Sarcopenia
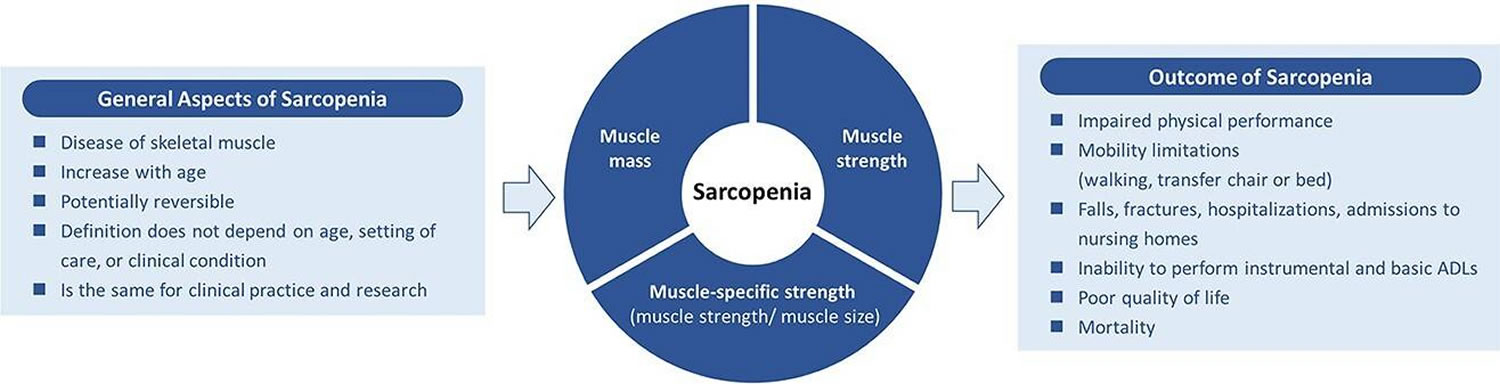
Sarcopenia is a condition characterized by loss of muscle mass, strength, and function in older adults and it can affect both under- and overweight adults 1, 2, 3, 4, 5. The term “sarcopenia” was introduced into the medical literature by Irv Rosenberg in 1995 6 . At that time “sarcopenia” was defined as an abnormal loss of muscle associated with aging and was validated in 2001 to predict functional decline 7. However, Manini and Clark in 2012 8 pointed out that it was muscle power and not muscle mass that was the predominant feature that led to loss of functional status. In 2010, the European Working Group on Sarcopenia defined sarcopenia as low muscle mass together with low muscle function (strength or performance) 9. Subsequently, other international groups developed similar definitions for sarcopenia focusing on walking speed or distance walked in 6 minute or grip strength in persons with lean muscle mass 10, 11, 12. A number of studies have confirmed the validity of these definitions and it was recently demonstrated that cutoffs for the definitions need to be ethnically sensitive 13, 14, 15, 16. Based on the available literature, it would appear that sarcopenia is present in 5 to 10% of persons 65 years of age or older 17, 18, 19. Today, the definition for “sarcopenia” lacks global and widely accepted definition that can be routinely used in clinical settings, even though an ICD10-CM (International Classification of Diseases, Tenth Revision, Clinical Modification) M62.84 code for sarcopenia exists 2, 20, 16. Although the ICD10-CM M62.84 code for sarcopenia is available in some countries such as Australia and the United States 21, the World Health Organization (WHO) has yet to include an ICD-10 code for sarcopenia in its version. A global definition of sarcopenia would ease the adoption of an ICD-10 code for sarcopenia by WHO, which, in turn, would increase clinical recognition of this condition worldwide.
The lack of a single, standard definition of sarcopenia has led to several issues, resulting in variable prevalence estimates and conflicting treatment decisions 22, 23, 2. First, research into the prevalence, incidence, and causes and consequences of sarcopenia is often difficult to harmonize, as disparate definitions can lead to widely different estimates of prevalence 24 or can identify different important consequences of sarcopenia. Second, the lack of a single definition has clinical implications because those seeing patients may be uncertain as to which measures or cut-off points to use when evaluating patients 2. Third, the lack of a unified definition for sarcopenia has impeded the development of clinical care pathways for sarcopenia 2. While there is evidence that, even in the oldest individual, non-pharmacological interventions such as resistance-based exercise can increase muscle strength 25, 26, 27 and multicomponent exercise interventions (aerobic, strength and balance/flexibility) can reduce the risk of mobility disability 28, 29, there are few specific exercise or dietary prescriptions in regular clinical use to treat sarcopenia. There is also a stymied development of pharmacological treatments for sarcopenia.
Sarcopenia is a part of normal aging, and occurs even in master athletes, although it is clearly accelerated by physical inactivity 30. Sarcopenia most commonly affects elderly and sedentary populations and in people who have comorbidities that affect the musculoskeletal system or impair physical activity 5. Sarcopenia is different from muscle loss (cachexia) caused by inflammatory disease, or from the weight loss and attendant muscle wasting caused by starva-tion or advanced disease 31. Sarcopenia is increasingly recognized as a correlate of ageing and is associated with increased likelihood of adverse outcomes including falls, fractures, frailty and mortality 32. Sarcopenia is universal with advanced age. That is, reduced muscle mass and strength are evident in all elderly persons compared to young, healthy, physically active young adults 30. If the sarcopenia progresses beyond a threshold of functional requirements, it leads to disability and frailty, and this can occur independently of any disease-induced frailty 33, 34. Sarcopenia is the most important cause of frailty in older persons 35, 36, 37, 38, 39. Superimposed illness will accelerate the loss of muscle mass, and thus increase the risk of disability, frailty, and death 30. In addition, there is a close association between sarcopenia and bone loss and hip fracture‐osteosarcopenia 40, 41. Sarcopenia has also been found to be a major reason for poor outcomes in persons with diabetes mellitus 42, 43.
