Purpura fulminans
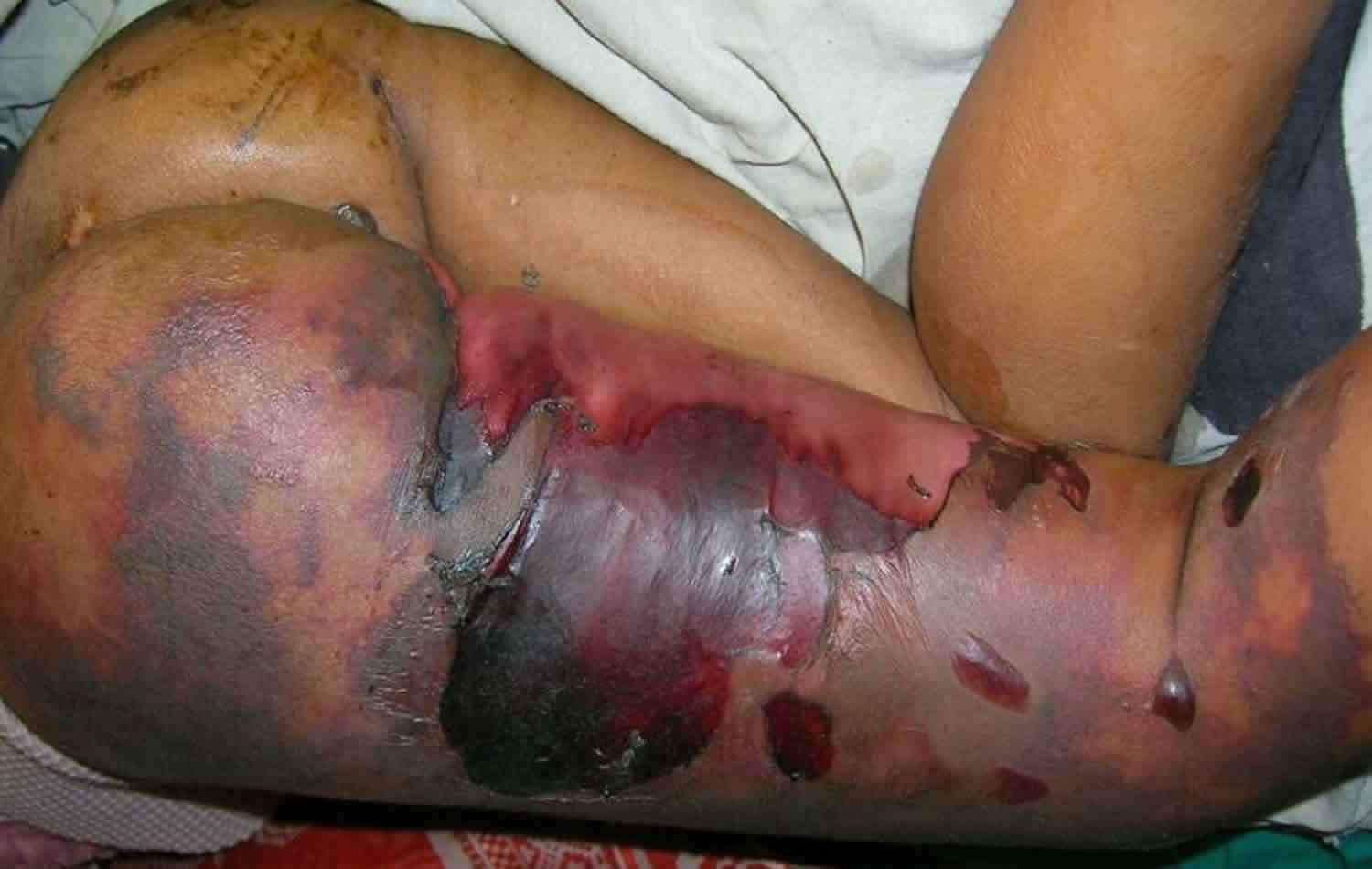
Purpura fulminans is an acute purpuric rash characterized by coagulation of the microvasculature or disseminated intravascular coagulation (DIC), which leads to purpuric lesions and skin necrosis. Purpura fulminans is the acute onset of often rapidly progressing cutaneous hemorrhage and necrosis caused by dermal vascular thrombosis and disseminated intravascular necrosis. Purpura fulminans is a true dermatological emergency and requires immediate diagnosis and management 1. Patients are often acutely ill with fever, have hemorrhage from multiple sites, and may be hypotensive. It is rapidly progressive and is often accompanied by disseminated intravascular coagulation and circulatory collapse. It occurs in neonates, children, and adults. There are 3 forms of purpura fulminans with a classification scheme based on the triggering mechanism. The hereditary neonatal purpura fulminans with severe protein C deficiency occurs in about 1:1,000,000 live births 2. Acute infectious purpura fulminans can be seen in up to 10% to 20% of patients who develop meningococcal septicemia. Meningococcus and Streptococcus pneumoniae were identified as the most common bacterial triggers, and varicella was the most common viral trigger. Acute infectious purpura fulminans has been found to be more common in patients who are physically or functionally asplenic. The idiopathic post-infectious purpura fulminans is very rare with only a few hundred cases reported.
