What is necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is the most frequent and lethal acquired disease of the gastrointestinal tract of premature infants, affecting newborn babies at a rate of 1–3 per 1000 births per year in North America 1. Necrotizing enterocolitis (NEC) is characterized by submucosal edema and hemorrhage, infiltration of the intestinal wall by neutrophils, disruption of the intestinal villus architecture, and in severe cases, full thickness necrosis or intestinal wall perforation 1. Bowel perforation occurs in one third of the affected infants 2. Although 5% to 25% of necrotizing enterocolitis cases occur in term infants, necrotizing enterocolitis is primarily a disease of preterm infants with the majority of cases occurring in very low birth weight infants (infants with birth weight < 1500 g) 3.
Necrotizing enterocolitis is categorized into three different stages, with clinical symptoms varying from feeding intolerance to severe cardiovascular compromise, coagulopathy, and peritonitis with or without pneumoperitoneum 4.
Classic early clinical signs of necrotizing enterocolitis include abdominal distension, feeding intolerance, and bloody stool in infants around 1 week old. Abdominal radiographs can demonstrate pneumatosis intestinalis and/or portal venous gas (see Figure 1) 5. Although the cause of NEC is not entirely known, milk feeding and bacterial growth play a role 6.
In North America, necrotizing enterocolitis occurs in about 7% of infants born between 500 and 1500 g 5 which translates into an incidence of around 1.1 per 1000 live births 1. Necrotizing enterocolitis has a mortality rate of approximately 30 %; lower birth weight infants and infants who require surgical treatment of necrotizing enterocolitis experience a higher mortality rate than larger babies or infants in whom necrotizing enterocolitis can be managed medically 7. Necrotizing enterocolitis costs the United States health care system over one billion dollars per year 7 with an average cost for surgical necrotizing enterocolitis between 300,000 and 600,000 dollars per patient 8. In addition to the immediate morbidity and economic costs associated with necrotizing enterocolitis, the disease results in long term sequel in around 25% of the time, such as neurodevelopmental delays or short gut syndrome 9.
Despite several decades of experience in treating patients with necrotizing enterocolitis 5, the overall mortality and approach to treatment have remained largely unchanged since the initial descriptions of the disease several decades ago 10.
Although the cause of necrotizing enterocolitis is not entirely known, milk feeding and bacterial growth play a role 6.
Necrotizing enterocolitis is a disease that occurs predominately in premature infants; the likelihood of developing necrotizing enterocolitis is inversely proportional to birth weight and gestational age 11. Interestingly, the onset of necrotizing enterocolitis appears to be most related to post gestational age (corrected postnatal age) as opposed to actual postnatal age. The peak incidence of necrotizing enterocolitis seems to occur at approximately 31 weeks post conceptual age. This highlights the relationship between host development and the development of necrotizing enterocolitis 12. There are several key differences between the preterm and the term neonate that contribute to the increased propensity of preterm neonates to develop necrotizing enterocolitis. The gastrointestinal tract of the preterm neonate demonstrates decreased intestinal barrier function 13, an impaired intestinal immune defense system 14, and an increased inflammatory propensity 15. Furthermore, the immune system of a preterm neonate is less developed than a baby born at term. In all neonates both the adaptive and the innate components of the immune system are immature owing to reduced physical barriers and impaired and delayed function of most cell types 16. Compared to term neonates, preterm infants have a stunted immune system possessing a smaller quantity of monocytes and neutrophils. The quality of these cells is also impaired with a reduced ability to kill pathogens. In addition, preterm neonates’ ability to produce cytokines is lowered translating into limited T cell activation (Table 1) 17.
Table 1. Comparison of the Term and Premature Neonatal Immune System
| Term | Preterm |
|---|---|
| ↓ Physical barriers | ↓↓↓ Physical barriers |
| ↑ Effectiveness of immune cells to target pathogens | ↓ Number of monocytes and neutrophils |
| ↓ Overall ability to produce cytokines | |
| ↓ T cell activation | |
| ↓ Number of natural killer cells | |
| ↓ Bactericidal/permability-increasing protein | ↓↓ Bactericidal/permability-increasing protein |
| ↓ Passive Immunity (level of IgG depends on transplacental transfer and thus increases with gestation age) |
Footnote: ↑ indicates increased; ↓ indicates decreased
[Source 18]Factors linked to increased necrotizing enterocolitis incidence 19
Factors related to the infant
- Prematurity (highest risk with lowest gestational age)
- Very low birth weight (<1,500 g)
- Low Apgar score at 5 min
- Formula feeding
- Mechanical ventilation
- Congenital defects
- Congenital heart disease
- Patent ductus arteriosus
- Gastroschisis
- Pharmacological interventions
- Indomethacin
- Histamine H2 receptor antagonists
- Prolonged empirical antibiotic use (≥5 days)
- Concomitant use of indomethacin and glucocorticoids
- Indomethacin tocolysis
- Anemia
Factors related to the mother
- HIV-positive status
- Illicit drug abuse (including opiates, cannabinoids and cocaine)
- Chorioamnionitis
- Vaginal delivery
Necrotizing enterocolitis stages
Despite considerable research, preventive strategies have remained elusive for several decades, reflecting the lack of a clear delineation of what constitutes the diagnosis of classic necrotizing enterocolitis. Thus, the term “necrotizing enterocolitis” often reflects a spectrum of intestinal conditions that differ with respect to pathogenesis and the strategies required for prevention and treatment.
Three forms of neonatal intestinal injury occur most often: conditions primarily seen in term infants, spontaneous intestinal perforations, and classic necrotizing enterocolitis. Although necrotizing enterocolitis is considered to be a disease that primarily affects preterm infants, necrotizing enterocolitis–like symptoms also occur in term and late preterm infants. In these more mature neonates, the disease usually occurs in the first week after birth, but it differs from that seen in preterm infants in that it is more often associated with other problems, such as maternal illicit drug use, intestinal anomalies (e.g., aganglionosis or atresias), congenital heart disease, and perinatal stress that may affect mesenteric blood flow 20. Among preterm infants, spontaneous intestinal perforations have at times been categorized as necrotizing enterocolitis but probably represent a different disease entity with a different pathogenesis 21. Spontaneous intestinal perforation usually occurs in the first several days after birth and is not associated with enteral feeding. This disorder is characterized by only minimal intestinal inflammation and necrosis, as evidenced by low levels of serum inflammatory cytokines. It has been associated with the administration of indomethacin and with glucocorticoids such as dexamethasone or hydrocortisone 22.
The lack of universally reliable diagnostic criteria makes it difficult to establish the diagnosis. A systematic description of necrotizing enterocolitis, the staging system described by Bell et al. 23, was first published in 1978 and subsequently refined 24. This system includes three stages 24:
- Bell stage 1 (Mild) criteria are highly nonspecific findings and may include feeding intolerance, mild abdominal distention, or both.
- Bell stage 2 (Moderate) criteria are radiographic findings such as pneumatosis intestinalis, which may be hard to detect on radiographs.
- One of the most important criteria for Bell stage 3 (Severe) is a perforated viscus, which may or may not be associated with intestinal necrosis and which could, in fact, be a spontaneous intestinal perforation or dissected air from the pleural cavity.
Furthermore, whether necrosis is actually present may not be clear in individual patients, since peritoneal drains may be placed without direct visualization and histopathological evaluation 25.
