Maggot therapy
Maggot therapy also called larval therapy, is the application of live fly larvae to wounds in order to aid in wound debridement (cleaning), disinfection and/or healing 1. Maggot therapy is a simple and successful method for cleansing infected and necrotic wounds. A maggot infestation on a living vertebrate host is called myiasis. When that infestation is limited to a wound, it is called wound myiasis. Maggot therapy is basically a therapeutic wound myiasis, controlled in ways that optimize efficacy and safety. Scientists control wound myiasis by carefully selecting the species and strain of fly, the species most commonly used is the blowfly Lucilia (Phaenicia) sericata, disinfecting the larvae, using special dressings to maintain the larvae on the wound, and integrating quality control measures throughout the process 1.
The use of maggots has become increasingly important in the treatment of nonhealing wounds, particularly those infected with the multidrug-resistant pathogen, methicillin-resistant Staphylococcus aureus (MRSA). Indeed, it has been shown that excretions/secretions from the blowfly Lucilia sericata (Lucilia (Phaenicia) sericata) exhibit potent, thermally stable, protease-resistant antibacterial activity against in vitro MRSA 2. The application of sterile Lucilia sericata larvae to an infected nonhealing wound results in the removal of necrotic tissue, disinfection, rapid elimination of infecting microorganisms, and enhancement of the healing process 3.
Debridement refers to the removal of dead, damaged, or infected tissue to improve the healing potential of the remaining healthy tissue. In order to debride necrotic tissue, larvae (ie, maggots) produce a mixture of proteolytic enzymes, including collagenase, that breaks down the necrotic tissue to a semi-liquid form to be absorbed and digested. Debridement is facilitated by wound disturbance as the larvae crawl around the tissue using their mouthhooks 4.
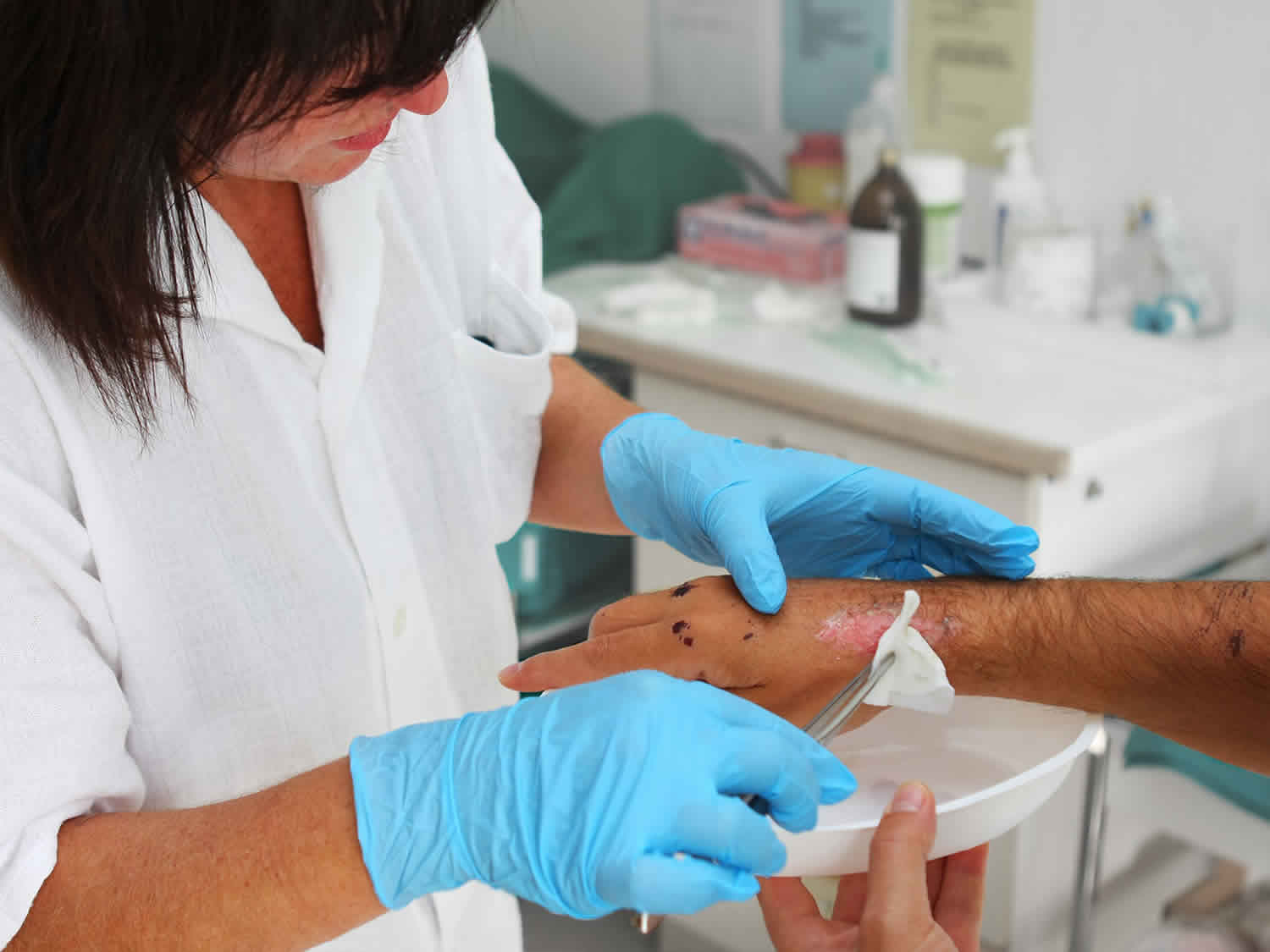
Maggot therapy is administered by applying sterilized fly larvae to the wound at a density of 5 to 8 per cm² 5. To apply larval therapy, a wound-sized hole is cut from a hydrocolloid dressing, a self-adhesive wafer with a semipermeable outer membrane. This both protects the skin from irritation by the maggot’s proteolytic enzymes and forms the base of the adhesive dressing. The sterile maggots are then moved from their container to a special piece of nylon netting placed on a nonwoven swab to draw away moisture. The netting is then bunched up to create a cage for the larvae, placed on the wound, and secured to the hydrocolloid dressing by waterproof adhesive tape. The dressing is finally covered with a simple absorbent pad held in place with adhesive tape or a bandage 3. Maggots are kept over the wound for cycles of about 48 hours; two 48-hour cycles are usually applied each week 5.
The most noticeable change in maggot-treated wounds is debridement: the dead (necrotic or gangrenous), infected tissues and debris are removed from the wound, and the wound bed is left looking clean and healthy. But ever since maggot therapy became a common practice 6, careful observers also noted other effects on the wounds: microbial killing (disinfection) and hastened wound healing (growth stimulation) 1.
