Post inflammatory hypopigmentation
Post inflammatory hypopigmentation also called post inflammatory hypomelanosis, is an acquired form of partial or total loss of skin pigmentation that occurs secondary to an exogenous or endogenous insult to the skin 1. Post-inflammatory hypopigmentation is a common cause of acquired hypopigmentary skin disorders. Post-inflammatory hypopigmentation can be a result of cutaneous inflammation, injury or dermatological treatment. Post-inflammatory hypopigmentation can be secondary to any cutaneous inflammatory conditions (eg., atopic eczema, seborrheic dermatitis, discoid eczema, contact dermatitis, psoriasis, pityriasis lichenoides chronica, lichen striatus, pityriasis rosea), infections (eg., Tinea versicolor, Herpes Zoster, Syphilis), procedures (eg., cryotherapy, dermabrasion) and injury (eg, a thermal burn) 1. The distribution and severity of skin pigment loss is related to the extent and degree of the inflammation. Post inflammatory hypopigmentation can occur in anyone but is more common in darker-skinned individuals, possibly because of the color contrast with their normal skin. There is no gender difference in the incidence of postinflammatory hypopigmentation.
Most cases of postinflammatory hypopigmentation improve spontaneously within weeks or months if the primary cause has been effectively treated; however, it can be permanent if there is complete destruction of melanocytes 1.
The most important key in its management is to identify and treat the primary cause. Current treatment options include topical medication, phototherapy and laser. Further studies are required to determine the underlying mechanisms and efficacy of each treatment. Lastly, there are limited data regarding the pathogenesis, natural course and treatment of postinflammatory hypopigmentation.
Post inflammatory hypopigmentation key points
- Many types of skin inflammatory conditions and injuries are associated with postinflammatory hypopigmentation.
- Some inflammatory conditions have a tendency to develop postinflammatory hypopigmentation rather than hyperpigmentation, including pityriasis lichenoides chronica and lichen striatus.
