Refsum syndrome
Refsum syndrome also called “classic Refsum disease” or “adult Refsum disease”, is an inherited neurocutaneous syndrome that is characterized by absence of the sense of smell (anosmia) and early-onset retinitis pigmentosa that causes vision loss, which are both universal findings with variable combinations of neuropathy, deafness, ataxia, and ichthyosis 1. Retinitis pigmentosa affects the retina, the light-sensitive layer at the back of the eye. Vision loss occurs as the light-sensing cells of the retina gradually deteriorate. The first sign of retinitis pigmentosa is usually a loss of night vision, which often becomes apparent in childhood. Over a period of years, the disease disrupts side (peripheral) vision and may eventually lead to blindness.
Vision loss and anosmia are seen in almost everyone with Refsum disease, but other signs and symptoms vary. About one-third of affected individuals are born with bone abnormalities of the hands and feet. Features that appear later in life can include progressive muscle weakness and wasting; poor balance and coordination (ataxia); hearing loss; and dry, scaly skin (ichthyosis). Additionally, some people with Refsum disease develop an abnormal heart rhythm (arrhythmia) and related heart problems that can be life-threatening.
Refsum disease is characterized biochemically by the accumulation of phytanic acid in plasma and tissues 2. Patients with Refsum disease are unable to degrade phytanic acid because of a deficient activity of phytanoyl-CoA hydroxylase (PhyH), a peroxisomal enzyme catalyzing the first step of phytanic acid alpha-oxidation 2. Refsum disease can be classified as a peroxisome biogenesis disorder 2. Adult Refsum disease is inherited as an autosomal recessive trait and is characterized by altered peroxisome assembly, resulting in multiple peroxisome enzyme deficiencies, complex developmental sequelae, and progressive disabilities 3.
Infantile Refsum disease is a peroxisome biogenesis disorder 4. Infantile Refsum disease is one of the less severe of Zellweger spectrum disorders, a group of peroxisomal biogenesis disorders resulting from a generalized peroxisomal function impairment 5. Increased plasma levels of very long chain fatty acids (VLCFA) and phytanic acid are biomarkers used in infantile Refsum disease diagnosis. Furthermore, an increased plasma level of phytanic acid is known to be associated with neurologic damage. Treatment of infantile Refsum disease is symptomatic and multidisciplinary.
Onset of symptoms ranges from age seven months to older than age 50 years. Cardiac arrhythmia and heart failure caused by cardiomyopathy are potentially severe health problems that develop later in life.
Refsum disease is caused by mutations in the phytanoyl-CoA hydroxylase (PHYH) and the PTS2 receptor (PEX7) genes. This disorder is inherited in an autosomal recessive mode. A single peroxisomal enzyme defect that causes deficiency of alpha-oxidation leads to accumulation of phytanic acid in blood and tissues of patients with Refsum disease. The cytotoxic effect of phytanic acid seems to be due to a combined action of Ca2+ regulation, mitochondrial depolarization, and increased reactive oxygen species generation in brain cells.
The diagnosis of Refsum disease is suspected on the basis of clinical findings and a plasma phytanic acid concentration greater than 200 µmol/L in most affected individuals. Confirmation of the diagnosis requires either (1) molecular genetic testing to identify biallelic pathogenic variants in either PHYH (encoding phytanoyl-CoA hydroxylase), which accounts for more than 90% of Refsum disease, or PEX7 (encoding the PTS2 receptor), which accounts for less than 10% of Refsum disease; or (2) enzyme analysis to identify deficiency of either phytanoyl-CoA hydroxylase enzyme activity or the peroxisome-targeting signal type 2 receptor.