Sarcopenia signs and symptoms include weakness, fatigue, loss of energy, balance problems, and trouble walking and standing. Muscle loss or weakness can lead to falls, broken bones, and other serious injuries and can affect a person’s ability to care for oneself. Older age, getting little or no exercise, and poor nutrition may increase the risk of sarcopenia. Sarcopenia may also occur in people with cancer.
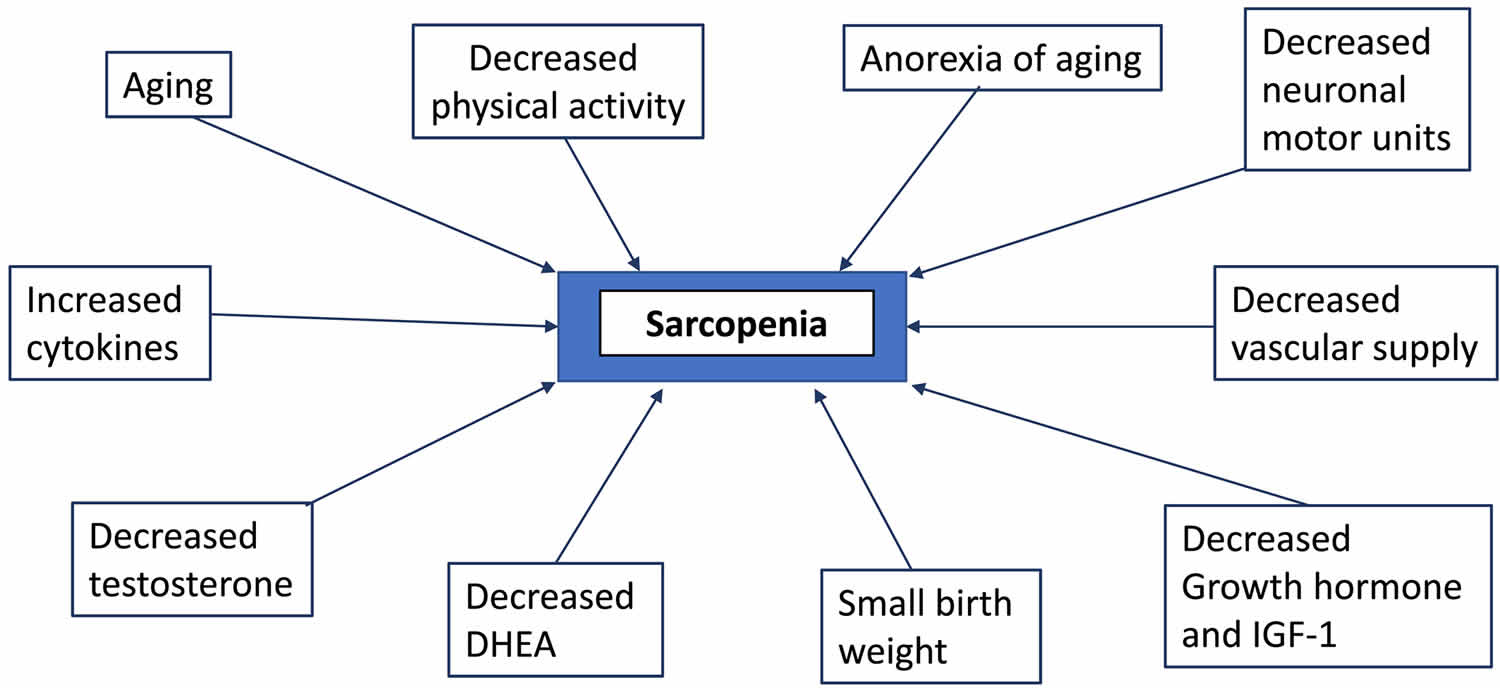
The term, sarcopenia, was first coined in 1988 by Irwin Rosenberg at a meeting in Albuquerque, New Mexico, to refer to muscle wasting of the older people 44. Its etymological origins are two Greek words: sarx for flesh and penia for reduced or deficiency. Baumgartner et al. 45 proposed an operational definition of sarcopenia in 1998. Utilizing dual energy X‐ray absorptiometry (DXA) to measure lean soft tissue, the authors defined sarcopenia as being <2 standard deviations (SDs) of appendicular muscle mass (ASM, kg) per height squared (m²) below the mean of a young reference group. Using this criterion, Baumgartner et al. 45 showed that the prevalence and the severity of sarcopenia significantly increased with age and that it was associated with physical disability. In 2002, Janssen et al. 46, using bioelectrical impedance analysis (BIA), showed that in the Third National Health and Nutrition Examination Survey (NHANES III), functional impairment was three times as likely in persons with an estimated lean mass below 2 SDs of the mean. Baumgartner et al 47, found that in older persons with obesity, those who had lost muscle mass had worse outcomes than those who had maintained their muscle mass. They coined the term ‘sarcopenic obesity’ for this condition. By the early 2000s, it was recognized that there are numerous causes of age‐related sarcopenia, including malnutrition, loss of motor units innervating muscle (α-motor neuron input), systemic inflammation-driven erosion of muscle mass, oxidative stress, decline in anabolic hormones, increased body fat and the ‘anorexia of aging’ coupled with a decrease in physical activity and disease burden are likely to culminate in sarcopena (see Figure 4 below) 48. At this stage, it was recognized that there were both primary sarcopenia (age related) and secondary sarcopenia (disease related, as with diabetes mellitus, cancer, chronic obstructive pulmonary disease, or heart failure) 49.