Table 2. Bell’s staging and suggested management for necrotizing enterocolitis
| Bell’s stage | Severity | Clinical signs and symptoms | Radiological | Treatment |
|---|---|---|---|---|
| I | Mild necrotizing enterocolitis, suspected necrotizing enterocolitis | Mild systemic signs and intestinal signs | Nonspecific |
|
| II | Moderate necrotizing enterocolitis |
| Pneumatosis intestinalis, portal venous gas |
|
| III | Advanced necrotizing enterocolitis |
| Pneumoperitoneum |
|
Abbreviation: NEC = necrotizing enterocolitis
[Source 19]Another classification system used to define necrotizing enterocolitis more specifically is published in the Vermont Oxford Network Manual of Operations 26. This manual describes clinical and radiographic findings, with one or more of each type of finding (clinical or radiographic) required to establish a diagnosis of necrotizing enterocolitis. The clinical findings include bilious gastric aspirate or emesis, abdominal distention, and occult gross blood in the stool, with the absence of anal fissures. The imaging findings include pneumatosis intestinalis, hepatobiliary gas, and pneumoperitoneum. However, the Vermont–Oxford diagnostic approach has shortcomings similar to those of the criteria described by Bell et al. 23, since severe necrotizing enterocolitis requiring surgery can develop in patients even though pneumatosis intestinalis or portal gas has not been detected on imaging. These patients may only have abdominal distention, without intraluminal bowel gas, on presentation 27. Thus, the ominous progression of the disease may be missed, with a failure to intervene early enough. A more reliable staging approach that allows for aggressive preventive measures is needed, but it will probably require the development of biomarkers that accurately predict the full expression of necrotizing enterocolitis.
Necrotizing enterocolitis symptoms
Necrotizing enterocolitis is categorized into three different stages, with clinical symptoms varying from feeding intolerance to severe cardiovascular compromise, coagulopathy, and peritonitis with or without pneumoperitoneum 4.
The typical neonate with necrotizing enterocolitis is a premature infant who is thriving, yet suddenly presents with feeding intolerance, abdominal distension, bloody stools and signs of sepsis (that is, changes in heart rate, respiratory rate, temperature and blood pressure) 5. An important consideration in the diagnosis of necrotizing enterocolitis is the gestational age at which these symptoms present, owing to the existence of an inverse relationship between gestational age and the onset and severity of symptoms in patients with necrotizing enterocolitis 28. Specifically, an infant born at ~27 weeks of gestation will typically present with necrotizing enterocolitis at ~4–5 weeks of age and has a substantially higher risk of necrotizing enterocolitis development than an infant born at closer to 37 weeks of gestation, for whom onset typically occurs within the first 2 weeks after birth 29. A late onset of necrotizing enterocolitis in the most premature infants might be related to delayed microbial colonization of the gut and establishment of virulent microbial agents, in part owing to the use of broad-spectrum antibiotics and prolonged hospital stay 30. Signs of sepsis can be associated with high gastric residuals (defined as the volume that remains in the stomach before the next enteral feeding) of ≥2 ml/kg or >50% of the previous feeding volume, which could indicate the presence of feeding intolerance 31. Although feeding intolerance is the most common early gastrointestinal symptom associated with necrotizing enterocolitis 32, some controversy persists as to the use of gastric residuals as an objective measure and their predictive value in the context of the disease progression, owing to the inherent variability in sampling gastric contents through a small nasogastric or orogastric tube, as well as to the lack of standardization in the procedure of obtaining gastric aspirates 33.
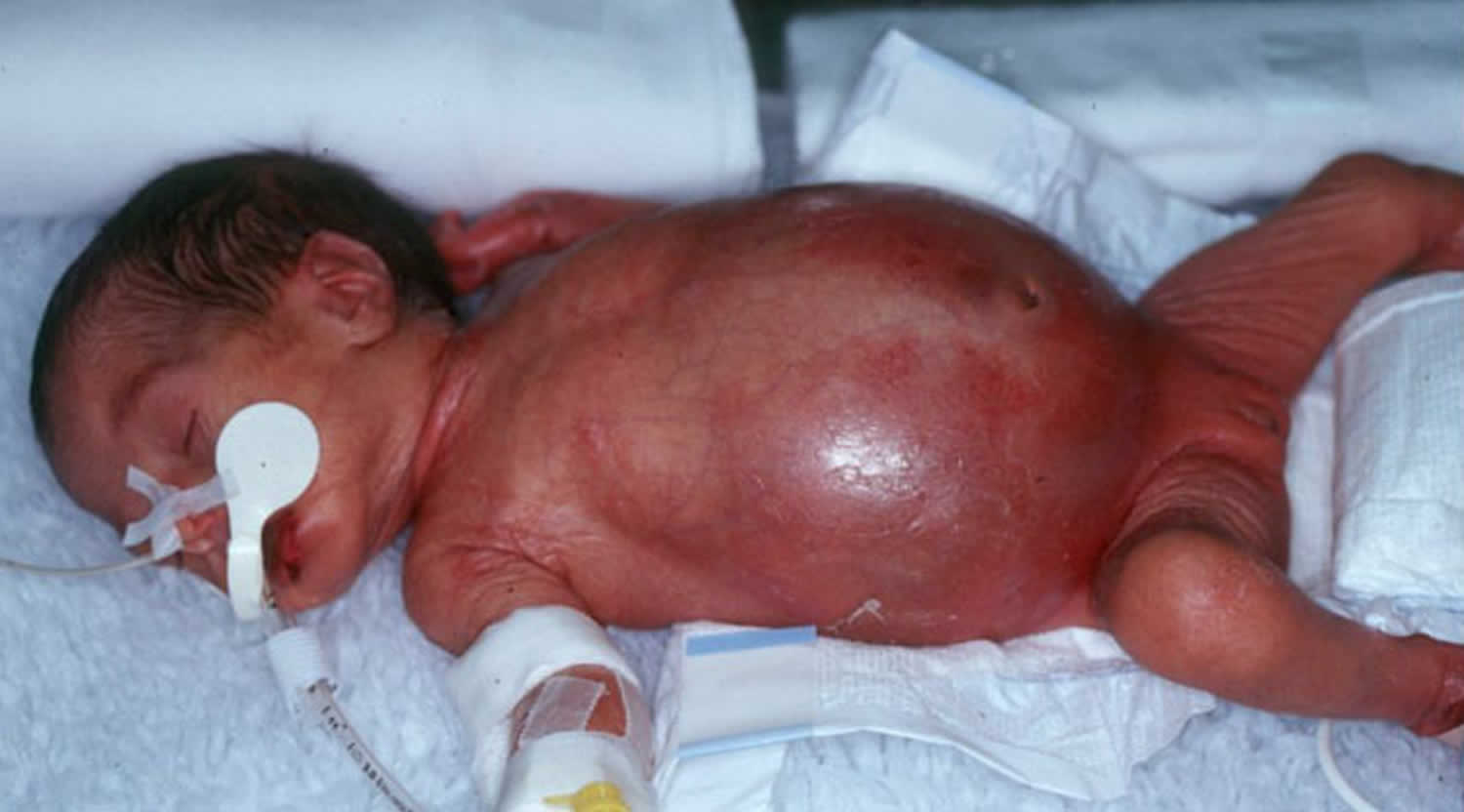
The most typical initial signs and symptoms of “classic” necrotizing enterocolitis in a preterm infant include feeding intolerance, abdominal distention (Figure 1A), and bloody stools after 8 to 10 days of age 5. The pathognomonic findings on abdominal radiography are pneumatosis intestinalis, portal venous gas, or both (Figure 1B) 5. Early imaging signs that should raise the suspicion of necrotizing enterocolitis include dilated loops of bowel, a paucity of gas, and gas-filled loops of bowel that are unaltered on repeated examinations. Extraluminal air (“free air”) outside the bowel is a sign of advanced necrotizing enterocolitis. Symptoms may progress rapidly, often within hours, from subtle signs to abdominal discoloration, intestinal perforation, and peritonitis, leading to systemic hypotension that requires intensive medical support, surgical support, or both (Figure 1C) 5.
