Multiple organ dysfunction syndrome
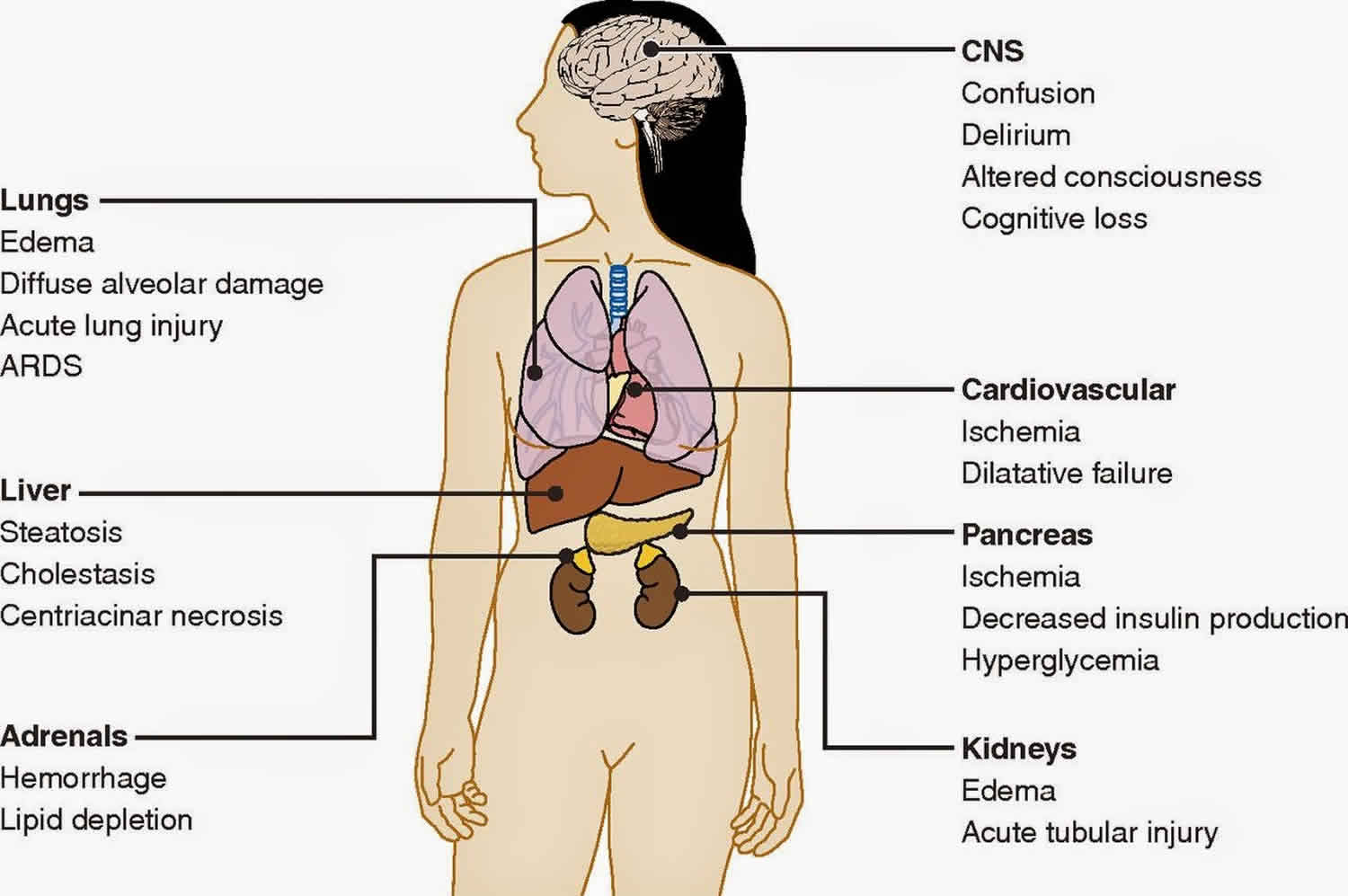
Multiple organ dysfunction syndrome (MODS) also called multi-organ failure, multiple systems organ failure, or through some of its more prominent manifestations, as the acute respiratory distress syndrome (ARDS) or disseminated intravascular coagulation (DIC), is one of the most common syndromes of critical illness and the leading cause of mortality among critically ill patients 1. Multiple organ dysfunction syndrome can be defined as the development of potentially reversible physiologic derangement involving 2 or more organ systems not involved in the disorder that resulted in ICU admission, and arising in the wake of a potentially life-threatening physiologic insult 2. Multiple organ dysfunction syndrome is a continuum, with incremental degrees of physiologic derangements in individual organs; it is a process rather than a single event 3. Alteration in organ function can vary widely from a mild degree of organ dysfunction to completely irreversible organ failure. The degree of organ dysfunction has a major clinical impact.
Multiple organ dysfunction syndrome is the clinical consequence of a dysregulated inflammatory response, triggered by clinically diverse factors with the main pillar of management being invasive organ support. During the last years, the advances in the clarification of the molecular pathways that trigger, mitigate, and determine the outcome of multiple organ dysfunction syndrome have led to the increasing recognition of multiple organ dysfunction syndrome as a distinct disease entity with distinct etiology, pathophysiology, and potential future therapeutic interventions. Given the lack of effective treatment for multiple organ dysfunction syndrome, its early recognition, the early intensive care unit admission, and the initiation of invasive organ support remain the most effective strategies of preventing its progression and improving outcomes.
Multiple organ dysfunction syndrome is as poorly understood as it is prevalent. Even its terminology merits comment. Although originally described as multiple organ failure, it is evident that normal physiologic function of the failing organ systems can be restored in survivors. Thus characterization of the process as multiple organ dysfunction is more appropriate. And although the syndrome involves the dysfunction of many organs, it also affects physiologic systems not classically thought of as organs, including the hematologic system, immune system, or the endocrine system. Finally, although it is described as a syndrome, its clinical course and causes are highly variable, and there is only the most general form of consensus regarding the organs whose dysfunction comprises the syndrome, or the criteria that should be used to describe this dysfunction.
Organ system specific support is the most important reason for the ICU, and it is not surprising, therefore, that the need for such support has become a model for describing the clinical course of the critically ill patient. The observation that critically ill patients die, not as a result of the progression of the disorder that precipitated ICU admission, but of a complex series of physiologic derangements that develop following resuscitation and management in the ICU was first made in the 1960’s. Baue, in 1975, published a landmark editorial in which he commented on the striking similarity of the post mortem findings in patients dying in an ICU and suggested that it was not the failure of a single system, but the concomitant failure of multiple interdependent organ systems that was the unsolved problem in critical care. Subsequent reports highlighted the important role of occult, uncontrolled infection in the pathogenesis of multiple organ dysfunction syndrome, although control of infection did not necessarily result in reversal of the physiologic derangements, nor was infection universally present in patients with the syndrome.
Multiple organ dysfunction syndrome pathophysiology
The pathophysiology of multiple organ dysfunction syndrome is characterized by a severe, systemic, somewhat uncontrolled inflammatory process that leads to multiple organ or system dysfunctions 4. Local and systemic responses are initiated by tissue damage. Respiratory failure is common in the first 72 hours after the original insult. Following this one might see hepatic failure (5-7 days), gastrointestinal bleeding (10-15 days), and renal failure (11-17 days).
Malignant intravascular inflammation
Sepsis has been referred to as a process of malignant intravascular inflammation. Normally, a potent, complex, immunologic cascade ensures a prompt protective response to microorganism invasion in humans. A deficient immunologic defense may allow infection to become established; however, an excessive or poorly regulated response may harm the host through maladaptive release of indigenously generated inflammatory compounds
Lipid A and other bacterial products release cytokines and other immune modulators that mediate the clinical manifestations of sepsis. Interleukins, tumor necrosis factor (TNF)-α, interferon gamma (IFN-γ), and other colony-stimulating factors are produced rapidly within minutes or hours after interactions of monocytes and macrophages with lipid A.
