Melanoma skin cancer
Melanoma also called malignant melanoma or cutaneous melanoma, is the most serious type of skin cancer that begins in the melanocytes of the skin (a type of skin cells that produce melanin and melanin gives your skin its color) (see Figure 1 below). Melanomas can develop anywhere on the skin, but they are more likely to start on the trunk (chest and back) in men and on the legs in women. The neck and face are other common sites. Having darkly pigmented skin lowers your risk of melanoma at these more common sites, but anyone can get melanoma on the palms of the hands, soles of the feet, or under the nails. Melanomas in these areas make up a much larger portion of melanomas in African Americans than in whites. Melanoma can also form in your eyes and, rarely, inside your body, such as in your nose, mouth, throat, genitals and anal area, but these are much less common than melanoma of the skin. While melanoma is much less common than some other types of skin cancer, it’s more dangerous because it’s much more likely to spread to other parts of the body than the other two skin cancer types (basal cell carcinoma, squamous cell carcinoma) if not caught and treated early.
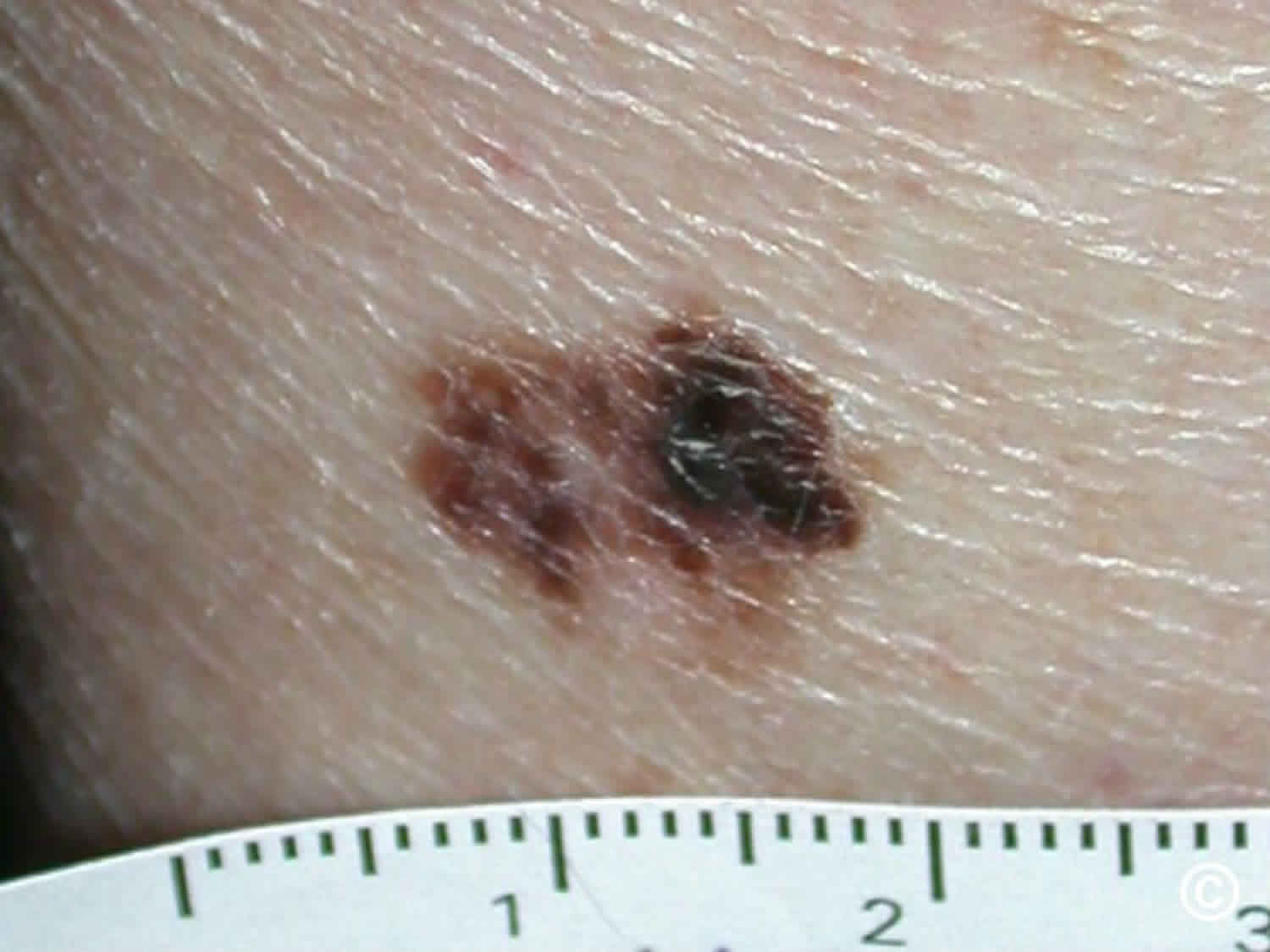
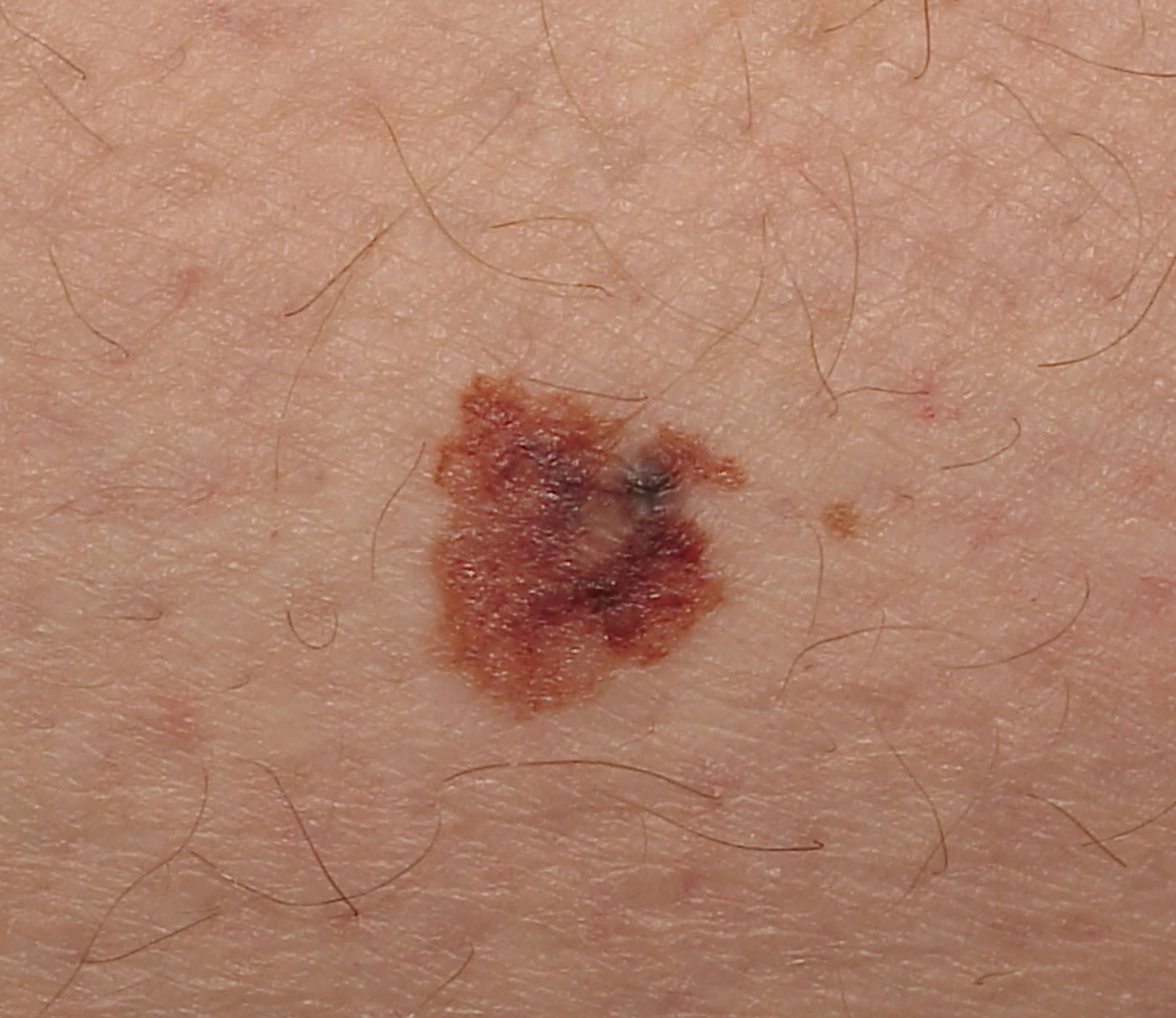
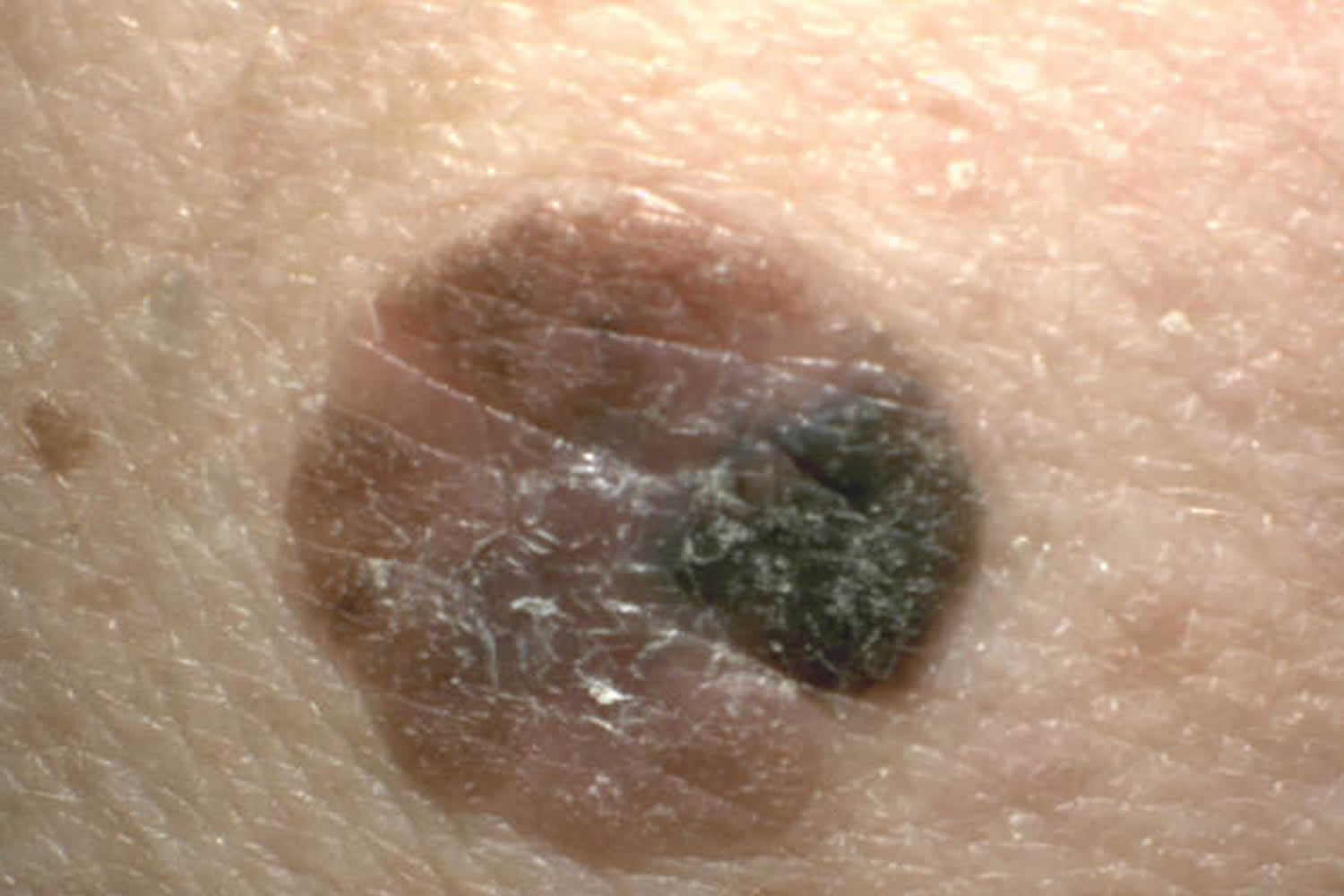
Melanoma may appear on the skin suddenly without warning but also can develop within an existing mole. The first sign of melanoma is often a mole that changes size, shape or color. Most melanoma cells still make melanin, so melanoma tumors are usually brown or black. But some melanomas do not make melanin and can appear pink, tan, or even white.
The ABCDE checklist can be used to check for the main warning signs of melanoma:
- A is for asymmetrical shape. Look for moles with irregular shapes, such as two very different-looking halves. Melanomas usually have 2 very different halves and are an irregular shape
- B is for irregular border. Look for moles with irregular, notched or scalloped borders — characteristics of melanomas. Melanoma often has an irregular appearance, however, if a symmetrical lesion continues to grow out of proportion to the patient’s other moles, especially if aged > 45, then melanoma must be considered
- C is for changes in color. Look for growths that have many colors (a mix of 2 or more colors) or an uneven distribution of color.
- D is for diameter. Look for new growth in a mole larger than 1/4 inch (about 6 millimeters).
- Melanoma grow at different rates – even if the lesion is not changing, if it looks suspicious or if you notice any skin changes that seem unusual make an appointment with your doctor.
- E is for evolving. Look for changes over time, such as a mole that grows in size or that changes color or shape. Moles may also evolve to develop new signs and symptoms, such as new itchiness or bleeding.
It is also important to note that cancerous (malignant) moles vary greatly in appearance. Some may show all of the changes listed above, while others may have only one or two unusual characteristics.
Melanoma accounts for only about 1% of skin cancers but causes a large majority of skin cancer deaths 1. The overall incidence of melanoma have been rising rapidly over the past few decades, but this has varied by age. The risk of melanoma increases as people age. The average age of people when melanoma skin cancer is diagnosed is 65. But melanoma is not uncommon even among those younger than 30 (especially young women). Melanoma is more common in men overall, but before age 50 the rates are higher in women than in men. Melanoma rates in the United States doubled from 1988 to 2019, and worldwide, the number of melanoma diagnoses are expected to increase by more than 50% by 2040 2.
The American Cancer Society’s estimates for melanoma in the United States for 2022 are 2:
- New cases: About 99,780 new melanomas will be diagnosed (about 57,180 in men and 42,600 in women).
- Deaths: About 7,650 people are expected to die of melanoma (about 5,080 men and 2,570 women).
- 5-Year Relative Survival: 93.7%. Relative survival is an estimate of the percentage of patients who would be expected to survive the effects of their cancer. It excludes the risk of dying from other causes. Because survival statistics are based on large groups of people, they cannot be used to predict exactly what will happen to an individual patient. No two patients are entirely alike, and treatment and responses to treatment can vary greatly.
- Percentage of All Cancer Deaths: 1.3%.
- Melanoma is more common in men than women and among individuals of fair complexion and those who have been exposed to natural or artificial sunlight (such as tanning beds) over long periods of time. There are more new cases among whites than any other racial/ethnic group.
- Rate of New Cases and Deaths per 100,000: The rate of new cases of melanoma of the skin was 21.5 per 100,000 men and women per year. For melanoma of the skin, death rates are higher among the middle-aged and elderly. The death rate was 2.2 per 100,000 men and women per year. These rates are age-adjusted and based on 2015–2019 cases and deaths.
- Lifetime Risk of Developing Cancer: Approximately 2.1 percent of men and women will be diagnosed with melanoma of the skin at some point during their lifetime, based on 2017–2019 data.
- In 2019, there were an estimated 1,361,282 people living with melanoma of the skin in the United States.
Melanoma of the skin represents 5.2% of all new cancer cases in the U.S. Melanoma is more than 20 times more common in whites than in African Americans. Overall, the lifetime risk of getting melanoma is about 2.6% (1 in 38) for whites, 0.1% (1 in 1,000) for Blacks, and 0.6% (1 in 167) for Hispanics 1. The risk for each person can be affected by a number of different factors.
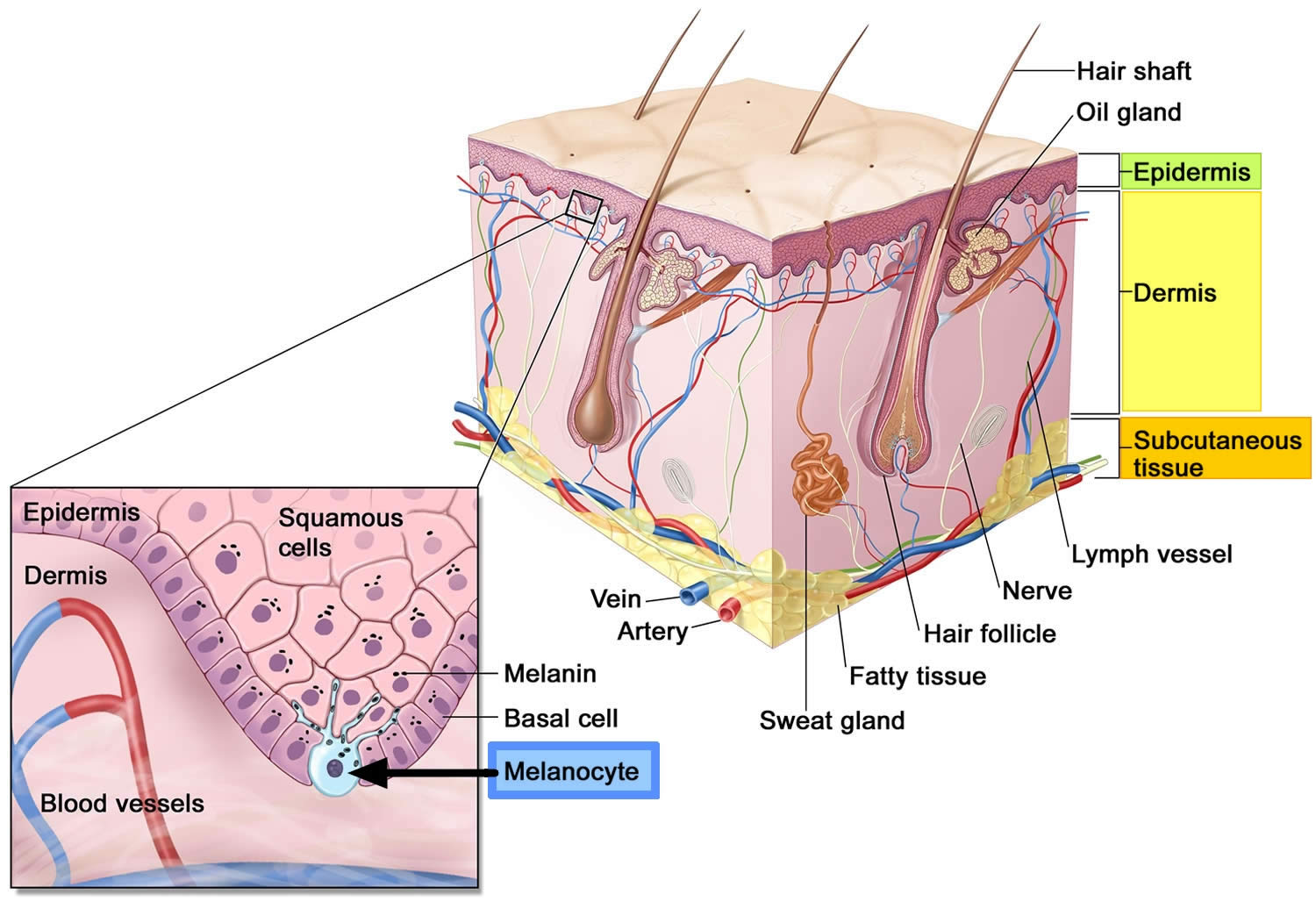
Normal melanocytes are found in the basal layer of the epidermis (the outer layer of skin) (see Figure 1). Melanocytes produce a protein called melanin, which protects skin cells by absorbing ultraviolet (UV) radiation. Melanocytes are found in equal numbers in black and white skin, but melanocytes in black skin produce much more melanin. People with dark brown or black skin are very much less likely to be damaged by UV radiation than those with white skin.
Non-cancerous growth of melanocytes results in moles (benign melanocytic nevi) and freckles (ephelides and lentigines). The cancerous growth of melanocytes results in melanoma. Melanoma is described as:
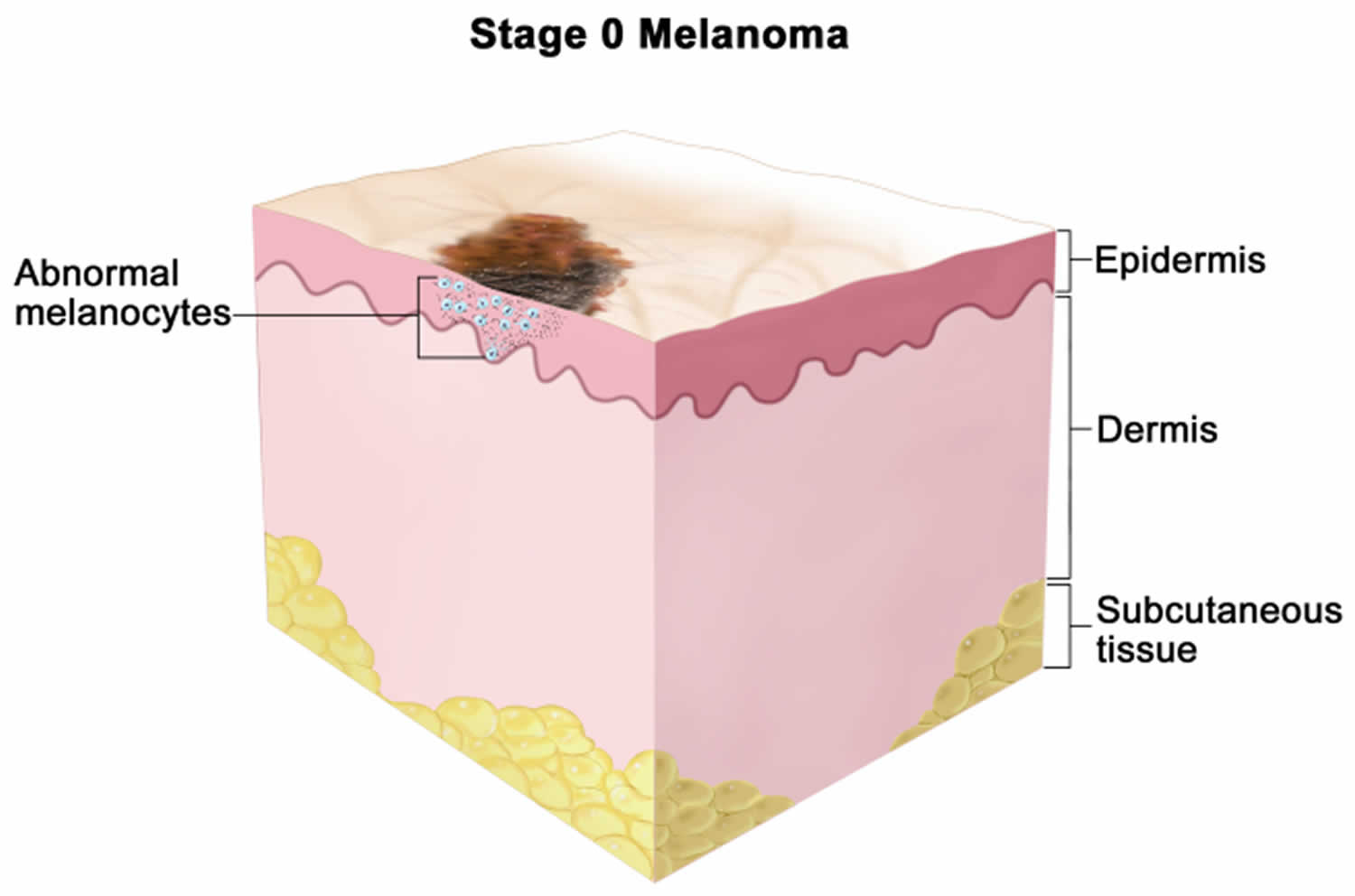
- In situ melanoma, if the skin cancer is confined to the epidermis
- Invasive melanoma, if the skin cancer has spread into the dermis
- Metastatic melanoma, if the skin cancer has spread to other tissues.
Most melanomas are brought to a doctor’s attention because of signs or symptoms a person is having. If you have an abnormal area on your skin that might be cancer, your doctor will examine it and might do tests to find out if it is melanoma, another type of skin cancer, or some other skin condition. If melanoma is found, other tests may be done to find out if it has spread to other areas of the body.
The best treatment for your melanoma depends on the size and stage of cancer, your overall health, and your personal preferences. Treatment for early-stage melanomas usually includes surgery to remove the melanoma. A very thin melanoma may be removed entirely during the biopsy and require no further treatment. Otherwise, your surgeon will remove the cancer as well as a border of normal skin and a layer of tissue beneath the skin. For people with early-stage melanomas, this may be the only treatment needed.
If melanoma has spread beyond the skin, treatment options may include:
- Surgery to remove affected lymph nodes. If melanoma has spread to nearby lymph nodes, your surgeon may remove the affected nodes. Additional treatments before or after surgery also may be recommended.
- Immunotherapy. Immunotherapy is a drug treatment that helps your immune system to fight cancer. Your body’s disease-fighting immune system might not attack cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process. Immunotherapy is often recommended after surgery for melanoma that has spread to the lymph nodes or to other areas of the body. When melanoma can’t be removed completely with surgery, immunotherapy treatments might be injected directly into the melanoma.
- Targeted therapy. Targeted drug treatments focus on specific weaknesses present within cancer cells. By targeting these weaknesses, targeted drug treatments can cause cancer cells to die. Cells from your melanoma may be tested to see if targeted therapy is likely to be effective against your cancer. For melanoma, targeted therapy might be recommended if the cancer has spread to your lymph nodes or to other areas of your body.
- Radiation therapy. This treatment uses high-powered energy beams, such as X-rays and protons, to kill cancer cells. Radiation therapy may be directed to the lymph nodes if the melanoma has spread there. Radiation therapy can also be used to treat melanomas that can’t be removed completely with surgery. For melanoma that spreads to other areas of the body, radiation therapy can help relieve symptoms.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. Chemotherapy can be given intravenously, in pill form or both so that it travels throughout your body. Chemotherapy can also be given in a vein in your arm or leg in a procedure called isolated limb perfusion. During this procedure, blood in your arm or leg isn’t allowed to travel to other areas of your body for a short time so that the chemotherapy drugs travel directly to the area around the melanoma and don’t affect other parts of your body.
