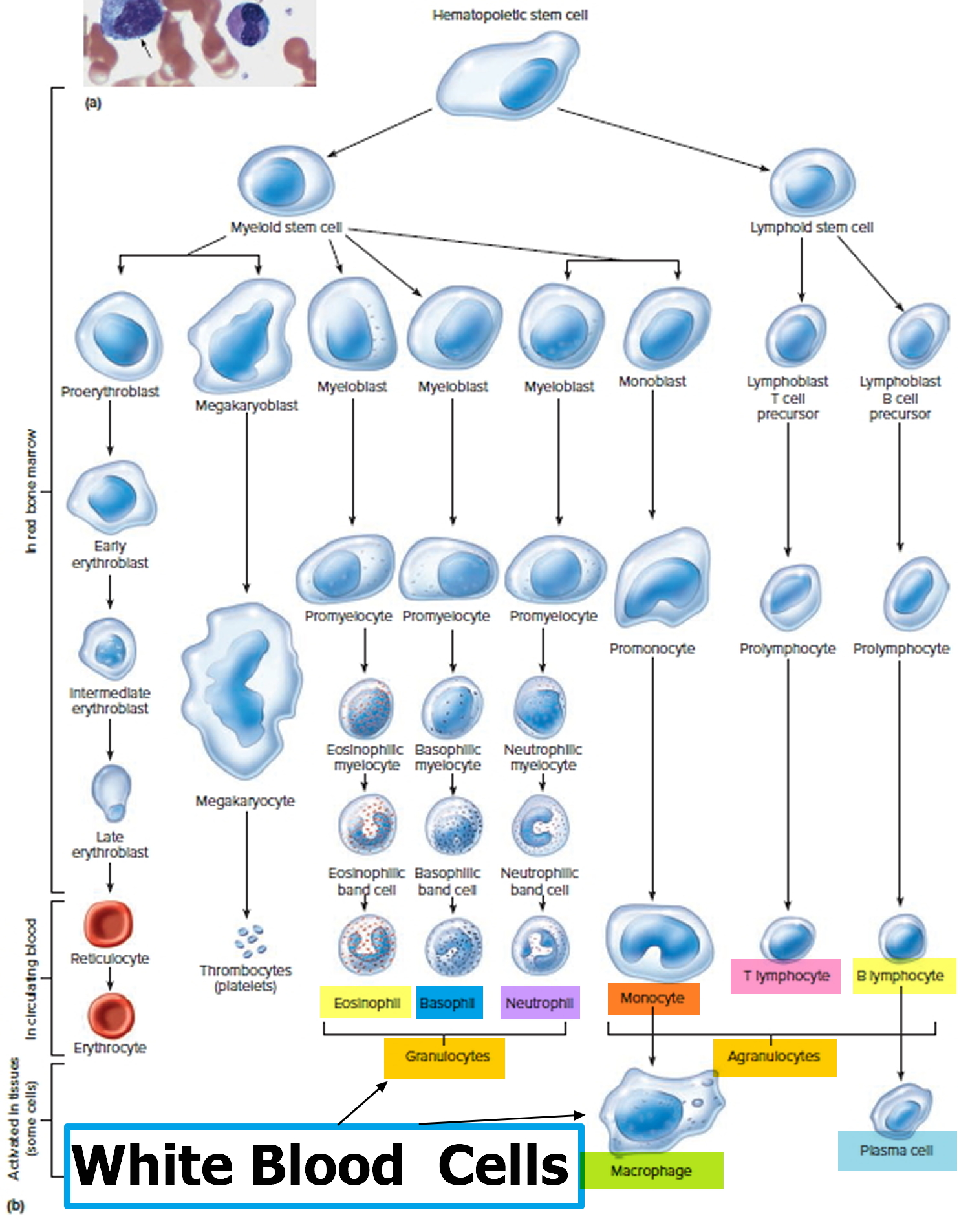
The life cycle of leukocytes includes development and differentiation, storage in the bone marrow, margination within the vascular spaces, and migration to tissues. Stem cells in the bone marrow produce cell lines of erythroblasts, which become red blood cells; megakaryoblasts, which become platelets; lymphoblasts; and myeloblasts. Lymphoblasts develop into various types of T and B cell lymphocytes. Myeloblasts further differentiate into monocytes and granulocytes, a designation that includes neutrophils, basophils, and eosinophils (Figure 2 and 3). Once white blood cells have matured within the bone marrow, 80% to 90% remain in storage in the bone marrow. This large reserve allows for a rapid increase in the circulating white blood cell count within hours. A relatively small pool (2% to 3%) of leukocytes circulate freely in the peripheral blood 2; the rest stay deposited along the margins of blood vessel walls or in the spleen. Leukocytes spend most of their life span in storage. Once a leukocyte is released into circulation and peripheral tissues, its life span ranges from two to 16 days, depending on the type of cell.
