Paneth cells
Paneth cells are secretory cells found at the base of small intestinal crypts (Lieberkühn crypts of the small intestine) and provide host defense against microbes in the small intestine. Paneth epithelial cells secrete granules containing alpha-defensins (α-defensins, an enzyme that destroy certain bacteria) when exposed to bacteria or bacterial antigens and maintain the intestinal environment by clearing enteric pathogens and regulating the composition of the intestinal microbiota 1. In addition to alpha-defensins, Paneth cells secrete lysozyme and phospholipase A2, both of which have clear antimicrobial activity. This battery of secretory molecules gives Paneth cells a potent arsenal against a broad spectrum of agents, including bacteria, fungi and even some enveloped viruses. The permanent bacterial residents of the intestinal lumen, called the intestinal flora, manufacture some essential vitamins, which the intestines absorb. Vitamin K is one substance produced by the intestinal bacteria. However, Paneth cell secretory responses remain debatable and the mechanisms that regulate the secretion are not well understood 1.
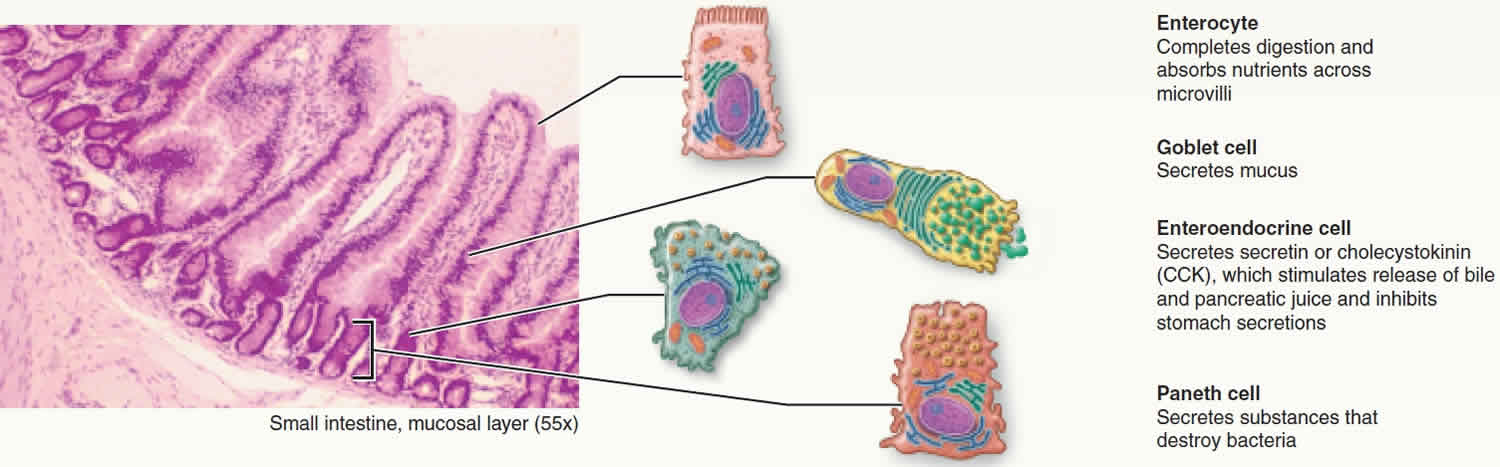
The small intestine absorbs luminal nutrients and also provides innate mucosal immune mechanisms that protect and prevent infection and invasion by certain pathogens 2. Epithelial cells that line the small intestine form a barrier consisting of intestinal epithelial stem cells and four major lineages of differentiated cells, including absorptive enterocytes, enteroendocrine cells, goblet cells, and Paneth cells that are oriented along the villus-crypt axis (see Figure 1) 3. Paneth cells, which occupy the base of small intestinal crypts with Lgr5+ (leucine-rich repeat containing G protein-coupled receptor 5) intestinal epithelial stem cells, contribute to innate enteric immunity by releasing secretory granules rich in varied host defense peptides, e.g., α-defensins, in response to bacteria and bacterial antigens such as lipopolysaccharide 4. On the contrary, it was reported that Paneth cells do not respond to luminal bacterial antigens directly but that an uncharacterized immune cell releases interferon gamma (IFN-γ) and that IFN-γ is what stimulates Paneth cell secretion 5. Therefore, Paneth cell secretory responses to bacterial stimuli have been controversial.
