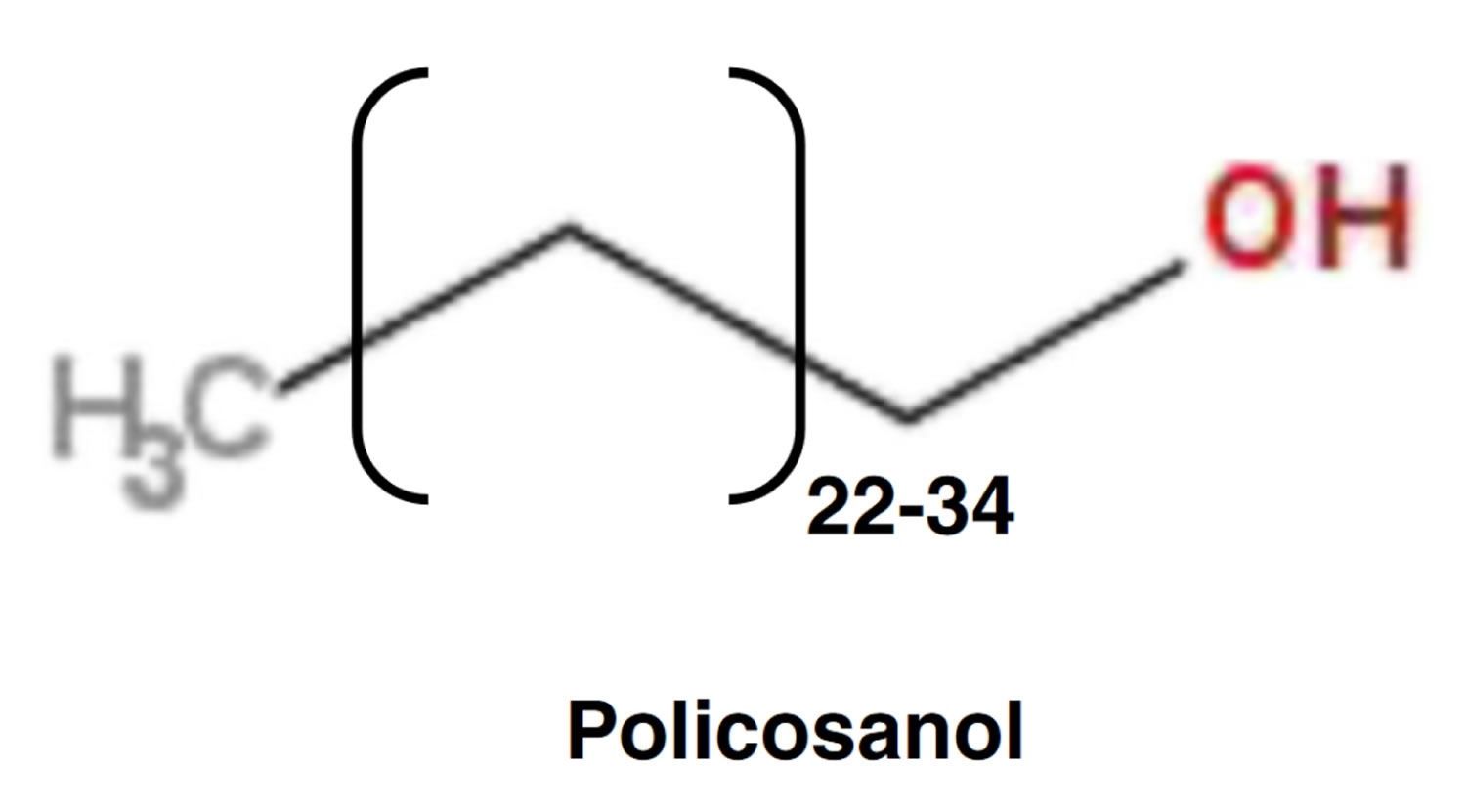
What is policosanol
Policosanol also known as sugar cane wax alcohol, is mixture of eight aliphatic primary alcohols originally purified from sugar cane wax (Saccharum officinarum L.), which has been heavily researched in Cuba in several human populations for its cholesterol-lowering properties 1. Policosanols may also be obtained from a diversity of natural food sources including bees wax, sugar cane wax, rice bran wax, white wax, corn bran wax, wheat germ, epidermis of some plants, rhizome, and grains 2. Policosanol contains a mixture of eight primary aliphatic alcohols (24-34 carbons in length) extracted from sugar cane (Saccharum officinarum) wax with octacosanol, triacontanol, dotriacontanol, hexacosanol and tetratriacotanol as main constituents 3. Octacosanol is the predominant alcohol, comprising approximately 63% of the mixture. Other important constituents include triacontanol (13%) and hexacosanol (6%). Minor components include tetracosanol, heptacocosanol, nonacosanol, dotriacontanol, and tetratriacontanol. Consumption of policosanol can reportedly improve blood lipids, policosanol elevates high-density lipoprotein (HDL or “good” cholesterol) levels and reduces low-density lipoprotein (LDL or “bad” cholesterol) levels and oxidation in hypercholesterolemic humans 4. In addition, policosanol also has significant antiplatelet effects in both humans and animal models. Policosanol also decreases platelet aggregation, decreases smooth muscle proliferation, and improves symptoms of cardiovascular disease. Policosanol decreases levels of thromboxane A2 and may increase levels of prostacycline 5. Large policosanol doses can inhibit platelet aggregation induced by arachidonic acid and collagen but not by adenosine diphosphate 6. Policosanol’s antiplatelet mechanism of action differs from that of aspirin, and potentiates the antithrombotic effects of aspirin 7. In humans, 20 mg of policosanol taken daily has a similar effect to 100 mg of aspirin daily on platelet adhesiveness; however, policosanol does not significantly affect coagulation time 7.
