What is renal papillary necrosis
Renal papillary necrosis is a disorder of the kidneys in which all or part of the renal papillae die. Renal papillary necrosis is characterized by coagulative necrosis of the renal medullary pyramids and papillae brought on by several associated conditions and toxins that exhibit synergism toward the development of ischemia 1. Renal papillary necrosis is seen in patients who take excessive amounts of analgesics such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) such as motrin or ibuprofen. Renal papillary necrosis is also seen in diabetes. The clinical course of renal papillary necrosis varies depending on the degree of vascular impairment, the presence of associated causal factors, the overall health of the patient, the presence of bilateral involvement, and, specifically, the number of affected papillae.
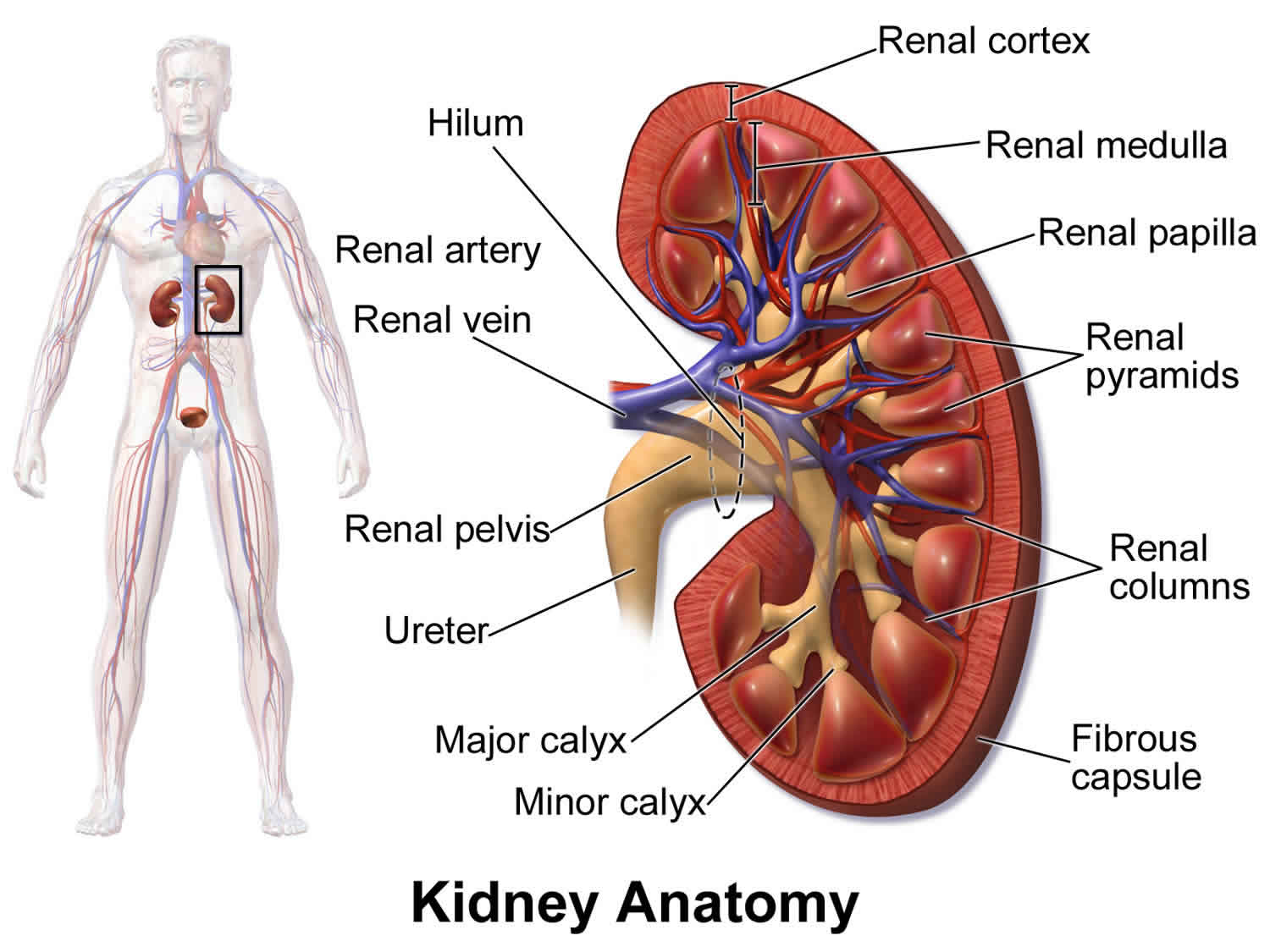
The renal papilla is the location where the renal pyramids in the medulla empty urine into the minor calyx in the kidney. Histologically it is marked by medullary collecting ducts converging to form a papillary duct to channel the fluid.