Although no specific laboratory markers have been validated in making the diagnosis of necrotizing enterocolitis, neutropenia and thrombocytopenia are often present 34. Consideration of alternative diagnoses is critical for infants who present with necrotizing enterocolitis and in whom overlapping signs and symptoms might be present, including those who have spontaneous intestinal perforation, ileus secondary to sepsis, sensitivity to cow milk, food protein intolerance, ischaemic bowel disease associated with heart disease or haematological disturbances (for example, polycythaemia).
Figure 1. Necrotizing Enterocolitis clinical and radiographic features
Footnote:
Panel A shows an infant with a shiny, distended abdomen with periumbilical erythema (redness).
Panel B the radiograph shown in the upper arrow points to portal venous gas, and the lower arrow points to a ring of intramural gas, which is indicative of pneumatosis intestinalis.
In Panel C, the arrow points to an area of necrotic bowel in an infant with necrotizing enterocolitis found upon surgical exploration.
[Source 5]Necrotizing enterocolitis long term effects
The outcome of children with necrotizing enterocolitis is characterized by high overall morbidity ranging from 20–50%, as patients experience recurrence, intestinal strictures, short bowel syndrome, growth delay and neurodevelopmental impairment 35. Infants with necrotizing enterocolitis have longer hospitalization stays, increased risk of death before discharge and accrue higher financial costs compared with premature infants without necrotizing enterocolitis 36. In the long term, patients who survive necrotizing enterocolitis are frequently affected by neurodevelopmental impairment, demonstrated by their impaired performance in cognitive and developmental assessments such as the Bayley Scales of Infant Development, the Griffiths Quotient and the Stanford–Binet test 37, underscoring the far-reaching sequelae of this disease 38. A detailed list of complications and outcomes is presented in Table 3.
Table 3. Complications and outcomes in patients with necrotizing enterocolitis
| Type of complication or outcome | Incidence | Associated factors |
|---|---|---|
| Recurrence | 4–10% | Nonoperative management, congenital heart disease |
| Mortality | 15–63% |
|
| Intestinal strictures | 12–35% |
|
| Stoma complications | 50% |
|
| Short Bowel Syndrome | 20–35% |
|
| Neurodevelopmental impairment | 30–50% | Necrotizing enterocolitis vs. no necrotizing enterocolitis (OR: 1.82). Surgical necrotizing enterocolitis versus medical necrotizing enterocolitis (OR: 2.34) |
| Growth delay | 10% |
|
Footnotes:
NEC = necrotizing enterocolitis
OR = Odds ratio is a measure of association between an exposure and an outcome.
OR=1 Exposure does not affect odds of outcome
OR>1 Exposure associated with higher odds of outcome
OR<1 Exposure associated with lower odds of outcome
Necrotizing enterocolitis causes
Despite decades of investigation into the pathophysiology of necrotizing enterocolitis, it still not well defined. The importance of bacterial colonization in the development of necrotizing enterocolitis was recognized decades ago by Santulli et al. 39. Despite this no single causative agent has been identified. As such, most theories on the pathogenesis of necrotizing enterocolitis focus on not a specific pathogen but a generalized microbial imbalance of intestinal flora called dysbiosis 40. One evolving school of thought is that the disruption of normal neonatal intestinal bacterium, or microbiome, induces a proinflammatory state, allowing bacterial translocation across intestinal epithelia 41. In 2016 Nino et al. 19 eloquently proposed a “unifying hypothesis for the development of necrotizing enterocolitis: that the intestine of the premature neonate exists in a hyper-reactive state relative to the full-term intestine, which favors necrotizing enterocolitis development upon colonization with an appropriate microbial milieu in a patient with a permissive genetic background”. At present, necrotizing enterocolitis is thought to develop in the premature infant in the setting of bacterial colonization, often after administration of non-breast milk feeds, and disease onset is thought to be due in part to a baseline increased reactivity of the premature intestinal mucosa to microbial ligands as compared with the full-term intestinal mucosa 19. The increased reactivity leads to mucosal destruction and impaired mesenteric perfusion and partly reflects an increased expression of the bacterial receptor Toll-like receptor 4 (TLR4) in the premature gut, as well as other factors that predispose the intestine to a hyper-reactive state in response to colonizing microorganisms 19. The increased expression of TLR4 in the premature gut reflects a surprising role for this molecule in the regulation of normal intestinal development through its effects on the Notch signalling pathway 19.
Toll like receptor 4 (TLR4) plays a critical role in the development of necrotizing enterocolitis – its activation leads to mucosal injury and reduced epithelial repair 18. Furthermore, Toll-like receptor 4 (TLR4) is upregulated in the premature gut as compared to the gut of the full term neonate 42. Toll-like receptor 4 (TLR4) has an important role in the regulation of normal gut development in utero; levels of TLR4 expression typically fall throughout gestation 43. As a result, TLR4 levels are high in preterm neonate. When the gut is subsequently colonized with numerous gram negative bacteria, there are deleterious consequences of exaggerated TLR4 signaling including increased release of proinflammatory cytokines, increased enterocyte apoptosis, and impaired mucosal healing. In addition, bacterial translocation through the gut mucosa activate TLR4 on the endothelia of the intestinal vasculature, resulting in reduction of blood flow and development of intestinal ischemia and necrosis 9. A 2017 study by Hui et al. 44 demonstrated increased pro-inflammatory cytokines and enhanced expression of TLR4 in resected intestinal samples from 28 to 29 week old infants with necrotizing enterocolitis.
In addition to increased TLR4 signaling there are other factors that predispose the premature gut to the development of necrotizing enterocolitis (Figure 2). The premature gut displays decreased digestion, decreased nutrient absorption 45 and impaired intestinal motility 46. It also has a high baseline level of cellular endoplasmic reticulum stress. This increases the likelihood of apoptosis in the intestinal epithelium. Furthermore, there are decreased physical barriers in the premature gut, with a decreased number of mucus-producing goblet cells 47, immature tight junctions 48, and increased microvascular tone in the intestinal mesentery 49. Although outside of the scope of this review, in addition to the TLR4 pathway, other pathways and cell types are thought to be important in the development of necrotizing enterocolitis including platelet-activating factor and macrophages 50.
Figure 2. Factors that Predispose the Immature Gut to Necrotizing Enterocolitis
[Source 18]The microbiome in necrotizing enterocolitis
Several studies validate the notion that the microbiome of the neonate with necrotizing enterocolitis is fundamentally different from the microbiome of the neonate who is unaffected by necrotizing enterocolitis. However, there are a range of organisms implicated in these studies, further highlighting the lack of a single causative agent. Additionally, direct comparison of these studies are difficult due to limitations in 16S rRNA sequencing (speciation is dependent on the quality and length of the sequence, challenging primer design, and inability to distinguish between living and dead bacteria) and heterogeneous populations studied- including a wide range of post gestational ages at which necrotizing enterocolitis develops 51.
