What is river blindness
Onchocerciasis also called River Blindness, is a tropical disease caused by the parasitic roundworm Onchocerca volvulus. Onchocerciasis is transmitted through repeated bites by blackflies of the genus Simulium spp. 1. Onchocerciasis is called River Blindness because the blackfly that transmits the infection lives and breeds near fast-flowing streams and rivers and the infection can result in blindness. In addition to visual impairment or blindness, onchocerciasis causes skin disease, including nodules under the skin or debilitating itching. Worldwide onchocerciasis is second only to trachoma as an infectious cause of blindness.
In the human body, the adult Onchocerca volvulus worms produce embryonic larvae (microfilariae) that migrate to the skin (subcutaneous nodules), eyes (river blindness) and other organs. When a female blackfly bites an infected person during a blood meal, it also ingests microfilariae which develop further in the blackfly and are then transmitted to the next human host during subsequent bites. Repeated bites by infected flies increase the number of adult worms and larvae. Chronic skin onchocerciasis (onchodermatitis) causes itching, a rash with small pimples (papular rash), scarring, and thickened, leathery skin (lichenification). Other symptoms may develop over time and might include saggy skin (such as ”hanging groin”), patchy areas of much lighter colored skin (leopard skin), ichthyosis-like lesions (”lizard skin”), darkening of the skin, and very severe itching. Symptoms of an eye infection may include itchy eyes, red eyes, and sensitivity to light (photophobia). In some cases, the larvae may infect the optic nerve. As the common name suggests, infection of the eye by onchocerciasis can lead to vision loss and eventually blindness.
Persons with heavy Onchocerca volvulus parasitic worm infections will usually have one or more of three conditions: skin rash (usually itchy), eye disease, or nodules under the skin. The World Health Organization’s (WHO) expert committee on onchocerciasis estimates that at least 25 million people are infected and 123 million people live in areas that put them at risk of infection. About 300,000 people are blind because of the parasite and another 800,000 have visual impairment. Nearly 99% of infected persons live in Africa; the remainder lives in Yemen and six countries in the Americas. Onchocerciasis is commonly treated with an oral medicine called ivermectin, antibiotics such as rifampin, azithromycin, and doxycycline, and, another recently approved anti-parasitic drug, Moxidectin 2.
Onchocerciasis key facts 1:
- Onchocerciasis, commonly known as “River Blindness”, is caused by the parasitic worm Onchocerca volvulus.
- Onchocerciasis is transmitted to humans through exposure to repeated bites of infected blackflies of the genus Simulium
- Symptoms include severe itching, disfiguring skin conditions, and visual impairment, including permanent blindness.
- More than 99% of infected people live in 31 African countries. The disease also exists in some foci in Latin America and Yemen.
- The Global Burden of Disease Study estimated in 2017 that there were 20.9 million prevalent Onchocerca volvulus infections worldwide: 14.6 million of the infected people had skin disease and 1.15 million had vision loss
- Community-directed treatment with ivermectin is the core strategy to eliminate onchocerciasis in Africa. In the Americas the strategy is biannual large-scale treatment with ivermectin.
- Four countries have been verified by WHO as free of onchocerciasis after successfully implementing elimination activities for decades: Colombia, Ecuador, Mexico, and Guatemala
- By the end of 2017, three additional countries had stopped mass drug administration and completed 3 years of post-treatment surveillance in at least one transmission area: Bolivarian Republic of Venezuela, Uganda, and Sudan
- 1.8 million people live in areas that no longer require mass drug administration for onchocerciasis.
Figure 1. Onchocerciasis skin nodules

In what parts of the world am I more likely to get onchocerciasis?
Onchocerciasis is locally transmitted in 30 countries of Africa and in Yemen in the Middle East. Local transmission occurred in 13 focal areas in 6 countries in the Americas (Mexico, Guatemala, Ecuador, Colombia, Venezuela, and Brazil), but transmission has now stopped in 11 of these focal areas. Transmission continues in one focal area in Venezuela and one in Brazil. Onchocerciasis in casual travelers is rare; the infection is transmitted in remote rural areas and, unlike malaria, contracting onchocerciasis often requires more than one infectious bite. Thus, risk of infection is greater in adventure travelers, missionaries, and Peace Corps and other long-term volunteers who are likely to have more intense or sustained exposure to blackfly bites. Given the low rate of transmission in the Americas, the likelihood is very low that any travelers in this region (even missionaries and long-term volunteers) would ever get infected.