- Skin biopsy can be of value to exclude dermatoses that present with hypopigmentation only, such as mycosis fungoides, sarcoidosis and leprosy.
- The most important step of management is to identify the cause of postinflammatory hypopigmentation. The hypopigmentation usually improves over time after the inflammation is ceased.
- Treatment options for postinflammatory hypopigmentation include topical tar, steroids, calcineurin inhibitors, phototherapy, excimer laser, fractional ablative CO2 laser, grafting and camouflage.
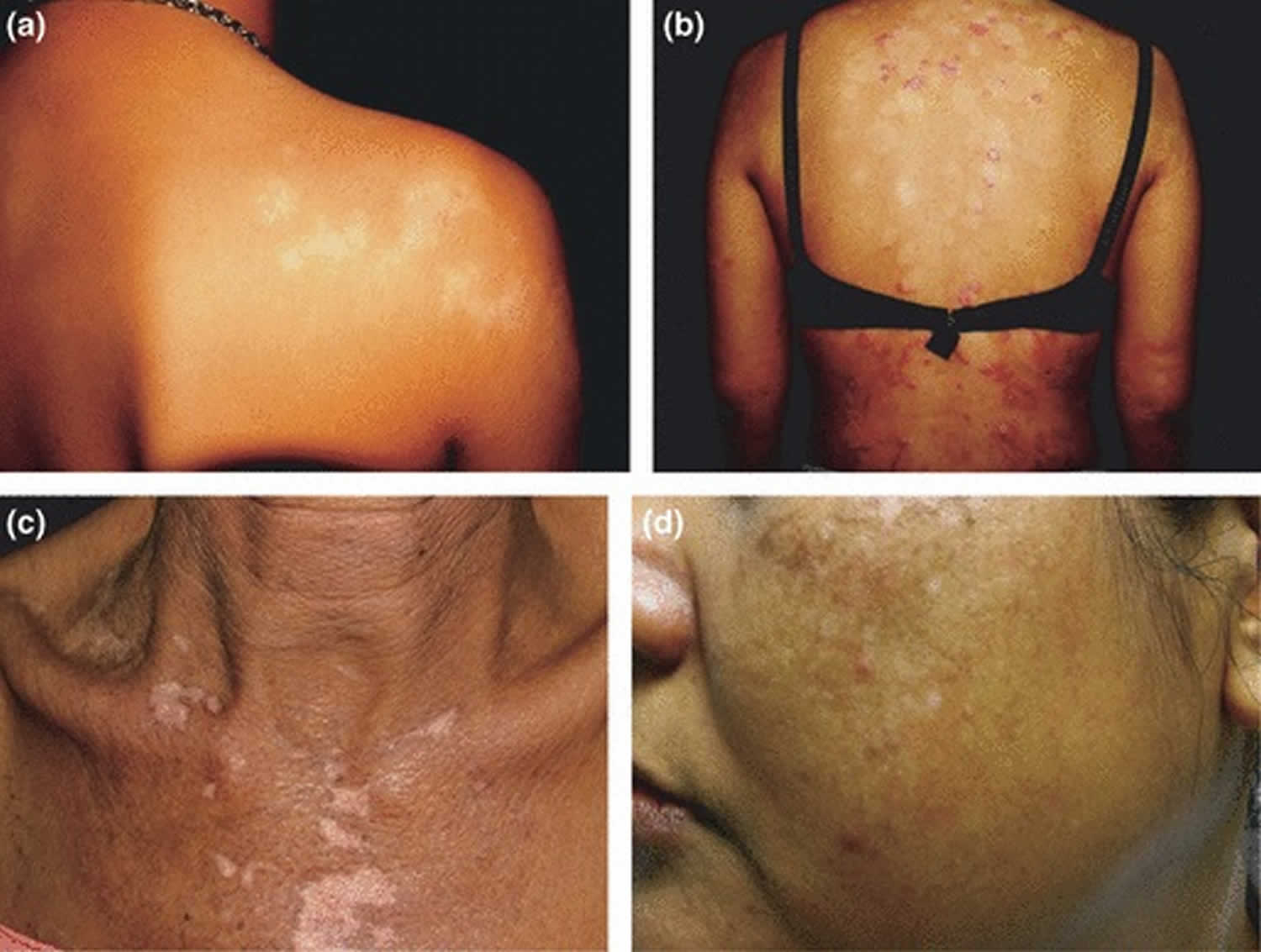
Figure 1. Post inflammatory hypopigmentation
Footnote: Postinflammatory hypopigmentation caused by (a) lichen striatus, showing linear distribution of hypopigmented lesions along Blaschko lines; (b) psoriasis, showing multiple well‐demarcated hypopigmented lesions a similar size and shape to the original psoriasis lesions. (c) Depigmentation secondary to discoid lupus erythematosus. The lesion is obvious in dark‐complexioned skin because of the colour contrast. (d) Hypopigmented and depigmented lesions secondary to low fluence Q‐switched 1064‐nm Nd:YAG laser therapy for melasma. The configuration of the lesions corresponds to the size and shape of the laser spot.
[Source 1 ]Post inflammatory hypopigmentation causes
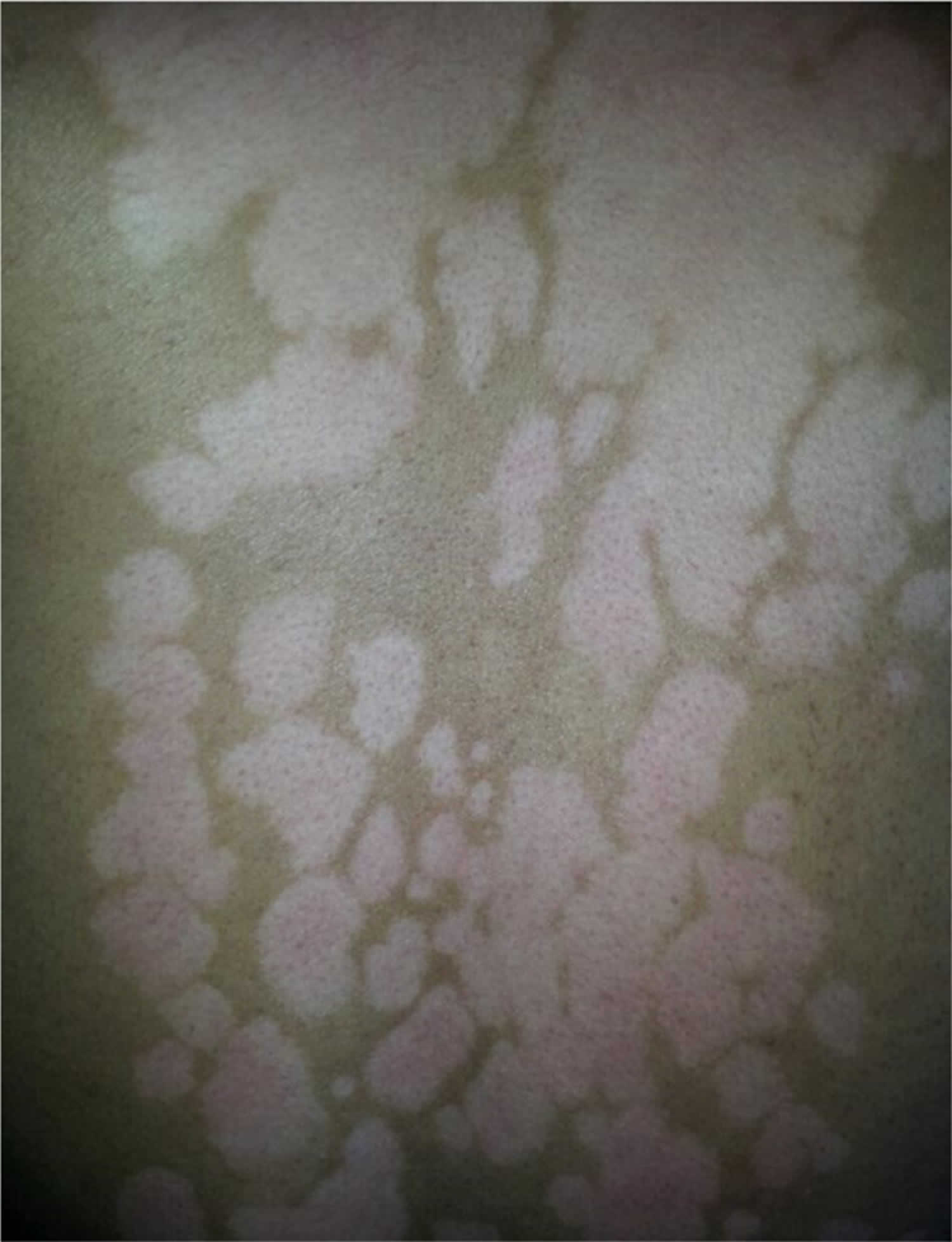
Many skin inflammatory conditions can lead to postinflammatory hypopigmentation. Some, such as pityriasis lichenoides chronica and lichen striatus, tend to induce post-inflammatory hypopigmentation rather than hyperpigmentation. Cutaneous injuries from burns, irritants and dermatological procedures (e.g., chemical peels, dermabrasion, cryotherapy, laser therapy) can also lead to postinflammatory hypopigmentation.
Post-inflammatory hypopigmentation causes:
- Inflammatory skin diseases
- Allergic contact dermatitis
- Atopic dermatitis
- Chronic graft versus host reaction
- Cutaneous lupus erythematosus
- Dermatitis (eczema)
- Discoid lupus erythematosus
- Insect‐bite reactions