Previous studies incorporated policosanol into the core of HDL enhanced HDL functions via enhancement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer protein (CETP) inhibition 8. Policosanol supplementation for 9 weeks in zebrafish had serum lipid-lowering and HDL-C-elevating effects via CETP inhibition; policosanol also ameliorated fatty liver changes 9. Policosanol supplementation in Korean participants raised serum HDL-C (HDL cholesterol or “good” cholesterol) and enhanced HDL functionality to inhibit oxidation and glycation of LDL (low-density lipoprotein or “bad” cholesterol) and HDL 10. Policosanol therapy for 8 weeks in healthy female subjects who had prehypertension resulted in a reduction in blood pressure and visceral fat amount which were accompanied by lowering of serum total cholesterol and triglyceride levels as well as increased HDL cholesterol (“good” cholesterol) levels via inhibition of serum CETP activity by elevating HDL/apoA-I contents and enhancing HDL functionalities, including cholesterol efflux and insulin secretion 11. Eight weeks of policosanol supplementation in spontaneously hypertensive rats resulted in remarkable decreases of blood pressure in a dose-dependent manner 12. In addition to increasing the HDL cholesterol (“good” cholesterol) level, long-term (24 weeks) consumption of policosanol lowered blood pressure while enhancing the athero-protective functions of HDL as well as its antioxidant, anti-glycation, and anti-atherosclerotic activities 13.
Kim and colleagues 14 recently reported that daily consumption of policosanol by young smoker (n = 7) and middle-aged male participants (n = 11) for 8 weeks resulted in a lowering of systolic blood pressure up to 4%. The serum triglyceride levels exhibited a reduction up to 28 and 26% from the baseline values in the young nonsmoker (n = 7) and middle-aged participants. Nonetheless, the percentage of HDL-C in total cholesterol was elevated in all male participants (young nonsmoker 36%; young smoker 35%; middle-aged male 8%) after 8 weeks of policosanol consumption 14.
Policosanol mechanisms of action
Policosanol appears to cause decreased synthesis and increased degradation of 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA), the rate-limiting step in cholesterol synthesis 15. This is different than the mechanism of action of statin drugs, which work by competitively inhibiting HMG-CoA. Policosanol has also demonstrated improvement in LDL metabolism by increasing LDL binding, uptake, and degradation in human fibroblasts 16. LDL oxidation is thought to be a necessary step in the development of atherosclerosis. Studies on humans and rats show policosanol decreases in vitro LDL oxidation using multiple oxidation models 17. Another step in the formation of atherosclerotic plaques is an increase in smooth muscle proliferation. In rabbits, policosanol decreased neointimal formation, indicating decreased smooth muscle cell proliferation 18. In a comparative study, policosanol demonstrated a greater effect than lovastatin on neointimal formation 19. Policosanol decreases platelet aggregation by decreasing the synthesis of platelet-aggregating thromboxane B2 (TXB2), with no effect on prostacyclin (PGI2) 20. Studies demonstrate policosanol reduces platelet aggregation induced by a number of experimental substances 21, with dose-dependent increases from 10-50 mg/day. Policosanol alone at 20 mg/day was more effective than 100 mg aspirin at reducing platelet aggregation induced by ADP, and equally effective when induced by epinephrine and collagen 22. Despite decreased platelet aggregation, there was no increase in coagulation time when policosanol was taken alone; however, when combined with 100 mg/day aspirin, coagulation time increased.
Policosanol benefits
The beneficial physiological effects of policosanols include reducing platelet aggregation, endothelial damage, and foam cell formation 23, increasing muscle endurance 24, improving performance of coronary heart disease patients during exercise 25 and anti-arthritic and antioxidant properties 26. Furthermore, studies exploring the physiological activities of policosanol have focused on its role in decreasing the levels of low-density lipoprotein (LDL or “bad” cholesterol) and increasing the levels of high-density lipoprotein (HDL or “good” cholesterol) in the blood 27. In addition, policosanol derived from insect wax promotes skin wound healing in mice; exhibits antibacterial, anti-inflammatory, and analgesic properties; and is skin-safe 28. Furthermore, it has been reported to cause proliferation of human follicle dermal papilla cells 29 and promote hair growth in androgen-induced alopecia mice 30. However, the efficacy of policosanols depends on the purity and composition of the preparation 31; hence, policosanols obtained from different sources may have varied effects.
Although there have been many conflicting data and arguments about the cholesterol-lowering efficacy of policosanol 32, a recent meta-analysis 33 of randomized controlled trials from 22 studies including 1886 subjects concluded that policosanol could significantly reduce total cholesterol and low-density lipoprotein (LDL or “bad” cholesterol) and increase high-density lipoprotein (HDL or “good” cholesterol).
A number of reasonably well-designed, short- and long-term clinical trials found that policosanol significantly lowered both LDL and total cholesterol levels in patients with familial hypercholesterolemia (high blood cholesterol), patients with type 2 diabetes mellitus, postmenopausal women, and elderly patients. Some longer-term studies have shown that policosanol significantly raises HDL cholesterol levels. In addition, policosanol has shown promise in treating patients with intermittent claudication.
Placebo-controlled trials for treating hypercholesterolemia. Short-term (eight and six weeks), small randomized clinical trials in patients with familial hypercholesterolemia found that policosanol doses of 5 mg/ day and 5 mg twice daily, respectively, lowered LDL cholesterol by 17.7% and 21.5% and total cholesterol by 13.1% and 16.2%, respectively 34. Long-term studies (52 and 104 weeks) with type 2 hypercholesterolemic patients showed that policosanol 5 mg twice daily lowered LDL cholesterol by 27.5% and 24.8%, lowered total cholesterol by 16.3% and 18.3%, and raised HDL cholesterol by 25.9% and 11.2%, respectively 35. These four studies demonstrated that maximum LDL and total cholesterol reductions occur after six to eight weeks of policosanol use, whereas increases in HDL cholesterol require several months of therapy.