Despite these limitations studies investigating the microbiome of a neonate with necrotizing enterocolitis have been informative. Among those studies, Wang et al. 52 reported a study of 20 preterm infants from a single institution, 10 suffering from necrotizing enterocolitis and 10 without the disease. These patients included four twin pairs. Bacterial DNA from fecal samples were obtained and underwent sequencing of the 16S rRNA gene. All 20 infants had low levels of diversity in the intestinal bacterial colonization but patients with necrotizing enterocolitis had a significantly reduced level of diversity compared to unaffected neonates. They had an increase in the colonization of Gammaproteobacteria with a decrease in other bacterial species. Mai et al. 53 collected weekly stool samples from infants with a gestation age < 32 weeks or a birth weight ≤ 1250 g. They then used 16S rRNA sequencing to compare the diversity of the microbiota and the prevalence of specific bacteria in nine infants with necrotizing enterocolitis and nine matched controls. Patients with necrotizing enterocolitis has an increase in Proteobacteria and a decrease in Firmicutes between 1 week and < 72 hours prior to the detection of clinical necrotizing enterocolitis.
Investigators have also searched for a microbial pattern that appears prior to necrotizing enterocolitis onset. Morrow et al. 54 analyzed stool samples from infants < 29 weeks gestational age and compared infants who developed necrotizing enterocolitis to matched controls. Infants who developed necrotizing enterocolitis not only had lower diversity in their microbiome but distinct patterns. In postnatal days 4 to 9, infants who developed necrotizing enterocolitis were dominated by members of the Firmicutes phylum. During days 10 to 16, samples from the remaining necrotizing enterocolitis cases were dominated by Proteobacteria. Interestingly, infants with Firmicutes dysbiosis developed necrotizing enterocolitis earlier than infants with Proteobacteria dysbiosis. All infants with necrotizing enterocolitis lacked Propionibacterium and were preceded by either Firmicutes or Proteobacteria dysbiosis. However, it should be noted that 25% of controls had this phenotype as well. Multiple studies have shown that Proteobacteria can be associated with an increased incidence of necrotizing enterocolitis; a fact that has been validated in the 2017 meta-analysis of 14 previous studies of intestinal dysbiosis in preterm infants who subsequently developed necrotizing enterocolitis by Pammi and et al. 55.
Figure 3. Factors Impacting the Neonatal Gut Microbiome
Footnote: Factors contributing to the development of the neonatal microbiome include both prenatal factors such as the maternal microbiome, the microbiome of the amniotic fluid, the degree of prematurity and the mode of delivery, and postnatal exposures including antibiotics, diet, and acid suppressing medications
[Source 56]Prenatal development of the microbiome
PCR studies of amniotic fluid have estimated the prevalence of microbial invasion of the amniotic cavity to be more than 30–50% higher than previously detected by culture based methods 57. The placental basal plate was found to have a microbiome of its own with many commensal bacterial species including organisms from the phyla Firmicutes, Tenericutes, Proteobacteria, Bacteriodetes, and Fusobacteria 58. It is unclear whether this colonization has any impact on the neonatal GI tract but, given that the fetus swallows large volumes of amniotic fluid during gestation, it is logical that the fetal intestine would be exposed to amniotic fluid microbes 59. This notion is further supported by the findings of low levels of microbial DNA in first-pass meconium 60. Jimenez et al. 61 were able to isolate low numbers of Enterococcus, Staphylococcus, and Streptococcus in the umbilical blood from scheduled, elective cesarean sections. In a later study they tested the meconium from term infants prior to breast feeding and found similar organisms: Enterococcus, Staphylococcus, and Escherichia coli.
The impact of mode of delivery on the microbiome
In the United States the caesarean section rate continues to rise, reaching 33.1% in 2013 62. Several studies have demonstrated a difference in the microbiome of infants born via cesarean delivery compared to vaginally delivered neonates. Infants born via the vaginal canal are typically seeded with vaginal flora including Lactobacillus and Prevotella. In contrast, infants born via cesarean section are typically seeded with skin flora 63. Infants born via cesarean section display delayed onset of colonization of Bifidobacterium and Bacteroides with increased levels of colonization by the Enterobacteriaceae family 64. In 2011 Domingiuez-Bello et al. 65 used sequencing technology to demonstrate that the gastrointestinal microbiota of infants born vaginally were colonized with Lactobacillus, but infants born via cesarean delivery were colonized by bacteria typically found in skin and hospitals such as Staphylococcus and Acinetobacter. They later demonstrated that exposing neonates delivered via cesarean section to maternal vaginal fluids at birth could redirect the microbiome, making it similar to neonates delivered vaginally 66. Large numbers of epidemiologic studies have demonstrated compelling evidence suggesting a link between cesarean delivery and increased risk of obesity, asthma, allergies, immune deficiencies, and other atopic disease 67. However, to date, a direct link between delivery by cesarean and necrotizing enterocolitis has not been found. Prognostic studies indicate that cesarean section is a risk factor for necrotizing enterocolitis but this is likely correlated not causative 68.
Dietary impact on the microbiome
Multiple studies over several decades have demonstrated that enteral feeding with human milk as opposed to formula decreases the incidence of necrotizing enterocolitis 69. Breast milk contains immunoglobulins, cytokines, lactoferrin, and growth factors 59. Breast milk also contains glycoproteins that have been shown to decrease organ injury and inflammation in sepsis in mouse models 70). In addition human milk contains human milk oligosaccharides that stimulates the growth of “healthy” bacteria- Bifidobacteria and Bacteroides species both possess the proper enzymes to digest human milk oligosaccharides and metabolize them for energy. Human milk oligosaccharides are the third most abundant ingredient in breast milk 71. Human milk oligosaccharides may help to select for beneficial microbes by providing them with substrates for growth, allowing them to thrive. This may decrease the ability of opportunistic pathogenic microbes to gain a foothold in the neonatal gut 71. Furthermore, one way in which breast milk is thought to be beneficial is downregulation of TLR4 signaling 72.
In addition to helping shape the intestinal microbiome by nutrient selection, breast milk has its own microbiome which evolves over time. Initially colostrum contains Staphylococcus, Streptococcus, Lactobacillus and Weissella but over time the microbes are more consistent with maternal oral flora (Veillonella, Leptotrichia, and Prevotella). Interestingly, while milk samples from mothers who underwent elective cesarean sections varied in bacterial composition from milk samples from mothers who experienced vaginal delivery, the microbiome in the breast milk of mothers who underwent nonelective cesarean sections was similar to the microbiome of milk among mothers with vaginal deliveries. This suggests that maternal stress and hormones influence breast milk microbiome more directly than mode of delivery 73.
After birth, breast fed infants are first colonized with aerobic or facultative anaerobic bacteria followed by a bloom of anaerobic bacteria. Formula fed infants’ gastrointestinal microbiomes differ by having fewer anaerobes and a plethora of gram negative bacteria 74 and have increased levels of Enterobacteriaceae, Bacteroides, and Clostridium in their stools compared to infants who receive breast milk. The effect on breast versus formula feeding on the levels of the Bifidobacterium species are less clear with some studies finding significantly reduced amounts in formula fed infants and other studies showing no difference at all 75.