Inflammatory mediator release becomes a self-stimulating process, and release of other such mediators, including interleukin (IL)-1, platelet activating factor, IL-2, IL-6, IL-8, IL-10, and nitric oxide (NO), further increases cytokine levels. This leads to continued activation of polymorphonuclear leukocytes, macrophages, and lymphocytes; proinflammatory mediators recruit more of these cells. All of these processes create a state of destructive immunologic dissonance.
Sepsis is described as an autodestructive process that permits extension of the normal pathophysiologic response to infection to involve otherwise normal tissues and results in multiple organ dysfunction syndrome. Organ dysfunction or organ failure may be the first clinical sign of sepsis, and no organ system is immune from the consequences of the inflammatory excesses of sepsis. Mortality increases as organ failure increases.
Although uncontrolled, once multiple organ dysfunction syndrome develops systemic evidence of both proinflammatory and anti-inflammatory up-regulation are usually present, suggesting that failure of host defense homeostasis is the final pathway from sepsis to multiple organ dysfunction syndrome, rather than simple hypotension-induced end-organ injury, as may occur with hemorrhagic shock. Survival from severe sepsis with multiple organ dysfunction syndrome is usually associated with a generalized reduction in both the proinflammatory and anti-inflammatory response.
A novel hypothesis has recently emerged that survival from severe sepsis requires a generalized down-regulation of the body’s immune response, energetic functions, and associated organ performance. Thus, multiple organ dysfunction syndrome may by the host’s adaptive response to overwhelming inflammation, allowing inflammation to clear without causing permanent end-organ harm. As discussed below, all organs reveal a generalized hyporesponsiveness that is clearly abnormal in health but may mark a survival strategy in severe sepsis.
Dysfunction of organ systems
Circulatory derangement
Significant derangement in autoregulation of circulation is typical of sepsis. Vasoactive mediators cause vasodilatation and increase microvascular permeability at the site of infection. nitric oxide plays a central role in the vasodilatation of septic shock. Also, impaired secretion of vasopressin may occur, which may permit persistence of vasodilatation.
Changes in both systolic and diastolic ventricular performance occur in sepsis. Through the use of the Frank-Starling mechanism, cardiac output often is increased to maintain blood pressure in the presence of systemic vasodilatation. Patients with preexisting cardiac disease are unable to increase their cardiac output appropriately.
Regionally, sepsis interferes with the normal distribution of systemic blood flow to organ systems. Consequently, core organs may not receive appropriate oxygen delivery, and the result is what is known as regional hypoperfusion.
Microcirculation is the key target organ for injury in sepsis since vascular endothelium is universally affected by the circulating inflammatory mediators. Although it is unclear if microcirculatory abnormalities are the cause or an innocent bystander of the end-organ injury, clear microvascular dysfunction is seen. A decrease in the number of perfused capillaries is seen, although with application of vasodilator therapies, full microvascular recruitment occurs. Mitochondrial dysfunction also occurs and is often associated with reduced mitochondrial transmembrane potential gradients, which are necessary to drive oxidative phosphorylation. The end result is an apparent inability of end-organs to extract oxygen maximally.
Debate continues as to whether this failure of energy metabolism is an adaptive cytoprotective mechanism similar to hibernation or reflects primary mitochondrial pathology. These are areas of active research but do not presently translate into clear clinical practice guidelines. Increased capillary endothelial permeability leads to widespread protein-rich tissue edema.
Septic shock and systemic inflammatory response syndrome are characterized by reversible myocardial depression, which can prove resistant to catecholamine and fluid administration. Circulating “myocardial depressant factor”—probably representing the synergistic effects of TNF-α, IL-1β, other cytokines, and nitric oxide—is implicated in pathogenesis. The two characteristics of this acute stress myocardial depression are impaired adrenergic responsiveness and diastolic dysfunction leading to relative catecholamine resistance and small rather than dilated hearts. Macrovascular myocardial ischemia and hypoperfusion are unlikely contributors.
In severe sepsis and septic shock, microcirculatory dysfunction and mitochondrial depression cause regional tissue distress, and regional dysoxia therefore persists. This condition is termed microcirculatory and mitochondrial distress syndrome 5. Sepsis-induced inflammatory autoregulatory dysfunction persists, and oxygen need is not matched by supply, leading to multiple organ dysfunction syndrome.
Redistribution of intravascular fluid volume resulting from reduced arterial vascular tone, diminished venous return from venous dilation, and release of myocardial depressant substances causes hypotension.
Pulmonary dysfunction
Endothelial injury in the pulmonary vasculature leads to disturbed capillary blood flow and enhanced microvascular permeability, resulting in interstitial and alveolar edema. Neutrophil entrapment within the pulmonary microcirculation initiates and amplifies the injury to alveolar capillary membranes. Acute lung injury and acute respiratory distress syndrome (ARDS) are frequent manifestations of these effects. Indeed, sepsis and pneumonia are the most common causes of ARDS.
Gastrointestinal dysfunction
The gastrointestinal (GI) tract may help propagate the injury of sepsis. Overgrowth of bacteria in the upper gastrointestinal tract may be aspirated into the lungs, producing nosocomial or aspiration pneumonia. The normal barrier function of the gut may be affected, allowing translocation of bacteria, endotoxins, and normal digestive proteases into the systemic circulation and extending the septic response.
Septic shock can cause paralytic ileus that can lead to a delay in the institution of enteral feeding. Excess nitric oxide production is thought to be the causative agent of sepsis-induced ileus. The optimal level of nutritional intake is interfered with in the face of high protein and calorie requirements. Narcotics and muscle relaxants can further worsen gastrointestinal tract motility.
Liver dysfunction
As a consequence of the role the liver plays in host defense, the abnormal synthetic functions caused by liver dysfunction can contribute to both the initiation and progression of sepsis. The reticuloendothelial system of the liver acts as a first line of defense in clearing bacteria and their products; liver dysfunction leads to a spillover of these products into systemic circulation.
Liver failure (“shocked liver”) can be manifested by elevations in liver enzymes and bilirubin, coagulation defects, and failure to excrete toxins such as ammonia, which lead to worsening encephalopathy.
Renal dysfunction
Acute kidney injury often accompanies sepsis. Different etiologies for acute kidney injury have been reported, and the cause is typically thought to be multifactorial 6. The mechanism of acute kidney injury is complex but likely involves a decrease in effective intravascular volume resulting from systemic hypotension, direct renal vasoconstriction, release of cytokines, and activation of neutrophils by endotoxins and other peptides, which contribute to renal injury. Still, most animal studies show that renal blood flow is increased, not decreased, in sepsis, though associated with impaired tubular function and a lack of significant histologic evidence of tubular injury.
Central nervous system dysfunction
Involvement of the central nervous system (CNS) in sepsis produces encephalopathy and peripheral neuropathy. The pathogenesis is poorly defined but is probably related to systemic hypotension, which can lead to brain hypoperfusion.
Coagulopathy
Subclinical coagulopathy, signaled by a mild elevation of the thrombin time (TT) or activated partial thromboplastin time (aPTT) or a moderate reduction in the platelet count, is extremely common; however, overt disseminated intravascular coagulation (DIC) may also develop. Protease-activated receptors (PARs), especially PAR 1, form the molecular link between coagulation and inflammation; PAR1 exerts cytoprotective effects when stimulated by activated protein C or low-dose thrombin but exerts disruptive effects on endothelial-cell barrier function when activated by high-dose thrombin 7.
Mechanisms of organ dysfunction and injury
The precise mechanisms of cell injury and resulting organ dysfunction in sepsis are not fully understood. multiple organ dysfunction syndrome is associated with widespread endothelial and parenchymal cell injury, some of which can be explained by the following 4 proposed mechanisms.
