Megalencephaly
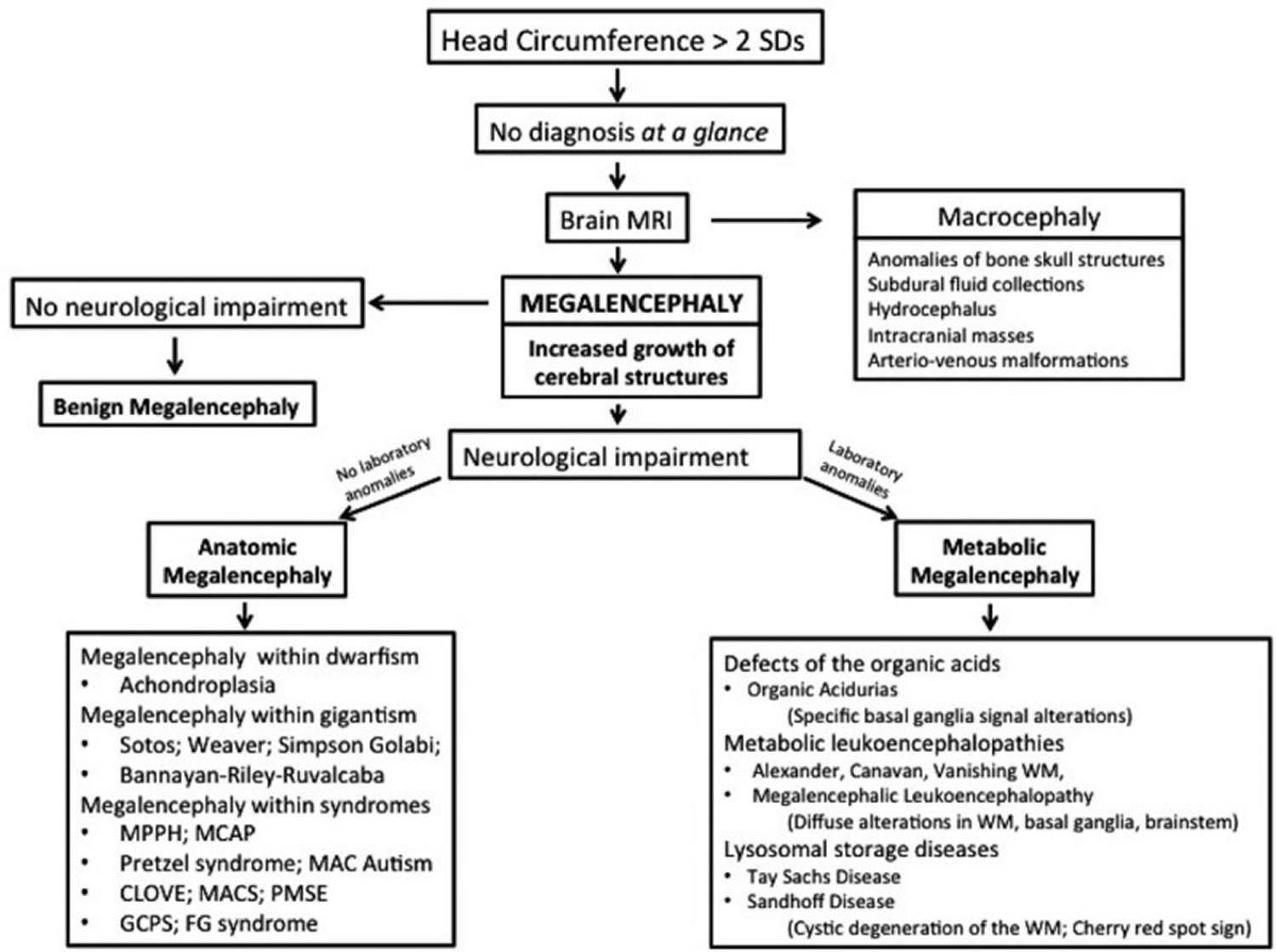
Megalencephaly also called macrencephaly, is a congenital condition in which an infant or child has an abnormally large, heavy, and usually malfunctioning brain (and head size). By definition, the brain weight or size is greater than average for the age and gender of the child and the occipitofrontal circumference is 2 or more standard deviation (SD) above the mean for age and gender 1. Megalencephaly is different from an increased head circumference or macrocephaly, which doesn’t necessarily indicate abnormality 2. A distinction between megalencephaly and macrocephaly has been proposed despite the fact that these terms encompass individuals with a large head and, in some cases, with similar neurologic manifestations including intellective disability of various degree, epileptic seizures, and motor impairment 3. Nevertheless they differ widely in causal events, cerebral structural anomalies, approach in the work-up, treatment and prognosis for which a clinical distinction is justified 4. Macrocephaly is referred to individuals in whom the brain enlargement is secondary to events inside the brain such as intracranial masses, abnormal ventricular dilatation, hydrocephalus ex-vacuo, and increase of bone skeletal structures. On the other hand, megalencephaly refers to anomalous structural cerebral events such as ineffective molecular control of neuronal growth during the various stage of the brain development or to inborn errors of metabolism 5. There are examples where the adverse events causing megalencephaly and macrocephaly may co-exist but semantic distinction among these conditions is suitable 6.
Distinction between megalencephaly and macrocephaly is useful because they represent clinical expression of different disorders with different approaches in clinical work-up, overall prognosis, and treatment 7. Sometimes, this distinction is not performed, and the terms are not appropriately referred in scientific papers but instead are used interchangeably 8. Megalencephaly defines an increased growth of cerebral structures related to dysfunctional anomalies during the various steps of brain development in the neuronal proliferation and/or migration phases or as a consequence of postnatal abnormal events that cause excessive cerebral growth 8. In contrast, in macrocephaly, the increased head circumference is linked to various events that can result in an increase of orbito-frontal head circumference for age, including anomalies of bone skull structures, subdural fluid collections, hydrocephalus, intracranial masses, and arteriovenous malformations 8. However, sometimes, adverse events resulting in both megalencephaly and macrocephaly may coexist in the same individual.
Megalencephaly is maintained to be prognostically more severe than macrocephaly and has been reported more frequently in the group of patients with intellective delay, epilepsy, and drug-resistant epilepsy 8.
In a clinical scenario, distinction between megalencephaly and macrocephaly is useful in terms of diagnosis, further testing, and overall prognosis for the patient and family 9. In clinical practice, the distinction between megalencephaly and macrocephaly relies on neuroimaging studies to identify enlarged cerebral structures or associated anomalies 10.
Megalencephaly is a sign of several uncommon congenital conditions and several molecular genetic mutations and can be differentiated into three groups: idiopathic, metabolic, or anatomic. Anatomic megalencephaly also called developmental megalencephaly, manifests with neurologic signs and may be isolated (“non-syndromic”) or part of complex syndromes, such as Megalencephaly-Capillary Malformation-Polymicrogyria (MCAP) and macrocephaly-cutis marmorata telangiectasica (M-CMTC) 3, leukodystrophies and neurofibromatosis 11. A differential diagnosis between idiopathic megalencephaly and anatomic, non-syndromic megalencephaly is not simple prior to the onset of clinical signs. Developmental delays, although usually mild and moderate, and dysmorphisms, if not severe, may be useful for a diagnosis.
Head enlargement may be evident at birth or the head may become abnormally large in the early years of life. Megalencephaly is thought to be related to a disturbance in the regulation of cell production in the brain. In normal development, neuron proliferation – the process in which nerve cells divide to form new generations of cells – is regulated so that the correct number of cells is produced in the proper place at the appropriate time. In a megalencephalic brain, too many cells are produced either during development or progressively as part of another disorder, such as one of the neurofibromatoses or leukodystrophies. Symptoms of megalencephaly include delayed development, seizures, and corticospinal (brain cortex and spinal cord) dysfunction. Megalencephaly affects males more often than females. Unilateral megalencephaly or hemimegalencephaly is a rare condition that is characterized by the enlargement of one side of the brain. Children with this disorder may have a large, asymmetrical head accompanied by seizures, partial paralysis, and impaired cognitive development. Megalencephaly is different from macrocephaly also called megacephaly or megalocephaly, which describes a big head, and which doesn’t necessarily indicate abnormality. Large head size is passed down through the generations in some families.
Brain MRI malformative anomalies were uncommonly reported in children with megalencephaly 3. This confirms the hypothesis of Berg and Dobyns 5, who maintain that genetic anomalies affecting brain development in the first steps of neuronal growth may be the cause of cerebral involvement; structural cerebral anomalies my not always be noticeable in brain imaging techniques. This cohort of children exhibits a set of clinical signs consisting of an abnormally large head circumference (above the 97th percentile), a mild-to-moderate intellectual disability, epileptic seizures in 5 cases and, in some cases, minor dysmorphism especially facial. In the absence of signs suggestive of known syndromes, a diagnosis of anatomic, non-syndromic megalencephaly was made. Nine of the ten patients did not shows structural abnormality of the brain, in the group with epilepsy four of the five patients failed to exhibit structural anomalies in their brain MRI. Only in one case the researchers found a cerebral cortex dysplasia and micropolygyria. Among the children with epilepsy the EEG was abnormal and indicative of epilepsy diagnosis in all their patients; dysmorphisms were noted in three children; these conditions were not severe and mainly affected the face.