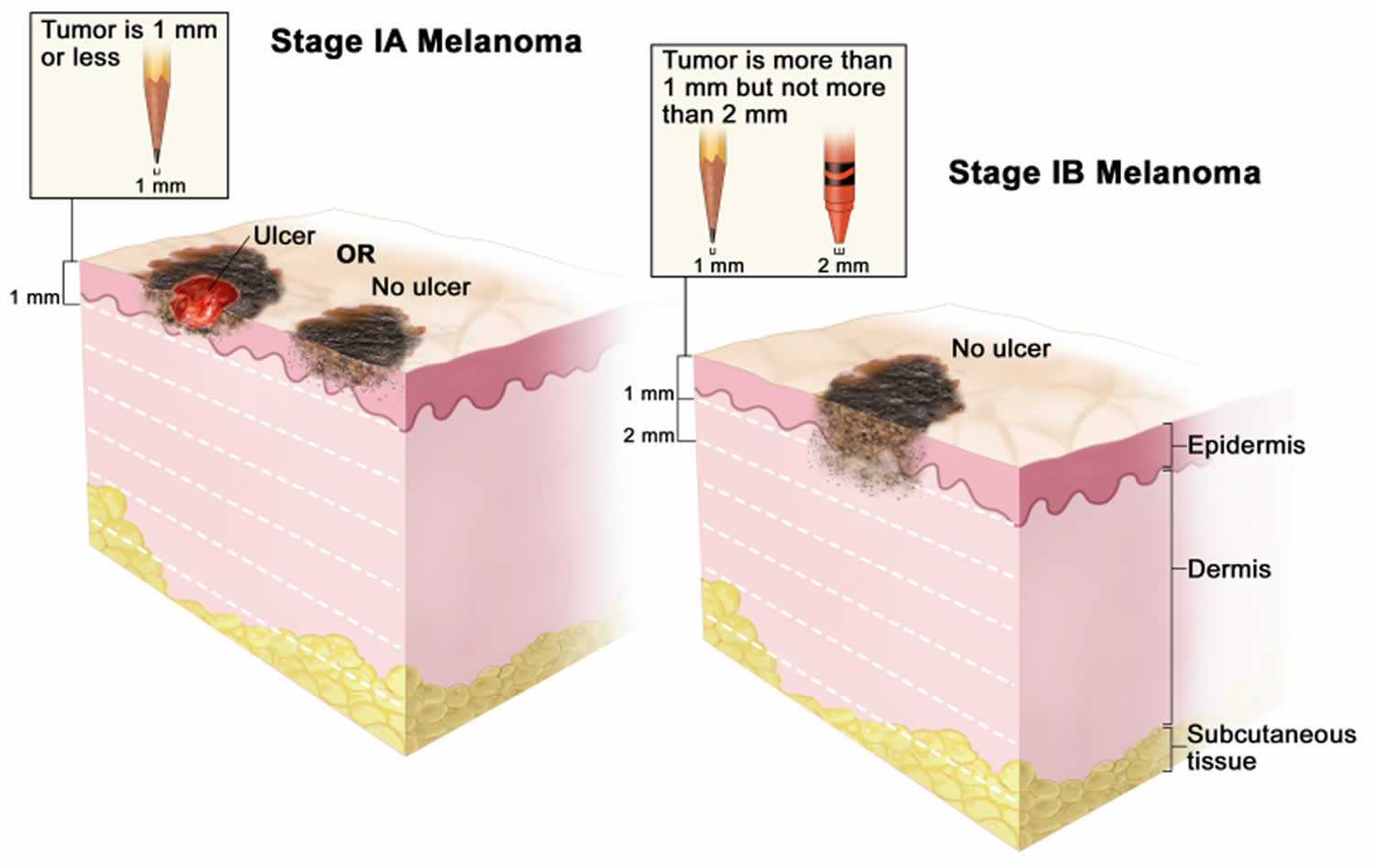
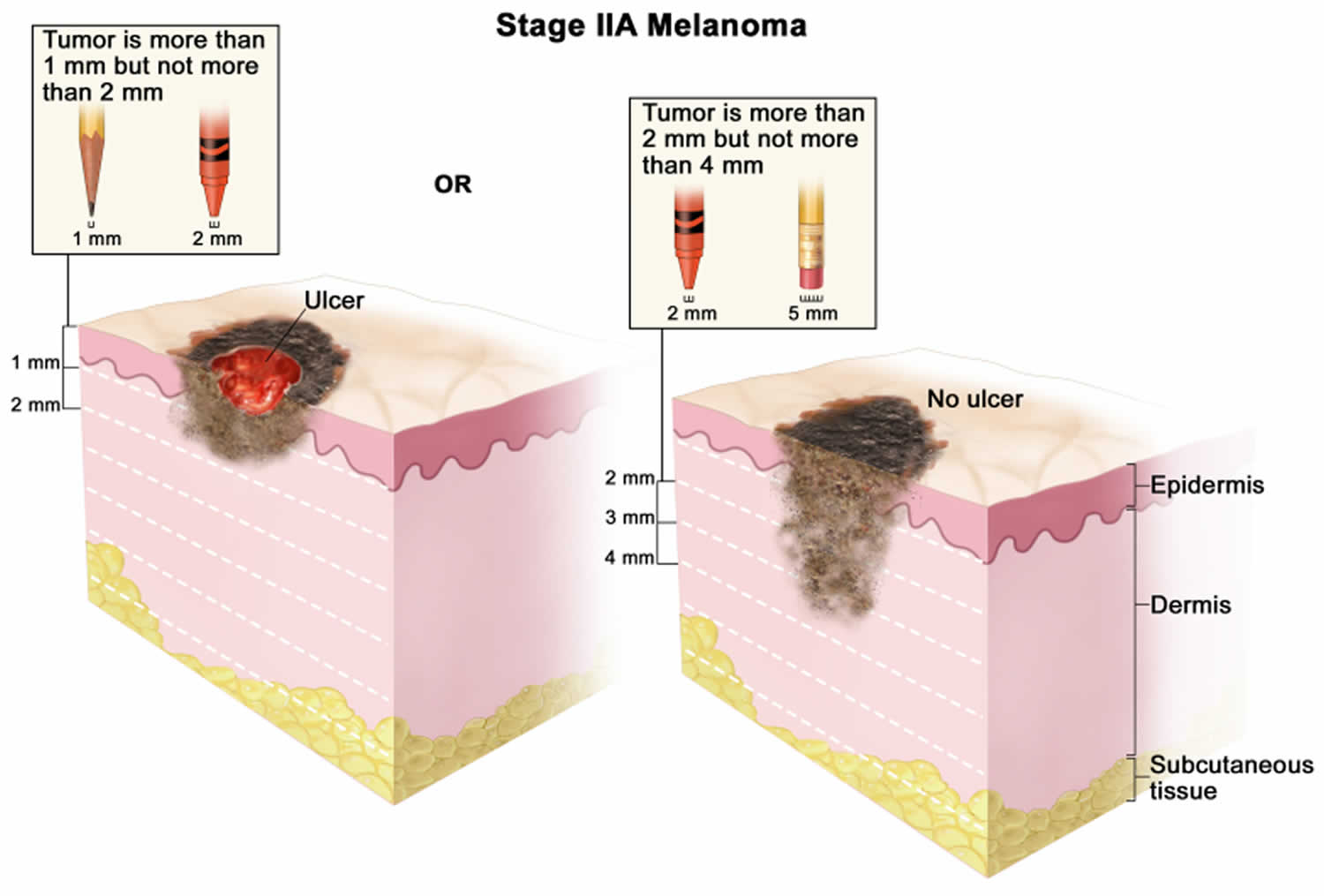
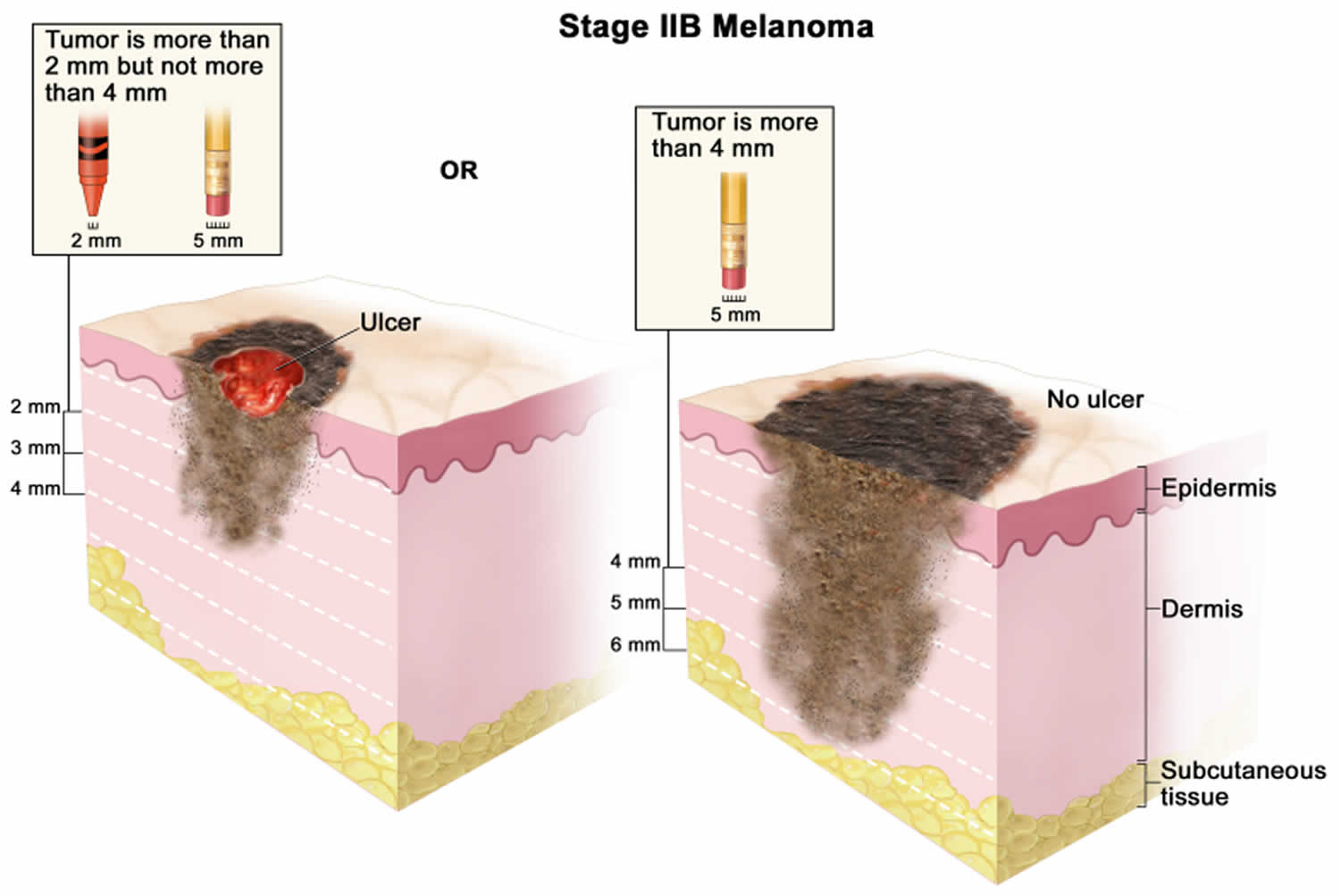
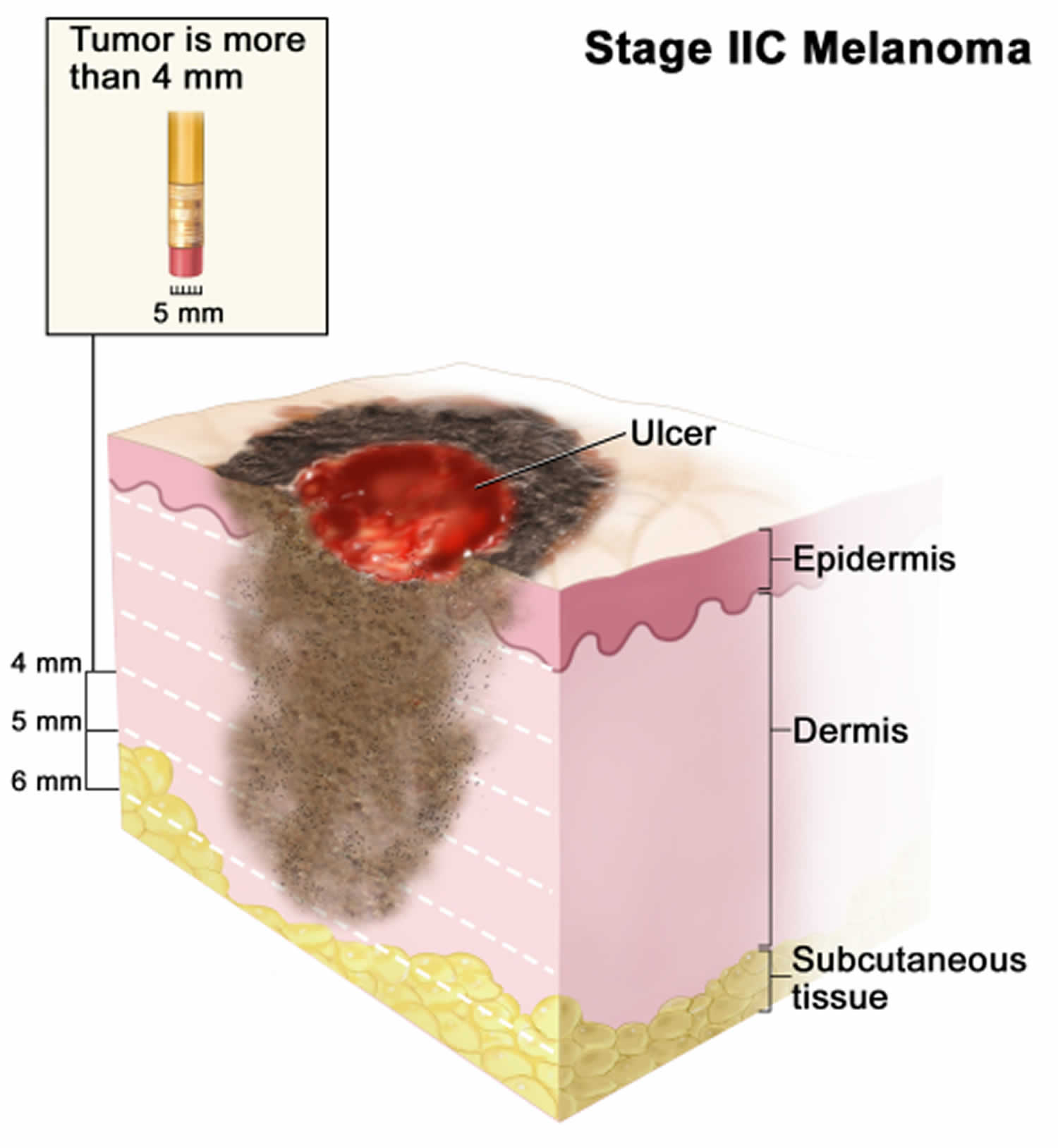
Figure 1. Anatomy of the skin
Footnote: Anatomy of the skin, showing the epidermis, dermis, and subcutaneous tissue. Melanocytes are in the layer of basal cells at the deepest part of the epidermis.
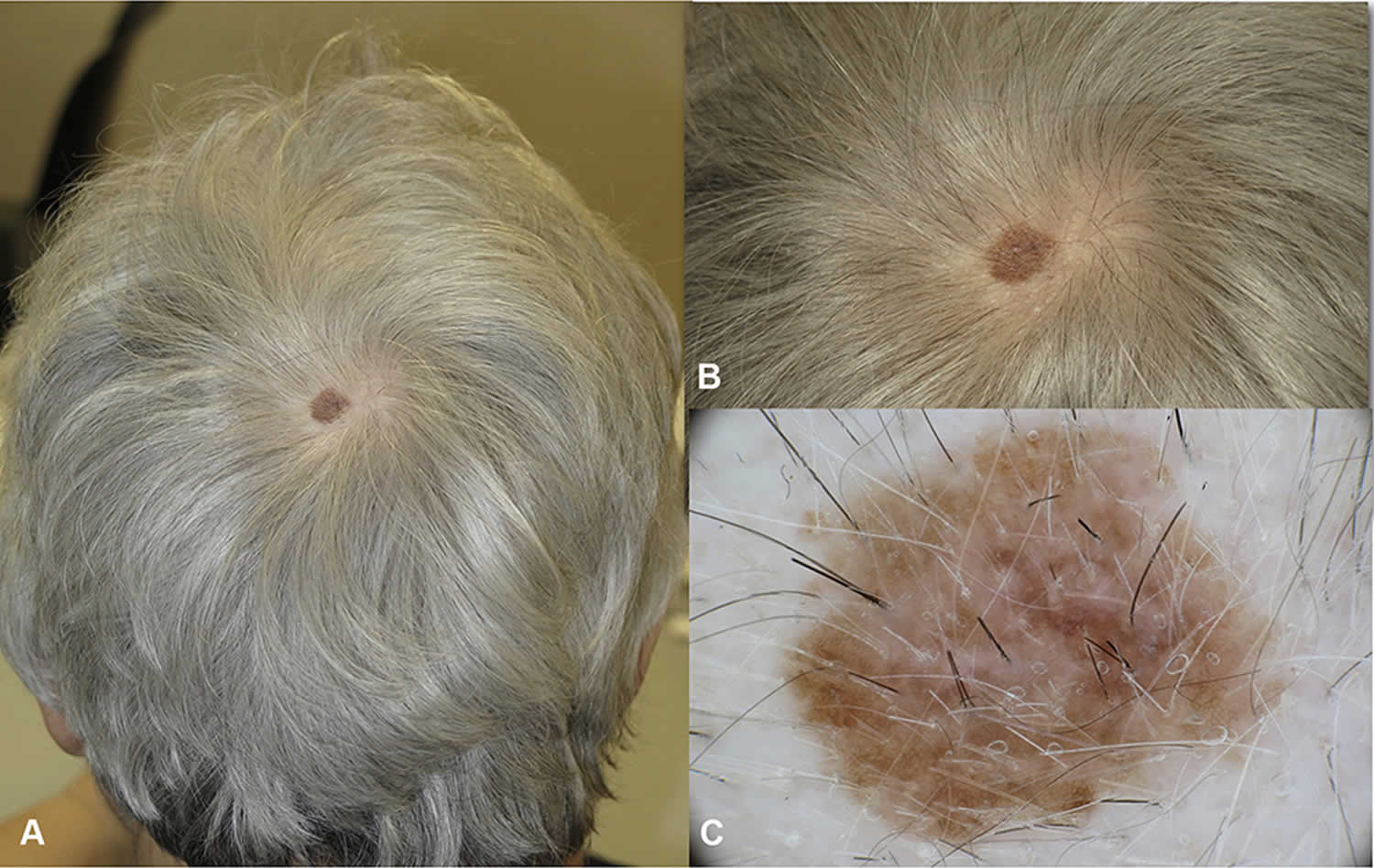
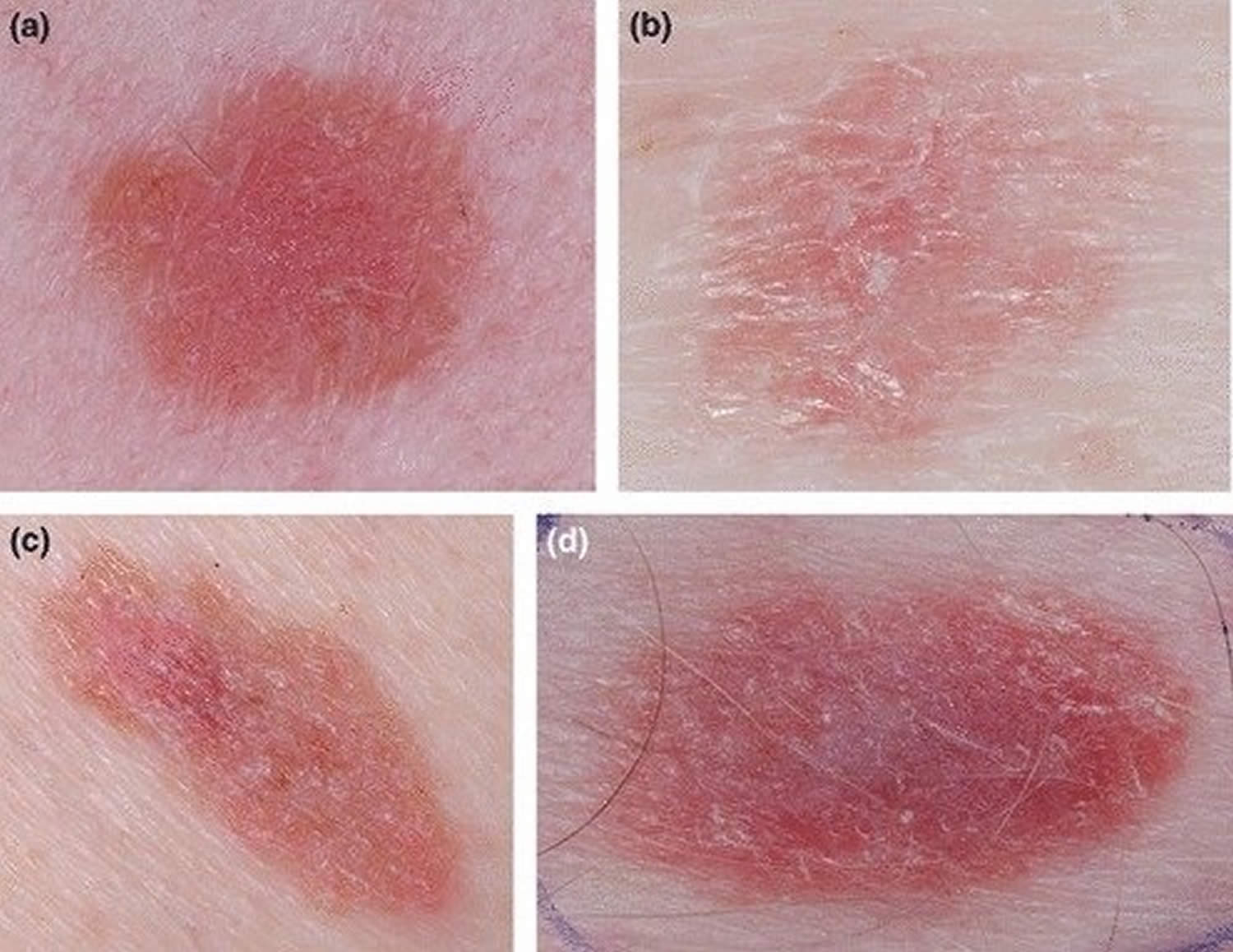
Figure 2. Melanoma on scalp
Footnote: In situ melanoma (or Stage 0 melanoma) of the vertex in a 68 year-old woman. (A) The lesion was arising in hair bearing scalp, the patient was not aware of the macule which was noticed by the hair dresser. (B) Close up clinical image, a flat brown macule 1 cm in diameter. (C and D) In dermoscopy atypical network and regression are detected. No non-prevalent benign pattern.
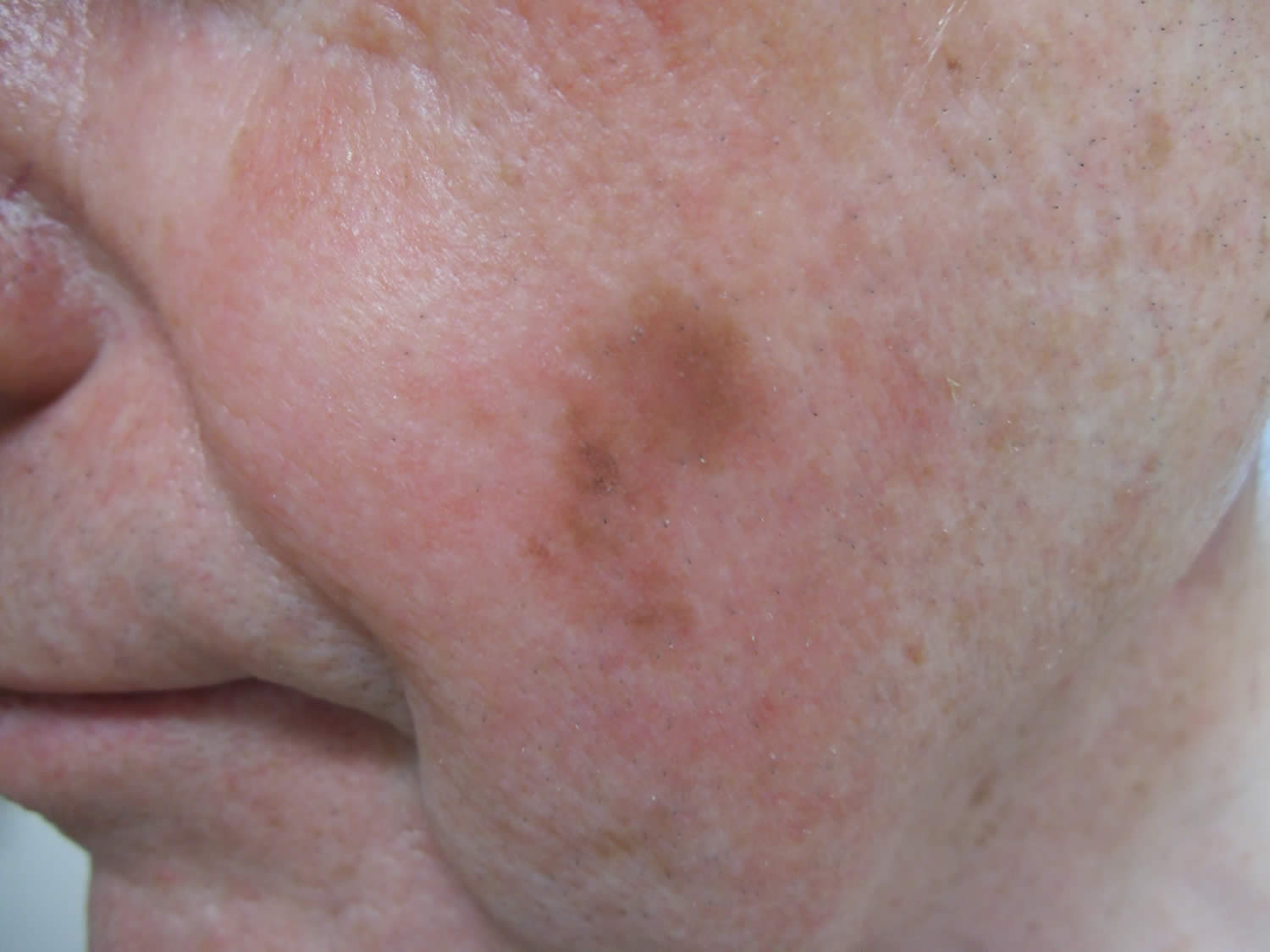
[Source 3 ]Figure 3. Melanoma on face
[Source 4 ]Skin cancer types
Skin cancer is a cancer that occurs when your skin cells grow abnormally and out of control, usually from too much exposure to ultraviolet (UV) radiation from the sun. This uncontrolled growth of abnormal cells forms a tumor in the skin. Tumors are either benign (non-cancerous), or malignant (cancerous tumors that spread through the body, causing damage).
Skin cancers are named according to the cells in which they form. There are 3 main types:
- Basal cell carcinoma (BCC) begins in the lower segment of cells of the epidermis (your outer layer of skin) called the basal cell layer. Basal cell carcinoma (BCC) tend to grow slowly, and rarely spread to other parts of the body.
- Squamous cell carcinoma (SCC) grows from the flat cells found in the top layer of your epidermis. Squamous cell carcinoma (SCC) can grow quickly on the skin over several weeks or months. Bowen’s disease is an early form of squamous cell carcinoma (SCC) that hasn’t grown beyond the top layer of skin.
- Melanoma grows from cells called melanocytes — cells that give your skin its color. Melanoma is the rarest type of skin cancer (accounting for 1 to 2% of cases) but is considered the most serious because it can spread quickly (metastasize) throughout the body.
Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are also called non-melanoma skin cancers. Basal cell carcinoma (BCC) represents more than 2 in 3 non-melanoma skin cancers, and around 1 in 3 are squamous cell carcinoma (SCC). There are other types of non-melanoma skin cancers, but they are rare.
Other types of non-melanoma skin cancers include:
- Merkel cell carcinoma
- Kaposi sarcoma
- Cutaneous (skin) lymphoma
- Skin adnexal tumors (tumors that start in hair follicles or skin glands)
- Various types of sarcomas
Together, these types account for less than 1% of all skin cancers.
Melanoma types
The American Joint Committee on Cancer (AJCC) identifies five different forms of extraocular melanoma 5:
- Lentigo maligna melanoma,
- Superficial spreading melanoma,
- Nodular melanoma,
- Acral-lentiginous melanoma,
- Mucosal lentiginous melanoma.
Eighty to 85% of melanomas are lentigo maligna melanoma, superficial spreading melanoma, or nodular melanoma. These different forms of melanoma represent distinct pathologic entities that have different clinical and biologic characteristics. The differential clinical features of these common types of melanoma are summarized in Table 1.
Table 1. Common types of melanoma
| Type of Melanoma | Common Locations | Median Age (years) | Sex Predilection | Duration | Identifying Features of Radial Growth Phase* |
|---|---|---|---|---|---|
| Lentigo maligna melanoma | Sun-exposed surfaces (head and neck most common) | 70 | None | 5 – 15 years | Flat. Shades of tan to black Frequent areas of hypopigmentation |
| Superficial spreading melanoma | All body surfaces | 56 | Males: head, neck, trunk Females: lower legs | 1 – 5 years | Flat to slightly raised Irregular margins. Shades of brown, black, pink. Areas of hypopigmentation |
| Nodular melanoma | All body surfaces | 49 | None overall Males: head, neck, trunk | 1 month – 2 years | None |
| Acral-lentiginous melanoma | Volar and subungual areas | 59 | Slight female predominance | 2 months – 10 years | Tan to dark-brown macule |
| Mucosal lentiginous melanoma | Oral, ocular, and genital mucosa | 56 | Slight male preponderance but varies from area to area | 4 – 20 years | Tan to dark-brown macular area |
Footnote: * The invasive tumors in each melanoma subtype are basically similar, varying from low convex to polypoid in shape and from dark blue-black to light tan or even (amelanotic) reddish pink in color. They are usually hairless and may be ulcerated.