Hypoxic hypoxia
The septic circulatory lesion disrupts tissue oxygenation, alters the metabolic regulation of tissue oxygen delivery, and contributes to organ dysfunction. Microvascular and endothelial abnormalities contribute to the septic microcirculatory defect in sepsis. The reactive oxygen species, lytic enzymes, and vasoactive substances (eg, nitric oxide and endothelial growth factors) lead to microcirculatory injury, which is compounded by the inability of the erythrocytes to navigate the septic microcirculation.
Direct cytotoxicity
Endotoxin, TNF-α, and nitric oxide may cause damage to mitochondrial electron transport, leading to disordered energy metabolism. This is called cytopathic or histotoxic anoxia, an inability to utilize oxygen even when it is present.
Apoptosis
Apoptosis (programmed cell death) is the principal mechanism by which dysfunctional cells are normally eliminated. The proinflammatory cytokines may delay apoptosis in activated macrophages and neutrophils, but other tissues (eg, gut epithelium), may undergo accelerated apoptosis. Therefore, derangement of apoptosis plays a critical role in the tissue injury of sepsis.
Immunosuppression
The interaction between proinflammatory and anti-inflammatory mediators may lead to an imbalance between them. An inflammatory reaction or an immunodeficiency may predominate, or both may be present.
Host response and other factors influencing outcome
Clinical characteristics that relate to the severity of sepsis include the host response to infection, the site and type of infection, the timing and type of antimicrobial therapy, the offending organism, the development of shock, the underlying disease, the patient’s long-term health condition, and the number of failed organs. Factors that lead to sepsis and septic shock may not be essential in determining the ultimate outcome.
The host response to sepsis is characterized by both proinflammatory responses and anti-inflammatory immunosuppressive responses. The direction, extent, and duration of these reactions are determined by both host factors (eg, genetic characteristics, age, coexisting illnesses, medications) and pathogen factors (eg, microbial load, virulence) 8.
Inflammatory responses are initiated by interaction between pathogen-associated molecular patterns expressed by pathogens and pattern recognition receptors expressed by host cells at the cell surface (toll-like receptors [TLRs] and C-type lectin receptors [CLRs]), in the endosome (TLRs), or in the cytoplasm (retinoic acid inducible gene 1–like receptors [RLRs] and nucleotide-binding oligomerization domain–like receptors [NLRs]) 8.
The consequence of exaggerated inflammation is collateral tissue damage and necrotic cell death, which results in the release of damage-associated molecular patterns, so-called danger molecules that perpetuate inflammation at least in part by acting on the same pattern-recognition receptors triggered by pathogens 8.
Multiple organ dysfunction syndrome prevention
Patients with impaired host defense mechanisms are at greatly increased risk for sepsis and multiple organ dysfunction syndrome. The main causes are chemotherapeutic drugs, malignancy, severe trauma, burns, diabetes mellitus, renal or hepatic failure, old age, ventilatory support, and invasive catheters.
One way of helping to prevent severe sepsis is to avoid invasive catheters or remove them as soon as possible. Prophylactic antibiotics in the perioperative phase, particularly after gastrointestinal surgery, may be beneficial. Use of topical antibiotics around invasive catheters and as part of a dressing for patients with burns is helpful. Maintenance of adequate nutrition, administration of pneumococcal vaccine to patients who have undergone splenectomy, and early enteral feeding are other preventive measures.
Topical or systemic antibiotics have been given to prevent sepsis and multiple organ dysfunction syndrome in high-risk patients. The use of nonabsorbable antibiotics in the stomach to prevent translocation of bacteria and occurrence of bacteremia has been a controversial issue. Numerous trials have been performed over the years using either topical antibiotics alone or a combination of topical and systemic antibiotics.
A systematic review by Nathens presented no benefit in medical patients but a reduced mortality in surgical trauma patients 9. The beneficial effect was from a combination of systemic and topical antibiotics, predominantly involving reduction of lower respiratory tract infections in patients who were treated.
Table 1 summarizes ICU interventions for which there is evidence of significant benefit, reflected in reduced organ dysfunction, or improved ICU survival. The list is a sampling that is of necessity inadequate. Any intervention that can prevent death or bring physiologic benefit to critically ill patients might reasonably be included; on the other hand, rigorous evaluation of most commonly accepted ICU interventions has not been undertaken.
Table 1. The prevention of multiple organ dysfunction syndrome in critical illness
| Target system | Convincing evidence | Controversial or investigational |
|---|---|---|
| Lung | Pressure or volume limited ventilation to minimize barotrauma and volutrauma | Liquid ventilation, non-physiologic modes of ventilation (high frequency, oscillation) |
| Cardiovascular | Restrict transfusion of packed red cells when hemoglobin is > 70 | Supranormal oxygen delivery; non-crystalloid fluids; SwanGanz catheterization |
| Renal | Avoidance of nephrotoxins | Continuous veno-venous hemofiltration |
| Gastrointestinal | Stress ulcer prophylaxis with H2 blockers rather than sucralfate | Gastric tonometry |
| Enteral nutrition | ||
| Hematologic | DVT prophylaxis | Anticoagulant therapies such as anti-thrombin III |
| Immunologic | SDD (Selective Decontamination of the Digestive Tract) | Anti-cytokine and other mediator-targeted therapies |
| Endocrine | Corticosteroids in late sepsis |
Multiple organ dysfunction syndrome symptoms
Symptoms of sepsis are usually nonspecific and include fever, chills, and constitutional symptoms of fatigue, malaise, anxiety, or confusion 10. These symptoms are not pathognomonic for infection and may also be observed in a wide variety of noninfectious inflammatory conditions. In addition, they may be absent in patients with serious infections, especially in elderly individuals.
Because systemic inflammatory response syndrome (SIRS), sepsis, septic shock, and multiple organ dysfunction syndrome represent a clinical continuum, the specific features exhibited in any given case depend on where the patient falls on that continuum.
Fever is a common feature of sepsis. Fever of infectious origin results from resetting the hypothalamus so that heat production and heat loss are balanced to maintain a higher temperature. An abrupt onset of fever usually is associated with a large infectious load.
Chills are a secondary symptom associated with fever and result from increased muscular activity in an attempt to produce heat and thereby raise the body temperature to the level required to reset the hypothalamus.
Sweating occurs when the hypothalamus returns to its normal set point and senses that the body temperature is above the desired level. Perspiration is stimulated to offload excess body heat through evaporative cooling.
Altered mental function is often observed. Mild disorientation or confusion is especially common in elderly individuals. More severe manifestations include apprehension, anxiety, and agitation, and in some cases, coma may eventually ensue. The mechanism by which mental function is altered is not known, but altered amino acid metabolism has been proposed as a cause of metabolic encephalopathy.
Hyperventilation with respiratory alkalosis is a common feature of sepsis. Stimulation of the medullary ventilatory center by endotoxins and other inflammatory mediators has been proposed as the cause of hyperventilation.
The following localizing symptoms are some of the most useful clues to the etiology of both fever and sepsis:
- Head and neck infections – Earache, sore throat, sinus pain, or swollen lymph glands
- Chest and pulmonary infections – Cough (especially if productive), pleuritic chest pain, and dyspnea
- Abdominal and gastrointestinal (GI) infections – Abdominal pain, nausea, vomiting, and diarrhea
- Pelvic and genitourinary (GU) infections – Pelvic or flank pain, vaginal or urethral discharge, urea, frequency, urgency
- Bone and soft tissue infections – Focal pain or tenderness, focal erythema, edema
Organ dysfunction in a critically ill patient can be described in one of two ways: 1) as the clinical intervention that was employed to support the failing organ system (mechanical ventilation, hemodialysis, inotropic or vasopressor agents, parenteral nutrition etc) or 2) as the acute physiologic derangement that made such support necessary. The first descriptions of the syndrome generally counted the number of failing systems, using as descriptors, the need for clinical intervention. More recently several similar descriptive scales have been developed, based on the quantification of organ dysfunction as a numeric scale. Each uses the same six organ systems to characterize multiple organ dysfunction syndrome – the respiratory, cardiovascular, renal, hepatic, neurologic, and hematologic systems. They differ in minor ways with respect to the selected parameters to describe cardiovascular dysfunction, and in the timing and weighting of the variables selected. The Multiple Organ Dysfunction (MOD) score, a scale that uses physiologic variables exclusively is presented in Table 2.