A 12-week randomized clinical trial involving 29 patients with hypercholesterolemia and type 2 diabetes mellitus who were taking 5 mg of policosanol twice daily yielded similar results: LDL and total cholesterol were lowered 21.7% and 16.9%, respectively 36.
A larger randomized clinical trial involving 244 postmenopausal women with familial hypercholesterolemia found that, in successive groups receiving 12-week policosanol regimens of 5 and 10 mg/day, policosanol lowered LDL cholesterol by 17.7% and 25.2% and total cholesterol by 12.6% and 16.7%, respectively 37. In addition, their HDL cholesterol levels increased by 16.5% and 29.3%, respectively 37.
A randomized clinical trial of 437 patients with familial hypercholesterolemia and more than two additional risk factors for coronary artery disease (coronary heart disease) received 5 mg/day of policosanol or placebo for 12 weeks, then 5 mg twice daily for an additional 12 weeks 38. The 5- and 10-mg policosanol regimens reduced LDL cholesterol by 18.2% and 25.6% and total cholesterol by 13.0% and 17.4%, respectively 38. Patients’ HDL cholesterol levels increased by 15.5% and 28.4%, respectively, respectively 38.
A subsequent, 24-week randomized clinical trial by the same authors, involving 179 elderly patients (ages 60-78 years) with familial hypercholesterolemia and at high risk for coronary heart disease, found that successive 12-week daily policosanol doses of 5 mg, then 10 mg, significantly reduced patients’ LDL cholesterol by 16.9% and 24.4% and total cholesterol by 12.8% and 16.2%, respectively 39. HDL cholesterol levels increased by 14.6% and 29.1% 39.
Comparative trials for treating hypercholesterolemia. Fifty-three patients with hypercholesterolemia and type 2 diabetes mellitus were randomized to receive either 10 mg of policosanol or 20 mg of lovastatin daily for 12 weeks 40. Similar results were obtained with each group; policosanol lowered LDL and total cholesterol by 20.4% and 14.2%, respectively, from base line, while lovastatin lowered LDL and total cholesterol by 16.8% and 14.0%, respectively, from base line 40. Policosanol increased HDL cholesterol levels by 7.5% and lovastatin lowered HDL cholesterol by 2.8% (not significant).
Sixty-eight elderly (60-80 years old) patients with familial hypercholesterolemia and multiple risk factors for coronary artery disease were randomized to take policosanol or pravastatin 10 mg daily for eight weeks 41. Again, similar results were obtained with each group; policosanol lowered LDL and total cholesterol by 19.3% and 13.9% from base line, respectively, while pravastatin lowered LDL and total cholesterol by 15.6% and 11.8% from base line, respectively 41.
Another eight-week trial of 53 elderly patients with hypercholesterolemia compared the effectiveness of 5 mg of policosanol twice daily with 5 mg of simvastatin twice daily 42. In the policosanol group, LDL and total cholesterol were lowered by 17.9% and 14.7%, respectively, whereas LDL and total cholesterol were lowered by 19.8% and 15.2%, respectively, in the simvastatin group 42.
Placebo-controlled trials for treating intermittent claudication. Sixty-two patients with intermittent claudication were randomized to receive policosanol 10 mg twice daily or placebo for six months 43. Compared with placebo, policosanol significantly increased both the mean initial claudication distance (measured using a treadmill) from 132.5 ± 13.5 to 205.7 ± 36.3 meters and the absolute claudication distance increased from 229.5 ± 22.0 to 365.4 ± 46.9 meters, while no changes were noted from base line in the placebo group 43.
A two-year study of 56 patients by the same research group found similar results 44. Again, the treatment group received 10 mg of policosanol twice daily or placebo. Treadmill walking distances were measured at 6, 12, 18, and 24 months. After 6 months of therapy, policosanol significantly increased both the mean initial claudication distance from 125.9 ± 8.7 to 201.1 ± 24.8 meters and the absolute claudication distance from 219.5 ± 14.1 to 380.7 ± 50.2 meters, compared with placebo 44. These beneficial effects seemed to improve as the study progressed, with the mean 24-month initial claudication distance increasing from a base line of 125.9 ± 8.7 to 333.5 ± 28.6 meters and the absolute claudication distance increasing from 219.5 ± 14.1 to 648.9 ± 54.1 meters, compared to placebo.
Policosanol and high cholesterol
The majority of policosanol research is on patients with type 2 hypercholesterolemia. Fifteen randomized, placebo-controlled, double-blind studies have shown positive results 45, 46, 47. Significant decreases in total cholesterol (TC) (8-23%), LDL (11.3-27.5%), LDL/HDL (15.3-38.3%), and total cholesterol/HDL (9.1-30.5%) were observed in all trials. Of the 13 trials measuring HDL, seven showed significant increases and in six HDL was unchanged. Doses ranged from 2-40 mg/day, with decreases in total cholesterol, LDL, LDL/HDL and total cholesterol/HDL and increases in HDL being dose-dependent up to 20 mg/day, with no further benefit at 40 mg/day. However, 40 mg/day significantly decreased triglycerides (TG), which was not seen withlower doses 46.
Policosanol was effective in three studies on patients with type 2 diabetes mellitus and hypercholesterolemia 48, 49, 50. All three trials used policosanol 5 mg twice daily for 12 weeks. Total cholesterol was reduced by 14-29 percent, LDL was reduced by 20-44 percent, LDL/HDL ratio was reduced by 24-52 percent, and HDLwas increased by 8-24 percent. No adverse effect on glycemic control was noted in any of the studies. In trials comparing policosanol with lovastatin (20 mg/day), policosanol performed significantly better at raising HDL and lowering the LDL/HDL ratio 51.