How is onchocerciasis spread?
The disease spreads by the bite of an infectious blackfly. When a blackfly bites a person who has onchocerciasis, microscopic worm larvae (called microfilariae) in the infected person’s skin enter and infect the blackfly. The larvae develop over 2 weeks in the fly to a stage that is infectious to humans. An infectious blackfly will typically drop larvae when biting a person. The larvae then penetrate the skin to infect the person. Because the worms reproduce only in humans but need to complete some of their development inside the blackfly, the intensity of human infection (number of worms in an individual) is related to the number of infectious bites sustained by an individual. Blindness is usually seen in the setting of longstanding and intense infection.
Can I take medication to prevent onchocerciasis?
No, there is neither a vaccine nor recommended drug available to prevent onchocerciasis.
Who is most at risk for onchocerciasis?
Those most at risk are people who live in areas where the parasite is spread followed by adventure travelers, missionaries, and Peace Corps volunteers who are exposed for long periods of time (generally more than 3 months) to blackfly bites in areas where the parasite exists. The disease is most intensely transmitted in remote rural African agricultural villages which are located near rapidly flowing streams.
What is the impact of onchocerciasis?
Onchocerciasis is the second leading infectious cause of blindness and can cause debilitating and disfiguring skin disease. However, the worldwide burden of onchocerciasis has been considerably reduced as the result of very successful disease control programs led by the World Health Organization (WHO). These programs are based on control of the blackfly population and/or mass administration to affected communities of an oral drug called ivermectin (Mectizan™), that is donated by Merck & Co., Inc. As a result of these programs, millions of people are at greatly reduced risk of debilitating itching, disfigurement, and blindness caused by onchocerciasis. Unfortunately, many people still do not have access to prevention and treatment measures.
How soon after infection will I have symptoms of onchocerciasis?
It can take up to one year for the larvae (also called microfilariae) to develop into an adult inside the human body and between 10 and 20 months before larvae can be found in the skin. Each adult female worm, which can live from 10–15 years, can produce millions of new larvae during her lifetime. As it is the larvae that cause most of the symptoms of onchocerciasis, most people feel well until after the adults start producing large numbers of new larvae.
What should I do if I think I may have onchocerciasis?
If you think you might have onchocerciasis, see your health care provider who may order skin biopsies (“snips”) or blood tests looking for antibodies to the parasite. However, examination of skin snips does not always show the parasites and positive blood tests do not necessarily indicate that you are still infected with Onchocerca volvulus.
Onchocerciasis epidemiology
Onchocercal infections are found in tropical climates. The main burden is in 30 countries in sub-Saharan Africa, though the Onchocerca volvulus parasite is found in limited areas in the Americas and in Yemen in the Middle East. In the Americas, transmission of onchocerciasis has been interrupted in 11 of the 13 regions. Interventions to limit transmission stopped in Colombia in 2008, in Ecuador in 2010, in Mexico in 2012, and in Guatemala in 2012. Transmission and interventions to limit transmission continue in one area in Venezuela and one area in Brazil. The World Health Organization (WHO) estimates that at least 25 million people are infected with Onchocerca volvulus worldwide; of these people 300,000 are blind and 800,000 have some sort of visual impairment. Some 123 million people are at risk for becoming infected with the parasite.
The people most at risk for acquiring onchocerciasis are those who live near streams or rivers where there are Simulium blackflies. Most of the areas where the blackflies are found are rural agricultural areas in sub-Saharan Africa. More than 99% of infected people live in 31 countries in sub-Saharan Africa: Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Republic of Congo, Côte d’Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Ethiopia, Gabon, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Malawi, Mali, Mozambique, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, South Sudan, Sudan, Togo, Uganda, United Republic of Tanzania. Usually, many bites are needed before being infected, so people who travel for short periods of time (generally less than 3 months) to areas where the parasite is found have a low chance of becoming infected with Onchocerca volvulus. Those travelers to at-risk areas most likely to become infected are long-term missionaries, Peace Corps and other long-term volunteers, and field researchers.