Table 2. The multiple organ dysfunction (MOD) score
| Organ system | 0 | 1 | >2 | 3 | 4 |
|---|---|---|---|---|---|
| Respiratorya | |||||
| (PO2/FIO2 Ratio) | > 300 | 226–300 | 151–225 | 76–150 | ≤ 75 |
| Renalb | |||||
| (Serum Creatinine) | ≤ 100 | 101–200 | 201–350 | 351–500 | > 500 |
| Hepaticc | |||||
| (Serum Bilirubin) | ≤ 20 | 21–60 | 61–120 | 121–240 | > 240 |
| Cardiovasculard | |||||
| (R/P Ratio) | ≤ 10.0 | >10.1–15.0 | 15.1–20.0 | 20.1–30.0 | > 30.0 |
| Hematologice | |||||
| (Platelet count) | > 120 | 81–120 | 51–80 | 21–50 | ≤ 20 |
| Neurologicf | |||||
| (Glasgow Coma Score) | 15 | 13–14 | 10–12 | 7–9 | ≤ 6 |
Footnotes:
a) The PO2/FIO2 ratio is calculated without reference to the use or mode of mechanical ventilation, and without reference to the use or level of PEEP.
b) The serum creatinine level is measured in μmol/liter, without reference to the use of dialysis.
c) The serum bilirubin level is measured in μmol/liter.
d) The R/P ratio is calculated as the product of the heart rate and right atrial (central venous) pressure, divided by the mean arterial pressure: R/P ratio = (heart rate x central venous pressure) / mean blood pressure
e) The platelet count is measured in platelets/mL 10-3
f) The Glasgow Coma Score is preferably calculated by the patient’s nurse, and is scored conservatively (for the patient receiving sedation or muscle relaxants, normal function is assumed unless there is evidence of intrinsically altered mentation).
Physical examination
The physical examination focuses first on the general condition of the patient. Assess the patient’s overall hemodynamic condition to search for signs of hyperperfusion. Look for signs suggestive of a focal infection. An acutely ill, toxic appearance is a common feature in patients with serious infections.
The vital signs may suggest sepsis, even if fever is absent. As noted (see above), tachypnea is common; tachycardia with an increased pulse pressure also is common.
Measure the body temperature accurately. Because oral temperatures are often unreliable, rectal temperatures should be obtained.
Investigate signs of systemic tissue perfusion. In the early stages of sepsis, cardiac output is well maintained or even increased. Along with the effects of vasodilatory mediators, this may result in warm skin, warm extremities, and normal capillary refill. As sepsis progresses, stroke volume and cardiac output fall. Patients begin to manifest signs of poor distal perfusion, including cool skin, cool extremities, and delayed capillary refill.
The following physical signs suggest focal, usually bacterial, infection:
- Central nervous system (CNS) infection – Profound depression in mental status and meningismus
- Head and neck infections – Inflamed or swollen tympanic membranes, sinus tenderness, pharyngeal exudates, stridor, cervical lymphadenopathy
- Chest and pulmonary infections – Localized rales or evidence of consolidation
- Cardiac infections – Regurgitant valvular murmur
- Abdominal and gastrointestinal infections – Focal tenderness, guarding or rebound, rectal tenderness, or swelling
- Pelvic and genitourinary infections – Costovertebral angle tenderness, pelvic tenderness, cervical motion pain, and adnexal tenderness
- Bone and soft tissue infections – Focal erythema, edema, infusion, and tenderness
- Skin infections – Petechiae and purpura
Multiple organ dysfunction syndrome diagnostic criteria
Defining any medical condition in the absence of a “gold standard” diagnostic test is challenging. The challenges in defining a syndrome such as multiple organ dysfunction syndrome are even greater, as its causation is multifactorial and the underlying biology is incompletely understood. Nonetheless, its study is enhanced by refinement of definitions and diagnostic criteria as new knowledge permits. The first set of multiple organ dysfunction syndrome diagnostic criteria in children were suggested by Wilkinson and colleagues in 1987 11. The list was changed in 1996 by Proulx 12, in which multiple organ dysfunction syndrome was defined as the simultaneous dysfunction of at least two organ systems. The organs and systems considered were respiratory, cardiovascular, neurological, hematological, renal, hepatic, and gastrointestinal. In 2005, an international pediatric sepsis consensus conference developed a new set of diagnostic criteria (Table 2) 13.
Despite their strengths, both sets of diagnostic criteria have weaknesses. For example, the number of organ systems that were considered is arbitrary; seven organ systems were considered in 1996, while only six were considered in 2005. Additionally, there are also problems with respect to the individual criteria within each organ system. For example, Goldstein 13 stated that respiratory dysfunction could only be diagnosed if any of the following criteria are met:
- PaO2/FiO2 < 300 mmHg in the absence of cyanotic heart disease or preexisting lung disease or
- PaCO2 > 65 mmHg or 20 mmHg over baseline PaCO2 or
- Proven need† for > 0.50 FiO2 to maintain saturation ≥ 92% or
- Need for non-elective invasive or non-invasive mechanical ventilation
This list of diagnostic criteria has not been scientifically validated, and whether these four criteria each have the same diagnostic importance remains to be determined. Moreover, some criteria for other organs are not adjusted to age (e.g., hemoglobin level, white blood cell (WBC) count, serum urea nitrogen (BUN), and creatinine). Another problem is that we only need to observe two simultaneous dysfunctional organs to diagnose multiple organ dysfunction syndrome, regardless of the pathophysiology of the organ dysfunction. This may not always be optimal. Finally, the same diagnostic importance, the same weight, is given to organ systems with different associated mortality risks. Although that is acceptable as diagnostic criteria, data suggest that individual organ system failures may be associated with different risks. For example, Leteurtre demonstrated that the risk of death is at least two times higher with neurological dysfunction than it is with respiratory dysfunction 14.
Table 3. Diagnostic criteria of multiple organ dysfunction syndrome according to Goldstein et al 13. Multiple organ dysfunction syndrome is defined as the concurrent dysfunction of two or more systems. Each organ failure or dysfunction is defined by meeting one or more criteria of each organ system.
| Respiratory dysfunction.§ |
|---|
|
| Cardiovascular dysfunction. Despite administration of intravenous fluid bolus ≥ 40 mL/kg in 1 hr:
|
Hematological dysfunction.
|
Neurological dysfunction.
|
Hepatic dysfunction.
|
Renal dysfunction.
|
Footnotes:
†Proven need assumes O2 requirement was tested by decreasing flow with subsequent increase in flow if required.
‡ALT = alanine transaminase (SGPT)
ΨIn postoperative patients, this requirement can be met if the patient has developed an acute inflammatory or infectious process in the lungs that prevents him or her from being extubated.
§Acute respiratory distress syndrome (ARDS) by these criteria must include a PaO2/FiO2 ratio ≤ 200 mm Hg, bilateral infiltrates, acute onset, and no evidence of left heart failure. Acute lung injury (ALI) is defined identically except the PaO2/FiO2 ratio is 201–300 mm Hg.