The intestinal lumen is chronically exposed to microorganisms, including pathogens, that are introduced orally. Therefore, the fate of Paneth cells after secreting granules, i.e., whether they remain depleted, undergo apoptosis, or replenish granules in preparation for additional microbial exposure is a key element in their life cycle. Although it was reported that secreted synaptic vehicles from the synaptic terminal of neurons are recycled 6, the regeneration of secretory granules after release from Paneth cells has not been explored previously. Some scientists have found that Paneth cells, after depleting their granule stores by secretion in response to carbamyl choline (carbachol), become refilled with newly generated granules ex vivo within 21 hours 1. In addition, Paneth cells that have resynthesized their granule contents respond to a secondary carbamyl choline (carbachol) stimulus with a comparably robust release of granules, evidence that individual Paneth cells prepare for multiple microbial exposures in contributing to innate enteric immunity. The dynamics of granulogenesis observed at high resolution visualized vesicular transport starting from the periphery of the Golgi near the nucleus toward the apical Paneth cell surface, within 21 hours of primary carbamyl choline (carbachol) stimulation. These findings suggest that Paneth cells also may repeatedly secrete and replenish their secretory granules in vivo to contribute continuously to innate host defense against luminal enteric microorganisms 1.
Cells closely involved in the innate immune response with respect to inflammatory bowel disease (IBD) include blood cells, macrophages, dendritic cells, Paneth cells, and goblet cells, which are also involved in the pathology of inflammatory bowel disease 7. These cells have been found to play important roles in the development of abnormalities, including maintaining homeostasis against stress at the cellular level and modulating autophagy 8.
Figure 1. Paneth cells
Paneth cells function
Paneth cells at the base of small intestinal crypts secrete granules containing α-defensins in response to bacteria and maintain the intestinal environment by clearing enteric pathogens and regulating the composition of the intestinal microbiota. However, Paneth cell secretory responses remain debatable and the mechanisms that regulate the secretion are not well understood. Paneth cells, which occupy the base of small intestinal crypts with Lgr5+ (leucine-rich repeat containing G protein-coupled receptor 5) intestinal epithelial stem cells, contribute to innate enteric immunity by releasing secretory granules rich in varied host defense peptides, e.g., α-defensins, in response to bacteria and bacterial antigens such as lipopolysaccharide 4. On the contrary, it was reported that Paneth cells do not respond to luminal bacterial antigens directly but that an uncharacterized immune cell releases interferon gamma (IFN-γ) and that IFN-γ is what stimulates Paneth cell secretion 5. Therefore, Paneth cell secretory responses to bacterial stimuli have been controversial.