Figure 1. Onchocerciasis map of the distribution 2017
Onchocerciasis transmission
The Simulium blackflies that transmit the Onchocerca volvulus parasite bite during the day. Female blackflies need to ingest blood for ovulation, so they feed on humans. If a blackfly bites an infected person, onchocerciasis larvae can be ingested by the blackfly after which they migrate to the flight muscles. The larvae develop inside the blackfly and become infective for humans in about 10 to 12 days. They migrate to the biting parts of the fly where they can be transmitted back to humans when it bites again.
Humans become infected when blackflies deposit Onchocerca infective larvae into the skin when biting to extract blood. Once inside the human body, the larvae mature into adults in around 3 months to 1 year. Most adult female worms live in fibrous nodules under the skin and sometimes near muscles and joints. Adult male worms are usually found near the female worms. Nodules form around the worms as part of the interaction between the parasite and its human host. Inside the nodules the worms are relatively safe from the human immune response. As adults, female worms produce thousands of new larvae daily. The larvae become detectable in the skin 10 to 20 months after the initial infection. The adult worms can live up to 15 years inside the human body, and their larvae have a lifespan up to 2 years.
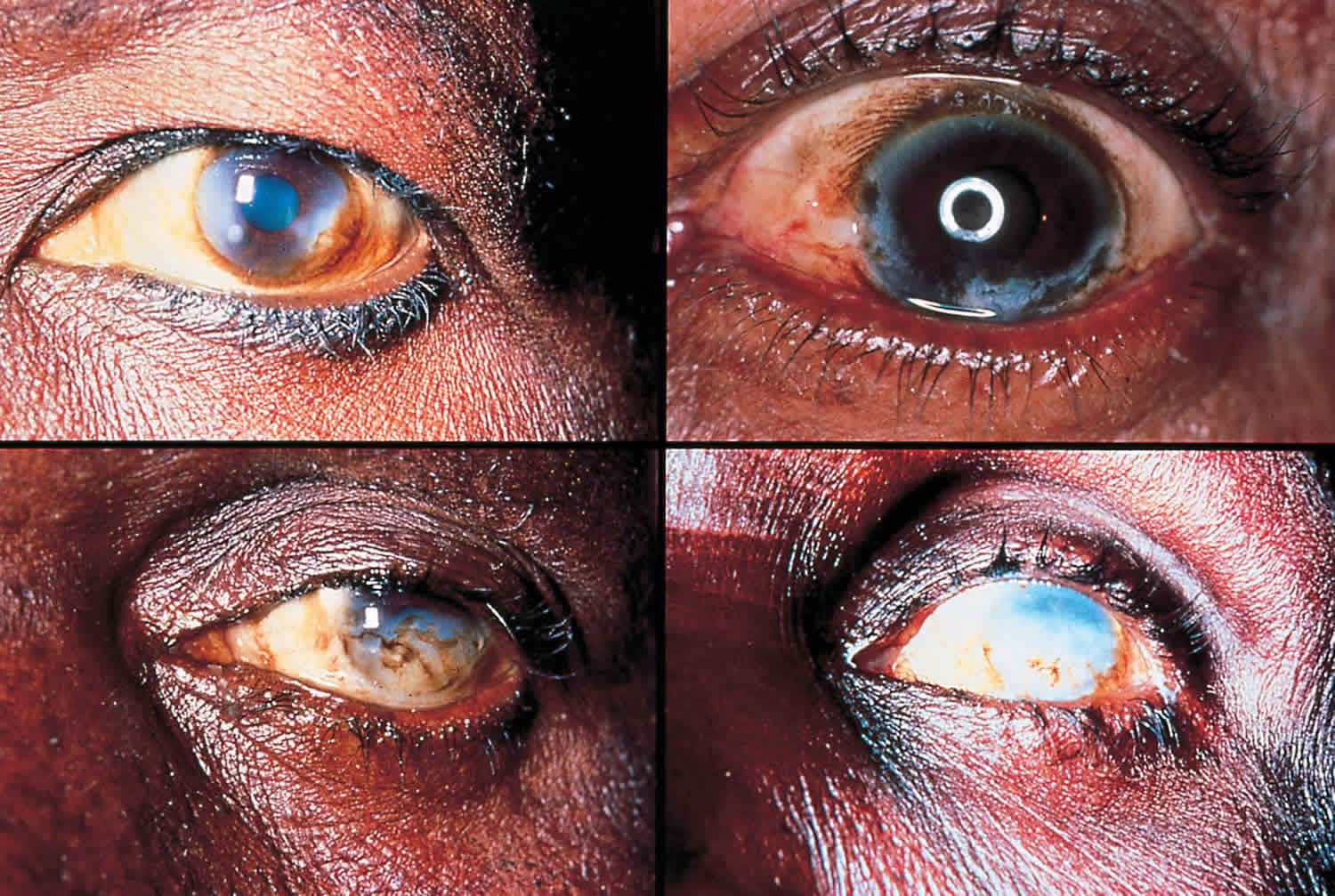
Some people do not experience symptoms while infected with Onchocerca volvulus, as the larvae can migrate through the human body without provoking a response from the immune system. But many people do have symptoms, which include itchy skin rashes, nodules under the skin, and vision changes. There can be non-painful swelling of lymph glands, but this is not common. Most symptoms of onchocerciasis are caused by the body’s response to dead or dying larvae. The inflammation caused in the skin, in addition to causing itching, can result in long-term damage to the skin. This can cause changes in the color of the skin that result in a “leopard skin” appearance, and can cause thinning of the skin with loss of elastic tissue that gives the skin a “cigarette-paper” appearance and can contribute to conditions such as “hanging groin.”. The inflammation caused by larvae that die in the eye results initially in reversible lesions on the cornea that without treatment progress to permanent clouding of the cornea, resulting in blindness. There can also be inflammation of the optic nerve resulting in vision loss, particularly peripheral vision, and eventually blindness.