Purpura fulminans mortality rate has been decreasing with supportive care, improved management of secondary complications, and some targeted treatments, but purpura fulminans remains a disabling condition often resulting in major amputations in those who survive.
Purpura fulminans causes
Purpura fulminans is a rapidly evolving syndrome of skin microvascular thrombosis and hemorrhagic necrosis. It is considered a sign of certain systemic diseases rather than a disease unto itself. Purpura fulminans occurs in 3 clinical settings:
- Neonatal purpura fulminans is associated with a hereditary deficiency of the anticoagulants protein C, protein S, and antithrombin III 3. These proteins are vitamin-K dependent cofactors which are pro-fibrinolytic. Protein C is one of the major inhibitors of the coagulation system which when activated inhibits factor Va and VIIIa which in turn down-regulate thrombin synthesis. It manifests very early in life and treatment is aimed at these deficiencies 4. Neonates typically present with massive venous and arterial thrombosis of the skin and other organs within 5 days of birth.
- Acute infectious purpura fulminans is the most common type and is associated with an acquired deficiency of protein C. It manifests as a skin finding in the most severe septic patients (usually sepsis with endotoxin producing gram-negative bacteria) as well as in necrotizing fasciitis with a predilection to certain infectious agents 5. The mechanism involves a disruption of the coagulation balance. Bacterial endotoxin triggers consumption of proteins C and S and antithrombin III. This pro-coagulative state leads to thromboses of dermal vessels and is associated with disseminated intravascular coagulation. The skin lesions may present early as petechial rashes. These rapidly progress to larger ecchymotic areas. Later in the course, hemorrhagic bullae may form which contribute to the classic hard eschars characteristic of purpura fulminans.
- Idiopathic purpura fulminans or acquired purpura fulminans is the rarest form of the disease, is thought to be a post-infectious autoimmune disorder often following an initiating febrile illness occurs approximately 7 to 10 days after an infectious disease, which later leads to rapidly progressive purpura. Idiopathic purpura fulminans has been associated with the development of anti-protein S antibodies. These antibodies bind to protein S and get excreted. This causes to a transient protein S deficiency which leads to hypo-activation of the protein C pathway and a hypercoagulable state similar to what was described above. Idiopathic purpura fulminans usually involves the skin (usually varicella or scarlet fever) 6.
All types of purpura fulminans involve dysfunction of hemostasis with a shift to a disease state with overwhelming procoagulation.
Purpura fulminans symptoms
The skin findings of purpura fulminans have a characteristic appearance and evolution. Purpura fulminans begins with erythema, which develops irregular central areas of blue-black hemorrhagic necrosis. In some cases, vesicles and bulla form. The affected skin initially is painful and indurated, but in the later stages, there may be a total loss of sensation. Secondary infection of gangrenous tissue may occur. Necrosis may extend to deeper tissues.
In general, the history and physical exam for purpura fulminans should be aimed at early recognition and determination of the underlying cause. Make sure to thoroughly examine the patient’s skin, especially in patients who could be presenting with necrotizing fasciitis. Early in the diagnosis of associated diseases, the skin findings may be subtle, so it is important to keep this diagnosis in your differential. Petechial rash or bruising in a neonate or septic patient should trigger consideration of purpura fulminans. Components of a patient’s history include a history of severe infections or trauma as well previous splenectomy. Because of the association with disseminated intravascular coagulation (DIC) patients may experience bleeding from multiple sites including mucous membranes, areas of trauma or the rectum. Because of the association with severe septic shock, the patient may experience symptoms of end-organ damage such as hematuria, oliguria, and respiratory distress. Pain out of proportion to exam should always make one consider necrotizing fasciitis.