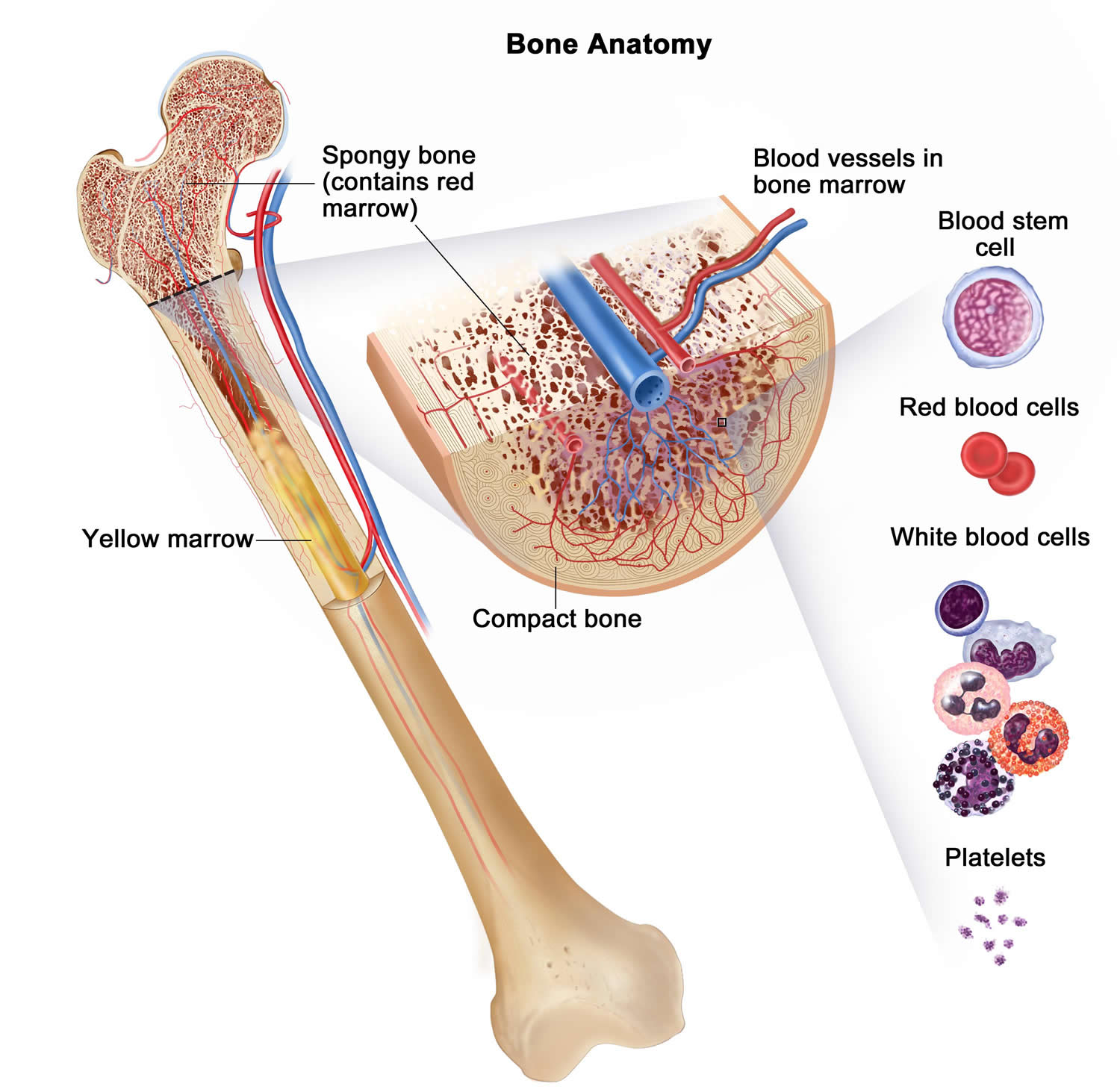
Figure 2. Bone marrow anatomy
Footnote: Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
Figure 3. White blood cells development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell
Figure 4. White blood cells development
Leukocytosis normal range
The normal range for white blood cell (WBC) counts changes with age and pregnancy (Table 1) 5. Healthy newborn infants may have a white blood cell (WBC) count from 13,000 to 38,000 per mm³ (13.0 to 38.0 × 109 per L) at 12 hours of life 1. By two weeks of age, this decreases to approximately 5,000 to 20,000 per mm³ (5.0 to 20.0 × 109 per L), and gradually declines throughout childhood to reach adult levels of 4,500 to 11,000 per mm³ (4.5 to 11.0 × 109 per L) by about 21 years of age 5. There is also a shift from relative lymphocyte to neutrophil predominance from early childhood to the teenage years and adulthood 6. During pregnancy, there is a gradual increase in the normal white blood cell (WBC) count (third trimester 95% upper limit = 13,200 per mm³ [13.2 × 109 per L] and 99% upper limit = 15,900 per mm3 [15.9 × 109 per L]), and a slight shift toward an increased percentage of neutrophils 7. In one study of afebrile postpartum patients, the mean white blood cell (WBC) count was 12,620 per mm3 (12.62 × 109 per L) for women after vaginal deliveries and 12,710 per mm3 (12.71 × 109 per L) after cesarean deliveries. Of note, positive bacterial cultures were not associated with leukocytosis or neutrophilia, making leukocytosis an unreliable discriminator in deciding which postpartum patients require antibiotic therapy 8. Patients of black African descent tend to have a lower white blood cell (WBC) count (by 1,000 per mm³ [1.0 × 109 per L]) and lower absolute neutrophil counts 9.
Table 1. White Blood Cell Count Variation with Age and Pregnancy
| Patient characteristic | Normal total leukocyte count |
|---|---|
Newborn infant | 13,000 to 38,000 per mm3 (13.0 to 38.0 × 109 per L) |
Infant two weeks of age | 5,000 to 20,000 per mm3 (5.0 to 20.0 × 109 per L) |
Adult | 4,500 to 11,000 per mm3 (4.5 to 11.0 × 109 per L) |
Pregnant female (third trimester) | 5,800 to 13,200 per mm3 (5.8 to 13.2 × 109 per L) |
Differentiation by Type of White Blood Cell