Figure 2. Simulium blackfly
Onchocerciasis life cycle
Onchocerciasis is caused by Onchocerca volvulus nematodes (roundworms) that inhabit subcutaneous tissues. (#1) During a blood meal, an infected blackfly (genus Simulium) introduces third-stage filarial larvae onto the skin of the human host, where they penetrate into the bite wound. In subcutaneous tissues the larvae (#2) develop into adult filariae, which commonly reside in nodules in subcutaneous connective tissues (#3). Adults can live in the nodules for approximately 15 years. Some nodules may contain numerous male and female worms. Females measure 33 to 50 cm in length and 270 to 400 μm in diameter, while males measure 19 to 42 mm by 130 to 210 μm. In the subcutaneous nodules, the female worms are capable of producing microfilariae for approximately 9 years. The microfilariae, measuring 220 to 360 µm by 5 to 9 µm and unsheathed, have a life span that may reach 2 years. They are occasionally found in peripheral blood, urine, and sputum but are typically found in the skin and in the lymphatics of connective tissues (#4). A blackfly ingests the microfilariae during a blood meal (#5). After ingestion, the microfilariae migrate from the blackfly’s midgut through the hemocoel to the thoracic muscles (#6). There the microfilariae develop into first-stage larvae (#7) and subsequently into third-stage infective larvae (#8). The third-stage infective larvae migrate to the blackfly’s proboscis (#9) and can infect another human when the fly takes a blood meal (#1).
Figure 3. Onchocerciasis life cycle
Onchocerciasis prevention
Blackflies bite during the day. The best prevention is avoiding being bitten by infected blackflies by using insecticides that contain N,N-Diethyl-meta-toluamide (DEET) on exposed skin, wearing long sleeve shirts and pants, and wearing permethrin treated clothing.
There are no vaccines or medications available to prevent becoming infected with Onchocerciasis. The best prevention efforts include personal protection measures against biting insects. This includes wearing insect repellant such as N,N-Diethyl-meta-toluamide (DEET) on exposed skin, wearing long sleeves and long pants during the day when blackflies bite, and wearing permethrin- treated clothing. For a description of the CDC’s information for preventing insect bites, refer to CDC’s Yellow Book. Mass distribution of ivermectin, donated by Merck & Co., Inc., to all people living in many areas where river blindness is found is being given to control onchocerciasis.