[Source 5 ]Superficial spreading melanoma
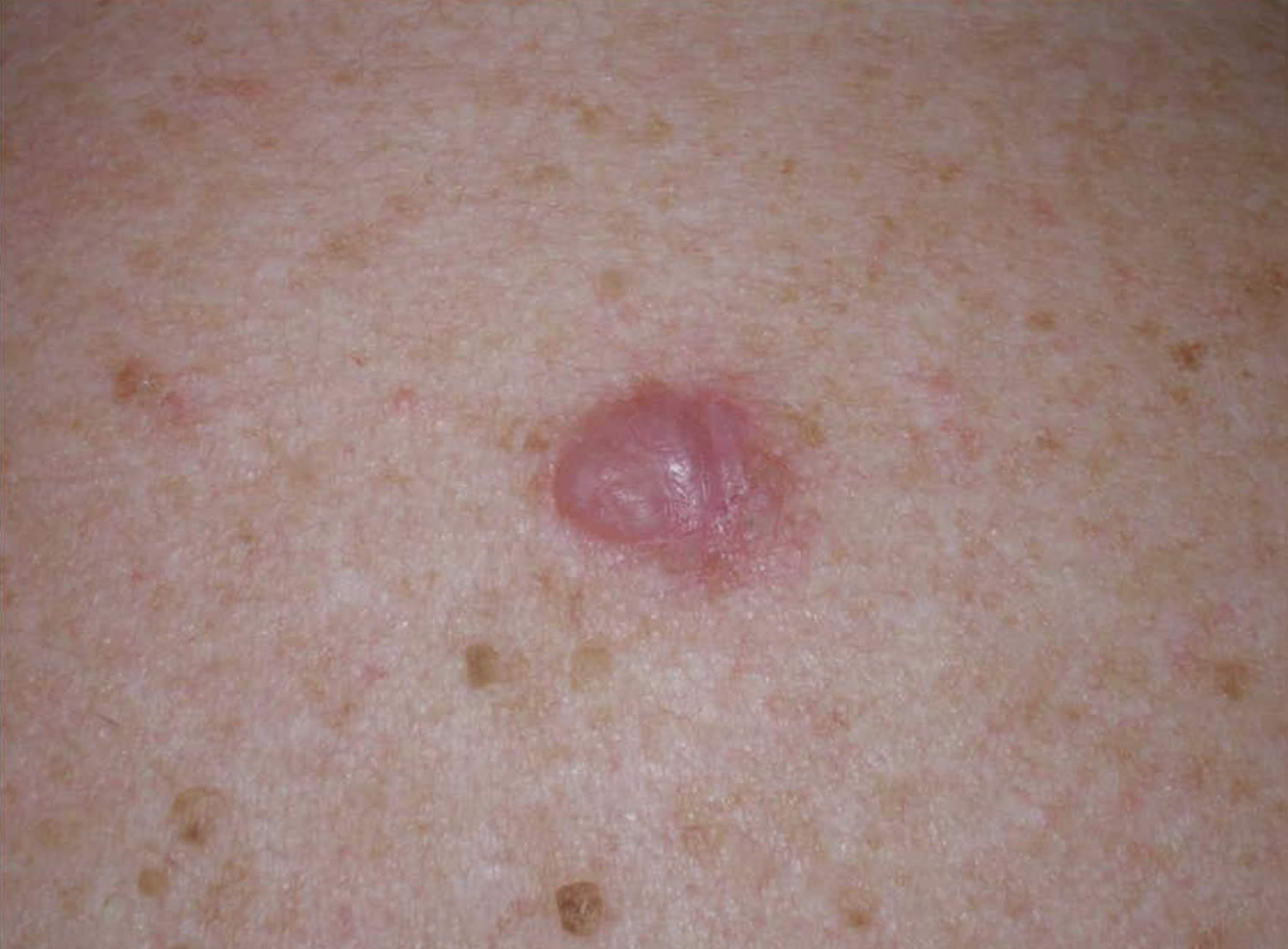
Superficial spreading melanoma is the most common type of melanoma, accounting for approximately 70% of all melanomas 5. Superficial spreading melanoma is a form of melanoma in which the malignant cells tend to stay within the epidermis (‘in situ’ phase) for a prolonged period (months to decades) 6. At first, superficial spreading melanoma grows horizontally in the skin – this is known as the radial growth phase, presenting as a slowly-enlarging flat area of discolored skin.
Superficial spreading melanoma generally arises in a preexisting lesion and is the lesion most commonly associated with pigmented dysplastic nevus syndrome. Superficial spreading melanoma may have a relatively long natural history. Typically, diagnosis follows an increased rate of change in a precursor lesion that had exhibited minor change over several years. Early in their evolution, superficial spreading melanomas usually appear flat, with irregular borders. Notching of the border is particularly characteristic. The lesions are usually multicolored with shades of tan, brown, black, red, and white. Amelanotic areas often represent areas of regression. As the lesion grows, it may develop an irregular surface. superficial spreading melanomas tend to occur throughout adulthood, with a peak incidence in the fifth decade of life. Most commonly, they occur on the head, neck, and trunk in males and on the extremities in females.
An unknown proportion of superficial spreading melanoma become invasive, that is, the melanoma cells cross the basement membrane between the epidermis and dermis and malignant melanocytes enter the dermis 6. A rapidly-growing nodular melanoma can arise within superficial spreading melanoma and proliferate deeply within the skin.
The initial treatment of a superficial spreading melanoma is excision; the lesion should be completely excised with a 2 mm margin of normal tissue. Further treatment depends mainly on the Breslow thickness of the lesion. Wide local excision may necessitate flap or graft closure of the wound. Occasionally, the pathologist will report incomplete excision of the melanoma, despite wide clinical margins. This means further surgery or radiotherapy will be recommended to ensure the tumour has been completely removed.
Figure 4. Superficial spreading melanoma
Nodular melanoma
Nodular melanomas are a faster-developing type of melanoma that can quickly grow downwards into the deeper layers of skin (vertical growth) if not removed. Nodular melanoma can penetrate deep within the skin within a few months of its first appearance. Nodular melanoma is the second most common growth pattern, comprising 10 to 15% of all cutaneous melanomas. Nodular melanomas usually appear as a changing lump (nodule) on the skin that might be black to red in color. Nodular melanomas often grow on previously normal skin and most commonly grow on the head and neck, chest or back.
Nodular melanoma may develop on any body surface area but most commonly is diagnosed on the trunk of men. Bleeding or oozing is a common symptom.
The main risk factors for nodular melanoma are:
- Increasing age
- Previous invasive melanoma or melanoma in situ
- Many melanocytic nevi (moles)
- Multiple (>5) atypical nevi (abnormal-looking moles)
- Fair skin that burns easily
It is less strongly associated with sun exposure than superficial spreading and lentigo maligna types of melanoma.
Nodular melanoma is thought to be biologically more aggressive than superficial spreading melanoma. Clinically, the lesion is dark and most often uniform in color. Histologically, nodular melanoma is notable for the complete absence of melanocytic abnormalities in the adjacent epidermis. Approximately 5% of nodular melanoma are amelanotic. Amelanotic nodular melanoma may have a symmetric appearance but occasionally becomes polypoid or cauliflower in appearance. nodular melanoma does not have a radial growth phase and is associated with rapid evolution to vertical growth and invasion of the dermis. For this reason, nodular melanomas tend to be thicker, more high-risk lesions.
The initial treatment of primary melanoma is to cut it out; the lesion should be completely excised with a 2-3 cm margin of normal tissue. Further treatment depends mainly on the Breslow thickness of the lesion.
After initial excision biopsy; the radial excision margins, measured clinically from the edge of the melanoma. This may necessitate a flap or graft to close the wound. Occasionally, the pathologist will report incomplete excision of the melanoma, despite wide margins. This means further surgery or radiotherapy will be recommended to ensure the tumor has been completely removed.
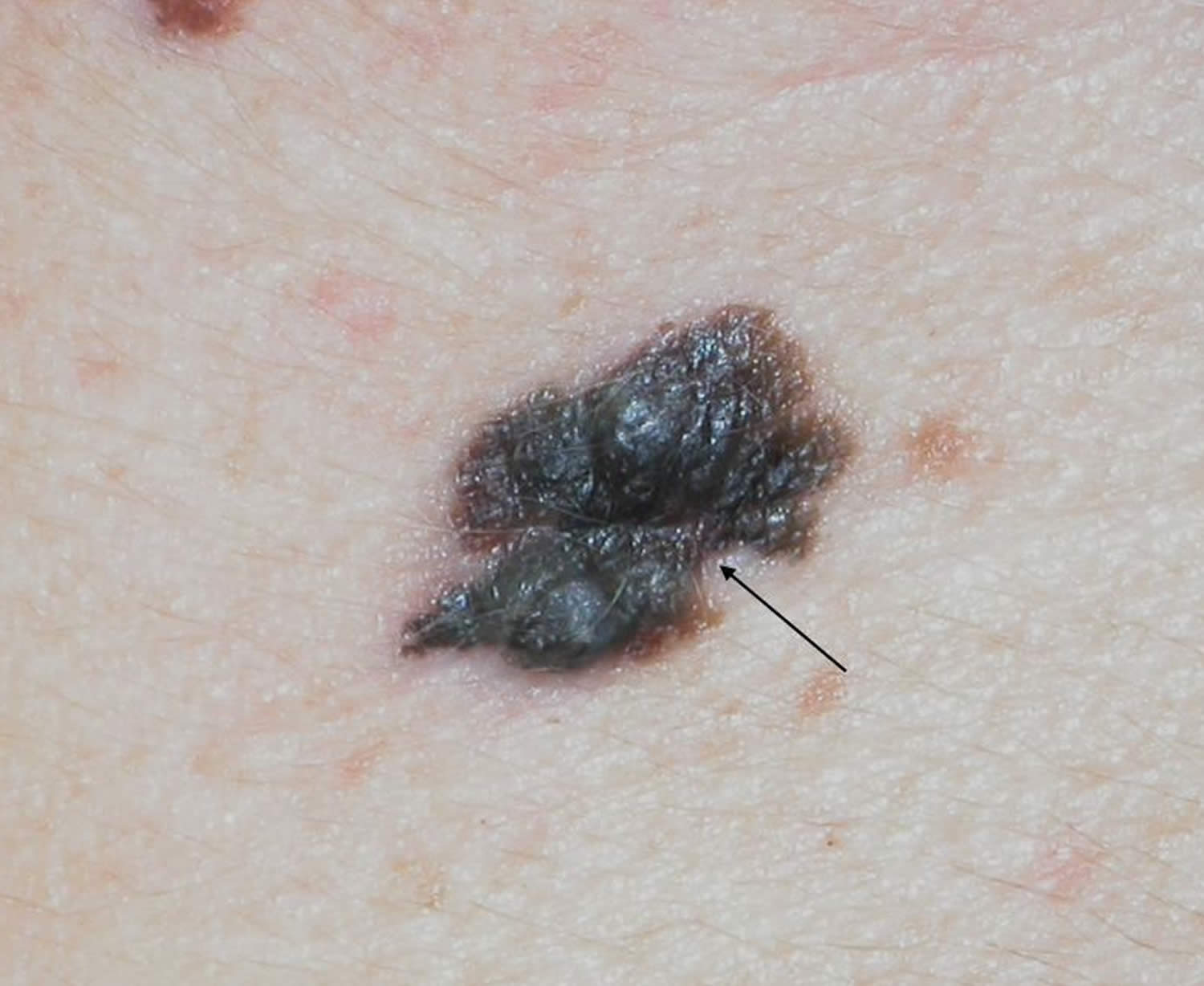
Figure 5. Nodular melanoma
Footnote: A dense, black, irregular lesion with multiple nodular areas and an irregular notched border (arrow)
[Source 7 ]Lentigo maligna melanoma
Lentigo maligna melanomas most commonly affect older people, particularly those who have spent a lot of time outdoors. Lentigo maligna melanoma constitutes approximately 10% of all melanomas, arises from lentigo maligna (melanotic freckle of Hutchinson or precancerous melanosis of Dubreuilh) 5. Lentigo maligna melanoma is found most commonly on sun-exposed skin such as the face in elderly individuals (median age, 70 years). Clinically, the lesions are generally large (3 to 4 cm in diameter) and flat with irregular borders, in variable shades of tan to dark brown. Hypopigmented areas in the lesion represent areas of regression. The precursor lesion, lentigo maligna, usually has been present for long periods (5 to 15 years) prior to the development of invasive melanoma.
To start with, lentigo maligna melanomas are flat and develop sideways in the surface layers of skin. Lentigo maligna melanomas look like a freckle, but they’re usually larger, darker and stand out more than a normal freckle. Lentigo maligna melanomas can gradually get bigger and may change shape. At a later stage, they may grow downwards into the deeper layers of skin and can form lumps (nodules).
Lentigo maligna melanoma should be completely removed surgically. If possible, there should be a 1 cm margin of normal skin around the tumor, but the margin may depend on the site of the lesion and how close it is to important structures like the mouth, eye or nose. If the local lymph nodes are enlarged due to melanoma, they should also be completely removed, which entails a major surgical procedure under general anaesthetic.
Figure 6. Lentigo maligna melanoma
Acral lentiginous melanoma
Acral lentiginous melanoma is a type of malignant melanoma originating on palms, soles of foot and under the nail (subungual melanoma). Acral lentiginous melanoma is a form of melanoma characterized by its site of origin: palm, sole, or beneath the nail (subungual melanoma). Acral lentiginous melanoma represents approximately 3 to 8% of all melanomas. Acral lentiginous melanoma does not represent the most common type of melanoma in any racial group in United States-based studies 8. Acral lentiginous melanoma is more common on feet than on hands. It can arise de novo in normal-appearing skin, or it can develop within an existing melanocytic nevus (mole). Acral lentiginous melanoma constitutes a substantially higher proportion of melanomas in dark-skinned individuals such as 70% of African Americans, 46% of Asians, and Hispanics. The majority of acral lentiginous melanoma lesions are tan to dark brown macule that are large (3 cm in diameter) with an irregular borders, but in advanced lesions it may be ulcerating or may present as a fungating mass. The majority arise in people over the age of 40 (median age, 59 years). Acral lentiginous melanoma is equally common in males and females. Subungual melanoma (melanoma beneath the nail) most commonly occurs on the great toe or thumb.
Although similar in clinical appearance to lentigo maligna melanoma, acral lentiginous melanoma is a biologically much more aggressive lesion, with a relatively short evolution to the vertical growth phase.
The cause or causes of acral lentiginous melanoma are unknown. It is not related to sun exposure.
Acral lentiginous melanoma starts as a slowly-enlarging flat patch of discolored skin. At first, the malignant cells remain within the tissue of origin, the epidermis — the in situ phase of melanoma, which can persist for months or years.
Acral lentiginous melanoma becomes invasive when the melanoma cells cross the basement membrane of the epidermis, and malignant cells enter the dermis. A rapidly-growing nodular melanoma can also arise within acral lentiginous melanoma and proliferate more deeply within the skin.
The initial treatment of primary melanoma is to cut it out; the lesion should be completely excised with a 2–3 mm margin of healthy tissue. Further treatment depends mainly on the Breslow thickness of the lesion. A flap or graft may be needed to close the wound. In the case of acral lentiginous and subungual melanoma, this may include partial amputation of a digit. Occasionally, the pathologist will report incomplete excision of the melanoma, despite wide margins requiring further surgery or radiotherapy to ensure the tumor has been completely removed.
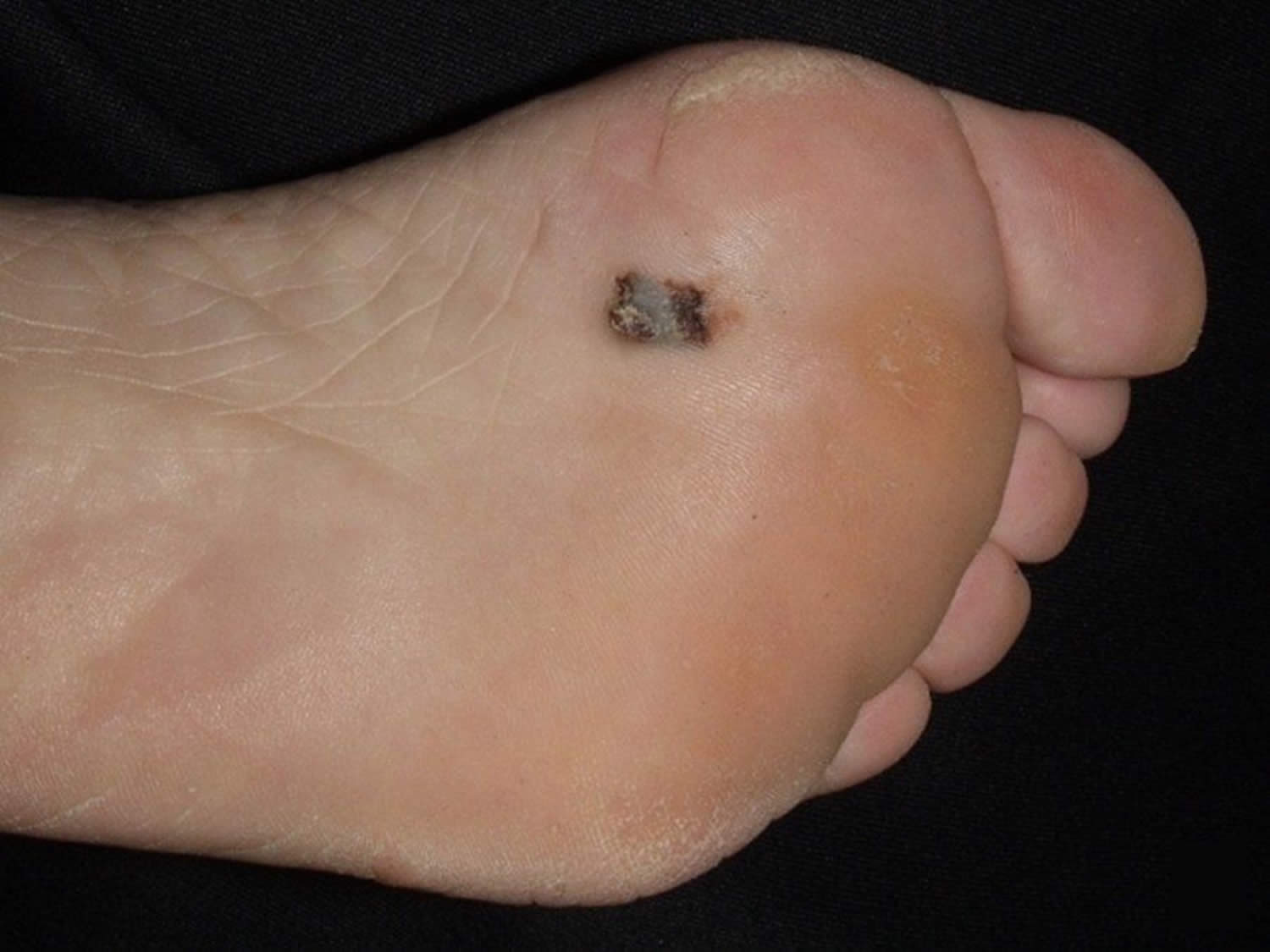
Figure 7. Acral lentiginous melanoma
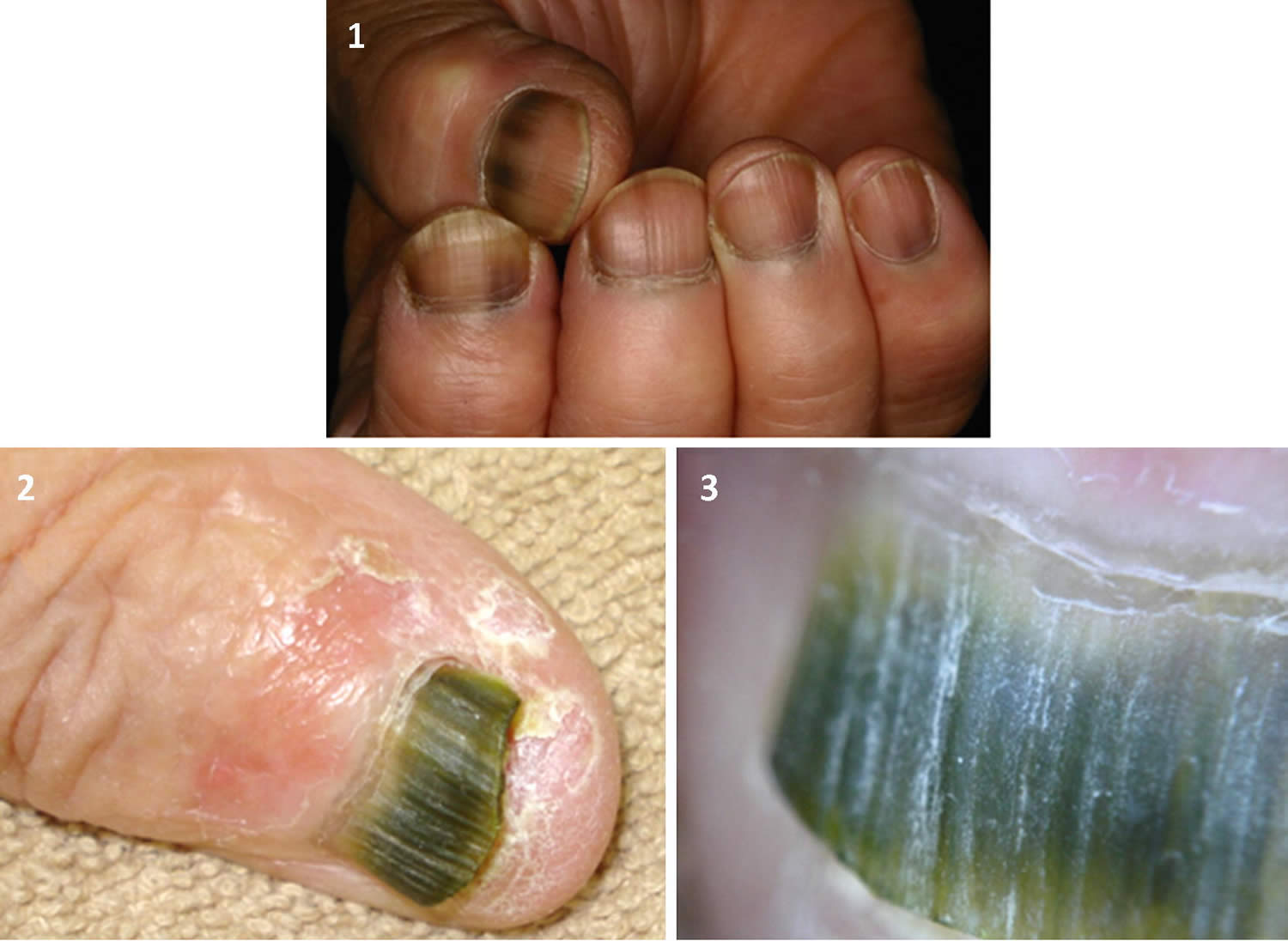
Subungual melanoma
Subungual melanoma is a melanoma of the nail matrix (malignant proliferation of melanocytes in the nail matrix). Subungual melanoma is usually a variant of acral lentiginous melanoma. Typically, subungual melanoma presents clinically as a pigmented streak in the nail plate, which slowly expands at the proximal border and may extend to involve the adjacent nail fold (Hutchinson sign). Subungual melanoma is most often diagnosed in the 60 to 70-year-old age group.
Melanoma of the nail unit is rare. While occurring equally in all racial groups, it accounts for around 0.7–3.5% of malignant melanomas in white-skinned populations and up to 75% of dark-skinned and Asian populations.
Subungual melanoma is the most common type of melanoma diagnosed in deeply pigmented individuals, probably due to this population’s low incidence of cutaneous melanoma, due to the melanin pigment protection from ultraviolet (UV) radiation.
In contrast to cutaneous melanoma, melanoma of the nail does not appear to be related to sun exposure. Melanoma of the nail unit originates from activation and proliferation of melanin producing melanocytes of the nail matrix.
Injury or trauma may be a factor, accounting for the greater incidence in the big toe and thumb (75–90% of cases).
The management plan for melanoma of the nail unit will usually require a multidisciplinary melanoma team to direct further investigations and treatment. The mainstay of management of melanoma of any kind is excision.
Figure 8. Subungual melanoma
[Source 9 ]Amelanotic melanoma
Amelanotic melanomas is a form of melanoma in which the malignant cells have little or no color, but may occasionally be pink or red, or have light brown or grey edges. The term ‘amelanotic’ is often used to indicate lesions that are only partially devoid of pigment while truly amelanotic melanoma where lesions lack all pigment is rare 10.
Amelanotic melanoma accounts for approximately 1–8% of all melanomas. The incidence of truly amelanotic melanoma is difficult to estimate, given that many hypopigmented lesions are labelled as amelanotic 10.
Risk factors for developing amelanotic melanoma include 11:
- Increasing age — although amelanotic melanoma accounts for a significant proportion of melanoma in young children 12
- Sun-exposed skin — particularly in older people with chronic photodamage.
The melanoma cells in amelanotic melanoma cannot produce mature melanin granules, which results in lesions that lack pigment. To account for the lack of pigment, three models have been proposed 11:
- Amelanotic melanoma may be a poorly differentiated subtype of typical melanoma.
- Amelanotic melanoma may be a de-differentiated melanoma that has lost its normal phenotype.
- Amelanotic melanoma cells may retain their melanocytic identity but gain the ability to form different phenotypes (multipotency) 13.
Amelanotic melanoma is treated in the same way that a pigmented melanoma is treated.
The prognosis of amelanotic melanoma is similar to that of pigmented melanomas 11. Prognostic factors include the Breslow thickness of the melanoma at the time of excision (this is considered to be the most important factor), the location of the lesion, patient age, and sex. Importantly, because of their atypical clinical features, amelanotic melanomas may have a delay in their diagnosis and, consequently, are often more advanced than pigmented melanomas when diagnosed 14.
The risk of metastasis is directly related to the Breslow thickness, with thicker melanomas being more likely to metastasise. The 2008 Clinical practice guidelines for the management of melanoma in Australia and New Zealand report that metastases are rare for thin melanomas (< 0.75 mm), with the risk increasing to 5% for melanomas 0.75–1.00 mm thick. Melanomas thicker than 4.0 mm have a significantly higher risk of metastasis of 40% 15.
Figure 9. Amelanotic melanoma
Footnotes: Clinical characteristics of amelanotic melanomas that are not of the nodular subtype. (a, b) Scaly, erythematous macules and patches, with a relatively circular to oval (c, d) symmetric shape, regular border and disruption of skin markings.
[Source 16 ]Mucosal melanoma
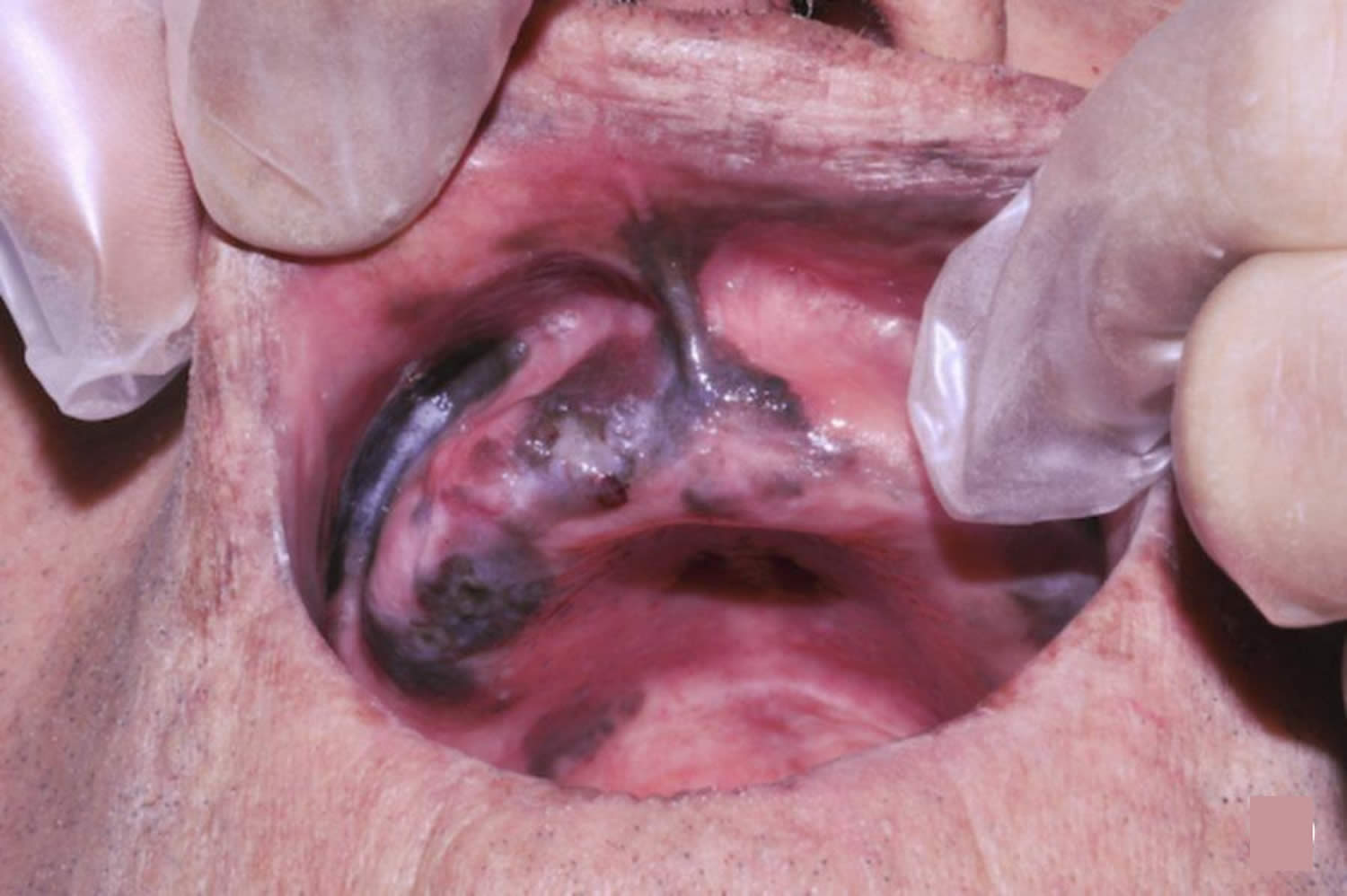
Melanoma usually develops in the skin and is called cutaneous melanoma (cutaneous means skin). But rarely, melanoma can start in the mucous membrane – this is the layer of tissue that covers the inside surface of parts of the body such as the mouth or vagina. Mucosal melanoma is a rare type of melanoma that occurs on mucosal surfaces. Mucous membranes are moist surfaces that line cavities within the body. This means that mucosal melanoma can be found in the respiratory tract, gastrointestinal tract or genitourinary tract. Mucosal melanomas have a poor prognosis, as most patients develop metastases despite aggressive therapy 17.