Changes in the normal distribution of types of white blood cells (WBCs) can indicate specific causes of leukocytosis (Table 2) 10. Although the differential of the major types of white blood cells (WBCs) is important for evaluating the cause of leukocytosis, it is sometimes helpful to think in terms of absolute, rather than relative, leukopenias and leukocytoses. To calculate the absolute cell count, the total leukocyte count is multiplied by the differential percentage. For example, with a normal white blood cell (WBC) count of 10,000 per mm³ (10.0 × 109 per L) and an elevated monocyte percentage of 12, the absolute monocyte count is 12% or 0.12 times the white blood cell (WBC) count of 10,000 per mm³, yielding 1,200 per mm³ (1.2 × 109 per L), which is abnormally elevated.
Table 2. Normal White Blood Cell Distribution
| White blood cell line | Normal percentage of total leukocyte count |
|---|---|
Neutrophils | 40 to 60% |
Lymphocytes | 20 to 40% |
Monocytes | 2 to 8% |
Eosinophils | 1 to 4% |
Basophils | 0.5 to 1% |
Leukocytosis causes
Leukocytosis, an increase in the number of white blood cells to more than 11,000 cells per microliter (11.0 × 109 per L) of blood, is often caused by the normal response of the body to help fight an infection, or to some drugs such as corticosteroids. However, an increase in the number of white blood cells is also caused by cancers of the bone marrow (such as leukemia) or by the release of immature or abnormal white blood cells from the bone marrow into the blood.
The most common type of leukocytosis is neutrophilia (an increase in the absolute number of mature neutrophils to greater than 7,000 per mm³ [7.0 × 109 per L]), which can arise from infections, stressful conditions, chronic inflammation, medication use, and other causes (see Table 3 below) 2. Lymphocytosis (when lymphocytes make up more than 40% of the white blood cell (WBC) count or the absolute count is greater than 4,500 per mm3 [4.5 × 109 per L]) can occur in patients with pertussis, syphilis, viral infections, hypersensitivity reactions, and certain subtypes of leukemia or lymphoma. Lymphocytosis is more likely to be benign in children than in adults 6. Epstein-Barr virus infection, tuberculosis or fungal disease, autoimmune disease, splenectomy, protozoan or rickettsial infections, and malignancy can cause monocytosis (monocytes make up more than 8% of the white blood cell (WBC) count or the absolute count is greater than 880 per mm³ [0.88 × 109 per L]) 10.