- Wear appropriate clothing. Travelers can minimize areas of exposed skin by wearing long-sleeved shirts, long pants, boots, and hats. Tucking in shirts, tucking pants into socks, and wearing closed shoes instead of sandals may reduce risk. Repellents or insecticides, such as permethrin, can be applied to clothing and gear for added protection.
- Bed nets. When accommodations are not adequately screened or air conditioned, bed nets are essential in providing protection and reducing discomfort caused by biting insects. If bed nets do not reach the floor, they should be tucked under mattresses. Bed nets are most effective when they are treated with a pyrethroid insecticide. Pretreated, long-lasting bed nets can be purchased before traveling, or nets can be treated after purchase. Effective, treated nets may also be available in destination countries. Nets treated with a pyrethroid insecticide will be effective for several months if they are not washed. Long-lasting pretreated nets may be effective for much longer.
- Insecticides and spatial repellents. More spatial repellent products are becoming commercially available. These products, containing active ingredients such as metofluthrin and allethrin, augment aerosol insecticide sprays, vaporizing mats, and mosquito coils that have been available for some time. Such products can help to clear rooms or areas of mosquitoes (spray aerosols) or repel mosquitoes from a circumscribed area (coils, spatial repellents). Although many of these products appear to have repellent or insecticidal activity under particular conditions, they have not yet been adequately evaluated in peer-reviewed studies for their efficacy in preventing vectorborne disease. Travelers should supplement the use of these products with repellent on skin or clothing and using bed nets in areas where vector-borne diseases are a risk or biting arthropods are noted. Since some products available internationally may contain pesticides that are not registered in the United States, it may be preferable for travelers to bring their own. Insecticides and repellent products should always be used with caution, avoiding direct inhalation of spray or smoke.
REPELLENTS FOR USE ON SKIN AND CLOTHING
CDC has evaluated information published in peer-reviewed scientific literature and data available from EPA to identify several types of EPA-registered products that provide repellent activity sufficient to help people reduce the bites of disease-carrying mosquitoes. Products containing the following active ingredients typically provide reasonably long-lasting protection:
- DEET (chemical name: N,N-diethyl-m-toluamide or N,N-diethyl-3-methyl-benzamide). Products containing DEET include, but are not limited to, Off!, Cutter, Sawyer, and Ultrathon.
- Picaridin (KBR 3023 [Bayrepel] and icaridin outside the US; chemical name: 2-(2-hydroxyethyl)-1-piperidinecarboxylic acid 1-methylpropyl ester). Products containing picaridin include, but are not limited to, Cutter Advanced, Skin So Soft Bug Guard Plus, and Autan (outside the US).
- Oil of lemon eucalyptus (OLE) or PMD (chemical name: para-menthane-3,8-diol), the synthesized version of OLE. Products containing OLE and PMD include, but are not limited to, Repel and Off ! Botanicals. This recommendation refers to EPA-registered products containing the active ingredient OLE (or PMD). “Pure” oil of lemon eucalyptus (essential oil not formulated as a repellent) is not recommended; it has not undergone similar, validated testing for safety and efficacy and is not registered with EPA as an insect repellent.
- IR3535 (chemical name: 3-[N-butyl-N-acetyl]-aminopropionic acid, ethyl ester). Products containing IR3535 include, but are not limited to, Skin So Soft Bug Guard Plus Expedition and SkinSmart.
- 2-undecanone (chemical name: methyl nonyl ketone). The product BioUD contains 2-undecanone.
EPA characterizes the active ingredients DEET and picaridin as “conventional repellents” and OLE, PMD, IR3535, and 2-undecanone as “biopesticide repellents,” which are either derived from or are synthetic versions of natural materials.
Precautions when Using Insect Repellents
Travelers should take the following precautions:
- Apply repellents only to exposed skin or clothing, as directed on the product label. Do not apply repellents under clothing.
- Never use repellents over cuts, wounds, or irritated skin.
- When using sprays, do not spray directly on face—spray on hands first and then apply to face. Do not apply repellents to eyes or mouth, and apply sparingly around ears.
- Wash hands after application to avoid accidental exposure to eyes or ingestion.