Figure 1. Megalencephaly
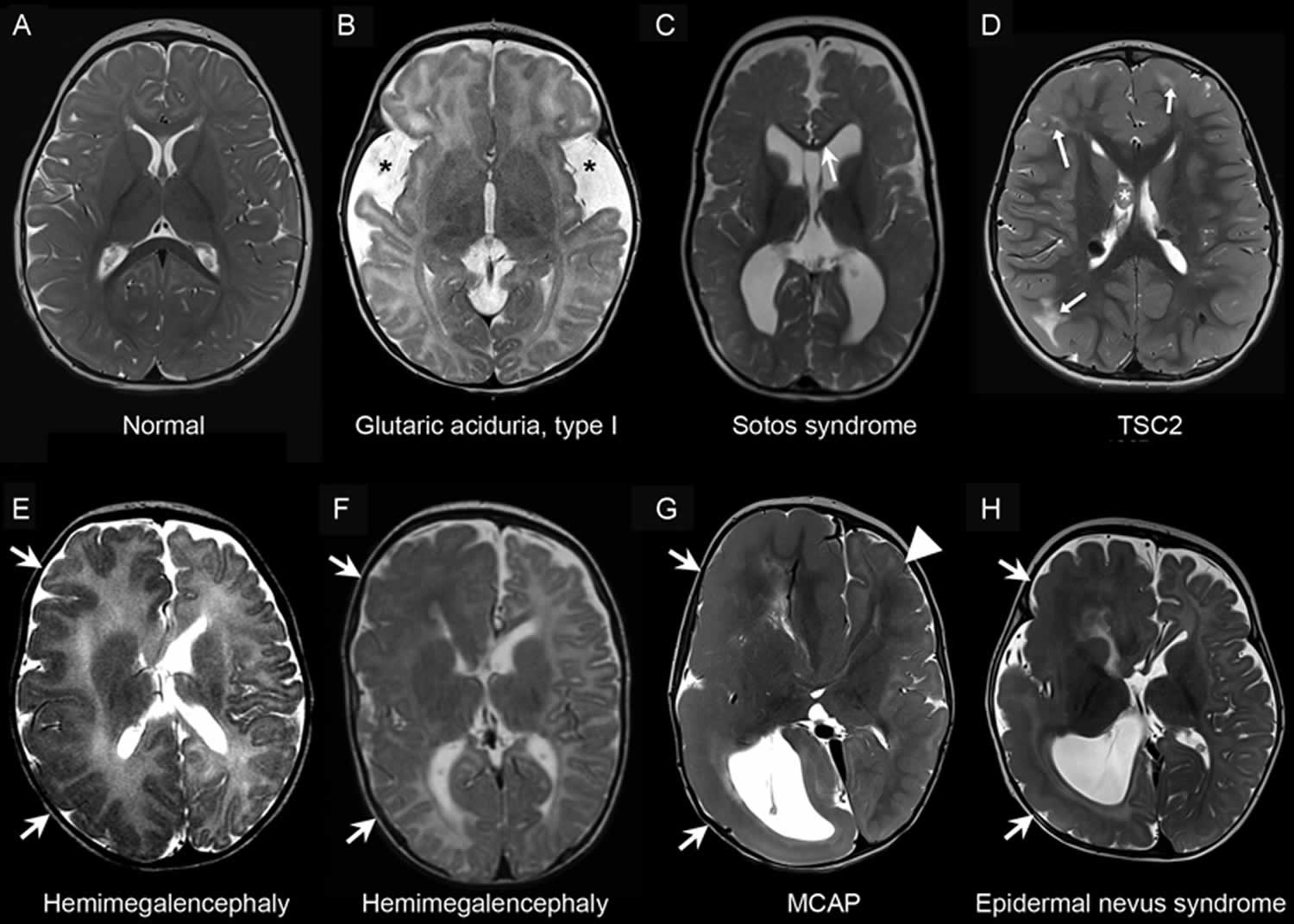
Footnote: Neuroimaging of metabolic and developmental megalencephaly. Representative axial T2-weighted magnetic resonance images: (A) A neurologically normal 1-year-old individual with symmetric hemispheres and normal myelination pattern for age. (B) An individual with glutaric academia, type I with typical features including enlarged extra-axial spaces and hypoplasia of the temporal lobes (asterisks), and delayed myelination pattern. (C) An individual with Sotos syndrome showing dolichocephaly and thinning of the corpus callosum (arrow). (D) An individual with tuberous sclerosis complex as a result of a TSC2 mutation. Note typical features including the multiple cortical tubers in the bilateral frontal and right parietal lobes (arrows), and the subependymal giant cell astrocytoma (SEGA) (asterisk). (E) Hemimegalencephaly of unknown etiology, with symmetric enlargement of one hemisphere of the brain (arrows). (F) AKT3 mutation resulting in asymmetric hemimegalencephaly, with arrows indicating enlargement of one hemisphere of the brain. (G) An individual with megalencephaly-capillary malformation–polymicrogyria syndrome (MCAP) showing hemimegalencephaly (arrows) and an abnormal gyration pattern on the contralateral side as well (arrowhead). (H) An individual with epidermal nevus syndrome that demonstrates asymmetric enlargement of one hemisphere (arrows).
[Source 10 ]Can megalencephaly be associated with other conditions?
Yes. Megalencephaly may be an isolated occurrence with normal cerebral structure, or associated with larger conditions or syndromes such as megalencephaly-capillary malformation syndrome, leukodystrophies and neurofibromatosis 11.
Benign familial megalencephaly
Benign familial megalencephaly also called benign familial macrocephaly or Cole-Hughes syndrome.
Asch and Myers 12 described 5 males in 2 generations of a family with occipitofrontal head circumferences greater than 2 standard deviation (SD) above the mean. All were neurologically and mentally normal. A maternal uncle of the first generation was said to have a large head. All were dolichocephalic. By sonographic studies the ventricular system was enlarged in 3 of the 5. Similar families were reported by Platt and Nash 13 and by Day and Shutt 14.
Arbour et al. 15 measured head size in the parents and sibs of 23 patients with a head circumference more than 2 SD above the mean and with no evidence of hydrocephalus or syndromic associations. In 12 of the 23, some degree of psychomotor impairment was present. It was found that head circumference of parents and sibs had a mean significantly greater than the population norm and a unimodal distribution. Probands with psychomotor impairment had bigger heads, and more had a history of birth difficulty than did unimpaired probands. They noted that macrocephaly in a parent or sib of an unborn child may present a risk for birth injury to that child.
In the course of a clinical study of Sotos syndrome, Cole and Hughes 16 found that 6 of 79 probands who failed to fit that phenotype showed remarkable similarities to each other and to some of their first- and second-degree relatives. In addition to macrocephaly, clinical features included typical facies characterized by square outline with frontal bossing, ‘dished-out’ midface, biparietal narrowing, and long philtrum. Birth weight and length were normal or near normal with subsequent obesity. Cole and Hughes 16 were uncertain as to whether this represented a new entity or benign familial macrocephaly.
Diaz-Rodriguez et al. 17 reported a mother and son with benign familial macrocephaly who displayed the characteristic square facial appearance with frontal bossing and dished-out midfacies. There was also an unaffected sister.
The pedigree pattern in the familial megalencephaly described by Asch and Myers 12 suggested male-limited autosomal dominant inheritance.
Arbour et al. 15 concluded that the usual genetic basis for nonsyndromic macrocephaly is multifactorial with a polymorphic genetic basis, rather than autosomal dominance. A risk of recurrence appeared to be much more lower than it would be on the assumption of autosomal dominant inheritance.
MPPH syndrome
MPPH syndrome is short for megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome, is a rare disorder that primarily affects the development of the brain. Affected individuals are born with an unusually large brain and head size (megalencephaly). The head and brain continue to grow rapidly during the first 2 years of life. MPPH syndrome is also associated with a brain abnormality called bilateral perisylvian polymicrogyria (BPP). The surface of the brain normally has many ridges or folds, called gyri. In people with bilateral perisylvian polymicrogyria, an area of the brain called the perisylvian region develops too many gyri, and the folds are irregular and unusually small. Other brain abnormalities, including a buildup of fluid in the brain (hydrocephalus), have also been reported in people with MPPH syndrome.
The problems with brain development cause a variety of neurological signs and symptoms. People with MPPH syndrome have delayed development and intellectual disability that ranges from mild to severe. About half of affected individuals develop recurrent seizures (epilepsy) beginning early in childhood. People with MPPH syndrome also have difficulty coordinating movements of the mouth and tongue (known as oromotor dysfunction), which leads to drooling, difficulty swallowing (dysphagia), and a delay in the production of speech (expressive language).
Polydactyly is a condition in which a person has more than five fingers per hand. Extra digits may be poorly developed and attached by a small stalk, or may be well-formed and have function 18. About half of people with MPPH syndrome have an extra finger or toe on one or more of their hands or feet (polydactyly). The polydactyly is described as postaxial because it occurs on the same side of the hand or foot as the pinky finger or little toe.
The brain abnormalities characteristic of MPPH syndrome are also found in a closely related condition called megalencephaly-capillary malformation syndrome (MCAP). However, megalencephaly-capillary malformation syndrome includes abnormalities of small blood vessels in the skin (capillary malformations) and several other features that are not usually part of MPPH syndrome.
Mutations in at least three different genes have been identified that cause MPPH including PIK3R2, AKT3, and CCND2. Most cases of MPPH syndrome are new (de novo) in families with no prior history 19, 20. MPPH syndrome appears to be a rare disease. About 60 affected individuals have been described in the medical literature 21.
The diagnosis of MPPH syndrome is based on physical examination, imaging studies, and genetic testing 1. Treatment is based on the signs and symptoms present in each person.