Mucosal melanomas are most often found in the head and neck, in the eyes, mouth, nasopharynx and larynx, but they can also arise throughout the gastrointestinal tract, anus and vagina. Mucosal melanoma makes up approximately 1.1% of all melanomas, but are usually more complicated because of late diagnosis due to their less visible locations and because they are often amelanotic – meaning they are not pigmented.
The peak age of diagnosis of mucosal melanoma is between 70 and 79. However, younger people have also been known to develop mucosal melanoma, especially of the oral cavity.
Mucosal melanoma of the genital tract is more common in females.
Subtypes of mucosal melanoma are based on the tissue in which they arise.
Possible places where mucosal melanoma can start include the:
- Respiratory tract
- Nasal cavity
- Paranasal sinuses
- Oral cavity
- Gastrointestinal tract
- Transitional zone of anal canal (the line where the normal skin meets the mucous membrane)
- Genitourinary tract
- Vulva
- Vagina
The signs and symptoms of mucosal melanoma largely depend on its location. Therefore, there are a wide variety of symptoms that patients may experience.
While there are many suggested risk factors for mucosal melanoma, there is only weak evidence for all, and none that are widely accepted. About 25% of mucosal melanomas have been linked with problems with a gene called KIT 17. Genes are the templates used for making protein, and mutations in the KIT gene cause the production of a mutant protein. The KIT gene can also be over-expressed, which means that there is more of the KIT protein being made than usual. Both the mutation and over-expression of the KIT gene have been associated with mucosal melanoma.
Possible risk factors include:
- Oral mucosal melanoma
- Smoking
- Ill-fitting dentures
- Ingested/inhaled environmental carcinogens
- Vulvar melanoma
- Chronic inflammatory disease
- Viral infections
- Chemical irritants
- Genetic factors
- Anorectal melanoma
- Human immunodeficiency virus (HIV)
The best form of treatment for mucosal melanoma is wide local excision of the lesion. This may not always be possible, as the melanoma may be located on an important anatomical structure, or be too large to excise safely.
Due to the likelihood of the same melanoma recurring, surgical resection is often combined with radiotherapy. Radiotherapy is also a consideration for patients who are not suitable for surgery.
Figure 10. Mucosal melanoma of the buccal mucosa
Ocular melanoma
Ocular melanoma also called eye melanoma, uveal melanoma or choroid melanoma 18. Uveal melanoma starts in the uvea. The uvea is the middle layer of the eye and has 3 parts:
- Iris (the colored part)
- Ciliary body
- Choroid
Most uveal melanomas develop in the choroid part of the uvea (choroid melanoma).
Ocular melanoma accounts for about 3.7% of all melanomas 19. Primary ocular melanoma is the most common primary malignant tumor of the eye in adults 20. In the US incidence of ocular melanoma is 6 per million, compared with 153.5 for cutaneous melanoma 19. Incidences of uveal and conjunctival melanomas in the US are 4.9 and 0.4 per million, respectively 19. It is more common among men, with incidence of 6.8 per million, compared with 5.3 per million in women (male to female rate ratio 1.29) 19. In Australia ocular melanoma shows higher rates among men older than 65 years and among residents of rural areas, with incidence of 8 per million in men, and 6.1 per million in women 21.
Ocular melanoma rates are 8-10 times higher among whites compared with blacks, but although obvious this difference is less pronounced compared to cutaneous melanoma which shows 16 times higher rates among whites 19. In contrast to other ocular melanomas, conjunctival melanoma rates are 2.6 times higher in whites than in blacks, which is similar with that of mucosal melanomas 22.
Incidence of ocular melanoma is increasing with age, with a peak in seventh and eighth decade of life 19. In contrast to uveal melanoma which incidence has remained stable over last three decades 23, conjunctival melanoma has shown an increase in incidence, especially among white men and older than 60 years 24.
In its early stages, ocular melanoma may not cause any symptoms. Because most melanomas develop in the part of the eye you cannot see, you may not know that you have a melanoma.
When ocular melanoma symptoms do occur, they can include:
- a dark spot on the iris or conjunctiva
- blurred or distorted vision or a blind spot in your side vision
- the sensation of flashing lights
- a change in the shape of the pupil
The main indicators of ocular melanoma in 90 patients described in a Canadian study were 25:
- partial loss of visual field (in 33% of patients)
- sun sensitivity (in 20%)
- blurred vision (in 20%)
- incidental finding on eye examination (in 17%)
Conjunctival melanoma presents as an increasingly irregular pigmented lesion on the external eye. Other symptoms may include a protruding eye, change in colour of the iris, red or painful eye, and retinal detachment.
It is not clear why eye melanomas develop. Scientists do know that people born with certain growths in or on the eye (nevi or moles), as well as those with lighter colored eyes, are at a greater risk for developing ocular melanoma.
Ocular melanoma occurs when the DNA of the pigment cells of the eye develop errors. These errors cause the cells to multiply out of control. The mutated cells collect in or on the eye and form a melanoma.
Certain factors increase your risk for developing melanoma. These include:
- exposure to natural sunlight or artificial sunlight (such as from tanning beds) over long periods of time may cause a melanoma on the surface of the eye (conjunctival melanoma)
- having light-colored eyes (blue or green eyes)
- older age
- Caucasian descent
- having certain inherited skin conditions, such as dysplastic nevus syndrome, which cause abnormal moles
- having abnormal skin pigmentation involving the eyelids and increased pigmentation on the uvea; and
- having a mole in the eye or on the eye’s surface
Because ocular melanoma may not cause any symptoms at first, the disease is often detected during a routine eye exam.
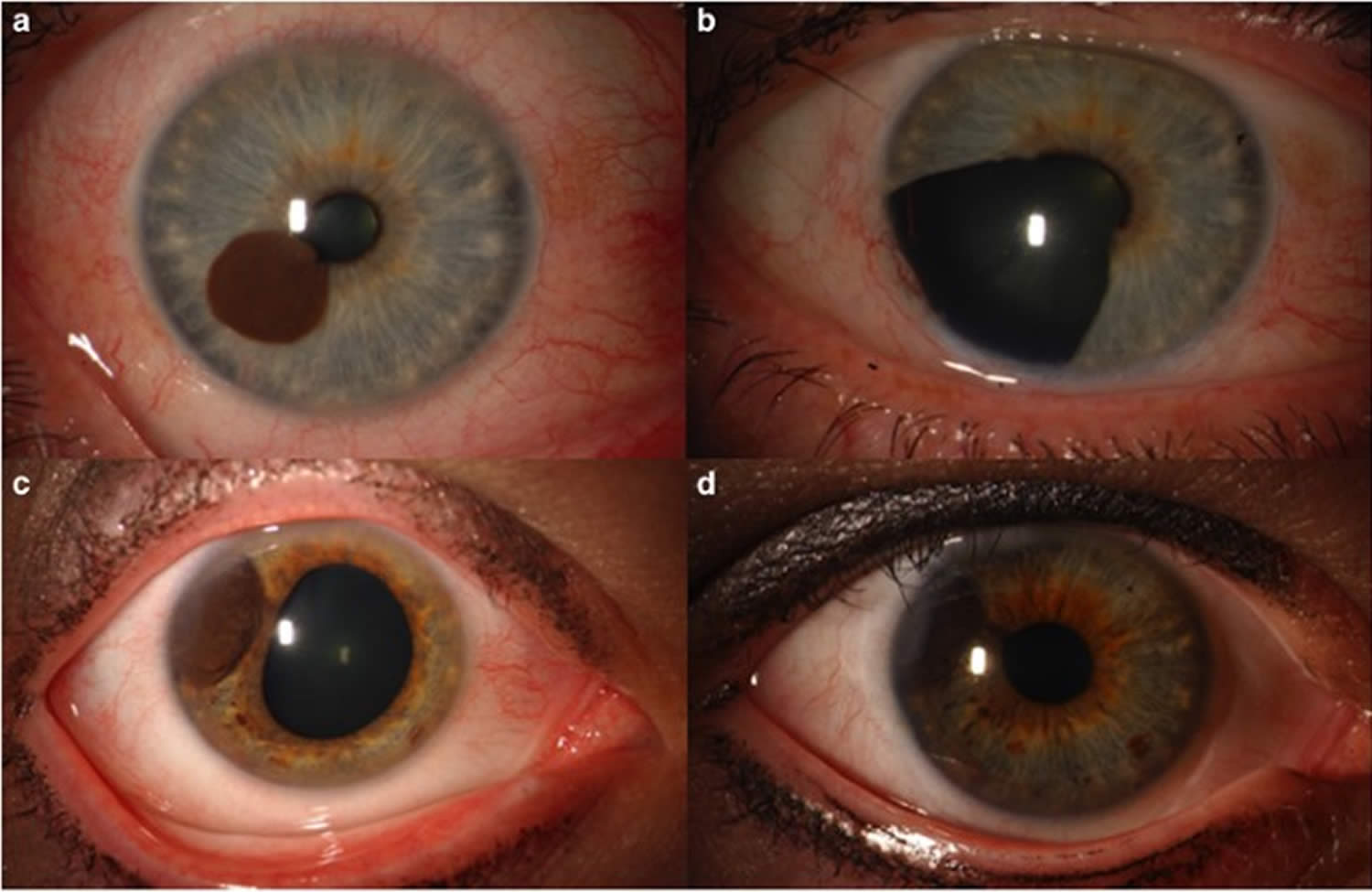
Figure 11. Ocular melanoma (iris melanoma)
Footnote: (a) Iris melanoma in the mid-zone of iris (b) treated by iridectomy. (c) Iris melanoma at the root of iris and (d) treated with iodine-125 plaque radiotherapy.
[Source 26 ]If your ophthalmologist suspects that you have ocular melanoma, he or she may recommend more tests. These may include:
- Ultrasound examination of the eye. An ultrasound examination of the eye is a procedure in which high-energy sound waves (ultrasound) are bounced off the internal tissues of the eye to make echoes. Eye drops are used to numb the eye and a small probe that sends and receives sound waves is placed gently on the surface of the eye. The echoes make a picture of the inside of the eye. The resulting image allows the ophthalmologist to measure the size of the melanoma.
- Fluorescein angiography. This procedure uses a dye injected into your arm, which travels into your eye. A special camera then takes pictures of the inside of your eye to see if there is any blockage or leakage.
- Fundus autofluorescence. This test uses a special kind of camera that makes areas of damage reveal themselves as small points of light in a photograph.
- Optical coherence tomography also known as OCT, this imaging test takes highly detailed pictures of the inside of your eye.
- Biopsy. If your ophthalmologist thinks you have a conjunctival melanoma, he or she may perform a biopsy. This is when the growth is removed from the surface of the eye. The tissue is then tested and examined in a laboratory. Biopsies are not usually needed to diagnose ocular melanoma, but may reveal information about the tumor and if it might spread to other parts of the body.
Your ophthalmologist may refer you to another specialist to do more tests to determine whether the melanoma has spread (metastasized). These tests may need to be repeated regularly for many years.
If you are diagnosed with ocular melanoma, your treatment options will vary. Treatment depends on:
- the location and size of the melanoma
- and your general health
Generally, treatment options fall into two categories: radiation and surgery.
- Ocular melanoma radiation. In radiation therapy, various types of radiation are used to kill the melanoma or keep it from growing. The most common type of radiation therapy used for ocular melanoma is called plaque radiation therapy. Radioactive seeds are attached to a disk, called a plaque, and placed directly on the wall of the eye next to the tumor. The plaque, which looks like a tiny bottle cap, is often made of gold. This helps protect nearby tissues from damage from the radiation directed at the tumor. Temporary stitches hold the plaque in place for four or five days, before it is removed. Radiation therapy can also be delivered by a machine. This machine directs a fine beam of radioactive particles to your eye. This type of radiation therapy is often done over the course of several days. For smaller tumors, laser, heat energy, or both may be used for treatment.
- Ocular melanoma surgery. Depending on the size and location of the melanoma, surgery may be recommended. The surgery may involve removing the tumor and some of the healthy tissue of the eye surrounding it. For larger tumors, tumors that cause eye pain, and for tumors involving the optic nerve, the surgery may involve removing the entire eye (enucleation). After the eye is removed, an implant is put in its place and attached to the eye muscles, so that the implant can move. Once you are healed from the surgery, you will be fitted with an artificial eye (prosthesis). It will be custom painted to match your existing eye.
- Both radiation and surgery can damage the vision in your eye.
Conjunctival melanoma treatment
For melanoma on the surface of the eye, treatment can include chemotherapy eye drops, surgery, freezing treatment, and radiation.
You should talk to your ophthalmologist about how treatment may affect your vision. He or she can also explain the options available to you to help with any vision loss.
Unfortunately metastatic melanoma remains the leading cause of death among patients with ocular melanoma. The extent of systemic spread and tumor burden determines the average length of survival after liver metastases have been detected. Metastatic disease was diagnosed at a 25% and 34% rate at 5-years and 10-years respectively in the Collaborative Ocular Melanoma Study (COMS) with the liver affected 89% of the time. The mortality rate after diagnosis of metastatic disease was 80% at 1 year and 92% at 2 years 27.
Desmoplastic melanoma
Desmoplastic melanoma is a rare type of invasive melanoma accounting for about 0.4% to 4% of all melanomas 28, 29. Desmoplastic melanoma can develop anywhere on your body but it’s most common in the head and neck area. It’s often the same color as your skin and can look like a scar. Desmoplastic melanoma is more common in men than women, with a male to female ratio of approximately 1.7-2 to 1 30. Mean age at diagnosis is 66 to 69 years, which is considerably older than that described for nondesmoplastic melanoma (approximately 60 years) 31.
- Desmoplastic melanoma often involves nerve fibers, when it is called neurotropic melanoma.
- The malignant cells within the dermis are surrounded by fibrous tissue.
- Desmoplastic melanoma sometimes arises underneath a lentigo maligna, a type of melanoma in situ.
The main risk factors for desmoplastic melanoma are 32:
- Increasing age
- Previous invasive melanoma or melanoma in situ
- Fair skin that burns easily
- Sun damaged skin.
Desmoplastic melanoma tends to occur in areas of chronic sun exposure. The most common location is the head and neck (50% of cases), followed by the trunk (20%-25%) and extremities (20%-25%) 33. Desmoplastic melanoma can, however, arise in areas not chronically exposed to the sun, such as mucous membranes 34 and acral sites 35.
Desmoplastic melanoma usually presents as a firm, nonpigmented papule or plaque with poorly defined borders in sun-damaged skin. Malignant melanoma is suspected initially in just 27% of cases 36. Clinically, desmoplastic melanoma is often confused with a benign skin lesion, such as scar tissue, dermatofibroma, neurofibroma, and intradermal melanocytic nevus, or with a malignant nonmelanocytic skin tumor such as basal cell carcinoma and squamous carcinoma.
There are also clinical differences between pure and mixed desmoplastic melanomas 37. An epidermal component in the form of lentigo maligna, lentigo maligna melanoma or superficial spreading melanoma appears to be present in 80% to 100% of mixed desmoplastic melanomas 33. Lesions suspicious for lentigo maligna melanoma should therefore be palpated to check for a firm subcutaneous nodule indicative of desmoplastic melanoma 33.
Figure 12. Desmoplastic melanoma
Footnote: Clinical image of a desmoplastic melanoma in a 53-year-old woman who presented with a firm, pink interscapular tumor initially thought to be a keloid lesion. Biopsy showed a pure desmoplastic melanoma with a thickness of 8.5 mm.
[Source 33 ]It is essential to diagnose desmoplastic melanoma accurately. A careful clinical history is important, which includes noting previous treatments received for non-resolving lesions. Palpation is an important step, as a large majority of desmoplastic melanoma are indurated. Clinical diagnosis is aided by dermoscopy and skin biopsy (usually excision biopsy). Depending on the thickness and proportion of desmoplasia within the invasive melanoma, sentinel lymph node biopsy, imaging studies and blood tests may be advised.
As desmoplastic melanoma is commonly found on the head and neck, and with lentigo maligna, it is possible that if such lesions are only partially biopsied, the desmoplastic melanoma may be missed. Reflectance confocal microscopy may be useful to help guide the site of the biopsy.
Dermoscopy can be helpful in distinguishing desmoplastic melanoma from other skin lesions. The most frequently observed dermatoscopic features of desmoplastic melanoma are:
- Melanocytic features in about 50% (pigmented globules or network)
- Asymmetrical structure and colours
- Regression features: scar-like areas, grey dots
- Multiple colours
- Atypical or polymorphous vascular pattern
- Crystalline structures.
If the skin lesion is suspicious of desmoplastic melanoma, it should be urgently cut out (excision biopsy).
The pathological diagnosis of melanoma can be very difficult. Histopathological features of desmoplastic melanoma include:
- A paucicellular atypical spindle cell proliferation, separated by fibrocollagenous stroma within the dermis or subcutaneous fat.
- There is overlying melanoma in-situ, typically of lentigo maligna type, in approximately 75% of cases.
- Nerve infiltration or neurotropism is found in about half of desmoplastic melanomas.
- Lymphocytic nodular aggregates may be seen in association with desmoplastic melanoma, but are not diagnostic.
Desmoplastic melanoma is categorized into either ‘pure’ or ‘mixed’ subtypes, which reflect the extent of desmoplasia. The mixed subtypes include a component of spindle cell melanoma.
- Pure subtype: ≥ 90% desmoplasia
- Mixed subtype: < 90% desmoplasia
Immunohistochemical stains for melanoma may be helpful.
- S100 and Sox 10 are usually positive.
- HMB-45 and Melan-A are usually negative.
- p75 nerve growth factor is a marker for spindle cell melanoma.
The initial treatment of a desmoplastic melanoma is surgical excision 32.
- For pure desmoplastic melanoma, a 2 cm margin of normal tissue may be recommended, whatever its thickness.
- For mixed desmoplastic melanoma, margins follow guidelines for other forms of invasive melanoma (1–2 cm, depending on Breslow thickness).
Adjuvant radiation therapy may be considered for pure desmoplastic melanoma, Breslow thickness > 4 mm, perineural invasion or positive surgical margins.
Current evidence suggests that desmoplastic melanoma behaves differently to conventional melanoma 38, 39. Desmoplastic melanoma appears to be associated with a higher risk of local recurrence and a lower rate of lymph node metastasis 40. The risk of lymph node involvement seems to be lower than in non-desmoplastic melanomas of a similar thickness; variable rates have been reported for sentinel lymph node involvement (0% to 18.2% depending on the series) 38. It is difficult to accurately predict the prognosis of desmoplastic melanoma, as conflicting data have been reported and many studies do not distinguish between pure and mixed variants. Most recent studies, however, have not found significant differences in survival between patients with desmoplastic melanomas and non-desmoplastic melanomas of a similar thickness 38, 39, 41. The impression that pure desmoplastic melanoma is less likely than mixed desmoplastic melanoma to spread to distant sites and therefore has better survival rates has not been consistently demonstrated. Maurichi et al. 41 observed significant differences in overall survival between patients with mixed and pure desmoplastic melanoma (61.3% vs. 79.5%), but their findings have not been corroborated by subsequent studies 42, 43. Distant metastases, which are mostly located in the lung, have been linked to previous recurrences and deep lesions 44.
Most studies have shown that desmoplastic melanoma has a high risk of local recurrence (approximately 10%-14%), particularly in the case of mixed desmoplastic melanomas 41, 45, 46. Some authors have attributed the more local aggressive nature of pure desmoplastic melanoma to its later diagnosis (it is a rare tumor with an atypical presentation) and to the high frequency of perineural invasion and inadequate surgical margins 36.
A number of factors might explain the more aggressive behavior of desmoplastic melanoma. Shi et al. 40, in a retrospective study of 3657 desmoplastic melanoma cases, found that male sex and an age of older than 68 years were independent predictors of worse overall and disease-free survival. Although these findings have some support in the literature, other authors have not detected any differences in disease-free survival 47. Perineural invasion has also been proposed as a poor prognostic factor in desmoplastic melanoma 48 and has been seen to significantly correlate with greater Breslow thickness 36.
There are conflicting reports about prognostic indicators and outcomes for desmoplastic melanoma, due to its rarity, differences in pathological interpretation, and differing study designs/methodology. The results of the three studies are described below.
- 5-year overall survival rates were better for 280 patients with localised desmoplastic melanoma (90%, median Breslow thickness 2.5 mm) compared to 7767 patients with non-desmoplastic melanoma (82%, median Breslow thickness 1.6 mm).
- A study of 1129 desmoplastic melanoma patients in the United States (1992–2007) reported a 5-year specific survival rate of 85% and 10-year survival of 80%. Older age, anatomic site of the head and neck and tumour thickness > 2 mm, ulceration, lymph node involvement and non-receipt of surgery were associated with lower survival.
- A study of 1,735 desmoplastic melanoma patients, also in the United States (1988–2006), reported overall survival at five years to be 65% and that wide local excision was associated with increased survival. Traditional prognostic factors such as Breslow thickness, nodal positivity, and ulceration did not predict survival in this group of patients.
Spindle cell melanoma
Spindle cell melanoma is a rare histological variant of malignant melanoma, characterized by the presence of spindle-shaped melanocytes arranged in sheets and fascicles 49. The diagnosis of spindle cell melanoma is challenging, as spindle cell melanoma may occur anywhere on the body and frequently mimics amelanotic lesions, including scarring and inflammation 50. On microscopy, it is often mistaken for other skin and soft tissue cancers with spindle cell morphologies 51. Desmoplastic melanoma is a variant of spindle cell melanoma where there are varying proportions of spindle cells and desmoplastic cells present in the histology. The actual incidence of spindle cell melanoma is unknown. Studies have suggested that between 1% and 14% of melanomas are of the spindle cell variant (including desmoplastic melanoma) 52.
Spindle cell melanomas more commonly occur in Caucasian men, affecting men and women at a ratio of 1.6:1–1.9:1 respectively. The average age at diagnosis is 50–80 years 53.
Spindle cell melanoma frequently presents as a non-specific amelanotic (non-pigmented) nodule on the patient’s trunk, head, or neck 54. Spindle cell melanoma may also first present as widespread melanoma metastases. Delays in diagnosis can occur due to its non-specific features and atypical presentation histologically 51.
Immunohistochemistry is a helpful tool in distinguishing spindle cell melanoma from other sarcomas and carcinomas 55. However, diagnosis remains a challenge as a number of sarcomas share some morphological and immunohistochemical features with spindle cell melanoma 56. Differentiation of spindle cell melanoma from desmoplastic melanoma is difficult because both melanomas are characterized by atypical, spindled, malignant melanocytes. However, the size of spindle cell collagen areas and the immunohistochemical markers, S100, MelanA and Tyrosinase, allow differential diagnosis 54. Therefore, the integration of clinical and histological assessment is essential for the diagnosis of spindle cell melanoma 57. Diagnosis of spindle cell melanoma is often delayed until patients exhibit advanced-stage disease, typically with widespread metastasis and poor treatment outcomes 58.
The treatment for spindle cell melanoma is similar to that for other forms of melanoma. Surgical excision is the first step in management 51.
Although the tendency for nodal involvement is low, the majority of spindle cell melanomas present with advanced disease, with a worse prognosis being seen in Caucasian men aged over 66 years 54.
Advanced disease with nodal and distal metastasis is associated with worse outcomes in spindle cell melanoma, as are higher grades of tumours, which have been associated with poorer disease-specific survival and overall survival 51.
Figure 13. Spindle cell melanoma
Spitzoid melanoma
Spitzoid melanoma is a malignant melanoma that is histologically similar to a benign skin lesion, Spitz nevus. Spitzoid melanoma presents as a changing and enlarging papule or nodule. It can be amelanotic (nonpigmented, red) or pigmented (brown, black or blue). Advanced Spitzoid melanoma may be crusted and ulcerated. It is most often located on the head or extremities.
Spitzoid melanoma is often round in shape and uniform in color. Therefore, it does not follow the commonly used ABCDE criteria of melanoma (Asymmetry, Border irregularity, Color variation, Diameter over 6 mm in greatest dimension) 59. Spitzoid melanoma can arise de novo (a new lesion), or within an existing Spitz nevus 60.
When the histological diagnosis is uncertain, the diagnosis of Spitzoid Melanoma of Uncertain Malignant Potential (STUMP) may be made.
Spitzoid melanomas are more common in adults than in children, but due to a very low rate of other cutaneous melanoma subtypes in children, the incidence of spitzoid melanomas is relatively high in children 60.
Spitzoid melanomas can occur in any ethnic group and on any body location 61.
Spitzoid melanoma is diagnosed on skin biopsy of an enlarging nodule.
Spitzoid melanoma should be completely excised with a margin of normal tissue 62. The recommended clinical margins depend on the measured thickness of the tumor, as with other melanomas. The specimen should be carefully examined under the microscope.
In thicker tumors, a sentinel lymph node biopsy may be recommended to determine whether the melanoma has spread. The role of sentinel lymph node biopsy biopsy is controversial in the management of atypical spitzoid melanocytic lesions and the reliability of any associated prognostic information unclear. Patients with spitzoid melanoma and positive sentinel lymph node biopsy have been shown to have a more indolent disease course than those with positive sentinel lymph node biopsy and non-spitzoid melanomas.
The prognosis of spitzoid melanoma in adults is similar to other melanomas of comparable thickness 61. In a study comparing ages of patients with spitzoid melanoma, it was found that there was an 88% 5-year survival rate in children (ages 0–10) with metastatic spitzoid melanoma, compared to a lower, 49% 5-year survival in children aged 11–17 years 63. The prognosis of children with spitzoid melanomas is better than that observed in adults 64.
Figure 14. Spitzoid melanoma
Footnotes: (a) Clinically, hypomelanotic rapid growing tumor on a 14 year old girl. (b) Dermoscopy: Polymorphic vascular pattern, with dotted vessels, milky red areas and remnants of pigment at the periphery, central shiny white structures. Spitzoid melanoma, Breslow 1.9 mm. (c) Clinically, amelanotic pink bump on the lower limb of a 3 year old girl. (d) Dermoscopy: Polymorphic vascular pattern, milky red areas and globules and shiny white structures. Spitzoid melanoma Breslow 5.5 mm. (e) Clinically ulcerated amelanotic bump on the lower limb of a 17 year old girl. (f) Dermoscopy: Vascular pattern showing dotted vessels and milky red globules, and ulceration. Spitzoid melanoma, Breslow 1.9 mm.
[Source 65 ]Melanoma signs and symptoms
Melanomas can develop anywhere on your body. Melanoma skin cancer most often develops in areas that have had exposure to the sun, such as your back, legs, arms and face. Melanomas can also occur in areas that don’t receive much sun exposure, such as the soles of your feet, palms of your hands and fingernail beds. These hidden melanomas are more common in people with darker skin.
The first melanoma signs and symptoms often are:
- A change in an existing mole
- The development of a new pigmented or unusual-looking growth on your skin. See your doctor as soon as possible if you notice changes in a mole, freckle or patch of skin, particularly if the changes happen over a few weeks or months.
- Melanoma doesn’t always begin as a mole. It can also occur on otherwise normal-appearing skin.
Melanoma can appear anywhere on your body, but they most commonly appear on the back in men and on the legs in women.
Melanoma can also develop underneath a nail, on the sole of the foot, in the mouth or in the genital area, but these types of melanoma are rare.
- Melanomas are uncommon in areas that are protected from sun exposure, such as the buttocks and the scalp.
- In most cases, melanomas have an irregular shape and are more than 1 color.
- The mole may also be larger than normal and can sometimes be itchy or bleed.
- Look out for a mole that gradually changes shape, size or color.
Melanoma found at typical skin sites arises as melanoma in situ, superficial spreading melanoma, or nodular melanoma. Lentigo maligna (and lentigo maligna melanoma) is predominantly a skin cancer of the head and neck, occasionally affecting the trunk and limbs.
Superficial (thin) forms of melanoma initially spread out within the epidermis. If all the melanoma cells are confined to the epidermis it is termed a melanoma in situ. When the cancerous cells have grown through the basement membrane and into the dermis it is known as invasive melanoma and is termed a superficial spreading melanoma. Superficial spreading melanoma is the most common type of invasive melanoma and accounts for over 50% of all melanoma. Nodular melanoma, presenting as a firm papule, nodule or plaque accounts for 20-25% of cases of melanoma and is the most aggressive type, it is more common in males and often presents in the fifth or sixth decade, but can occur at any age.
To help you identify characteristics of unusual moles that may indicate melanomas or other skin cancers, think of the letters ABCDE. The ABCDE checklist can be used to check for the main warning signs of melanoma:
- A is for asymmetrical shape. Look for moles with irregular shapes, such as two very different-looking halves. Melanomas usually have 2 very different halves and are an irregular shape
- B is for irregular border. Look for moles with irregular, notched or scalloped borders — characteristics of melanomas. Melanoma often has an irregular appearance, however, if a symmetrical lesion continues to grow out of proportion to the patient’s other moles, especially if aged > 45, then melanoma must be considered
- C is for changes in color. Look for growths that have many colors (a mix of 2 or more colors) or an uneven distribution of color.
- D is for diameter. Look for new growth in a mole larger than 1/4 inch (about 6 millimeters).
- Melanoma grow at different rates – even if the lesion is not changing, if it looks suspicious or if you notice any skin changes that seem unusual make an appointment with your doctor.