- Lichen planus
- Lichen sclerosus
- Lichen striatus
- Lymphomatoid papulosis
- Pityriasis lichenoides chronica
- Psoriasis
- Sarcoidosis
- Scleroderma
- Stevens–Johnson syndrome
- Infections
- Chickenpox
- Herpes zoster
- Impetigo
- Onchocerciasis
- Pinta
- Pityriasis versicolor
- Syphilis
- Procedure‐related
- Chemical peels
- Cryotherapy
- Dermabrasion
- Laser
- Miscellaneous
- Thermal burns.
Patients with atopic dermatitis (atopic eczema) may present with postinflammatory hypopigmentation. The pigmentary changes are more common and intense if potent topical corticosteroids are used. Vitiligo‐like depigmentation has been reported as a consequence of severe atopic dermatitis 2.
Lichen striatus is another common cause of postinflammatory hypopigmentation, with an incidence of up to 59% 3. The dermatosis resolves spontaneously within 2 years, leaving a transient hypopigmentation, especially in dark‐skinned people. In addition, the inflammatory phase may be undetectable, and hypopigmentation may be the sole feature.
In many dark‐skinned patients, pityriasis lichenoides chronica may present with extensive hypopigmentation with few characteristic scaly papular lesions 4.
Pigmentary changes are common after thermal burns and freezing. In superficial burns, postinflammatory hyperpigmentation commonly occurs, whereas deep burns may produce postinflammatory hypopigmentation 5. Melanocytes are very sensitive to cold, and irreversible damage can occur at −4 to −7°C 6. After skin freezing, transient hypopigmentation is seen, caused by the blockage of melanin transfer from melanocytes to keratinocytes, probably because keratinocytes and melanocytes are separated by edema. Thereafter, melanocytes migrate into the lesion, resulting in an area of hypopigmentation with a hyperpigmented rim. The pigmentary changes persist for at least 6 months. After prolonged freezing, there is hypopigmentation with absence of melanosomes in keratinocytes, which may be due to a decrease in melanocyte number, reduction in melanosome synthesis or block in melanosome transfer 7.