Figure 2. MPPH syndrome
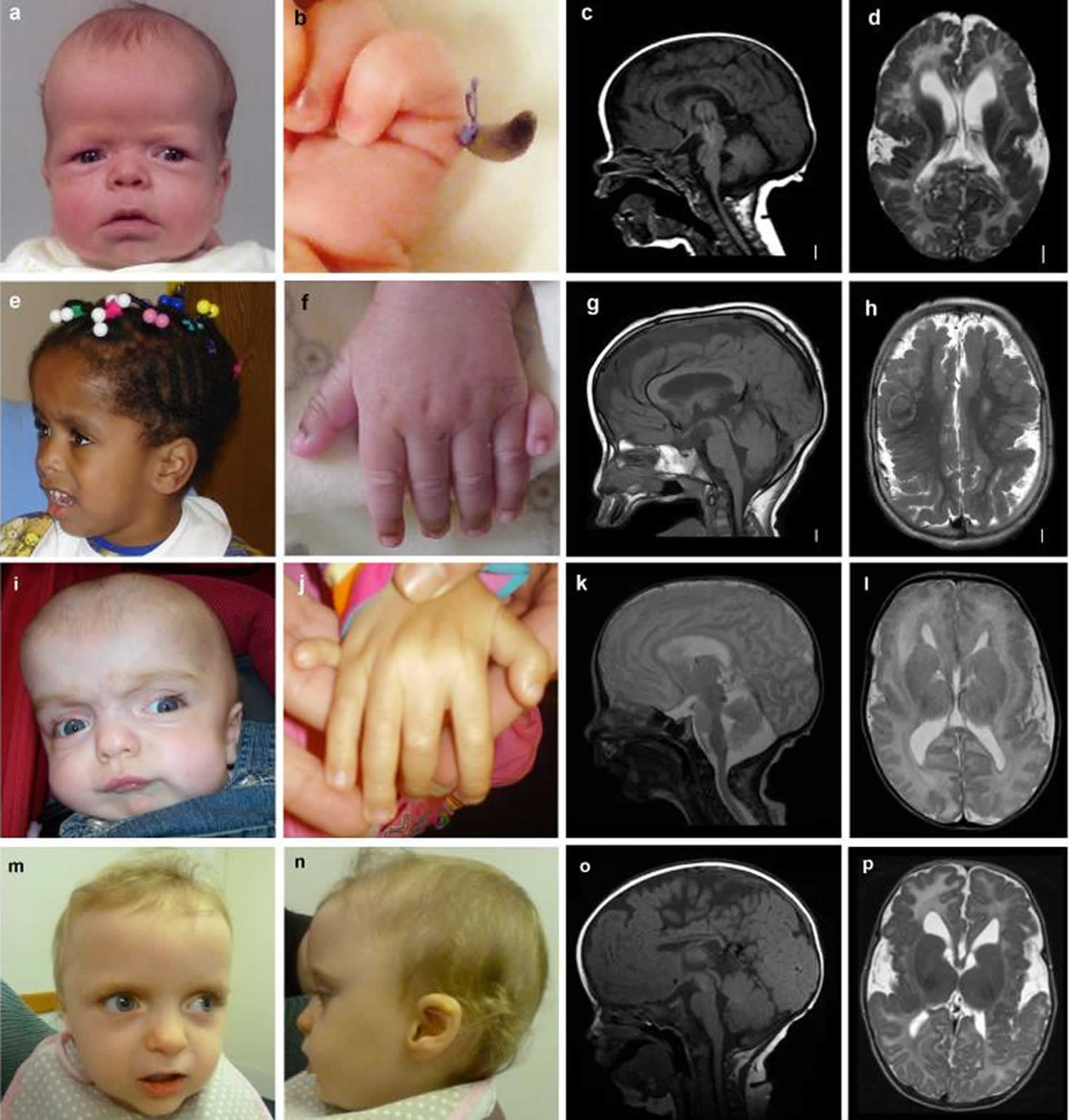
Footnote: Images of children with MPPH syndrome and CCND2 gene mutations. Photos and brain MRI of 4 affected individuals are shown: (a, e, i, m, n) Photographs demonstrate macrocephaly with prominent forehead, and (b, f, j) postaxial polydactyly of the hands. (c, g, k, o) T1- or T2-weighted midsagittal images demonstrate large brain size in relation to facial structures, (d, h, l, p) while axial images show polymicrogyria that appears most severe in the perisylvian regions, but also involves other regions.
[Source 19 ]What is polymicrogyria?
Polymicrogyria is a condition characterized by abnormal development of the brain before birth. Specifically, the surface of the brain develops too many folds which are unusually small. The signs and symptoms associated with the condition vary based on how much of the brain and which areas of the brain are affected; however, affected people may experience recurrent seizures (epilepsy); delayed development; crossed eyes; problems with speech and swallowing; and muscle weakness or paralysis. Bilateral forms (affecting both sides of the brain) tend to cause more severe neurological problems. Polymicrogyria can result from both genetic and environmental causes. It may occur as an isolated finding or as part of a syndrome. Treatment is based on the signs and symptoms present in each person 22.
What are the signs and symptoms of polymicrogyria?
A wide variety of symptoms may be observed in people with polymicrogyria, depending on the areas of the brain implicated and whether or not it is part of a larger syndrome. Signs and symptoms may include 23:
- Developmental delay
- Crossed eyes
- Epilepsy
- Paralysis of the face, throat, and tongue
- Difficulty with speech and swallowing
- Drooling
Can polymicrogyria be associated with other conditions?
Yes. Polymicrogyria may be an isolated occurrence or it may be a part of a larger condition, chromosome abnormality, and/or syndrome. “Syndrome” is a term used to describe a condition that is characterized by a particular collection of symptoms. Examples of associated syndromes, include Aicardi syndrome, Zellweger syndrome, and Smith-Lemli-Opitz syndrome 24.
What is polydactyly?
Polydactyly is a condition in which a person has more than five fingers per hand. Extra digits may be poorly developed and attached by a small stalk, or may be well-formed and have function 18.
What kind of polydactyly is typically seen in people with MPPH syndrome?
People with MPPH syndrome have post axial polydactyly, meaning the extra digit is on the outside of the little toe(s) or finger(s), and often times involves both hands and feet 11.
Can polydactyly be associated with other conditions?
Yes. Polydactyly can occur on its own (e.g. familial polydactyly) or may be a part of a larger condition, chromosome abnormality, and/or syndrome. Examples of associated syndromes, include Carpenter syndrome, Ellis-van Creveld syndrome, Laurence-Moon-Biedl syndrome, Rubinstein-Taybi syndrome, and Smith-Lemli-Opitz syndrome. It can also occur in association with chromosomal abnormalities such as Trisomy 13 18.
What is congenital hydrocephalus?
Congenital hydrocephalus is when a child is born with an excessive accumulation of cerebrospinal fluid (CSF) in the brain 25. CSF is a clear fluid that surrounds the brain and spinal cord. This excess fluid causes an abnormal widening of spaces in the brain called ventricles (ventriculomegalia) and can create a harmful pressure on brain tissue 25. Symptoms of hydrocephalus vary and may include an unusually large head with thin, transparent scalp, bulging forehead with increased spaces between the bones of the skull (fontanelles), and a downward gaze. Other symptoms may include seizures, abnormal reflexes, slow heartbeat and respiratory rate, headaches, vomiting, irritability, weakness, and visual problems 26.
It is caused by genetic and non-genetic factors. The most common cause of congenital hydrocephalus are variations (mutations) in the L1CAM gene, where there is a narrow passageway between the third and fourth ventricles (aqueductal stenosis). Other causes include mutations in many other genes, brain and/or spinal cord malformations, infections, bleeding inside the cavities of the brain (intraventricular hemorrhage), trauma, exposition to certain drugs (teratogens) or a congenital tumor of the brain 25. Congenital hydrocephalus can be an isolated malformation or be part of a syndrome where there are other associated malformations 25. It is most often treated by surgically inserting a shunt system to transport the excess CSF and allow for re-absorption. If left untreated, blindness and continuing mental deterioration may occur 25.
Hydrocephalus may be subdivided according to the particular defect that exists in the brain and whether the cerebrospinal fluid pressure is high or normal 25:
- Communicating hydrocephalus is when there is no blockage (obstruction) in the ventricules but the fluid is not absorbed readily, or there is too much fluid to be absorbed.
- Noncommunicating (obstructive) hydrocephalus is when there is a blockage of the CSF causing widening (dilation) of the pathways that are located upstream of the block, resulting in an increased pressure inside the brain.
There are also 2 other forms of hydrocephalus that usually affect only adults 25:
- Normal-pressure hydrocephalus is where the ventricules are expanded but the pressure inside the nervous system is normal.
- Hydrocephalus ex-vacuo occurs when stroke or traumatic injury cause damage to the brain and the brain tissue may shrink.
Hydrocephalus may also be classified in congenital or acquired. Acquired hydrocephalus develops at the time of birth or at some point afterward and may be caused by injury or disease 25.
Can hydrocephalus be associated with other conditions?
Yes. Hydrocephalus can occur due to a number of causes including head injuries, strokes, and infections. It can also be a part of a larger condition, chromosome abnormality, and/or syndrome 27.
MPPH syndrome causes
MPPH syndrome can be caused by mutations in at least three different genes, the AKT3, CCND2 and PIK3R2 genes. It is not known exactly how mutations within these genes causes MPPH syndrome; however, studies show that they are involved in a number of different functions in the body including vascular, limb, and brain development as well as regulation of growth 19, 20. The proteins produced from all three genes are involved in a chemical signaling pathway called the PI3K-AKT-mTOR pathway. This signaling influences many critical cell functions, including the creation (synthesis) of new proteins, cell growth and division (proliferation), and the survival of cells. The PI3K-AKT-mTOR pathway is essential for the normal development of many parts of the body, including the brain.