Renal papillary necrosis can lead to secondary infection of desquamated necrotic foci, deposition of calculi, and/or separation and eventual sloughing of papillae, with impending acute urinary tract obstruction. Multiple sloughed papillae can obstruct their respective calyces or can congregate and embolize to more distal sites (eg, ureteropelvic junction, ureter, ureterovesical junction). Previously undiagnosed congenital anomalies (eg, partial ureteropelvic junction obstruction) can provide a narrowed area where the sloughed papilla can nest and obstruct.
Renal papillary necrosis is potentially disastrous and, in the presence of bilateral involvement or an obstructed solitary kidney, may lead to renal failure. The infectious sequelae of renal papillary necrosis are more serious if the patient has multiple medical problems, particularly diabetes mellitus.
Researchers recognize 2 pathologic forms of renal papillary necrosis—the medullary form and the papillary form. Renal papillary necrosis is classified as focal (ie, involving only the tip of the papilla) or diffuse (ie, involving the whole papilla and areas of the medulla), depending primarily on the patient’s degree of impaired vasculature. Renal papillary necrosis may simply affect a single papilla, or the entire kidney may be grossly involved. Once again, renal papillary necrosis is more often a bilateral process; many of the predisposing factors are systemic. Renal papillary necrosis never involves the entire medulla; the disease is always strictly limited to the inner, more distal zone of the medulla and the papilla.
Papillary necrosis causes
Generally, any condition associated with ischemia predisposes an individual to papillary necrosis. Important general considerations include shock, massive fluid sequestration (eg, as in pancreatitis), dehydration, hypovolemia, and hypoxia. Certain conditions have a known association with renal papillary necrosis, and the underlying mechanism of these conditions is ischemia, which ultimately leads to renal papillary necrosis.
A useful mnemonic device for the conditions associated with renal papillary necrosis is POSTCARDS, which stands for the following:
- Pyelonephritis
- Obstruction of the urinary tract
- Sickle cell hemoglobinopathies, including sickle cell trait
- Tuberculosis
- Cirrhosis of the liver, chronic alcoholism
- Analgesic abuse
- Renal transplant rejection, radiation
- Diabetes mellitus
- Systemic vasculitis
More than half the patients with renal papillary necrosis have 2 or more of these causative factors. Thus, renal papillary necrosis in most patients is multifactorial in origin, and physicians must consider the pathogenesis of renal papillary necrosis a combination of detrimental factors that overlap and operate in concert to cause renal papillary necrosis.
One of the most common and most preventable risk factors is the use of analgesics. A classic risk factor is phenacetin, with its highly toxic metabolite, p-phenetidin. Recently, however, the rising popularity of nonsteroidal anti-inflammatory drugs (NSAIDs), particularly those that inhibit cyclooxygenases (ie, COX-1, COX-2) has led to a relatively high frequency of adverse events in patients at risk for renal papillary necrosis.
In healthy individuals in whom renal arterial blood flow is not compromised, NSAIDs have little effect unless they are used in excess. This is mostly true because the kidney is not relying on the vasodilatory effects of prostaglandin to supply adequate perfusion. However, in patients who are predisposed to renal hypoperfusion, local prostaglandin synthesis protects the glomeruli and tubules from ischemia. The inhibition of prostaglandin synthesis by NSAIDs that inhibit COX-1 and, as recently reported, COX-2, removes this protective mechanism and predisposes the kidney to further renal hypoperfusion and, ultimately, ischemia. An extremely important precaution is to strictly monitor patients with prior renal disease or any of the above-mentioned etiologic conditions when prescribing NSAIDs.
