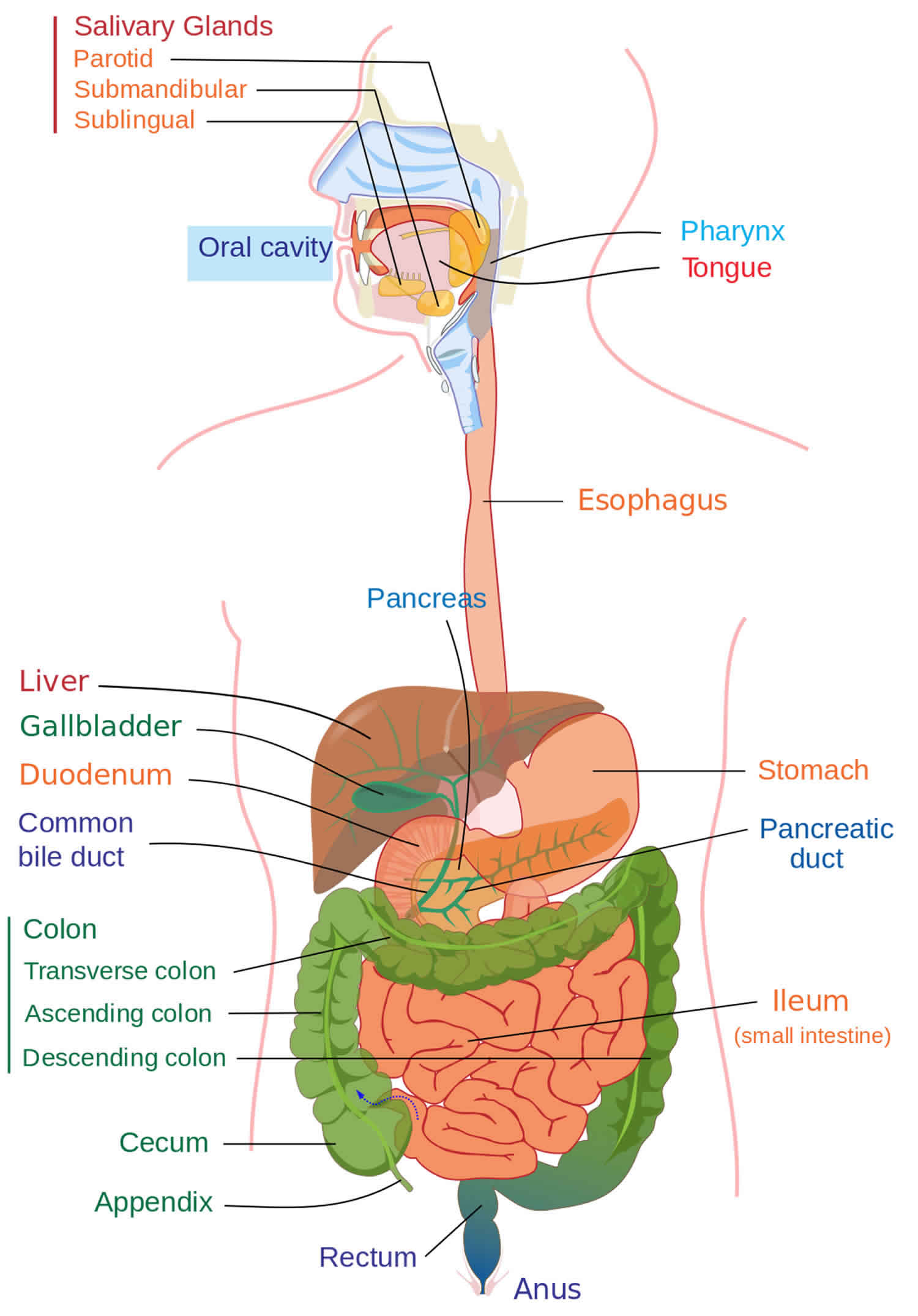
Protein losing enteropathy
Protein-losing enteropathy is an abnormal loss of protein from the gastrointestinal tract due to different causes 1. Protein-losing enteropathy can also refer to the inability of the digestive tract to absorb proteins. Protein losing enteropathy occurs when the loss of proteins through the gastrointestinal tract exceeds the synthesis of proteins by the body, leading to hypoproteinemia. Normally most of the proteins entering the gut are degraded into amino acids and are reabsorbed. In conditions causing inflammation and erosions of the gastrointestinal tract, the mucosal permeability increases, leading to excessive leakage of serum proteins into the gut, and poor reabsorption. This state leads to hypoproteinemia. In diseases causing increased lymphatic pressure and lymphatic obstruction, there is an increased leak of lymph into the gastrointestinal tract and decreased absorption of chylomicrons, resulting in a deficiency of fat-soluble vitamins and protein loss 2. Protein-losing enteropathy should be suspected in patients with low serum proteins and in whom other causes of hypoproteinemia have been ruled out 2.