Scientific evidence for all three actions – debridement (cleaning of debris), disinfection, and hastened wound healing – has been slow in coming. The first controlled clinical trials were not begun until 1990 7, and it was not until 2004 that the U.S Food and Drug Administration (FDA) first granted marketing clearance to medicinal maggots (Medical Maggots; Monarch Labs, Irvine, CA) as a medical device 8. The indications for that product were limited to debridement. Clinical evidence of maggot-induced disinfection and growth stimulation was not strong enough to convince regulators at that time. But today, numerous clinical and laboratory studies demonstrate antimicrobial and/or growth-promoting activity. Some clinical studies do not demonstrate these effects; instead, they leave doctors with doubts about the clinical significance of the wound healing activities that scientists see in most other clinical and laboratory studies.
Do maggots clean wounds?
Several comprehensive reviews have been published over the past decade 9, 10, 11. The resulting body of literature provided both laboratory and clinical evidence to support all three actions associated with maggot therapy: debridement, disinfection, and growth stimulation 1. Nonsupportive data were also available, though less commonly. The best way to consider the role of maggots in wound healing may be to first review the wound healing process in general and then to separately summarize the literature concerning each major wound healing effect of the maggots.
Wound healing and the chronic wound
Wound healing is classically described as 4 distinct but overlapping physiological phases of repair and rebuilding 12:
- Homeostasis;
- Inflammation;
- Proliferation;
- Remodeling and maturing.
With each phase, new cells are recruited into the area to perform the work, or cells already present alter their activity to secrete new cytokines or perform new duties, in response to changing conditions in the wound (bleeding, hypoxia, alterations in cytokine concentrations, etc.). When no longer needed, the cells undergo apoptosis and are removed or engulfed by other cells (i.e., macrophages). Normally, these four waves in the healing process progress quickly and smoothly, one into the next. But occasionally healing may stagnate, and the wound is said to be chronic. Wound healing may be trapped at any phase (or even while undergoing a combination of phases), but typically it is within the inflammatory phase: dead, infected debris may not be adequately removed from the wound bed, and/or it might not be possible for the body to eradicate the local infection, and/or the proteases and other destructive products of inflammation by clearing the newly formed cellular and extracellular matrix as fast as it is being laid down. It is in this context that debridement, disinfection, or cellular proliferation and migration are so important, for they can push the stagnant wound into the next phase of healing.
Debridement
Of the three described actions of maggot therapy, debridement (physical and chemical) is the best studied. Each maggot is capable of removing 25 mg of necrotic material from the wound within just 24 hours 13.
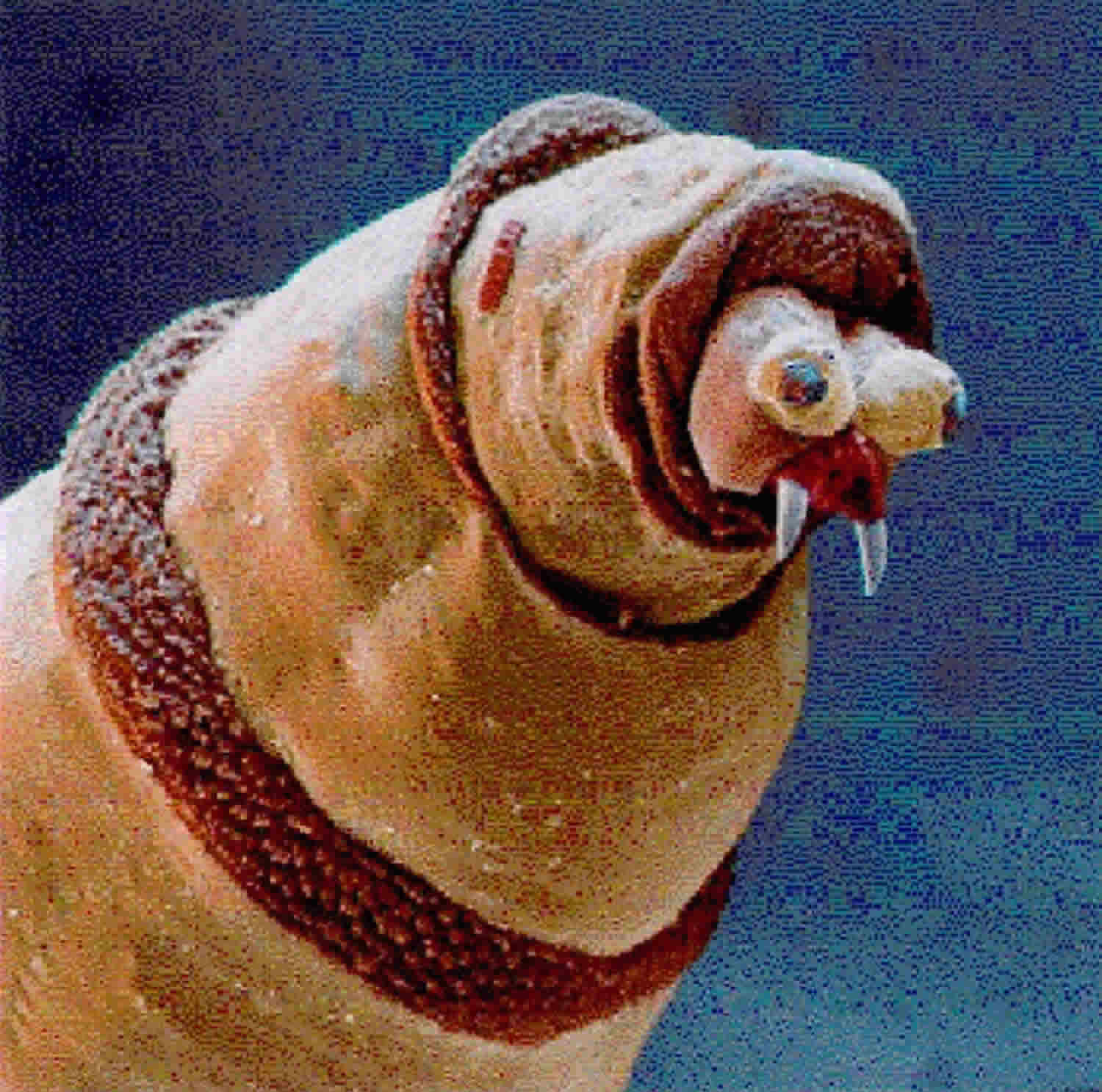
The physical mechanics of maggot debridement 11 are readily apparent to anyone who has seen the larvae under the microscope. Larvae are covered by minute spines which scrape along the wound base as the maggots crawl about, loosening debris as does a surgeon’s rasper or file (Figure 1). The mandibles, in the form of “mouth hooks,” are used to help pull the maggot’s body forward as it crawls and to probe every nook and cranny for food or shelter. The maggot does not “bite off” pieces of tissue, but it rather secretes and excretes its digestive enzymes (alimentary secretions and excretions or ASE), the consequence of which is that digestion begins in the wound bed, outside of the maggot’s own body. The necrotic tissue liquefies, and the maggots can then easily imbibe it. The physical movement of the maggot over the wound, plowing the tissue and spreading its alimentary secretions and excretions as it goes, contributes significantly to the debridement effort. The physical action of the maggot over the wound is a primary reason given by the FDA for classifying medicinal maggots as a medical device and not a simple drug. Experiments performed by Barnes et al 14 have demonstrated that the blowfly Lucilia sericata (Lucilia (Phaenicia) sericata) larvae excretions/secretions are able to inhibit bacteria growth in both stationary and exponential phases. For these reasons, maggot debridement was approved by the US Food and Drug Administration in 2004 15.