- Children should not handle repellents. Instead, adults should apply repellents to their own hands first, and then gently spread on the child’s exposed skin.
- Avoid applying directly to children’s hands. After returning indoors, wash your child’s treated skin and clothing with soap and water or give the child a bath.
- Use just enough repellent to cover exposed skin or clothing. Heavy application and saturation are generally unnecessary for effectiveness. If biting insects do not respond to a thin film of repellent, apply a bit more.
- After returning indoors, wash repellent-treated skin with soap and water or bathe. Wash treated clothing before wearing it again. This precaution may vary with different repellents—check the product label.
If a traveler experiences a rash or other reaction, such as itching or swelling, from an insect repellent, the repellent should be washed off with mild soap and water and its use discontinued. If a severe reaction has occurred, a local poison-control center should be called for further guidance, if feasible. Travelers seeking health care because of the repellent should take the repellent to the doctor’s office and show the doctor. Permethrin should never be applied to skin but only to clothing, bed nets, or other fabrics as directed on the product label.
Children and Pregnant Women
Most repellents can be used on children aged >2 months. Protect infants aged <2 months from mosquitoes by using an infant carrier draped with mosquito netting with an elastic edge for a tight fit. Products containing OLE specify that they should not be used on children aged <3 years. Other than the safety tips listed above, EPA does not recommend any additional precautions for using registered repellents on children or on pregnant or lactating women.
Onchocerciasis symptoms
Infected persons may be without symptoms. Onchocerciasis is an eye and skin disease. Those with symptoms will usually have one or more of the three manifestations: skin rash (usually itchy), eye disease, and nodules under the skin. Symptoms are caused by the microfilariae, which move around the human body in the subcutaneous tissue and induce intense inflammatory responses when they die. Infected people may show symptoms such as severe itching and various skin changes. In most cases, nodules under the skin form around the adult worms.
The most serious manifestation consists of lesions in the eye that can lead to visual impairment and permanent blindness.
Onchocerciasis diagnosis
The diagnosis of onchocerciasis can be difficult in light infections, which are more common in persons who have travelled to but are not residents of affected areas. There are multiple ways that the diagnosis can be made:
- The most common method of diagnosis is the skin snip. A 1- to 2- mg shaving or biopsy of the skin is done to identify larvae, which emerge from the skin when it is put in physiologic solutions (e.g. normal saline). Typically 6 snips are taken from different areas of the body. Polymerase chain reaction (PCR) of the skin can allow for diagnosis if the larvae are not visualized.
- In patients with nodules in the skin, the nodule can be surgically removed and examined for adult worms.
- Infections in the eye can be diagnosed with a slit-lamp examination of the anterior part of the eye where the larvae or the lesions they cause are visible.
- Antibody tests have been developed to test for infection, though they are not widely available in the United States. These tests cannot distinguish between past and current infections, so they are not as useful in people who lived in areas where the parasite exists, but they are useful in visitors to these areas.
- Some of the tests are general tests for infection with any filarial parasite and some are more specific to onchocerciasis.
The gold standard test for the diagnosis of onchocerciasis remains the skin snip biopsy. The biopsy is performed using a sclerocorneal biopsy punch or by elevating a small cone of skin (3 mm in diameter) with a needle and shaving it off with a scalpel. This will result in the removal of around 2 mg of tissue. The tissue is then incubated in normal saline at room temperature for 24 hours to allow the microfilariae (larvae) to emerge. The microfilariae can then be identified microscopically. The sites for the skin snip are usually over the iliac crest, the scapula, and the lower extremities. Six snips provide the most diagnostic sensitivity. Although the skin snip is highly specific, its sensitivity can be limited in the pre-patent stage of infection, which can last 1 to 1.5 years, and in low intensity infections. Performing polymerase chain reaction (PCR) of the skin snip can increase the sensitivity in these two situations, though this is not commercially available.
If a patient has skin nodules caused by onchocercal infection, nodulectomy allows for the identification of macrofilariae (adult worms) in the tissue. Slit lamp eye exam can be used to visualize microfilariae in individuals with eye disease.