Postinflammatory hypopigmentation is also a possible complication of chemical peels. The use of Baker phenol peel in the past was associated with porcelain‐white (alabaster) skin. The likelihood of hypopigmentation depends on the quantity of phenol applied, the level of occlusion, skin type (Fitzpatrick type 1 has a greater likelihood) and existing photodamage 8. Savant 9 reported a study on dermabrasion in 65 patients with different facial conditions; 41 had permanent hypopigmentation.
Laser resurfacing commonly induces hypopigmentation, which seems to be related to the depth of resurfacing, and it may be permanent. It usually occurs 3–10 months after the procedure. In one study 10, the incidence was up to 22% after CO2 laser resurfacing. For pigment‐specific laser, the rates of hypopigmentation after treatment of nevus of Ota with Q‐switch ruby, Q‐switch alexandrite, Q‐switch neodymium:yttrium–aluminium–garnet (Nd:YAG) and a Q‐switch alexandrite/Q‐switch Nd:YAG combination are 16.8%, 10.5%, 7.6% and 40%, respectively. Factors associated with higher risk include the number of treatment sessions and the absorption spectrum of melanin; melanin absorbs ruby laser (694 nm) better than alexandrite laser (755 nm) or QS‐Nd:YAG laser (1064 nm) 11. Pigmentary changes have also been associated with alexandrite laser hair removal. Weisberg 12 reported seven patients who developed similar pigmentary changes, described as initial hyperpigmented rings, followed by a wafer‐like crust, hypopigmentation and finally resolution within 2 weeks to 6 months.
Postinflammatory hypopigmentation pathogenesis
There is limited information about the mechanism and pathogenesis of postinflammatory hypopigmentation. The variation in individual response to cutaneous inflammation or trauma is not well understood. Ruiz‐Maldonado 13 proposed the term ‘individual chromatic tendency’ to describe this variation. Melanocytes can react with normal, increased or decreased melanin production in response to cutaneous inflammation or trauma. The chromatic tendency is genetically determined, and inherited in an autosomal dominant pattern. People with weak melanocytes, which have high susceptibility to damage, are more likely to develop hypopigmentation, whereas those with strong melanocytes tend to develop hyperpigmentation. However, dark‐skinned people do not always have strong melanocytes, and those with weak melanocytes are prone to develop hypopigmentation.
Melanogenesis is a complex process, which includes melanin synthesis, transport and release to keratinocytes. It is controlled by multiple mediators (e.g., growth factors, cytokines) acting on melanocytes, keratinocytes and fibroblasts. Through the release of these mediators, cutaneous inflammation may cause aberration of melanogenesis. A study using histopathological examination of hypopigmented lesions occurring after laser resurfacing found variation in the quantity of epidermal melanin and number of melanocytes. It is suggested that hypopigmentation may result from inhibition of melanogenesis rather than destruction of melanocytes 14; however, severe inflammation may lead to loss of melanocytes or even melanocyte death, and thus permanent pigmentary changes.
Post inflammatory hypopigmentation differential diagnosis
The differential diagnosis of postinflammatory hypopigmentation includes vitiligo, chemical leucoderma, pityriasis alba, progressive macular hypomelanosis, pityriasis versicolor, leprosy, sarcoidosis, hypopigmented lesions in acantholytic disorders, hypopigmented lesions in extramammary Paget disease, hypopigmented mycosis fungoides, infundibulomatosis, and hypopigmentation from medication, especially potent topical corticosteroids and intralesional corticosteroids. These conditions can be differentiated by clinical findings (e.g., epidermal changes, induration, presence of scales and lesion distribution) and histopathological examination 15.
Post inflammatory hypopigmentation signs and symptoms
The size and shape of hypopigmented lesions usually correlate with the distribution and configuration of the original inflammatory dermatosis, and the colour ranges from hypopigmentation to depigmentation (see Figure 1a–c). Complete depigmentation is commonly seen in cases of severe atopic dermatitis and discoid lupus erythematosus, and is more obvious in patients with darker skin. Pigmentary changes sometimes coexist with the original inflammatory lesions, making the diagnosis straightforward. However, in some conditions, the inflammatory phase is not always present, and hypopigmentation may be the only feature. Thus, repeated examinations are required to identify the primary inflammatory dermatosis. Pigmentary changes caused by pigment‐specific laser are seen as small white macules that match the size and shape of the laser spot (see Figure 1d).