Hobson 16. was one of the first investigators to systematically demonstrate proteolytic activity of Lucilia sericata larval digestive enzymes. Vistnes et al. 17 used animal models to demonstrate that the maggots’ digestive enzymes were capable of dissolving necrotic tissue and identified several proteases. More recent studies of larval alimentary secretions and excretions help scientists see just how these proteolytic enzymes fit into the context of debridement and wound healing, for they now know that alimentary secretions and excretions include a wide array of matrix metalloproteinases (MMPs), including at least the trypsin-like and chymotrypsin-like serine proteases, an aspartyl proteinase, and an exopeptidase-like matrix metalloproteinase, active across a wide pH range 18.
It is important to recognize that humans produce at least 23 different matrix metalloproteinases (MMPs) which not only degrade extracellular protein but also regulate a wide variety of cellular processes through activation (or deactivation) of signaling molecules and/or their receptors 19. Matrix metalloproteinases (MMPs) play critical roles in all phases of tissue repair and wound healing, including hemostasis, thrombosis, inflammatory cell activation, collagen degradation, fibroblast and keratinocyte migration, and tissue remodeling. Disturbances in wound healing can occur when one group of proteases is deficient or out of balance with another.
Telford et al. 18 demonstrated that some of the maggot’s proteases are resistant to human wound protease inhibitors. At least one of these chymotrypsin-like proteases has now been produced recombinantly in Escherichia coli 20 and could soon enter clinical trials as a purified debriding enzyme.
Larval secretions also contain deoxyribonuclease (DNAse), able to degrade both microbial DNA and also human DNA in necrotic debris 21. DNAse may play an important role not only in debridement but also in inhibiting microbial growth and biofilm.
The wealth of case reports and case series in the literature suggests that most clinicians are impressed by the debridement efficacy of medicinal maggots. Controlled studies of maggot debridement are less common, but quite worthy of examination.
In a prospective study of spinal cord injury patients with chronic, nonhealing pressure ulcers, patients were monitored for 3-4 weeks while receiving standard wound care (whatever modality was prescribed by the surgically led wound care team), followed by 3-4 weeks of maggot therapy 7. Tissue quality and wound size were assessed weekly. Maggot debridement of necrotic tissue was achieved in less than 14 days (average of 10 days), but none of the control wounds were more than 50% debrided, even after 4 weeks of treatment.
In a cohort of 63 patients with 92 pressure ulcers, followed for at least 8 weeks while receiving either standard wound care (as prescribed by the hospital’s wound care team), or maggot therapy (two 48- to 72-hour cycles per week), maggot-treated wounds were debrided four times faster than control wounds (0.8 cm²/week versus 0.2 cm²/week) 22.
In a similar cohort of 18 diabetic subjects with 20 nonhealing neuropathic and neuroischemic foot ulcers 23, maggot-treated wounds were 50% debrided within an average of 9 days, but control wounds did not achieve that level of debridement until an average of 29 days. Within 2 weeks, maggot-treated wounds were left with only 7% necrotic tissue (0.9 cm²) compared to 39% necrotic tissue (3.1 cm²) in the control group and all maggot-treated wounds were completely debrided within 4 weeks, while most control wounds were still over 33% covered with necrotic tissue.
Wayman and colleagues 24 randomized 12 venous stasis leg ulcer subjects to receive either maggot debridement therapy or their standard of care (hydrogel). In this randomized controlled trial, the six wounds in the maggot debridement therapy arm were debrided faster than the six wounds in the control arm, with all of the maggot-debrided wounds completely debrided after just one 2-3-day treatment, compared to only 4 of the control wounds completely debrided after a month of therapy.
In a larger clinical trial of maggot therapy for venous stasis ulcers, this time designed to look for maggot-associated wound healing, Dumville and colleagues 25 enrolled 263 subjects to receive either standard (“free-range”) maggot debridement, maggot debridement using “Biobags” (a patented ravioli-like pouch containing the live larvae), or their standard of care, hydrogel, and compression dressings. All subjects received compression dressings, except during maggot debridement. Time to debridement differed significantly between the three groups (25.38). The median time to debridement was 14 days with free-range larvae, 28 days with bagged larvae, and 72 days for the control arm.
Unfortunately, very few studies have compared free range with bagged maggots, though such a study could be a valuable mechanism for evaluating the relative importance of the maggot’s physical versus chemical activity 1. Most, though not all, laboratory studies comparing free range versus contained maggots have suggested that maggots in direct contact with the wound are more effective, at least for debridement, than maggots separated from the wound by their containment dressings 26. To date, only one clinical study was designed to compare the difference between these two methods of maggot therapy. In this prospective clinical trial, Steenvoorde and colleagues 27 enrolled 64 patients with 69 chronic, necrotic wounds. Patients were treated with either free range or contained maggot debridement therapy, depending on maggot availability and clinician preference. The investigators monitored 8 specific outcome measures: (1) complete healing without any other intervention; (2) complete healing by secondary intervention (e.g., split-skin graft); (3) wound free from infection and less than one-third of the initial size; (4) wound clean but not decreased in size; (5) no difference in wound size or character; (6) wound worsened; (7) minor amputation was still required (e.g., partial toe amputation); and (8) major amputation was still required. Their analysis revealed better outcomes in the free range group compared to the contained maggots group, despite the fact that the free range technique required fewer maggot applications and fewer total number of maggots per treatment. The authors concluded that containment of maggots reduced the effectiveness and efficiency of maggot debridement therapy, probably by preventing contact with, and/or complete access to, the wound bed.
Figure 1. Scanning electron micrograph of Lucilia (Phaenicia) sericata maggot
[Source 28 ]Disinfection
The natural habitat of Lucilia sericata larvae is in rotting organic matter such as a corpse or excrement. Therefore, it should be no surprise that this maggot would be well-protected from infection. Early on, scientists believed that ingestion was the primary method by which the maggots cleared the wounds of infection 13, and subsequent researchers demonstrated that highly effective killing does indeed occur in the gut 29. Greenberg hypothesized that antimicrobial compounds might be produced in the gut by symbiotic microbes such as Proteus mirabilis, and, in 1986, Erdmann and Khalil identified and isolated two antibacterial substances (phenylacetic acid and phenylacetaldehyde) from the Proteus mirabilis that they isolated from the gut of a related blowfly larva: Cochliomyia hominivorax 30.
Antimicrobial killing also occurs outside the maggot’s gut, and the extracorporeal secretion/excretion of antimicrobial compounds may even be responsible for most of the maggot’s antimicrobial activity 31. Some early researchers believed that wound disinfection was largely due to the physical “washing-out” (lavage) of microbes from the wound bed during maggot therapy, by the fluid secreted by both the maggots (alimentary secretions and excretions) and the host (“wound exudate”). They also pointed to the antimicrobial activity of ammonia-containing byproducts of the maggots’ digestion of tissue proteins and the resulting alkalinized wound bed 6.