Mutations in the AKT3, CCND2, or PIK3R2 gene increase the activity of their respective proteins or prevent the proteins from being broken down when they should. As a result, chemical signaling through the PI3K-AKT-mTOR pathway is enhanced, which increases cell growth and division. In the brain, the increased number of cells leads to rapid and abnormal brain growth starting before birth. The rapid growth disrupts the structure and function of the developing brain. It is less clear how increased PI3K-AKT-mTOR signaling contributes to polydactyly, although the extra digits are probably related to abnormal cell proliferation in the developing hands and feet. CCND2 and PIK3R2 gene mutations are more likely to cause polydactyly than are AKT3 gene mutations.
MPPH syndrome inheritance pattern
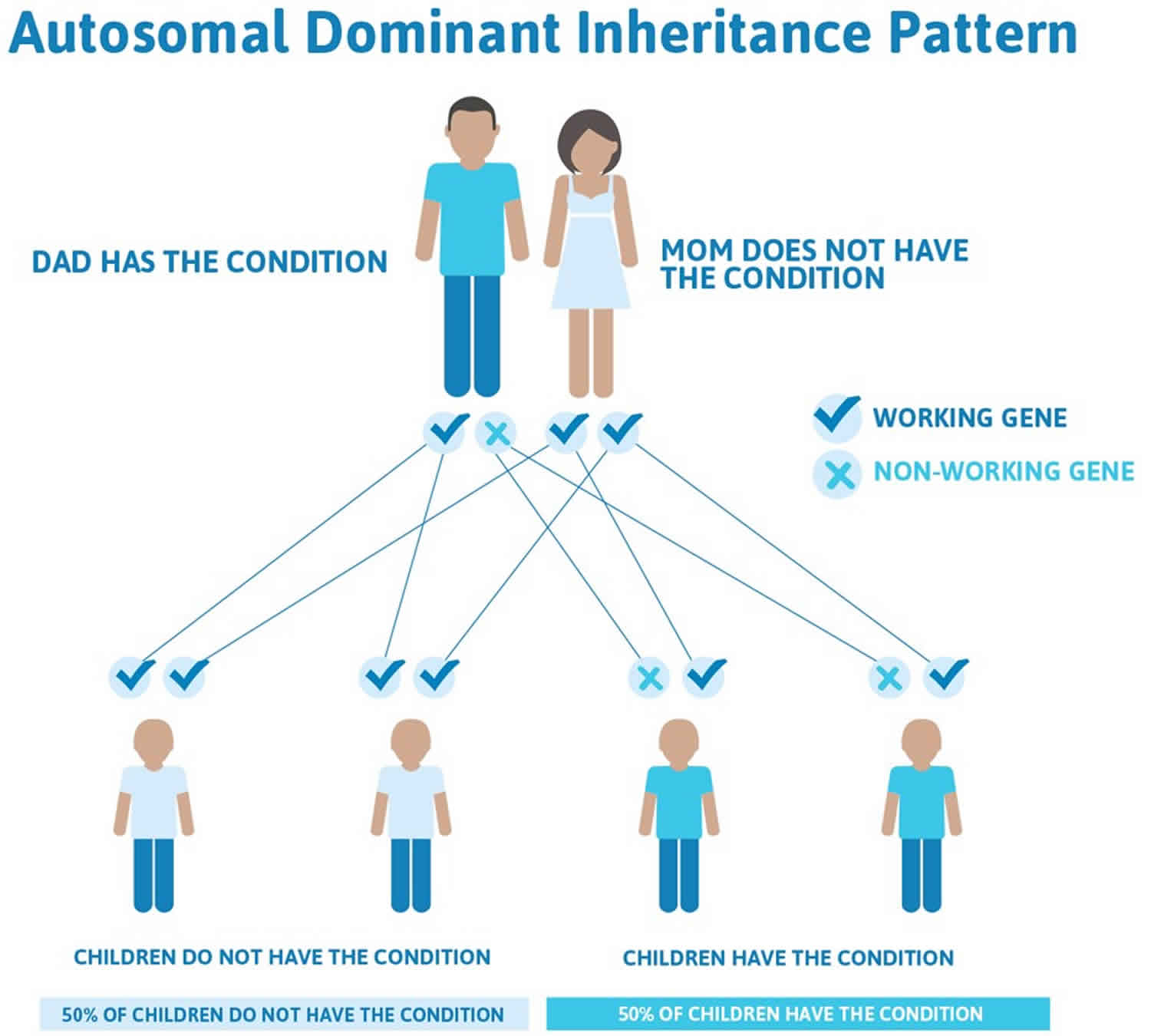
This condition is considered autosomal dominant, which means one copy of the altered gene in each cell is sufficient to cause the disorder.
Almost all cases of this condition result from new (de novo) gene mutations that occur during the formation of reproductive cells (eggs or sperm) or in early embryonic development. These cases occur in people with no history of the disorder in their family.
In a small number of cases, people with MPPH syndrome have inherited the altered gene from an unaffected parent who has a mutation only in their sperm or egg cells. This phenomenon is called germline mosaicism.
Rarely, MPPH syndrome can also result from somatic mosaicism, in which some of an affected person’s cells have a gene mutation and others do not. The genetic changes, which are called somatic mutations, arise randomly in one cell during embryonic development. As cells continue to divide, only cells arising from the first abnormal cell will have the mutation.
Figure 3. MPPH syndrome autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
MPPH syndrome symptoms
MPPH syndrome is characterized by the presence of multiple birth defects and developmental delay. Classic signs and symptoms of MPPH syndrome include polymicrogyria (the surface of the brain develops too many folds which are unusually small), megalencephaly, intellectual disability, seizures, polydactyly, and hydrocephalus 28. Other features might include characteristic facial features, low muscle tone (hypotonia), and impaired vision 29. Additional signs and symptoms reported in the medical literature include thin corpus callosum, psychomotor impairment (i.e., slowing down of physical reactions, movements, and speech), impaired vision (including cortical visual impairment and blindness), feeding difficulties (occasionally requiring gastrostomy tube placement), connective tissue symptoms including skin elasticity, mild characteristic facial differences, macrosomia (often at birth), and infantile spasms 30.
Additional clinical features 28:
Seen in <5 individuals each
- Congenital cardiovascular defects (including ventricular septal defect, atrial septal defect)
- Thyroid problems (including hypothyroidism, Hashimoto thyroiditis)
- Renal anomalies (e.g., duplicated renal collecting system)
Seen in one individual only
MPPH syndrome diagnosis
The clinical diagnosis of MPPH syndrome can be established in individuals with the two core features: megalencephaly and bilateral perisylvian polymicrogyria 28. The molecular diagnosis of MPPH syndrome is established in a proband (index case) with some of the suggestive clinical and imaging features and the identification of a heterozygous pathogenic variant in one of three genes: AKT3, CCND2, or PIK3R2. While most individuals with MPPH syndrome have a germline pathogenic variant in one of these three genes, some have a somatic mosaic pathogenic variant (most commonly reported in PIK3R2). To date 62 individuals with features of MPPH syndrome have been reported with either a clinical diagnosis (presence of the two core clinical and imaging findings: megalencephaly and polymicrogyria) , and/or a molecularly confirmed diagnosis 28.
MPPH syndrome treatment
Neurosurgical complications (hydrocephalus and cerebellar tonsillar ectopia). Findings warranting neurosurgical referral include rapidly enlarging head circumference, obstructive hydrocephalus, symptoms of increased intracranial pressure, and progressive or symptomatic cerebellar tonsillar ectopia (CBTE) or Chiari malformation. Early treatment of hydrocephalus may reduce the risk for progressive cerebellar tonsillar ectopia, but data to determine the most appropriate neurosurgical management are lacking.
Feeding difficulties such as chewing and swallowing difficulties and dysphagia require evaluation by a feeding specialist and/or gastroenterologist to promote early identification and prompt intervention which may include dietary modification and/or placement of a gastrostomy tube.
Speech therapy is indicated for difficulties with speech, swallowing, and feeding.
Epilepsy may require long-term antiepileptic treatment.
Developmental delays. Initiation of physical, occupational, and speech therapy is recommended within the first year of life.
Surveillance: Given the limited number of individuals reported with MPPH syndrome, no formal surveillance guidelines exist; however, recommended surveillance includes the following:
- Follow-up with a pediatric neurologist, at least every six months until age six years, and annually thereafter.
- Brain MRI to detect hydrocephalus and/or cerebellar tonsillar ectopia; provisionally recommended every six months from birth to age two years, and annually from age two to six years. In older individuals, the frequency should be based on prior results and clinical findings, with particular attention to apnea or other abnormal patterns of respiration, headaches, changes in gait, or other neurologic problems.
- Long-term neurologic follow up is recommended for management of epilepsy.
- Routine follow up with a developmental pediatrician is appropriate, given the high risk for developmental delays and/or intellectual disability.
MPPH syndrome prognosis
Megalencephaly is maintained to be prognostically more severe than macrocephaly and has been reported more frequently in the group of patients with intellective delay, epilepsy, and drug-resistant epilepsy 8.
Most people with MPPH syndrome have:
- Developmental delay
- Slow learning and intellectual disability
- Seizures (epilepsy)
- Abnormalities of muscle tone are common, particularly reduced muscle tone in infancy.
- Large head (megalencephaly)
- Characteristic appearance with a broad, prominent forehead
- Polymicrogyria, an abnormal organization of the grey matter (cortex). Polymicrogyria can affect smaller or larger parts of the cortex, which is at the surface of the brain
- Extra fingers and/or toes (polydactyly) are seen in a proportion of children with MPPH syndrome.
Megalencephaly causes
Megalencephaly is classically defined into 3 groups, according to the cause: idiopathic or benign, metabolic, and anatomic 8. Benign or idiopathic megalencephaly refers to children who have an abnormally large head with no neurological impairment. An increased head circumference is often reported in one or both the parents. In these individuals, the head circumference gradually increases from infancy to become stable at around 18 months of age or more with normal cerebral development 33.