- E is for evolving. Look for changes over time, such as a mole that grows in size or that changes color or shape. Moles may also evolve to develop new signs and symptoms, such as new itchiness or bleeding.
It is also important to note that cancerous (malignant) moles vary greatly in appearance. Some may show all of the changes listed above, while others may have only one or two unusual characteristics.
Nodular melanoma can be brown, black, blue-black, and up to 50% are hypomelanotic (pink-red or skin-colored). The surface can be smooth, rough, or crusted. Nodular melanoma often lacks dermoscopic clues, and the diagnosis should be considered in any skin lesion demonstrating all of EFG:
- E = Elevation, and
- F = Firmness to touch, and
- G = Growth. Persistent growth for over one month
Hidden melanomas
Melanomas can also develop in areas of your body that have little or no exposure to the sun, such as the spaces between your toes and on your palms, soles, scalp or genitals 66. These are sometimes referred to as hidden melanomas because they occur in places most people wouldn’t think to check. When melanoma occurs in people with darker skin, it’s more likely to occur in a hidden area.
Hidden melanomas include:
- Melanoma under a nail also known as acral-lentiginous melanoma, is a rare form of melanoma that can occur under a fingernail or toenail. It can also be found on the palms of the hands or the soles of the feet. It’s more common in people of Asian descent, black people and in others with dark skin pigment.
- Melanoma in the mouth, digestive tract, urinary tract or vagina. Mucosal melanoma develops in the mucous membrane that lines the nose, mouth, esophagus, anus, urinary tract and vagina. Mucosal melanomas are especially difficult to detect because they can easily be mistaken for other far more common conditions.
- Melanoma in the eye also known as ocular melanoma. Ocular melanoma most often occurs in the uvea — the layer beneath the white of the eye (sclera). The most common type of ocular melanoma is uveal or choroidal melanoma, which grows at the back of the eye. Very rarely, it can grow on the thin layer of tissue that covers the front of the eye (the conjunctiva) or in the colored part of the eye (the iris). Noticing a dark spot or changes in vision can be signs of eye melanoma, although it’s more likely to be diagnosed during a routine eye examination.
The following should be considered as an urgent referral (2-week wait) for possible melanoma 7:
- Lips – irregular shape, atypical color (black, or > one color / shade of color), a firm papule/nodule
- Oral / genital – any new or changing pigmented lesion. Have a low threshold for referral as an accurate history is usually absent (check local guidelines for where to refer)
Melanoma causes
Melanoma occurs when something goes wrong in the melanin-producing cells (melanocytes) that give color to your skin.
Normally, skin cells develop in a controlled and orderly way — healthy new cells push older cells toward your skin’s surface, where they die and eventually fall off. But when some cells develop DNA damage, new cells may begin to grow out of control and can eventually form a mass of cancerous cells. DNA is the chemical in each of your cells that makes up your genes, which control how your cells function. You usually look like your parents because they are the source of your DNA. But DNA affects more than just how you look.
Some genes control when your cells grow, divide into new cells, and die:
- Genes that help cells grow, divide, and stay alive are called oncogenes.
- Genes that keep cell growth in check, repair mistakes in DNA, or cause cells to die at the right time are called tumor suppressor genes.
Cancers can be caused by DNA mutations (or other types of changes) that keep oncogenes turned on, or that turn off tumor suppressor genes. These types of gene changes can lead to cells growing out of control. Changes in several different genes are usually needed for a cell to become a cancer cell.
Just what damages DNA in skin cells and how this leads to melanoma isn’t clear. It’s likely that a combination of factors, including environmental and genetic factors, causes melanoma. Still, doctors believe exposure to ultraviolet (UV) radiation from the sun and from tanning lamps and beds is the leading cause of melanoma.
Ultraviolet radiation (UVR) is a major carcinogen. It damages the skin by producing reactive oxygen species (free radicals) that damage proteins, lipids, RNA and DNA. According to the two-hit model, where cancer is a result of accumulation mutations to the DNA, the development of skin cancer depends on an individual’s genetic makeup (particularly their MC1R signalling polymorphisms — that determine skin and hair color and sun sensitivity) plus two factors.
- Ultraviolet radiation (UVR) causes tumor initiation through mutations in DNA.
- Ultraviolet radiation (UVR) is a tumor promoter and causes progressive tumor growth.
UV light doesn’t cause all melanomas, especially those that occur in places on your body that don’t receive exposure to sunlight. This indicates that other factors may contribute to your risk of melanoma. These melanomas often have different gene changes than those in melanomas that develop in sun-exposed areas, such as changes in the C-KIT (or just KIT) gene.
The most common change in melanoma cells is a mutation in the BRAF oncogene, which is found in about half of all melanomas. Other genes that can be affected in melanoma include NRAS, CDKN2A, and NF1. Usually only one of these genes is affected 67.
Less often, people inherit gene changes from a parent that clearly raise their risk of melanoma. Familial (inherited) melanomas most often have changes in tumor suppressor genes such as CDKN2A (also known as p16) or CDK4 that prevent them from doing their normal job of controlling cell growth. This could eventually lead to cancer 67.
Some people, such as those with xeroderma pigmentosum (XP), inherit a change in one of the XP (ERCC) genes, which normally help to repair damaged DNA inside the cell. Changes in one of these genes can lead to skin cells that have trouble repairing DNA damaged by UV rays, so these people are more likely to develop melanoma, especially on sun-exposed parts of the body.
Melanoma skin cancer risk factors
A risk factor is anything that raises your risk of getting a disease such as cancer. Many risk factors for melanoma have been found, but it’s not always clear exactly how they might cause cancer.
Factors that may increase your risk of melanoma include:
- Fair skin. Having less pigment (melanin) in your skin means you have less protection from damaging UV radiation. If you have blond or red hair, light-colored eyes, and freckle or sunburn easily, you’re more likely to develop melanoma than is someone with a darker complexion. But melanoma can develop in people with darker complexions too, including Hispanic people and black people.
- A history of sunburn. One or more severe, blistering sunburns can increase your risk of melanoma.
- Excessive ultraviolet (UV) light exposure. Exposure to UV radiation, which comes from the sun and from tanning lights and beds, can increase the risk of skin cancer, including melanoma. The pattern and timing of the UV exposure may play a role in melanoma development. For example, melanoma on the trunk (chest and back) and legs has been linked to frequent sunburns (especially in childhood). This might also have something to do with the fact that these areas aren’t constantly exposed to UV light. Some evidence suggests that melanomas that start in these areas are different from those that start on the face, neck, and arms, where the sun exposure is more constant. And different from either of these are melanomas on the palms of the hands, soles of the feet, or under the nails (known as acral lentiginous melanomas), or on internal surfaces such as the mouth and vagina (mucosal melanomas), where there has been little or no sun exposure.
- Ultraviolet radiation (UVR):
- Damages skin (sunburn, premature skin aging and cancer)
- Damages eyes (keratitis and cataracts)
- Suppresses immunity in the skin and internally
- Predisposes to bacterial and viral skin infection
- Exacerbates pre-existing actinic keratoses
- Prevents innate immunity from rejecting skin tumors
- Ultraviolet radiation (UVR):
- Living closer to the equator or at a higher elevation. People living closer to the earth’s equator, where the sun’s rays are more direct, experience higher amounts of UV radiation than do those living farther north or south. In addition, if you live at a high elevation, you’re exposed to more UV radiation.
- Having many moles or unusual moles. A mole also known as a nevus, is a benign (non-cancerous) pigmented tumor. Babies are not usually born with moles; they often begin to appear in children and young adults. Most moles will never cause any problems, but having more than 50 ordinary moles on your body indicates an increased risk of melanoma. Also, having an unusual type of mole increases the risk of melanoma. Known medically as dysplastic nevi or atypical moles, these tend to be larger than normal moles and have irregular borders and a mixture of colors. Dysplastic nevi can appear on skin that is exposed to the sun as well as skin that is usually covered, such as on the buttocks or scalp. Dysplastic nevi often run in families. A small percentage of dysplastic nevi may develop into melanomas. But most dysplastic nevi never become cancer, and many melanomas seem to arise without a pre-existing dysplastic nevus.
- Dysplastic nevus syndrome (atypical mole syndrome): People with this inherited condition have many dysplastic nevi. If at least one close relative has had melanoma, this condition is referred to as familial atypical multiple mole and melanoma syndrome (FAMMM). People with familial atypical multiple mole and melanoma syndrome (FAMMM) have a very high lifetime risk of melanoma, so they need to have very thorough, regular skin exams by a dermatologist (a doctor who specializes in skin problems). Sometimes full body photos are taken to help the doctor recognize if moles are changing and growing. Many doctors recommend that these patients be taught to do monthly skin self-exams as well.
- Congenital melanocytic nevi: Moles present at birth are called congenital melanocytic nevi. The lifetime risk of melanoma developing in congenital melanocytic nevi is estimated to be between 0 and 5%, depending on the size of the nevus. People with very large congenital nevi have a higher risk, while the risk is lower for those with small nevi. For example, the risk for melanoma is very low in congenital nevi smaller than the palm of the hand, while those that cover large portions of back and buttocks (“bathing trunk nevi”) have significantly higher risks. Congenital nevi are sometimes removed by surgery so that they don’t have a chance to become cancer. Whether doctors advise removing a congenital nevus depends on several factors including its size, location, and color. Many doctors recommend that congenital nevi that are not removed should be examined regularly by a dermatologist and that the patient should be taught how to do monthly skin self-exams. Again, the chance of any single mole turning into cancer is very low. However, anyone with lots of irregular or large moles has an increased risk for melanoma.
- A family history of melanoma. Your risk of melanoma is higher if one or more of your first-degree relatives (parents, brothers, sisters, or children) has had melanoma. Around 10% of all people with melanoma have a family history of the disease. The increased risk might be because of a shared family lifestyle of frequent sun exposure, a family tendency to have fair skin, certain gene changes (mutations) that run in a family, or a combination of these factors. Most experts don’t recommend that people with a family history of melanoma have genetic testing to look for mutations that might increase risk, as it’s not yet clear how helpful this is. Rather, experts advise that they do the following:
- Have regular skin exams by a dermatologist
- Thoroughly examine their own skin once a month
- Be particularly careful about sun protection and avoiding manmade UV rays (such as those from tanning beds)
- Personal history of melanoma or other skin cancers. A person who has already had melanoma has a higher risk of getting melanoma again. People who have had basal or squamous cell skin cancers are also at increased risk of getting melanoma.
- Weakened immune system. A person’s immune system helps fight cancers of the skin and other organs. People with weakened immune systems (from certain diseases or medical treatments) are more likely to develop many types of skin cancer, including melanoma. Your immune system may be impaired if you take medicine to suppress the immune system, such as after an organ transplant, or if you have a disease that impairs the immune system, such as AIDS.
- Being older. Melanoma is more likely to occur in older people, but it is also found in younger people. In fact, melanoma is one of the most common cancers in people younger than 30 (especially younger women). Melanoma that runs in families may occur at a younger age.
- Being male. In the United States, men have a higher rate of melanoma than women, although this varies by age. Before age 50, the risk is higher for women; after age 50 the risk is higher in men.
- Xeroderma pigmentosum. Xeroderma pigmentosum (XP) is a rare, inherited condition that affects skin cells’ ability to repair damage to their DNA. People with XP have a high risk of developing melanoma and other skin cancers when they are young, especially on sun-exposed areas of their skin.
The main risk factors for developing the most common type of melanoma (superficial spreading melanoma) include:
- Increasing age
- Previous invasive melanoma or melanoma in situ
- Previous basal cell carcinoma (BCC) or squamous cell carcinoma (SCC)
- Many melanocytic nevi (moles)
- Multiple (>5) atypical nevi (large or histologically dysplastic nevi)
- A strong family history of melanoma with 2 or more first-degree relatives affected
- White/fair skin that burns easily
- Parkinson disease also increases the risk of melanoma.
These risk factors are not relevant to rare types of melanoma.
Familial melanoma
Members of certain families are more susceptible to the development of the genetic abnormalities associated with melanoma. As a result, these family members have an exceedingly high risk of developing melanoma. Approximately 8 to 12% of all cases of cutaneous melanoma occur in persons with familial predisposition to the development of melanoma.
Familial human melanoma is characterized by an increased risk of developing primary melanoma, a higher incidence of multiple primary melanomas, and an earlier age at onset 68. These characteristics are common to many other heritable malignant and premalignant conditions such as retinoblastoma, Gardner’s syndrome, xeroderma pigmentosum, and nevoid-basal cell carcinoma 69. In individuals with familial melanoma, the locus for cutaneous malignant melanoma-dysplastic nevus resides on the distal portion of the short arm of chromosome 1. Bale and colleagues 70 suggest that the assignment of the locus for cutaneous melanoma to a region of the human genome frequently involved in karyotypic abnormalities in melanoma cells demonstrates that chromosomal deletion may represent the second event in a recessive model of tumorigenesis. Two genetic lesions relating to p16 and the cell cycle regulating cyclin (CDK4) have been identified in familial melanoma; in these patients, the predisposition to melanoma appears to be associated with an increased risk of developing pancreatic cancer and perhaps other cancers 71. The p53 tumor suppressor gene, although overexpressed in melanoma, may only be a sign of genetic disruption and is not an initiating event 72. All patients and their families should undergo complete skin surveys as part of their routine medical care 73.
Evidence for the genetic cause of melanoma comes from the recognition that chromosomal alterations involving the short arm of chromosome 1 and both arms of chromosomes 6 and 7 have been identified in melanomas and cell lines derived from either primary melanomas or metastases 70. Evidence suggests a 9p21 deletion in familial melanoma 74 and consistent lesions of 9p have become apparent 75. Common losses also occur on chromosomes 10 and 8p, along with gains in copy number for chromosomes 7, 8, 6p, 19, 20, 17, and 2 76.
Melanoma skin cancer prevention
You can reduce your risk of melanoma and other types of skin cancer if you:
- Avoid the sun during the middle of the day. Ultraviolet radiation (UVR) is the most important preventable risk factor for skin cancer. For many people in North America, the sun’s rays are strongest between about 10 a.m. and 4 p.m. Schedule outdoor activities for other times of the day, even in winter or when the sky is cloudy. You absorb UV radiation year-round, and clouds offer little protection from damaging rays. Avoiding the sun at its strongest helps you avoid the sunburns and suntans that cause skin damage and increase your risk of developing skin cancer. Sun exposure accumulated over time also may cause skin cancer.
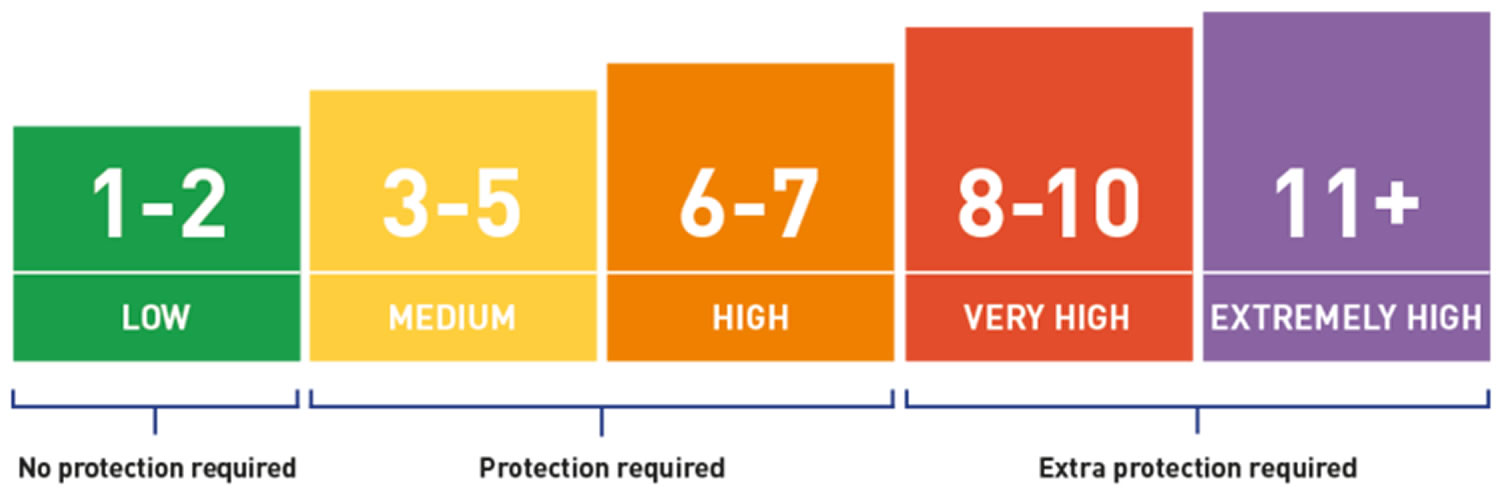
- Wear broad spectrum sunscreen year-round. Use a broad-spectrum sunscreen with at least SPF30+, even on cloudy days. Apply sunscreen generously, and reapply every two hours — or more often if you’re swimming or perspiring.
- Wear protective clothing. Cover your skin with dark, tightly woven clothing that covers your arms and legs, and a broad-brimmed hat, which provides more protection than does a baseball cap or visor. Some companies also sell protective clothing. A dermatologist can recommend an appropriate brand. Don’t forget sunglasses. Look for those that block both types of UV radiation — UVA and UVB rays.
- If you are going to be in the sun, “Slip! Slop! Slap! and Wrap” catchphrase can help you remember some of the key steps you can take to protect yourself from UV rays:
- Slip on a shirt.
- Slop on sunscreen.
- Slap on a hat.
- Wrap on sunglasses to protect the eyes and sensitive skin around them.
- If you are going to be in the sun, “Slip! Slop! Slap! and Wrap” catchphrase can help you remember some of the key steps you can take to protect yourself from UV rays:
- Protect children from the sun. Children need special attention, since they tend to spend more time outdoors and can burn more easily. Parents and other caregivers should protect children from excess sun exposure by using the steps above. Children need to be taught about the dangers of too much sun exposure as they become more independent.
- Avoid tanning lamps and beds. Tanning lamps and beds emit UV rays and can increase your risk of skin cancer. Many people believe the UV rays of tanning beds are harmless. This is not true. Tanning lamps give off UV rays, which can cause long-term skin damage and can contribute to skin cancer. Tanning bed use has been linked with an increased risk of melanoma, especially if it is started before a person is 30. Most dermatologists (skin doctors) and health organizations recommend not using tanning beds and sun lamps.
- Become familiar with your skin so that you’ll notice changes. Examine your skin often for new skin growths or changes in existing moles, freckles, bumps and birthmarks. With the help of mirrors, check your face, neck, ears and scalp. Examine your chest and trunk and the tops and undersides of your arms and hands. Examine both the front and back of your legs and your feet, including the soles and the spaces between your toes. Also check your genital area and between your buttocks.
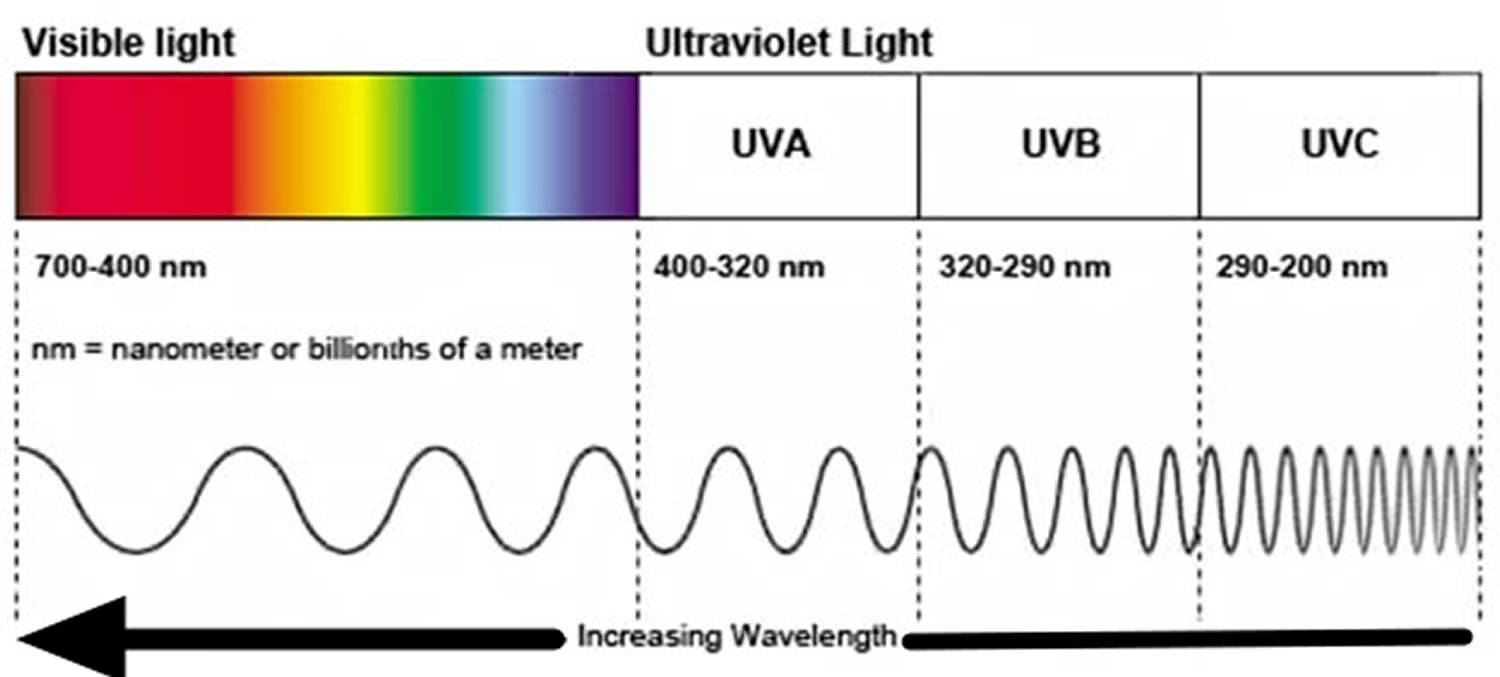
What is ultraviolet radiation?
Ultraviolet radiation (UVR) forms part of the electromagnetic spectrum — the energy emitted from the sun. Ultraviolet radiation (UVR) is categorized according to wavelength, which ranges from 100–400 nm 77.
- Ultraviolet radiation (UVR) is involved in tanning, accelerated skin ageing, ocular damage, and the development of skin cancer.
- Ultraviolet radiation (UVR) also has beneficial effects due to the production of vitamin D in the skin and its immune-modulating effects on inflammatory skin diseases.
Artificial sources of UV include tanning beds and mercury vapor lighting; some halogen, fluorescent, and incandescent lights; and some forms of lasers.
Figure 15. Ultraviolet radiation
Ultraviolet A radiation
- Ultraviolet A radiation (UVA) is long wavelength UVR (wavelength: 315–400 nm).
- UVA passes through the whole atmosphere and accounts for 95% of UVR reaching the Earth’s surface.
- It penetrates through an individual’s epidermis into the dermis.
- UVA has lower energy than ultraviolet B radiation (UVB) and causes less damage per photon.
- It contributes to sunburn, ageing skin, and skin cancer through oxidative injury (cell damage from free radicals).
- UVA stimulates epidermal melanin oxidization (immediate tanning) and melanin production (delayed tanning), which protect the skin by absorbing UVR within the epidermis.
Ultraviolet B radiation
- Ultraviolet B radiation (UVB) is medium wavelength UVR (wavelength: 280–315 nm).
- Most solar UVB is filtered by the atmosphere.
- It penetrates into an individual’s epidermis but not into their dermis.
- UV-B has high energy and damages epidermal cell DNA.
- It stimulates melanin production.
- UV-B is the main cause of sunburn and some forms of skin cancer.
Ultraviolet C radiation
- Ultraviolet C radiation (UVC) is short-wavelength UVR (wavelength: < 280 nm).
- Solar UV-C is completely absorbed by the atmosphere and doesn’t reach the earth’s surface.
- UV-C can be emitted by artificial sources of UVR (eg, by welding arcs and mercury vapor lamps).
- Although shorter UVR wavelengths do not penetrate through the skin, UV-C has higher energy than UVB and injures the skin surface.
- Exposure to UV-C can lead to severe but superficial sunburn.
What affects UV exposure?
The strength of the sun’s UV rays reaching the ground depends on a number of factors, such as:
- Time of day: UV rays are strongest in the middle of the day, between 10 am and 4 pm.
- Season of the year: UV rays are stronger during spring and summer months. This is less of a factor near the equator.
- Distance from the equator (latitude): UV exposure goes down as you get further from the equator.
- Altitude: More UV rays reach the ground at higher elevations.
- Cloud cover: The effect of clouds can vary, but it’s important to know that UV rays can get through to the ground, even on a cloudy day.
- Reflection off surfaces: UV rays can bounce off surfaces like water, sand, snow, or pavement, leading to an increase in UV exposure.
The UV Index
The Ultraviolet Index (UVI) is a measure of the strength of solar ultraviolet radiation (UVR) at the earth’s surface and ranges from 1 to 11+. The higher the ultraviolet index (UVI), the greater the potential for damage to the skin and eyes, and the shorter the time before skin damage (manifested as skin reddening or sunburn). The US National Weather Service and the Environmental Protection Agency (EPA) have developed the UV Index, which gives you an idea of how strong the UV light is in your area on any given day. A higher number means greater risk of exposure to UV rays and a higher chance of sunburn and skin damage that could ultimately lead to skin cancer.
An easy way to tell how much UV exposure you are getting is to look for your shadow:
- If your shadow is taller than you are (in the early morning and late afternoon), your UV exposure is likely to be lower.
- If your shadow is shorter than you are (around midday), you are being exposed to higher levels of UV radiation. Seek shade and protect your skin and eyes.
The UV Index is part of many weather forecasts throughout the country. Further information about the UV Index, as well as your local UV Index forecast, can be found on the EPA’s website at https://www.epa.gov/sunsafety/uv-index-1
Figure 16. Ultraviolet index scale
UV Index Scale 78:
- Level 1 to 2 (UV Low): No protection needed. You can safely stay outside using minimal sun protection.
- Levels 3 to 5 (Moderate UV): The yellow band, numbers three to five, indicates moderate UV levels. Protection is required when spending long periods outside. Seek shade during late morning through mid-afternoon. When outside, generously apply broad-spectrum SPF30+ or higher sunscreen on exposed skin, and wear protective clothing, a wide-brimmed hat, and sunglasses.
- Levels 6 and 7 (High UV): Protection is essential at levels six and seven, represented by an amber band. Seek shade during late morning through mid-afternoon. When outside, generously apply broad-spectrum SPF30+ or higher sunscreen on exposed skin, and wear protective clothing, a wide-brimmed hat, and sunglasses.
- Levels 8 to 10 (Very High UV): When UV levels are in the very high red band, eight to ten, it’s recommended people seek shade between 11 am and 4 pm, slip, slop, slap and wrap and make sure they reapply broad-spectrum SPF30+ sunscreen at least every two hours on exposed skin.
- Level 11+ (Extreme UV): Levels of 11 or higher, shown in a purple band, are regarded as extreme. Reschedule activities for the early morning and evening. Full protection is essential between 11 am and 4 pm.
The UV Index is affected by:
- The angle of the sun: the higher the sun in the sky, the higher the ultraviolet index (UVI). This means that ultraviolet radiation (UVR) levels vary with the time of the day and the time of year.
- Latitude: the closer to equatorial regions, the higher the ultraviolet index (UVI).
- Cloud cover: Ultraviolet index (UVI) is highest on a clear day, but even with cloud cover, ultraviolet index (UVI) can be high. And with scattered cloud, it can be even higher if the sun is unobscured by clouds, because of reflection from cloud edges.
- Altitude: the thinner atmosphere at higher altitudes means less ultraviolet radiation (UVR) is absorbed. In clean air, ultraviolet index (UVI) increases by about 5% per cent per km increase in altitude. In polluted areas, the gradient can be much greater.
- Ozone: Ozone is a trace gas that forms the ozone layer within the stratosphere, which is part of the atmosphere around the Earth. Ozone absorbs some ultraviolet radiation (UVR) that would otherwise reach the earth’s surface.
- Reflection: ultraviolet radiation (UVR) is reflected by different surfaces; fresh snow can reflect as much as 80% of UVR, sand can reflect about 15%, and sea foam about 25%.
Broad Spectrum sunscreens
Sunscreen protects the skin from absorbing ultraviolet (UV) rays. Sunscreen products contain one or more UV filters as active ingredients. There are two kinds of UV rays that can affect the skin: UVA and UVB. Per U.S. Food and Drug Administration (FDA) regulations, sunscreen can only be called “broad spectrum” if it protects against both UVA and UVB. The SPF on sunscreens stands for Sun Protection Factor and refers to the level of sunburn protection provided by the sunscreen product. All sunscreens are tested to measure the amount of UV radiation exposure it takes to cause sunburn when using a sunscreen vs. when not using a sunscreen. The higher the SPF, the greater the protection is from UV radiation, and the greater the sunburn protection.
Here’s how your sun protection looks with each level of SPF:
- SPF 15 – filters about 93% of UVB rays
- SPF 30 – filters about 97% of UVB rays
- SPF 50 – filters about 98% of UVB rays
- SPF 100 – filters about 99% of UVB rays
Because SPF values are determined from a test that measures protection against sunburn caused by UVB radiation, SPF values only indicate a sunscreen’s protection from UVB—the kind of radiation that causes sunburn, damages the skin, and can contribute to developing melanoma.