Impact of antibiotics on the microbiome
Antibiotic exposure has a large impact on the neonatal microbiome delaying the colonization of beneficial bacteria and reducing the diversity of the intestinal microbiome, both factors which are thought to predispose the neonate to necrotizing enterocolitis 59. Years of research and numerous studies have demonstrated that use of antibiotics may be associated with development of necrotizing enterocolitis 76. Alexander et al. demonstrated there was a direct correlation between duration of antibiotics and risk of developing necrotizing enterocolitis among infants without culture-proven sepsis 77. For more detailed review of the topic, Esaiassen et al. published a meta-analysis in 2017 demonstrating the same: prolonged antibiotic exposure in uninfected preterm infants is associated with an increased risk of necrotizing enterocolitis and/or death 78.
Impact of acid suppression on the microbiome
Acid suppression therapy has a known impact on the preterm microbiome. Gupta et al. 79 demonstrated that the use of H2 blockers in premature infants shifts the microflora pattern towards Proteobacteria and limits the diversity of the fecal microbiome. These alterations may predispose an infant to necrotizing enterocolitis. Romaine et al. 80 performed a retrospective cohort study and found that the use of H2 blockers are associated with increased risk of the combined outcome of death, necrotizing enterocolitis, or sepsis in hospitalized very low birth weight infants.
Strategies for necrotizing enterocolitis prevention
Given that necrotizing enterocolitis occurs in a well-defined population of patients — that is, those who are premature — there might be benefit in identifying specific preventive strategies that, if administered successfully to the appropriate patients, could reduce the incidence of necrotizing enterocolitis. In this regard, there has been tremendous interest in developing specific nutritional and pharmacological strategies to reduce the incidence of necrotizing enterocolitis.
Nutritional approaches for necrotizing enterocolitis prevention: the use of breast milk
Multiple randomized clinical trials have now validated the empirical observation that breast milk statistically significantly reduces the incidence of necrotizing enterocolitis 81. Human milk contains a variety of beneficial bioactive factors, among which several have been shown to reduce necrotizing enterocolitis incidence and progression81. Below is a list of human milk components and the experimental evidence supporting their protective effects. Considerable research efforts have been deployed to identify these critical factors in the hope that new preventive strategies can be developed 82. Although the precise mechanisms by which breast milk protects against necrotizing enterocolitis are not yet fully understood, emerging experimental evidence suggests that breast milk inhibits TLR4 signalling by preventing glycogen synthase kinase 3β activity 83. Consequently, breast-milk-mediated downregulation of TLR4 signalling can reverse the inhibition in intestinal stem cell proliferation and mucosal healing, which are themselves inhibited by TLR4 83. Moreover, these effects were shown to be partially dependent upon activation of epidermal growth factor receptor signalling 83. Whether the development of necrotizing enterocolitis in association with formula feeding represents the presence of an injurious component in infant formula, or the deficiency of a protective agent only present in breast milk remains to be determined37,69,124. The lack of availability of human breast milk (which can arise for a number of reasons, such as insufficient production by the mother of an infant) remains a major challenge in neonatal care 81, and has led to the use of donor breast milk as a potential substitute or supplement to formula-feeding. Multiple reports support the use of donor human milk as a potentially effective strategy for reducing the incidence of necrotizing enterocolitis 84. For those instances in which no human breast milk is available, emphasis has been placed on determining the best evidence-based strategies for formula-feeding 32. Although no specific feeding regimen (that is, composition, volume and rate of feeding) has been validated to prevent necrotizing enterocolitis 32, the use of standardized feeding guidelines (for example, patient-specific orders with set thresholds to manage feeding intolerance) 85 have been implemented in multiple centres and have been proven to be effective to reduce the incidence and severity of the disease 85.
Necrotizing enterocolitis-protective factors in human milk
- Nitrate and/or nitrite and antioxidant factors
- L-arginine
- Human milk oligosaccharides and prebiotics
- Lactoferrin
- Secretory IgA
- Platelet-activating factor acetylhydrolase
- Growth factors: –
- Epidermal growth factor
- Heparin-binding EGF-like growth factor
- Transforming growth factor β2
- Erythropoietin180
Necrotizing enterocolitis treatment
Despite considerable advances in neonatal care, necrotizing enterocolitis remains a devastating disease that lacks a cure. Current management is largely nonspecific and includes the administration of broad-spectrum antibiotics, initiation of bowel rest and the provision of fluid and inotropic support to maintain cardiorespiratory function 35. Surgical intervention is required in up to 50% of the necrotizing enterocolitis cases in large, population-based and hospital-based multicentre studies coordinated by neonatal research networks 8 and typically includes the removal of necrotic intestine. In rare cases, the placement of a peritoneal drain and abdominal irrigation might be sufficient.
Definitive necrotizing enterocolitis may require medical or surgical management based on the clinical presentation (Table 4). Medical intervention typically includes abdominal decompression, bowel rest, broad-spectrum intravenous antibiotics, and intravenous hyperalimentation. Surgical interventions are generally required in patients with intestinal perforation or deteriorating clinical or biochemical status (e.g., shock or a decreasing platelet count, neutrophil count, or both). Surgical procedures may involve drain placement, exploratory laparotomy with resection of diseased bowel, and enterostomy with creation of a stoma.
Two commonly used methods for treating advanced necrotizing enterocolitis with intestinal perforation are laparotomy and primary peritoneal drainage without laparotomy. The relative benefits of these methods have been controversial. Two large multicenter studies attempted to address this controversy 86, 87. The first concluded that the type of procedure does not influence survival or other clinically important early outcomes 86. The second study also showed no significant differences in outcomes between the groups, but it showed that infants treated with peritoneal drainage very often required a subsequent laparotomy 87. Further analysis of data from the latter study examined whether peritoneal drainage improved the patient’s immediate clinical status, and it showed no improvement when peritoneal drainage was used for this purpose 88. In addition, a systematic review of several studies suggested mortality was increased by more than 50% with peritoneal drainage as compared with laparotomy 89. Follow-up examinations at 18 to 22 months in infants who had undergone surgery for necrotizing enterocolitis in the neonatal period showed a significantly reduced risk of death or neurodevelopmental impairment among those who had undergone a laparotomy as compared with those who had undergone peritoneal drainage 90. These studies indicate that once surgery is required, the outcome may be poor, a finding that underscores the need for effective prevention.
Table 4. Diagnostic Criteria for and Treatment of Necrotizing Enterocolitis
| Diagnosis and Signs and Symptoms | Treatment Strategy |
|---|---|
| Suspected necrotizing enterocolitis | |
| Abdominal distention without radiographic evidence of pneumatosis intestinalis, portal venous gas, or free intraperitoneal air | Close clinical observation for increased abdominal distention and feeding intolerance |
| Unexpected onset of feeding intolerance | Consideration of bowel decompression and brief discontinuation of feeding (e.g., 24 hr); abdominal radiograph (anteroposterior and left lateral decubitus); monitoring of white cell, differential, and platelet counts (sudden decreases suggest progression of disease); consideration of blood cultures and short course of intravenous antibiotics |
| Definitive medical necrotizing enterocolitis | |
| Abdominal distention with pneumatosis intestinalis, portal venous gas, or both | Bowel decompression and discontinuation of enteral feedings for approximately 7–10 days |
| Other radiographic signs such as fixed, dilated loops of intestine and ileus patterns are not pathognomonic but should be treated as such | Close monitoring of white-cell, differential, and platelet counts (sudden decreases suggest progression of disease); blood culture and intravenous antibiotics for 7–10 days; close monitoring of abdominal radiographs (anteroposterior and left lateral decubitus); notification of surgical team |
| Surgical necrotizing enterocolitis | |
| Free intraperitoneal air on abdominal radiograph after initial medical signs and symptoms | Exploratory laparotomy with resection if necessary |
| Persistent ileus pattern, abdominal distention, and radiographs that show an absence of bowel gas, coupled with deteriorating clinical and laboratory values (e.g., decreasing neutrophil and platelet counts) | Placement of drain |
Therapeutic alteration of the neonatal microbiome
Research over the last decade has demonstrated the importance of the gut microbiome on human health and disease. Microbiome alterations have been associated with a vast array of diseases ranging from cardiovascular disease to colorectal cancer, obesity, diabetes, and rheumatoid arthritis 91. Furthermore, microbiome manipulation has already proven beneficial in the treatment of clostridium difficile infection 92 and has demonstrated promising results in the treatment of inflammatory bowel disease 93 and in experimental models of obesity 94.