Metabolic megalencephaly
Some inborn errors of metabolism may manifest with megalencephaly. Diagnosis of this disorder is based on specific neurological features associated with the megalencephaly. Familial history of similar disorders with a recessive hereditary pattern, either autosomal or sex-linked, or a history of marriages among relatives may be suggestive for a metabolic disorder. The clinical course is clearly progressive with a more or less rapid neurological impairment, which may also involve other organs, including the eyes, heart, spleen and liver, skin, and muscles. In these patients, increased intracranial pressure may also be present.
Physical examination in pediatric patients with megalencephaly and metabolic disorders show signs of neurological impairment, such as large or tense fontanels, enlarged sutures, the sun-setting sign, hypotonia, irritability, or, in older children, neurodevelopment delay, lethargy, and/or seizures. In these patients, the physical examination should be extended to the other organs; hepatosplenomegaly and/or other anomalous manifestations may indicate the diagnosis of neurometabolic disease. Laboratory findings are mandatory for a diagnosis of metabolic impairment through the research of enzyme or chemical defect, which can be observed in serum, urine, cerebrospinal fluid, and tissue culture. Some of these patients may die before the head circumference ever reaches the maximum level.
Three groups of metabolic disorders have been suggested to be associated with megalencephaly 34:
- defects of the organic acids,
- metabolic leukoencephalopathies or “metabolic encephalopathies”, and
- lysosomal storage diseases.
Defects of organic acids
Defects of organic acids include glutaric aciduria type 1 (gene defect GCDH) and L–2-hydroxyglutaric aciduria (gene defect L2HG). Glutaric aciduria type 1 is caused by deficiency of glutaryl-CoA dehydrogenase involved in the pathway of lysine to hydroxylysine and L-tryptophan. Abnormal head circumference is present since birth and is the earliest and most distinctive sign of this disorder. The enzymatic defect causes an accumulation of glutaric acid and 3-hydroxyglutaric acid that interferes in the energetic metabolism and oxidative stress, provoking neuronal impairment 35. Untreated patients present with dystonic movement disorders in infancy. Brain MRI presents with widening of Sylvian fissure (batwing appearance), diffuse white matter signal abnormalities, and bilateral high signal in the basal ganglia. Fronto-temporal atrophy has also been reported 36. The pathological features are characterized by striatal injury consequent to encephalopathic crises associated with frequent infectious episodes 37.
L-2-hydroxyglutaric aciduria includes a group of neurometabolic disorders in which the metabolic defect causes an elevated elimination of hydroxyglutaric acid in the urine. L-2-hydroxyglutaric aciduria is the most common and severe form that manifests with neurological impairment, large head, cerebellar sign, and epileptic seizures 38. The MRI images reported by Steenweg et al 39 displayed a cerebral white matter involvement that mainly affected the frontal and subcortical regions. The abnormal subcortical white matter anomalies show a mildly swollen appearance with an initial aspect partially multifocal. Neuropathologic examination shows mild cortical neuronal loss with intense gliosis, spongiosis, and vacualitation of the neuronal cells. Subcortical white matter contains numerous hyperplastic astrocytes with severe demyelinization and cystic cavities 40
D-2-hydroxyglutaric aciduria is caused by recessive mutation in D2HGDH (type 1) or by dominant gain-of-function mutations in IDH2 (Type 2). In this form, the megalencephaly is often present and is associated with cardiomyopathy, hypotonia, and intellectual delay. Brain MRI shows confluent subcortical white matter lesions that spread centrifugally with atrophy of the cerebellar vermis and involvement of dentate nuclei 41.
Metabolic encephalopathies
Canavan disease is caused by deficiency of aspartoacylase that leads to the accumulation of N-acetylaspartic acid (NAA) in the brain and in white matter. In this disorder, there is a degeneration of myelin in the phospholipid layer that isolates the axon. The NAA in a high concentration results in myelin vacuolization and astrocyte swelling. The clinical features in the infantile form are characterized by a rapidly increasing head circumference, severe hypotonia, and irritability with an onset at around 3 to 6 months of age. Subsequently, feeding difficulties with poor growth become more evident as the delayed milestones. Hypertonia, joint stiffness, and seizures develop rapidly, and most of the patients die in the first decade of life. MRI reveals diffuse white matter degeneration mainly involving cerebral hemispheres with extensive thickening of the white matter. Diagnosis is made in the presence of high levels of urinary NAA 42. Pathologic studies show spongy degeneration of white matter with no specific morphologic changes.
Alexander disease has been the first identified primary genetic disorder of astrocytes and is caused by mutations in the gene encoding glial fibrillary acidic protein (GFAP). Progressive neurological impairment, megalencephaly, and a typical MRI pattern are classically recognized as diagnostic. The brain MRI shows characteristic symmetric and extensive abnormalities with frontal predominance and relative sparing of occipital and temporal white matter 43.
Van der Knaap et al 44, in a report concerning the brain MRI of patients with Alexander disease, have identified 5 criteria, 4 of which are remarkable, for the diagnosis of the disease: an extensive cerebral white matter change with frontal predominance; a periventricular rim with high signal on T1-weighted images and low signal on T2-weighted images; abnormalities of basal ganglia and thalami; brain stem abnormalities; and contrast enhancement of particular gray and white matter structures. The pathological findings show a brain that is typically too large and too heavy; extensive paucity of myelin is found in the hemispheres in which frontal lobes are more affected than the others. The presence of Rosenthal fibers throughout the central nervous system (CNS) is the pathological hallmark of the disorder. A notable increase of astrocytes filled with Rosenthal fibers is reported to mainly affect the subpial, perivascular, and subependymal pattern. Rosenthal fibers are mainly found in the outer, subpial layers of the cerebral cortex, in frontal white matter, and near the periventricular area 45.
Megalencephalic leukoencephalopathy (MLC) with subcortical cysts associated with MLC1 and HEPACAM gene mutations and leukoencephalopathy with vanishing white matter, which is linked to mutations in EIFB1, EIFB2, EIFB3, EIFB4, and EIFB5 (eukaryote translational initiation factor B1-B5) genes, have been reported in patients presenting an abnormal head circumference. In MLC, the large head may be present at birth, but more often it appears during the first year of life. The degree of abnormal head circumference may reach 4 to 6 SDs above the average. After the first year, the head growth tends to normalize and to stabilize within the 98th percentile. Patients present with hypotonia, cognitive delay, and seizures 46. The brain MRI shows diffusely abnormal and mildly swollen white matter. Subcortical cysts are almost invariably present in the anterior temporal region and often in the frontal-parietal region. The subcortical cysts tend to increase in size and number 47.
In leukoencephalopathy with vanishing white matter, the clinical signs begin to appear during early childhood with spasticity and motor incoordination. The pathological findings show a gray matter of normal structure, while the white matter appears rarefied with a small number of axons and U-fibers and presence of small cavities in the white matter. The presence of foamy oligodendrocytes is typical of this disorder. The brain MRI displays reversal of signal intensity of the white matter. Recovery sequences and holes in the white matter are found 48.
Lysosomal storage diseases
Lysosomal storage diseases are also associated with an abnormally large brain. Tay–Sachs disease (TSD) is a disorder due to mutations in the HEXA gene located on chromosome 15q23–24. Two forms of β-hexosaminidase are recognized: β-hexosaminidase A [HEXA] is a heterodimer comprised of an α-acid and a β subunit, while β-hexosaminidase B [HEXB] is formed by two β subunits. The clinical signs begin at the age of 6 months with a progressive delay of the developmental milestones and hypotonia. A large head becomes apparent by 1 year of age due to an abnormal content of the ganglioside GM2, which accumulates in the brain. Short stature, progressive spasticity, and seizures with visual degeneration and deafness are the presenting clinical signs. Cherry red spots at the fundoscopic examination are useful for diagnosis. Pathologic examination displays massively increased weight and volume of the brain, which may weigh more than twice than a normal brain. Cystic degeneration of the cerebral white matter (status spongiosus) and atrophy of the cerebellar hemispheres are frequently observed 49.
Sandhoff disease is linked to an inherited deficiency of HEXA and HEXB that are needed for the degradation of the neuronal membrane components (ganglioside GM2, its derivate GA2, the glycolipid globoside). The HEXB gene is located on chromosome 5q13. As for TSD, signs begin at the age of 6 months with large head, seizures, cherry red spots, and doll-like facies 50. In the brain, an amount of GM2 asialoganglioside that is 300 times more than normal is found in affected patients 49.
Large head circumference above the normal has been reported in patients with other metabolic disorders, but they do not have all the characteristics to be included in the group of megalencephaly, for example, Mucopolisaccharidoses (MPS) type 1-Hurler (gene mutation IDLIA), MPS II-Hunter syndrome (IDS gene), and MPS III-Sanfilippo syndrome (SGSH, HAGLN, HGSNAF, GNS genes). In these patients, the head circumference is usually normal at birth, and it gradually increases in the first years of life (usually between 1 and 3 years). The increased head circumference in Type I-Hurler and type II-Hunter is linked to chronic communicating hydrocephalus, progressive cerebral storage of glycosaminoglycans, enlarged cerebral perivascular spaces, and neuroinflammation. In MPS III, the increase of head circumference is noticed in the first decade, but there is a progressive decrease, and in adulthood, the head circumference, due to progressive brain atrophy, returns to a normal range.
Volumetric analysis of cortical gray matter, cortical white matter, corpus callosum, and frontal lobes in MPS II, as reported by Yund et al 51, notes that the mean volumes are larger, but they are not statistically significant different than controls. In a large study of 118 patients with MPS III by de Ruijter et al 52, mean head circumference SDs according to age was 0.88 for all patients (boy 0.71, girl 1.1). The data reported by Yund et al 51 confirmed our experience of several patients followed in the metabolic center of the University of Catania.