Currently, there is no internationally agreed-upon test for measuring UVA protection in human skin. An estimate is made by a laboratory test in which the proportion of radiation passing through a measured amount of sunscreen is determined.
To get the most protection, you want a broad-spectrum sunscreen—these protect against UVB and UVA rays. UVA rays are long enough to reach the skin’s dermal layer, damaging collagen, and elastic tissue. That layer is also where the cells that stimulate skin darkening are found; that’s why UVA rays are considered the dominant tanning rays. (UVA rays are what is found in tanning beds and other tanning devices.) Though many people still think a tan looks healthy, it’s actually a sign of DNA damage—the skin darkens in an imperfect attempt to prevent further injury, which can lead to the cell mutations that can lead to melanoma.
The Melanoma Foundation recommends that you use a broad-spectrum sunscreen of SPF 30 or higher daily on all exposed skin and reapply it at least every two hours, as well as after swimming, sweating, or towel drying 79.
Melanoma diagnosis
Tests and procedures used to diagnose melanoma include:
- Physical exam. Your doctor will ask questions about your health history and examine your skin to look for signs that may indicate melanoma. If you are being seen by your primary doctor and melanoma is suspected, you may be referred to a dermatologist, a doctor who specializes in skin diseases, who will look at the area more closely. Along with a standard physical exam, many dermatologists use a technique called dermoscopy (also known as dermatoscopy, epiluminescence microscopy [ELM] or surface microscopy) to see spots on the skin more clearly. The doctor uses a dermatoscope, which is a special magnifying lens and light source held near the skin. Sometimes a thin layer of alcohol or oil is used with this instrument. The doctor may take a digital photo of the spot.
- Removing a sample of tissue for testing (skin biopsy). To determine whether a suspicious skin lesion is melanoma, your doctor may recommend removing a sample of skin for testing. The sample is sent to a lab for examination. What type of biopsy procedure your doctor recommends will depend on your particular situation. Most often doctors recommend removing the entire growth when possible. One common technique, the punch biopsy, is done with a circular blade that’s pressed into the skin around the suspicious mole. Another technique, called an excisional biopsy, uses a scalpel to cut away the entire mole and a small margin of healthy tissue around it.
- Shave (tangential) biopsy. For this type of biopsy, the doctor shaves off the top layers of the skin with a small surgical blade. Bleeding from the biopsy site is stopped by applying an ointment, a chemical that stops bleeding, or a small electrical current to cauterize the wound. A shave biopsy is useful in diagnosing many types of skin diseases and in sampling moles when the risk of melanoma is very low. Shave (tangential) biopsy is not generally used if a melanoma is strongly suspected unless the biopsy blade will go deep enough to get below the suspicious area. Otherwise, if it is a melanoma, the biopsy sample may not be thick enough to measure how deeply the cancer has invaded the skin.
- Punch biopsy. For a punch biopsy, the doctor uses a tool that looks like a tiny round cookie cutter to remove a deeper sample of skin. The doctor rotates the punch biopsy tool on the skin until it cuts through all the layers of the skin. The sample is removed and the edges of the biopsy site are often stitched together.
- Excisional and incisional biopsies. To examine a tumor that might have grown into deeper layers of the skin, the doctor may use an excisional (or less often, an incisional) biopsy.
- An excisional biopsy removes the entire tumor (along with a small margin of normal skin around it). This is usually the preferred method of biopsy for suspected melanomas if it can be done, although this isn’t always possible.
- An incisional biopsy removes only a portion of the tumor.
- For these types of biopsies, a surgical knife is used to cut through the full thickness of skin. A wedge or sliver of skin is removed for examination, and the edges of the cut are usually stitched together.
- “Optical” biopsies. Some newer types of biopsies, such as reflectance confocal microscopy, can be done without needing to remove samples of skin. Reflectance confocal microscopy is used widely in Europe, and it’s now available in some centers in the US. It may be especially useful for people with many unusual moles, as it can cut down on the number of skin biopsies these people need. Reflectance confocal microscopy might also be helpful in determining the edges of a melanoma, which could help during surgery. This technique will likely become more widely available in the coming years.
- Adhesive patch testing. Adhesive patch testing is a newer technique now being studied. Instead of cutting into the skin to get a biopsy sample, a sticky patch is placed over the suspicious area. When it’s removed it takes some of the top layers of skin with it, which can then be tested for certain gene changes that are often linked with melanoma. If one of these gene changes is found, a standard biopsy of the area can then be done. If no gene changes are found, a biopsy isn’t needed, and the area can be watched instead.
Biopsies of melanoma that may have spread
Biopsies of areas other than the skin may be needed in some cases. For example, if melanoma has already been diagnosed on the skin, nearby lymph nodes may be biopsied to see if the cancer has spread to them.
Rarely, biopsies may be needed to figure out what type of cancer someone has. For example, some melanomas can spread so quickly that they reach the lymph nodes, lungs, brain, or other areas while the original skin melanoma is still very small. Sometimes these tumors are found with imaging tests (such as CT scans) or other exams even before the melanoma on the skin is discovered. In other cases, they may be found long after a skin melanoma has been removed, so it’s not clear if it’s the same cancer.
In still other cases, melanoma may be found somewhere in the body without ever finding a spot on the skin. This may be because some skin lesions go away on their own (without any treatment) after some of their cells have spread to other parts of the body. Melanoma can also start in internal organs, but this is very rare, and if melanoma has spread widely throughout the body, it may not be possible to tell exactly where it started.
When melanoma has spread to other organs, it can sometimes be confused with a cancer starting in that organ. For example, melanoma that has spread to the lung might be confused with a primary lung cancer (cancer that starts in the lung).
Special lab tests can be done on the biopsy samples that can tell whether it is a melanoma or some other kind of cancer. This is important because different types of cancer are treated differently.
Biopsies of suspicious areas inside the body often are more involved than those used to sample the skin.
Fine needle aspiration (FNA) biopsy
FNA biopsy is not used on suspicious moles. But it may be used, for example, to biopsy large lymph nodes near a melanoma to find out if the melanoma has spread to them.
For this type of biopsy, the doctor uses a syringe with a thin, hollow needle to remove very small pieces of a lymph node or tumor. The needle is smaller than the needle used for a blood test. A local anesthetic is sometimes used to numb the area first. This test rarely causes much discomfort and does not leave a scar.
If the lymph node is just under the skin, the doctor can often feel it well enough to guide the needle into it. For a suspicious lymph node deeper in the body or a tumor in an organ such as the lung or liver, an imaging test such as ultrasound or a CT scan is often used to help guide the needle into place.
FNA biopsies are not as invasive as some other types of biopsies, but they may not always collect enough of a sample to tell if a suspicious area is melanoma. In these cases, a more invasive type of biopsy may be needed.
Surgical (excisional) lymph node biopsy
This procedure can be used to remove an enlarged lymph node through a small incision (cut) in the skin. A local anesthetic (numbing medicine) is generally used if the lymph node is just under the skin, but the person may need to be sedated or even asleep (using general anesthesia) if the lymph node is deeper in the body.
This type of biopsy is often done if a lymph node’s size suggests the melanoma has spread there but an FNA biopsy of the node wasn’t done or didn’t find any melanoma cells.
Sentinel lymph node biopsy
If melanoma has been diagnosed and has any concerning features (such as being at least a certain thickness), a sentinel lymph node biopsy is often done to see if the cancer has spread to nearby lymph nodes, which in turn might affect treatment options. This test can be used to find the lymph nodes that are likely to be the first place the melanoma would go if it has spread. These lymph nodes are called sentinel nodes (they stand sentinel, or watch, over the tumor, so to speak).
To find the sentinel lymph node (or nodes), a doctor injects a small amount of a radioactive substance into the area of the melanoma. After giving the substance time to travel to the lymph node areas near the tumor, a special camera is used to see if it collects in one or more sentinel lymph nodes. Once the radioactive area has been marked, the patient is taken for surgery, and a blue dye is injected in the same place the radioactive substance was injected. A small incision is then made in the marked area, and the lymph nodes are then checked to find which one(s) became radioactive and turned blue. These sentinel nodes are removed and looked at under a microscope.
If there are no melanoma cells in the sentinel nodes, no more lymph node surgery is needed because it is very unlikely the melanoma would have spread beyond this point. If melanoma cells are found in the sentinel node, the remaining lymph nodes in this area are typically removed and looked at as well. This is known as a lymph node dissection.
If a lymph node near a melanoma is abnormally large, a sentinel node biopsy probably won’t be needed. The enlarged node is simply biopsied.
Lab tests of biopsy samples
Samples from any biopsies will be sent to a lab, where a doctor called a pathologist will look at them under a microscope for melanoma cells. Often, skin samples are sent to a dermatopathologist, a doctor who has special training in looking at skin samples.
If the doctor can’t tell for sure if melanoma cells are in the sample just by looking at it, special lab tests will be done on the cells to try to confirm the diagnosis. These might include:
- Immunohistochemistry (IHC)
- Fluorescence in situ hybridization (FISH)
- Comparative genomic hybridization (CGH)
- Gene expression profiling (GEP)
If melanoma is found in the samples, the pathologist will look at certain important features such as the tumor thickness and mitotic rate (the portion of cells that are actively dividing). These features help determine the stage of the melanoma, which in turn can affect treatment options and prognosis (outlook).
Testing for gene changes
For some people with melanoma, biopsy samples may be tested to see if the cells have mutations (changes) in certain genes, such as the BRAF gene. About half of melanomas have BRAF mutations. Some drugs used to treat advanced melanomas are only likely to work if the cells have BRAF mutations, so this test is important in helping to determine treatment options. Tests for changes in other genes, such as C-KIT, might be done as well.
A newer lab test known as DecisionDx-Melanoma looks at certain gene expression patterns in melanoma cells to help show if early-stage melanomas are likely to spread.
The DecisionDx-Melanoma test divides melanomas into 2 main groups, based on their gene patterns:
- Class 1 tumors have a lower risk of spreading.
- Class 2 tumors have a higher risk of spreading.
The DecisionDx-Melanoma test might help tell if someone with early-stage melanoma should get a sentinel lymph node biopsy (SLNB) or additional treatment, or if they need to be followed more closely after treatment to look for signs of recurrence. Doctors are still studying the best of use of this test.
Tests of other genes and gene patterns are now being studied as well.
Imaging tests
Imaging tests use x-rays, magnetic fields, or radioactive substances to create pictures of the inside of the body. They are used mainly to look for the possible spread of melanoma to lymph nodes or other organs. These tests are not needed for most people with very early-stage melanoma, which is very unlikely to have spread.
Imaging tests can also be done to help determine how well treatment is working or to look for possible signs of cancer coming back (recurring) after treatment.
- Chest x-ray. This test might be done to help determine if melanoma has spread to the lungs, although a CT scan of the chest (see below) is often done instead.
- Ultrasound. Ultrasound uses sound waves to create images of the inside of your body on a computer screen. This test might be used to look at the lymph nodes near the tumor, especially if it’s not clear if they’re enlarged based on a physical exam. Ultrasound is typically fairly quick and easy to do, and it doesn’t expose you to radiation.
- Ultrasound-guided needle biopsy: Ultrasound can also be used to help guide a biopsy needle into a suspicious lymph node.
- Computed tomography (CT) scan. The CT scan uses x-rays to make detailed, cross-sectional images of your body. Unlike a regular x-ray, CT scans can show the detail in soft tissues (such as internal organs). This test can show if any lymph nodes are enlarged or if organs such as the lungs or liver have suspicious spots, which might be from the spread of melanoma.
- CT-guided needle biopsy: CT scans can also be used to help guide a biopsy needle into a suspicious area within the body.
- Magnetic resonance imaging (MRI) scan. MRI scans use radio waves and strong magnets instead of x-rays to create detailed images of parts of your body. MRI scans can be very helpful in looking at the brain and spinal cord.
- Positron emission tomography (PET) scan. A PET scan can help show if the cancer has spread to lymph nodes or other parts of the body. It is most useful in people with more advanced stages of melanoma. For this test, you are injected with a slightly radioactive form of sugar, which collects mainly in cancer cells. A special camera is then used to create a picture of areas of radioactivity in the body.
- PET/CT scan: Many centers have special machines that do both a PET and CT scan at the same time (PET/CT scan). This lets the doctor compare areas of higher radioactivity on the PET scan with the more detailed appearance of that area on the CT scan.
Blood tests
Blood tests aren’t used to diagnose melanoma, but some tests may be done before or during treatment, especially for more advanced melanomas. Doctors often test blood for levels of a substance called lactate dehydrogenase (LDH) before treatment. If the melanoma has spread to distant parts of the body, a high LDH level is a sign that the cancer may be harder to treat. This can affect the stage of the cancer.
Other tests of blood cell counts and blood chemistry levels may be done in a person who has advanced melanoma to see how well the bone marrow (where new blood cells are made), liver, and kidneys are working before and during treatment.
Melanoma stages
If you receive a diagnosis of melanoma, the next step is to determine the extent (stage) of the cancer. This process is called staging. Melanoma staging means finding out if the melanoma has spread from its original site in the skin. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics. Melanoma staging can be very complex, so if you have any questions about the stage of your cancer or what it means, ask your doctor to explain it to you in a way you understand.
To assign a stage to your melanoma, your doctor will:
- Determine the thickness. The thickness of a melanoma is determined by carefully examining the melanoma under a microscope and measuring it with a special tool. The thickness of a melanoma helps doctors decide on a treatment plan. In general, the thicker the tumor, the more serious the disease. Thinner melanomas may only require surgery to remove the cancer and some normal tissue around it. If the melanoma is thicker, your doctor may recommend additional tests to see if the cancer has spread before determining your treatment options.
- See if the melanoma has spread to the lymph nodes. If there’s a risk that the cancer has spread to the lymph nodes, your doctor may recommend a procedure known as a sentinel node biopsy. During a sentinel node biopsy, a dye is injected in the area where your melanoma was removed. The dye flows to the nearby lymph nodes. The first lymph nodes to take up the dye are removed and tested for cancer cells. If these first lymph nodes (sentinel lymph nodes) are cancer-free, there’s a good chance that the melanoma has not spread beyond the area where it was first discovered.
- Look for signs of cancer beyond the skin. For people with more-advanced melanomas, doctors may recommend imaging tests to look for signs that the cancer has spread to other areas of the body. Imaging tests may include X-rays, CT scans and positron emission tomography (PET) scans. These imaging tests generally aren’t recommended for smaller melanomas with a lower risk of spreading beyond the skin.
Other factors may go into determining the risk that the cancer may spread (metastasize), including whether the skin over the area has formed an open sore (ulceration) and how many dividing cancer cells (mitoses) are found when looking under a microscope.
Melanoma is staged using the Roman numerals 0 through IV (4). Some stages are split further, using capital letters (A, B, etc.). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage 4, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
At stage 0 (melanoma in situ) and stage 1, a melanoma is small and has a very successful treatment rate. But the higher the numeral, the lower the chances of a full recovery. By stage 4, the cancer has spread beyond your skin to other organs, such as your lungs or liver.
Breslow thickness
Breslow thickness is reported for invasive melanomas. The Breslow thickness is measured vertically in millimeters (mm) from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement. Breslow thickness is measured in millimeters (mm) with a small ruler, called a micrometer. A specialist doctor called a pathologist uses the special ruler with a microscope when looking at your tissue sample in the laboratory. The Breslow thickness is a strong predictor of outcome; the thicker the melanoma, the more likely it is to metastasize (spread). Doctors use the Breslow thickness in another staging system for melanoma called the TNM staging system.
Clark level of invasion
The Clark level indicates the anatomic plane of melanoma invasion. The Clark scale is a way of measuring how deeply the melanoma has grown into the skin and which levels of the skin are affected. You can see the main layers of the skin in Figure 1 above. The deeper the Clark level, the greater the risk of metastasis (secondary spread). The Clark level is useful in predicting outcome in thin tumors, and less useful for thicker ones in comparison to the value of the Breslow thickness.
Table 2. The Clark scale has 5 levels
| Level | Characteristics |
|---|---|
| Level 1 | In situ melanoma – the melanoma cells are only in the outer layer of the skin (the epidermis) |
| Level 2 | Melanoma has invaded papillary dermis (superficial dermis) |
| Level 3 | Melanoma cells are touching the next layer down known as the reticular dermis (deep dermis) |
| Level 4 | Melanoma has invaded reticular dermis |
| Level 5 | Melanoma has invaded subcutaneous tissue |
TNM Staging system
Most melanoma specialists refer to the American Joint Committee on Cancer (AJCC) TNM system, which is based on 3 key pieces of information:
- The extent of the main (primary) tumor (T): How deep has the cancer grown into the skin? Is the cancer ulcerated?
- Tumor thickness: The thickness of the melanoma is called the Breslow thickness. In general, melanomas less than 1 millimeter (mm) thick (about 1/25 of an inch) have a very small chance of spreading. As the melanoma becomes thicker, it has a greater chance of spreading.
- Ulceration: Ulceration is a breakdown of the skin over the melanoma. Melanomas that are ulcerated tend to have a worse outlook.
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or distant organs ? (Melanoma can spread almost anywhere in the body, but the most common sites of spread are the lungs, liver, brain, bones, and the skin or lymph nodes in other parts of the body.)
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
Table 3. American Joint Committee on Cancer (AJCC) TNM Staging System
| AJCC Stage | Melanoma Stage Description | |
|---|---|---|
| 0 | This stage is also known as melanoma in situ. The cancer is confined to the epidermis, the outermost skin layer (Tis). It has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). | |
| 1 | The tumor is no more than 2 mm (2/25 of an inch) thick and might or might not be ulcerated (T1 or T2a). The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0) | |
| 2 | The tumor is more than 2 mm thick (T2b or T3) and may be thicker than 4 mm (T4). It might or might not be ulcerated. The cancer has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0). | |
| 3A | The tumor is no more than 2 mm thick and might or might not be ulcerated (T1 or T2a). The cancer has spread to 1 to 3 nearby lymph nodes, but it is so small that it is only seen under the microscope (N1a or N2a). It has not spread to distant parts of the body (M0). | |
| 3B | There is no sign of the primary tumor (T0) AND: The cancer has spread to only one nearby lymph node (N1b) OR It has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor (without reaching the nearby lymph nodes) (N1c) It has not spread to distant parts of the body (M0). | |
| OR | ||
| The tumor is no more than 4 mm thick and might or might not be ulcerated (T1, T2, or T3a) AND: The cancer has spread to only one nearby lymph node (N1a or N1b) OR It has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor (without reaching the nearby lymph nodes) (N1c) OR It has spread to 2 or 3 nearby lymph nodes (N2a or N2b) It has not spread to distant parts of the body (M0). | ||
| 3C | There is no sign of the primary tumor (T0) AND: The cancer has spread to 2 or more nearby lymph nodes, at least one of which could be seen or felt (N2b or N3b) OR It has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor, and it has reached the nearby lymph nodes (N2c or N3c) OR It has spread to nearby lymph nodes that are clumped together (N3b or N3c) It has not spread to distant parts of the body (M0). | |
| OR | ||
| The tumor is no more than 4 mm thick, and might or might not be ulcerated (T1, T2, or T3a) AND: The cancer has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor, and it has reached nearby lymph nodes (N2c or N3c) OR The cancer has spread to 4 or more nearby lymph nodes (N3a or N3b), or it has spread to nearby lymph nodes that are clumped together (N3b or N3c) It has not spread to distant parts of the body (M0). | ||
| OR | ||
| The tumor is more than 2 mm but no more than 4 mm thick and is ulcerated (T3b) OR it is thicker than 4 mm but is not ulcerated (T4a). The cancer has spread to one or more nearby lymph nodes AND/OR it has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor (N1 or higher). It has not spread to distant parts of the body. | ||
| OR | ||
| The tumor is thicker than 4 mm and is ulcerated (T4b) AND: The cancer has spread to 1 to 3 nearby lymph nodes, which are not clumped together (N1a/b or N2a/b) OR It has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor, and it might (N2c) or might not (N1c) have reached 1 nearby lymph node) It has not spread to distant parts of the body (M0). | ||
| 3D | The tumor is thicker than 4 mm and is ulcerated (T4b) AND: The cancer has spread to 4 or more nearby lymph nodes (N3a or N3b) OR It has spread to nearby lymph nodes that are clumped together (N3b) It has spread to very small areas of nearby skin (satellite tumors) or to skin lymphatic channels around the tumor, AND it has spread to at least 2 nearby lymph nodes, or to lymph nodes that are clumped together (N3c) OR It has not spread to distant parts of the body (M0). | |
| 4 | The tumor can be any thickness and might or might not be ulcerated (any T). The cancer might or might not have spread to nearby lymph nodes (any N). It has spread to distant lymph nodes or to organs such as the lungs, liver or brain (M1). | |
Footnotes: The staging system in the table above uses the pathologic stage also called the surgical stage. This is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away (or at all), the cancer will be given a clinical stage instead. This is based on the results of physical exams, biopsies, and imaging tests. The clinical stage will be used to help plan treatment. Sometimes, though, the cancer has spread farther than the clinical stage estimates, so it may not predict a person’s outlook as accurately as a pathologic stage. If your cancer has been clinically staged, it is best to talk to your doctor about your specific stage.
[Source 80 ]Stage 0 melanoma (melanoma in situ)
Stage 0 melanoma is also known as melanoma in situ. Abnormal melanocytes are confined to the epidermis, the outermost layer of the skin (Tis). It has not spread to nearby lymph nodes (N0) or to distant parts of the body (M0).
[Source 81 ]Stage 1 melanoma
Stage 1 melanoma. In stage 1A, the tumor is not more than 1 millimeter thick, with or without ulceration (a break in the skin). In stage 1B, the tumor is more than 1 mm but not more than 2 millimeters thick, without ulceration. Skin thickness is different on different parts of the body.
[Source 82 ]Stage 2A melanoma
In stage 2A melanoma the tumor is more than 1 mm but not more than 2 millimeters thick, with ulceration (a break in the skin); OR it is more than 2 but not more than 4 millimeters thick, without ulceration. Skin thickness is different on different parts of the body.
[Source 83 ]Stage 2B melanoma
In stage 2B melanoma the tumor is more than 2 mm but not more than 4 millimeters thick, with ulceration (a break in the skin); OR it is more than 4 millimeters thick, without ulceration. Skin thickness is different on different parts of the body.
[Source 84 ]Stage 2C melanoma
In stage 2C melanoma the tumor is more than 4 millimeters thick, with ulceration (a break in the skin). Skin thickness is different on different parts of the body.
[Source 85 ]Stage 3 melanoma
Stage 3 melanoma generally means that cancer cells have spread to the lymph nodes close to where the melanoma started (the primary tumor). Or it has spread to an area between the primary tumor and the nearby lymph nodes. The tumor may be any thickness, with or without ulceration.
Stage 3 melanoma is divided into four levels – A, B, C and D depending on where the cancer has spread to, such as if there are only satellite or in-transit metastases or if there is melanoma in one or several lymph nodes.
- Stage 3A – the primary melanoma is no more than 2 mm thick. It may or may not be ulcerated. It has spread to nor more than 3 lymph nodes detected by pathology (not palpable nodes). It has not spread to distant sites.
- Stage 3B – Either the primary melanoma site cannot be found and it has spread to only one lymph node or it has spread to very small areas of nearby skin (satellite metastasis) or lymphatic vessels, without spreading to distant sites; OR The melanoma primary is no more than 4 mm and may or may not be ulcerated. It has spread to up to three lymph nodes or to very small areas of nearby skin (satellite tumours) or lymphatic channels. It has not spread to distant sites.
- Stage 3C – The primary melanoma site cannot be found AND it has spread to up to 4 or more lymph nodes OR it has spread to 2 or more lymph nodes and to very small areas of nearby skin (satellite metastasis) or lymphatic vessels, without spreading to distant sites;
- OR The melanoma primary is no more than 4 mm and may or may not be ulcerated. It has spread to one or more lymph nodes or to very small areas of nearby skin (satellite tumours) or lymphatic channels or lymph nodes clumped together. It has not spread to distant sites;
- OR The melanoma is between 2.1 mm and 4 mm and may or may not be ulcerated AND has spread to one or more lymph nodes or has spread to very small areas of nearby skin (satellite tumours) or lymphatic channels or lymph nodes clumped together. It has not spread to distant sites;
- OR The melanoma is thicker than 4 mm, is ulcerated has spread to no more than 3 lymph nodes or to very small areas of nearby skin (satellite tumours) or lymphatic channels. It has not spread to distant sites.
- Stage 3D – The primary melanoma is thicker than 4 mm, is ulcerated AND has spread to 4 or more lymph nodes OR has spread to very small areas of nearby skin (satellite tumours) or lymphatic channels. It has not spread to distant sites. This area is further divided into satellite or in-transit metastases. Satellite metastases are cancer cells that have spread very close to the primary tumor (within 2 cm). In-transit metastases are cancer cells that have spread further than 2 cm but before the nearest lymph node.
In some cases, the primary tumor can’t be found but there are melanoma cells in the lymph nodes or nearby area.
Stage 4 melanoma
Stage 4 melanoma is also known as metastatic melanoma or advanced melanoma, the cancer has spread to other parts of the body, such as the brain, spinal cord, lung, liver, gastrointestinal tract, bone, muscle, and/or distant lymph nodes. Cancer may have spread to places in the skin far away from where it first started.
[Source 86 ]Melanoma treatment
The best treatment for your melanoma depends on the size and stage of cancer, your overall health, and your personal preferences.
Treatment for early-stage melanomas
Treatment for early-stage melanomas usually includes surgery to remove the melanoma. A very thin melanoma may be removed entirely during the biopsy and require no further treatment. Otherwise, your surgeon will remove the cancer as well as a border of normal skin and a layer of tissue beneath the skin. For people with early-stage melanomas, this may be the only treatment needed.
Surgery for melanoma
Surgery is the main treatment option for most melanomas, and usually cures early-stage melanomas.
Wide local excision
Doctors diagnose melanoma by removing the abnormal mole or area of skin (skin biopsy). They send it to the laboratory to check if it’s a melanoma and how thick it is.
It’s likely you’ll have a second operation to remove more tissue if you’re diagnosed with melanoma. This is called a wide local excision. Your doctor removes a larger area of healthy skin and tissue from around where the melanoma was. This helps to reduce the risk of the melanoma coming back. This fairly minor operation will cure most thin melanomas.
How much tissue you have removed depends on:
- whether melanoma cells are in the surrounding skin and tissue
- how deep the melanoma has grown into the tissue beneath the skin
- the position of the melanoma on your body
- whether the surgery will affect your movement afterwards (for example, if the melanoma is close to a joint)
Local anesthesia is injected into the area to numb it before the excision. The site of the tumor is then cut out, along with a small amount of normal skin around the edges (called the margin). The wound is usually stitched back together afterward. This will leave a scar.
The removed sample is then viewed with a microscope to make sure that no cancer cells were left behind at the edges of the skin that was removed.
Wide excision differs from an excisional biopsy. The margins are wider because the diagnosis is already known. The recommended margins vary depending on the thickness of the tumor. Thicker tumors need larger margins (both at the edges and in the depth of the excision).
The margins can also vary based on where the melanoma is on the body and other factors. For example, if the melanoma is on the face, the margins may be smaller to avoid large scars or other problems. Smaller margins might increase the risk of the cancer coming back, so be sure to discuss the options with your doctor.
The National Institute for Health and Care Excellence (NICE) recommend that for :
- Stage 0 melanoma (melanoma in situ), your doctor removes at least 0.5cm of tissue around the melanoma
- Stage 1 melanoma, the surgeon removes at least 1 cm of tissue around the melanoma
- Stage 2 melanoma, the surgeon removes at least 2 cm of tissue around the melanoma
You might have a skin graft or flap to cover the wound if your doctor needs to remove a large area of skin.
Most people who have a wide local excision don’t need a skin graft or flap. The area can heal up well without one.
- Skin graft. For a skin graft, your surgeon removes a thin sheet of skin from somewhere else on your body (the donor site). They then place it over the area where the melanoma was. The donor skin is usually taken from somewhere where it won’t be too obvious, such as your inner thigh or behind your ear. At first the area looks like a graze. It may feel quite sensitive or painful at first. The skin grows back quite quickly, usually over a couple of weeks. The skin graft is very delicate while it heals. It’s vital that the graft is not damaged during this time. Try not to knock it. You might have antibiotics to help prevent an infection.
- Skin flap. For a skin flap, your surgeon takes some skin with its own blood supply from an area next to where the melanoma was. There are different types of skin flaps and sometimes you may need more than one operation for the type of skin flap you’re having. Your surgeon explains what you need before you have the operation. Skin flaps are often used for the face. Your surgeon will do their best to make sure the cuts (incisions) they make during the operation fit in with the natural lines of your face so that any scars are hidden.
Mohs surgery
In some situations, Mohs surgery also known as Mohs micrographic surgery, might be an option. This type of surgery is used more often for some other types of skin cancer, but not all doctors agree on using it for melanoma.
Mohs surgery is done by a specially trained dermatologist or surgeon. In this procedure, the skin (including the melanoma) is removed in very thin layers. Each layer is then looked at with a microscope. If cancer cells are seen, the doctor removes another layer of skin. This is repeated until a layer shows no signs of cancer. This is a slow process, often taking several hours, but it means that more normal skin near the tumor can be saved, which can help the area look better after surgery.