Given the link between gut dysbiosis and necrotizing enterocolitis, it is logical then, that future prevention and treatment of the disease will also include a component of microbiome manipulation and altering the microbiome is a promising target for future therapies 95. A 2014 Cochrane review of randomized and quasi-randomized trials found that enteral supplementation of probiotics prevents severe necrotizing enterocolitis and all cause mortality in preterm infants 96. In 2016 Denkel et al. found that dual-strain probiotics reduced necrotizing enterocolitis and mortality in preterm infants in a German newborn intensive care unit 97. However, the evidence regarding probiotics is difficult to interpret. Although the meta-analyses of probiotics usage have shown a beneficial effect, not all individual randomized control trials have demonstrated the same. Trials are difficult to generalize as many use a different study design, differing probiotics, and differing infant diets and feeding times 98. The strain of probiotics used is likely to be important. The PiPs trial did not demonstrate any benefit with routine administration of Bifidobacterium breve 99. Furthermore, there are conflicting opinions regarding giving live bacteria to particular vulnerable preterm neonates.
There are three major options for an approach to microbiome-based therapies: additive, subtractive, or modulatory therapies. Additive therapy includes the manipulation of the microbiome by supplementing the microbiome of the host with either specific strains of organisms or groups of natural or engineered microorganisms. Subtractive therapy involves the removal of specific deleterious members of the microbiome to cure disease. Modulatory therapies involve administration of nonliving agents, called prebiotics, to modify the composition or activity of the host microbiome 100.
However, before probiotics can routinely be used in the prevention of necrotizing enterocolitis, dose, strain, and timing of administration need to be standardized. Probiotics might require regulatory approval for use in the neonate before they can become standard of care. In addition to commercially available probiotics the development of genetically engineered probiotics are underway, although this process is still in its infancy. Bacterial cells could be altered to allow recombinant expression of therapeutic biomolecules. This would overcome issues with bioavailability and drug inactivation with oral administration. Protein synthesis of the therapeutics could be tied to conditions associated with the disease 100.
Quantitative metagenomics can be used to directly map the human gut microbiome. In the future this could be used for risk detection 101. Current efforts are aimed at risk detection of chronic diseases, but given the association between gut dysbiosis and necrotizing enterocololitis, and the knowledge that certain bacterial strains appear more frequently in patients who develop necrotizing enterocolitis, this strategy could be applied to the disease in the future. At risk preterm infants would be good targets for microbiome analysis. Microbiome patterns thought to be associated with an increased risk for the development of necrotizing enterocolitis could then be ideal candidates for microbiome alteration.
- Necrotizing enterocolitis: contemporary management and outcomes. Papillon S, Castle SL, Gayer CP, Ford HR. Adv Pediatr. 2013; 60(1):263-79. https://www.advancesinpediatrics.com/article/S0065-3101(13)00012-1/pdf[↩][↩][↩]
- Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Current Opinion in Infectious Diseases 2003;16(4):349‐55.[↩]
- Kosloske AM. Epidemiology of necrotizing enterocolitis. Acta Paediatrica. Supplement 1994;396:2‐7.[↩]
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7.[↩][↩]
- Neu J, Walker WA. Necrotizing Enterocolitis. The New England journal of medicine. 2011;364(3):255-264. doi:10.1056/NEJMra1005408. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3628622/[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No.: CD005496. DOI: 10.1002/14651858.CD005496.pub4[↩][↩]
- Unraveling the enigma that is neonatal necrotizing enterocolitis. McElroy SJ. J Perinatol. 2014 Oct; 34(10):729-30. https://www.ncbi.nlm.nih.gov/pubmed/25263723/[↩][↩]
- Outcomes and costs of surgical treatments of necrotizing enterocolitis. Stey A, Barnert ES, Tseng CH, Keeler E, Needleman J, Leng M, Kelley-Quon LI, Shew SB. Pediatrics. 2015 May; 135(5):e1190-7.[↩][↩]
- Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Niño DF, Sodhi CP, Hackam DJ. Nat Rev Gastroenterol Hepatol. 2016 Oct; 13(10):590-600.[↩][↩]
- Outcomes and costs of surgical treatments of necrotizing enterocolitis. Stey A, Barnert ES, Tseng CH, Keeler E, Needleman J, Leng M, Kelley-Quon LI, Shew SB. Pediatrics. 2015 May; 135(5):e1190-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4411777/[↩]
- Necrotizing enterocolitis among neonates in the United States. Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. J Perinatol. 2003 Jun; 23(4):278-85.[↩]
- Pathogenesis of NEC: Impact of an altered intestinal microbiome. Neu J, Pammi M. Semin Perinatol. 2017 Feb; 41(1):29-35.[↩]
- Xing T, Camacho Salazar R, Chen Y-H. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers. 2017;5(4):e1356901. doi:10.1080/21688370.2017.1356901. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5788446/[↩]
- Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Lu P, Sodhi CP, Jia H, Shaffiey S, Good M, Branca MF, Hackam DJ. Am J Physiol Gastrointest Liver Physiol. 2014 Jun 1; 306(11):G917-28.[↩]
- The nitric oxide synthase 2 pathway is targeted by both pro- and anti-inflammatory treatments in the immature human intestine. Ferretti E, Tremblay E, Thibault MP, Grynspan D, Burghardt KM, Bettolli M, Babakissa C, Levy E, Beaulieu JF. Nitric Oxide. 2017 Jun 1; 66():53-61[↩]
- Neonatal infectious diseases: evaluation of neonatal sepsis. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Pediatr Clin North Am. 2013 Apr; 60(2):367-89.[↩]
- Melville JM, Moss TJM. The immune consequences of preterm birth. Frontiers in Neuroscience. 2013;7:79. doi:10.3389/fnins.2013.00079. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3659282/[↩]
- Denning N-L, Prince JM. Neonatal intestinal dysbiosis in necrotizing enterocolitis. Molecular Medicine. 2018;24:4. doi:10.1186/s10020-018-0002-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6016883/[↩][↩][↩]
- Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nature reviews Gastroenterology & hepatology. 2016;13(10):590-600. doi:10.1038/nrgastro.2016.119. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5124124/[↩][↩][↩][↩][↩][↩][↩]