However, there is no absolute level of lean mass, body cell mass, or muscle mass at which one can definitely say that sarcopenia is present 30. Such a definition would be an important advance. In reaching such a definition, one should consider two important and generally agreed-upon concepts in relation to lean body mass. First, there is a direct structure ± function link between muscle mass and strength, in that more muscle generally equals greater strength and vice versa. However, the function defining the relationship between muscle loss and strength loss is not the same as that applying to muscle and strength gain 30.
Making the clinical diagnosis of sarcopenia is difficult for the following reasons. There is no absolute level of lean mass, body cell mass, or muscle mass for comparison. There is no generally accepted clinical test to diagnose sarcopenia. Finally, there is no accepted threshold of functional decline at which sarcopenia is implied. Dual-energy x-ray absorptiometry (DXA) is a well-established, low-radiation technique used to assess body composition and provides reproducible estimates of appendicular skeletal lean mass 50. It is acknowledged that the accuracy of DXA for assessing muscle mass in people of different ages and different pathological conditions may vary. Moreover, DXA (in contrast to CT-scan and MRI) cannot assess intra-muscular fat, which turns out to be of increasing importance in terms of the quality of muscle and associations with clinical outcomes. Bearing these limitations in mind, DXA is still considered as the procedure of choice for routine clinical assessment 32. Using DXA, appendicular skeletal lean mass (ASM) is measured as the sum of the non-bone and non-fat mass of the four limbs. To adjust for body size, a skeletal muscle index (SMI) is derived as ASM/height². Thresholds of skeletal muscle index (SMI) at two standard deviations (SDs) below the mean skeletal muscle index (SMI) of young male and female reference groups have been proposed as gender-specific cut-off points for sarcopenia. This results in two thresholds, proposed by the European Working Group on Sarcopenia (EWGSOP) 51, the first of 5.5 kg/m² for women and 7.26 kg/m² 52 for men and the second of 5.67 kg/m² for women and 7.25 kg/m² for men 53, depending on the reference group on which these cut-off have been established. Using a different approach, the Function NIH sarcopenia project 54 has also recently defined cut-offs for appendicular lean mass adjusted for body mass index (BMI), giving values of < 0.512 for women and < 0.789 for men. However, it should be pointed that these cut-offs might also be modified according to ethnicity 55.
Bio-electrical impedance analysis (BIA) is a method which estimates the volume of fat and lean body mass based on the relationship between the volume of a conductor and its electrical resistance. The method is not expensive, requires no specialized staff and is relatively easy to use in clinical practice, both on ambulatory subjects or on hospitalized patients. Moreover, reference values have been established for older individuals 51. Even if the method’s accuracy has been challenged and has been reported to overestimate muscle mass and underestimate fat mass 56, it is possible to use some adjustment equations to obtain valid measurements 57.
Possible therapeutic strategies include increased protein intake and aggressive resistance-based exercise programs, but long-term randomized controlled trials are needed to evaluate the efficacy of these modalities. Hormonal supplementation may help if levels are low. Countermeasures should have the goals of maintaining adequate total body mass and protein intake. Physical activity incorporating resistance training is probably the most effective countermeasure to sarcopenia.
Pharmacologic interventions such as growth hormone or testosterone, which increase lean mass (and evidently muscle mass as well) do not alter strength much, while progressive resistance training, which causes large increases in strength, can do so with little evident muscle hypertrophy, at least in the first few months of training 30. For example, the strength and vastus lateralis area (measured by CT scan) lost over a 12 years follow-up period in seven healthy men, and the amount gained during a 12 week period of intensive resistance training 58. It is clear that there is hysteresis in this system: the decline and the regain occur at vastly different rates. Thus, defining sarcopenia solely on compositional terms may be useful and attractive for research purposes, but it may be simplistic in explaining functional changes caused by treatment of sarcopenia 30.