Additionally, note that a short course of NSAIDs has caused papillary necrosis and nonoliguric renal failure in otherwise healthy individuals as young as age 17 years. A case such as this may be an anomaly, but caution is warranted when prescribing NSAIDs, and adequate hydration is recommended.
These precautions should also be extended to patients receiving specific COX-2 inhibitors. Touted as being safer than COX-1 inhibitors because they spare the gastrointestinal tract, COX-2 inhibitors have been shown to significantly decrease renal medullary prostaglandin levels. Therefore, care should be exercised when administering even the COX-2 inhibitors to patients with a predisposition to renal disease or renal papillary necrosis, and otherwise healthy patients should maintain adequate hydration and avoid physiologic stress while on these medications.
Recently, multiple publications have described indinavir-induced renal papillary necrosis. In one study, diagnosis was delayed as the initial symptoms were attributed to suspected urinary tuberculosis. These studies demonstrate the necessity of renal function monitoring during HIV treatment above that of calculus monitoring 2.
Patients with known or suspected renal papillary necrosis should limit or completely avoid the use of analgesics. Other nephrotoxic medications should also be avoided. The routine use of indwelling catheters should be discouraged except when clinically indicated. Clean intermittent catheterization, although more time-consuming, is equally effective for the purpose of monitoring output is less likely to cause nosocomial urinary tract infection.
In patients with obstructed collecting systems who are hemodynamically unstable, obtunded, and floridly septic, avoiding any retrograde instrumentation of the ureter, such as stent placement, should be seriously considered. Unnecessary retrograde instrumentation is discouraged because of the risk of irrigating purulent or contaminated urinary inoculum from the lower urinary tract into the renal pelvis and pyelovenous system, exacerbating urosepsis.
In general, patients who are more ill should preferably be treated with antegrade percutaneous nephrostomy placement. However, an uncorrectable concomitant coagulopathy may preclude any percutaneous procedures, considering the potential morbidity of renal hemorrhage. Attempt correction of any coexisting coagulopathy prior to percutaneous nephrostomy.
In patients with hemodynamic instability who have a grossly infected and obstructed collecting system with an uncorrectable coagulopathy, the problem is more dire and a truly effective treatment remains elusive. These patients are probably best treated with retrograde stent placement to avoid a potentially lethal renal hemorrhage. In the severely ill patient, perform the quickest and safest procedure to establish drainage of the hydronephrotic section. This means that carefully considered retrograde procedures are sometimes the preferred modalities.
Broad-spectrum intravenous antibiotics administered immediately prior to any retrograde study may have a protective effect.
Papillary necrosis symptoms
Symptoms of renal papillary necrosis may include:
- Back pain or flank pain
- Bloody, cloudy, or dark urine
- Tissue pieces in the urine
Other symptoms that may occur with renal papillary necrosis:
- Fever and chills
- Painful urination
- Needing to urinate more often than usual (frequent urination) or a sudden, strong urge to urinate (urgency)
- Difficulty starting or maintaining a urine stream (urinary hesitancy)
- Urinary incontinence
- Urinating large amounts
- Urinating often at night
Renal papillary necrosis has a variable clinical course that ranges from a chronic, protracted, and relapsing form to an acute, rapidly progressive form. The acute progressive form is particularly rare, but the effects are devastating, resulting in death from septicemia and renal failure. Patients with the more common chronic form may remain asymptomatic until diagnosed incidentally through the appearance of a ring shadow on a radiographic image, by the passage of sloughed papillae in the urine, or during autopsy. The symptomatic form manifests as episodes of pyelonephritis and hydronephrosis, and it mimics nephrolithiasis.
The most common presenting symptoms in symptomatic patients include fever and chills, flank and/or abdominal pain, and hematuria. Acute renal failure with oliguria or anuria is rare; when these symptoms develop, the disease may be fulminant, requiring dialysis and potentially resulting in death.
If renal function deteriorates suddenly in a patient with confirmed diabetes or in a patient with a known history of chronic obstruction and/or pyelonephritis, consider the diagnosis of papillary necrosis, even if the patient is asymptomatic.