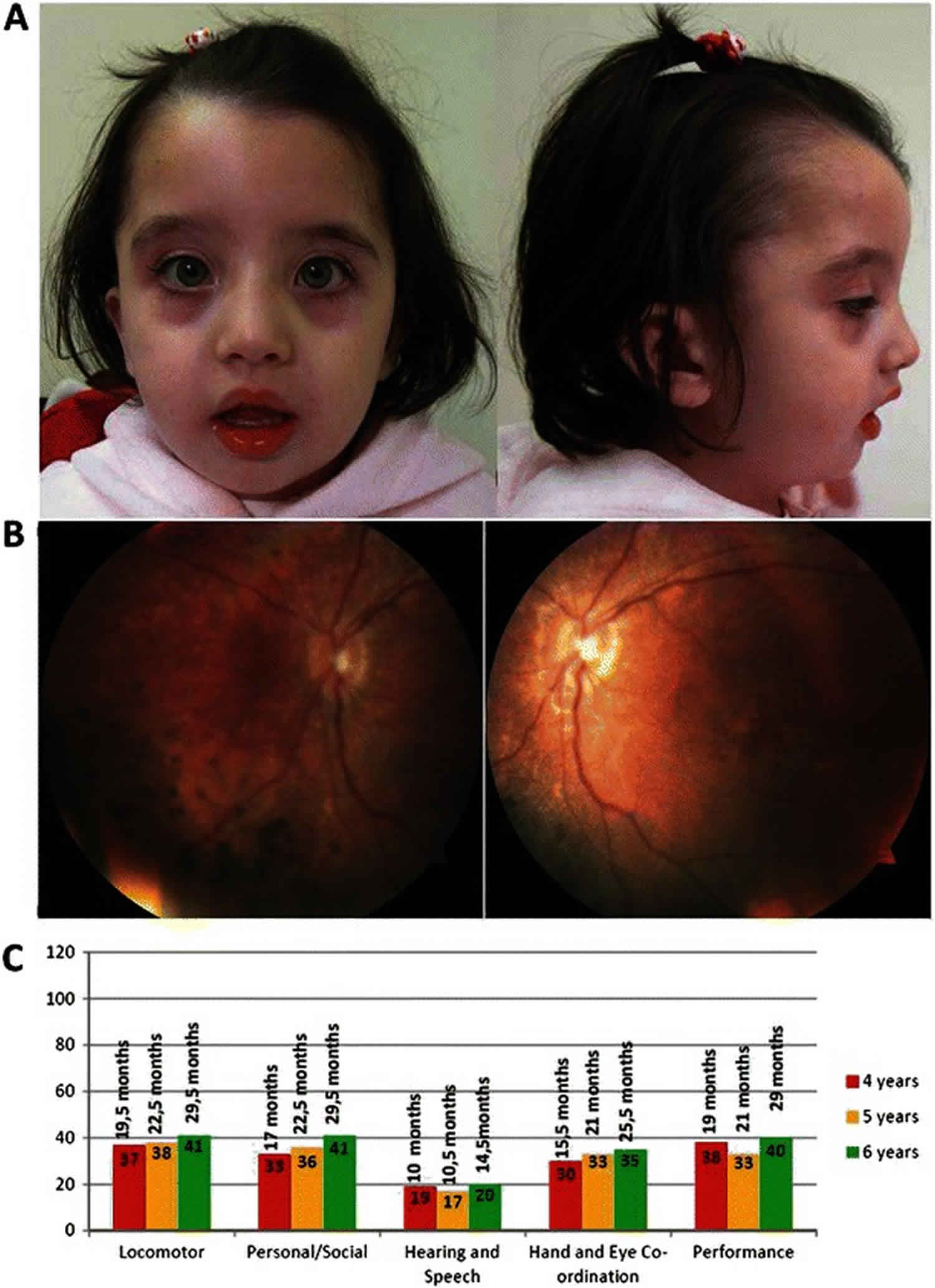
Figure 1. Infantile Refsum disease
Footnote: (a) Facial dysmorphic features observed in the proband, at 4 years old, included high forehead, absent orbital ridges, and micrognathia. (b) Bilateral retinographies, obtained at 4 years old, showing diffuse pigment epithelium changes in the retinal mid-periphery, as well as mottled appearance in the macular area, compatible with retinitis pigmentosa. (c) Performance level (age of development in months) in five subscales of the Griffiths Mental Development Scales at 4, 5 and 6 years of age.
[Source 5 ]Refsum disease causes
More than 90 percent of all cases of Refsum disease result from mutations in the phytanoyl-CoA hydroxylase (PHYH) gene. The remaining cases are caused by mutations in a gene called PTS2 receptor (PEX7).