Abbreviations: aPTT = activated partial thromboplastin time; ARDS = acute respiratory distress syndrome; CNS = central nervous system; DIC = disseminated intravascular coagulation; FI O2 = fraction of inspired oxygen; PEEP = positive end-expiratory pressure; PT = prothrombin time; BP = Blood pressure; SD = Standard deviation
[Source 15 ]Table 4. Age-specific vital signs and laboratory parameters
| Age-Groupa | Heart rate (beats/min)b | Respiratory rate (breaths/min) | Leukocyte count (leukocytes × 103/mm3)b | Systolic blood pressure (mmHg)b | |
|---|---|---|---|---|---|
| Tachycardia | Bradycardia | ||||
| 0 day–1 week | >180 | <100 | >50 | >34 | <59 |
| 1 week–1 month | >180 | <100 | >40 | >19.5 or <5 | <75 |
| 1 month–1 year | >180 | <90 | >34 | >17.5 or <5 | <75 |
| 2–5 years | >140 | NA | >22 | >15.5 or <6 | <74 |
| 6–12 years | >130 | NA | >18 | >13.5 or < 4.5 | <83 |
| 13– < 18 years | >110 | NA | >14 | >11 or < 4.5 | <90 |
Footnote: Lower values for heart rate, leukocyte count, and systolic blood pressure are for the 5th percentile and upper values are for 95th percentile.
a) Modified from: Parker MM. Pathophysiology of cardiovascular dysfunction in septic shock. New Horiz.1998;6:130–138.
b) Modified from: Rudolph CD, Rudolph AM (Eds). Rudolph’s Pediatrics – 21st Edition. The McGraw-Hill Companies Inc. New York, NY. 2002
Multiple organ dysfunction syndrome diagnosis
Laboratory tests are useful in cases of suspected sepsis or septic shock to assess the general hematologic and metabolic condition of the patient. The microbiologic studies provide results that may indicate occult bacterial infection or bacteremia and identify the causative pathogen or pathogens.
Various imaging modalities are employed to diagnose clinically suspected focal infection, detect the presence of a clinically occult focal infection, and evaluate complications of sepsis and septic shock.
Imaging studies
In patients with severe sepsis, a chest radiograph should be obtained because the clinical examination is unreliable for diagnosing pneumonia. Clinically occult infiltrates have been detected by routine use of chest radiography in adults who are febrile without localizing symptoms or signs and in patients who are febrile and neutropenic without pulmonary symptoms. Supine and upright or lateral decubitus abdominal films may be useful when an intra-abdominal source is suspected.
Ultrasonography is the imaging modality of choice when a biliary tract infection is suspected of being the source of sepsis.
Computed tomography (CT) is the imaging modality of choice for excluding an intra-abdominal abscess or a retroperitoneal source of infection. A CT scan of the head should be obtained when there is evidence of increased intracranial pressure (papilledema), when factors suggesting focal mass lesions (eg, focal defects, previous sinusitis or otitis, recent intracranial surgery) are present, or before lumbar puncture (LP) when meningitis is suspected.
When clinical evidence of a deep soft tissue infection exists (eg, crepitus, bullae, hemorrhage, or a foul-smelling exudate), a plain radiograph should be obtained. The presence of soft tissue gas and the spread of infection beyond the clinically detectable disease may necessitate surgical exploration.
Laboratory studies
A complete blood cell (CBC) count with differential should be obtained. An adequate hemoglobin concentration is necessary to ensure oxygen delivery in shock; hemoglobin should be maintained at a level of 8 g/dL.
Acute-phase reactants and platelets usually increase at the onset of any serious stress. With persistent sepsis, the platelet count will fall, and disseminated intravascular coagulation (DIC) may develop.
The white blood cell (WBC) differential and the WBC count may predict the existence of a bacterial infection. In adults who are febrile, a WBC count higher than 15,000/µL or a neutrophil band count higher than 1500/µL is associated with a high likelihood of bacterial infection 16.
A metabolic assessment should be performed with measurement of serum electrolytes, including magnesium, calcium, phosphate, and glucose, at regular intervals. Renal and hepatic function should be assessed with measurement of serum creatinine, blood urea nitrogen (BUN), bilirubin, alkaline phosphate, and alanine aminotransferase (ALT).
Arterial blood gas testing is indicated.
Measurement of serum lactate provides an assessment of tissue hypoperfusion. Elevated serum lactate indicates that significant tissue hypoperfusion exists with the shift from aerobic to anaerobic metabolism. This signals a worse degree of shock and a higher mortality.
Coagulation status should by assessed by measuring the prothrombin time (PT) and the activated partial thromboplastin time (aPTT). Patients with clinical evidence of coagulopathy require additional tests to detect the presence of DIC.
Although indiscriminate use of blood cultures has low utility, blood culture is the primary modality for facilitating the diagnosis of intravascular infections (eg, endocarditis) and infections of indwelling intravascular devices. Two populations—people who abuse intravenous (IV) drugs and patients with prosthetic heart valves—are at high risk for endocarditis.
Patients at risk for bacteremia include adults who are febrile with elevated WBC or neutrophil band counts, elderly patients who are febrile, and patients who are febrile and neutropenic. These populations have a 20-30% incidence of bacteremia. The incidence of bacteremia is at least 50% in patients with sepsis and evidence of end-organ dysfunction.
A urinalysis and a urine culture should be ordered for every patient who is in a septic state. Urinary infection is a common source of sepsis, especially in elderly individuals. Adults who are febrile without localizing symptoms or signs have a 10-15% incidence of occult urinary tract infection (UTI).
Secretions or tissue for Gram stain and culture should be obtained from sites of potential infection. Generally, the Gram stain is the only available test for immediately documenting the presence of bacterial infection and guiding the choice of initial antibiotic therapy.
Other diagnostic procedures
When meningitis or encephalitis is suspected, lumbar puncture must be performed on an urgent basis. In patients with an acute fulminant presentation, rapid onset of septic shock, and severe impairment of mental status, bacterial meningitis must be ruled out by means of lumbar puncture.
Procedures such as cardiac monitoring, noninvasive blood pressure monitoring, and pulse oximetry are necessary because patients often require admission to the intensive care unit (ICU) for invasive monitoring and support. Supplemental oxygen is provided during initial stabilization and resuscitation.
In all patients in septic shock, adequate venous access for volume resuscitation is necessary. A central venous line can also be used to monitor central venous pressure for assessment of intravascular volume status.
An indwelling urinary catheter used to monitor urinary output can serve as a marker for adequate renal perfusion and cardiac output.
Patients in whom septic shock associated with acute lung injury or right-sided heart failure require either right-heart catheterization with a pulmonary artery (Swan-Ganz) catheter or a transpulmonary thermodilution device (eg, PiCCO, Vigileo) to guide therapy. These catheters provide an assessment of the volume status of a patient who is in a septic state. Cardiac output measurements can be obtained. Furthermore, determination of mixed venous oxygenation from the pulmonary artery catheter is helpful in determining the status of tissue oxygenation.
Dynamic hemodynamic monitoring devices using pulse pressure and stroke volume variation are used in some centers when the patients are in sinus rhythm and on mechanical ventilation without spontaneous breathing to define volume responsiveness and assess dynamic arterial tone, both useful in deciding on resuscitation treatment options.
Most patients who are in a septic state experience respiratory distress secondary to severe sepsis or as a manifestation of septic shock. Pulmonary dysfunction of sepsis (ie, acute respiratory distress syndrome [ARDS]) also may occur. These patients need intubation and mechanical ventilation for optimum respiratory support.