Amputation
In uncommon situations where the melanoma is on a finger or toe and has grown deeply, part or all of that digit might need to be amputated.
Lymph node dissection
If you have swollen lymph nodes near to the melanoma that your doctor can feel, you usually have an ultrasound scan and biopsy. Your doctor takes a sample from the nodes using a fine needle (fine needle aspiration) or a core needle biopsy to check for cancer cells. You might have surgery to remove the lymph nodes in the area if cancer has spread there. This is a lymph node dissection.
In this operation, the surgeon removes all of the lymph nodes in the region near the primary melanoma tumor. For example, if the melanoma is on a leg, the surgeon would remove the nodes in the groin region on that side of the body, which is where melanoma cells would most likely travel to first.
Once the diagnosis of melanoma is made from the skin biopsy, the doctor will examine the lymph nodes near the melanoma. Depending on the thickness and location of the melanoma, this may be done by physical exam, or by imaging tests (such as ultrasound or CT or PET scans) to look at nodes that are not near the body surface.
If the nearby lymph nodes are abnormally hard or large, and a fine needle aspiration (FNA) biopsy or excisional biopsy finds melanoma in a node or nodes, a lymph node dissection is usually done.
If the lymph nodes are not enlarged, a sentinel lymph node biopsy may be done, particularly if the melanoma is thicker than 1 mm. (See Tests for Melanoma Skin Cancer for a description of this procedure.) If the sentinel lymph node does not contain cancer, then there is no need for a lymph node dissection because it’s unlikely the melanoma has spread to the lymph nodes. If the sentinel lymph node contains cancer cells, removing the remaining lymph nodes in that area with a lymph node dissection is usually advised. This is called a completion lymph node dissection.
It’s not clear if a lymph node dissection can cure melanomas that have spread to the nodes. This is still being studied. Still, some doctors feel it might prolong a patient’s life and at least avoid the pain that may be caused by cancer growing in these lymph nodes.
A full lymph node dissection can cause some long-term side effects. One of the most troublesome can be lymphedema. Lymph nodes in the groin or under the arm normally help drain fluid from the limbs. If they are removed, fluid may build up. This can cause limb swelling, which may or may not go away. If severe enough, it can cause skin problems and an increased risk of infections in the limb. Elastic stockings or compression sleeves can help some people with this condition.
Lymphedema, along with the pain from the surgery itself, is a main reason why lymph node dissection is not done unless the doctor feels it is really necessary. Sentinel lymph node biopsy, however, is unlikely to have this effect. It’s important to discuss the risks of side effects with your doctor before having either of these procedures.
Treatment for melanomas that have spread beyond the skin
If melanoma has spread beyond the skin, treatment options may include:
- Surgery to remove affected lymph nodes. If melanoma has spread to nearby lymph nodes, your surgeon may remove the affected nodes. Additional treatments before or after surgery also may be recommended.
- Immunotherapy. Immunotherapy is a drug treatment that helps your immune system to fight cancer. Your body’s disease-fighting immune system might not attack cancer because the cancer cells produce proteins that help them hide from the immune system cells. Immunotherapy works by interfering with that process. Immunotherapy is often recommended after surgery for melanoma that has spread to the lymph nodes or to other areas of the body. When melanoma can’t be removed completely with surgery, immunotherapy treatments might be injected directly into the melanoma.
- Targeted therapy. Targeted drug treatments focus on specific weaknesses present within cancer cells. By targeting these weaknesses, targeted drug treatments can cause cancer cells to die. Cells from your melanoma may be tested to see if targeted therapy is likely to be effective against your cancer. For melanoma, targeted therapy might be recommended if the cancer has spread to your lymph nodes or to other areas of your body.
- Radiation therapy. This treatment uses high-powered energy beams, such as X-rays and protons, to kill cancer cells. Radiation therapy may be directed to the lymph nodes if the melanoma has spread there. Radiation therapy can also be used to treat melanomas that can’t be removed completely with surgery. For melanoma that spreads to other areas of the body, radiation therapy can help relieve symptoms.
- Chemotherapy. Chemotherapy uses drugs to kill cancer cells. Chemotherapy can be given intravenously, in pill form or both so that it travels throughout your body. Chemotherapy can also be given in a vein in your arm or leg in a procedure called isolated limb perfusion. During this procedure, blood in your arm or leg isn’t allowed to travel to other areas of your body for a short time so that the chemotherapy drugs travel directly to the area around the melanoma and don’t affect other parts of your body.
Immunotherapy
Immunotherapy is the use of medicines to help the body’s natural defence system (immune system) to recognize and destroy melanoma cells more effectively. You have immunotherapy if your melanoma is BRAF negative. If your melanoma is BRAF positive, you may have targeted cancer drugs or immunotherapy. Several types of immunotherapy can be used to treat melanoma.
The immunotherapy drugs for melanoma are:
- Ipilimumab (Yervoy)
- Pembrolizumab (Keytruda)
- Atezolizumab (Tecentriq)
- Nivolumab (Opdivo)
You might have a combination of drugs such as nivolumab and ipilimumab.
Immune checkpoint inhibitors
An important part of the immune system is its ability to keep itself from attacking normal cells in the body. To do this, it uses “checkpoints,” which are proteins on immune cells that need to be turned on (or off) to start an immune response. Melanoma cells sometimes use these checkpoints to avoid being attacked by the immune system. But these drugs target the checkpoint proteins, helping to restore the immune response against melanoma cells.
Pembrolizumab (Keytruda) and nivolumab (Opdivo) are drugs that target PD-1, a protein on immune system cells called T cells that normally help keep these cells from attacking other cells in the body. By blocking PD-1, these drugs boost the immune response against melanoma cells. This can often shrink tumors and help people live longer.
- Pembrolizumab (Keytruda) and nivolumab (Opdivo) can be used to treat melanomas:
- That can’t be removed by surgery
- That have spread to other parts of the body
- After surgery (as adjuvant treatment) for certain stage 2 or 3 melanomas to try to lower the risk of the cancer coming back
- These drugs are given as an intravenous (IV) infusion, typically every 2 to 6 weeks, depending on the drug and why it’s being given.
Atezolizumab (Tecentriq) is a drug that targets PD-L1, a protein related to PD-1 that is found on some tumor cells and immune cells. Blocking this protein can help boost the immune response against melanoma cells.
- Atezolizumab (Tecentriq) can be used along with cobimetinib and vemurafenib in people with melanoma that has the BRAF gene mutation, when the cancer can’t be removed by surgery or has spread to other parts of the body.
- Atezolizumab (Tecentriq) is given as an intravenous (IV) infusion every 2 to 4 weeks.
Ipilimumab (Yervoy) is another drug that boosts the immune response, but it has a different target. Ipilimumab (Yervoy) blocks CTLA-4, another protein on T cells that normally helps keep them in check.
- Ipilimumab (Yervoy) can be used to treat melanomas that can’t be removed by surgery or that have spread to other parts of the body. It might also be used for less advanced melanomas after surgery (as an adjuvant treatment) in some situations, to try to lower the risk of the cancer coming back.
- When used alone, this drug doesn’t seem to shrink as many tumors as the PD-1 inhibitors, and it tends to have more serious side effects, so usually one of those other drugs is used first. Another option in some situations might be to combine this drug with one of the PD-1 inhibitors. This can increase the chance of shrinking the tumors (slightly more than a PD-1 inhibitor alone), but it can also increase the risk of side effects.
- Ipilimumab (Yervoy) is given as an intravenous (IV) infusion, usually once every 3 weeks for 4 treatments (although it may be given for longer when used as an adjuvant treatment).
Relatlimab targets LAG-3, another checkpoint protein on certain immune cells that normally helps keep the immune system in check.
- Relatlimab is given along with the PD-1 inhibitor nivolumab (in a combination known as Opdualag). It can be used to treat melanomas that can’t be removed by surgery or that have spread to other parts of the body.
- Relatlimab is given as an intravenous (IV) infusion, typically once every 4 weeks.
Side effects of immune checkpoint inhibitors
- Some of the more common side effects of these drugs can include fatigue, cough, nausea, skin rash, poor appetite, constipation, joint pain, and diarrhea.
- Other, more serious side effects occur less often.
- Infusion reactions: Some people might have an infusion reaction while getting these drugs. This is like an allergic reaction, and can include fever, chills, flushing of the face, rash, itchy skin, feeling dizzy, wheezing, and trouble breathing. It’s important to tell your doctor or nurse right away if you have any of these symptoms while getting these drugs.
- Autoimmune reactions: These drugs remove one of the safeguards on the body’s immune system. Sometimes the immune system responds by attacking other parts of the body, which can cause serious or even life-threatening problems in the lungs, intestines, liver, hormone-making glands, kidneys, or other organs.
It’s very important to report any new side effects to someone on your health care team as soon as possible. If serious side effects do occur, treatment may need to be stopped and you might be given high doses of corticosteroids to suppress your immune system.
Talimogene laherparepvec (oncolytic virus therapy)
Doctors might use another type of immunotherapy called talimogene laherparepvec (T- VEC or Imlygic) also known as oncolytic virus therapy, which they inject directly into the melanomas in the skin or lymph nodes that can’t be removed with surgery. Talimogene laherparepvec (T- VEC or Imlygic) is a weakened form of the cold sore virus. The changed virus grows in the cancer cells and destroys them. Talimogene laherparepvec (T- VEC or Imlygic) also works by helping the immune system to recognize and attack cancer cells.
You might have T-VEC for melanoma that can’t be removed with surgery or has spread to certain areas of the body such as the lymph nodes or skin. It treats the tumor it is injected into but may also have an effect on tumors nearby. The virus is injected directly into the tumors, typically every 2 weeks. This treatment can sometimes shrink these tumors, and might also shrink tumors in other parts of the body.
Some of the common side effects include:
- flu-like symptoms including a high temperature, sore throat, shivering and muscle aches
- high blood pressure
- headaches and dizziness
- bleeding
- tummy pain
- diarrhoea and constipation
- feeling or being sick
- skin problems such as a rash
- tiredness (fatigue)
Doctors used to use two other types of immunotherapy drugs called interferon and interleukin-2 (IL-2) to treat melanoma. They don’t use these drugs very often any more.
Interleukin-2 (IL-2)
Interleukins are proteins that certain cells in the body make to boost the immune system in a general way. Lab-made versions of interleukin-2 (IL-2) are sometimes used to treat melanoma. They are given as intravenous (IV) infusions, at least at first. Some patients or caregivers may be able to learn how to give injections under the skin at home.
- For advanced melanomas: Interleukin-2 (IL-2) can sometimes shrink advanced melanomas when used alone. It is not used as much as in the past, because the immune checkpoint inhibitors are more likely to help people and tend to have fewer side effects. But interleukin-2 (IL-2) might be an option if these drugs are no longer working.
- For some earlier-stage melanomas: Melanomas that have reached the nearby lymph nodes are more likely to come back in another part of the body, even if all of the cancer is thought to have been removed. Interleukin-2 (IL-2) can sometimes be injected into the tumors (known as intralesional therapy) to try to prevent this. Side effects are similar but tend to be milder when IL-2 is injected directly into the tumor.
When deciding whether to use interleukin-2 (IL-2), patients and their doctors need to take into account the potential benefits and side effects of this treatment.
Side effects of interleukin-2 (IL-2) can include flu-like symptoms such as fever, chills, aches, severe tiredness, drowsiness, and low blood cell counts. In high doses, interleukin-2 (IL-2) can cause fluid to build up in the body so that the person swells up and can feel quite sick. Because of this and other possible serious side effects, high-dose interleukin-2 (IL-2) is given only in the hospital, in centers that have experience with this type of treatment.
Bacille Calmette-Guerin (BCG) vaccine
Bacille Calmette-Guerin (BCG) is a germ related to the one that causes tuberculosis. BCG doesn’t cause serious disease in humans, but it does activate the immune system. The BCG vaccine can be used to help treat stage 3 melanomas by injecting it directly into tumors, although it isn’t used very often.
Imiquimod cream
Imiquimod (Zyclara) is a drug that is put on the skin as a cream. It stimulates a local immune response against skin cancer cells. For very early (stage 0) melanomas in sensitive areas on the face, some doctors may use imiquimod if surgery might be disfiguring. It might also be an option for some melanomas that have spread along the skin.
The cream is usually applied 2 to 5 times a week for around 3 months. Some people have serious skin reactions to this drug. Imiquimod is not used for more advanced melanomas.
Targeted therapy
Targeted therapy drugs target parts of melanoma cells that make them different from normal cells. Targeted drugs work differently from standard chemotherapy drugs, which basically attack any quickly dividing cells. Targeted drugs can be very helpful in treating melanomas that have certain gene changes.
Drugs that target cells with BRAF gene changes
About half of all melanomas have changes (mutations) in the BRAF gene. Melanoma cells with these changes make an altered BRAF protein that helps them grow. Some drugs target this and related proteins, such as the MEK proteins. If you have melanoma that has spread beyond the skin, a biopsy sample of it will likely be tested to see if the cancer cells have a BRAF mutation. Drugs that target the BRAF protein (BRAF inhibitors) or the MEK proteins (MEK inhibitors) aren’t likely to work on melanomas that have a normal BRAF gene.
Most often, if a person has a BRAF mutation and needs targeted therapy, they will get both a BRAF inhibitor and a MEK inhibitor, as combining these drugs often works better than either one alone.
- BRAF inhibitors. Vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) are drugs that attack the BRAF protein directly.
- These drugs can shrink or slow the growth of tumors in some people whose melanoma has spread or can’t be removed completely.
- Dabrafenib can also be used (along with the MEK inhibitor trametinib) after surgery in people with stage 3 melanoma, where it can help lower the risk of the cancer coming back.
- These drugs are taken as pills or capsules, once or twice a day.
- Common side effects can include skin thickening, rash, itching, sensitivity to the sun, headache, fever, joint pain, fatigue, hair loss, and nausea. Less common but serious side effects can include heart rhythm problems, liver problems, kidney failure, severe allergic reactions, severe skin or eye problems, bleeding, and increased blood sugar levels. Some people treated with these drugs develop new squamous cell skin cancers. These cancers are usually less serious than melanoma and can be treated by removing them. Still, your doctor will want to check your skin often during treatment and for several months afterward. You should also let your doctor know right away if you notice any new growths or abnormal areas on your skin.
- MEK inhibitors. The MEK gene works together with the BRAF gene, so drugs that block MEK proteins can also help treat melanomas with BRAF gene changes. MEK inhibitors include trametinib (Mekinist), cobimetinib (Cotellic), and binimetinib (Mektovi).
- These drugs can be used to treat melanoma that has spread or can’t be removed completely.
- Trametinib can also be used along with dabrafenib after surgery in people with stage 3 melanoma, where it can help lower the risk of the cancer coming back.
- Again, the most common approach is to combine a MEK inhibitor with a BRAF inhibitor. This seems to shrink tumors for longer periods of time than using either type of drug alone.
- Some side effects (such as the development of other skin cancers) are actually less common with the combination.
- MEK inhibitors are pills taken once or twice a day.
- Common side effects can include rash, nausea, diarrhea, swelling, and sensitivity to sunlight. Rare but serious side effects can include heart lung, or liver damage; bleeding or blood clots; vision problems; muscle damage; and skin infections.
- Drugs that target cells with C-KIT gene changes. A small portion of melanomas have changes in the C-KIT gene that help them grow. These changes are more common in melanomas that start in certain parts of the body:
- On the palms of the hands, soles of the feet, or under the nails (known as acral melanomas)
- Inside the mouth or other mucosal (wet) areas
- In areas that get chronic sun exposure
- Some targeted drugs, such as imatinib (Gleevec) and nilotinib (Tasigna), can affect cells with changes in C-KIT. If you have an advanced melanoma that started in one of these places, your doctor may test your melanoma cells for changes in the C-KIT gene, which might mean that one of these drugs could be helpful.
Drugs that target different gene changes are also being studied in clinical trials.
Chemotherapy
Chemotherapy (chemo) uses drugs that kill cancer cells. Chemotherapy might be used to treat advanced melanoma after other treatments have been tried, but it’s not often used as the first treatment because newer forms of immunotherapy and targeted drugs are typically more effective. Chemo is usually not as helpful for melanoma as it is for some other types of cancer, but it can shrink tumors in some people.
Several chemo drugs can be used to treat melanoma:
- Dacarbazine (also called DTIC)
- Temozolomide
- Nab-paclitaxel
- Paclitaxel
- Cisplatin
- Carboplatin
Some of these drugs are given alone, while others are more often combined with other drugs. It’s not clear if using combinations of drugs is more helpful than using a single drug, but it can add to the side effects.
Doctors give chemo in cycles, with each period of treatment followed by a rest period to give the body time to recover. Each chemo cycle typically lasts for a few weeks.
Isolated limb perfusion (ILP) and isolated limb infusion (ILI)
These are ways of giving chemotherapy that are sometimes used to treat melanoma that is confined to an arm or leg but that can’t be removed with surgery. The idea with this approach is to keep the chemo in the limb and not allow it to reach other parts of the body, where it could cause more side effects.
This is done during a surgical procedure. The blood flow of the arm or leg is separated from the rest of the body, and a high dose of chemotherapy is circulated through the limb for a short period of time. This lets doctors give high doses to the area of the tumor without exposing other parts of the body to these doses.
To do this, a tube is placed into the artery that feeds blood into the limb, and a second tube is placed into the vein that drains blood from it.
- For isolated limb perfusion (ILP), the artery and vein are first surgically separated from the circulation to the rest of the body, and are then hooked up to tubes going to a special machine in the operating room.
- For isolated limb infusion (ILI), long tubes (catheters) are inserted through the skin and into the artery and vein. This method is less complex and takes less time, and it might not require general anesthesia (where you are in a deep sleep).
In either case, a tourniquet is tied around the limb to help make sure the chemo doesn’t enter the rest of the body. Chemotherapy (usually with a drug called melphalan) is infused into the blood in the limb through the artery. This is done by the machine in isolated limb perfusion (ILP), and by using a syringe in isolated limb infusion (ILI). During the treatment session, the blood exits the limb through the tube in the vein, the chemo is added, and then the blood is returned back to the limb through the tube in the artery. During isolated limb perfusion (ILP), the drug can also be heated by the machine to help the chemo work better. By the end of the treatment the drug is washed out of the limb, and the tubes are removed (and for isolated limb perfusion (ILP) the blood vessels are stitched back together) so that the circulation is returned to normal.
Chemo drugs can cause side effects. These depend on the type and dose of drugs given and how long they are used. The side effects of chemo can include:
- Hair loss
- Mouth sores
- Loss of appetite
- Nausea and vomiting
- Diarrhea or constipation
- Increased risk of infection (from having too few white blood cells)
- Easy bruising or bleeding (from having too few blood platelets)
- Fatigue (from having too few red blood cells)
These side effects usually go away once treatment is finished. There are often ways to lessen side effects. For example, drugs can help prevent or reduce nausea and vomiting. Be sure to ask your doctor or nurse about drugs to help reduce side effects.
Some chemo drugs can have other side effects. For example, some drugs can damage nerves, which can lead to symptoms (mainly in the hands and feet) such as pain, burning or tingling sensations, sensitivity to cold or heat, or weakness. This condition is called peripheral neuropathy. It usually goes away once treatment is stopped, but for some people it can last a long time.
Be sure to talk with your cancer care team about what to expect in terms of side effects. While you are getting chemo, report any side effects to your medical team so that they can be treated promptly. In some cases, the doses of chemo may need to be reduced or treatment may need to be delayed or stopped to prevent side effects from getting worse.
Radiation therapy
Radiation therapy also known as radiotherapy uses high-energy rays (such as x-rays) or particles to kill cancer cells. Radiation therapy is not needed for most people with melanoma on the skin, although it might be useful in certain situations:
- Radiation therapy might be an option to treat very early stage melanomas, if surgery can’t be done for some reason.
- Radiation can also be used after surgery for an uncommon type of melanoma known as desmoplastic melanoma.
- Sometimes, radiation is given after surgery in the area where lymph nodes were removed, especially if many of the nodes contained cancer cells. This is to try to lower the chance that the cancer will come back.
- Radiation can be used to treat melanoma that has come back after surgery, either in the skin or lymph nodes, or to help treat distant spread of the disease.
- Radiation therapy is often used to relieve symptoms caused by the spread of the melanoma, especially to the brain or bones. Treatment with the goal of relieving symptoms is called palliative therapy. Palliative radiation therapy is not expected to cure the cancer, but it might help shrink it or slow its growth for a time to help control some of the symptoms.
The type of radiation most often used to treat melanoma, known as external beam radiation therapy (EBRT), focuses radiation from a source outside of the body on the cancer. Treatment is much like getting an x-ray, but the radiation is stronger. The procedure itself is painless. Each treatment lasts only a few minutes, although the setup time – getting you into place for treatment – usually takes longer. The treatment schedule can vary based on the goal of treatment and where the melanoma is. Before treatments start, your radiation team will take careful measurements to find the correct angles for aiming the radiation beams and the proper dose of radiation. This planning session is called simulation.
Stereotactic radiosurgery (SRS) is a type of radiation therapy that can sometimes be used for tumors that have spread to the brain. Despite the name, there is no actual surgery. High doses of radiation are aimed precisely at the tumor(s) in one or more treatment sessions. There are 2 main ways to give stereotactic radiosurgery (SRS):
- In one version, a machine called a Gamma Knife® focuses about 200 thin beams of radiation on the tumor from different angles over a few minutes to hours. The head is kept in the same position by placing it in a rigid frame.
- In another version, a linear accelerator (a machine that creates radiation) that is controlled by a computer moves around the head to deliver radiation to the tumor from many different angles over a few minutes. The head is kept in place with a head frame or a plastic face mask.
These treatments can be repeated if needed.
Stereotactic body radiation therapy (SBRT) is similar to stereotactic radiosurgery (SRS) (using a linear accelerator), but it can be used to treat tumors in other parts of the body.
Side effects of radiation therapy are usually limited to the area getting radiation. Common side effects can include:
- Sunburn-like skin problems
- Changes in skin color
- Hair loss where the radiation enters the body
- Fatigue
- Nausea (if radiation is aimed at the abdomen)
Often these go away after treatment.
Radiation therapy to the brain can sometimes cause memory loss, headaches, trouble thinking, or reduced sexual desire. Usually these symptoms are minor compared with those caused by a tumor in the brain, but they might still affect your quality of life.
Surgery for metastatic melanoma
If melanoma has spread (metastasized) from the skin to other organs such as the lungs or brain, the cancer is very unlikely to be curable by surgery. Even when only 1 or 2 areas of spread are found by imaging tests such as CT or MRI scans, there are likely to be others that are too small to be found by these scans.
Surgery is sometimes done in these circumstances, but the goal is usually to try to control the cancer rather than to cure it. If 1 or even a few metastases are present and can be removed completely, this surgery may help some people live longer. Removing metastases in some places, such as the brain, might also help prevent or relieve symptoms and improve a person’s quality of life.
If you have metastatic melanoma and your doctor suggests surgery as a treatment option, be sure you understand what the goal of the surgery would be, as well as its possible benefits and risks.
Stage 0 melanoma treatment
Stage 0 melanoma (melanoma in situ) has not grown deeper than the top layer of the skin (the epidermis). It is usually treated by surgery (wide excision) to remove the melanoma and a small margin of normal skin around it. The removed sample is then sent to a lab to be looked at with a microscope. If cancer cells are seen at the edges of the sample, a second, wider excision of the area may be done.
Some doctors may consider the use of imiquimod cream (Zyclara) or radiation therapy instead of surgery, although not all doctors agree with this.
For melanomas in sensitive areas on the face, some doctors may use Mohs surgery or even imiquimod cream if surgery might be disfiguring, although not all doctors agree with these uses.
Stage 1 melanoma treatment
Stage 1 melanoma is typically treated by wide excision (surgery to remove the melanoma as well as a margin of normal skin around it). The width of the margin depends on the thickness and location of the melanoma. Most often, no other treatment is needed.
Some doctors may recommend a sentinel lymph node biopsy to look for cancer in nearby lymph nodes, especially if the melanoma is stage 1B or has other characteristics that make it more likely to have spread. You and your doctor should discuss this option.
If the sentinel lymph node biopsy does not find cancer cells in the lymph nodes, then no further treatment is needed, although close follow-up is still important.
If cancer cells are found on the sentinel lymph node biopsy, a lymph node dissection (removal of all lymph nodes near the cancer) might be recommended. Another option might be to watch the lymph nodes closely by getting an ultrasound of the nodes every few months.
If the sentinel lymph node biopsy found cancer, adjuvant (additional) treatment with immune checkpoint inhibitors or targeted therapy drugs (if the melanoma has a BRAF gene mutation) might be recommended to try to lower the chance the melanoma will come back. Other drugs or perhaps vaccines might also be options as part of a clinical trial.
Stage 2 melanoma treatment
Wide excision (surgery to remove the melanoma and a margin of normal skin around it) is the standard treatment for stage 2 melanoma. The width of the margin depends on the thickness and location of the melanoma. Because the melanoma may have spread to nearby lymph nodes, many doctors recommend a sentinel lymph node biopsy as well. This is an option that you and your doctor should discuss.
If a sentinel lymph node biopsy is done and does not find cancer cells in the lymph nodes, then sometimes no further treatment is needed, but close follow-up is still important.
For certain stage 2 melanomas, the immune checkpoint inhibitor pembrolizumab (Keytruda) might be given after surgery to help reduce the risk of the cancer returning.
If the sentinel lymph node biopsy finds that the sentinel node contains cancer cells (which changes the cancer stage to stage 3 – see below), then a lymph node dissection (where all the lymph nodes in that area are surgically removed) might be done right away. Adjuvant (additional) treatment with immune checkpoint inhibitors or targeted therapy drugs (if the melanoma has a BRAF gene mutation) might be recommended to try to lower the chance the melanoma will come back. Other drugs or perhaps vaccines might also be options as well as part of a clinical trial.
In other cases where the sentinel lymph node biopsy finds cancer, the lymph nodes might be watched closely with an ultrasound of the nodes every few months, instead of doing a lymph node dissection right then. Your doctor will discuss the best options with you depending on the details of your situation.
Stage 3 melanoma treatment
These cancers have already reached the lymph nodes when the melanoma is first diagnosed. Surgical treatment for stage 3 melanoma usually requires wide excision of the primary tumor as in earlier stages, along with lymph node dissection.
After surgery, (additional) adjuvant treatment with immune checkpoint inhibitors or with targeted therapy drugs (for cancers with BRAF gene changes) may help lower the risk of the melanoma coming back. Other drugs or perhaps vaccines may also be recommended as part of a clinical trial to try to reduce the chance the melanoma will come back. Another option is to give radiation therapy to the areas where the lymph nodes were removed, especially if many of the nodes contain cancer.
If melanoma tumors are found in nearby lymph vessels in or just under the skin (known as in-transit tumors), they are removed, if possible. Other options might include injections of the T-VEC vaccine (Imlygic), Bacille Calmette-Guerin (BCG) vaccine, or interleukin-2 (IL-2) directly into the melanoma; radiation therapy; or applying imiquimod cream. For melanomas on an arm or leg, another option might be isolated limb perfusion or isolated limb infusion (infusing just the limb with chemotherapy). Other possible treatments might include targeted therapy drugs (for melanomas with a BRAF or C-KIT gene change), immunotherapy, or chemotherapy.
Some people with stage 3 melanoma might not be cured with current treatments, so they may want to think about taking part in a clinical trial of newer treatments.
Stage 4 melanoma treatment
Stage 4 melanomas is also known as metastatic melanoma or advanced melanoma, mean the melanoma have already spread (metastasized) to distant lymph nodes or other areas of the body. Skin tumors or enlarged lymph nodes causing symptoms can often be removed by surgery or treated with radiation therapy.
Metastases in internal organs are sometimes removed, depending on how many there are, where they are, and how likely they are to cause symptoms. Metastases that cause symptoms but cannot be removed may be treated with radiation, immunotherapy, targeted therapy, or chemotherapy.
The treatment of widespread melanomas has changed in recent years as newer forms of immunotherapy and targeted drugs have been shown to be more effective than chemotherapy.
Immunotherapy drugs called checkpoint inhibitors are typically the first drugs tried, especially in people whose cancer cells do not have BRAF gene changes. These drugs can shrink tumors for long periods of time in some people. Options might include:
- Pembrolizumab (Keytruda) or nivolumab (Opdivo)
- Nivolumab combined with relatlimab (Opdualag)
- Nivolumab or pembrolizumab, plus ipilimumab (Yervoy)
Combinations of checkpoint inhibitors might be more effective, although they’re also more likely to result in serious side effects. People who get any of these drugs need to be watched closely for serious side effects..
In about half of all melanomas, the cancer cells have BRAF gene changes. If a BRAF gene change is found, treatment with newer targeted therapy drugs – typically a combination of a BRAF inhibitor and a MEK inhibitor – might be a good option. Immune checkpoint inhibitors such as pembrolizumab or nivolumab might be another option, as well as a combination of targeted drugs plus the immune checkpoint inhibitor atezolizumab (Tecentriq).
Doctors are now studying if targeted therapy or immunotherapy is better as the first treatment. But there might be situations where it makes sense to use one instead of the other. For example, the targeted drugs are more likely to shrink tumors quickly, so they might be preferred in cases where this is important. In either case, if one type of treatment isn’t working, the other can be tried.