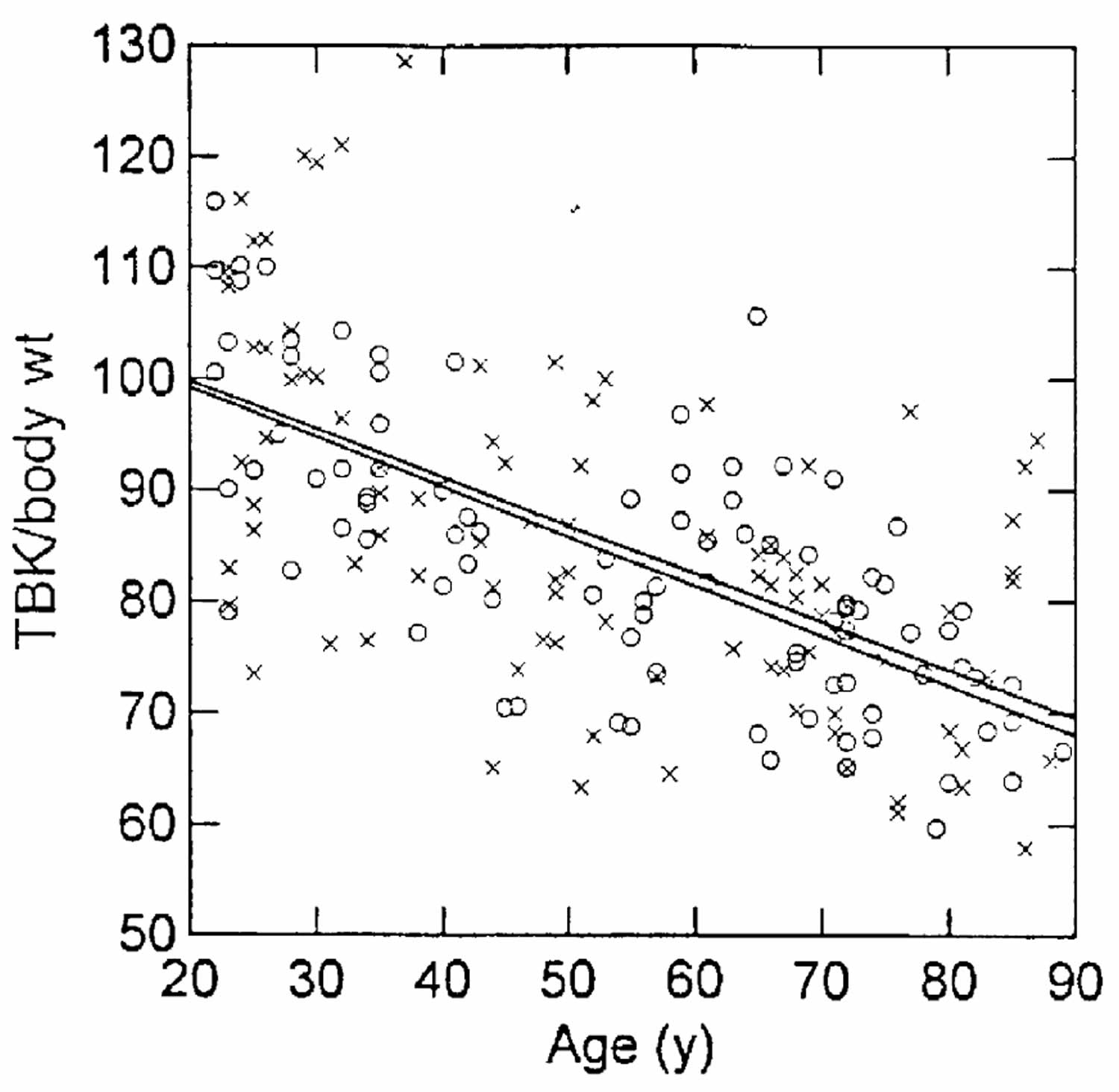
Second, there is reasonable evidence that there is a limit on how much lean body mass can be lost before death supervenes. The available data, based on starvation 59, AIDS patients 60, and critical illness 61, suggest that loss of more than about 40% of baseline lean mass is fatal. `Baseline’ is a slippery concept here, because again absolute mass is not explanatory – basketball players do not necessarily outlive jockeys, but rather the amount of loss as a function of the baseline mass that the individual started with. Reference Man and Woman are one benchmark, based on a few cadaver studies in generally healthy persons 62. Kehayias et al 63 defined baseline as the mean for adults aged 20 ± 30 years; no healthy subjects were found below approximately 70% of that standard, and there was a steady decline in body cell mass for both men and women across age groups between 30 and 100 years (see Figure 2).
The latter point also raises the issue of the importance of sarcopenia as an indicator of reduced protein stores for times of stress 30. It is well accepted that during illness, gluconeogenesis increases in importance, while ketogenesis is relatively suppressed, so that protein is burned for energy in excess of the levels seen in starvation adaptation 30. Given the anorexia caused by acute illness, and by the iatrogenic limitation on dietary intake that often obtains in hospitals, endogenous protein stores are crucial in determining the availability of metabolic substrate to cope with the illness, and thus the ability to survive it. Therefore, it is no wonder that elderly, sarcopenic patients fare worse than young, healthy adults for almost all diseases. Tellado et al 61 have shown that measurement of body cell mass was the only independent determinant of survival in intensive care unit patients in multivariate analysis, removing the significance of univariate predictors such as albumin, age, and even diagnosis. Thus, the metabolic significance of sarcopenia in illness should be considered independently of its functional impact during times of better health, as both are important to the survival and well-being of elderly persons.
Figure 1. Sarcopenia definition (conceptual definition of sarcopenia)
[Source 2 ]Figure 2. Lean body mass
Footnote: Body cell mass or lean body mass, measured as total body potassium (TBK) per kg body weight, as a function of age in a cross-sectional study. Data are expressed as a percentage of the reference 20 ± 30-year-old groups for men (O) and women (x) separately.
[Source 63 ]Primary sarcopenia (sarcopenia of aging)
In 2010, the European Working Group on Sarcopenia for Older Persons 64 recommended a new operational definition of sarcopenia of aging, i.e. the presence of low muscle mass together with low muscle function (strength or performance). Over the last decade, numerous other consensus groups have agreed to this revision to the meaning of sarcopenia of aging 65. However, these groups all used different cut‐offs to define sarcopenia of aging, highlighting the fact that different cut‐offs are necessary for different ethnic groups 66.