Multiple organ dysfunction syndrome treatment
Treatment of patients with septic shock has the following three major goals:
- To resuscitate the patient from septic shock, using supportive measures to correct hypoxia, hypotension, and impaired tissue oxygenation
- To identify the source of infection and treat it with antimicrobial therapy, surgery, or both
- To maintain adequate organ system function, guided by cardiovascular monitoring, and to interrupt the pathogenesis of multiple organ dysfunction syndrome
Current management principles used in addressing these goals include the following:
- Early recognition
- Early hemodynamic resuscitation
- Early and adequate antibiotic therapy
- Source control
- Continued hemodynamic support
- Corticosteroids (refractory vasopressor-dependent shock)
- Tight glycemic control
- Proper ventilator management with low tidal volume in patients with acute respiratory distress syndrome (ARDS)
The major focus of resuscitation from septic shock is supporting cardiac and respiratory functions. To prevent multiple organ dysfunction syndrome, these patients require a very close monitoring and institution of appropriate therapy for major organ function. Problems encountered in these patients include the following:
- Temperature control – Fever generally requires no treatment, except in patients with limited cardiovascular reserve, because of increased metabolic requirements; antipyretic drugs and physical cooling methods, such as sponging or cooling blankets, may be used to lower the temperature
- Metabolic support – Patients with septic shock develop hyperglycemia and electrolyte abnormalities; serum glucose should be kept in normal range with insulin infusion; regular measurement and correction of electrolyte deficiency (including hypokalemia, hypomagnesemia, hypocalcemia and hypophosphatemia) is recommended
- Anemia and coagulopathy – Hemoglobin as low as 7 g/dL is well tolerated and does not warrant transfusion unless the patient has poor cardiac reserve or demonstrates evidence of myocardial ischemia; thrombocytopenia and coagulopathy are common in sepsis and do not necessitate replacement with platelets or fresh frozen plasma, unless the patient develops active clinical bleeding
- Renal dysfunction – Closely monitor urine output and renal function in all patients with sepsis; any abnormalities of renal function should prompt attention to adequacy of circulating blood volume, cardiac output, and blood pressure; correct these if they are inadequate
- Nutritional support – Early nutritional support is of critical importance in patients with septic shock; the enteral route is preferred unless the patient has an ileus or other abnormality; gastroparesis is observed commonly and can be treated with motility agents or placement of a small bowel feeding tube
Recognition of septic shock requires identification of features of the systemic inflammatory response syndrome (SIRS)—mental changes, hyperventilation, distributive hemodynamics, hyperthermia or hypothermia, and a reduced, elevated, or left-shifted white blood cell (WBC) count—along with the existence of a potential source of infection.
Patients in septic shock require immediate cardiorespiratory stabilization with large volumes of intravenous (IV) fluids, infusion of vasoactive drugs, and, often, endotracheal intubation and mechanical ventilation.
Empiric IV antimicrobial therapy should be immediately directed toward all potential infectious sources.
The drugs used for hemodynamic support of patients with sepsis have adverse effects on splanchnic circulation. Accordingly, the ideal hemodynamic therapy in these patients has not been determined. After adequate fluid resuscitation, therapy with dopamine may be initiated, followed by norepinephrine when dopamine fails. Alternatively, therapy may be initiated with norepinephrine, with dobutamine used if inotropic support is needed. The use of epinephrine as a single agent in septic shock is not recommended.
Manipulation of oxygen delivery by increasing the cardiac index has either yielded no improvement or has worsened morbidity and mortality. Routine use of hemodynamic drugs to raise cardiac output to supranormal levels is not recommended.
Drotrecogin alfa (activated protein C) was the only widely accepted drug specific to the therapy of sepsis. However, in a clinical trial (PROWESS-SHOCK trial), this agent failed to show a survival benefit for patients with severe sepsis and septic shock. The results of the trial led to the withdrawal of drotrecogin alfa from the worldwide market on October 25, 2011. The adverse side effect of drotrecogin alfa is bleeding.
Lactic acidosis of septic shock usually causes anion gap metabolic acidosis. Administration of bicarbonate has the potential to worsen intracellular acidosis. Correction of acidemia with sodium bicarbonate has not been proved to improve hemodynamics in critically ill patients with increased blood lactate levels. Nevertheless, bicarbonate therapy has been used in cases where the pH is less than 7.20 or the bicarbonate level is lower than 9 mmol/L, though no data to support this practice exist.
The pathogenesis of septic shock and multiple organ dysfunction syndrome derives from mediators produced because of the immune response of the host. Despite encouraging data from animal studies, immunosuppressive agents, such as high-dose corticosteroids, have not shown any benefit in humans.
The Surviving Sepsis Campaign recommends that glucose levels in the septic patient should be kept at less than 150 mg/dL.
Research has focused on modifying the host response to sepsis via a number of approaches, including the following:
- Antibodies against gram-negative endotoxin
- Gamma globulins
- Monoclonal antibodies against tumor necrosis factor
- Blockade of eicosanoid production
- Blockade of interleukin (IL)–1 activity
- Inhibition of nitric oxide (NO) synthase
These approaches have met with modest success in animal experiments, but at present, they cannot be recommended for general use in humans.
General management is as follows 17:
- Protocolized, quantitative resuscitation of patients with sepsis-induced tissue hypoperfusion
- Goals during the first 6 hours of resuscitation are (1) central venous pressure 8-12 mm Hg, (2) mean arterial pressure (MAP) above 65 mm Hg, (3) urine output above 0.5 mL/kg/h, (4) central venous (superior vena cava) or mixed venous oxygen saturation 70% or 65%, respectively
- In patients with elevated lactate levels, targeting resuscitation to normalize lactate as rapidly as possible
- Screening for sepsis and performance improvement
Diagnosis is as follows 17:
- Cultures as clinically appropriate before antimicrobial therapy if no significant delay (45 min) in the start of antimicrobial(s)
- At least 2 sets of blood cultures obtained before antimicrobial therapy
- Imaging studies performed promptly to confirm a potential source of infection
Antimicrobial therapy is as follows 17:
- Administration of effective intravenous antimicrobials within the first hour of recognition of septic shock and severe sepsis without septic shock as the goal of therapy
- Initial empiric anti-infective therapy of one or more drugs that have activity against all likely pathogens (bacterial and/or fungal or viral) and that penetrate in adequate concentrations into tissues presumed to be the source of sepsis
- Antimicrobial regimen should be reassessed daily for potential deescalation
- Use of low procalcitonin levels or similar biomarkers to assist the clinician in the discontinuation of empiric antibiotics in patients who initially appeared septic but have no subsequent evidence of infection
- Combination empirical therapy for neutropenic patients with severe sepsis and for patients with difficult-to-treat, multidrug-resistant bacterial pathogens
- Duration of therapy typically 7-10 days; longer courses may be appropriate in patients who have a slow clinical response, undrainable foci of infection, bacteremia with Staphylococcus aureus, some fungal and viral infections, or immunologic deficiencies (including neutropenia)
- Antiviral therapy initiated as early as possible in patients with severe sepsis or septic shock of viral origin
- Antimicrobial agents should not be used in patients with severe inflammatory states determined to be of noninfectious cause
Source control is as follows 17:
- A specific anatomical diagnosis of infection requiring consideration for emergent source control be sought and diagnosed or excluded as rapidly as possible
- Intervention be undertaken for source control within the first 12 hours after the diagnosis is made, if feasible
Infection prevention is as follows 17:
- Selective oral decontamination and selective digestive decontamination should be introduced and investigated as a method to reduce the incidence of ventilator-associated pneumonia; this infection control measure can then be instituted in healthcare settings and regions where this methodology is found to be effective
- Oral chlorhexidine gluconate be used as a form of oropharyngeal decontamination to reduce the risk of ventilator-associate pneumonia in ICU patients with severe sepsis
Choice of resuscitation fluid is as follows 17:
- Surviving Sepsis Campaign: Recommend using crystalloids as the initial fluid of choice in the resuscitation of severe sepsis and septic shock and recommend against the use of hydroxyethyl starches (HES). Albumin can be used in the fluid resuscitation of severe sepsis and septic shock when patients require substantial amounts of crystalloids.
Empiric antimicrobial therapy
Initial selection of particular antimicrobial agents is empiric and is based on an assessment of the patient’s underlying host defenses, the potential sources of infection, and the most likely pathogens.
Antibiotics must be broad-spectrum and must cover gram-positive, gram-negative, and anaerobic bacteria because all of these classes of organisms produce identical clinical pictures. Administer antibiotics parenterally in doses high enough to achieve bactericidal serum levels. Many studies have found that clinical improvement correlates with the achievement of serum bactericidal levels rather than with the number of antibiotics administered.