With advanced molecular and biochemical methods now at our disposal, many researchers over the past two decades have focused their attention on isolating antimicrobial proteins and other biochemicals produced by Lucilia sericata 32. Often, the isolated molecules were more active against gram positive bacteria than gram negatives, but sometimes this was merely a matter of dose and potency 33. Antimicrobial activity has been seen even against highly antibiotic-resistant bacteria 34 and against the protozoan Leishmania parasite 35. Kawabata et al. 36 demonstrated that the antimicrobial activity could be modified by exposure to microbial challenges (as is the case with many innate immunodefense peptides).
By 2010, Cerovský et al. 37 completely sequenced the 40-residue defensin-like antimicrobial peptide now called: “lucifensin.” Altincicek and Vilcinskas 38 used suppression subtractive hybridization to show that 65 Lucilia sericata genes upregulated in response to septic challenge (cuticular puncture) with lipopolysaccharide. Valachová and colleagues 39 demonstrated that lucifensin expression was increased in response to microbial ingestion only in the fat body; lucifensin was expressed in the salivary glands throughout the larval period and not significantly affected by microbial ingestion.
Even more antimicrobial molecules are likely to be discovered in the coming years. Numerous antimicrobial molecules have already been isolated in other blow flies, including the antibacterial peptide diptericin from Phormia terraenovae 40 and the antiviral alloferons from Calliphora vicina 41, the latter of which has already been commercialized.
Maggots also fight bacteria in their more resistant form: biofilm. In contrast to free living (“planktonic”) individual bacteria, biofilm is a structured community of one or more species of bacterial cells, living closely in an enclosed, protective, self-produced polymeric matrix, and adherent to an inert or living surface 42. Antibiofilm activity is valuable because biofilm is highly resistant to the penetration and successful activity of the human immune system and antibiotics. Biofilm is a particularly difficult problem in chronic wounds. One of the most powerful tools we have against biofilm is physically eroding it (i.e., brushing our teeth to rid ourselves of dental biofilm). Many therapists prescribe brushing to rid a wound of biofilm. It is reasonable to assume that the maggots are helping to rid a wound of biofilm simply by crawling over it with their rough bodies. What was particularly surprising, though, was the discovery that maggot alimentary secretions and excretions is capable of dissolving biofilm and inhibiting the growth of new biofilm 43. This has been shown at least for Staphylococcus aureus and Pseudomonas aeruginosa biofilm.
There should be no more doubt that maggots secrete and excrete potent antimicrobial compounds. But what is the evidence that maggots bring about clinically relevant disinfection? Numerous case reports have purported wound disinfection following maggot therapy, but controlled clinical evidence of maggot-induced antimicrobial activity has been sparse, until recently. In a prospective clinical trial of maggot therapy for chronic leg ulcers, Contreras-Ruiz and colleagues 44 randomized 19 subjects to either maggot therapy or conventional debridement and compression therapy and found that maggot-treated wounds had significantly reduced bacterial counts compared to control wounds. The maggot-treated group displayed more anxiety and wound odor during treatment, but no greater pain or other adverse events.
In Tantawi et al.’s case series 45, 13 diabetic ulcers in 10 subjects similarly demonstrated significant decreases in the number of microbial species and the colony counts after maggot therapy. In an observational study by Bowling and colleagues 46, 13 sequentially enrolled stable diabetic patients with MRSA-colonized ulcers, not already receiving MRSA-specific antibiotics, were debrided with maggot therapy. Semiquantitative cultures were taken at baseline and before each cycle of maggot debridement therapy. The mean duration of maggot debridement therapy was less than 3 weeks (one treatment per week), and the authors noted that this was far less than the duration of conventional antibiotic treatment for MRSA. By the end of maggot debridement, MRSA colonization was eliminated from all but 1 of the 13 ulcers (efficacy = 92%); no complications or patient complaints were encountered.
When reviewing their patients, Steenvoorde and Jukema 47 also found decreased colony counts of gram positive organisms following maggot therapy, but they found increased counts of gram negatives. Their results may have resulted from the decreased competition by gram positive microbes. The study authors speculated that higher doses may be necessary for effective gram negative killing.
Armstrong et al. 48 probably best addressed the clinical relevancy of maggot-induced disinfection by designing a case-control study of maggot therapy for lower extremity wounds in hospice patients and recording the antibiotics prescribed by the patients’ primary clinicians, as a measure of clinically significant infection. This study revealed significantly fewer days of antibiotics compared to controls, over a 6-month observation period, indicating that the patients were cleared of their infection faster and remained infection free longer.
Not all clinical studies of maggot-induced disinfection have demonstrated such positive results. Dumville et al.’s 267-subject randomized controlled trial of maggot therapy for venous stasis wounds 25 did not demonstrate any significant difference between the time-dependent decreasing bacterial burden in maggot-treated patients versus control patients, nor any significant difference in the number of MRSA-colonized wounds that were cleared. But then, as the authors pointed out, there were so few patients with MRSA that the study was not adequately powered to see any likely difference. What’s more, looking for significant population differences in colonizing bacteria may not truly be an appropriate endpoint if we are really more concerned with clinical infections.
Growth stimulation
Evidence of maggot-induced tissue growth or wound healing now comes from both laboratory and clinical studies and also suggests both mechanical and biochemical pathways. Among the early theories about maggot-induced wound healing were that the simple removal of debris and microbial killing 49 or the action of crawling over the clean wound bed 50 might be enough to stimulate wound healing. Scientists now know that both of these hypotheses likely contribute to wound healing: physical and electrical stimulation of healthy cells can induce the release of host growth factors, and any meaningful reduction in debris and biofilm or microbial population likely decreases inflammation and promotes wound healing. Some investigators believed that the alkalinity of maggot-treated wounds, along with the isolated allantoin and urea-containing compounds, was responsible for wound healing 51. In fact, today, allantoin and urea are components of many cosmetics.
With recent advances in cellular biology and chemistry, we now know that maggot alimentary secretions and excretions stimulates the proliferation of fibroblasts 52 and endothelial tissue (unpublished data), increases angiogenesis 53, and enhances fibroblast migration over model wound surfaces 54. Biopsies of maggot-treated wounds reveal profound angiogenesis 55. Using remittance spectroscopy to evaluate patients before and after maggot therapy, Wollina and colleagues 56 found that vascular perfusion and tissue oxygenation surrounding the wound actually increased following maggot therapy. Zhang and colleagues 57 are currently seeing evidence that maggot extracts may even stimulate the growth of neural tissue.
Early clinical reports of maggot-induced wound healing were merely case studies or series; but beginning in the 1990’s, controlled comparative trials of maggot therapy began to appear. These were small, due to a lack of funding and support; but they showed the promising results needed to propel maggot therapy into the scientific limelight and justified larger and more definitive studies. In a prospective study of spinal cord injury patients with chronic, nonhealing pressure ulcers, patients were followed for 3-4 weeks while receiving standard wound care (whatever modality was prescribed by the surgically led wound care team), followed by 3-4 weeks of maggot therapy 7. Tissue quality and wound size were assessed and photographed weekly. The average wound size (cm²) increased weekly during control therapy but decreased by over 20% per week with maggot therapy. Debridement of necrotic tissue was achieved in just 10 days with maggot therapy. None of the control wounds were debrided by more than 50%, even with 4 weeks of treatment.