Large head has been anecdotally described in patients with Krabbe disease, but as reported by Barone et al 53 in 11 patients with classic infantile and late-onset Krabbe disease, no patients with an abnormally large head have been found.
Anatomic megalencephaly
This group of disorders manifests with developmental megalencephaly linked to a single gene mutation involving early brain cellular growth, migration, or replication. Mutation in the mammalia Target of Rapamyicin (mTOR), mitogen-activated protein kinase, originally called “extracellular signal-regulated kinases” (MAPK/ERK), and Sonic hedgehog (SHH) pathways have been frequently reported as pathogenetic events causing megalencephaly as a single anomaly or in association with other body structural anomalies 33.
Megalencephaly within dwarfism
Achondroplasic patients have a mutation at the FGF3 gene, codon 380. In these patients, the cranium is disproportionately large relative to height, but it is unusual to reach level above 2 SDs. The patients present with a prominent forehead, the nasal bridge is moderately flat, and the chest is narrow. Most of these signs may be present at birth. The cognitive aspect is normal, but cerebral complication may be frequent 54.
Megalencephaly within gigantism (more often associated with ventricular megalencephaly)
Sotos syndrome (also known as cerebral gigantism) is a prenatal and postnatal overgrowth syndrome linked to a mutation of the gene encoding the nuclear receptor set domain containing protease 1 (NSD1) on chromosome 5q35. Patients present with tall stature, large head, distinctive craniofacial features, gait dyspraxia, seizures, and developmental delay. Some of these patients also present an auto- and hetero-aggressivity causing severe damage. Height tends to normalize after puberty, whereas the head circumference persists to be large 55.
Brain neuroimaging shows abnormalities involving the cavum septum pellucidum and cavum vergae, hypoplasia of corpus callosum, enlarged subarachnoidal spaces, ventricle dilatation, slight hypodensity of the white matter, and cerebral atrophy 55. Schaefer and Buehler 56 reported a neuroradiologic study performed in 40 patients affected by Sotos syndrome. Most of the anomalies were found in the ventricular system with ventricles enlarged, and prominence of the trigone and occipital horns, extracerebral supratentorial, and increased posterior fossa fluid spaces; gray matter heterotopias, periventricular leukomalacia, periventricular leukomalacia, cavum septum pellucidum, and corpus callosum anomalies were also reported.
Phenotypic overlap between Sotos and Weaver syndrome has been widely reported. In Weaver syndrome, tall stature has been reported as the most common feature observed in 90% and intellectual disability in 80% of patients 57. Additional clinical features include camptodactyly, soft and doughy skin, umbilical hernia, and a low hoarse cry.
Simpson–Golabi–Behmal syndrome (SGBS) types I and II show clinical signs of multiple congenital abnormalities, including pre-post natal overgrowth, distinctive craniofacial features, large head, and organomegaly. Other anomalies may be observed involving the skeletal system, heart, CNS, kidney, and gastrointestinal tract 58. Genomic rearrangements and point mutations involving the glycan-3 gene (GPC3) at Xq26 are the causal events associated with SGBS. Large head has been reported in about 70% of children with SGBS. MRI shows midline defect, such as abnormal corpus callosum, central lipomata, and hydrocephalia 58. Craniosynostosis may be frequently present.
Bannayan–Riley–Ruvalcaba syndrome (BRRS) is a well-known disorder linked to a germline mutation of PTEN. This syndrome, with its allelic disorders including the Cowden syndrome, manifests with an overgrowth of connective tissue and multiple benign hamartomatous lesions and megalencephaly. The Cowden syndrome is associated with a high risk for thyroid, breast, and endometrial cancers. Patients with BRRS present a large head, intestinal polyposis, lipomas, and pigmented penile macules 59. Bhargava et al 60 reported a brain neuroradiologic study in 7 patients affected by BRRS. They found the presence of white matter cysts localized in the parietal lobe in all the patients, whereas cysts were also reported in the frontal lobe in 3 and in 1 in the temporal lobe, respectively. The cysts were predominantly surrounded by white matter t 2 hyperintensities. Both these syndromes are enclosed in the spectrum of PTEN hamartoma tumor syndromes linked to the germline mutations in the tumor suppressor PTEN gene located in chromosome 10q23.3 61.
Megalencephaly within syndromes
Recently, megalencephaly as a presenting sign has been reported in two syndromes, which represent a typical example of anatomic brain overgrowth: megalencephaly–polymicrogyria–polydactily–hydrocephalus (MPPH) and megalencephaly-capillary malformation (MCAP). De novo germline mutations in APT3 and PIK3R2 have been reported in patients with MPPH syndrome. This syndrome is characterized by abnormally increased head sizes, reaching levels above 10 SDs. Ventriculomegaly that may progress to hydrocephalus, cerebelllar tonsillar ectopia, and polymicrogyria are other associated anomalies. Cutaneous lesions are accompanying features consisting of capillary malformations and variable connective tissue dysplasia. Mild focal or segmental body overgrowth, together with finger or toe syndactily and postaxial polydactily may be present 34. MCAP has many features overlapping the MPPH. It has been also linked to the PIK3CA gene. The genes PIK3R2, ATK3, and PIK3CA are members of the phosphatidyl inositol-3kinase of the AKT pathway and are present in all the developmental brain cellular activities, including apoptosis. MPPH shows many of the same features as the MCAP, including the severe megalencephaly, with the exception of the postaxial polydactyly, which is more common and more typical in the MPPA syndrome 62.
Pretzel syndrome
Polyhydramnios, megalencephaly, and symptomatic epilepsy syndrome (PMSE), which is also called Pretzel syndrome, is characterized by infantile-onset epilepsy, neurocognitive delay, and craniofacial dysmorphism. PMSE is caused by homozygous deletion of exons 9 to 13 of the LYK5/STRADA gene that encodes the pseudokinase STRADA, an upstream inhibitor of mammalian target of rapamycin complex 1 63. Polyhydramnios is frequently reported: around 80% of the cases reported present with this anomaly. The psychomotor disturbances are very impressive with cognitive delay, early seizures, and muscle involvement that are hypotrophic. The affected child lies in a particular position termed a “Pretzel-like posture.” Atrial septal defect is reported in one-third of the patients. The large head commonly present in this child as reported by brain MRI is linked to the presence of extracerebral fluid or hydrocephalus in association with excessive cerebral growth. Histopathologic evidence of heterotopic neurons in subcortical white matter and subependymal regions were reported by Puffenberger et al 64 wherein a single postmortem neuropathologic study showed megalencephaly, ventriculomegaly, cytomegaly, and extensive vacuolization and astrocytosis of white matter. A constitutive activation of mTORC1 signaling pathway was reported by the authors in the frontal cortex, basal ganglia, hippocampus, and spinal cord.
Other syndromes
PTEN gene mutations have been observed in patients with increased head circumference and autistic behavior. Butler et al 65 reported 18 individuals who presented a germline PTEN mutation with X-linked intellectual disability, large head, and neurobehavioral features of autistic spectrum disorder. In these patients (13 males), the head circumerence increase ranged from 2.5 to 8.0 SDs. Large head and autism has been reported in patients with RAB1q mutation 34.
Congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) syndrome has been reported in association with CNS malformations and seizures 66. Among the patients reported by Gucev et al 66, a newborn girl presented with massive lymphatic truncal vascular malformation with cutaneous venous anomaly and overgrown feet and splayed toes. Cranial computed tomography (CT) showed encephalomalacia, widening of ventricles and the sulci, and hemimegalencephaly. Patients with CLOVE syndrome and megalencephaly have also been reported 66, but megalencephaly is not a prominent sign in this syndrome.
Macrocephaly, alopecia, cutis laxa, and scoliosis is a rare condition with an autosomal recessive inheritance. Mutations in RIN2 (chromosome 20p11.23) have been linked to this disorder 67. RIN2 gene encodes the RAS and RAB interactor protein 2, which is involved in cell trafficking. The presenting clinical features consist of progressive facial coarsening, gingival hypertrophy, severe scoliosis, sparse hair, cutis laxa, and joint hyperlaxity 68.
PMSE 64 is linked to homozygous 7-kilobase deletion in LYK5, which encodes STE20-related adaptor protein, a pseudokinase functionally necessary for proper localization and function of serine/threonine kinase 11 (a.k.a. LKB1). The LYK5 deletion is associated with polyhydramionis, preterm labor, and distinctive craniofacial features in addition to megalencephaly and multifocal seizures. Puffenberger et al 64 reported 16 patients of whom 4 (38%) died during childhood due to status epilepticus, congestive heart failure, and hypernatremic dehydratation. A pathologic examination carried out in a single patient disclosed the presence of megalencephaly, ventriculomegaly, cytomegaly, and extensive vacuolization and astrocytosis in the white matter.
Greig cephalo-polysyndactyly syndrome (GCPS) is linked to a mutation involving the GLI3 protein, which is a zinc finger transcription factor that is expressive in early development. Patients present with preaxial or postaxial polydactily with or without syndactily and craniofacial features, including large head and hypertelorism. Abnormalities located in the corpus callosum have been reported 69. Démurger et al 70 state that macrocephaly was found in 32 of 53 (60%) patients with GCPS, but in 7 of 18 patients (39%), a severe ventricular dilatation was present.
Acrocallosal syndrome is an autosomal recessive disorder that manifests with corpus callosum agenesis, facial dysmorphism, postaxial polydactily of the hands as well as preaxial polydactyly of the feet. The disorder is linked to a mutation of the KIF7 gene that encodes a molecule within the sonic hedgehog 71. The reported large head has been related to enlarged ventricles 72 and therefore should not be included in the group of megalencephaly as with the GCPS.