- Necrotizing enterocolitis in full-term or near-term infants: risk factors. Martinez-Tallo E, Claure N, Bancalari E. Biol Neonate. 1997; 71(5):292-8.[↩]
- Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? Gordon PV, Swanson JR, Attridge JT, Clark R. J Perinatol. 2007 Nov; 27(11):661-71.[↩]
- Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll BJ, National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 2001 Jan 11; 344(2):95-101.[↩]
- Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Ann Surg. 1978 Jan; 187(1):1-7.[↩][↩]
- Necrotizing enterocolitis: treatment based on staging criteria. Walsh MC, Kliegman RM. Pediatr Clin North Am. 1986 Feb; 33(1):179-201. https://www.ncbi.nlm.nih.gov/pubmed/3081865/[↩][↩]
- Potential confounder of NEC clinical trials. Swanson JR, Attridge JT, Gordon PV. J Perinatol. 2009 Mar; 29(3):256-7; author reply 257-8.[↩]
- Peritoneal drainage does not stabilize extremely low birth weight infants with perforated bowel: data from the NET Trial. Rees CM, Eaton S, Khoo AK, Kiely EM, Members of NET Trial Group., Pierro A. J Pediatr Surg. 2010 Feb; 45(2):324-8; discussion 328-9.[↩]
- Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, Faingold R, Taylor G, Gerstle JT. Radiographics. 2007 Mar-Apr; 27(2):285-305.[↩]
- Low birthweight, gestational age, need for surgical intervention and gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Elfvin A, Dinsdale E, Wales PW, Moore AM. Acta Paediatr. 2015 Aug; 104(8):771-6.[↩]
- Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, Canadian Neonatal Network. Pediatrics. 2012 Feb; 129(2):e298-304.[↩]
- Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Collado MC, Cernada M, Neu J, Pérez-Martínez G, Gormaz M, Vento M. Pediatr Res. 2015 Jun; 77(6):726-31.[↩]
- Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Cobb BA, Carlo WA, Ambalavanan N. Pediatrics. 2004 Jan; 113(1 Pt 1):50-3.[↩]
- Good M, Sodhi CP, Hackam DJ. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol. 2014;10:875–884.[↩][↩][↩]
- Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Pediatr Neonatol. 2014 Oct; 55(5):335-40.[↩]
- Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Res. 2014 Jul; 76(1):100-8[↩]
- Necrotizing enterocolitis: contemporary management and outcomes. Papillon S, Castle SL, Gayer CP, Ford HR. Adv Pediatr. 2013; 60(1):263-79.[↩][↩]
- Stey A, Barnert ES, Tseng C-H, et al. Outcomes and Costs of Surgical Treatments of Necrotizing Enterocolitis. Pediatrics. 2015;135(5):e1190-e1197. doi:10.1542/peds.2014-1058. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4411777/[↩]
- Neonatal necrotizing enterocolitis. Henry MC, Moss RL. Semin Pediatr Surg. 2008 May; 17(2):98-109. https://www.ncbi.nlm.nih.gov/pubmed/18395659/[↩]
- Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R, NICHD Neonatal Research Network. Pediatrics. 2005 Mar; 115(3):696-703. https://www.ncbi.nlm.nih.gov/pubmed/15741374/[↩]
- Acute necrotizing enterocolitis in infancy: a review of 64 cases. Sántulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, Blanc WA, Berdon WE. Pediatrics. 1975 Mar; 55(3):376-87.[↩]
- Development of the Neonatal Intestinal Microbiome and Its Association With Necrotizing Enterocolitis. Elgin TG, Kern SL, McElroy SJ. Clin Ther. 2016 Apr; 38(4):706-15. https://www.ncbi.nlm.nih.gov/pubmed/26852144/[↩]
- Patel RM, Denning PW. Intestinal Microbiota and Its Relationship with Necrotizing Enterocolitis. Pediatric research. 2015;78(3):232-238. doi:10.1038/pr.2015.97. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4655440/[↩]
- Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Hackam DJ, Afrazi A, Good M, Sodhi CP. Clin Dev Immunol. 2013; 2013():475415.[↩]
- Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. J Immunol. 2009 Jan 1; 182(1):636-46.[↩]
- Immunoregulation effects of different γδT cells and toll-like receptor signaling pathways in neonatal necrotizing enterocolitis. Hui L, Dai Y, Guo Z, Zhang J, Zheng F, Bian X, Wu Z, Jiang Q, Guo M, Ma K, Zhang J. Medicine (Baltimore). 2017 Feb; 96(8):e6077.[↩]
- Breast-feeding improves gut maturation compared with formula feeding in preterm babies. Reisinger KW, de Vaan L, Kramer BW, Wolfs TG, van Heurn LW, Derikx JP. J Pediatr Gastroenterol Nutr. 2014 Dec; 59(6):720-4.[↩]
- Stem Cell Factor/Kit Signal Insufficiency Contributes to Hypoxia-Induced Intestinal Motility Dysfunctions in Neonatal Mice. Ren H, Han J, Li Z, Xiong Z. Dig Dis Sci. 2017 May; 62(5):1193-1203.[↩]
- Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Deplancke B, Gaskins HR. Am J Clin Nutr. 2001 Jun; 73(6):1131S-1141S.[↩]
- The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. Shock. 2007 Feb; 27(2):124-33.[↩]
- The role of the intestinal microcirculation in necrotizing enterocolitis. Watkins DJ, Besner GE. Semin Pediatr Surg. 2013 May; 22(2):83-7.[↩]
- Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. MohanKumar K, Namachivayam K, Chapalamadugu KC, Garzon SA, Premkumar MH, Tipparaju SM, Maheshwari A. Pediatr Res. 2016 Jun; 79(6):951-61.[↩]
- Hosny M. Updating on gut microbiota and its relationship with the occurrence of necrotizing enterocolitis – ScienceDirect. Hum Microbiome J. 2017;4:14–19. doi: 10.1016/j.humic.2016.09.002.[↩]
- 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. ISME J. 2009 Aug; 3(8):944-54.[↩]
- Fecal microbiota in premature infants prior to necrotizing enterocolitis. Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. PLoS One. 2011; 6(6):e20647.[↩]
- Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Morrow AL, Lagomarcino AJ, Schibler KR, Taft DH, Yu Z, Wang B, Altaye M, Wagner M, Gevers D, Ward DV, Kennedy MA, Huttenhower C, Newburg DS. Microbiome. 2013 Apr 16; 1(1):13.[↩]
- Pammi M, Cope J, Tarr PI, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5:31. doi:10.1186/s40168-017-0248-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5343300/[↩]
- Vongbhavit K, Underwood MA. Prevention of Necrotizing Enterocolitis Through Manipulation of the Intestinal Microbiota of the Premature Infant. Clinical therapeutics. 2016;38(4):716-732. doi:10.1016/j.clinthera.2016.01.006. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4902014/[↩]
- Diversity of microbes in amniotic fluid. DiGiulio DB. Semin Fetal Neonatal Med. 2012 Feb; 17(1):2-11.[↩]
- The placenta harbors a unique microbiome. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. Sci Transl Med. 2014 May 21; 6(237):237ra65[↩]
- The altered gut microbiome and necrotizing enterocolitis. Torrazza RM, Neu J. Clin Perinatol. 2013 Mar; 40(1):93-108.[↩][↩][↩]