Coverage directed against anaerobes is particularly important in the treatment of patients with intra-abdominal or perineal infections. Antipseudomonal coverage is indicated in patients with neutropenia or burns.
Patients who are immunocompetent generally can be treated with a single drug that provides broad-spectrum coverage, such as a third-generation cephalosporin. However, patients who are immunocompromised usually must be treated with 2 broad-spectrum antibiotics that provide overlapping coverage. Within these general guidelines, no single combination of antibiotics is clearly superior to any other.
Vasopressor therapy
When proper fluid resuscitation fails to restore hemodynamic stability and tissue perfusion, initiate therapy with vasopressor agents. The agents used are norepinephrine, epinephrine, vasopressin, dopamine, and phenylephrine. These drugs maintain adequate blood pressure during life-threatening hypotension and preserve perfusion pressure for optimizing flow in various organs. Maintain the mean BP required for adequate splanchnic and renal perfusion (mean arterial pressure [MAP] of 65 mm Hg) on the basis of clinical indices for organ perfusion.
Norepinephrine is the first-choice vasopressor. Epinephrine (added to and potentially substituted for norepinephrine) can be used when an additional agent is needed to maintain adequate blood pressure. Vasopressin at 0.03 units/minute can be added to norepinephrine with the intent of either raising MAP or decreasing norepinephrine dosage. Dopamine as an alternative vasopressor agent to norepinephrine is used only in highly selected patients (eg, patients with low risk of tachyarrhythmias and absolute or relative bradycardia). Phenylephrine is not recommended in the treatment of septic shock, except in circumstances when norepinephrine is associated with serious arrhythmias, cardiac output is known to be high and blood pressure is persistently low, or as salvage therapy when combined inotrope/vasopressor drugs and low-dose vasopressin have failed to achieve MAP target 17.
Norepinephrine
Norepinephrine is a potent alpha-adrenergic agonist with minimal beta-adrenergic agonist effects. It can successfully increase blood pressure in patients who are in a septic state and remain hypotensive after fluid resuscitation and dopamine. Doses range from 0.2-1.35 µg/kg/min; doses as high as 3.3 µg/kg/min have been used because alpha-receptor down-regulation may occur in sepsis.
In patients with sepsis, indices of regional perfusion (eg, urine flow and lactate concentration) have improved after norepinephrine infusion. In recent controlled trials, no significant difference was noted in the rate of death between patients with shock who were treated with dopamine and those who were treated with norepinephrine; the use of dopamine was associated with a greater number of adverse events, which were mostly cardiac arrhythmias 18.
Accordingly, use norepinephrine early, and do not withhold it as a last resort. Norepinephrine therapy appears to have no effects on splanchnic oxygen consumption and hepatic glucose production, provided adequate cardiac output is maintained.
Epinephrine
Epinephrine can increase MAP by increasing the cardiac index, stroke volume, systemic vascular resistance, and heart rate. It may increase oxygen delivery and consumption and decreases splanchnic blood flow. Administration of epinephrine is associated with an elevation of systemic and regional lactate concentrations.
The use of epinephrine is recommended in patients who are unresponsive to traditional agents. The undesirable effects of this agent include increased lactate concentration, potential production of myocardial ischemia and arrhythmias, and reduced splanchnic flow.
Dopamine
A precursor of norepinephrine and epinephrine, dopamine has varying effects, depending on the dose administered. A dose lower than 5 µg/kg/min results in vasodilation of renal, mesenteric, and coronary beds. At a dose of 5-10 µg/kg/min, beta1 -adrenergic effects induce an increase in cardiac contractility and heart rate. At doses of about 10 µg/kg/min, alpha-adrenergic effects lead to arterial vasoconstriction and an increase in blood pressure.
Dopamine is only partially effective in increasing MAP in patients who are hypotensive with septic shock after volume resuscitation. The blood pressure increases primarily as a result of an inotropic effect, which is useful in patients who have concomitant reduced cardiac function. The undesirable effects are tachycardia, increased pulmonary shunting, potentially decreased splanchnic perfusion, and increased pulmonary artery occlusion pressure (pulmonary wedge pressure).
Renal-dose dopamine
In healthy volunteers, infusion of dopamine at low doses (0.5-2 mg/kg/min) increases both renal blood flow and the glomerular filtration rate by selective stimulation of renal dopaminergic receptors. However, “beneficial” effects of such renal-dose dopamine in sepsis are unsubstantiated. Multiple studies have not demonstrated a beneficial effect with prophylactic or therapeutic low-dose dopamine administration in patients who are critically ill.
Administering low-dose dopamine does not protect the patient from developing acute renal failure, and there is no evidence that it preserves mesenteric profusion. Consequently, routine use of this practice is not recommended. Aggressively resuscitating patients with septic shock, maintaining adequate perfusion pressure, and avoiding excessive vasoconstriction are effective measures for protecting the kidneys.
Phenylephrine
Phenylephrine is a selective alpha1 -adrenergic receptor agonist that is primarily used in anesthesia to increase blood pressure. Although the data are limited, phenylephrine has been found to increase MAP in patients with sepsis who are hypotensive with an increase in oxygen consumption and potential to reduce cardiac output. Phenylephrine may be a good choice when tachyarrhythmias limit therapy with other vasopressors.
Angiotensin II
Angiotensin II targets the renin-angiotensin-aldosterone system (RAAS), a powerful mediator of arterial blood pressure. Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial was conducted to determine whether the addition of angiotensin II to background vasopressors would improve blood pressure in patients with catecholamine-resistant vasodilatory shock 19. In that study, 321 patients with vasodilatory shock were randomized to receive either angiotensin II (163 patients) or placebo (158 patients). Enrolled patients had shock despite receiving more than 0.2 μg/kg/min of norepinephrine or another vasopressor in a similar dose. The primary endpoint was a response with respect to MAP at hour 3 after the start of infusion, with response defined as an increase from baseline of at least 10 mm Hg or an increase to at least 75 mm Hg, without an increase in the dose of background vasopressors. The primary endpoint was reached by more patients in the angiotensin II group than in the placebo group. At 48 hours, the mean improvement in the cardiovascular Sequential Organ Failure Assessment (SOFA) score was greater in the angiotensin II group than in the placebo group. There was no statistically significant difference in mortality between the two groups.
Role of inotropic therapy
Although myocardial performance is altered during sepsis and septic shock, cardiac output is usually maintained in patients with sepsis who have undergone volume resuscitation. Data from the 1980s and 1990s suggested a linear relation between oxygen delivery and oxygen consumption (pathologic supply dependency), indicating that oxygen delivery was likely insufficient to meet the metabolic needs of the patient.
However, subsequent investigations challenged the concept of pathologic supply dependency and the practice of elevating cardiac index and oxygen delivery (hyperresuscitation) on the grounds that these interventions have not been shown to improve patient outcome. However, if there is inadequate cardiac index, MAP, mixed venous oxygen saturation, and urine output despite optimal volume resuscitation and vasopressor therapy, a trial of dobutamine infusion up to 20 µg/kg/min be administered or added to vasopressor therapy.
Surgical drainage and debridement
Patients with infected foci should be taken for definitive surgical treatment after initial resuscitation and administration of antibiotics. When an infected focus persists, there is little to be gained from spending hours on attempting to stabilize the patient.
Infectious processes require expeditious surgical drainage or debridement for source control, even if the patient does not appear stable. Without emergency surgical treatment, the patient’s condition may not improve.
Recombinant Human Activated Protein C Therapy
Activated protein C is an endogenous protein that not only promotes fibrinolysis and inhibits thrombosis and inflammation but also may modulate the coagulation and inflammation of severe sepsis. Sepsis reduces the level of protein C and inhibits conversion of protein C to activated protein C. Administration of recombinant activated protein C inhibits thrombosis and inflammation, promotes fibrinolysis, and modulates coagulation and inflammation.