The signs and symptoms of Refsum disease result from the abnormal buildup of a type of fatty acid called phytanic acid. This substance is obtained from the diet, particularly from beef and dairy products. It is normally broken down through a process called alpha-oxidation, which occurs in cell structures called peroxisomes. These sac-like compartments contain enzymes that process many different substances, such as fatty acids and certain toxic compounds.
Mutations in either the PHYH or PEX7 gene disrupt the usual functions of peroxisomes, including the breakdown of phytanic acid. As a result, this substance builds up in the body’s tissues. The accumulation of phytanic acid is toxic to cells, although it is unclear how an excess of this substance affects vision and smell and causes the other specific features of Refsum disease.
Refsum disease inheritance pattern
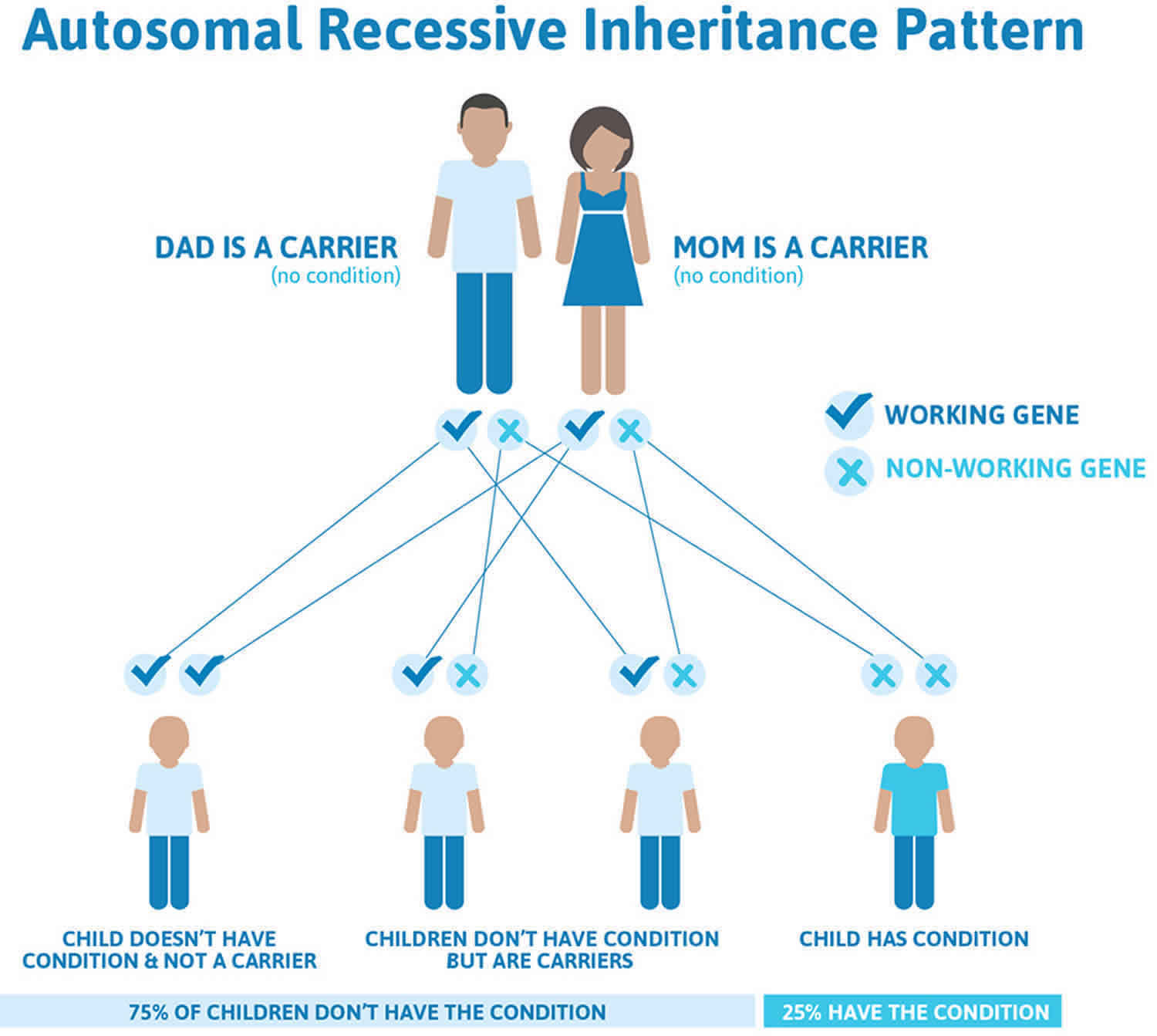
This condition is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