Towards the end of last year, two consensus articles on sarcopenia of aging were published. One was an update by the European Working Group on Sarcopenia (EWGSOP2) 67 and the other was on the management of sarcopenia of aging by the International Clinical Practice Guidelines for Sarcopenia (ICFSR) 68. The European Working Group on Sarcopenia (EWGSOP2) requires low muscle strength as a key characteristic of low muscle quality and the presence of low muscle quantity to confirm the diagnosis. If a person also has functional impairment, confirmed with a physical performance measure 69, this is characterized as severe sarcopenia. The authors recommended measuring muscle strength with either grip strength or the chair stand test. Muscle mass can be measured by DXA, magnetic resonance imaging, or computed tomography. Either gait speed, the Short Physical Performance Battery (SPPB), the Timed Up and Go test, or the 400‐m‐walk can be used for the assessment of physical performance. The Short Physical Performance Battery (SPPB) takes about 10 min to complete 70. Participants presenting a score ≤8 points have been described as having a poor physical performance 51.
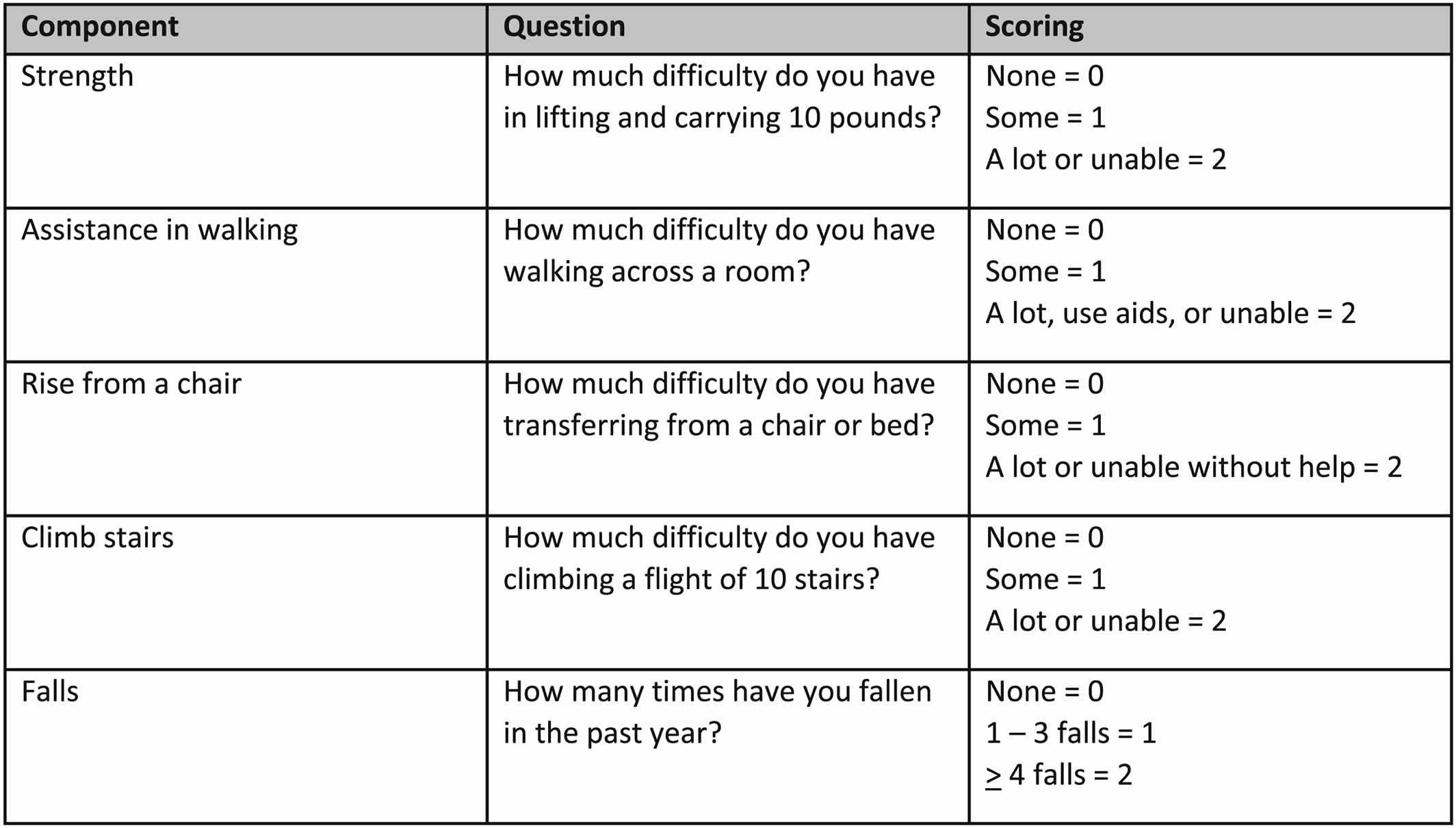
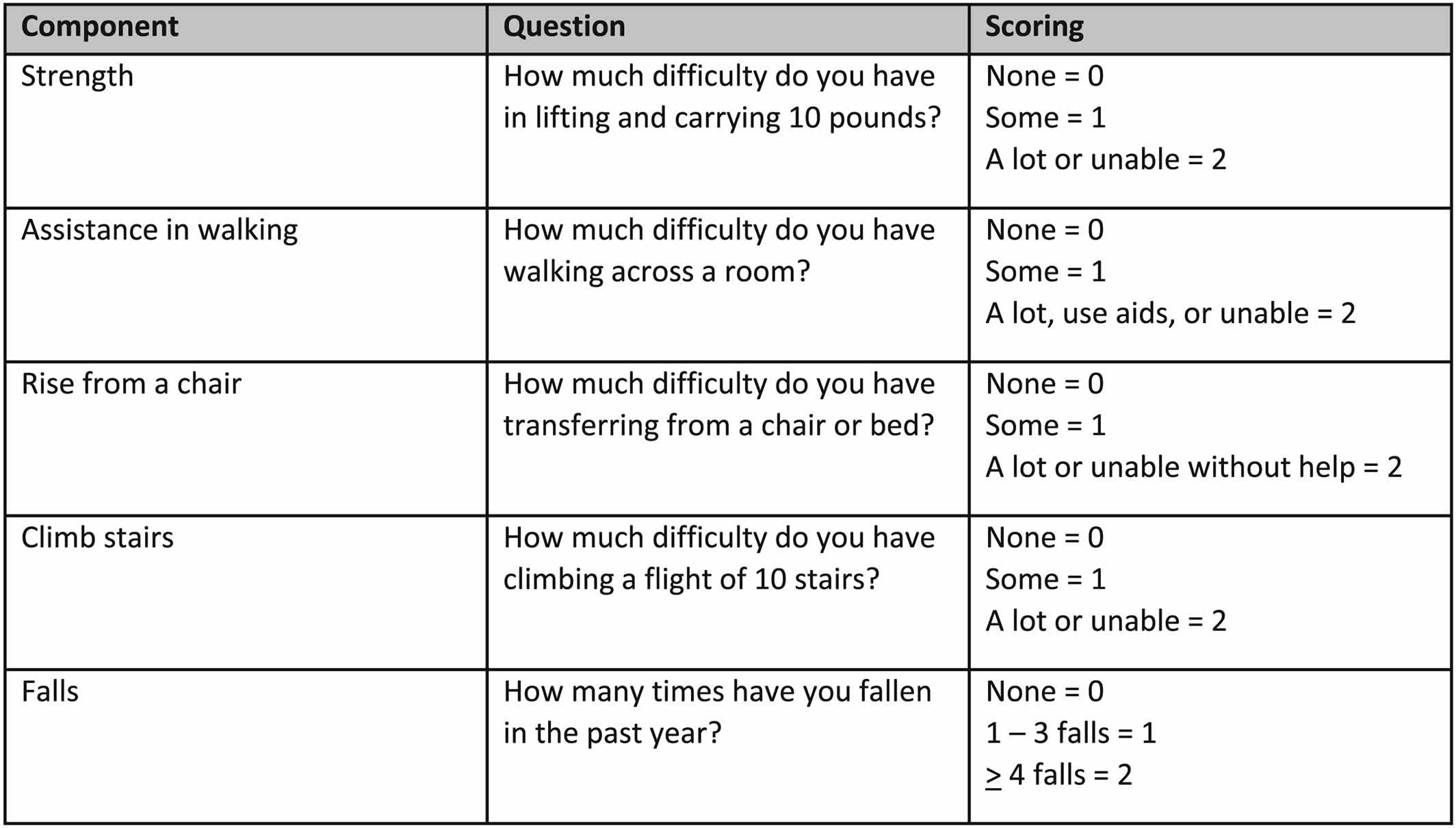
Recognizing the limited time available during a typical visit to a health care professional, the European Working Group on Sarcopenia also suggested that case finding should be used to identify older persons at risk for sarcopenia. They recommended the use of clinical symptoms usually associated with sarcopenia or the SARC‐F (Figure 2), a questionnaire with five questions, which has high specificity, albeit low sensitivity, to identify persons with sarcopenia 71. The SARC‐F has been translated into multiple languages. The SARC‐F is also recommended by the International Clinical Practice Guidelines for Sarcopenia (ICFSR) for screening 68. The specificity of the SARC‐F can be improved by measuring calf circumference as well 72. The Ishii screening test (age, grip strength, and calf circumference) is recommended as an alternative screening test 73. However, this already includes grip strength, which is a core measure of sarcopenia.