A cohort of 63 patients with 92 pressure ulcers was prospectively followed for at least 8 weeks while receiving either standard wound care (as prescribed by the hospital’s wound care team) or maggot therapy (two 48- to 72-hour cycles per week) 22. In patients with bilateral wounds, only one was treated with maggot therapy, and patients were allowed to select that one. Therefore, maggot-treated wounds tended to be larger (22 cm2 versus 14 cm²) and deeper (35% down to bone in the maggot therapy group; 8% in the control group). Nevertheless, 4- and 8-week healing rates were significantly better for maggot-treated wounds than control wounds, as was the weekly decrease in surface area and the rate of granulation tissue growth over the base of the wound.
The wound healing rate, based on studies by Gilman 58 and Margolis et al. 59, was defined as the change in surface area divided by the mean circumference over time. Four and eight-week healing rates have repeatedly been shown to be accurate surrogates for wound healing in general, although they have not been accepted as substitutes for complete wound closure in clinical trials.
Indeed, twice as many wounds in the maggot-treated group completely healed during the period of observation (39% within an average of 12 weeks versus 21% within an average of 13.4 weeks). But most patients were not followed more than 10 weeks, and this difference was not statistically significant.
In another cohort of 18 diabetic subjects with 20 nonhealing neuropathic and neuroischemic foot ulcers, six wounds were treated with conventional therapy, six with maggot therapy, and eight with conventional therapy first and then maggot therapy 23. As in the pressure ulcer patients, 4- and 8-week healing rates were significantly better for maggot-treated wounds than control wounds, as was the weekly change in surface area and the rate of granulation tissue growth over the base of the wound. Repeated measures ANOVA indicated that treatment rendered was the only factor associated with these differences.
In Armstrong’s retrospective case-control study of lower extremity wounds in nonambulatory hospice patients 48, in which the researchers demonstrated significantly better infection control and fewer amputations required in the maggot-treated group, the difference in wound healing rates between the maggot-treated group (57% healed) and the control group (33% healed) was not statistically significant. In this study population, the probability of healing may have had more do to with the patients’ underlying circulatory compromise, malnutrition, and poor physiologic health than with the treatments rendered. For those wounds that did heal, wound healing was much faster in the maggot-treated wounds than in the control wounds (18 weeks versus 22 weeks).
In the 140-subject randomized controlled trial by Markevich and colleagues 60, wounds treated with maggot therapy were ultimately covered with more granulation tissue and were smaller in size than the wounds in the control study arm. This 10-day long clinical trial failed to show any significant difference in wound healing between the maggot debridement therapy arm (60% healed by day 10) and the control arm (34% healed by day 10), but it is generally believed that the lack of any significant difference may be due to the fact that this 10-day debridement study was much too short to detect any meaningful wound healing. Indeed, 60% healing of diabetic foot ulcers in only 10 days instead of 10 weeks is, by itself, quite impressive.
Many in the wound care community looked with excitement at the study by Dumville et al. 25, intended to evaluate maggot-induced wound healing in venous stasis ulcers. This randomized controlled trial demonstrated significantly faster debridement in the maggot therapy arms, but did not demonstrate any significantly faster healing in those subjects. Several reasons may explain this, including the simple fact that the maggots may not expedite healing in any clinically meaningful way. Alternatively, as the authors pointed out, their study may have been too small to demonstrate the difference, given that there were less than 100 subjects in each of 3 arms. Some believe that the reason that no greater wound healing was seen in the maggot-treated arms was related to the study design, which used a “maggot debridement” protocol rather than a “maggot growth promotion” protocol 61. In this study, maggot therapy was stopped as soon as wounds were debrided (treatment day number 15, on average, for the free range maggot therapy group) and was never administered to those patients again, even if their wounds deteriorated over the subsequent 7 months that it took, on average, to heal 62.
Indeed, maggot-associated wound healing and antimicrobial activity is likely short-lived after the maggots are removed. Sherman and Shimoda 63 reported successful wound healing without infection or dehiscence in patients surgically closed 1–21 days following maggot debridement to be 100%, compared to wounds debrided without maggot debridement therapy or those debrided with maggot debridement therapy more than 21 days before closure, which healed successfully only 68% of the time.
Many clinicians intuitively feel that faster debridement brings faster wound healing. After all, the wound cannot heal if infected, necrotic tissue and debris are occupying the center of the wound. Yet, it has been difficult to find any large randomized controlled trial that demonstrates this to be true 64. Perhaps the problem has been that chronic wounds often reacquire infection or biofilm; and additional tissue may die, requiring redebridement. Addressing the on-going need for wound cleaning and disinfection is the paradigm behind “maintenance debridement,” and appears to be gaining support as an important strategy for treating wounds 65.
If this paradigm is correct, it would explain why maggot therapy continued beyond the point of gross debridement has been associated with faster wound healing 22. It may be true that no one single method of maintenance debridement is faster than another. But maggot therapy is one of the few highly effective methods of debridement which can safely and inexpensively be continued throughout the healing process, which may explain why it remains one of the methods of maintenance debridement best associated with faster wound healing.
Miscellaneous actions
Platelets, neutrophils, and monocytes/macrophages are among the first cells recruited to the young wound when they remain beyond their usefulness and contributed to an unending inflammatory phase that can interfere with or even prevent the wound from moving forward in the healing process. Maggot secretions have recently been found to affect the activity of these cells in ways that decrease inflammation. While this can be thought of as a subset of actions which promote wound healing, they are separated out for the purpose of this discussion because these actions may also play important roles in disinfection, if not also debridement.
Exposing unstimulated human neutrophils to crude L. sericata salivary gland extract, Pecivova and colleagues 66 measured no effect on superoxide generation or myeloperoxidase (MPO) release. But when opsonized zymosan stimulated neutrophils were exposed to high concentrations of the salivary gland extract, superoxide generation and MPO release were significantly reduced. The researchers concluded that medicinal maggots might aid in wound healing by decreasing the generation of proinflammatory factors in this way, while still maintaining normal phagocytosis or apoptosis.
van der Plas et al. 67 monitored cyclic AMP (cAMP) in human neutrophils before and after exposure to L. sericata alimentary secretions and excretions and then again in human monocytes 68. Their findings of elevated cAMP and suppressed proinflammatory responses (without a measurable decrease in antimicrobial activity) led the authors to conclude that the larval secretions were moving the monocytes and neutrophils forward from the proinflammatory phase and into the angiogenic phase of wound healing 69.
Cazander and colleagues 70 recently discovered that maggot alimentary secretions and excretions reduced complement activation in healthy and immune-activated (postoperative) human sera by as much as 99.9% by breaking down C3 and C4 proteins.