The parents of some individuals with Werner syndrome have been closely related by blood (consanguineous). In these cases, if both parents carry the same disease gene, there is a higher-than-normal risk that their children may inherit the two disease genes necessary for the development of the disease.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 2 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 2. Refsum disease autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Refsum disease symptoms
Refsum disease or adult Refsum disease, is suspected in individuals with late childhood-onset retinitis pigmentosa and variable combinations of the following clinical findings (listed in descending order of frequency):
- Anosmia
- Sensory motor neuropathy
- Hearing loss
- Ataxia
- Ichthyosis
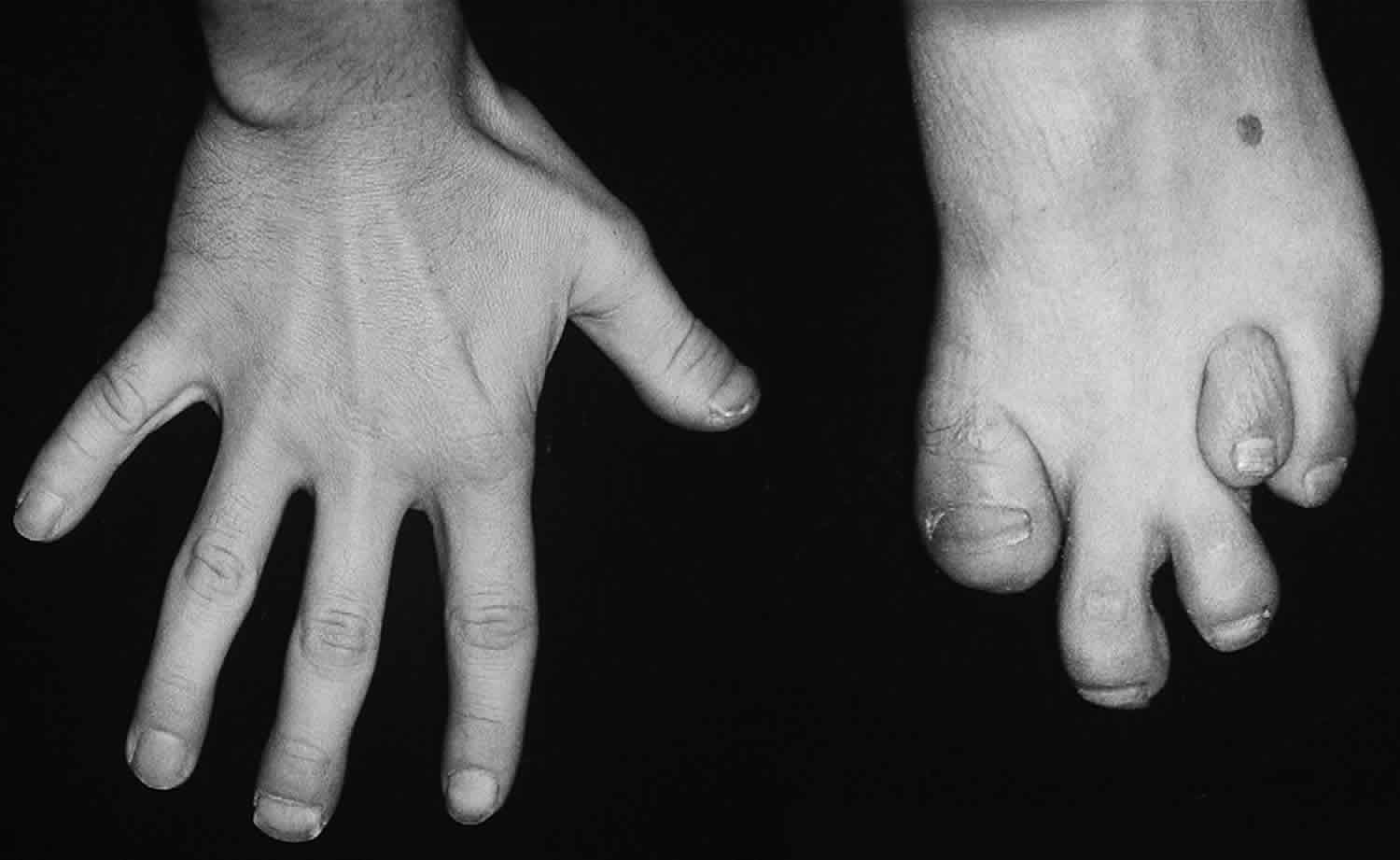
- Short metacarpals and metatarsals present from birth (~35% of individuals)
- Cardiac arrhythmias and cardiomyopathy
It should be noted that: (1) the full constellation of signs and symptoms is rarely seen in an affected individual; (2) most features develop with age.