Table 3. Nonmalignant Causes of Neutrophilia
| Cause | Distinguishing features | Evaluation |
|---|---|---|
| Patient characteristics | Pregnancy, obesity, race, age | Reference appropriate WBC count by age or pregnancy trimester Compare WBC count to recent baseline (if available) |
| Infection | Fever, system-specific symptoms Physical examination findings | Obtain system-specific cultures and imaging (e.g., sputum cultures, chest radiography) Consider empiric antibiotics Consider use of other biomarkers, such as CRP and procalcitonin |
| Reactive neutrophilia | Exercise, physical stress (e.g., postsurgical, febrile seizures), emotional stress (e.g., panic attacks), smoking | Confirm with history |
| Chronic inflammation | Rheumatic disease, inflammatory bowel disease, granulomatous disease, vasculitides, chronic hepatitis | Obtain personal and family medical history Consider erythrocyte sedimentation rate and CRP levels, specific rheumatology laboratories Consider subspecialist consultation (e.g., rheumatology, gastroenterology) |
| Medication induced | Corticosteroids, beta agonists, lithium, epinephrine, colony-stimulating factors | Confirm with history; consider discontinuation of medication, if warranted |
| Bone marrow stimulation | Hemolytic anemia, immune thrombocytopenia, bone marrow suppression recovery, colony-stimulating factors | Complete blood count differential; compare with baseline values (if available) Examine peripheral smear Consider reticulocyte and lactate dehydrogenase levels Consider flow cytometry, bone marrow examination, hematology/oncology consultation |
| Splenectomy | History of trauma or sickle cell disease | Confirm with history |
| Congenital | Hereditary/chronic idiopathic neutrophilia, Down syndrome, leukocyte adhesion deficiency | Obtain family, developmental history Consider hematology/oncology, genetics, and immunology consultations |
Footnote: After patient characteristics, causes are listed in approximate order of frequency.
Abbreviations: CRP = C-reactive protein; WBC = white blood cell.
[Source 1 ]Eosinophilia (eosinophil absolute count greater than 500 per mm³ [0.5 × 109 per L]), although uncommon, may suggest allergic conditions such as asthma, urticaria, atopic dermatitis or eosinophilic esophagitis, drug reactions, dermatologic conditions, malignancies, connective tissue disease, idiopathic hypereosinophilic syndrome, or parasitic infections, including helminths (tissue parasites more than gut-lumen parasites) 11. Isolated basophilia (number of basophils greater than 100 per mm³ [0.1 × 109 per L]) is rare and unlikely to cause leukocytosis in isolation, but it can occur with allergic or inflammatory conditions and chronic myelogenous leukemia (Table 4) 12.
Table 4. Selected Conditions Associated with Elevations in Certain White Blood Cell Types
| White blood cell line | Conditions that typically cause elevations |
|---|---|
Basophils | Allergic conditions, leukemias |
Eosinophils | Allergic conditions, dermatologic conditions, eosinophilic esophagitis, idiopathic hypereosinophilic syndrome, malignancies, medication reactions, parasitic infections |
Lymphocytes | Acute or chronic leukemia, hypersensitivity reaction, infections (viral, pertussis) |
Monocytes | Autoimmune disease, infections (Epstein-Barr virus, fungal, protozoan, rickettsial, tuberculosis), splenectomy |
Neutrophils | Bone marrow stimulation, chronic inflammation, congenital, infection, medication induced, reactive, splenectomy |
Nonmalignant causes
A reactive leukocytosis, typically in the range of 11,000 to 30,000 per mm³ (11.0 to 30.0 × 109 per L), can arise from a variety of etiologies. Any source of stress can cause a catecholamine-induced demargination of white blood cells, as well as increased release from the bone marrow storage pool. Examples include surgery, exercise, trauma, burns, and emotional stress 13. One study showed an average increase in white blood cells of 2,770 per mm³ (2.77 × 109 per L) peaking on postoperative day 2 after knee or hip arthroplasty 14. Medications known to increase the white blood cell (WBC) count include corticosteroids, lithium, colony-stimulating factors, beta agonists, and epinephrine. During the recovery phase after hemorrhage or hemolysis, a rebound leukocytosis can occur.