Acute ureteral obstruction from sloughed papillae manifests as flank pain and colic due to hydronephrosis or pyonephrosis; hematuria is invariably present. Pyonephrosis or secondary acute or relapsing pyelonephritis compounds the presentation with fever, chills, prostration, and sepsis.
Papillary necrosis complications
Health problems that may result from renal papillary necrosis include:
- Kidney infection
- Kidney stones
- Kidney cancer, especially in people who take a lot of pain medicines
Necrotic papillae represent a fertile environment for the deposition of both infectious organisms and lithogenic sediment. This necrotic deposition can lead to the development of florid pyelonephritis, perirenal abscesses, and sepsis. Calculous formation compounds the necrosis because certain bacteria thrive within the calculi. Calculi can also propagate, which may lead to further obstruction, increased pyelovenous pressure, and worsened ischemia.
Always consider sloughed papillae as a cause of ureteral obstruction in the differential diagnoses of flank pain, colic, and hematuria, especially when no calculi are visible and particularly in patients with diabetes.
The development of transitional cell carcinoma of the renal pelvis or calyces is a serious complication, particularly in patients with papillary necrosis associated with analgesic abuse.
Papillary necrosis diagnosis
Laboratory Studies
The general diagnostic studies include a urinalysis (ie, routine, microscopic), a complete blood cell count, a complete metabolic panel, and prothrombin time and activated partial thromboplastin time determinations. If patients have concomitant fever, obtain urine and blood cultures under sterile conditions. If patients are prostrate and obtunded, measure arterial blood gases and perform standard electrocardiography and chest radiography. If acute obstruction is suspected, perform renal ultrasonography or another radiographic evaluation and request an immediate consultation with a urologist.
Patients who present with hematuria, even if diagnostic interventions indicate papillary necrosis, require a full urologic workup for their hematuria because they may have a concomitant bladder tumor or similar lesion.
- Perform a routine and microscopic urinalysis from properly collected specimens (ie, sterile catheterization, clean-catch midstream).
- Perform a urine culture obtained via sterile catheterization or clean-catch midstream.
- Obtain a urine cytology study on a voided specimen.
- Perform an imaging study, preferably with intravenous contrast, to evaluate the upper urinary tract. Use CT scanning or intravenous urography (IVU), depending on preference. Perform the imaging study prior to cystoscopy because if the study is limited or incomplete, a urologist may need to perform bilateral retrograde pyelography (RPG) in addition to routine cystoscopy. The test of choice to evaluate the upper tracts of patients with contraindications to intravenous contrast is a bilateral retrograde pyelography.
- If necessary, perform a cystoscopy (ie, flexible or rigid) with bilateral retrograde pyelograms.
- Ureteroscopy may be indicated if the retrograde pyelogram reveals a filling defect in either collecting system. Do not dismiss any persistent collecting system filling defect as a sloughed papilla or blood clot until certain it is not a urothelial papillary tumor or radiolucent stone.
The most common urinalysis findings include proteinuria, pyuria, bacteriuria, and low urine-specific gravity. More than 50% of patients develop leukocytosis and azotemia.
An acutely elevated serum creatinine may be the result of either a bilateral or unilateral process. This process can be obstructive or may be the manifestation of some toxic, metabolic, or inflammatory insult.
Patients with known or possible obstruction require an urgent consultation with a urologist.
If the clinical picture is suggestive, investigate for any of the conditions associated with renal papillary necrosis, including pyelonephritis, obstructed urinary tract, hemoglobinopathies, tuberculosis, liver cirrhosis, analgesic abuse, renal transplant rejection, and diabetes mellitus.
Clinical findings may also prompt performing hemoglobin electrophoresis, a subdermal tuberculin test, liver function tests, serum ammonium measurements, serum and urine salicylate and acetaminophen levels, a hemoglobin A1c measurement, and cyclosporin or tacrolimus levels.
Renal papillary necrosis radiology
Plain radiography
- Standard radiography of the abdomen that visualizes the span of the kidneys, ureters, and bladder is very good for visualizing radiopaque calculi and may offer hints as to whether the patient has 2 kidneys. However, this imaging modality neither yields information on the integrity of the urinary tract nor helps to diagnose hydronephrosis or to elucidate kidney function.