Two studies with a total of 300 patients indicate policosanol is effective in post-menopausal women with hyperlipidemia 52. Both studies started with policosanol 5 mg daily, which was later increased [at week 8 in one study 53 and week 12 in the other 51] to 10 mg daily for a period of eight or 12 more weeks. At the end of the policosanol 5-mg portion, total cholesterol, LDL, LDL/HDL, and total cholesterol/HDL decreased by 13-20 percent, 17-18 percent, 17.0-17.2 percent, and 16.3-16.7 percent, respectively, whereas HDL was unchanged in one trial and increased by 16.5 percent in the other. At the end of the policosanol 10-mg/day period policosanol supplementation resulted in decreased total cholesterol, LDL, LDL/HDL, and total cholesterol/HDL by 17-20 percent, 25-28 percent, 27-30 per-cent, and 21-27 percent, respectively, and increased HDL 7-29 percent. Significantly more side effects were seen in the placebo group in each trial.
In comparative trials policosanol generated lipid profiles similar to simvastatin 54, pravastatin 55, lovastatin 56, probucol 57, acipimox 58 and atorvastatin 59. First, two trials on patients with type II hypercholesterolemia, comparing low dose simvastatin (5 or 10 mg/day) and moderate dose policosanol (5 or 10 mg/day), demonstrated that both substances greatly improved lipid profiles with no significant differences in results or side effects between the groups 60. Second, policosanol (10 mg/day) compared favorably to low-dose pravastatin (10 mg/day) in patients with type II hypercholesterolemia in two studies 61. In one trial, policosanol-treated patients had significantly greater decreases in LDL, LDL/HDL, total cholesterol/HDL, and increases in HDL 61, while in another trial policosanol-treated patients had significantly greater increases in HDL 62. The pravastatin group had more side effects in both studies. A study comparing policosanol to lovastatin in patients with type 2 diabetes and hypercholesterolemia (type II) found policosanol (10 mg/day) is more effective at lowering LDL/HDL and increasing HDL than 10 mg/day lovastatin, with significantly fewer side effects 63. In addition, in patients with type II hypercholesterolemia and concomitant coronary risk factors, policosanol (10 mg/day) decreased LDL/HDL and increased HDL more effectively than 20 mg/day lovastatin, with fewer side effects 64. Policosanol (5 mg twice daily) also compared favorably to probucol (500 mg twice daily) at reducing total cholesterol, LDL, and triglyceride in patients with type II hypercholesterolemia 65. Again, policosanol (10 mg/day) compared favorably to acipimox (750 mg/day), a niacin derivative, in regard to total cholesterol, LDL, LDL/HDL ,total cholesterol/HDL, and HDL, with fewer side effects 66. Lastly, policosanol was significantly less effective than atorvastatin (Lipitor) in reducing both LDL and total cholesterol, although it was similar in reducing both atherogenic ratios and triglyceride. Atorvastatin, however, significantly increased creatine phosphokinase (CPK) and creatinine, whereas policosanol significantly reduced alanine aminotransferase (AST), glucose and creatine phosphokinase levels 59. These studies suggest a therapeutic benefit to policosanol in type II hypercholesterolemia, while presenting no adverse effects on the liver.
In a trial to determine whether policosanol could safely be used for patients with altered liver function tests, 46 patients with primary hypercholesterolemia and elevated liver enzymes were treated with policosanol (5 or 10 mg/day) or placebo for 12 weeks. Both 5 and 10 mg policosanol significantly lowered lipids and reduced serum levels of ALT, suggesting improvement in liver function 67.
Policosanol and intermittent claudication
Two studies demonstrated positive results using policosanol for patients with intermittent claudication. In 62 patients treated with 10 mg policosanol twice daily for six months,the distance individuals could walk on a treadmill before noticing claudication symptoms increased 63.1 percent and absolute distance to being unable to walk any further increased 65.1 percent, while placebo had no effect on walking distances. Policosanol also improved lower extremity symptoms of coldness and pain compared to placebo 68. In a two-year follow-up study with 56 patients, improvements were progressive throughout the study, with the distance walked before initial claudication symptoms improving 60.1 percent after six months and 187.8 percent after 24 months. Absolute walking distance increased 81 percent after six months and 249 percent after 24 months. Policosanol also significantly decreased symptoms of claudication and increased the ankle/arm pressure ratio at 12 and 24 months. Even more impressive, significantly more patients in the placebo group experienced serious vascular events (8 patients with 10 total serious adverse events), while none were experienced in the policosanol group 69.
Recently, intermittent claudication was investigated in comparative double-blind studies with lovastatin or ticlopidine 70. Policosanol significantly increased the initial and absolute claudication distances in both studies, surpassing ticlopidine (a platelet-aggregation inhibitor) in one study, while significantly out-competing lovastatin (which had minimal effect) in the other study.
Policosanol and ischemic heart disease
Forty-five patients with documented ischemic heart disease were placed on 5 mg policosanol twice daily, 5 mg policosanol twice daily plus 125 mg aspirin or 125 mg aspirin for 20 months 71. The policosanol groups showed an insignificantly lower percentage of patients with functional progression of ischemia and a significantly greater partial regression of ischemia. Furthermore, exercise capacity and left ventricular function improved significantly in the policosanol groups compared to the aspirin-only group. Both policosanol groups were more effective than aspirin alone, but policosanol plus aspirin therapy was more effective than policosanol alone.There were four vascular events in aspirin alone (1 fatal myocardial infarction, 2 unstable angina, 1 cardiac failure), one in the group taking policosanol alone (non-fatal myocardial infarction), and none in the combined group. A follow-up study on the same patients examined treadmill exercise ECG-testing performance 72. Those taking policosanol demonstrated decreases in cardiovascular functional class, rest- and exercise-induced angina, cardiac events, and ischemic ST-segment response. These benefits were greatest in the policosanol plus aspirin group. In addition, policosanol showed an increase in maximum oxygen uptake, a decline in double product (peak heart rate times peak systolic blood pressure), and an increase in aerobic functional capacity compared to placebo.