Post inflammatory hypopigmentation diagnosis
Examination under Wood lamp accentuates the lesion, and helps distinguish between hypopigmented and depigmented lesions. In addition, it may help to exclude some conditions (e.g., progressive macular hypomelanosis displays punctiform red fluorescence, whereas pityriasis versicolor is coppery‐orange). Confocal laser scanning microscopy may allow distinction between different hypomelanotic conditions, based on the melanin content and distribution patterns. Melanophages have been found in postinflammatory hypopigmentation but not in vitiligo and naevus depigmentosus. However, the contents of melanin and dermal papillary rings vary with the degree of the inflammation 16.
Histopathology of postinflammatory hypopigmentation shows nonspecific findings, including decreased epidermal melanin, variable degrees of superficial lymphohistiocytic infiltration, and presence of melanophages in the upper dermis. In addition, there may be some histopathological evidence that can help to establish the diagnosis of the cause of postinflammatory hypopigmentation, such as in lupus erythematosus 17. Even if the biopsy shows nonspecific findings, it is still useful in excluding many dermatoses that present with hypopigmentation only, such as mycosis fungoides, sarcoidosis and leprosy.
Post inflammatory hypopigmentation treatment
The most important step of management is to identify the cause. The hypopigmentation due to inflammatory skin disorders and infections usually resolves by itself over weeks to months once the underlying disorder has been effectively treated. There is no effective treatment for hypopigmentation due to scarring. The response of vitiligo to therapy is highly variable.
To prevent iatrogenic hypopigmentation, dermatological and cosmetic procedures should be performed carefully, especially in high‐risk patients.
Twice‐daily application of a medium‐potency topical steroid in combination with a tar‐based preparation has been used to treat postinflammatory hypopigmentation, although the mechanisms behind this are currently not well understood. The steroid may affect inflammatory cells responsible for the inflammation 18, while the tar may photodynamically induce melanogenesis 19. A preparation of combined steroid and tar is more effective in stimulating melanogenesis 18.
Topical pimecrolimus cream was reported to be beneficial in an open‐label, pilot trial for the treatment of seborrhoeic dermatitis with associated postinflammatory hypopigmentation in dark‐skinned patients 20. The regimen consisted of twice‐daily application of 1% pimecrolimus cream for 16 weeks. The degree of improvement, assessed by a mexameter, was greatest during the first 2 weeks after the application.
Sun or ultraviolet (UV) exposure may help in repigmentation when there are functional melanocytes in the affected area; however, overexposure may enhance the colour contrast as a result of tanning of surrounding skin. Topical application of 0.1% 8‐methoxypsoralen, 0.5–1% coal tar or anthralin followed by sun exposure can be helpful in the restoring the pigment 13. Various regimens of topical photochemotherapy (topical psoralen UVA; PUVA) have been used to treat postinflammatory hypopigmentation caused by various conditions, with favourable results. The regimen consists of topical application of 0.001–0.5% 8‐methoxypsoralen in aquaphor or hydrophilic ointment to the affected area for 20–30 min, followed by UVA exposure 1–3 times per week at an initial dose of 0.2–0.5 J/cm², increasing by 0.2–0.5J/cm² weekly 18.
The 308‐nm excimer laser may be used to stimulate pigmentation in hypopigmented scars, and had a response rate of 60–70% after nine biweekly treatments. However, regular subsequent treatment is needed every 1–4 months to maintain the results 21. For extensive involvement, narrowband UVB phototherapy or oral PUVA may be used 2–3 times weekly. The number of treatment sessions required is higher for repigmenting vitiligo lesions 18. The ablative fractional CO2 laser has been reported to be effective in the treatment of hypopigmentation associated with CO2 laser resurfacing 22.
In depigmented lesions with total loss of melanocytes, epidermal or melanocyte grafting may be considered 23. Various methods of camouflage including high‐coverage makeup, tanning products and tattooing may be an alternative option.
Post inflammatory hypopigmentation prognosis
Minimal hypopigmentation usually resolves within a few weeks, but severe hypopigmentation and depigmentation associated with lupus erythematosus, scleroderma or burn may require years to become repigmented, and may be permanent.