Symptoms develop progressively and slowly with neurologic (eg, mild peripheral intermittent neuropathy, tinnitus, anosmia) and ophthalmic (eg, failing vision, night blindness as a result of progressive retinitis pigmentosa) manifestations.
Ichthyosis may accompany, but most often follows, the occurrence of the above symptoms.
Neurologic/ophthalmologic signs are as follows:
- Partial intermittent sensorimotor polyneuropathy
- Cataract
- Nystagmus
- Retinitis pigmentosa
- Anosmia
- Concentric constriction of the visual fields
- Sensorineural deafness
Signs resulting from cerebellar ataxia are as follows:
- Progressive weakness
- Foot drop
- Loss of balance
Cardiomyopathy with a serious conduction defect is a life-threatening sign 6.
Hepatic/renal symptoms are clinically silent despite fatty degeneration.
An ichthyosiform desquamation occurs, resembling a mild acquired ichthyosis vulgaris with a fine, white scaling that is noticeable over the lower trunk but also affects the limbs. Ichthyotic symptoms may range from mild hyperkeratosis of the palms and soles to severe scaling of lamellar ichthyosis type observed on the trunk.
Skeletal defects (noticed in some patients) are not related directly to phytanic acid levels. These defects occur in 35-75% of cases. The knees, elbows, and short tubular bones of the hands and feet are affected (see image below); in particular, the terminal phalanx of the thumb also is affected. Enamel defects have been described in a case report 7.
Refsum disease diagnosis
More than 90% of individuals with Refsum disease have deficiency of phytanoyl-CoA hydroxylase encoded by PHYH gene and fewer than 10% have a deficiency of the PTS2 receptor encoded by PEX7 1.
Establishing the diagnosis first requires analysis of phytanic acid concentration in plasma or serum and then either molecular genetic testing or enzyme analysis (if molecular genetic testing is not available or results are ambiguous).
In Refsum disease, the phytanic acid level in the blood is increased. The normal range is equal to or less than 0.2 mg/dL; however, phytanic acid levels are 10-50 mg/dL or even higher in patients with Refsum disease. Associated laboratory findings are summarized in Table 1. Note that alpha-methylacyl-CoA racemase (AMACR) deficiency, the major disorder in the differential diagnosis of Refsum disease, is included in Table 1 for comparison. When present, normal plasma phytanic acid levels essentially rule out the two forms of Refsum disease.
Table 1. Comparison of peroxisomal metabolites in the two forms of Refsum disease and alpha-methylacyl-CoA racemase (AMACR) deficiency
| Forms of Refsum Disease | AMACR Deficiency | Normal | ||
|---|---|---|---|---|
| Phytanoyl-CoA Hydroxylase Deficiency | PTS2 Receptor Deficiency | |||
| Gene | PHYH | PEX7 | AMACR | — |
| Plasma phytanic acid concentration 1 | >200 µmol/L 2 | >200 µmol/L 2 | >20 µmol/L | <10 µmol/L |
| Plasma pristanic acid concentration | <2 µmol/L | <2 µmol/L | >20 µmol/L | <3.0 µmol/L |
| Phytanic acid / pristanic acid ratio | Elevated | Elevated | Decreased | Normal |
| Plasma pipecolic acid concentration | Mildly elevated in 20% | Normal | Normal | Normal |
| Erythrocyte plasmalogen concentration 1 | Normal | Decreased to normal | Normal | Normal |
| Di- & trihydroxycholestanoic acid | Normal | Normal | Elevated | Normal |
Footnotes: Plasma very-long-chain fatty acids (VLCFA) are normal in all three conditions.