Atherosclerotic lesions resulting in carotid-vertebral atherosclerosis improved in a studyof 22 patients given 10 mg/day policosanol for one year 73. Carotid-vertebral atherosclerosis assessed using Doppler-ultrasound showed progression of disease in three of 11 patients on placebo and no patients on policosanol. Disease regression occurred in six of 11 patients on policosanol and one on placebo. Neither of these values reached statistical significance; however, when a progression/regression ratio was calculated it did reach statistical significance for improvement with policosanol.
Policosanol (2 mg/day) improved abnormal rest and stress ECG patterns, and decreased symptoms of angina in a single-blind, 14-month, placebo-controlled trial in 23 middle-aged patients with primary or marginal hypercholesterolemia. No patient had a new coronary event, but significantly more patients (5/12) in the policosanol group with stable angina or silent ischemia had improved coronary symptoms and/or rest and stress ECG patterns, compared to placebo (0/11). Policosanol-treated patients also had no deterioration in symptoms or ECG patterns, while three of 11 placebo-treated patients deteriorated 74.
Policosanol dosage
The suggested starting dosage of policosanol for treatment of high blood cholesterol (hypercholesterolemia) is 2-10 mg daily, taken with the evening meal. Liver synthesis of cholesterol is thought to occur primarily at night; therefore, once-daily doses of policosanol should be taken in the evening. However, maximum reductions should be seen at policosanol 5-20 mg/day. Policosanol dosages exceeding 10 mg/day are usually given in divided doses with meals. Daily doses as high as 40 mg have been studied; however, maximum clinical benefits appear to be obtained with 20 mg/day (greater than 20 mg/day seems to offer no further benefit) 75; however, higher policosanol doses (40mg/day) may be indicated for lowering triglycerides. A prudent recommendation would be to start with policosanol 5 mg daily and increase to 10 mg twice daily or more if needed.
The recommended policosanol dosage for intermittent claudication is 10 mg twice daily with meals.
No dosage reduction is necessary in patients with compromised liver function. No studies have been conducted with patients with compromised renal function 76.
Policosanol side effects
Policosanol is a relatively nontoxic and useful agent for reducing LDL and total cholesterol in patients with type 2 or diabetes-related hypercholesterolemia. Long-term use may increase levels of HDL cholesterol (“good” cholesterol). While the lack of numerous independent clinical trials precludes the use of policosanol as a first-line agent for treating hypercholesterolemia or intermittent claudication, available safety and efficacy data indicate that it may be useful as a second-line agent in patients who cannot use 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors and other lipid-lowering medications.
Policosanol appears to be well tolerated and safe when given long-term (clinical trials up to three years) 77. In post-marketing studies looking at 27,879 patients, the most significant adverse effects were weight loss (1.8% of patients), polyuria (0.7%), headache (0.6%), insomnia (0.5%), or polyphagia (0.5%) 77. Only 22 patients had to discontinue treatment because of side effects. Other reported side effects include nervousness, somnolence, dizziness, erythema, excitability, hypotension, hypertension, pruritis, skin rash, nausea, epigastric pain, diarrhea, constipation, and bleeding from the nose and gums 76. In clinical trials there were either no significant differences in adverse events or significantly more adverse events in placebo groups compared to policosanol. Increases in liver enzymes and creatine kinase have not been reported with policosanol.
Several animal studies using policosanol doses up to 500 mg/kg (rat model) did not reveal any significant drug, reproductive, or mutagenic toxicity 78. Toxicity studies in rats, dogs, mice, and monkeys have shown policosanol to be non-toxic and not carcinogenic at doses 1,500-times the normal human dosage 79. Reproductive studies on rats and mice show policosanol at 1,500-times the normal human dose has no adverse effect on fertility, reproduction, teratogenesis, or development 80.
Because of policosanol’s effects on platelet adhesiveness, policosanol can have additive effects with all anticoagulant and antiplatelet medications. Cumulative data from long-term clinical trials have not indicated drug interactions or additive toxicity with calcium-channel blockers, angiotensin-converting-enzyme inhibitors, -blockers, diuretics, nitrates, nonsteroidal antiinflammatory agents, anxiolytics, antidepressants, neuroleptics, oral hypoglycemic agents, digoxin, thyroid hormones, and antiulcer medications 76. However, formal drug interaction studies in humans have not been performed.
Policosanol should be avoided in pregnant and lactating women until further research can be performed to assure its safety in this population.
Since the mechanism of action has not been clearly defined, policosanol should not be given concurrently with HMG-CoA reductase inhibitors until further research can prove the safety of using both medications concurrently.
Policosanol drug interactions
Policosanol inhibits platelet aggregation and may enhance the effect of other anticoagulant medications. When combined with aspirin, policosanol increased coagulation time in humans 81.