- Thus, plain radiography is not paramount because it is generally not diagnostic for renal papillary necrosis. Much better radiographic tools are available for this purpose.
CT scanning
If the clinical scenario suggests acute obstruction, CT scanning is the imaging modality of choice, mostly because it is extremely accurate for diagnosing calculi—one of the prime differential diagnoses of a sloughed papilla.
A CT scan also shows the entire anatomy of the collecting system and easily reveals hydronephrosis, inflammatory changes, and purulent collections, all without the administration of intravenous contrast. With the administration of contrast and delayed films, if necessary, clinicians can easily visualize filling defects. Contrast images also provide a good, albeit unquantified, estimate of cortical function.
CT scans can also be used to accurately diagnose renal papillary necrosis. Historically, subsequent verification via IVU was required. However, Lang et al have shown that they can identify papillary and medullary necrosis at an early and reversible stage using multiphasic helical CT scanning. [14, 15] When adequately treated with antibiotics, reperfusion improved in approximately 60% of patients within 3 months.
When intravenous contrast is contraindicated, CT scanning without contrast may be ideal for diagnosing acute obstruction, estimating renal function, and, most importantly, excluding nephrolithiasis or ureterolithiasis. Ultrasonography has similar capabilities but, without high-grade obstruction, is not as sensitive for diagnosing calculi. Although less expensive and less invasive (ie, no radiation exposure), ultrasonography is operator-dependent and less sensitive for diagnosing calculi. A bilateral retrograde pyelography is preferred in patients with contraindications to intravenous contrast and in those in whom the urinary tract must indispensably be opacified.
CT findings include (1) small kidneys, (2) ring shadows in the medullae, (3) contrast-filled clefts in the renal parenchyma, and (4) renal pelvic filling defects.
Lang et al 3 describe the ischemic changes of early medullary and papillary necrosis as “a circumscribed, yet often poorly marginated area of diminished enhancement in the tip of the medullary period.” They claim these changes can be seen on scans taken in the early corticomedullary phase but are best seen on scans taken in the nephrographic phase.
Intravenous urography with nephrotomography
This modality provides an excellent display of the anatomy; even very minor morphological changes in the urinary tract are precisely documented.
Intravenous urography (IVU) is typically the imaging method of choice for diagnosing renal papillary necrosis, although it has its limitations. Clear IVU imaging largely depends on a paucity of stool or bowel gas, which is usually not the case, meaning that images can be obscured. Additionally, approximately 15% of calculi are not radiopaque; thus, Intravenous urography (IVU) is not the best initial test in patients who present with colic, in whom stones are more common and who require a different workup and treatment plan. In addition, in severe cases, renal function may be so poor that diagnostic changes cannot be demonstrated. Lastly, IVU is contraindicated in patients with azotemia and in patients with coexisting diseases, particularly allergy, asthma, dehydration, diabetes mellitus, thyrotoxicosis, and plasmocytoma. With the advances in CT imaging and the limitations of intravenous urography (IVU), many clinicians and radiologists consider CT scan the imaging modality of choice for renal papillary necrosis.
If, for any reason, IVU is not the best choice, contrast-enhanced CT scanning, with its far superior contrast resolution, may demonstrate necrotic detached papillae within medullary cavities, thus establishing the diagnosis.
IVU findings include (1) shrinkage and irregularity of papillae, with consequent widening of calyceal fornices, creating what are described as hooks and spurs; (2) desquamated papilla in situ, demarcated by contrast material as a ring shadow, often in a triangular shape (commonly referred to as the ring sign); (3) a calix without a papilla; (4) a partially calcified filling defect in the renal pelvis (ie, sequestered papilla); and (5) contrast-containing rice-grain–sized cavities in the papilla, which are pathognomonic for the medullary form of renal papillary necrosis.
Renal ultrasonography
This imaging modality is safe, quick, inexpensive, noninvasive, and diagnostic for hydronephrosis, certain anomalies, and stones large enough to provide a shadow. It is also operator-dependent, which should be taken into consideration.