Maggots in wound treatment
Experiments performed by Barnes et al 14 have demonstrated that the blowfly Lucilia sericata (Lucilia (Phaenicia) sericata) larvae excretions/secretions are able to inhibit bacteria growth in both stationary and exponential phases. For these reasons, maggot debridement was approved by the US Food and Drug Administration in 2004 15.
Prete 71 demonstrated that hemolymph and alimentary secretions of larvae were growth stimulatory for in vitro human fibroblasts. Both factors increased the proliferation of fibroblasts stimulated by epidermal growth factor or interleukin 6. Clinical observations provided evidence for growth stimulation in chronic wounds 72.
Lucilia sericata larvae digest necrotic tissue and pathogens; they discriminate between necrotic and healthy (granulating) tissue. This technique is rapid and selective, although some of the evidence to support its use is still derived from anecdotal reports 73. Depending on the size and depth of the wound, 50 to 1000 sterile maggots, about 24 to 48 hours old, are applied 2 to 4 times per week and left on for a period of 24 to 72 hours 15. Several papers have described the utility of maggot debridement therapy 74, though there is only 1 randomized, specific Lucilia sericata clinical debridement trial using maggot therapy 75. Clinical studies have demonstrated maggot therapy to be safe and effective in patients both with and without diabetes and for many problematic wounds, including pressure ulcers, venous stasis leg ulcers, wound bed preparation prior to surgical closure, and a variety of other traumatic, infectious, and vascular wounds 76.
Wayman et al 77 compared the cost of larval therapy with hydrogel dressings in the treatment of necrotic venous ulcers. Twelve patients with sloughy venous ulcers were randomized to receive either larval therapy or the control hydrogel therapy. Effective debridement occurred with a maximum of 1 larval application in all 6 experimental patients; 2 of the 6 hydrogel patients still required dressings at 1 month. The median cost of treatment of the larval group was £78.64 compared with £136.23 for the control group. Thus, this study confirmed both the clinical efficacy and cost effectiveness of maggot therapy in the debridement of sloughy venous ulcers 77.
- Sherman RA. Mechanisms of maggot-induced wound healing: what do we know, and where do we go from here?. Evid Based Complement Alternat Med. 2014;2014:592419. doi:10.1155/2014/592419 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3976885[↩][↩][↩][↩][↩]
- Bexfield A, Nigam Y, Thomas S, Ratcliffe NA. Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microb Infect. 2004;6(14):1297–1304.[↩]
- Whitaker IS, Twine C, Whitaker MJ, Welck M, Brown CS, Shandall A. Larval therapy from antiquity to the present day: mechanisms of action, clinical applications and future potential. Postgrad Med J. 2007;83(980):409–413.[↩][↩]
- Valachova I, Takac P, Majtan J. Midgut lysozymes of Lucilia sericata – new antimicrobials involved in maggot debridement therapy. Insect Mol Biol. 2014;23(6):779–787.[↩]
- Larval Therapy for Chronic Cutaneous Ulcers. Wounds. 2017;29(12):367-373. https://www.medscape.com/viewarticle/891517[↩][↩]
- W. S. Baer, “The treatment of chronic osteomyelitis with the maggot (larva of the blow fly),” Journal of Bone and Joint Surgery, vol. 13, pp. 438–475, 1931.[↩][↩]
- R. A. Sherman, F. Wyle, and M. Vulpe, “Maggot therapy for treating pressure ulcers in spinal cord injury patients,” The Journal of Spinal Cord Medicine, vol. 18, no. 2, pp. 71–74, 1995.[↩][↩][↩]
- FDA, “510(k) Premarket Notification,” Medical Maggots, K033391, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=13466[↩]
- Y. Nigam, A. Bexfield, S. Thomas, and N. A. Ratcliffe, “Maggot therapy: the science and implication for CAM part I—history and bacterial resistance,” Evidence-Based Complementary and Alternative Medicine, vol. 3, no. 2, pp. 223–227, 2006.[↩]
- Y. Nigam, A. Bexfield, S. Thomas, and N. A. Ratcliffe, “Maggot therapy: the science and implication for CAM part II—Maggots combat infection,” Evidence-Based Complementary and Alternative Medicine, vol. 3, no. 3, pp. 303–308, 2006.[↩]
- R. A. Sherman, K. Y. Mumcuoglu, M. Grassberger, and T. I. Tantawi, “Maggot therapy,” in Biotherapy—History, Principles and Practice: A Practical Guide to the Diagnosis and Treatment of Disease Using Living Organisms, M. Grassberger, R. A. Sherman, O. S. Gileva, C. M. H. Kim, and K. Y. Mumcuoglu, Eds., pp. 5–29, Springer Science+Business Media, Dordrecht, The Netherlands, 2013.[↩][↩]
- J. Stechmiller and G. Schultz, “Bench science advances for chronic wound care,” in Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, D. L. Krasner, G. T. Rodeheaver, and R. G. Sibbald, Eds., pp. 67–73, HMP Communications, Malvern, Pa, USA, 4th edition, 2007.[↩]
- K. Y. Mumcuoglu, “Clinical applications for maggots in wound care,” The American Journal of Clinical Dermatology, vol. 2, no. 4, pp. 219–227, 2001.[↩][↩]
- Barnes KM, Dixon RA, Gennard DE. The antibacterial potency of the medicinal maggot, Lucilia sericata (Meigen): variation in laboratory evaluation. J Microbiol Meth. 2010; 82(3):234–237.[↩][↩]
- Parnés A, Lagan KM. Larval therapy in wound management: a review. Int J Clin Pract. 2007;61(3):488–493.[↩][↩][↩]
- R. P. Hobson, “On an enzyme from blow-fly larvae (Lucilia sericata) which digests collagen in alkaline solution,” Biochemical Journal, vol. 25, pp. 1458–1463, 1931.[↩]
- L. M. Vistnes, R. Lee, and G. A. Ksander, “Proteolytic activity of blowfly larvae secretions in experimental burns,” Surgery, vol. 90, no. 5, pp. 835–841, 1981.[↩]
- G. Telford, A. P. Brown, R. A. M. Seabra et al., “Degradation of eschar from venous leg ulcers using a recombinant chymotrypsin from Lucilia sericata,” The British Journal of Dermatology, vol. 163, no. 3, pp. 523–531, 2010.[↩][↩]
- S. E. Gill and W. C. Parks, “Metalloproteinases and their inhibitors: regulators of wound healing,” International Journal of Biochemistry and Cell Biology, vol. 40, no. 6-7, pp. 1334–1347, 2008.[↩]