An early publication by the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group demonstrated that administration of recombinant human activated protein C (drotrecogin alfa) resulted in lower mortality (24.7%) in the treatment group than in the placebo group (30.8%) 20. Treatment with drotrecogin alfa was associated with a 19.4% relative reduction in the risk of death and a 6.1% absolute reduction in the risk of death.
After that early publication, the efficacy and safety of drotrecogin alfa were widely debated. Drotrecogin alfa was withdrawn from the worldwide market on October 25, 2011, after analysis of the PROWESS-SHOCK clinical trial, in which the drug failed to demonstrate a statistically significant reduction in 28-day all-cause mortality in patients with severe sepsis and septic shock 21. Trial results observed a 28-day all-cause mortality of 26.4% in patients treated with drotrecogin alfa, compared with 24.2% in the placebo group.
Corticosteroid therapy
Despite the theoretical and experimental animal evidence supporting the use of large doses of corticosteroids in those with severe sepsis and septic shock, all randomized human studies of this practice (except a single study from 1976) found that corticosteroids did not prevent the development of shock, reverse the shock state, or improve 14-day mortality. Therefore, routine use of high-dose corticosteroids in patients with severe sepsis or septic shock is not indicated.
Although further research is required to address this issue definitively, hydrocortisone can be given at 200-300 mg/day for up to 7 days or until vasopressor support is no longer required for patients with refractory septic shock.
Trials have demonstrated positive results from administration of stress-dose corticosteroids to patients in severe and refractory shock 22. Large clinical trials have documented a clear benefit of hydrocortisone plus fludrocortisone for adults with septic shock, reducing the time on ventilator and the severity of acute kidney injury, along with overall lower Sequential Organ Failure Assessment (SOFA) scores 23. Thus, it is reasonable to provide stress-dose steroid coverage plus mineralocorticoid supplement to septic shock patients, and especially those who have the possibility of adrenal suppression.
The following key points summarize current use of corticosteroids in septic shock:
- Older, traditional trials of corticosteroids in sepsis probably failed to show good results because they used high doses and did not select patients appropriately
- Subsequent trials with low-dose (physiologic) dosages in select patient populations (vasopressor-dependent patients and those with potential relative adrenal insufficiency) reported improved outcomes
- Corticosteroids should be initiated for patients with vasopressor-dependent septic shock
A cosyntropin stimulation test may be useful to identify patients with relative adrenal insufficiency, defined as failure to raise levels above 9 µg/dL.
Tight glycemic control
A protocolized approach to blood glucose management in ICU patients with severe sepsis is recommended, commencing insulin dosing when 2 consecutive blood glucose levels are of more than 180 mg/dL. This approach should target an upper blood glucose level equal or more than 180 mg/dL rather than an upper target blood glucose of equal or less than 110 mg/dL 17.
Multiple organ dysfunction syndrome prognosis
Regardless of how multiple organ dysfunction syndrome is characterized, it is apparent that the risk of ICU death increases as the severity of organ dysfunction – whether the number of failing organs (Table 5), or the overall degree of dysfunction increases. Mortality from multiple organ dysfunction syndrome remains high. Mortality from ARDS alone is 40-50%; once additional organ system dysfunction occurs, mortality increases as much as 90%. Several clinical trials have demonstrated a mortality ranging from 40% to 75% in patients with multiple organ dysfunction syndrome arising from sepsis.
The poor prognostic factors are advanced age, infection with a resistant organism, impaired host immune status, and poor prior functional status. Development of sequential organ failure despite adequate supportive measures and antimicrobial therapy is a harbinger of a poor outcome.
There is a graded severity from systemic inflammatory response syndrome to sepsis, severe sepsis, and septic shock, with associated 28-day mortality rates of approximately 10%, 20%, 20-40%, and 40-60%, respectively 24.
A multicenter prospective study published in the Journal of the American Medical Association reported a mortality of 56% during ICU stay 25. Of all deaths, 27% occurred within 2 days of the onset of severe sepsis, and 77% of all deaths occurred within the first 14 days. The risk factors for early mortality in this study were a higher severity of illness score, the presence of 2 or more acute organ failures at the time of sepsis, shock, and a low blood pH (< 7.3).
Lobo et al 26 determined that multiple organ dysfunction syndrome is the primary cause of death in high-risk patients after surgery; the risk factors for death due to multiple organ failure should be considered in determining risk stratification.
For patients who survive sepsis and multiple organ dysfunction syndrome, the road to recovery is often long and challenging. Post hospital discharge, patients may have physical, emotional, and cognitive consequences. In addition, these patients have a higher risk of repeat sepsis episodes. Aggressive rehabilitation programs, including psychological treatments, may be helpful 27.
Table 5. Prognosis in multiple organ failure
| Number of failing systems | Mortality (%) |
|---|---|
| 0 | < 10 |
| 1 | 0–30 |
| 2 | 20–50 |
| 3 | 40–80 |
| 4 | 60–100 |
| 5 or more | > 80 |
- Gourd, N. M., & Nikitas, N. (2019). Multiple Organ Dysfunction Syndrome. Journal of Intensive Care Medicine. https://doi.org/10.1177/0885066619871452[↩]
- Marshall JC. The multiple organ dysfunction syndrome. In: Holzheimer RG, Mannick JA, editors. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt; 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6868[↩][↩][↩][↩]
- Multiple organ dysfunction syndrome in sepsis. https://emedicine.medscape.com/article/169640-overview[↩]
- Pediatric sepsis and multiple organ dysfunction syndrome. Curr Opin Pediatr. 2001 Jun;13(3):247-53. DOI:10.1097/00008480-200106000-00006[↩]
- Harrois A, Huet O, Duranteau J. Alterations of mitochondrial function in sepsis and critical illness. Curr Opin Anaesthesiol. 2009 Apr. 22(2):143-9.[↩]
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005 Aug 17. 294(7):813-8.[↩]
- Ruf W. New players in the sepsis-protective activated protein C pathway. J Clin Invest. 2010 Sep. 120(9):3084-7.[↩]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013 Aug 29. 369(9):840-51.[↩][↩][↩]
- Nathens AB, Marshall JC. Selective decontamination of the digestive tract in surgical patients: a systematic review of the evidence. Arch Surg. 1999 Feb. 134(2):170-6.[↩]
- Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009 Jan. 37(1):96-104.[↩]
- Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111:324–328.[↩]
- Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037.[↩]
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8.[↩][↩][↩]
- Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197.[↩]
- Watson RS, Crow SS, Hartman ME, Lacroix J, Odetola FO. Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med. 2017;18(3_suppl Suppl 1):S4–S16. doi:10.1097/PCC.0000000000001047 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5334773[↩][↩]
- Nelson DP, Lemaster TH, Plost GN, Zahner ML. Recognizing sepsis in the adult patient. Am J Nurs. 2009 Mar. 109(3):40-5; quiz 46.[↩]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb. 41(2):580-637.[↩][↩][↩][↩][↩][↩][↩][↩]
- Patel GP, Grahe JS, Sperry M, et al. Efficacy and safety of dopamine versus norepinephrine in the management of septic shock. Shock. 2010 Apr. 33(4):375-80.[↩]
- Khanna A, English SW, Wang XS, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017 Aug 3. 377 (5):419-430.[↩]
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8. 344(10):699-709.[↩]
- Angus DC. Drotrecogin alfa (activated) … a sad final fizzle to a roller-coaster party. Crit Care. 2012 Feb 6. 16(1):107.[↩]
- Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999 Apr. 27(4):723-32.[↩]
- Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018 Mar 1. 378 (9):809-818.[↩]
- Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000. 26 Suppl 1:S64-74.[↩]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995 Sep 27. 274(12):968-74.[↩]
- Lobo SM, Rezende E, Knibel MF, et al. Early determinants of death due to multiple organ failure after noncardiac surgery in high-risk patients. Anesth Analg. 2011 Apr. 112(4):877-83[↩]
- Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA. 2018 Jan 2. 319 (1):62-75.[↩]