In Opitz–Kaveggia syndrome, also known as “FG syndrome,” the mutation involves the MED12 gene, which is located on Xq13 and is a member of the large mediator complex; it has a relevant role in RNA polymerase II transcription. The mutations of MED12 can cause, in addition to the FG syndrome, the Lujan syndrome and Ohdo syndrome 73. Affected male patients present distinctive facial appearance, mental retardation, large head, imperforate anus, and hypotonia. In the classical report of Opitz and Kaveggia 74, the syndrome was designed as a multiple congenital anomaly syndrome with signs of relative macrocephaly, broad and flat thumbs, emperforate anus, hypotonia, and moderately severe cognitive delay.
Nguyen et al 75 reported a new autosomal-recessive neurological condition characterized by megalencephaly, thick corpus callosum, and severe intellectual disability. The boy presented with neonatal overgrowth of the occipital-frontal circumference at the 90th percentile, +3 SDs at the age of 14 years and 66.5 cm at 18 years of age. Facial features included a long face with high forehead, prognatism, and long and thin feet and hands. Severe myopia, thick corpus callosum, enlarged white matter, and small cerebellum were also reported. The syndrome seems to be caused by a homozygous nonsense variant in the HERC1 protein that is an ubiquitin ligase that interacts with tuberous sclerosis complex 2, an upstream negative regulator of the mTOR pathway.
Grotto et al 76 reported 5 patients affected by severe intellectual disability linked to BRWD3 nonsense mutation, p.Tyr131, and compared the clinical presentations to those of 4 patients previously reported. The main symptoms consisted of intellectual disability that ranged from mild to moderate (9/9) and speech delay (8/9); also, a large head was present in 7 of 9 patients. Among these, one patient presented with neonatal overgrowth and large head; at the age of 12 years, the weight, length, and head circumference measurements were +3.5 to 4.5 SDs. In this group of patients, dysmorphic features were also present, including high forehead, hypertelorism, short palpebral fissures, anteverted nares, pointed chin, broad hands and feet, joint laxity, genu valgum, and small penis.
Developmental delay labeled macrocephaly syndrome in a large Amish kindred with an autosomal recessive inheritance was described and related to homozygous or compound heterozygous mutations in the KPTN gene encoding kaptin 77.
Megalencephaly symptoms
Megalencephaly may cause no symptoms or be associated with developmental delay, seizures, and neurological problems. Megalencephaly is more common in males than females 2.
Among all the forms of megalencephaly, the idiopathic form is the most frequent and is not associated with neurological manifestations, intellectual disabilities or other symptoms involving other parts of the body 3. In idiopathic megalencephaly, the child’s head circumference increases gradually until an age of 18 months and then becomes more stable during the course of development. The children’s parental occipitofrontal circumference measurements were within normal ranges. This situation is opposite to what is typically reported in the literature for benign megalencephaly (i.e., a large parental head is frequently reported) 78.
Megalencephaly diagnosis
By definition, both in megalencephaly and macrocephaly, the measurement of head circumference is reported to be 2 standard deviations (SDs) above the age-related mean 8. A proper measure of the head circumference should be performed by putting the tape measure along the most prominent diameter of the occiput and the mid forehead; then, the results of the measurement must be checked with the head circumference growth charts, according to the age, gender, and height parameters 79. Correlation with the maternal and paternal head circumference is useful. The newborn brain is reported to weigh about 370 g and increases about 4-fold from infancy to childhood till reaching an adult’s weight of about 1500 g 80.
Figure 4. Diagnostic flowchart for increased head circumference in children
[Source 8 ]Megalencephaly treatment
There is no standard treatment for megalencephaly. Treatment will depend upon the disorder with which the megalencephaly is associated and will address individual symptoms and disabilities.
Megalencephaly prognosis
The prognosis for infants and children with megalencephaly depends upon the underlying cause and the associated neurological disorders. The prognosis for children with hemimegalencephaly is poor.
- Mirzaa GM, Conway RL, Gripp KW, Lerman-Sagie T, Siegel DH, deVries LS, Lev D, Kramer N, Hopkins E, Graham JM Jr, Dobyns WB. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A. 2012 Feb;158A(2):269-91. doi: 10.1002/ajmg.a.34402 https://doi.org/10.1002/ajmg.a.34402[↩][↩]
- Megalencephaly Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Megalencephaly-Information-Page[↩][↩]
- Massimino, Carmela & Marino, Silvia & Giallongo, Alessandro & Praticò, Andrea & Gangi, Gloria & Filosco, Federica & Oliva, Claudia & Lombardo, Giulia & Pavone, Piero. (2020). Non-syndromic megalencephaly and epilepsy: Our findings. Brain and Nerves. 5. 10.15761/JBN.1000125[↩][↩][↩][↩]
- Winden KD, Yuskaitis CJ, Poduri A (2015) Megalencephaly and Macrocephaly. Semin Neurol 35: 277-287.[↩]
- Berg AT, Dobyns WB (2015) Progress in autism and related disorders of brain development. Lancet Neurol 14: 1069-1170.[↩][↩]
- Pavone P, Praticò AD, Rizzo R, Corsello G, Ruggieri M, et al. (2017) A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Medicine (Baltimore) 96: e6814.[↩]
- Winden KD, Yuskaitis CJ, Poduri A. Megalencephaly and macrocephaly. Semin Neurol 2015;35:277–87.[↩]
- Pavone P, Praticò AD, Rizzo R, et al. A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Medicine (Baltimore). 2017;96(26):e6814. doi:10.1097/MD.0000000000006814 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500017[↩][↩][↩][↩][↩][↩][↩][↩]
- Williams CA, Dagli A, Battaglia A. Genetic disorders associated with macrocephaly. Am J Med Genet A 2008; 146A (15) 2023-2037[↩]
- Megalencephaly and Macrocephaly. Semin Neurol 2015; 35(03): 277-287 DOI: 10.1055/s-0035-1552622 https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0035-1552622[↩][↩]
- Ariana Kariminejad, Farid Radmanesh, Ali-reza Rezayi, Seyed-Hasan Tonekaboni, Joseph G. Gleeson. Megalencephaly-Polymicrogyria-Polydactyly-Hydrocephalus Syndrome A Case Report. Journal of Child Neurology. May 2013; 28(5):651-657. http://www.ncbi.nlm.nih.gov/pubmed/22859694[↩][↩][↩]
- Asch, A. J., Myers, G. J. Benign familial macrocephaly: report of a family and review of the literature. Pediatrics 57: 535-539, 1976.[↩][↩]
- Platt, M., Nash, A. Benign familial megalencephaly. (Abstract) Pediat. Res. 6: 426 only, 1972.[↩]
- Day, R. E., Shutt, W. H. Normal children with large heads–benign familial megalencephaly. Arch. Dis. Child. 54: 512-517, 1979.[↩]
- Arbour, L., Watters, G. V., Hall, J. G., Fraser, F. C. Multifactorial inheritance of non-syndromic macrocephaly. Clin. Genet. 50: 57-62, 1996.[↩][↩]
- Cole, T. R. P., Hughes, H. E. Autosomal dominant macrocephaly: benign familial macrocephaly or a new syndrome? Am. J. Med. Genet. 41: 115-124, 1991.[↩][↩]
- Diaz-Rodriguez, M., Becerra-Solano, L. E., Toscano-Flores, J. J., Banuelos-Robles, O., Duran-Gonzalez, J., Ramirez Duenas, M. L. Benign familial macrocephaly in a mother-son pair. Genet. Counsel. 21: 85-89, 2010.[↩]
- Polydactyly. https://medlineplus.gov/ency/article/003176.htm[↩][↩][↩]
- Mirzaa G, Parry DA, Fry AE, et al. De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat Genet. 2014;46(5):510-515. doi:10.1038/ng.2948 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4004933[↩][↩][↩]
- Rivière JB, Mirzaa GM, O’Roak BJ, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44(8):934-940. Published 2012 Jun 24. doi:10.1038/ng.2331 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3408813[↩][↩]
- Megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. https://medlineplus.gov/genetics/condition/megalencephaly-polymicrogyria-polydactyly-hydrocephalus-syndrome[↩]
- Stutterd CA, Dobyns WB, Jansen A, et al. Polymicrogyria Overview. 2005 Apr 18 [Updated 2018 Aug 16]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1329[↩]
- Polymicrogyria. https://medlineplus.gov/genetics/condition/polymicrogyria[↩]
- Golden JA, Bonnemann CG. Developmental Structural Disorders. In: Goetz CG. Textbook of Clinical Neurology. Philadelphia, PA: Saunders; 2007[↩]
- Hydrocephalus Fact Sheet. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Hydrocephalus-Fact-Sheet[↩][↩][↩][↩][↩][↩][↩][↩]