A small portion of melanomas have changes in the C-KIT gene. These melanomas might be helped by targeted drugs such as imatinib (Gleevec) and nilotinib (Tasigna), although these drugs often stop working eventually.
Immunotherapy using interleukin-2 (IL-2) can help a small number of people with stage IV melanoma live longer, and it might be tried if immune checkpoint inhibitors aren’t working. Higher doses of IL-2 seem to be more effective, but they can also have more severe side effects, so they might need to be given in the hospital.
Chemotherapy can help some people with stage IV melanoma, but other treatments are usually tried first. Dacarbazine (DTIC) and temozolomide (Temodar) are the chemo drugs used most often, either by themselves or combined with other drugs. Even when chemotherapy shrinks these cancers, the cancer usually starts growing again within several months.
It’s important to carefully consider the possible benefits and side effects of any recommended treatment before starting it.
Because stage 4 melanoma is often hard to cure with current treatments, patients may want to think about taking part in a clinical trial. Many studies are now looking at new targeted drugs, immunotherapies, chemotherapy drugs, and combinations of different types of treatments.
Recurrent melanoma treatment
Treatment of melanoma that comes back after initial treatment depends on the stage of the original melanoma, what treatments a person has already had, where the melanoma comes back, and other factors.
Local recurrence
Melanoma might come back in the skin near the site of the original tumor, sometimes even in the scar from the surgery. In general, these local (skin) recurrences are treated with surgery similar to what would be recommended for a primary melanoma. This might include a sentinel lymph node biopsy (SLNB). Depending on the results of the sentinel lymph node biopsy, other treatments might be recommended as well.
In-transit recurrence
If melanoma recurs in nearby lymph vessels in or just under the skin (known as in-transit recurrence), it should be removed, if possible. Other options include injections of the T-VEC vaccine (Imlygic), Bacille Calmette-Guerin (BCG) vaccine, or interleukin-2 (IL-2) directly into the melanoma; radiation therapy; or applying imiquimod cream. For melanomas on an arm or leg, another option might be isolated limb perfusion or isolated limb infusion (infusing just the limb with chemotherapy). Other possible treatments might include targeted therapy (for melanomas with a BRAF or C-KIT gene change), immunotherapy, or chemotherapy.
Recurrence in nearby lymph nodes
If nearby lymph nodes weren’t all removed during the initial treatment, the melanoma might come back in these lymph nodes. Lymph node recurrence is treated by lymph node dissection if it can be done, sometimes followed by adjuvant (additional) treatments such as radiation therapy and/or immunotherapy or targeted therapy (for cancers with BRAF gene changes). If surgery is not an option, radiation therapy or systemic treatment (immunotherapy, targeted therapy, or chemo) can be used.
Recurrence in other parts of the body
Melanoma can also come back in distant parts of the body. Almost any organ can be affected. Most often, the melanoma will come back in the lungs, bones, liver, or brain. Treatment for these recurrences is generally the same as for stage 4 melanoma (see above). Melanomas that recur on an arm or leg may be treated with isolated limb perfusion/infusion chemotherapy.
Melanoma that comes back in the brain can be hard to treat. Single tumors can sometimes be removed by surgery. Radiation therapy to the brain (stereotactic radiosurgery or whole brain radiation therapy) may help as well. Systemic treatments (immunotherapy, targeted therapy, or chemo) might also be tried.
As with other stages of melanoma, people with recurrent melanoma may want to think about taking part in a clinical trial.
Melanoma prognosis
Your outlook (prognosis) depends on the stage of the melanoma when it was diagnosed. This means how deeply the melanoma has grown into the skin and whether it has spread. Survival is better for women than it is for men. Scientists don’t know exactly why this is. It may be because women are more likely to see a doctor about their melanoma at an earlier stage. Age can affect outlook and younger people have a better prognosis than older people. Your outlook may also be affected by where the melanoma is in the body.
Survival rates can give you an idea of what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding of how likely it is that your treatment will be successful. A relative survival rate compares people with the same type and stage of cancer to people in the overall population. For example, if the 5-year relative survival rate for a specific stage of melanoma of the skin is 93.7%, it means that people who have that cancer are, on average, about 93.7% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed 2.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for melanoma skin cancer in the United States, based on how far the cancer has spread 2. The SEER database, however, does not group cancers by American Joint Committee on Cancer (AJCC) TNM Staging System stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: There is no sign that the cancer has spread beyond the skin where it started.
- Regional: The cancer has spread beyond the skin where it started to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body, such as the lungs, liver, or skin on other parts of the body.
Table 4. 5-year relative survival rates for melanoma skin cancer
| SEER stage | 5-year relative survival rate |
|---|---|
| Localized | 99% |
| Regional | 68% |
| Distant | 30% |
| All SEER stages combined | 93% |
Footnotes:
- SEER = Surveillance, Epidemiology, and End Results. The SEER database is maintained by the National Cancer Institute (NCI), to provide survival statistics for different types of cancer.
- These numbers are based on people diagnosed with melanoma between 2011 and 2017
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, and other factors can also affect your outlook. For example, younger people tend to have a better outlook than older people, regardless of the stage. And people who have weakened immune systems, such as those who have had organ transplants or who are infected with HIV, are at greater risk of dying from their melanoma.
- People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Your doctor knows your situation, so ask them how these numbers might apply to you.
[Source 87 ]Survival statistics are available for each stage of melanoma. These figures are for men and women in England diagnosed between 2013 and 2017 88.
- Stage 1 melanoma: Almost everyone (almost 100%) will survive their cancer for 5 years or more after they are diagnosed.
- Stage 2 melanoma: 80 out of 100 people (80%) will survive their cancer for 5 years or more after diagnosis.
- Stage 3 melanoma: 70 out of 100 people (70%) will survive their cancer for 5 years or more after they are diagnosed.
- Stage 4 melanoma: The survival statistics for stage 4 melanoma don’t take into account the age of the people with melanoma. Statistics that do take into account the age (age-standardized statistics) are not available. Almost 30 out of 100 people (almost 30%) survive their cancer for 5 years or more after they are diagnosed.
Generally for people with melanoma in England:
- Almost all people (almost 100%) will survive their melanoma for 1 year or more after they are diagnosed
- Around 90 out of every 100 people (around 90%) will survive their melanoma for 5 years or more after diagnosis
- More than 85 out of every 100 people (more than 85%) will survive their melanoma for 10 years or more after they are diagnosed
Poor prognostic factors include the following 89:
- Tumor thickness (worse prognosis in thicker lesions)
- Evidence of tumor in regional lymph nodes (stage 3 disease)
- A higher number of positive lymph nodes
- Presence of distant metastasis (stage 4 disease)
- Anatomic site (trunk and/or face lesions have worse prognoses than extremity lesions)
- Presence of ulceration
- Presence of regression on histologic examination (controversial)
- Male sex
- Key Statistics for Melanoma Skin Cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html[↩][↩]
- Melanoma of the Skin — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/melan.html[↩][↩][↩][↩]
- Licata, G., Scharf, C., Ronchi, A., Pellerone, S., Argenziano, G., Verolino, P., & Moscarella, E. (2021). Diagnosis and Management of Melanoma of the Scalp: A Review of the Literature. Clinical, cosmetic and investigational dermatology, 14, 1435–1447. https://doi.org/10.2147/CCID.S293115[↩]
- Cutaneous Melanoma. A Pocket Guide for Diagnosis and Management. 2017, Chapter 3 – Diagnosis of Primary Melanoma. Pages 27-79. https://doi.org/10.1016/B978-0-12-804000-3.00003-X[↩]
- Morton DL, Essner R, Kirkwood JM, et al. Malignant Melanoma. In: Bast RC Jr, Kufe DW, Pollock RE, et al., editors. Holland-Frei Cancer Medicine. 5th edition. Hamilton (ON): BC Decker; 2000. Chapter 120. Available from: https://www.ncbi.nlm.nih.gov/books/NBK20896[↩][↩][↩][↩]
- Superficial spreading melanoma. https://dermnetnz.org/topics/superficial-spreading-melanoma[↩][↩]
- Melanoma (including melanoma in situ, superficial spreading melanoma, nodular melanoma). https://www.pcds.org.uk/clinical-guidance/melanoma-an-overview1[↩][↩]
- Fernandez-Flores A, Cassarino DS. Histopathological diagnosis of acral lentiginous melanoma in early stages. Ann Diagn Pathol. 2017 Feb;26:64-69. doi: 10.1016/j.anndiagpath.2016.08.005[↩]
- Teramoto Y., Martinez-Said H., Guo J., Garbe C. (2020) Acral Lentiginous Melanoma. In: Balch C. et al. (eds) Cutaneous Melanoma. Springer, Cham. https://doi.org/10.1007/978-3-030-05070-2_67[↩]
- Pizzichetta, M., Talamini, R., Stanganelli, I., Puddu, P., Bono, R., Argenziano, G., Veronesi, A., Trevisan, G., Rabinovitz, H. and Soyer, H. (2004), Amelanotic/hypomelanotic melanoma: clinical and dermoscopic features. British Journal of Dermatology, 150: 1117-1124. https://doi.org/10.1111/j.1365-2133.2004.05928.x[↩][↩]
- Amelanotic melanoma. https://dermnetnz.org/topics/amelanotic-melanoma[↩][↩][↩]
- Stefanaki, C., Chardalias, L., Soura, E., Katsarou, A. and Stratigos, A. (2017), Paediatric melanoma. J Eur Acad Dermatol Venereol, 31: 1604-1615. https://doi.org/10.1111/jdv.14299[↩]
- Cheung, W.L., Patel, R.R., Leonard, A., Firoz, B. and Meehan, S.A. (2012), Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. Journal of Cutaneous Pathology, 39: 33-39. https://doi.org/10.1111/j.1600-0560.2011.01808.x[↩]
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000 May;42(5 Pt 1):731-4. doi: 10.1067/mjd.2000.103981[↩]
- Clinical practice guidelines for the management of melanoma in Australia and New Zealand. Wellington: New Zealand Guidelines Group, 2008. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf[↩]
- Jaimes, Natalia & Braun, R.P. & Thomas, Luc & Marghoob, Ashfaq. (2011). Jaimes N, Braun RP, Thomas L et alClinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol 26:591-596. Journal of the European Academy of Dermatology and Venereology : JEADV. 26. 591-6. https://www.researchgate.net/profile/Luc-Thomas/publication/51139817_Jaimes_N_Braun_RP_Thomas_L_et_alClinical_and_dermoscopic_characteristics_of_amelanotic_melanomas_that_are_not_of_the_nodular_subtype_J_Eur_Acad_Dermatol_Venereol_26591-596/links/599fd7f5aca2724fca81aac1/Jaimes-N-Braun-RP-Thomas-L-et-alClinical-and-dermoscopic-characteristics-of-amelanotic-melanomas-that-are-not-of-the-nodular-subtype-J-Eur-Acad-Dermatol-Venereol-26591-596.pdf[↩]
- Mucosal melanoma. https://dermnetnz.org/topics/mucosal-melanoma[↩][↩]
- What is Ocular Melanoma? https://www.aao.org/eye-health/diseases/what-is-ocular-melanoma[↩]
- McLaughlin, C.C., Wu, X.-C., Jemal, A., Martin, H.J., Roche, L.M. and Chen, V.W. (2005), Incidence of noncutaneous melanomas in the U.S.. Cancer, 103: 1000-1007. https://doi.org/10.1002/cncr.20866[↩][↩][↩][↩][↩][↩]
- Jovanovic, P., Mihajlovic, M., Djordjevic-Jocic, J., Vlajkovic, S., Cekic, S., & Stefanovic, V. (2013). Ocular melanoma: an overview of the current status. International journal of clinical and experimental pathology, 6(7), 1230–1244. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3693189[↩]
- Vajdic CM, Kricker A, Giblin M, McKenzie J, Aitken J, Giles GG, Armstrong BK. Incidence of ocular melanoma in Australia from 1990 to 1998. Int J Cancer. 2003 May 20;105(1):117-22. doi: 10.1002/ijc.11057[↩]
- Hu DN, Yu G, McCormick SA, Finger PT. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol. 2008 Mar;145(3):418-423. doi: 10.1016/j.ajo.2007.10.022[↩]
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011 Sep;118(9):1881-5. doi: 10.1016/j.ophtha.2011.01.040[↩]
- Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003 Jun;135(6):800-6. doi: 10.1016/s0002-9394(02)02288-2[↩]
- Gallagher RP, Elwood JM, Rootman J, Threlfall WJ, Davis J. Symptoms and time to presentation and treatment in ocular melanoma: the Western Canada Melanoma Study. Can J Ophthalmol. 1988 Feb;23(1):11-3.[↩]
- Kaliki, S., Shields, C. Uveal melanoma: relatively rare but deadly cancer. Eye 31, 241–257 (2017). https://doi.org/10.1038/eye.2016.275[↩]
- Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, Kirkwood JM, Koh WJ, Robertson DM, Shaw JM, Straatsma BR, Thoma J; Collaborative Ocular Melanoma Study Group. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005 Dec;123(12):1639-43. doi: 10.1001/archopht.123.12.1639[↩]
- Feng, Z., Wu, X., Chen, V., Velie, E. and Zhang, Z. (2011), Incidence and survival of desmoplastic melanoma in the United States, 1992–2007. Journal of Cutaneous Pathology, 38: 616-624. https://doi.org/10.1111/j.1600-0560.2011.01704.x[↩]
- Quinn, M.J., Crotty, K.A., Thompson, J.F., Coates, A.S., O’Brien, C.J. and McCarthy, W.H. (1998), Desmoplastic and desmoplastic neurotropic melanoma. Cancer, 83: 1128-1135. https://doi.org/10.1002/(SICI)1097-0142(19980915)83:6<1128::AID-CNCR11>3.0.CO;2-7[↩]
- Xu, Zhe MDa; Yibulayin, Feiluore MDb; Shi, Ping MDa; Feng, Lei MDc,* Desmoplastic melanoma versus spindle cell melanoma, Medicine: July 2018 – Volume 97 – Issue 29 – p e11563 doi: 10.1097/MD.0000000000011563[↩]
- Wasif, N., Gray, R.J. and Pockaj, B.A. (2011), Desmoplastic melanoma – the step-child in the melanoma family?. J. Surg. Oncol., 103: 158-162. https://doi.org/10.1002/jso.21778[↩]
- Desmoplastic melanoma. https://dermnetnz.org/topics/desmoplastic-melanoma[↩][↩]
- Boada Garcia A, Quer Pi-Sunyer A, Richarz N, Jaka-Moreno A. Update on the Diagnosis and Management of Desmoplastic Melanoma. Actas Dermosifiliogr. 2022 Jan;113(1):47-57. English, Spanish. doi: 10.1016/j.ad.2021.06.004[↩][↩][↩][↩]
- Prasad, M.L., Patel, S.G. and Busam, K.J. (2004), Primary mucosal desmoplastic melanoma of the head and neck. Head Neck, 26: 373-377. https://doi.org/10.1002/hed.10384[↩]
- Desmoplastic melanoma of the hand: case reports and review of the literature. J Hand Surg Am., 20 (1995), pp. 873-876. https://doi.org/10.1016/S0363-5023(05)80447-2[↩]
- de Almeida, Luis Soares MD*; Requena, Luis MD†; Rütten, Arno MD‡; Kutzner, Heinz MD‡; Garbe, Claus MD§; Pestana, Dinis PhD¶; Gomes, Manuel Marques MD* Desmoplastic Malignant Melanoma: A Clinicopathologic Analysis of 113 Cases, The American Journal of Dermatopathology: June 2008 – Volume 30 – Issue 3 – p 207-215 doi: 10.1097/DAD.0b013e3181716e6b[↩][↩][↩]
- Busam, Klaus J MD*; Mujumdar, Urvi MPH†; Hummer, Amanda J MS†; Nobrega, Jennifer BA*; Hawkins, William G MD‡; Coit, Daniel G MD‡; Brady, Mary S MD‡ Cutaneous Desmoplastic Melanoma, The American Journal of Surgical Pathology: November 2004 – Volume 28 – Issue 11 – p 1518-1525 doi: 10.1097/01.pas.0000141391.91677.a4[↩]
- Nicolson, NG, Han, D. Desmoplastic melanoma. J Surg Oncol. 2019; 119: 208– 215. https://doi.org/10.1002/jso.25317[↩][↩][↩]
- Murali, R., Shaw, H.M., Lai, K., McCarthy, S.W., Quinn, M.J., Stretch, J.R., Thompson, J.F. and Scolyer, R.A. (2010), Prognostic factors in cutaneous desmoplastic melanoma. Cancer, 116: 4130-4138. https://doi.org/10.1002/cncr.25148[↩][↩]
- Desmoplastic melanoma: demographic and clinicopathological features and disease-specific prognostic factors. Oncol Lett., 17 (2019), pp. 5619-5627. https://doi.org/10.3892/ol.2019.10259[↩][↩]
- Maurichi, Andrea MD*; Miceli, Rosalba PhD†; Camerini, Tiziana PhD‡; Contiero, Paolo PhD§; Patuzzo, Roberto MD*; Tragni, Gabrina MD¶; Crippa, Federica MD*; Romanidis, Konstantinos MD*; Ruggeri, Roberta MD*; Carbone, Antonino MD¶; Santinami, Mario MD* Pure Desmoplastic Melanoma, Annals of Surgery: December 2010 – Volume 252 – Issue 6 – p 1052-1057 doi: 10.1097/SLA.0b013e3181efc23c[↩][↩][↩]
- Conic, Ruzica Z. MD*†; Ko, Jennifer MD‡; Allam, Sherihan H. MD, MSc†; Atanaskova-Mesinkovska, Natasha MD, PhD†§; Kovalyshyn, Ivanka DO†‡; Bergfeld, Wilma MD†‡; Gastman, Brian R. MD† Mixed Versus Pure Variants of Desmoplastic Melanoma, Annals of Plastic Surgery: March 2018 – Volume 80 – Issue 3 – p 277-281 doi: 10.1097/SAP.0000000000001225[↩]
- Laeijendecker, AE, El Sharouni, M-A, Sigurdsson, V, van Diest, PJ. Desmoplastic melanoma: The role of pure and mixed subtype in sentinel lymph node biopsy and survival. Cancer Med. 2020; 9: 671– 677. https://doi.org/10.1002/cam4.2736[↩]
- Lens, M., Newton-Bishop, J. and Boon, A. (2005), Desmoplastic malignant melanoma: a systematic review. British Journal of Dermatology, 152: 673-678. https://doi.org/10.1111/j.1365-2133.2005.06462.x[↩]
- Posther, K.E., Selim, M.A., Mosca, P.J. et al. Histopathologic Characteristics, Recurrence Patterns, and Survival of 129 Patients With Desmoplastic Melanoma. Ann Surg Oncol 13, 728–739 (2006). https://doi.org/10.1245/ASO.2006.03.091[↩]
- Jaroszewski DE, Pockaj BA, DiCaudo DJ, Bite U. The clinical behavior of desmoplastic melanoma. Am J Surg. 2001 Dec;182(6):590-5. doi: 10.1016/s0002-9610(01)00819-4[↩]
- Khan F, Strohl A, Allen PD, Doerr TD. Desmoplastic Melanoma of the Head and Neck: Incidence and Survival, 1992-2013. Otolaryngology–Head and Neck Surgery. 2017;157(4):648-656. doi:10.1177/0194599817725696[↩]
- Baer, S.C., Schultz, D., Synnestvedt, M. and Elder, D.E. (1995), Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer, 76: 2242-2247. https://doi.org/10.1002/1097-0142(19951201)76:11<2242::AID-CNCR2820761110>3.0.CO;2-I[↩]
- Winnepenninckx V, De Vos R, Stas M, van den Oord JJ. New phenotypical and ultrastructural findings in spindle cell (desmoplastic/neurotropic) melanoma. Appl Immunohistochem Mol Morphol. 2003 Dec;11(4):319-25. doi: 10.1097/00129039-200312000-00007[↩]
- Díaz A, Valera A, Carrera C, Hakim S, Aguilera P, García A, Palou J, Puig S, Malvehy J, Alos L. Pigmented spindle cell nevus: clues for differentiating it from spindle cell malignant melanoma. A comprehensive survey including clinicopathologic, immunohistochemical, and FISH studies. Am J Surg Pathol. 2011 Nov;35(11):1733-42. doi: 10.1097/PAS.0b013e318229cf66[↩]
- Xu, Z., Shi, P., Yibulayin, F., Feng, L., Zhang, H., & Wushou, A. (2018). Spindle cell melanoma: Incidence and survival, 1973-2017. Oncology letters, 16(4), 5091–5099. https://doi.org/10.3892/ol.2018.9247[↩][↩][↩][↩]
- Piao Y, Guo M, Gong Y. Diagnostic challenges of metastatic spindle cell melanoma on fine-needle aspiration specimens. Cancer. 2008 Apr 25;114(2):94-101. doi: 10.1002/cncr.23345[↩]
- Kim, J., Lazar, A. J., Davies, M. A., Homsi, J., Papadopoulos, N. E., Hwu, W. J., Bedikian, A. Y., Woodman, S. E., Patel, S. P., Hwu, P., & Kim, K. B. (2012). BRAF, NRAS and KIT sequencing analysis of spindle cell melanoma. Journal of cutaneous pathology, 39(9), 821–825. https://doi.org/10.1111/j.1600-0560.2012.01950.x[↩]
- Weissinger SE, Keil P, Silvers DN, Klaus BM, Möller P, Horst BA, Lennerz JK. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014 Apr;27(4):524-34. doi: 10.1038/modpathol.2013.162[↩][↩][↩]
- Tacha D, Qi W, Ra S, Bremer R, Yu C, Chu J, Hoang L, Robbins B. A newly developed mouse monoclonal SOX10 antibody is a highly sensitive and specific marker for malignant melanoma, including spindle cell and desmoplastic melanomas. Arch Pathol Lab Med. 2015 Apr;139(4):530-6. doi: 10.5858/arpa.2014-0077-OA[↩]
- Stowman AM, Mills SE, Wick MR. Spindle Cell Melanoma and Interdigitating Dendritic Cell Sarcoma: Do They Represent the Same Process? Am J Surg Pathol. 2016 Sep;40(9):1270-9. doi: 10.1097/PAS.0000000000000678[↩]
- Yeh I, Vemula SS, Mirza SA, McCalmont TH. Neurofibroma-like spindle cell melanoma: CD34 fingerprint and CGH for diagnosis. Am J Dermatopathol. 2012;34:668–670. doi: 10.1097/DAD.0b013e318244819a[↩]
- Rawandale, N. A., & Suryawanshi, K. H. (2016). Primary Spindle Cell Malignant Melanoma of Esophagus: An Unusual Finding. Journal of clinical and diagnostic research : JCDR, 10(2), OD03–OD4. https://doi.org/10.7860/JCDR/2016/16121.7188[↩]
- Lee, D.A., Cohen, J.A., Twaddell, W.S., Palacios, G., Gill, M., Levit, E., Halperin, A.J., Mones, J., Busam, K.J., Silvers, D.N. and Celebi, J.T. (2006), Are all melanomas the same?. Cancer, 106: 907-913. https://doi.org/10.1002/cncr.21686[↩]
- Kamino H. Spitzoid melanoma. Clin Dermatol. 2009 Nov-Dec;27(6):545-55. doi: 10.1016/j.clindermatol.2008.09.013[↩][↩]
- Zedek DC, McCalmont TH. Spitz nevi, atypical spitzoid neoplasms, and spitzoid melanoma. Clin Lab Med. 2011 Jun;31(2):311-20. doi: 10.1016/j.cll.2011.03.008[↩][↩]
- Spitzoid melanoma. https://dermnetnz.org/topics/spitzoid-melanoma[↩]
- Pol-Rodriquez, M., Lee, S., Silvers, D.N. and Celebi, J.T. (2007), Influence of age on survival in childhood spitzoid melanomas. Cancer, 109: 1579-1583. https://doi.org/10.1002/cncr.22584[↩]
- Kim, J. Y., Choi, J. E., Ahn, H. H., Kye, Y. C., & Seo, S. H. (2012). A case of spitzoid melanoma with lymph node metastasis in a child. Journal of Korean medical science, 27(4), 454–457. https://doi.org/10.3346/jkms.2012.27.4.454[↩]
- Carrera, C., Scope, A., Dusza, S. W., Argenziano, G., Nazzaro, G., Phan, A., Tromme, I., Rubegni, P., Malvehy, J., Puig, S., & Marghoob, A. A. (2018). Clinical and dermoscopic characterization of pediatric and adolescent melanomas: Multicenter study of 52 cases. Journal of the American Academy of Dermatology, 78(2), 278–288. https://doi.org/10.1016/j.jaad.2017.09.065[↩]
- Melanoma. https://www.mayoclinic.org/diseases-conditions/melanoma/symptoms-causes/syc-20374884[↩]
- What Causes Melanoma Skin Cancer? https://www.cancer.org/cancer/melanoma-skin-cancer/causes-risks-prevention/what-causes.html[↩][↩]
- Lynch, H. T., Frichot, B. C., 3rd, & Lynch, J. F. (1978). Familial atypical multiple mole-melanoma syndrome. Journal of medical genetics, 15(5), 352–356. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1013730/pdf/jmedgene00300-0024.pdf[↩]
- Clark WH Jr, Ainsworth AM, Bernardino EA, Yang CH, Mihm CM Jr, Reed RJ. The developmental biology of primary human malignant melanomas. Semin Oncol. 1975 Jun;2(2):83-103.[↩]
- Bale SJ, Dracopoli NC, Tucker MA, Clark WH Jr, Fraser MC, Stanger BZ, Green P, Donis-Keller H, Housman DE, Greene MH. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p. N Engl J Med. 1989 May 25;320(21):1367-72. doi: 10.1056/NEJM198905253202102. Erratum in: N Engl J Med 1991 Mar 28;324(13):925.[↩][↩]
- Ghiorzo P, Ciotti P, Mantelli M, Heouaine A, Queirolo P, Rainero ML, Ferrari C, Santi PL, De Marchi R, Farris A, Ajmar F, Bruzzi P, Bianchi-Scarrà G. Characterization of ligurian melanoma families and risk of occurrence of other neoplasia. Int J Cancer. 1999 Nov 12;83(4):441-8. doi: 10.1002/(sici)1097-0215(19991112)83:4<441::aid-ijc2>3.0.co;2-r[↩]
- Essner R, Kuo CT, Wang H, Wen DR, Turner RR, Nguyen T, Hoon DS. Prognostic implications of p53 overexpression in cutaneous melanoma from sun-exposed and nonexposed sites. Cancer. 1998 Jan 15;82(2):309-16. doi: 10.1002/(sici)1097-0142(19980115)82:2<317::aid-cncr10>3.0.co;2-1[↩]
- Lucchina LC, Barnhill RL, Duke DM, Sober AJ. Familial cutaneous melanoma. Melanoma Res. 1995 Dec;5(6):413-8. doi: 10.1097/00008390-199512000-00004[↩]
- Cannon-Albright LA, Goldgar DE, Meyer LJ, Lewis CM, Anderson DE, Fountain JW, Hegi ME, Wiseman RW, Petty EM, Bale AE, et al. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148-52. doi: 10.1126/science.1439824[↩]
- Fountain, J. W., Karayiorgou, M., Ernstoff, M. S., Kirkwood, J. M., Vlock, D. R., Titus-Ernstoff, L., Bouchard, B., Vijayasaradhi, S., Houghton, A. N., & Lahti, J. (1992). Homozygous deletions within human chromosome band 9p21 in melanoma. Proceedings of the National Academy of Sciences of the United States of America, 89(21), 10557–10561. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC50378/pdf/pnas01095-0592.pdf[↩]
- Bastian BC, LeBoit PE, Hamm H, Bröcker EB, Pinkel D. Chromosomal gains and losses in primary cutaneous melanomas detected by comparative genomic hybridization. Cancer Res. 1998 May 15;58(10):2170-5.[↩]
- Ultraviolet radiation and human health. https://dermnetnz.org/topics/ultraviolet-radiation-and-human-health[↩]
- UV Index Scale. https://www.epa.gov/sunsafety/uv-index-scale-0[↩]
- What is Sunscreen? https://www.aimatmelanoma.org/melanoma-101/prevention/sunscreen/[↩]
- Melanoma Skin Cancer Stages. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/melanoma-skin-cancer-stages.html[↩]
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®): Patient Version. 2021 Sep 3. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage 0 melanoma. Abnormal melanocytes…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65950/figure/CDR0000062713__383[↩]
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®): Patient Version. 2021 Sep 3. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage I melanoma. In stage…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65950/figure/CDR0000062713__384[↩]
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®): Patient Version. 2021 Sep 3. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage IIA melanoma. The tumor…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65950/figure/CDR0000062713__385[↩]
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®): Patient Version. 2021 Sep 3. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage IIB melanoma. The tumor…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65950/figure/CDR0000062713__386[↩]
- PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage IIC melanoma. The tumor…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65951/figure/CDR0000045137__6[↩]
- PDQ Adult Treatment Editorial Board. Melanoma Treatment (PDQ®): Patient Version. 2021 Sep 3. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage IV melanoma. Cancer has…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65950/figure/CDR0000062713__388[↩]
- Survival Rates for Melanoma Skin Cancer. https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html[↩]
- Melanoma skin cancer survival. https://www.cancerresearchuk.org/about-cancer/melanoma/survival[↩]
- Heistein JB, Acharya U. Malignant Melanoma. [Updated 2021 Nov 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470409[↩]