Manifestations of neonatal purpura fulminans may include the following:
- Development within the first 72 hours after birth
- Purpuric lesions over many different skin sites, including the perineal region, the flexor surface of the thighs, and abdominal skin
- Skin lesions soon enlarge and become vesiculated, producing hemorrhagic bullae with subsequent necrosis and black eschar formation
- Thrombocytopenia
- Possible signs of a urinary tract infection (UTI)
Manifestations of idiopathic purpura fulminans may include the following:
- Sudden development 7-10 days after the onset of the precipitating infection
- Progressively enlarging, well-demarcated purplish areas of hemorrhagic cutaneous necrosis with derangements in coagulation factors
- Erythematous macules that progress within hours to sharply defined areas of purpura
- Impaired perfusion of limbs and digits
- Major organ dysfunction (eg, lungs, heart, or kidneys)
The four primary features of acute infectious purpura fulminans are as follows:
- Large purpuric skin lesions
- Fever
- Hypotension
- Disseminated intravascular coagulation (DIC)
Purpura fulminans diagnosis
Purpura fulminans represents an imbalance in the coagulation system. Specific levels of antithrombin III, free protein C, and free and total protein S may help confirm the diagnosis especially in the neonatal form of the disease. Otherwise, the evaluation of patients with purpura fulminans mimics the evaluation of the underlying cause. Searching for inciting infection with labs, cultures, and imaging should follow sepsis guidelines. If the clinician is considering necrotizing fasciitis, white blood cell (WBC) count and a sodium level with or without the other components of the Laboratory Risk Indicator for Necrotizing Fasciitis score may help the surgeon decide on an early intervention. Because of the strong association with disseminated intravascular coagulation, one should also evaluate for thrombocytopenia, elevated coagulation factors (PT, PTT), increased d-dimer assay (or serum fibrin degradation products), and a decreasing fibrinogen level.
Table 1. Typical lab values in acute disseminated intravascular coagulopathy (DIC)
| Lab Test | Acute DIC | Reference Range |
| Platelet count | < 150,000/L | 150,000-450,000/L |
| Fibrinogen | >340 mg/dL | 170-340 mg/dL |
| Prothrombin time (PT) | >13 seconds | 9-13 seconds |
| Activated partial thromboplastin time (aPTT) | >35 seconds | 23-35 seconds |
| D-dimer | >250 ng/mL | 0-250 ng/mL |
Purpura fulminans treatment
The treatment of all types of purpura fulminans starts with supportive care and adequate hydration. This is important because of the widespread thrombosis associated with this disease can lead to damage of multiple end organs. As this commences, finding and treating the underlying cause is essential. Anticoagulation may be stared to prevent further necrosis. There may need for replacement of blood, factors, and platelets lost because of both the pro-coagulable state and disseminated intravascular coagulation (DIC). Finally, early surgical debridement for areas which have become necrotic has been shown to decrease mortality.
In the neonatal form of the disease hydration, platelet transfusion, followed by an assessment of protein C and S levels followed closely with fresh frozen plasma transfusions are the mainstay of treatment. Heparin and warfarin have been used as anticoagulants, and later protein C concentrate can be added if this deficiency is found 8.
The treatment of idiopathic purpura fulminans is similar to what is described above. Additionally, there may be a role for immunomodulation with corticosteroids.
In the acute infectious form, broad-spectrum antibiotics should include coverage of Neisseria meningitidis, Streptococcus, Staphylococcus, and Clostridia species. Often carbapenem or vancomycin with beta lactam-beta lactamase inhibitor combinations are used. Clindamycin is often included as it has specific properties which inhibit some of the toxins which allow this disease to progress. IVIg therapy is also used because of antibodies to these toxins. Activated protein C may be administered to reduce the inflammatory cascade, restore the coagulation balance which may decrease the progression of purpuric skin injury. In acute infectious purpura fulminans, the decision to anticoagulated is based on the occurrence of concurrent disseminated intravascular coagulation (DIC) 9.
In all forms of the disease repeated tissue assessments with debridement of affected areas are carried out as needed. Repeated surgeries are often required.
Neonatal purpura fulminans treatment
In a neonate with neonatal purpura fulminans, immediate treatment with platelet concentrate is recommended to reverse the thrombocytopenia and the bleeding manifestations. The neonate often develops disseminated intravascular coagulation (DIC). In the absence of signs of generalized bloodstream infection, deficiencies of the anticoagulant factors protein C, protein S, and antithrombin III (ATIII) remain important considerations. Consequently, the endogenous activity of these anticoagulant factors must be assessed by means of a chromogenic assay.