Protein losing enteropathy causes
There are many causes of protein-losing enteropathy. Conditions that cause serious inflammation in the intestines can lead to protein loss. Some of these are:
- Bacteria or parasite infection of the intestines
- Celiac sprue
- Crohn disease
- HIV infection
- Lymphoma
- Lymphatic obstruction in the gastrointestinal tract
- Intestinal lymphangiectasia
The three main groups of disorders that cause excess protein loss in stools are:
1) Primary Erosive/Ulcerative gastrointestinal Disorders
This group of conditions includes inflammatory bowel diseases (both ulcerative colitis and Crohn disease), gastrointestinal malignancies, any erosions or ulcers of stomach or duodenum, Clostridium difficile colitis, carcinoid syndrome, graft versus host disease.
2) Non-Erosive/Non-Ulcerative Gastrointestinal Disorders
This group includes topical Sprue, celiac disease, Menetrier disease, amyloidosis, cutaneous burns, eosinophilic gastroenteritis, bacterial overgrowth, Intestinal parasitic infections, Whipple disease, collagenous colitis, AIDS, mixed connective tissue diseases, systemic lupus erythematosus (SLE), rheumatoid arthritis 3.
3) Disorders Causing Increased Interstitial Pressure or Lymphatic Obstruction
This grouping can be due to primary intestinal lymphangiectasia, right-sided heart failure, constrictive pericarditis, congenital heart disease, Fontan procedure for single ventricle, cirrhosis with portal hypertension gastropathy, hepatic venous outflow obstruction, mesenteric tuberculosis or sarcoidosis, retroperitoneal fibrosis, lymphoenteric fistula, lymphoma, and thoracic duct obstruction 4.
Protein losing enteropathy symptoms
Protein losing enteropathy symptoms will depend on the disease that is causing the problem.
Protein losing enteropathy symptoms can include:
- Diarrhea
- Fever
- Abdominal pain
- Swelling
Loss of serum proteins leads to decreased oncotic pressure in capillaries, which, in turn, leads to peripheral edema (most common presenting symptom) due to transudation of fluids from capillaries to the subcutaneous tissue. Patients can also present with abdominal distension due to ascites and pleural effusions. In primarily gastrointestinal causes of protein loss, they can have diarrhea, bloating, abdominal pain, etc. This condition can cause loss of immunoglobulins and lymphocytes, which cause immunocompromised state leading to frequent infections, and also patients can get opportunistic infections. Patients with protein loss due to cardiac diseases can present with symptoms of heart failure like pitting edema, pleural effusion, shortness of breath, elevated jugular venous pressure.
Protein losing enteropathy complications
Complications depend mostly on the underlying disease causing protein-losing enteropathy. Patients with Inflammatory bowel disease can develop colon cancer, anemia, primary sclerosing cholangitis, and many other complications. Protein losing enteropathy due to ulcerative diseases can have a perforation of ulcer and peritonitis. Patients with Clostridium difficile colitis can develop toxic megacolon. Patients can develop a deficiency of micronutrients and vitamins (particularly fat-soluble vitamins). Due to the loss of immunoglobulins and lymphocytes, patients get prone to recurrent and opportunistic infections.
Protein losing enteropathy diagnostic tests
You may need tests that look at your intestinal tract. These may include a CT scan of the abdomen or an upper gastrointestinal (GI) bowel series.