Leukocytosis is one of the hallmarks of infection. In the acute stage of many bacterial infections, there are primarily mature and immature neutrophils; sometimes, as the infection progresses, there is a shift to lymphocyte predominance. The release of less-mature bands and metamyelocytes into the peripheral circulation results in the so-called “left shift” in the white blood cell (WBC) differential. Of note, some bacterial infections paradoxically cause neutropenias, such as typhoid fever, rickettsial infections, brucellosis, and dengue 15. Viral infections may cause leukocytosis early in their course, but a sustained leukocytosis is not typical, except for the lymphocytosis in some childhood viral infections.
An elevated white blood cell (WBC) count is a suggestive, but not definitive, marker of the presence of significant infection. For example, the sensitivity and specificity of an elevated white blood cell (WBC) count in diagnosing acute appendicitis are 62% and 75%, respectively 16. For diagnosing serious bacterial infections without a source in febrile children, the discriminatory value of leukocytosis is less than that of other biomarkers, such as C-reactive protein or procalcitonin 17. Although a white blood cell (WBC) count greater than 12,000 per mm³ (12.0 × 109 per L) is one of the criteria for the systemic inflammatory response syndrome (or sepsis when there is a known infection), leukocytosis alone is a poor predictor of bacteremia and not an indication for obtaining blood cultures 18.
Other acquired causes of leukocytosis include functional asplenia (predominantly lymphocytosis), smoking, and obesity. Patients with a chronic inflammatory condition, such as rheumatoid arthritis, inflammatory bowel disease, or a granulomatous disease, may also exhibit leukocytosis. Genetic causes include hereditary or chronic idiopathic neutrophilia and Down syndrome.
Malignant causes
Leukocytosis may herald a malignant disorder, such as an acute or chronic leukemia or a myeloproliferative disorder, such as polycythemia vera, myelofibrosis, or essential thrombocytosis 19. Many solid tumors may lead to a leukocytosis in the leukemoid range, either through bone marrow involvement or production of granulocyte colony-stimulating or granulocyte-macrophage colony-stimulating factors 20. Chronic leukemias are most commonly diagnosed after incidental findings of leukocytosis on complete blood counts in asymptomatic patients. Patients with features suggestive of hematologic malignancies require prompt referral to a hematologist/oncologist (Table 5) 21.
Table 5. Findings Suggestive of Hematologic Malignancies in the Setting of Leukocytosis
Symptoms |
Bruising/bleeding tendency |
Fatigue, weakness |