- Hydrocephalus in children: Physiology, pathogenesis, and etiology. https://www.uptodate.com/contents/hydrocephalus-in-children-physiology-pathogenesis-and-etiology[↩]
- Hydrocephalus. https://medlineplus.gov/hydrocephalus.html[↩]
- Mirzaa G. MPPH Syndrome. 2016 Nov 17. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK396098[↩][↩][↩][↩]
- Mirzaa GM, Conway RL, Gripp KW, Lerman-Sagie T, Siegel DH, deVries LS, Lev D, Kramer N, Hopkins E, Graham JM Jr, Dobyns WB. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A. 2012 Feb;158A(2):269-91. https://doi.org/10.1002/ajmg.a.34402[↩]
- Pisano T. Megalencephaly, polymicrogyria, and hydrocephalus (MPPH) syndrome: a new case with syndactyly. J Child Neurol. 2008[↩]
- Osterling WL, Boyer RS, Hedlund GL, Bale JF. Jr. MPPH syndrome: two new cases. Pediatr Neurol. 2011;44:370–3.[↩]
- Demir N, Peker E, Gülşen I, Kaba S, Tuncer O. Megalencephaly, polymicrogyria, polydactyly and hydrocephalus (mpph) syndrome: a new case with occipital encephalocele and cleft palate. Genet Couns. 2015;26:381–5.[↩]
- Winden KD, Yuskaitis CJ, Poduri A. Megalencephaly and macrocephaly. Semin Neurol 2015;35:277–87. https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0035-1552622[↩][↩]
- Mirzaa GM, Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. Am J Med Genet C Semin Med Genet 2014;166C:156–72.[↩][↩][↩]
- Jafari P, Braissant O, Bonafé L, et al. The unsolved puzzle of neuropathogenesis in glutaric aciduria type I. Mol Genet Metab 2011;104:425–37.[↩]
- Strauss KA, Puffenberger EG, Robinson DL, et al. Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Med Genet C Semin Med Genet 2003;121C:38–52.[↩]
- Kölker S, Christensen E, Leonard JV, et al. Diagnosis and management of glutaric aciduria type I: revised recommendations. J Inherit Metab Dis 2011;34:677–94.[↩]
- Steenweg ME, Jakobs C, Errami A, et al. An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study. Hum Mutat 2010;31:380–90.[↩]
- Steenweg ME, Salomons GS, Yapici Z, et al. L-2-Hydroxyglutaric aciduria: pattern of MR imaging abnormalities in 56 patients. Radiology 2009;251:856–65.[↩]
- Seijo-Martínez M, Navarro C, Castro del Río M, et al. L-2-hydroxyglutaric aciduria: clinical, neuroimaging, and neuropathological findings. Arch Neurol 2005;62:666–70.[↩]
- Nyhan WL, Shelton GD, Jakobs C, et al. D-2-hydroxyglutaric aciduria. J Child Neurol 1995;10:137–42.[↩]
- Matalon R, Michals-Matalon K. Canavan disease. In: Pagon RA, Adam MP, Ardinger HH, et al, editors. GeneReviews® [Internet]. Seattle, WA: University of Washington; 1993–2015. September 16, 1999.[↩]
- Rodriguez D. Leukodystrophies with astrocytic dysfunction. Handb Clin Neurol 2013;113:1619–28.[↩]
- van der Knaap MS, Naidu S, Breiter SN, et al. Alexander disease: diagnosis with MR imaging. AJNR Am J Neuroradiol 2001;22:541–52.[↩]
- Sawaishi Y. Review of Alexander disease: beyond the classical concept of leukodystrophy. Brain Dev 2009;31:493–8.[↩]
- van der Knaap MS, Scheper GC. Megalencephalic leukoencephalopathy with subcortical cysts. In: Pagon RA, Adam MP, Ardinger HH, et al, editors. GeneReviews® [Internet]. Seattle, WA: University of Washington; 1993–2015. August 11, 2003. [updated November 03, 2011].[↩]
- van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol 2006;5:413–23.[↩]
- Pronk JC, van Kollenburg B, Scheper GC, et al. Vanishing white matter disease: a review with focus on its genetics. Ment Retard Dev Disabil Res Rev 2006;12:123–8.[↩]
- Swaiman KF, Menkes JH, Devivo DC, Prensky AL. Swaiman KF, Wright FS. Metabolic disorders of the central nervous system. The Practice of Paediatric Neurology 2nd ed. Vol. ILondon: The C.V. Mosby Co; 1982. 575.[↩][↩]
- Krivit W, Desnick RJ, Lee J, et al. Generalized accumulation of neutral glycosphingolipids with GM2 ganglioside accumulation in the brain. Sandhoff’s disease (variant of Tay-Sachs disease). Am J Med 1972;52:763–70.[↩]
- Yund B, Rudser K, Ahmed A, et al. Cognitive, medical,;1; and neuroimaging characteristics of attenuated mucopolysaccharidosis type II. Mol Genet Metab 2015;114:170–7.[↩][↩]
- de Ruijter J, Broere L, Mulder MF, et al. Growth in patients with mucopolysaccharidosis type III (Sanfilippo disease). J Inherit Metab Dis 2014;37:447–54.[↩]
- Barone R, Brühl K, Stoeter P, et al. Clinical and neuroradiological findings in classic infantile and late-onset globoid-cell leukodystrophy (Krabbe disease). Am J Med Genet 1996;63:209–17.[↩]
- Bouali H, Latrech H. Achondroplasia: current options and future perspective. Pediatr Endocrinol Rev 2015;12:388–95.[↩]
- Mauceri L, Sorge G, Baieli S, et al. Aggressive behavior in patients with Sotos syndrome. Pediatr Neurol 2000;22:64–7.[↩][↩]
- Schaefer CB, Buehler BA. Saul RA, Phelan MC. Neuroanatomic Features of Sotos Syndrome. Proceedings of the Greenwood Genetic Center. Greenwood, SC: Greenwood Genetic Center; 1993. 36.[↩]
- Tatton-Brown K, Murray A, Hanks S, et al. Childhood Overgrowth Consortium, Rahman N. Weaver syndrome and EZH2 mutations: clarifying the clinical phenotype. Am J Med Genet A 2013;161A:2972–80.[↩]
- Tenorio J, Arias P, Martínez-Glez V, et al. Simpson-Golabi-Behmel syndrome types I and II. Orphanet J Rare Dis 2014;9:138.[↩][↩]
- Pilarski R, Stephens JA, Noss R, et al. Predicting PTEN mutations: an evaluation of Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome clinical features. J Med Genet 2011;48:505–12.[↩]
- Bhargava R, Au Yong KJ, Leonard N. Bannayan-Riley-Ruvalcaba syndrome: MRI neuroimaging features in a series of 7 patients. AJNR Am J Neuroradiol 2014;35:402–6.[↩]
- Piccione M, Fragapane T, Antona V, et al. PTEN hamartoma tumor syndromes in childhood: description of two cases and a proposal for follow-up protocol. Am J Med Genet A 2013;161A:2902–8.[↩]
- Mirzaa GM, Conway RL, Gripp KW, et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am J Med Genet A 2012;158A:269–91.[↩]
- Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sci Transl Med 2013;5: 182ra53[↩]
- Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain 2007;130:1929–41.[↩][↩][↩]
- Butler MG, Dasouki MJ, Zhou XP, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 2005;42:318–21.[↩]
- Gucev ZS, Tasic V, Jancevska A, et al. Congenital lipomatous overgrowth, vascular malformations, and epidermal nevi (CLOVE) syndrome: CNS malformations and seizures may be a component of this disorder. Am J Med Genet A 2008;146A:2688–90.[↩][↩][↩]
- Aslanger AD, Altunoglu U, Aslanger E, et al. Newly described clinical features in two siblings with MACS syndrome and a novel mutation in RIN2. Am J Med Genet A 2014;164A:484–9.[↩]
- Syx D, Malfait F, Van Laer L, et al. The RIN2 syndrome: a new autosomal recessive connective tissue disorder caused by deficiency of Ras and Rab interactor 2 (RIN2). Hum Genet 2010;128:79–88.[↩]
- Biesecker LG. The Greig cephalopolysyndactyly syndrome. Orphanet J Rare Dis 2008;3:10.[↩]
- Démurger F, Ichkou A, Mougou-Zerelli S, et al. New insights into genotype-phenotype correlation for GLI3 mutations. Eur J Hum Genet 2015;23:92–102.[↩]
- Ibisler A, Hehr U, Barth A, et al. Novel KIF7 mutation in a Tunisian boy with acrocallosal syndrome: case report and review of the literature. Mol Syndromol 2015;6:173–80.[↩]
- Speksnijder L, Cohen-Overbeek TE, Knapen MF, et al. A de novo GLI3 mutation in a patient with acrocallosal syndrome. Am J Med Genet A 2013;161A:1394–400.[↩]
- Graham JM, Jr, Schwartz CE. MED12 related disorders. Am J Med Genet A 2013;161A:2734–40.[↩]
- Opitz JM, Kaveggia EG. Studies of malformation syndromes of man 33: the FG syndrome. An X-linked recessive syndrome of multiple congenital anomalies and mental retardation. Z Kinderheilkd 1974;117:1–8.[↩]
- Nguyen LS, Schneider T, Rio M, et al. A nonsense variant in HERC1 is associated with intellectual disability, megalencephaly, thick corpus callosum and cerebellar atrophy. Eur J Hum Genet 2016;24:455–8.[↩]
- Grotto S, Drouin-Garraud V, Ounap K, et al. Clinical assessment of five patients with BRWD3 mutation at Xq21.1 gives further evidence for mild to moderate intellectual disability and macrocephaly. Eur J Med Genet 2014;57:200–6.[↩]
- Pajusalu S, Reimand T, Õunap K. Novel homozygous mutation in KPTN gene causing a familial intellectual disability-macrocephaly syndrome. Am J Med Genet A 2015;167A:1913–5.[↩]
- Mroske C, Rasmussen K, Shinde DN, Huether R, Powis Z, et al. (2015) Germline activating MTOR mutation arising through gonadal mosaicism in two brothers with megalencephaly and neurodevelopmental abnormalities. BMC Med Genet 16: 102.[↩]
- Sniderman A. Abnormal head growth. Pediatr Rev 2010;31:382–4.[↩]
- Blinkov SM, Glazer II. The Human Brain in Figures and Tables. A Quantitative Handbook. New York: Plenum Press; 1968.[↩]