May Hegglin anomaly
May-Hegglin anomaly is a rare, inherited, blood platelet disorder characterized by abnormally large and misshapen platelets (giant platelets), neutrophils with abnormal cytoplasmic inclusions and variable thrombocytopenia 1. The defect of the white blood cells consists of the presence of very small (2-5 micrometers) rods, known as Dohle bodies, in the fluid portion of the cell (cytoplasm). Some people with May-Hegglin anomaly may have no symptoms while others may have various bleeding abnormalities. In mild cases, treatment for May-Hegglin anomaly is not usually necessary. In more severe cases, transfusions of blood platelets may be necessary.
May-Hegglin anomaly is a rare blood platelet disorder that affects males and females in equal numbers. It occurs more often in people of Greek or Italian descent than among others. As of about 10 years ago, only about 170 cases were reported in the literature.
May-Hegglin anomaly is one of a family of five autosomal dominant, giant platelet disorders (macrothrombocytopenias), characterized by mutations in the MYH9 gene, each of which involves slight variants (alleles) of the same gene in the same location 2. The other giant platelet disorders related to May-Hegglin anomaly are Sebastian syndrome 3, Fechtner syndrome 4, Epstein syndrome 5 and the Alport-like syndrome with macrothrombocytopenia. Advances in the understanding of one of these syndromes may help in understanding the others.
May Hegglin anomaly causes
May-Hegglin anomaly is one of a family of macrothrombocytopenias characterized by mutations in the MYH9 gene, which is present at chromosomal region 22q12-13 and codes for nonmuscle myosin heavy-chain IIA 6. The Döhle like leukocyte inclusions in May-Hegglin anomaly are due to precipitation of myosin heavy chains in leukocytes.
The MYH9 gene provides instructions for making a protein called myosin-9. This protein is one part (subunit) of the myosin IIA protein.
There are three forms of myosin II, called myosin IIA, myosin IIB and myosin IIC. The three forms are found throughout the body and perform similar functions. They play roles in cell movement (cell motility); maintenance of cell shape; and cytokinesis, which is the step in cell division when the fluid surrounding the nucleus (the cytoplasm) divides to form two separate cells. While some cells use more than one type of myosin II, certain blood cells such as platelets and white blood cells (leukocytes) use only myosin IIA.
MYH9 gene mutations that cause MYH9-related disorder typically result in a nonfunctional version of the myosin-9 protein. The nonfunctional protein cannot properly interact with other subunits to form myosin IIA. Platelets and leukocytes, which only use myosin IIA, are most affected by a lack of functional myosin-9. It is thought that a lack of functional myosin IIA leads to the release of large, immature platelets in the bloodstream, resulting in a reduced amount of normal platelets. In leukocytes, the nonfunctional myosin-9 clumps together. These clumps of protein, called inclusion bodies, are a hallmark of MYH9-related disorder and are present in the leukocytes of everyone with this condition.