- D. I. Pritchard, G. Telford, M. Diab, and W. Low, “Expression of a cGMP compatible Lucilia sericata insect serine proteinase debridement enzyme,” Biotechnology Progress, vol. 28, no. 2, pp. 567–572, 2012.[↩]
- A. Brown, A. Horobin, D. G. Blount et al., “Blow fly Lucilia sericata nuclease digests DNA associated with wound slough/eschar and with Pseudomonas aeruginosa biofilm,” Medical and Veterinary Entomology, vol. 26, no. 4, pp. 432–439, 2012.[↩]
- R. A. Sherman, “Maggot versus conservative debridement therapy for the treatment of pressure ulcers,” Wound Repair and Regeneration, vol. 10, no. 4, pp. 208–214, 2002.[↩][↩][↩]
- R. A. Sherman, “Maggot therapy for treating diabetic foot ulcers unresponsive to conventional therapy,” Diabetes Care, vol. 26, no. 2, pp. 446–451, 2003.[↩][↩]
- J. Wayman, V. Nirojogi, A. Walker, A. Sowinski, and M. A. Walker, “The cost effectiveness of larval therapy in venous ulcers,” Journal of Tissue Viability, vol. 10, no. 3, pp. 91–94, 2000.[↩]
- J. C. Dumville, G. Worthy, J. M. Bland et al., “Larval therapy for leg ulcers (VenUS II): randomised controlled trial,” The British Medical Journal, vol. 338, article b773, 2009.[↩][↩][↩]
- F. A. S. Blake, N. Abromeit, M. Bubenheim, L. Li, and R. Schmelzle, “The biosurgical wound debridement: experimental investigation of efficiency and practicability,” Wound Repair and Regeneration, vol. 15, no. 5, pp. 756–761, 2007.[↩]
- P. Steenvoorde, C. E. Jacobi, and J. Oskam, “Maggot debridement therapy: free-range or contained? An in-vivo study,” Advances in Skin and Wound Care, vol. 18, no. 8, pp. 430–435, 2005.[↩]
- Fleischmann W., Grassberger M., and Sherman RA Therapy. A Handbook of Maggot-Assisted Wound Healing. Stuttgart: Thieme, 2004:93[↩]
- K. Y. Mumcuoglu, J. Miller, M. Mumcuoglu, M. Friger, and M. Tarshis, “Destruction of bacteria in the digestive tract of the maggot of Lucilia sericata (Diptera: Calliphoridae),” Journal of Medical Entomology, vol. 38, no. 2, pp. 161–166, 2001.[↩]
- G. R. Erdmann and S. K. W. Khalil, “Isolation and identification of two antibacterial agents produced by a strain of Proteus mirabilis isolated from larvae of the screwworm (Cochliomyia hominivorax) (Diptera: Calliphoridae),” Journal of Medical Entomology, vol. 23, no. 2, pp. 208–211, 1986.[↩]
- E. R. Pavillard and E. A. Wright, “An antibiotic from maggots,” Nature, vol. 180, no. 4592, pp. 916–917, 1957.[↩]
- A. A. Kruglikova and S. I. Chernysh, “Surgical maggots and the history of their use,” Entomology Review, vol. 93, no. 6, pp. 667–674, 2013.[↩]
- A. S. Andersen, D. Sandvang, K. M. Schnorr et al., “A novel approach to the antimicrobial activity of maggot debridement therapy,” Journal of Antimicrobial Chemotherapy, vol. 65, no. 8, pp. 1646–1654, 2010.[↩]
- L. Margolin and P. Gialanella, “Assessment of the antimicrobial properties of maggots,” International Wound Journal, vol. 7, no. 3, pp. 202–204, 2010.[↩]
- E. Polat, H. Cakan, M. Aslan et al., “Detection of anti-leishmanial effect of the Lucilia sericata larval secretions in vitro and in vivo on Leishmania tropica: first work,” Experimental Parasitology, vol. 132, no. 2, pp. 129–134, 2012.[↩]
- T. Kawabata, H. Mitsui, K. Yokota, K. Ishino, K. Oguma, and S. Sano, “Induction of antibacterial activity in larvae of the blowfly Lucilia sericata by an infected environment,” Medical and Veterinary Entomology, vol. 24, no. 4, pp. 375–381, 2010.[↩]
- V. Cerovský, J. Zdárek, V. Fucík, L. Monincová, Z. Voburka, and R. Bém, “Lucifensin, the long-sought antimicrobial factor of medicinal maggots of the blowfly Lucilia sericata,” Cellular and Molecular Life Sciences, vol. 67, no. 3, pp. 455–466, 2010.[↩]
- B. Altincicek and A. Vilcinskas, “Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata,” Insect Molecular Biology, vol. 18, no. 1, pp. 119–125, 2009.[↩]
- I. Valachová, J. Bohová, Z. Pálošová, P. Takáč, M. Kozánek, and J. Majtán, “Expression of lucifensin in Lucilia sericata medicinal maggots in infected environments,” Cell and Tissue Research, vol. 353, no. 1, pp. 165–171, 2013.[↩]
- J. L. Dimarcq, E. Keppi, B. Dunbar et al., “Insect immunity. Purification and characterization of a family of novel inducible antibacterial proteins from immunized larvae of the dipteran Phormia terranovae and complete amino-acid sequence of the predominant member, diptericin A,” European Journal of Biochemistry, vol. 171, no. 1-2, pp. 17–22, 1988.[↩]
- S. Chernysh, S. I. Kim, G. Bekker et al., “Antiviral and antitumor peptides from insects,” Proceedings of the National Academy of Sciences of the United States of America, vol. 99, no. 20, pp. 12628–12632, 2002.[↩]
- J. W. Costerton, P. S. Stewart, and E. P. Greenberg, “Bacterial biofilms: a common cause of persistent infections,” Science, vol. 284, no. 5418, pp. 1318–1322, 1999.[↩]
- G. Cazander, M. C. van de Veerdonk, C. M. J. E. Vandenbroucke-Grauls, M. W. J. Schreurs, and G. N. Jukema, “Maggot excretions inhibit biofilm formation on biomaterials,” Clinical Orthopaedics and Related Research, vol. 468, no. 10, pp. 2789–2796, 2010.[↩]
- J. Contreras-Ruiz, S. Arroyo-Escalante, Fuentes-Suarez, J. Adominguez-Cherit, C. Sosa-de-Martinez, and E. Maravilla-Franco, “Maggot therapy and infection control in venous ulcers: a comparative study,” in Proceedings of the Symposium on Advanced Wound Care (SAWC ’05), San Diego, Calif, USA, April 2005.[↩]
- T. I. Tantawi, Y. M. Gohar, M. M. Kotb, F. M. Beshara, and M. M. El-Naggar, “Clinical and microbiological efficacy of MDT in the treatment of diabetic foot ulcers,” Journal of Wound Care, vol. 16, no. 9, pp. 379–383, 2007.[↩]
- F. L. Bowling, E. V. Salgami, and A. J. M. Boulton, “Larval therapy: a novel treatment in eliminating methicillin-resistant Staphylococcus aureus from diabetic foot ulcers,” Diabetes Care, vol. 30, no. 2, pp. 370–371, 2007.[↩]
- P. Steenvoorde and G. N. Jukema, “The antimicrobial activity of maggots: in-vivo results,” Journal of Tissue Viability, vol. 14, no. 3, pp. 97–101, 2004.[↩]