Other tests you may need include:
- Colonoscopy
- Esophagogastroduodenoscopy (EGD)
- Small intestine biopsy
- Alpha-1-antitrypsin test (A1AT)
- Small bowel capsule endoscopy
- CT or MR enterography
Diagnosis of protein-losing enteropathy should be suspected in patients with hypoproteinemia once the other common causes like severe malnutrition, nephrotic syndrome, or chronic liver diseases have been ruled out. Since the protein loss in protein losing enteropathy occurs independent of their molecular weight, these patients have low albumin and low globulins in their serum. If there are isolated low serum albumin and normal serum globulins, then alternative causes should be considered.
Alpha 1 antitrypsin intestinal clearance is the primary and most common test performed to diagnose protein losing enteropathy. This protein has high molecular weight, is minimally degraded in the gut and is excreted intact. Its clearance is calculated by 24 hr stool collection and measuring alpha 1 antitrypsin in serum and stool.
- Alpha 1-antitrypsin clearance = (stool volume) x (stool alpha 1-antitrypsin)/ (serum alpha-1 antitrypsin).
The normal clearance of alpha 1 antitrypsin is under 13 ml/24 hours. Clearance greater than 27 ml/ day indicates increased gastrointestinal protein loss and points towards the diagnosis of protein losing enteropathy. Clearance is affected by diarrhea. Any condition causing diarrhea leads to increased clearance of alpha 1 antitrypsin, so the higher threshold of more than 56 ml/24 hr should be used in these conditions. Increased stomach acidity and decreased pH lead to increased degradation of alpha 1 antitrypsin, so the performance of this test should be while the patient is on antacids especially if there is suspicion of hypersecretory state causing increased protein loss. Gastrointestinal bleeding can also cause increased clearance of alpha 1 antitrypsin.
Other tests like 51Cr-labeled albumin clearance(considered gold standard), technetium 99 labeled serum albumin scintigraphy can also be performed if there is high clinical suspicion of protein losing enteropathy with negative alpha 1 antitrypsin clearance test. These tests have high sensitivity, but they are very cumbersome, expensive and not readily available so not routinely performed 5.
Once the diagnosis of protein losing enteropathy is established with increased alpha 1 antitrypsin clearance, further workup is needed to find the underlying etiology and to guide treatment. Important consideration should be given to the history and physical examination of the patient. Detailed history helps in narrowing down the list of conditions causing hypoproteinemia and protein losing enteropathy and guides the approach towards further work up. Basic labs like complete blood count with differential, liver function tests, renal function tests should be performed in all patients. In cases where symptoms suggest gastrointestinal causes of protein loss, tests for celiac disease, infectious work for chronic intestinal infections, appropriate imaging of abdomen and pelvis, upper and lower gastrointestinal endoscopies with biopsies, capsule endoscopies (if upper and lower endoscopies are unremarkable) should be performed. Autoimmune workup should be ordered if systemic lupus erythematosus (SLE) or rheumatoid arthritis is suspected. Echocardiogram and workup on the lines of heart failure should be done if cardiac causes of protein losing enteropathy is suspected. Fontan procedure in patients with congenital heart disease is a well-known cause of protein losing enteropathy and has been extensively studied.
Protein losing enteropathy treatment
Protein losing enteropathy treatment involves treating the underlying condition that caused protein-losing enteropathy. Besides this, dietary modifications also play a critical role in the management of protein-losing enteropathy. Diet rich in protein and medium chain triglycerides and low in fat is considered the best diet in this condition. Patients may require 2 to 3g/kg/day of protein. Replacement of micronutrients, electrolytes, and vitamin deficiencies should occur as appropriate.
If heart failure is the cause of protein losing enteropathy, then optimization of heart failure medications needs to be done. Diuretics are an option for symptoms of anasarca and fluid overload. Pericardiectomy can help in constrictive pericarditis. Treatment with immunosuppressive medications should be the approach in inflammatory bowel disease, systemic lupus erythematosus (SLE), rheumatoid arthritis, and other inflammatory conditions. Treat parasitic infections if they are the cause of protein losing enteropathy. Surgical resection can be a consideration in Menetrier disease 6 and refractory inflammatory bowel disease. Octreotide has been considered beneficial in primary intestinal lymphangiectasia and Menetrier disease by decreasing lymphatic pressure and reducing intestinal protein loss. Budesonide and corticosteroids may be helpful in eosinophilic gastroenteritis and some cases of protein losing enteropathy due to Fontan procedure 7.