Fever > 100.4°F (38°C) |
Immunosuppression |
Night sweats |
Unintentional weight loss |
Physical examination findings |
Lymphadenopathy |
Petechiae |
Splenomegaly or hepatomegaly |
Laboratory abnormalities |
Decreased red blood cell count or hemoglobin/hematocrit levels |
Increased or decreased platelet count |
Monomorphic lymphocytosis on peripheral smear |
Predominantly immature cells on peripheral smear |
White blood cell count > 30,000 per mm3 (30.0 × 109 per L), or > 20,000 per mm3 (20.0 × 109 per L) after initial management |
Leukocytosis diagnosis
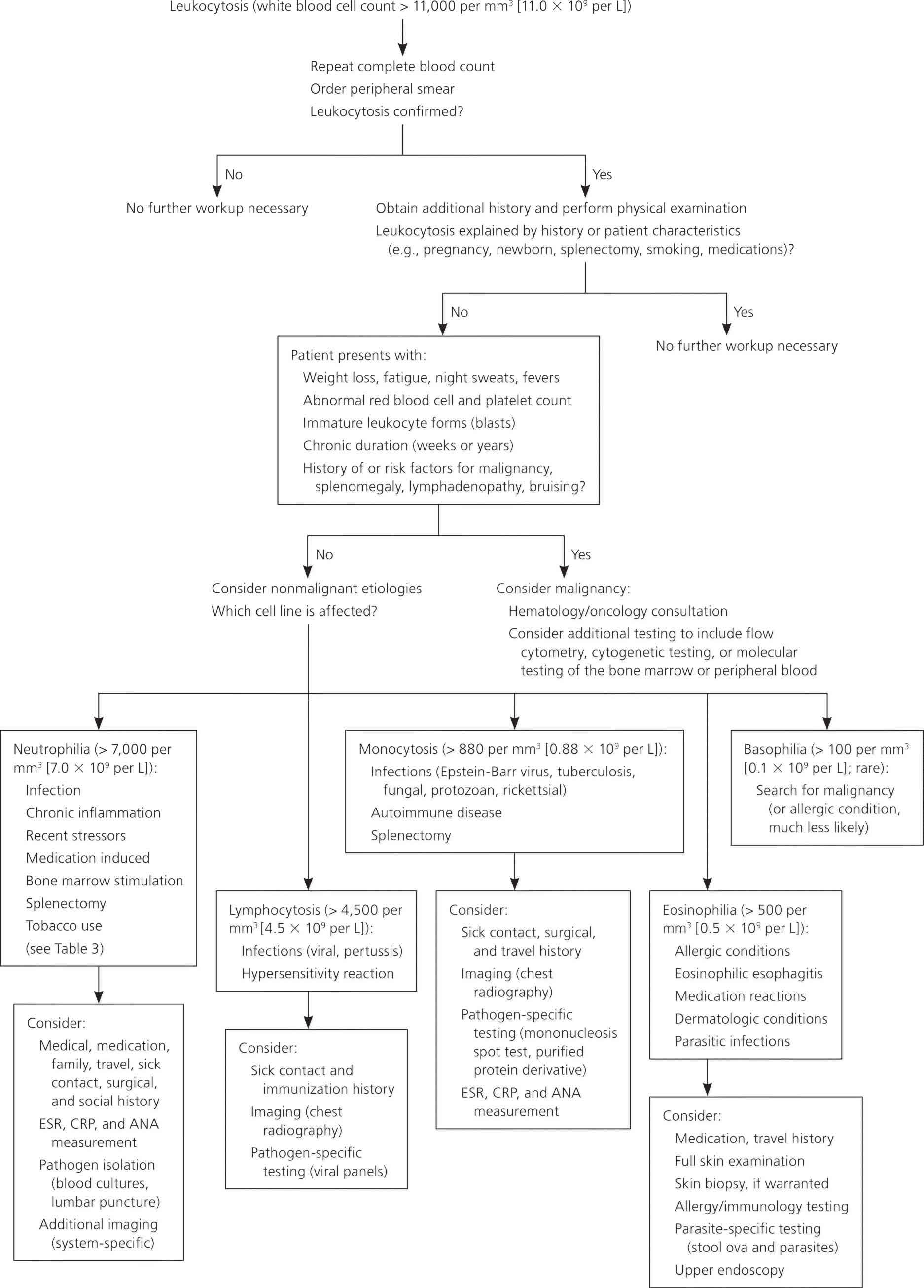
A systematic approach to patients with leukocytosis includes identifying historical clues that suggest potential causes (Figure 5). Fever and pain may accompany infections or malignancies; other constitutional symptoms, such as fatigue, night sweats, weight loss, easy bruising, or bleeding, might suggest malignancy 21. Previous diagnoses or comorbid conditions that cause chronic inflammation should be noted, as well as recent stressful events, medication use, smoking status, and history of splenectomy or sickle cell anemia. A history of an elevated white blood cell (WBC) count is important, because duration will help determine the likely cause. Leukocytosis lasting hours to days has a different differential diagnosis (e.g., infections, acute leukemias, stress reactions) than a case that persists for weeks to months (e.g., chronic inflammation, some malignancies).
The physical examination should note erythema, swelling, or lung findings suggestive of an infection; murmurs suggestive of infective endocarditis; lymphadenopathy suggestive of a lymphoproliferative disorder; or splenomegaly suggestive of chronic myelogenous leukemia or a myeloproliferative disorder; petechiae or ecchymoses; or painful, inflamed joints suggestive of connective tissue disease or infection.
Initial laboratory evaluation should include`a repeat complete blood count to confirm the elevated white blood cell (WBC) level, with differential cell counts and a review of a peripheral blood smear. The peripheral smear should be examined for toxic granulations (suggestive of inflammation), platelet clumps (which may be misinterpreted as white blood cells), the presence of immature cells, and uniformity of the white blood cells. On evaluation of a leukocytosis with lymphocyte predominance, a monomorphic population is concerning for chronic lymphocytic leukemia, whereas a pleomorphic (varying sizes and shapes) lymphocytosis is suggestive of a reactive process 22. With all forms of leukocytosis, concurrent abnormalities in other cell counts (erythrocytes or platelets) suggest a primary bone marrow process and should prompt hematology/oncology evaluation.