While bioelectrical impedance analysis (BIA) was not strongly supported by the European Working Group on Sarcopenia, to measure muscle mass, they recognize that its portability, affordability, and availability make bioelectrical impedance analysis (BIA) a feasible tool to estimate muscle mass in many care settings. Ultrasound of muscle such as the quadriceps is emerging as a potential tool to measure muscle quantity and, because it excludes intermuscular adipose tissue from the measurement, also muscle quality 74; a protocol for using ultrasounds in sarcopenia has recently been proposed by the European Geriatric Medicine Society 75.
There is increasing evidence that creatine dilution, implemented by ingesting a dose of the deuterium labelled isotope, may also offer an accurate approach for measuring muscle mass 76. Studies so far suggest creatine dilution estimates of muscle mass may have good correlations with functional outcomes 77. Nonetheless, its relevance and practicality in clinical settings remain to be determined.
The International Clinical Practice Guidelines for Sarcopenia (ICFSR) consensus made similar conditional recommendations utilizing the strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire for screening and applying either the original European Working Group on Sarcopenia or Foundation for NIH diagnostic criteria 68.
Figure 3. Sarcopenia questionnaire (SARC‐F questionnaire includes scoring)
Secondary sarcopenia
Malignant disease has been the most studied secondary sarcopenia, and international consensus definitions specific to cancer sarcopenia 78 are predicated on disease specific outcomes: mortality, complications of cancer surgery, and chemotherapy toxicity. Whether this secondary sarcopenia should be considered early cachexia or sarcopenia remains controversial 79, but it is becoming clearer that sarcopenia is only one of the different features of muscle changes during cancer cachexia. Owing to the prevalent use of computed tomography imaging in cancer diagnosis and follow‐up, secondary analysis of oncologic imaging for skeletal muscle cross‐sectional areas or volumes is the current standard for the quantification of muscle mass in this domain.