- D. G. Armstrong, P. Salas, B. Short et al., “Maggot therapy in “lower-extremity hospice” wound care: fewer amputations and more antibiotic-free days,” Journal of the American Podiatric Medical Association, vol. 95, no. 3, pp. 254–257, 2005.[↩][↩]
- W. Robinson and V. H. Norwood, “Destruction of pyogenic bacteria in the alimentary tract of surgical maggots implanted in infected wounds,” The Journal of Laboratory and Clinical Medicine, vol. 19, no. 6, pp. 581–586, 1934.[↩]
- J. Buchman and J. E. Blair, “Maggots and their use in the treatment of chronic osteomyelitis,” Surgery, Gynecology and Obstetrics, vol. 55, pp. 177–190, 1932.[↩]
- W. Robinson, “Stimulation of healing in non-healing wounds by allantoin occurring in maggot secretions and of wide biological distribution,” Journal of Bone and Joint Surgery, vol. 17, pp. 267–271, 1935.[↩]
- P. E. Prete, “Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy,” Life Sciences, vol. 60, no. 8, pp. 505–510, 1997.[↩]
- Z. Zhang, S. Wang, Y. Diao, J. Zhang, and D. Lv, “Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity,” Lipids in Health and Disease, vol. 9, article 24, 2010.[↩]
- A. G. Smith, R. A. Powis, D. I. Pritchard, and S. T. Britland, “Greenbottle (Lucilia sericata) larval secretions delivered from a prototype hydrogel wound dressing accelerate the closure of model wounds,” Biotechnology Progress, vol. 22, no. 6, pp. 1690–1696, 2006.[↩]
- R. A. Sherman, “Maggot therapy for foot and leg wounds,” International Journal of Lower Extremity Wounds, vol. 1, no. 2, pp. 135–142, 2002.[↩]
- U. Wollina, K. Liebold, W. Schmidt, M. Hartmann, and D. Fassler, “Biosurgery supports granulation and debridement in chronic wounds—clinical data and remittance spectroscopy measurement,” International Journal of Dermatology, vol. 41, no. 10, pp. 635–639, 2002.[↩]
- Z. Zhang, S. Wang, X. Tian, Z. Zhao, J. Zhang, and D. Lv, “A new effective scaffold to facilitate peripheral nerve regeneration: chitosan tube coated with maggot homogenate product,” Medical Hypotheses, vol. 74, no. 1, pp. 12–14, 2010.[↩]
- T. H. Gilman, “Parameter for measurement of wound closure,” Wounds, vol. 3, pp. 95–101, 1990.[↩]
- D. J. Margolis, E. A. Gross, C. R. Wood, and G. S. Lazarus, “Planimetric rate of healing in venous ulcers of the leg treated with pressure bandage and hydrocolloid dressing,” Journal of the American Academy of Dermatology, vol. 28, no. 3, pp. 418–421, 1993.[↩]
- Y. O. Markevich, J. McLeod-Roberts, M. Mousley, and E. Melloy, “Maggot therapy for diabetic neuropathic foot wounds: a randomized study,” in Proceedings of the 36th Annual Meeting of the European Association for the Study of Diabetes, Jerusalem, Israel, 2000.[↩]
- Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ 2009; 338 doi: https://doi.org/10.1136/bmj.b773[↩]
- D. McCaughan, N. Cullum, J. Dumville, and The VenUS II Team, “Patients’ perceptions and experiences of venous leg ulceration and their attitudes to larval therapy: an in-depth qualitative study,” Health Expectations, 2013.[↩]
- R. A. Sherman and K. J. Shimoda, “Presurgical maggot debridement of soft tissue wounds is associated with decreased rates of postoperative infection,” Clinical Infectious Diseases, vol. 39, no. 7, pp. 1067–1070, 2004.[↩]
- M. Bradley, N. Cullum, and T. Sheldon, “The debridement of chronic wounds: a systematic review,” Health Technology Assessment, vol. 3, no. 17, pp. 1–78, 1999.[↩]
- R. D. Wolcott, J. P. Kennedy, and S. E. Dowd, “Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds,” Journal of Wound Care, vol. 18, no. 2, pp. 54–56, 2009.[↩]
- J. Pecivova, T. Macickova, P. Takac, M. Kovacsova, D. Cupanikova, and M. Kozanek, “Effect of the extract from salivary glands of Lucilia sericata on human neutrophils,” Neuroendocrinology Letters, vol. 29, pp. 794–797, 2008.[↩]
- M. J. A. van der Plas, A. M. van der Does, M. Baldry et al., “Maggot excretions/secretions inhibit multiple neutrophil pro-inflammatory responses,” Microbes and Infection, vol. 9, no. 4, pp. 507–514, 2007.[↩]
- M. J. A. van der Plas, M. Baldry, J. T. van Dissel, G. N. Jukema, and P. H. Nibbering, “Maggot secretions suppress pro-inflammatory responses of human monocytes through elevation of cyclic AMP,” Diabetologia, vol. 52, no. 9, pp. 1962–1970, 2009.[↩]
- M. J. A. van der Plas, J. T. van Dissel, and P. H. Nibbering, “Maggot secretions skew monocyte-macrophage differentiation away from a pro-inflammatory to a pro-angiogenic type,” PLoS ONE, vol. 4, no. 11, Article ID e8071, 2009.[↩]
- G. Cazander, M. W. Schreurs, L. Renwarin, C. Dorresteijn, D. Hamann, and G. N. Jukema, “Maggot excretions affect the human complement system,” Wound Repair and Regeneration, vol. 20, no. 6, pp. 879–886, 2012.[↩]
- Prete PE. Growth effects of Phaenicia sericata larval extracts on fibroblasts: mechanism for wound healing by maggot therapy. Life Sci. 1997;60(8):505–510.[↩]
- Wollina U, Liebold K, Schmidt WD, Hartmann M, Fassler D. Biosurgery supports granulation and debridement in chronic wounds–clinical data and remittance spectroscopy measurement. Int J Dermatol. 2002;41(10):635–639.[↩]
- Eneroth M, van Houtum WH. The value of debridement and vacuum-assisted closure (V.A.C.) therapy in diabetic foot ulcers. Diabetes Metab Res Rev. 2008;24(Suppl 1):S76–S80.[↩]
- Tamura T, Cazander G, Rooijakkers SH, Trouw LA, Nibbering PH. Excretions/secretions from medicinal larvae (Lucilia sericata) inhibit complement activation by two mechanisms [published online ahead of print December 26, 2016]. Wound Repair Regen. 2017;25(1):41–50.[↩]
- Markevich YO, McLeod-Roberts J, Mousley M, Melloy E. Maggot therapy for diabetic neuropathic foot wounds. Diabetologia. 2000;43(Suppl 11): A15.[↩]
- Masiero FS, Thyssen PJ. Evaluation of conventional therapeutic methods versus maggot therapy in the evolution of healing of tegumental injuries in Wistar rats with and without diabetes mellitus [published online ahead of print March 15, 2016]. Parasitol Res. 2016;115(6):2403–2407.[↩]
- Wayman J, Nirojogi V, Walker A, Sowinski A, Walker MA. The cost effectiveness of larval therapy in venous ulcers. J Tissue Viability. 2000;10(3):91–94.[↩][↩]