More than 1 × 1014 bacteria live in the human intestinal lumen and harmonize with the host to create a normal intestinal microbiota of symbiotic microorganisms that contribute to maintaining intestinal homeostasis 9. Disruption of the intestinal microbiota induces dysbiosis (a term for a microbial imbalance) and is associated with various diseases such as inflammatory bowel disease, obesity and diabetes mellitus 10. In DEFA5+/+ mice, which express the human enteric α-defensin HD5 transgene in Paneth cells, the ileal microbiota contained significantly fewer Firmicutes and a higher percentage of Bacteroidetes compared to their wild-type FVB littermates 11. In contrast, the ileal microbiota of matrix metalloproteinase 7-null (Mmp7−/−) mice, which are deficient in activated luminal α-defensins (termed cryptdins), had a significantly greater percentage of Firmicutes and fewer Bacteroidetes compared to wild-type control mice 11. Alpha-defensins secreted into the small intestinal lumen have been recovered as intact, functional forms in the lumen of large intestine 12. Furthermore, scientists have shown that active cryptdins are detected not only in luminal contents of the intestine, but also in feces 13, suggesting that α-defensins also may influence the composition of the large intestinal microbiota. Masuda et al. 14 showed that cryptdin-4 had potent in vitro bactericidal activities against pathogenic bacteria and less activity against commensal species, suggesting that the peptide may regulate the composition of the intestinal microbiota. Taken together, secreted Paneth cell α-defensins have a role in regulating the intestinal microbiota and thus contribute to intestinal homeostasis. Moreover, Paneth cell dysfunction is associated with certain diseases such as inflammatory bowel disease, obesity and enteropathy in graft-versus-host disease 15. In graft-versus-host disease model mice, loss of secreted α-defensins due to depletion of Paneth cell numbers is associated with subsequent dysbiosis, resulting in fatal sepsis 16. Furthermore, administered α-defensin partially prevents dysbiosis and improves graft-versus-host disease survival 17. These reports suggest that dysfunction of Paneth cell α-defensin secretion is a major factor in initiating dysbiosis and disease and that secretion of Paneth cell granules is a key contributor to maintaining the in vivo intestinal environment via controlling the intestinal microbiota. However, mechanisms that regulate Paneth cell granule secretion remain undefined, partly because quantitative ex vivo methods of evaluating secretion have not been applied to the problem.
Paneth cell metaplasia
Paneth cell metaplasia is defined as the presence of Paneth cells outside their normal site of origin which is the base of the Lieberkühn crypts of the small intestine 18. In the colon, a Paneth cells normally be found in the cecum and proximal parts of the colon. Their presence outside these sites in the gastrointestinal tract is regarded as pathologic and indicates metaplasia 19. Paneth cell metaplasia is described in a variety of pathologic conditions including some neoplasms 20 and cases of chronic inflammatory bowel disease 21. Notably, Paneth cell metaplasia, i.e. the unusual appearance of Paneth cells in the colon, has been reported in patients affected by chronic inflammatory bowel diseases such as Ulcerative Colitis 22. Paneth cells may also be rarely found in gastrointestinal, biliary and prostatic tumors 18.
Some authorities have found that Paneth cell metaplasia affects the prognosis of certain pre-neoplastic conditions and tumors. Furthermore, it was found by some investigators that the presence of Paneth cell metaplasia in distal colorectal adenoma is inversely associated with synchronous severely dysplastic adenoma or adenocarcinoma 23. Chen et al. 24 also found that the presence of Paneth cell metaplasia in cases of Barrett’s esophagitis is associated with a higher risk for disease progression. However, Kinoshita et al. 25 suggested that the presence of Paneth cell metaplasia may have anti-neoplastic and growth inhibiting effects and is hence associated with low risk of malignancy.
In general, the literature is still lacking regarding the significance and the prognosis of Paneth cell metaplasia. Needless to say, the presence of Paneth cell metaplasia in Krukenberg ovarian tumors is not clear and the impact of those cells on the prognosis of this advanced cancer needs further studies 18.