There are several points of relevance regarding age‐related and disease‐related loss of muscle mass. Loss of muscle mass with age occurs in a continuous fashion after reaching peak muscle mass in young adulthood (at about 30 years of age). A variety of longitudinal observational studies provide information on the rate of muscle loss per decade. The percentage loss of appendicular muscle mass per 10 years is of the order of ~5% in men and is usually reported to be somewhat lower in women. Chronic illness‐related muscle loss is also progressive; however, these are non‐linear and of a considerably greater magnitude than the values seen in aging. For example, cancers of advanced stage induce muscle loss over time that take an exponential course 80 with increasing intensity according to the disease progression, varying from 2% per 100 days to 15% per 100 days. Total cumulative loss in 12 months in colon cancer patients was 15.6%, equivalent to circa 30 years of aging 80; this is partially disease‐related but also in part a consequence of cancer surgery or systemic antineoplastic therapies which induce punctate short‐term losses. Acute illness requiring hospitalization is associated with even higher intensity of muscle loss than in cancer. In elective hip replacement surgery during an average of 5.6 ± 0.3 days of hospitalization, associated with significant decline in quadriceps (−3.4 ± 1.0%) and thigh muscle cross‐sectional area (CSA) (−4.2 ± 1.1%) in the non‐operated leg aging 81. This could be in part due to bed rest as 5 days of one‐legged knee immobilization using a full leg cast resulted in decline of quadriceps muscle cross‐sectional area from baseline of 3.5 ± 0.5%. Acute sarcopenia secondary to hospitalization or chronic disease exacerbations may be partially recoverable or may lead to heightened risk of developing sarcopenia at a young age 82.
Sarcopenia causes
Causes of sarcopenia are generally attributable to the natural processes of aging, which are not entirely understood and are multifactorial 10. Factors contributing to the development of sarcopenia include decreased Type 2 Fast-Twitch muscle fiber size and number and a decline in satellite cells 83, inactivity, obesity, insulin resistance, reduced androgen and growth factor serum concentrations, inadequate protein intake, and a blunted muscle protein synthesis response to protein meals or resistance exercise 84, 85, 86, 87, 88, 89, 90. A major component of muscle loss with aging is due to a loss of motor units innervating muscle 91. Over the lifespan there is a loss of approximately 25% of motor neurons innervating Type 2 Fast-Twitch muscle fibers 92. Damage to motor units can be detected by measuring
circulating C-terminal agrin fragment (CAF) 93. The accelerated loss of muscle mass that occurs in persons with diabetes mellitus is due to the decreased muscle innervation coupled with decreased blood flow to muscle 42, 94, 95.
Additionally, sarcopenia is associated with and may, in part, be caused by several chronic diseases that negatively affect the musculoskeletal system and physical activity 10. These include chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), chronic kidney disease (CKD), diabetes mellitus (DM), human immunodeficiency virus (HIV), and cancer 96, 97, 98, 99, 100, 101. Theoretically, the role of these disease processes on sarcopenia may exert themselves primarily through direct effects on muscle function, or secondarily through retardation of physical activity or caloric restriction 102. It should be noted that in the context of cancer, cachexia is more commonly assessed as an indicator of tissue loss than is sarcopenia, though the clinical presentations of both diseases demonstrate significant overlap 103.
Figure 4. Sarcopenia causes
Sarcopenia Pathophysiology
Generally, a significant decline of type 2 Fast-Twitch muscle fiber, but not type 1 Slow-Twitch muscle fibers are observed in sarcopenic patients 104. Muscles atrophy and are replaced by connective tissue, though the mechanism in sarcopenia may be different than that seen in other settings of “muscle atrophy”, since in younger individuals there is not an obvious problem with the satellite cells.
Several mechanisms of the underlying pathophysiology of sarcopenia have been described 5:
- Age-related declines in anabolic hormone serum concentrations: Normal physiological serum levels of anabolic hormones such as testosterone, human growth hormone (hGH), and insulin-like growth factor-1 (IGF-1) have been demonstrated to function in the development, maintenance, or rejuvenation of muscle tissue 105, 106, 107, 108. Age-related declines of anabolic hormones are observed in patients with sarcopenia and thus, support this underlying pathophysiology of sarcopenia 109.
- Insulin resistance with “sarcopenic obesity”: Aging patients often experience changes in body composition represented by increased fatty tissue alongside decreased muscle mass, coined as “sarcopenic obesity” 110. These changes are associated with metabolic dysfunction, including insulin resistance, leading to the accumulation of fat around internal organs 111. Additionally, insulin resistance is inversely associated with skeletal muscle mass 112. Such pathophysiology is likely mediated via dysfunction of insulin’s exerted effects on skeletal muscle – insulin resistance impairs the anti-proteolytic and muscle protein synthesis enhancing properties of the hormone on skeletal muscle tissue 113. Similarly, diminished lean body mass reduces uptake of glucose into skeletal muscle, further propagating insulin resistance 114, 115.
- Age-related neurodegeneration: Progressive neurodegeneration is a commonly observed phenomenon in aging populations 116, 91. Aging is accompanied by a decline of alpha motor neurons in the spinal cord, loss of peripheral nerve fibers, and reduced number of neuromuscular junctions 109, 117. Considering the role of the neurological system in muscle fiber recruitment, current evidence supports neurodegeneration as underlying pathophysiology for reduced muscle strength and size in sarcopenia 117.