Ulreich 4 could not duplicate his IVU-confirmed diagnosis of renal papillary necrosis when reviewing the sonograms of the same patients.
Vijayaraghavan et al 5 describe sonographic features of necrotic sloughed papillae representing filling defects in the ureter. In one third of their patients, necrosed papillae were visualized in cavities in the medullary region communicating with the calyces.
Ultrasonography findings may suggest the diagnosis late in the course of the disease but is not sensitive enough to be confirmatory in the earlier, more reversible phases of renal papillary necrosis.
Retrograde pyelography
This test is more invasive because it requires endoscopic access. Images may reveal a clubbed calyx or a filling defect in the ureter.
Precautions such as intravenous antibiotic prophylaxis must be taken because this procedure involves retrograde introduction of contrast, which can increase intrapelvic pressure and may lead to pyelovenous backflow of infectious material, thus predisposing the patient to sepsis. Gentle slow introduction of contrast decreases the likelihood of this complication, but intravenous antibiotics are warranted nonetheless.
Papillary necrosis treatment
There is no specific treatment for renal papillary necrosis. Treatment depends on the cause. For example, if analgesic nephropathy is the cause, your doctor will recommend that you stop using the medicine that is causing it. This may allow the kidney to heal over time.
Treatment for renal papillary necrosis consists of the following:
- Ameliorate the ischemia with hydration and alkalinization
- Treat the underlying cause of the renal papillary necrosis (eg, maintain normal glycemic state)
- Institute targeted antibiotic therapy
Because ischemia is such a prominent underlying factor in the development of renal papillary necrosis, promptly resuscitate patients and treat their hypoxia, if present. In addition, patients with acute disease may require broad-spectrum intravenous antibiotics, hydration, glycemic control, and urinary alkalinization. Cessation of analgesic abuse stabilizes and may improve renal function.
In patients without acute ureteral obstruction, treat the infectious and metabolic complications of renal papillary necrosis by replacing insensible losses, maintaining hydration, alkalinizing the urine, and administering antibiotics directed toward the pathogen (as revealed by culture or Gram stain and by observing for the development of obstruction or sepsis).
Patients with hematuria significant enough to cause an acute drop in their hematocrit level may require blood transfusions. Patients with sickle cell disease may require exchange transfusions, and patients with diabetes who have acute infectious complications and refractory hyperglycemia may require insulin therapy.
Acute obstruction with concomitant urinary tract infection is a urologic emergency that requires immediate percutaneous nephrostomy to relieve the obstruction, ureteral stent placement, or endoscopic retrieval of the obstructing sloughed papillae. Endoscopic retrieval is not recommended unless the offending papillae are crowning or extruding from the ureteral orifice; even then, the procedure is challenging. Retrograde pyelography and ureteroscopy are useful diagnostic tools, but consider these only when the patient is afebrile and after intravenous administration of antibiotics. Otherwise, placement of a ureteral stent would suffice, with retrograde instrumentation postponed until the patient is afebrile.
The recommended treatment is to drain the dilated collecting system either endoscopically or percutaneously. In patients with severe disease who are febrile and have smoldering sepsis, percutaneous nephrostomy is preferred because it does not require general anesthesia and carries a smaller risk of pyelovenous reflux and worsening sepsis. Cystoscopy and ureteral stent placement allow cystoscopic surveillance of the bladder, which is necessary if hematuria is the presenting symptom. However, in a patient with hydronephrosis, high fever, leukocytosis, and overt sepsis, the preferred treatment is to percutaneously drain the kidney. Perform diagnostic cystoscopy and retrograd pyelography (if necessary) later, when the patient’s situation is not so dire.
Nephrectomy may be life-saving in patients with overwhelming infection (ie, emphysematous pyelonephritis). Consider that papillary necrosis is primarily a bilateral disease, and these patients must be informed that this may result in progressive renal failure and possible dialysis dependency in the future.
In selected patients, ureteroscopic investigation of a ureteral filling defect may be warranted. A basket catheter can be introduced through the ureteroscope to extract the offending sloughed papilla. This is performed only in afebrile patients, after broad-spectrum intravenous antibiotics have been administered.