- Marinangeli CP, Jones PJ, Kassis AN, Eskin MN. Policosanols as nutraceuticals: fact or fiction. Crit Rev Food Sci Nutr. 2010 Mar;50(3):259-67. doi: 10.1080/10408391003626249[↩]
- Lin Y, Rudrum M, van der Wielen RP, Trautwein EA, McNeill G, Sierksma A, et al. Wheat germ policosanol failed to lower plasma cholesterol in subjects with normal to mildly elevated cholesterol concentrations. Metabolism. 2004;53(10):1309–1314. doi: 10.1016/j.metabol.2004.05.006[↩]
- Park, H. J., Yadav, D., Jeong, D. J., Kim, S. J., Bae, M. A., Kim, J. R., & Cho, K. H. (2019). Short-Term Consumption of Cuban Policosanol Lowers Aortic and Peripheral Blood Pressure and Ameliorates Serum Lipid Parameters in Healthy Korean Participants: Randomized, Double-Blinded, and Placebo-Controlled Study. International journal of environmental research and public health, 16(5), 809. https://doi.org/10.3390/ijerph16050809[↩]
- Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J. 2002 Feb;143(2):356-65. doi: 10.1067/mhj.2002.119997[↩]
- Arruzazabala ML, Valdez S, Mas R et al. Effect of policosanol successive dose increases on platelet aggregation in healthy volunteers. Pharmacol Res. 1996; 34:181-5.[↩]
- Carbajal D, Arruzazabala ML, Valdes S et al. Effect of policosanol on platelet aggregation and serum levels of arachidonic acid metabolites in healthy volunteers. Prostaglandins Leukot Essent Fatty Acids. 1998; 58:61-4.[↩]
- Arruzazabala ML, Valdez S, Mas R et al. Comparative study of policosanol, aspirin and the combination therapy policosanol-aspirin on platelet aggregation in healthy volunteers. Pharmacol Res. 1997; 36:293-7.[↩][↩]
- Lim SM, Yoo JA, Lee EY, Cho KH. Enhancement of High-Density Lipoprotein Cholesterol Functions by Encapsulation of Policosanol Exerts Anti-Senescence and Tissue Regeneration Effects Via Improvement of Anti-Glycation, Anti-Apoptosis, and Cholesteryl Ester Transfer Inhibition. Rejuvenation Res. 2016 Feb;19(1):59-70. doi: 10.1089/rej.2015.1712[↩]
- Lee EY, Yoo JA, Lim SM, Cho KH. Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality. Rejuvenation Res. 2016 Apr;19(2):149-58. doi: 10.1089/rej.2015.1745[↩]
- Kim JY, Kim SM, Kim SJ, Lee EY, Kim JR, Cho KH. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int J Mol Med. 2017 Apr;39(4):889-899. doi: 10.3892/ijmm.2017.2907[↩]
- Cho KH, Kim SJ, Yadav D, Kim JY, Kim JR. Consumption of Cuban Policosanol Improves Blood Pressure and Lipid Profile via Enhancement of HDL Functionality in Healthy Women Subjects: Randomized, Double-Blinded, and Placebo-Controlled Study. Oxid Med Cell Longev. 2018 Apr 16;2018:4809525. doi: 10.1155/2018/4809525[↩]
- Cho, K. H., Yadav, D., Kim, S. J., & Kim, J. R. (2018). Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules (Basel, Switzerland), 23(5), 1080. https://doi.org/10.3390/molecules23051080[↩]
- Kim SJ, Yadav D, Park HJ, Kim JR, Cho KH. Long-Term Consumption of Cuban Policosanol Lowers Central and Brachial Blood Pressure and Improves Lipid Profile With Enhancement of Lipoprotein Properties in Healthy Korean Participants. Front Physiol. 2018 Apr 24;9:412. doi: 10.3389/fphys.2018.00412[↩]
- Kim, J. Y., Kim, S. M., Kim, S. J., Lee, E. Y., Kim, J. R., & Cho, K. H. (2017). Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. International journal of molecular medicine, 39(4), 889–899. https://doi.org/10.3892/ijmm.2017.2907[↩][↩]
- Menendez R, Amor AM, Rodeiro I, et al.Policosanol modulates HMG-CoA reductaseactivity in cultured fibroblasts. Arch Med Res2001;32:8-12.[↩]
- Menendez R, Fernandez SI, Del Rio A, et al.Policosanol inhibits cholesterol biosynthesisand enhances low density lipoprotein process-ing in cultured human fibroblasts. Biol Res1994;27:199-203.[↩]
- Menendez R, Mas R, Amor MA, et al. Effectsof policosanol treatment on the susceptibilityof low density lipoprotein (LDL) isolated fromhealthy volunteers to oxidative modification invitro. Br J Clin Pharmacol 2000;50:255-262.[↩]
- Noa M, Mas R, Mesa R. Effect of policosanolon intimal thickening in rabbit cuffed carotidartery. Int J Cardiol 1998;67:125-132.[↩]
- Noa M, Mas R, Mesa R. A comparative studyof policosanol vs. lovastatin on intimalthickening in rabbit cuffed carotid artery.Pharmacol Res 2001;43:31-37.[↩]
- Carbajal D, Arruzazabala ML, Valdes S, MasR. Effect of policosanol on platelet aggrega-tion and serum levels of arachidonic acidmetabolites in healthy volunteers. Prostaglan-dins Leukot Essent Fatty Acids1998;58:61-64.[↩]
- Castano G, Mas R, Arruzazabala M, et al.Effects of policosanol and pravastatin on lipidprofile, platelet aggregation and endothelemiain older hypercholesterolemic patients. Int JClin Pharmacol Res1999;29:105-116.[↩]
- Arruzazabala ML, Valdes S, Mas R, et al.Comparative study of policosanol, and thecombination therapy policosanol-aspirin onplatelet aggregation in healthy volunteers.Pharmacol Res 1997;36:293-297.[↩]
- Carbajal D, Arruzazabala ML, Valdes S, Mas R. Effect of policosanol on platelet aggregation and serum levels of arachidonic acid metabolites in healthy volunteers. Prostaglandins Leukot Essent Fatty Acids. 1998;58(1):61–64. doi: 10.1016/s0952-3278(98)90130-2[↩]