As indicated by the history and examination findings, physicians should consider performing cultures of blood, urine, and joint or body fluid aspirates; rheumatology studies; a test for heterophile antibodies (mononucleosis spot test); and serologic titers. Radiologic studies may include chest radiography (to identify infections, some malignancies, and some granulomatous diseases) and, as indicated by history, computed tomography or bone scan. If hematologic malignancy is suspected, additional confirmatory testing may include flow cytometry, cytogenetic testing, or molecular testing of the bone marrow or peripheral blood.
If your have leukocytosis, the other tests might your doctor might order include:
Other general tests to check your health may include a comprehensive metabolic panel (CMP). Depending on your signs, symptoms, medical history, physical exam and suspected condition, your healthcare provider may choose to order a variety of other tests. A few general examples include:
- Bacterial infection: a culture of the affected area (e.g., urine culture, sputum culture, blood culture), strep test
- Viral infection: tests for mononucleosis, EBV
- Inflammation: CRP, ESR
- Autoimmune diseases: ANA
- Allergies: Allergy tests
- Leukemia: Bone marrow biopsy, immunophenotyping (e.g., flow cytometry), chromosome analysis
Figure 5. Algorithm for the evaluation of leukocytosis
Abbreviations: ANA = antinuclear antibodies; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate
[Source 1 ]Leukocytosis symptoms
A white blood cell (WBC) count is normally ordered as part of the complete blood count (CBC), which may be performed when an individual undergoes a routine health examination. The test may be done when someone has general signs and symptoms of an infection and/or inflammation such as:
- Fever, chills
- Body aches, pain
- Headache
- A variety of other signs and symptoms, depending on the site of suspected infection or inflammation
Testing may be performed when there are signs and symptoms that a health practitioner thinks may be related to a blood disorder, autoimmune disorder, or an immune deficiency.
A white blood cell (WBC) may be ordered often and on a regular basis to monitor an individual who has been diagnosed with an infection, blood or immune disorder or another condition affecting white blood cells (WBCs). It may also been ordered periodically to monitor the effectiveness of treatment or when a particular therapy is known to affect white blood cells (WBCs), such as radiation or chemotherapy.
Leukocytosis treatment
Once the cause of your leukocytosis is found, your doctor can recommend ways to treat it. In most cases, treatment for leukocytosis is not necessary.
In extreme instances of hyperleukocytosis syndrome (e.g., acute leukemia), leukapheresis, hydration, and urine alkalinization to facilitate uric acid excretion are indicated; however, perform these treatments only in consultation with a hematologist, oncologist, or both. Direct treatment toward the underlying etiology.
Leukemic hyperleukocytosis may cause clinically significant complications when the WBC count exceeds 100,000/μL in acute myelogenous leukemia and 300,000/μL in acute lymphoblastic leukemia. Therefore, in patients with these findings, measures to reduce the WBC count are advisable. However, a decrease in leukocyte count that is too rapid carries a risk of severe tumor lysis syndrome and should be avoided.
Leukapheresis or exchange blood transfusion is a treatment of choice for this purpose, with hydration, urine alkalinization, and administration of allopurinol or rasburicase (uric acid oxydase) to reduce serum uric acid and minimize tumor lysis syndrome. When rasburicase is used, urine alkalinization is not recommended.
A study by Nguyen et al. 23 indicated that supportive care and conservative management can discourage early hyperleukocytosis-related morbidity and mortality in children with acute lymphoblastic leukemia, possibly negating the need for leukapheresis. A study by Choi et al. 24, meanwhile, found no evidence that in patients with acute leukemia (myelogenous or lymphoblastic) and hyperleukocytosis, leukapheresis improves early mortality rates or reduces the incidence of either tumor lysis syndrome or disseminated intravascular coagulopathy.
Promptly institute definitive treatment with appropriate chemotherapy. A study by Mamez et al. 25 indicated that the administration of oral hydroxyurea before chemotherapy can lower the rate of early death in hyperleukocytic patients with acute myelogenous leukemia. The study, which involved 160 patients, found the hospital mortality rate for patients who received pre-chemotherapy hydroxyurea to be 19%, compared with 34% for those who received no hydroxyurea prior to chemotherapy. However, the two treatment groups did not differ with regard to disease-free survival.