1. Measured by gas chromatography
2. Plasma phytanic acid concentration may vary considerably because phytanic acid intake is dependent on local diet and may be deceptively low in populations with lower intakes of saturated fatty acids and cholesterol.
Abbreviation: AMACR = α-methylacyl-CoA racemase.
Imaging Studies
Skeletal radiography is required to estimate bone changes.
MRI findings could reveal symmetrical signal changes involving the corticospinal tracts, cerebellar dentate nuclei, and corpus callosum 8.
Proton nuclear magnetic resonance (1 H-NMR) spectroscopy of blood plasma or serum lipid extracts can be used to correctly identify and quantify lipids, including unusual lipids in the blood of patients with inborn errors of lipid metabolism, which could be applicable in clinical diagnosis and follow up.
Other Tests
Phytanic oxidase activity estimation in skin fibroblast cultures is important.
Electrocardiogram (ECG) is helpful to rule out cardiac conduction defect.
UPSIT (University of Pennsylvania Smell Identification Test) reveals anosmia or grossly impaired smell function 9.
Urine acylcarnitine analysis by nuclear magnetic resonance electrospray ionization-MS/MS seems to be a new tool for the diagnosis of peroxisomal biogenesis disorders 10.
Skin biopsy
Skin biopsy shows features of ichthyosis vulgaris, such as moderate hyperkeratosis and acanthosis with thin granular layer. Variably sized vacuoles are visible in basal and suprabasal keratinocytes. The lipid stains performed on cryostat cut sections revealed presence of lipid accumulation in vacuoles. Nerve biopsy examination shows nerve demyelination with marked Schwann cell proliferation and onion bulb formation.
Ultrastructural skin examination reveals many intracellular non–membrane-bound vacuoles in the basal layer and less numerous in keratinocytes of suprabasal epidermis. Transmission electron microscopy does not reveal changes of keratohyalin observed in dominant ichthyosis vulgaris.
Refsum disease treatment
No curative therapy currently exists for Refsum disease.
The following treatment are indicated for adult Refsum disease:
- Dietary restriction of phytanic acid intake
- Avoidance of sudden weight loss
- Lifelong treatment with hydrating creams. Appropriate medications consist of skin care products (eg, keratolytics, emollients). Research has shown that enzymes responsible for the omega-hydroxylation of phytanic acid could be useful in Refsum disease treatment. Elevation of CYP4 enzymes could have a favorable role in the elimination of phytanic acid in persons with Refsum disease. Targeting CYP4 enzymes may represent a future therapeutic option 11.
- Regular care by a cardiologist for cardiac arrhythmias and cardiomyopathy in order to treat signs and symptoms properly with antiarrhythmic and cardiogenic supportive drugs
- Plasmapheresis: The main indication for plasmapheresis in patients with Refsum disease is a severe or rapidly worsening clinical condition 12. A minor indication is failure of dietary management to reduce a high plasma phytanic acid level 13. Cascade filtration may be an alternative for plasmapheresis. It is as efficient as plasmapheresis and eliminates the need for albumin replacement 14.
- Because the pupils do not dilate well if at all, other measures, such as use of iris hooks, may be necessary to allow sufficient pupillary enlargement during cataract surgery. In addition, an anterior chamber lens with iris fixation may be necessary because the brittleness of the zonular fibers holding the lens capsule may not allow positioning of an intraocular lens in the capsular bag after cataract removal, a complication observed in one patient.
By restricting dietary intake of phytanic acid or eliminating phytanic acid by plasmapheresis or lipid apheresis, plasma phytanic acid concentrations can be reduced by 50% to 70%, typically to about 100 to 300 µmol/L. This reduction in plasma phytanic acid concentration successfully resolves symptoms of ichthyosis, sensory neuropathy, and ataxia in approximately that order. However, it is uncertain whether treatment affects the progression of the retinitis pigmentosa, anosmia, and deafness 15. Although data are limited, it appears that despite strict dietary treatment the retinitis pigmentosa is very slowly progressive.