- Kabir Y, Kimura S. Tissue distribution of (8-14C)-octacosanol in liver and muscle of rats after serial administration. Ann Nutr Metabol. 1995;39(5):279–284. doi: 10.1159/000177873[↩]
- Stüsser R, Batista J, Padrón R, Sosa F, Pereztol O. Longterm therapy with octacosanol improves treadmill exercise-ECG testing performance of coronary heart disease patients. Int J Clin Pharmacol Ther. 1998;36(9):469–473. doi: 10.1002/zaac.200700297[↩]
- Harrabi S, Ferchichi A, Bacheli A, Fellah H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018;17(1):1–7. doi: 10.1186/s12944-018-0682-z[↩]
- Sánchez-López J, Illnait-Ferrer J, Mas-Ferreiro R, Mendoza-Castaño S, Fernández-Dorta L, Mesa-Angarica M, et al. Long-term effect of policosanol on the functional recovery of non-cardioembolic ischemic stroke patients: a one year study. Rev Neurol. 2017;64(4):153–161.[↩]
- Ma JJ, Ma LY, Zhang H, Zhang ZQ, Wang YQ. The preparation and evaluation of efficacy on skin wound healing in mice of insect wax compound ointment. J Environ Entomol. 2018;40(6):1238–1247.[↩]
- Wang ZD, Feng Y, Li X, Ding WF, Chen XM. Effect of white wax and Policosanol from white wax tween aqueous solution on HFDPCS. For Res. 2017;30(1):41–45. doi: 10.13275/j.cnki.lykxyj.2017.01.006[↩]
- Wang ZD, LI X, Ding WF, Sun L, Feng Y. Mechanism of white wax on treating Seborrheic alopecia. Chin J Ethnomed Ethnopharm. 2019;28(10):17–21.[↩]
- Marinangeli CPF, Jones PJH, Kassis AN, Eskin MNA. Policosanols as Nutraceuticals: fact or fiction. Criti Rev Food Sci Nutr. 2010;50(3):259–267. doi: 10.1080/10408391003626249[↩]
- Francini-Pesenti F., Beltramolli D., Dall’Acqua S., Brocadello F. Effect of sugar cane policosanol on lipid profile in primary hypercholesterolemia. Phytotherapy Research. 2008;22(3):318–322. doi: 10.1002/ptr.2315[↩]
- Gong J., Qin X., Yuan F., et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: a meta-analysis of randomized controlled trials. Molecular Nutrition & Food Research. 2018;62(1) doi: 10.1002/mnfr.201700280[↩]
- Aneiros E, Mas R, Calderon B et al. Effect of policosanol in lowering cholesterol levels in patients with type II hypercholesterolemia. Curr Ther Res. 1995; 56:176-82.[↩]
- Canetti M, Moreiro M, Mas R et al. A two-year study on the efficacy and tolerability of policosanol in patients with type II hyperlipoproteinaemia. Int J Clin Pharmacol Res. 1995; 15:159-65.[↩]
- Torres O, Agreamonte AJ, Illnait J et al. Treatment of hypercholesterolemia in NIDDM with policosanol. Diabetes Care. 1995; 18:393-6.[↩]
- Castano G, Mas R, Fernandez L et al. Effects of policosanol on postmenopausal women with type II hypercholesterolemia. Gynecol Endocrinol. 2000; 14:187-95.[↩][↩]
- Mas R, Castano G, Illnait J et al. Effects of policosanol in patients with type II hypercholesterolemia and additional coronary risk factors. Clin Pharmacol Ther. 1999; 65:439-47.[↩][↩][↩]
- Castano G, Mas R, Fernandez J et al. Effects of policosanol in older patients with type II hypercholesterolemia and high coronary risk. J Gerontol. 2001; 56A: M186-92.[↩][↩]
- Crespo N, Illnait J, Mas R et al. Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res. 1999; 19:117-27.[↩][↩]
- Castano G, Mas R, Arruzazabala M et al. Effects of policosanol and pravastatin on lipid profile, platelet aggregation and endothelemia in older hypercholesterolemic patients. Int J Clin Pharmacol Res. 1999; 19:105-16.[↩][↩]
- Ortensi G, Gladstein J, Valli H et al. A comparative study of policosanol versus simvastatin in elderly patients with hypercholesterolemia. Curr Ther Res. 1997; 58: 390-401.[↩][↩]
- Castano G, Mas R, Roca J et al. A double-blind, placebo-controlled study of the effects of policosanol in patients with intermittent claudication. Angiology. 1999; 50: 123-30.[↩][↩]
- Castano G, Mas R, Fernandez L et al. A long-term study of policosanol in the treatment of intermittent claudication. Angiology. 2001; 52:115-25.[↩][↩]
- Castano G, Mas R, Fernandez JC, et al. Effectsof policosanol in older patients with type IIhypercholesterolemia and high coronary risk. J Gerontol 2001;56A:M186-M192.[↩]
- Castano G, Mas R, Fernandez L, et al. Effectsof policosanol 20 versus 40 mg/day in thetreatment of patients with type II hypercholes-terolemia: a 6-month double-blind study. Int J Clin Pharmacol Res 2001;21:43-57.[↩][↩]
- Castano G, Mas R, Fernandez JC, et al. Effectsof policosanol on older patients with hyperten-sion and type II hypercholesterolaemia. DrugsR D 2002;3:159-172.[↩]
- Crespo N, Alvarez R, Mas R, et al. Effects ofpolicosanol on patients with non-insulin-dependent diabetes mellitus and hypercholesterolemia: a pilot study. Curr Ther Res Clin Exp 1997;58:44-51.[↩]
- Torres O, Agramonte A, Illnait J, et al.Treatment of hypercholesterolemia in NIDDM with policosanol. Diabetes Care 1995;18:393-397.[↩]
- Crespo N, Illnait J, Mas R, et al. Comparative study of the efficacy and tolerability ofpolicosanol and lovastatin in patients withhypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res 1999;29:117-127.[↩]
- Castano G, Mas R, Fernandez L, et al. Effectsof policosanol and lovastatin in patients with intermittent claudication: a double-blind comparative pilot study. Angiology 2003;54:25-38.[↩][↩]
- Mirkin A, Mas R, Martinto M, et al. Efficacy and tolerability of policosanol in hypercholesterolemic postmenopausal women. Int J Clin Pharmacol Res 2001;21:31-41.[↩]