- Yokoi Y, Nakamura K, Yoneda T, et al. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci Rep. 2019;9(1):2710. Published 2019 Feb 25. doi:10.1038/s41598-019-39610-7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6389922[↩][↩][↩][↩]
- Chen L, Tuo B, Dong H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients. 2016;8:43. doi: 10.3390/nu8010043[↩]
- van der Flier LG, Clevers HS. Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145[↩]
- Tanabe H, et al. Mouse Paneth Cell Secretory Responses to Cell Surface Glycolipids of Virulent and Attenuated Pathogenic Bacteria. Infect. Immun. 2005;73:2312–2320. doi: 10.1128/IAI.73.4.2312-2320.2005[↩][↩]
- Farin HF, et al. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell–derived IFN-γ J. Exp. Med. 2014;211:1393–1405. doi: 10.1084/jem.20130753[↩][↩]
- Cousin MA, Nicholls DG. Synaptic Vesicle Recycling in Cultured Cerebellar Granule Cells: Role of Vesicular Acidification and Refilling. J. Neurochem. 1997;69:1927–1935. doi: 10.1046/j.1471-4159.1997.69051927.x[↩]
- Eriguchi Y., Nakamura K., Yokoi Y., Sugimoto R., Takahashi S., Hashimoto D., Teshima T., Ayabe T., Selsted M.E., Ouellette A.J. Essential role of IFN-γ in T cell-associated intestinal inflammation. JCI. Insight. 2018;20:pii:121886. doi: 10.1172/jci.insight.121886[↩]
- Iida T, Yokoyama Y, Wagatsuma K, Hirayama D, Nakase H. Impact of Autophagy of Innate Immune Cells on Inflammatory Bowel Disease. Cells. 2018;8(1):7. Published 2018 Dec 22. doi:10.3390/cells8010007 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6356773[↩]
- Mitsuoka T. Establishment of Intestinal Bacteriology. Biosci. Microbiota Food Health. 2014;33:99–116. doi: 10.12938/bmfh.33.99.[↩]
- Kang D-W, et al. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. Plos One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322[↩]
- Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010;11:76–82. doi: 10.1038/ni.1825.[↩][↩]
- Mastroianni JR, et al. Alternative Luminal Activation Mechanisms for Paneth Cell α-Defensins. J. Biol. Chem. 2012;287:11205–11212. doi: 10.1074/jbc.M111.333559[↩]
- Nakamura K, Sakuragi N, Ayabe T. A monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for detection of secreted α-defensin. Anal. Biochem. 2013;443:124–131. doi: 10.1016/j.ab.2013.08.021[↩]
- Masuda K, Sakai N, Nakamura K, Yoshioka S, Ayabe T. Bactericidal Activity of Mouse α-Defensin Cryptdin-4 Predominantly Affects Noncommensal Bacteria. J. Innate Immun. 2011;3:315–326. doi: 10.1159/000322037[↩]
- Salzman NH, Bevins CL. Dysbiosis—A consequence of Paneth cell dysfunction. Semin. Immunol. 2013;25:334–341. doi: 10.1016/j.smim.2013.09.006[↩]
- Eriguchi Y, et al. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2015;17:702–706. doi: 10.1111/tid.12423[↩]
- Hayase, E. et al. R-Spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J. Exp. Med.214, 3507–3518 (2017).[↩]
- Alrajban WA, Khubrani RA, Almalki MS, Almassri A, Alrikabi AC. Extensive Paneth cell metaplasia in an ovarian Krukenberg tumor: report of an unusual case and literature review. J Surg Case Rep. 2018;2018(12):rjy323. Published 2018 Dec 11. doi:10.1093/jscr/rjy323 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6289214[↩][↩][↩]
- Stappenbeck T. Paneth cell development, differentiation, and function: new molecular cues. Gastroenterology 2009;137:30–3.[↩]
- Park K, Chen Z, MacDonald T, Siddiqui J, Ye H, Erbersdobler A, et al. Prostate cancer with Paneth cell–like neuroendocrine differentiation has recognizable histomorphology and harbors AURKA gene amplification. Hum Pathol 2014;45:2136–43.[↩]
- Simmonds N, Furman M, Karanika E, Phillips A, Bates A. Paneth cell metaplasia in newly diagnosed inflammatory bowel disease in children. BMC Gastroenterol 2014;14:93.[↩]
- Shi J. Defensins and Paneth cells in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1284–1292.[↩]
- Mahon M, Xu J, Yi X, Liu X, Gao N, Zhang L. Paneth cell in adenomas of the distal colorectum is inversely associated with synchronous advanced adenoma and carcinoma. Sci Rep 2016;6:26129.[↩]
- Chen W, Frankel W, Cronley K, Yu L, Zhou X, Yearsley M. Significance of Paneth cell metaplasia in Barrett esophagus. Am J Clin Pathol 2015;143:665–71.[↩]
- Kinoshita H, Hayakawa Y, Koike K. Metaplasia in the stomach—precursor of gastric cancer? Int J Mol Sci 2017;18:2063.[↩]