- Vachiramon V, Thadanipon K. Postinflammatory hypopigmentation. Clin Exp Dermatol. 2011 Oct;36(7):708-14. https://doi.org/10.1111/j.1365-2230.2011.04088.x[↩][↩][↩][↩]
- Larregue M, Martin J, Bressieux JM et al. Vitiligoid achromias and severe atopic dermatitis. Apropos of 4 cases. Ann Dermatol Venereol 1985; 112: 589–600.[↩]
- Peramiquel L, Baselga E, Dalmau J et al. Lichen striatus. Clinical and epidemiological review of 23 cases. Eur J Pediatr 2006; 165: 267–9.[↩]
- Lane TN, Parker SS. Pityriasis lichenoides chronica in black patients. Cutis 2010; 85: 125–9.[↩]
- El‐Bishry MA, Nassar AM, El‐Maghraby MZ. Tattooing, a new hope for secondary leukoderma. Scand J Plast Reconstr Surg 1979; 13: 147–53.[↩]
- Gage AA, Meenaghan MA, Natiella JR, Greene GW Jr. Sensitivity of pigmented mucosa and skin to freezing injury. Cryobiology 1979; 16: 348–61.[↩]
- Burge SM, Bristol M, Millard PR, Dawber RP. Pigment changes in human skin after cryotherapy. Cryobiology 1986; 23: 422–32.[↩]
- Brody HJ. Complications of chemical resurfacing. Dermatol Clin 2001; 19: 427–38, vii–viii.[↩]
- Savant SS. Facial dermabrasion in acne scars and genodermatoses – a study of 65 patients. Indian J Dermatol Venereol Leprol 2000; 66: 79–84.[↩]
- Bernstein LJ, Kauvar AN, Grossman MC, Geronemus RG. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg 1997; 23: 519–25.[↩]
- Kono T, Nozaki M, Chan HH, Mikashima Y. A retrospective study looking at the long‐term complications of Q‐switched ruby laser in the treatment of nevus of Ota. Lasers Surg Med 2001; 29: 156–9.[↩]
- Weisberg NK, Greenbaum SS. Pigmentary changes after alexandrite laser hair removal. Dermatol Surg 2003; 29: 415–19.[↩]
- Ruiz‐Maldonado R, Orozco‐Covarrubias ML. Postinflammatory hypopigmentation and hyperpigmentation. Semin Cutan Med Surg 1997; 16: 36–43.[↩][↩]
- Grimes PE, Bhawan J, Kim J et al. Laser resurfacing‐induced hypopigmentation: histologic alterations and repigmentation with topical photochemotherapy. Dermatol Surg 2001; 27: 515–20.[↩]
- Verma S, Patterson JW, Derdeyn AS et al. Hypopigmented macules in an Indian man. Arch Dermatol 2006; 142: 1643–8.[↩]
- Xiang W, Xu A, Xu J et al. In vivo confocal laser scanning microscopy of hypopigmented macules: a preliminary comparison of confocal images in vitiligo, nevus depigmentosus and postinflammatory hypopigmentation. Lasers Med Sci 2010; 25: 551–8.[↩]
- Franca AF, de Souza EM. Histopathology and immunohistochemistry of depigmented lesions in lupus erythematosus. J Cutan Pathol 2010; 37: 559–64.[↩]
- Halder RM, Richards GM. Management of dyschromias in ethnic skin. Dermatol Ther 2004; 17: 151–7.[↩][↩][↩][↩]
- Urbanek RW. Tar vitiligo therapy. J Am Acad Dermatol 1983; 8: 755.[↩]
- High WA, Pandya AG. Pilot trial of 1% pimecrolimus cream in the treatment of seborrheic dermatitis in African American adults with associated hypopigmentation. J Am Acad Dermatol 2006; 54: 1083–8.[↩]
- Alexiades‐Armenakas MR, Bernstein LJ, Friedman PM, Geronemus RG. The safety and efficacy of the 308‐nm excimer laser for pigment correction of hypopigmented scars and striae alba. Arch Dermatol 2004; 140: 955–60.[↩]
- Tierney EP, Hanke CW. Treatment of CO2 laser induced hypopigmentation with ablative fractionated laser resurfacing: case report and review of the literature. J Drugs Dermatol 2010; 9: 1420–6.[↩]
- Falabella R. Postdermabrasion leukoderma. J Dermatol Surg Oncol 1987; 13: 44–8.[↩]