May Hegglin anomaly inheritance pattern
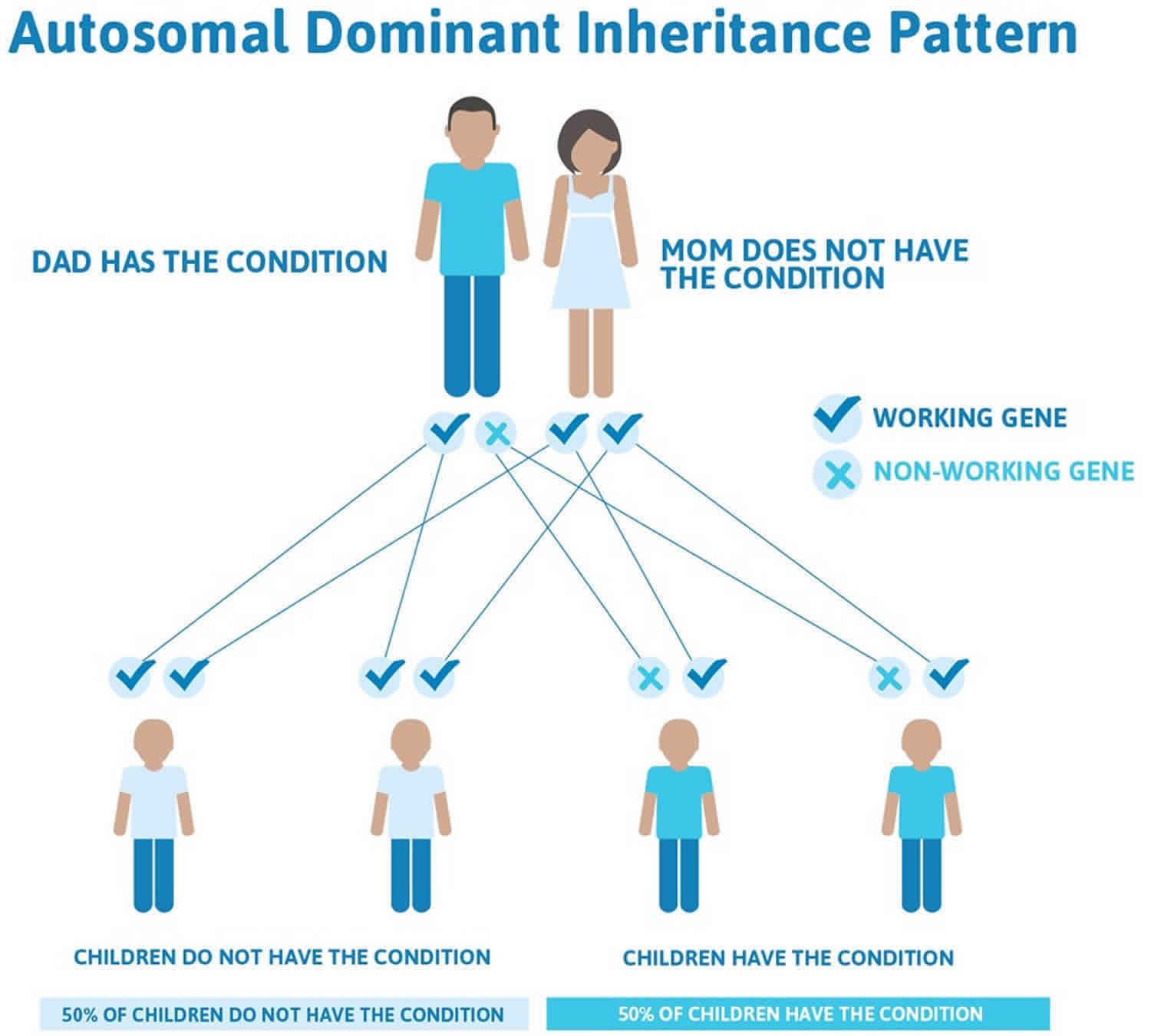
May-Hegglin anomaly is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder.
In most cases, an affected person inherits the mutation from one affected parent. Approximately 30 percent of cases result from new mutations in the gene and occur in people with no history of the disorder in their family.
In cases where the autosomal dominant condition does run in the family, the chance for an affected person to have a child with the same condition is 50% regardless of whether it is a boy or a girl. These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
- When one parent has the abnormal gene, they will pass on either their normal gene or their abnormal gene to their child. Each of their children therefore has a 50% (1 in 2) chance of inheriting the changed gene and being affected by the condition.
- There is also a 50% (1 in 2) chance that a child will inherit the normal copy of the gene. If this happens the child will not be affected by the disorder and cannot pass it on to any of his or her children.
Figure 1 illustrates autosomal dominant inheritance. The example below shows what happens when dad has the condition, but the chances of having a child with the condition would be the same if mom had the condition.
Figure 1. May Hegglin anomaly autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
May Hegglin anomaly symptoms
Some people with May-Hegglin anomaly may have symptoms at birth while others may have no symptoms throughout their lifetime. Symptoms may include red or purple colored spots on the skin (purpura), nose bleeds (epitaxis), excessive bleeding from the mouth during dental work, headaches, and/or muscle weakness on one side of the body due to bleeding within the brain (intracranial bleeding).
Excessive bleeding may occur in some people with May-Hegglin anomaly when steroid drugs used to treat another disorder are discontinued.
May Hegglin anomaly diagnosis
The diagnosis of May-Hegglin anomaly is made by specialized blood tests that reveal giant, oddly shaped platelets and characteristic cellular “inclusions” in certain white blood cells (leukocytes). The presence of inclusion bodies in leukocytes helps to distinguish May-Hegglin anomaly from immune-mediated thrombocytopenia. There also might be fewer platelets than normal (mild thrombocytopenia). Immunofluorescence study of neutrophil NMMHC-IIA can be helpful for diagnosis of May-Hegglin anomaly in patients without leukocyte inclusion bodies 7. Genetic studies for MYH9 gene mutation can confirm the diagnosis of May-Hegglin anomaly in uncertain cases. A comprehensive molecular evaluation includes a screening of 40 exons. It is hypothesized that genetic assessment can evaluate the risk of development of cataracts, deafness and kidney disease but this is debatable.