A high-calorie diet is necessary to avoid mobilization of stored lipids, including phytanic acid, into the plasma.
Postoperative care requires parenteral nutrition with solutions that do not contain phytanic acid – e.g., Intralipid® available in 10%, 20%, and 30% concentrations, which are all based on soybean oil and egg yolk phospholipid.
Refsum disease diet
Eliminate all sources of chlorophyll from the diet. The major dietary exclusions are green vegetables (source of phytanic acid) and animal fat (phytol). The aim of such dietary treatment is to reduce daily intake of phytanic acid from the usual level of 50 mg/d to less than 5 mg/d.
This change is accompanied by increased nerve conduction velocities, return of reflexes, and improvement in sensation and objective coordination.
Ichthyosis clears, and its recurrence may be a marker of rising phytanic acid level in blood.
Improvement in clinical status as a result of diet is due to the presence of alternative pathway oxidation omega-oxidation that is able to metabolize small amounts of phytanic acid.
Lifelong strict adherence to the diet is mandatory. A high carbohydrate intake should be provided to avoid a rapid weight loss as it metabolizes tissue phytanic acid. Periodic monitoring of fat-soluble vitamins, vitamin B-12, copper or selenium, and sodium should be performed 16.
Prevention of secondary complications
Treatment of hypertension may help in delaying cardiomyopathy, which inevitably leads to arrhythmias. It is probably better to avoid amiodarone as an anti-arrhythmic drug, because of the risk of hyperthyroidism, which results in catabolism and increased phytanic acid release from tissues if not recognized in time. This complication was observed in one individual.
Once cardiomyopathy has become difficult to treat, cardiac transplantation can be lifesaving. One individual with PHYH-related Refsum disease and one with PEX7-related Refsum disease have successfully received a donor heart.
Surveillance
The following are appropriate:
- Measurement of plasma phytanic acid levels every three to six months and more frequently during illnesses or increased stress that may lead to a catabolic state
- Annual ophthalmologic examination to identify vision loss resulting from cataracts, which are treatable
- Annual cardiac examination to identify cardiomyopathy and concomitant arrhythmias
Agents/Circumstances to Avoid
Avoid the following:
- All food products containing phytanic acid, such as ruminant (cow, sheep, and goat) products and certain fish (cod) products. Some nuts should also be avoided 17.
- Fasting, because stored lipids, including phytanic acid, are mobilized into the plasma
- Ibuprofen, because it is metabolized by AMACR and may interfere with the metabolism of phytanic acid
- Amiodarone because of the risk that hyperthyroidism would induce enhanced catabolism, with consequent increase of plasma phytanic acid.
Pregnancy Management
Because of the tendency for pregnancy to induce catabolism, it is extremely important to manage plasma phytanic acid concentration during pregnancy in women with Refsum disease.
Fairly rapid reduction of visual fields has been observed during the third trimester of pregnancy [BP Leroy, unpublished observations], possibly due to increased plasma phytanic acid concentration resulting from increased catabolism.
Therapies under investigation
At present, the potential of enzyme replacement therapy (ERT) similar to that for lysosomal storage diseases (e.g., Hurler syndrome [see MPS I], Fabry disease, and Gaucher disease) is under investigation. This may eventually replace dietary restrictions and plasma- or lipapheresis.
In the long run, gene therapy may be the treatment of choice, but many issues need to be resolved before it can be applied.
Search ClinicalTrials.gov (https://clinicaltrials.gov/) in the US and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/ctr-search/search) in Europe for access to information on clinical studies for a wide range of diseases and conditions.
Refsum disease prognosis
Prognosis in untreated patients generally is poor. Dysfunction of myelinated nerve fibers and the cardiac conduction system leads to central and peripheral neuropathic symptoms, impaired vision, and cardiac arrhythmias. The latter frequently are the cause of death. Reversible vestibular neuropathy has been described 18.
In early diagnosed and treated cases, phytanic acid decreases slowly, followed by improvement of the skin scaling and, to a variable degree, reversal of recent neurological signs. Retention of vision and hearing are reported. Attenuation of neurologic, ophthalmologic, and cardiac symptoms requires constant adherence to a suitable diet and plasmapheresis.