- Castano G, Mas R, Fernandez L, et al. Effects ofpolicosanol on postmenopausal women with type II hypercholesterolemia. Gynecol Endocrinol 2000;14:187-195.[↩]
- Ortensi G, Gladstein J, Valli H, Tesone PA. Acomparative study of policosanol versus simvastatin in elderly patients with hypercholesterolemia. Curr Ther Res Clin Exp 1997;58:390-401.[↩]
- Castano G, Mas R, Arruzazabala M, et al.Effects of policosanol and pravastatin on lipidprofile, platelet aggregation and endothelemiain older hypercholesterolemic patients. Int J Clin Pharmacol Res 1999;29:105-116.[↩]
- Mirkin A, Mas R, Martinto M, et al. Efficacyand tolerability of policosanol in hypercholesterolemic postmenopausal women. Int J Clin Pharmacol Res 2001;21:31-41.[↩]
- Pons P, Illnait J, Mas R, et al. A comparativestudy of policosanol versus probucol in patientswith hypercholesterolemia. Curr Ther Res Clin Exp 1997;58:26-35.[↩]
- Alcocer L, Fernandez L, Compos E, Mas R. Acomparative study of policosanol versusacipimox in patients with type II hypercholesterolemia. Int J Tissue React 1999;21:85-92.[↩]
- Castano G, Mas R, Fernandez L, et al. Compari-son of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs Aging 2003;20:153-163.[↩][↩]
- Illnait J, Castano G, Mas R, Fernandez JC. Acomparative study on the efficacy and tolerability of policosanol and simvastatin for treating type II hypercholesterolemia. Can J Cardiol 1997;13:342B.[↩]
- Benitez M, Romero C, Mas R, et al. A comparative study of policosanol versus pravastatin in patients with type II hypercholesterolemia. Curr Ther Res Clin Exp 1997;58:859-867.[↩][↩]
- Castano G, Mas R, Arruzazabala M, et al.Effects of policosanol and pravastatin on lipid profile, platelet aggregation and endothelemiain older hypercholesterolemic patients. Int J Clin Pharmacol Res 1999;29:105-116.[↩]
- Crespo N, Illnait J, Mas R, et al. Comparativestudy of the efficacy and tolerability ofpolicosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res 1999;29:117-127.[↩]
- Castano G, Mas R, Fernandez JC, et al. Efficacyand tolerability of policosanol compared with lovastatin in patients with type II hypercholesterolemia and concomitant coronary risk factors.Curr Ther Res Clin Exp 2000;61:137-146.[↩]
- Pons P, Illnait J, Mas R, et al. A comparative study of policosanol versus probucol in patients with hypercholesterolemia. Curr Ther Res Clin Exp 1997;58:26-35.[↩]
- Alcocer L, Fernandez L, Compos E, Mas R. A comparative study of policosanol versus acipimox in patients with type II hypercholesterolemia. Int J Tissue React 1999;21:85-92.[↩]
- Zardoya R, Tula L, Castano G, et al. Effects ofpolicosanol on hypercholesterolemic patients with abnormal serum biochemical indicators ofhepatic function. Curr Ther Res Clin Exp 1996;57:568-577.[↩]
- Castano G, Mas R, Roca J, et al. A double-blind,placebo-controlled study of the effects of policosanol in patients with intermittent claudication. Angiology 1999;50:123-130.[↩]
- Castano G, Mas R, Fernandez L, et al. A long-term study of policosanol in the treatment of intermittent claudication. Angiology 2001;52:115-125.[↩]
- Castano G, Mas R, Gamez R, et al. Effects ofpolicosanol and ticlopidine in patients with intermittent claudication: a double-blinded pilot comparative study. Angiology 2004;55:361-371.[↩]
- Batista J, Strusser R, Padron R, et al. Functionalimprovement in coronary artery disease after 20 months of lipid-lowering therapy with policosanol. Adv Ther 1996;13:137-148.[↩]
- Stusser R, Batista J, Padron R, et al. Long-termtherapy with policosanol improves treadmillexercise-ECG testing performance of coronary heart disease patients. Int J Clin Pharmacol Ther 1998;36:469-473.[↩]
- Batista J, Stusser R, Penichet M, Uguet E.Doppler-ultrasound pilot study of the effects of long-term policosanol therapy on carotid-vertebral atherosclerosis. Curr Ther Res Clin Exp 1995;56:906-914.[↩]
- Batista J, Stusser R, Saez F, Perez B. Effect of policosanol on hyperlipidemia and coronary heart disease in middle-aged patients. A 14-month pilot study. Int J Clin Pharmacol Ther 1996;34:134-137.[↩]
- Castano G, Mas R, Fernandez L et al. Effects of policosanol 20 versus 40 mg/day in the treatment of patients with type II hypercholesterolemia: a 6-month double-blind study. Int J Clin Pharmacol Res. 2001; 21:43-57.[↩]
- Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J. 2002; 143:356-65.[↩][↩][↩]
- Fernandez L, Mas R, Illnait J et al. Policosanol: results of a postmarketing surveillance of 27,879 patients. Curr Ther Res. 1998; 59:717-22.[↩][↩]
- Aleman CL, Mas R, Hernandez C et al. A 12-month study of policosanol oral toxicity in Sprague Dawley rats. Toxicol Lett. 1994; 70:77-87.[↩]
- Aleman CL, Noa M, Elias EC, et al. Carcinogenicity of policosanol in mice: an 18-month study.Food Chem Toxicol 1995;33:573-578.[↩]
- Rodriguez MD, Garcia H. Evaluation of peri-and post-natal toxicity of policosanol in rats.Teratog Carcinog Mutagen 1998;18:1-7.[↩]
- Arruzazabala ML, Valdes S, Mas R, et al. Comparative study of policosanol, and the combination therapy policosanol-aspirin on platelet aggregation in healthy volunteers.Pharmacol Res 1997;36:293-297.[↩]