May Hegglin anomaly treatment
The literature is conflicting, but most patients with May-Hegglin anomaly do not appear to have clinically significant bleeding problems, and specific treatment is not required. Corticosteroids and splenectomy are ineffective 8. In rare patients with severe bleeding, platelet transfusion may be required.
Patients with May-Hegglin anomaly who undergo normal vaginal or cesarean delivery do not appear to have a significantly increased risk of bleeding 9.
For patients with May-Hegglin anomaly scheduled for surgery, a careful personal and family history of bleeding tendency should be obtained and a manual platelet count performed to determine the actual risk for bleeding. Intravenous desmopressin acetate (DDAVP) may be valuable 10. A patient with May-Hegglin anomaly who successfully underwent craniotomy after desmopressin acetate infusion alone has been described 10. Routine prophylactic platelet transfusions are not usually indicated, though it is prudent to ensure that platelets are available in case unexpected bleeding occurs.
Depending on the degree of thrombocytopenia and family history, individuals may be at an increased risk for bleeding, and refraining from participation in contact or collision sports may be prudent.
A hematologist should be consulted to assist in the management of patients who are undergoing surgery or vaginal delivery and patients who have experienced severe trauma.
May-Hegglin anomaly prognosis
The rarity of May-Hegglin anomaly has led to conflicting literature regarding the risk for bleeding. Asymptomatic patients have been described 11; however, abnormal bleeding has also been documented 12. The bleeding risk is increased by taking drugs that decrease platelet function. The risk for excess bleeding with surgical procedures is unclear 10. Rare reports have described arterial thrombotic events associated with May-Hegglin anomaly, though the risk remains unclear 13.
- May-Hegglin anomaly. https://rarediseases.org/rare-diseases/may-hegglin-anomaly/[↩]
- Wang Y, Liu S, Zhang Y, Yang J. Myosin Heavy Chain 9: Oncogene or Tumor Suppressor Gene?. Med Sci Monit. 2019 Jan 31. 25:888-92.[↩]
- Greinacher A, Nieuwenhuis HK, White JG. Sebastian platelet syndrome: a new variant of hereditary macrothrombocytopenia with leukocyte inclusions. Blut. 1990 Nov. 61(5):282-8.[↩]
- Peterson LC, Rao KV, Crosson JT, White JG. Fechtner syndrome–a variant of Alport’s syndrome with leukocyte inclusions and macrothrombocytopenia. Blood. 1985 Feb. 65(2):397-406.[↩]
- Epstein CJ, Sahud MA, Piel CF, Goodman JR, Bernfield MR, Kushner JH. Hereditary macrothrombocytopathia, nephritis and deafness. Am J Med. 1972 Mar. 52(3):299-310.[↩]
- Seri M, Cusano R, Gangarossa S, et al. Mutations in MYH9 result in the May-Hegglin anomaly, and Fechtner and Sebastian syndromes. The May-Heggllin/Fechtner Syndrome Consortium. Nat Genet. 2000 Sep. 26(1):103-5.[↩]
- Untanu RV, Vajpayee N. May Hegglin Anomaly. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441952[↩]
- May-Hegglin Anomaly Treatment & Management. https://emedicine.medscape.com/article/956447-treatment[↩]
- Chabane H, Gallais Y, Pathier D, Tchernia G, Gaussem P. Delivery management in a woman with thrombocytopenia of the May-Hegglin anomaly type. Eur J Obstet Gynecol Reprod Biol. 2001 Nov. 99(1):124-5.[↩]
- Sehbai AS, Abraham J, Brown VK. Perioperative management of a patient with May-Hegglin anomaly requiring craniotomy. Am J Hematol. 2005 Aug. 79(4):303-8.[↩][↩][↩]
- Mayer K, Schildknecht O, von Felten A. [May-Hegglin anomaly: further studies on thrombocyte dysfunction]. Schweiz Med Wochenschr. 1997 Jun 28. 127(26):1134-40.[↩]
- Noris P, Spedini P, Belletti S, et al. Thrombocytopenia, giant platelets, and leukocyte inclusion bodies (May- Hegglin anomaly): clinical and laboratory findings. Am J Med. 1998 Apr. 104(4):355-60.[↩]
- Antonio G, Silvia V, Emanuela B, Fabrizio F. Thrombotic events in MYH9 gene-related autosomal macrothrombocytopenias (old May-Hegglin, Sebastian, Fechtner and Epstein syndromes). J Thromb Thrombolysis. 2011 Nov. 32(4):474-7.[↩]