Routine monitoring is advisable after initiation of treatment in the form of checking micronutrients and vitamin deficiencies, serum protein levels, and alpha 1 antitrypsin clearance.
Protein losing enteropathy diet
A low-fat diet with supplementation with medium-chain triglycerides is theoretically of benefit in patients with lymphangiectasias. However, in practice, ingesting a diet containing medium-chain triglycerides results in increased blood flow with no reduction in fecal protein loss 8.
Patients with celiac sprue typically respond to a gluten-free diet. A minority requires corticosteroids.
Protein losing enteropathy prognosis
Prognosis is variable and dependant on the underlying cause of protein-losing enteropathy. If there is a successful treatment of the underlying cause, then it can lead to complete resolution of protein losing enteropathy.
A retrospective study by John et al 9 indicated that the survival rate has increased for patients who develop protein-losing enteropathy as a complication of the Fontan procedure, an operation for the treatment of several types of congenital heart abnormalities. Although the 5-year mortality rate has been reported to be 50% for patients who develop protein-losing enteropathy following the surgery, the investigators found that in 42 such patients identified from Mayo Clinic clinical databases, the survival rate at 5 years after the diagnosis of enteropathy was 88% 9.
The survival rate tended to be lower, however, in patients with a high Fontan pressure, reduced ventricular function (ejection fraction < 55%), and a New York Heart Association functional classification of greater than 2. Treatments associated with a higher survival rate included spironolactone, octreotide, and sildenafil, as well as the creation of fenestrae and Fontan obstruction relief 9.
Mortality and morbidity
Morbidity and mortality of this condition directly relate to its cause, either primary gastrointestinal disease or a multisystem disorder.
- Nagra N, Dang S. Protein Losing Enteropathy. [Updated 2019 Jun 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542283[↩]
- Craven MD, Washabau RJ. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 2019 Mar;33(2):383-402.[↩][↩]
- Zubiaga Toro L, Ruiz-Tovar J, Castro MJ, Ortiz de Solórzano FJ, Luque de León E, Jiménez JM, Carbajo MÁ. Whipple disease after bariatric surgery: from malabsorption to malnutrition status. Nutr Hosp. 2019 Mar 07;36(1):238-241.[↩]
- Schumacher KR, Yu S, Butts R, Castleberry C, Chen S, Edens E, Godown J, Johnson J, Kemna M, Lin K, Lowery R, Simpson K, West S, Wilmot I, Gossett JG. Fontan-associated protein-losing enteropathy and post‒heart transplant outcomes: A multicenter study. J. Heart Lung Transplant. 2019 Jan;38(1):17-25.[↩]
- Chau TN, Mok MY, Chan EY, Luk WH, Lai KB, Li FT, Leung VK, Wong R. Evaluation of performance of measurement of faecal α(1)-antitrypsin clearance and technetium-99m human serum albumin scintigraphy in protein-losing enteropathy. Digestion. 2011;84(3):199-206.[↩]
- Gompertz M, Montenegro C, Bufadel ME, Defilippi C, Castillo J, Morales C. [Ménétrier disease. Report of one case]. Rev Med Chil. 2012 Sep;140(9):1174-8.[↩]
- John AS, Driscoll DJ, Warnes CA, Phillips SD, Cetta F. The use of oral budesonide in adolescents and adults with protein-losing enteropathy after the Fontan operation. Ann. Thorac. Surg. 2011 Oct;92(4):1451-6.[↩]
- Lee HJ, Rha MY, Cho YY, Kim ER, Chang DK. A case of protein supplement effect in protein-losing enteropathy. Clin Nutr Res. 2012 Jul. 1(1):94-8.[↩]
- John AS, Johnson JA, Khan M, et al. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol. 2014 Jul 8. 64(1):54-62.[↩][↩][↩]