- Wanders RJA, Waterham HR, Leroy BP. Refsum Disease. 2006 Mar 20 [Updated 2015 Jun 11]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1353[↩][↩]
- Refsum disease. https://emedicine.medscape.com/article/1114720-overview[↩][↩][↩]
- Braverman NE, D’Agostino MD, Maclean GE. Peroxisome biogenesis disorders: Biological, clinical and pathophysiological perspectives. Dev Disabil Res Rev. 2013 Jun. 17(3):187-96.[↩]
- Aubourg P, Wanders R. Peroxisomal disorders. Handb Clin Neurol. 2013. 113:1593-609.[↩]
- Sá MJ, Rocha JC, Almeida MF, et al. Infantile Refsum Disease: Influence of Dietary Treatment on Plasma Phytanic Acid Levels. JIMD Rep. 2016;26:53–60. doi:10.1007/8904_2015_487 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4864718[↩][↩]
- Koh JT, Jeong BC, Kim JH, et al. Changes underlying arrhythmia in the transgenic heart overexpressing Refsum disease gene-associated protein. Biochem Biophys Res Commun. 2004 Jan 2. 313(1):156-62.[↩]
- Tran D, Greenhill W, Wilson S. Infantile refsum disease with enamel defects: a case report. Pediatr Dent. 2011 May-Jun. 33(3):266-70.[↩]
- Cakirer S, Savas MR. Infantile Refsum disease: serial evaluation with MRI. Pediatr Radiol. 2005 Feb. 35(2):212-5.[↩]
- Gibberd FB, Feher MD, Sidey MC, Wierzbicki AS. Smell testing: an additional tool for identification of adult Refsum’s disease. J Neurol Neurosurg Psychiatry. 2004 Sep. 75(9):1334-6.[↩]
- Duranti G, Boenzi S, Rizzo C, et al. Urine acylcarnitine analysis by ESI-MS/MS: a new tool for the diagnosis of peroxisomal biogenesis disorders. Clin Chim Acta. 2008 Dec. 398(1-2):86-9.[↩]
- Edson KZ, Rettie AE. CYP4 Enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid ?-hydroxylase activities. Curr Top Med Chem. 2013. 13(12):1429-40.[↩]
- Harari D, Gibberd FB, Dick JP, Sidey MC. Plasma exchange in the treatment of Refsum’s disease (heredopathia atactica polyneuritiformis). J Neurol Neurosurg Psychiatry. 1991 Jul. 54(7):614-7.[↩]
- Sá MJ, Rocha JC, Almeida MF, Carmona C, Martins E, Miranda V, et al. Infantile Refsum Disease: Influence of Dietary Treatment on Plasma Phytanic Acid Levels. JIMD Rep. 2015 Aug 25.[↩]
- Gutsche HU, Siegmund JB, Hoppmann I. Lipapheresis: an immunoglobulin-sparing treatment for Refsum’s disease. Acta Neurol Scand. 1996 Sep. 94(3):190-3.[↩]
- Gibberd FB, Wierzbicki AS. Heredopathia atactica polyneuritiformis: Refsum’s disease. In: Klockgether R, ed. Handbook of Ataxia Disorders. New York, NY: Marcel Dekker; 2000:235-56.[↩]
- Baldwin EJ, Harrington DJ, Sampson B, Feher MD, Wierzbicki AS. Safety of long-term restrictive diets for peroxisomal disorders: vitamin and trace element status of patients treated for Adult Refsum Disease. Int J Clin Pract. 2016 Mar. 70 (3):229-35.[↩]
- Brown PJ, Mei G, Gibberd FB, Burston D, Mayne PD, McClinchy JE, Sidey MC. Diet and Refsum’s disease. The determination of phytanic acid and phytol in certain foods and application of these knowledge to the choice of suitable convenience foods for patients with Refsum’s disease. J Hum Nutr Diet. 1993;6:295–305.[↩]
- Taylor RL, Jankelowitz SK, Young AS, Sullivan D, Halmagyi GM, Welgampola MS. Reversible vestibular neuropathy in adult Refsum disease. Neurology. 2018 May 8. 90 (19):890-892.[↩]