With a provisional diagnosis of purpura fulminans due to protein C deficiency, fresh frozen plasma (FFP) transfusion must be started. The fresh frozen plasma can later be replaced with low-molecular-weight heparin (LMWH). Subsequently, oral anticoagulation with warfarin must be instituted. Debridement of the dead tissue is mandatory. The protein C, protein S, and antithrombin III genes must be analyzed both in the patient and in the parents.
These patients require long-term oral anticoagulation, which, if well tolerated, may be sufficient to permit them to remain free of coagulopathy throughout life 10. If the genetic assays reveal a defect in the protein C or antithrombin III genes, the protein C or antithrombin III concentrates may be used to correct this coagulation disorder.
In 2018 guidelines released by the American Society of Hematology on the management of venous thromboembolism, the guidelines panel remarked that protein C replacement offers better long-term effectiveness than does anticoagulation in pediatric patients with purpura fulminans arising from homozygous protein C deficiency (although the panel also cited a concern that protein C therapy may be prohibitively expensive). In addition, the American Society of Hematology guidelines suggested that during an acute episode in such pediatric patients, treatment involve anticoagulation not by itself but in combination with protein C replacement 11.
Idiopathic purpura fulminans treatment
In 1995, Sheridan et al 12 described a management strategy for idiopathic purpura fulminans with multiple organ failure in children. In three cases in the report, the purpura fulminans involved a large percentage of the patients’ body surface areas. One patient, a 15-year-old boy, had skin lesions on 55% of his body surface area. Another patient, an 11-month-old girl, was affected on 25% of her body surface area. The third patient, a 2-year-old boy, had evidence of purpura fulminans on 55% of his body surface area. The pathogenesis of purpura fulminans was not known but probably involved acute transient decreases in protein C, protein S, or antithrombin III 12.
The successful management of meningococcal sepsis in these patients was facilitated by early diagnosis and aggressive antibiotic therapy 12. Management of purpura fulminans was particularly challenging in these cases because the children had evidence of multiple organ failure.
To better understand the current management of idiopathic purpura fulminans, seven burn centers performed a 10-year retrospective chart review of patients who were diagnosed with idiopathic purpura fulminans 13. A total of 70 patients were identified, with a mean patient age of 13 years. Neisseria meningitidis 14 was the most common pathogen identified in infants and adolescents, while Streptococcus species predominated in adults.
In the patients studied, acute management consisted of antibiotic treatment, volume resuscitation, and ventilatory and inotropic support 13. Protein C replacement was performed in only 9% of the cases. One fourth of the patients required amputation of all of the extremities. When performed early, fasciotomies may reduce the depth of soft tissue involvement and the extent of amputation. Although the overall mortality in this study was only 13%, the surgeons believed this number to be inaccurately low because it did not reflect the number of patients who succumbed to sepsis in facilities outside the multicenter study group.
In general, the authors recommend a conservative approach to treatment of idiopathic purpura fulminans that includes excising gangrenous areas after they have been demarcated from purpuric and indeterminate zones. In the presence of infection, however, early aggressive surgical debridement is essential to prevent invasive wound sepsis. When compartment syndrome is suspected in patients with tense limbs and distal ischemia, early fasciotomy is recommended. If established gangrene is present, conservative amputation is warranted.
Manco-Johnson and Knapp-Clevenger 15 described the use of activated protein C (APC) in a 14-year-old girl with protein C deficiency due to idiopathic purpura fulminans. At the end of the activated protein C infusions, all skin lesions of purpura fulminans were resolved. The patient experienced no adverse reactions to activated protein C. The authors concluded that activated protein C is safe and effective for the treatment of purpura fulminans with severe genetic protein C deficiency.
Recognition of the pathophysiologic mechanism of idiopathic purpura fulminans provides a rational basis for treatment with immediate heparinization and infusion of fresh frozen plasma 16. In some cases complicated by major vessel thrombosis, the use of tissue plasminogen activator (tPA) may reduce thromboembolic complications.
Acute infectious purpura fulminans treatment
Meningococcemia and infection due to Staphylococcus aureus lead to acute infectious purpura fulminans. Patients with these infections have remarkably reduced levels of activated protein C as a result of dysfunction of the endothelial protein C activation pathway. Activated protein C not only acts as an anticoagulant but also serves as an important modulator of the inflammatory response.
Patients who present with acute infectious purpura fulminans should receive broad-spectrum intravenous antibiotic therapy with activity against a variety of pathogens, including Neisseria meningitidis, streptococci, and methicillin-resistant staphylococcus aureus (MRSA).