There are antibody tests that can assist in the diagnosis of onchocerciasis, though many are not available outside the research setting. There is a general screen for any filarial infection (including Wuchereria, Brugia, Loa, and Mansonella infections) that is available in some specialty diagnostic labs. Because the test is highly sensitive, it is useful in determining if an individual has had filarial infection, but it is not specific enough to identify which filarial infection. As with any antibody test, the results indicate only that the patient has been exposed to the disease, but it cannot determine if the patient has an active infection. This distinction is less important in symptomatic travelers, but it limits the usefulness of the test in persons from endemic areas. One advantage of the test is that it can pick up evidence of infection in the pre-patent stage of infection. There are several Onchocerca-specific serologic tests in existence, such as the OV-16 antigen antibody test and the OV luciferase immunoprecipitation system (LIPS) assay, but these are currently only available in the research setting and are not approved for diagnosis in the United States.
In general the diagnosis of Onchocerciasis infection should be made with skin snip. However, when skin snips are negative and clinical suspicion of infection is high, the general antibody test could be used in an attempt to exclude infection. If the general antibody test were positive, then it might be necessary to consider performing additional skin snips and/or seeking additional diagnostic information by enlisting the assistance of researchers who perform antibody tests.
Onchocerciasis treatment
People who are found to be infected with river blindness should be treated in order to prevent the long-term skin damage and blindness. The recommended treatment is ivermectin, which will need to be given every 6 months for the life span of the adult worms or for as long as the infected person has evidence of skin or eye infection. Ivermectin has been shown to reduce the occurrence of blindness and to reduce the occurrence and severity of skin symptoms. Ivermectin kills the microfilariae (larvae) and prevents them from causing damage, but not the adult worms (macrofilariae). There is a promising new treatment using doxycycline that kills the adult worms by killing the Wolbachia bacteria on which the adult worms depend in order to survive. If you are infected, it is possible that your doctor will want to treat you both with the ivermectin and the new treatment doxycycline. Before any treatment is begun, however, you need to make sure that you are not also infected with Loa loa, another filarial parasite found in central Africa that is sometimes found in the same areas where Onchocerca volvulus is found, because Loa loa can be responsible for severe side effects to the medications used to treat onchocerciasis.
Note on treatment in patients co-infected with Loa loa: Patients with Loa loa co-infection should not be treated for onchocerciasis without consulting an expert on loaiasis due to the risk of a fatal encephalitic reaction to ivermectin. Treatment of co-infected people with doxycycline has only been studied in persons with Loa counts of <8000 microfilariae per mL.
Table 1. Treatments for Onchocerciasis
| Usage/Drug | Adult Dose | Pediatric dose |
|---|---|---|
| To kill microfilariae: ivermectin | 150 mcg/kg orally in one dose every 6 months | 150 mcg/kg orally in one dose every 6 months |
| To kill adult worms: doxycycline* | 200 mg orally daily for 6 weeks | 200 mg orally daily for 6 weeks |
Footnote: * Doxycycline is not standard therapy, but several studies support its use and safety. Treatment with ivermectin should be given one week prior to treatment with doxycycline in order to provide symptom relief to the patient. If the patient cannot tolerate 200 mg PO daily of doxycycline, 100mg PO daily is sufficient to sterilize female Onchocerca.
- Oral ivermectin is available for human use in the United States.
- Doxycycline is available for human use in the United States.
Ivermectin
- Note on Treatment in Pregnancy
- Ivermectin is pregnancy category C. Data on the use of ivermectin in pregnant women are limited, though the available evidence suggests no difference in congenital abnormalities in the children of women who were accidentally treated during mass prevention campaigns with ivermectin compared with those who were not. The World Health Organization (WHO) excludes pregnant women from mass prevention campaigns that use ivermectin. However, the risk of treatment in pregnant women who are known to have an infection needs to be balanced with the risk of disease progression in the absence of treatment.
- Pregnancy Category C: Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal, or other) and there are no controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus.
- Note on Treatment During Breastfeeding
- Ivermectin is excreted in low concentrations in human milk. Ivermectin should be used in breast-feeding women only when the risk to the infant is outweighed by the risk of disease progress in the mother in the absence of treatment.