- Evaluation of Patients with Leukocytosis. Am Fam Physician. 2015 Dec 1;92(11):1004-1011. https://www.aafp.org/afp/2015/1201/p1004.html[↩][↩][↩][↩][↩][↩]
- Cerny J, Rosmarin AG. Why does my patient have leukocytosis? Hematol Oncol Clin North Am. 2012;26(2):303–319, viii.[↩][↩][↩]
- Jain R, Bansal D, Marwaha RK. Hyperleukocytosis: emergency management. Indian J Pediatr. 2013;80(2):144–148.[↩]
- LaFleur-Brooks, M. (2008). Exploring Medical Language: A Student-Directed Approach (7th ed.). St. Louis, Missouri, US: Mosby Elsevier. p. 398. ISBN 978-0-323-04950-4.[↩]
- Hoffman R, Benz EJ Jr, Silberstein LE, Heslop H, Weitz J, Anastasi J. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, Pa.: Elsevier/Saunders; 2013:table 164–20.[↩][↩][↩]
- Chabot-Richards DS, George TI. Leukocytosis. Int J Lab Hematol. 2014;36(3):279–288.[↩][↩]
- Lurie S, Rahamim E, Piper I, Golan A, Sadan O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;136(1):16–19.[↩]
- Dior UP, Kogan L, Elchalal U, et al. Leukocyte blood count during early puerperium and its relation to puerperal infection. J Matern Fetal Neonatal Med. 2014;27(1):18–23.[↩]
- Lim EM, Cembrowski G, Cembrowski M, Clarke G. Race-specific WBC and neutrophil count reference intervals. Int J Lab Hematol. 2010;32(6 pt 2):590–597.[↩]
- Berliner N. Leukocytosis and leukopenia. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, Pa.: Elsevier/Saunders; 2012.[↩][↩][↩]
- Ustianowski A, Zumla A. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 2012;26(3):781–789.[↩]
- Munker R. Leukocytosis, leukopenia, and other reactive changes of myelopoiesis. In: Munker R, Hiller E, Glass J, Paquette R, eds. Modern Hematology: Biology and Clinical Management. 2nd ed. Totowa, N.J.; Humana Press; 2007.[↩]
- Iskandar JW, Griffeth B, Sapra M, Singh K, Giugale JM. Panic-attack-induced transient leukocytosis in a healthy male: a case report. Gen Hosp Psychiatry. 2011;33(3):302.e11–302.e12[↩]
- Deirmengian GK, Zmistowski B, Jacovides C, O’Neil J, Parvizi J. Leukocytosis is common after total hip and knee arthroplasty. Clin Orthop Relat Res. 2011;469(11):3031–3036[↩]
- Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13(11):1328–1340.[↩]
- Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, Lee CC. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 2013;100(3):322–329.[↩]
- Yo CH, Hsieh PS, Lee SH, et al. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without source: a systematic review and meta-analysis. Ann Emerg Med. 2012;60(5):591–600.[↩]
- Dellinger RP, Levy MM, Rhodes A, et al.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.[↩]
- Davis AS, Viera AJ, Mead MD. Leukemia: an overview for primary care. Am Fam Physician. 2014;89(9):731–738.[↩]
- Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115(17):3919–3923.[↩]
- Racil Z, Buresova L, Brejcha M, et al. Clinical and laboratory features of leukemias at the time of diagnosis: an analysis of 1,004 consecutive patients. Am J Hematol. 2011;86(9):800–803.[↩][↩]
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program. 2012;2012:475–484.[↩]
- Nguyen R, Jeha S, Zhou Y, et al. The Role of Leukapheresis in the Current Management of Hyperleukocytosis in Newly Diagnosed Childhood Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2016 May 17.[↩]
- Choi MH, Choe YH, Park Y, et al. The effect of therapeutic leukapheresis on early complications and outcomes in patients with acute leukemia and hyperleukocytosis: a propensity score-matched study. Transfusion. 2018 Jan. 58 (1):208-16 [↩]
- Mamez AC, Raffoux E, Chevret S, et al. Pre-treatment with oral hydroxyurea prior to intensive chemotherapy improves early survival of patients with high hyperleukocytosis in acute myeloid leukemia. Leuk Lymphoma. 2016 Feb 5. 1-8.[↩]