- Sensitive Quantitative Analysis of the Meconium Bacterial Microbiota in Healthy Term Infants Born Vaginally or by Cesarean Section. Nagpal R, Tsuji H, Takahashi T, Kawashima K, Nagata S, Nomoto K, Yamashiro Y. Front Microbiol. 2016; 7():1997.[↩]
- Is meconium from healthy newborns actually sterile? Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM. Res Microbiol. 2008 Apr; 159(3):187-93[↩]
- Mistry K, Fingar KR, Elixhauser A. Variation in the Rate of Cesarean Section Across U.S. Hospitals, 2013: Statistical Brief #211. 2016 Sep. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK396065[↩]
- Cesarean delivery may affect the early biodiversity of intestinal bacteria. Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. J Nutr. 2008 Sep; 138(9):1796S-1800S.[↩]
- Dynamics of infant gut microbiota are influenced by delivery mode and gestational duration and are associated with subsequent adiposity. Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, Brüssow H, Lee YS, Yap F, Chong YS, Godfrey KM, Holbrook JD, GUSTO Study Group. MBio. 2015 Feb 3; 6;1.[↩]
- Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Gastroenterology. 2011 May; 140(6):1713-9.[↩]
- Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, Mendez K, Knight R, Clemente JC. Nat Med. 2016 Mar; 22(3):250-3.[↩]
- Cesarean section and chronic immune disorders. Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Pediatrics. 2015 Jan; 135(1):e92-8.[↩]
- Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. BMC Pediatr. 2017 Apr 14; 17(1):105.[↩]
- An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM, Laroia N, Ehrenkranz RA, Dudell G, Cristofalo EA, Meier P, Lee ML, Rechtman DJ, Lucas A. J Pediatr. 2010 Apr; 156(4):562-7.e1.[↩]
- Treatment with milk fat globule epidermal growth factor-factor 8 (MFG-E8) reduces inflammation and lung injury in neonatal sepsis. Hansen LW, Yang WL, Bolognese AC, Jacob A, Chen T, Prince JM, Nicastro JM, Coppa GF, Wang P. Surgery. 2017 Aug; 162(2):349-357.[↩]
- Development of the Neonatal Intestinal Microbiome and Its Association With Necrotizing Enterocolitis. Elgin TG, Kern SL, McElroy SJ. Clin Ther. 2016 Apr; 38(4):706-15.[↩][↩]
- Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. He Y, Lawlor NT, Newburg DS. Adv Nutr. 2016 Jan; 7(1):102-11.[↩]
- The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. Am J Clin Nutr. 2012 Sep; 96(3):544-51.[↩]
- Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Le Huërou-Luron I, Blat S, Boudry G. Nutr Res Rev. 2010 Jun; 23(1):23-36.[↩]
- Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, Hodgkinson AJ. Appl Environ Microbiol. 2013 May; 79(9):3040-8.[↩]
- Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Abdel Ghany EA, Ali AA. Ann Saudi Med. 2012 Sep-Oct; 32(5):521-6.[↩]
- Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. Alexander VN, Northrup V, Bizzarro MJ. J Pediatr. 2011 Sep; 159(3):392-7.[↩]
- Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. Esaiassen E, Fjalstad JW, Juvet LK, van den Anker JN, Klingenberg C. J Antimicrob Chemother. 2017 Jul 1; 72(7):1858-1870.[↩]
- Histamine-2 receptor blockers alter the fecal microbiota in premature infants. Gupta RW, Tran L, Norori J, Ferris MJ, Eren AM, Taylor CM, Dowd SE, Penn D. J Pediatr Gastroenterol Nutr. 2013 Apr; 56(4):397-400.[↩]
- Safety of histamine-2 receptor blockers in hospitalized VLBW infants. Romaine A, Ye D, Ao Z, Fang F, Johnson O, Blake T, Benjamin DK Jr, Cotten CM, Testoni D, Clark RH, Chu VH, Smith PB, Hornik CP, Best Pharmaceuticals for Children Act – Pediatric Trials Network. Early Hum Dev. 2016 Aug; 99():27-30.[↩]
- Breastfeeding and the use of human milk. Section on Breastfeeding. Pediatrics. 2012 Mar; 129(3):e827-41.[↩][↩][↩]
- Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Pammi M, Abrams SA. Cochrane Database Syst Rev. 2015 Feb 20; (2):CD007137[↩]
- Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, Lu P, Branca MF, Ma C, Prindle T Jr, Mielo S, Pompa A, Hodzic Z, Ozolek JA, Hackam DJ. Mucosal Immunol. 2015 Sep; 8(5):1166-79.[↩][↩][↩]
- Formula versus donor breast milk for feeding preterm or low birth weight infants. Quigley M, McGuire W. Cochrane Database Syst Rev. 2014 Apr 22; (4):CD002971.[↩]
- Necrotizing enterocolitis risk: state of the science. Gephart SM, McGrath JM, Effken JA, Halpern MD. Adv Neonatal Care. 2012 Apr; 12(2):77-87; quiz 88-9.[↩][↩]
- Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354:2225–34.[↩][↩]
- Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248:44–51.[↩][↩]
- Rees CM, Eaton S, Khoo AK, Kiely EM. Peritoneal drainage does not stabilize extremely low birth weight infants with perforated bowel: data from the NET Trial. J Pediatr Surg. 2010;45:324–8.[↩]
- Sola JE, Tepas JJ, III, Koniaris LG. Peritoneal drainage versus laparotomy for necrotizing enterocolitis and intestinal perforation: a meta-analysis. J Surg Res. 2010;161:95–100.[↩]
- Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics. 2006;117(4):e680–e687.[↩]
- The gut microbiome in health and in disease. Shreiner AB, Kao JY, Young VB. Curr Opin Gastroenterol. 2015 Jan; 31(1):69-75.[↩]
- Fecal transplantation for the treatment of Clostridium difficile infection. Brandt LJ. Gastroenterol Hepatol (N Y). 2012 Mar; 8(3):191-4.[↩]
- Therapeutic Manipulation of the Microbiome in IBD: Current Results and Future Approaches. Hansen JJ, Sartor RB. Curr Treat Options Gastroenterol. 2015 Mar; 13(1):105-20.[↩]
- The New Era of Treatment for Obesity and Metabolic Disorders: Evidence and Expectations for Gut Microbiome Transplantation. Jayasinghe TN, Chiavaroli V, Holland DJ, Cutfield WS, O’Sullivan JM. Front Cell Infect Microbiol. 2016; 6():15.[↩]
- Prevention of Necrotizing Enterocolitis Through Manipulation of the Intestinal Microbiota of the Premature Infant. Vongbhavit K, Underwood MA. Clin Ther. 2016 Apr; 38(4):716-32.[↩]
- Cochrane in context: Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No.: CD005496. DOI: 10.1002/14651858.CD005496.pub4 https://doi.org/10.1002/ebch.1977[↩]
- Protective Effect of Dual-Strain Probiotics in Preterm Infants: A Multi-Center Time Series Analysis. Denkel LA, Schwab F, Garten L, Geffers C, Gastmeier P, Piening B. PLoS One. 2016; 11(6):e0158136.[↩]
- Reducing Incidence of Necrotizing Enterocolitis. Patel AL, Panagos PG, Silvestri JM. Clin Perinatol. 2017 Sep; 44(3):683-700.[↩]
- Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR, Probiotics in Preterm Infants Study Collaborative Group. Lancet. 2016 Feb 13; 387(10019):649-60.[↩]
- Microbiome therapeutics – Advances and challenges. Mimee M, Citorik RJ, Lu TK. Adv Drug Deliv Rev. 2016 Oct 1; 105(Pt A):44-54.[↩][↩]
- The human gut microbiome impacts health and disease. Ehrlich SD. C R Biol. 2016 Jul-Aug; 339(7-8):319-23.[↩]