Patients who present with hematuria, even if all the diagnostic interventions indicate papillary necrosis, require a full urologic workup for their hematuria. A thorough evaluation of the urinary tract, as outlined in Lab Studies, limits the differential diagnoses of hematuria, excluding other possible causes. Attribute the hematuria to papillary necrosis only after performing the studies listed in Lab Studies and deeming the results negative.
Keep in mind that, if the patient’s system is acutely obstructed with possible pyonephrosis, retrograde studies such as retrograde pyelography and ureteroscopy are contraindicated because they are likely to cause or exacerbate sepsis from pyelovenous reflux of purulent material from the lower urinary tract. If this clinical scenario occurs, decompress the system with either a double-J ureteral stent or, preferably, a nephrostomy tube. Send any urine or pus obtained from these procedures for microscopic analysis, Gram stain, and culture. After proper decompression, administer systemic antibiotics with empiric coverage until the Gram stain and culture results are received. Once the patient responds systemically, with stable hemodynamics, no fever, no acidosis, and no leukocytosis, the urologist can proceed with the diagnostic workup.
If the infection rages and the patient does not improve despite supportive measures and proper antibiotic coverage, a nephrectomy may be life-saving. However, remember that the disease is usually bilateral.
Surgery may be indicated for associated anatomic anomalies that predispose patients to urinary stasis and recurrent urinary tract infections. Treatable conditions include the following:
- Calculi
- Ureteropelvic junction obstruction
- Vesicoureteral reflux
- Ureteral strictures
- Ureteroceles
If transitional cell carcinoma of the collecting system is identified, thoroughly evaluate the patient for metastatic disease. If metastases are not found, the proper treatment for presumed invasive transitional cell carcinoma of the upper urinary tract is radical nephroureterectomy, with removal of the entire transmural ureter and a cuff of bladder mucosa. Currenty, some physicians are resecting and staging tumors endoscopically and are treating selected patients more conservatively (ie, surveillance, if the tumor does not invade the muscle layer). Nevertheless, nephroureterectomy remains the criterion standard.
Papillary necrosis prognosis
The prognosis of renal papillary necrosis depends on the cause of the ischemic insult, the number of associated pathologic factors, the dispersal of the necrosis, the involvement of one or both kidneys, and the overall health of the patient. Elderly debilitated patients with multiple medical problems have a poor prognosis, as do patients with overwhelming sepsis and multiple comorbidities. The prognosis is generally worse in patients with diabetes, specifically those who are not compliant and who are prone to severe episodes of hyperglycemia because of the systemic nature of their disease.
Considering the synergistic nature of its predisposing factors, papillary necrosis may be avoided by controlling chronic diseases such as sickle cell disease, diabetes, and cirrhosis. Patients with such conditions should be careful to avoid excessive use of analgesics that are known to be associated with papillary necrosis. Patients who use such analgesics should be screened for signs and symptoms of urinary tract infections and/or urinary obstruction and treated accordingly.
When papillary necrosis arises unexpectedly (ie, in a patient with sepsis), the treatment focus should be as follows:
- Prevent urinary tract infections (eg, by avoiding unnecessary use of indwelling catheters)
- Maintain adequate hydration and homeostasis
- Avoid analgesics and other nephrotoxic medications
- Maintain tight glycemic control in patients with diabetes.
- Papillary Necrosis. https://emedicine.medscape.com/article/439586-overview[↩]
- Sutariya HC, Pandya VK. Renal Papillary Necrosis: Role of Radiology. J Clin Diagn Res. 2016 Jan. 10 (1):TD10-2[↩]
- Lang EK, Macchia RJ, Thomas R, Davis R, Ruiz-Deya G, Watson RA, et al. Multiphasic helical CT diagnosis of early medullary and papillary necrosis. J Endourol. 2004 Feb. 18(1):49-56.[↩]
- Ulreich S. Ultrasound in the evaluation of renal papillary necrosis [letter]. Radiology. 1983 Sep. 148(3):864.[↩]
- Vijayaraghavan SB, Kandasamy SV, Mylsamy A, Prabhakar M. Sonographic features of necrosed renal papillae causing hydronephrosis. J Ultrasound Med. 2003 Sep. 22(9):951-6; quiz 957-8.[↩]