- Note on Treatment in Pediatric Patients
- The safety of ivermectin in children who weigh less than 15kg has not been demonstrated. According to the WHO guidelines for mass prevention campaigns, children who are at least 90 cm tall can be treated safely with ivermectin. The WHO growth standard curves show that this height is reached by 50% of boys by the time they are 28 months old and by 50% of girls by the time they are 30 months old, many children less than 3 years old been safely treated with ivermectin in mass prevention campaigns, albeit at a reduced dose.
Doxycycline
- Note on Treatment in Pregnancy
- Doxycycline is in pregnancy category D. Doxycycline should not be used in pregnant women due to positive evidence of fetal risk. Doxycycline might be indicated in life-threatening situations where no other treatment is available.
- Pregnancy Category D: There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective).
- Note on Treatment During Lactation
- Doxycycline is excreted in breast milk. The American Academy of Pediatrics classifies doxycycline as compatible with breastfeeding, whereas the World Health Organization (WHO) advises to avoid doxycycline in lactating women unless used for malaria. Dental staining and inhibition of bone growth in the infant may occur. Doxycycline should be used during lactation only if the potential benefit of therapy to the mother justifies the known risk to the infant.
- Note on Treatment in Pediatric Patients
- Doxycycline is contraindicated in children age 8 years and younger as it may cause permanent discoloration of the teeth. The WHO recommends that doxycycline should be used only for life-threatening infections in children age 8 years and younger. Use of doxycycline in children age 8 and younger should be limited to instances when there are contraindications to the use of other appropriate antibiotics and the potential benefit justifies the known risk.
There is no evidence that prolonged daily treatment provides any benefit over annual treatment, as one dose results in a significant decrease in microfilarial load that lasts a year or more. Treatment with higher than recommended doses has an increased incidence of side effects and may even be harmful. Although ivermectin does not kill the macrofilariae, it does sterilize the females. There is evidence that treating people with ivermectin more frequently than once a year facilitates more rapid sterilization of the female and that treating a person who no longer lives in an endemic area more frequently, such as every 3 to 6 months, could result in a shorter duration of symptoms. Treatment for a patient who will not be returning to live in an endemic area should be given every 6 months (and dosing as frequent as every 3 months could be considered) for as long as there is evidence of continued infection. Evidence of continued infection would include skin symptoms such as pruritus, microfilariae in skin biopsies, and microfilariae on eye exam. Finding adult worms in nodules would not necessarily constitute evidence of the need for continued treatment, as the macrofilariae do not cause symptoms and ivermectin does not kill the macrofilariae. Treatment with ivermectin can cause mild symptoms associated with death of the microfilariae, such as increase itching, but there is no worsening of eye symptoms. Severe adverse reactions to ivermectin in the absence of Loa loa co-infection are rare.
An evolving treatment is doxycycline, which has been shown in studies to kill Wolbachia, an endosymbiotic rickettsia-like bacterium that appears to be required for the survival of the Onchocerca volvulus macrofilariae and for embryogenesis. Treatment with a 6-week course of doxycycline has been shown to kill more than 60% of the adult female worms and to sterilize 80 to 90% of the females 20 months after treatment. Doxycycline does not kill the microfilariae, so treatment with ivermectin would be needed to result in a more rapid decrease of symptoms. Most protocols that have examined the effectiveness of doxycycline had given treatment with ivermectin 4 to 6 months after treatment with doxycycline, so the safety of simultaneous treatment is not known. Treating with ivermectin one week prior to starting doxycycline would be reasonable. As doxycycline does not result in the rapid death of the parasites and as most of the mild side effects of ivermectin treatment are thought to be related to rapid release of Wolbachia antigens, the side effect profile of the medication when used for the treatment of onchocerciasis does not appear to be different than that of its use for other indications. There are limited data to suggest that treatment of onchocerciasis with doxycycline in patients co-infected with Loa loa is safe, but this is limited to one randomized controlled trial where only people with Loa microfilarial loads < 8,000 per mL were treated and one community-based study of doxycycline treatment in co-endemic areas in which Loa microfilarial loads were not determined.
Older treatments for onchocerchiasis, such as suramin and diethylcarbamazine should not be used. Suramin has multiple systemic toxicities that limit its use in the presence of other less toxic and effective therapies. Diethylcarbamazine accelerates the development of onchocercal blindness.