Consideration should also be given to early administration of activated protein C concentrates to minimize purpura skin injury and to reduce the inflammatory cascade before irreparable tissue injury occurs 17. Finally, because toxic shock syndrome is mediated by strong antigens, intravenous immunoglobulin (IVIg) therapy should be implemented because these preparations contain significant antibodies against the causative exotoxins 18.
Because patients with DIC are at risk for acute thrombosis and bleeding, it is difficult to predict which will occur in a specific patient. Given these inherent risks and the lack of evidence available, prophylactic anticoagulation is not currently indicated in the treatment of acute infectious purpura fulminans 19.
Hyperbaric oxygen therapy has rarely been used in the treatment of purpura fulminans and is not currently considered an important part of its therapy.
- Perera TB, Murphy-Lavoie HM. Purpura Fulminans. [Updated 2019 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532865[↩]
- Lamadrid-Zertuche AC, Garza-Rodríguez V, Ocampo-Candiani JJ. Pigmented purpura and cutaneous vascular occlusion syndromes. An Bras Dermatol. 2018 Jun;93(3):397-404.[↩]
- Findley T, Patel M, Chapman J, Brown D, Duncan AF. Acquired Versus Congenital Neonatal Purpura Fulminans: A Case Report and Literature Review. J. Pediatr. Hematol. Oncol. 2018 Nov;40(8):625-627.[↩]
- Irfan Kazi SG, Siddiqui E, Habib I, Tabassum S, Afzal B, Khan IQ. Neonatal Purpura Fulminans, a rare genetic disorder due to protein C deficiency: A case report. J Pak Med Assoc. 2018 Mar;68(3):463-465.[↩]
- Olivieri M, Huetker S, Kurnik K, Bidlingmaier C, Keil J, Reiter K, Hoffmann F. Purpura fulminans – It’s Not Always Sepsis. Klin Padiatr. 2018 Jul;230(4):225-226.[↩]
- Hale AJ, LaSalvia M, Kirby JE, Kimball A, Baden R. Fatal purpura fulminans and Waterhouse-Friderichsen syndrome from fulminant Streptococcus pneumoniae sepsis in an asplenic young adult. IDCases. 2016;6:1-4.[↩]
- Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009 Apr;145(1):24-33.[↩]
- Kizilocak H, Ozdemir N, Dikme G, Koc B, Celkan T. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J. Thromb. Thrombolysis. 2018 Feb;45(2):315-318.[↩]
- Colling ME, Bendapudi PK. Purpura Fulminans: Mechanism and Management of Dysregulated Hemostasis. Transfus Med Rev. 2018 Apr;32(2):69-76.[↩]
- Marlar RA, Montgomery RR, Broekmans AW. Diagnosis and treatment of homozygous protein C deficiency. Report of the Working Party on Homozygous Protein C Deficiency of the Subcommittee on Protein C and Protein S, International Committee on Thrombosis and Haemostasis. J Pediatr. 1989 Apr. 114(4 Pt 1):528-34.[↩]
- Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018 Nov 27. 2 (22):3292-316.[↩]
- Sheridan RL, Briggs SE, Remensnyder JP, Tompkins RG. Management strategy in purpura fulminans with multiple organ failure in children. Burns. 1996 Feb. 22(1):53-6.[↩][↩][↩]
- Warner PM, Kagan RJ, Yakuboff KP, Kemalyan N, Palmieri TL, Greenhalgh DG, et al. Current management of purpura fulminans: a multicenter study. J Burn Care Rehabil. 2003 May-Jun. 24(3):119-26.[↩][↩]
- de Risi-Pugliese T, Servy A, Decousser JW, et al. Skin biopsy PCR for rapid microbiological diagnosis in patients with purpura fulminans. Br J Dermatol. 2017 Mar 30.[↩]
- Manco-Johnson MJ, Knapp-Clevenger R. Activated protein C concentrate reverses purpura fulminans in severe genetic protein C deficiency. J Pediatr Hematol Oncol. 2004 Jan. 26(1):25-7.[↩]
- Levin M, Eley BS, Louis J, Cohen H, Young L, Heyderman RS. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995 Sep. 127(3):355-63.[↩]
- Smith OP, White B. Infectious purpura fulminans: caution needed in the use of protein c. Br J Haematol. 1999 Jul. 106(1):253-4.[↩]
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O’Rourke K, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome–a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999 Apr. 28(4):800-7.[↩]
- [Guideline] Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009 Apr. 145 (1):24-33.[↩]