- Age-related increase in inflammatory markers: Elevated levels of C-reactive protein (CRP), tumor necrosis factor-alpha (TNF), interleukin (IL)-6, and IL-1 (IL1-Ra) are observed in elderly populations with significant sarcopenia 118. The catabolic effects that may be exerted by these cytokines on skeletal muscle are well documented and may present a mechanism in which sarcopenia develops with age 119, 120, 121.
Decreased protein intake in the elderly also plays a role: 1/3 of men over the age of 60 eat less than the recommended dietary allowance (RDA) of 0.8 g/kg. A decline in exercise, a potent stimulus to protein synthesis, also contributes.
Katherine Tucker, professor and chair of the Department of Health Sciences at Northeastern University, explained that individuals’ dietary needs change with aging 122. Older adults may require less energy, experience less efficient absorption and utilization of many nutrients, and have different nutrient requirements due to chronic conditions and medications. These changes result in older adults needing a nutrient-dense diet. Unfortunately, it can be challenging for this population to obtain such a nutrient-dense diet because it involves overcoming barriers such as loss of appetite, changes in taste and smell, oral health decline, mobility constraints, and lower incomes.
Tucker concluded her presentation by recommending some dietary changes based on the available data. Older adults should be encouraged to eat:
- more fruits and vegetables, especially orange and dark green vegetables, to increase intakes of vitamin C, carotenoids, folate, vitamin B6, magnesium, potassium, and dietary fiber;
- more low-fat dairy to improve intakes of magnesium, calcium, potassium, and vitamins B12 and D;
- more whole grains, including more fortified breakfast cereals, to increase intakes of vitamin B6, crystalline vitamin B12, magnesium, and dietary fiber;
- fewer foods high in sugar, solid fats, sodium; and
- fewer refined grains.
Inadequate Intake
Data from the 2003–2004 the National Health and Nutrition Examination Survey (NHANES) were used by the Institute of Medicine Committee to Review Child and Adult Care Food Program Meal Requirements to identify the prevalence of inadequacy of protein and select nutrients among adults 60 years and older (see Table 1) 123. Using NHANES data for adults 70 years and older, Lichtenstein and colleagues 124 reported calcium, potassium, fiber, and vitamins D, E, and K as shortfall nutrients. In addition to those nutrients, Tucker emphasized the importance of adequate protein intake for prevention of sarcopenia and noted the controversy regarding the current recommendation for protein intake among older adults; should it be the same as the recommendation for younger adults or should it be higher?
Table 1. Estimated Prevalence of Inadequacy (%) of Protein and Selected Vitamins and Minerals Among Adults ≥ 60 Years Based on Usual Nutrient Intakes from NHANES 2003–2004
| Nutrient | Males | Females |
|---|---|---|
| Protein | 12 | 20 |
| Vitamin A | 54 | 43 |
| Vitamin C | 49 | 40 |
| Vitamin E | 92 | 98 |
| Thiamin | 6 | 12 |
| Riboflavin | 2.8 | 3.7 |
| Niacin | 1.8 | 4.6 |
| Vitamin B6 | 19 | 39 |
| Folate | 11 | 24 |
| Vitamin B12 | 2.4 | 9 |
| Phosphorus | 1.2 | 4.8 |
| Magnesium | 78 | 73 |
| Iron | 1 | 1.5 |
| Zinc | 26 | 21 |
Footnote: All nutrients in this table have an Estimated Average Requirement (EAR).
[Source 123 ]Tucker highlighted several nutrients of concern in the older adult population.
- Protein. The current Estimated Average Requirement for protein for all adults 19 years and older is 0.66 g/kg/day; however, Tucker indicated that a moderately higher protein intake (1.0–1.3 g/kg/day) may be required for older adults to maintain nitrogen balance due to decreased efficiency of protein synthesis and impaired insulin action. Need for increased protein intake is further supported by the Health, Aging, and Body Composition Study, which found that older adults with the highest intake of protein lost less lean body mass than those with lower protein intakes 125. However, there is some concern that higher protein intake may increase risk of toxicity or impaired renal function 126.
- Vitamin E. Vitamin E is important because of its role as an antioxidant and in immune function. There is some controversy over whether the current Recommended Daily Allowance (RDA), 15 mg of α-tocopherol, is too high, as very few individuals are able to meet this recommendation from diet alone. Vitamin E supplements increase α-tocopherol levels while reducing γ-tocopherol, so supplements may not be the healthiest option for increasing intake. Some literature suggests that other tocopherols (found in nuts, seeds, and plant oils) are also important 127; however, there are no current nutrient recommendations for other forms of vitamin E.
- Vitamin B12. Although the daily intake of total vitamin B12 does not appear to be low for most older adults, dietary intake data may underestimate the number of people who are vitamin B12 deficient given that atrophic gastritis and loss of stomach acid prevent some older adults from absorbing it. As a result, the Institute of Medicine 123 recommended that older adults get their vitamin B12 in crystalline form such as from fortified foods or supplements. The Framingham Offspring Study found that nonsupplement users had a higher prevalence of low B12 (less than 250 μmol/L) than those who were taking a supplement containing vitamin B12 128. Vitamin B12 deficiency can lead to peripheral neuropathy, balance disturbances, cognitive disturbances, physical disability, and increased risk of heart disease from high homocysteine. Tucker stated, “It’s critical that more attention be given to this important nutrient as many of these symptoms are nonspecific and not always diagnosed correctly” 128.
- Vitamin B6. Vitamin B6 is important for numerous metabolic reactions and health outcomes. Inadequacy may lead to high homocysteine and impaired immune function and has been associated with impaired cognitive function and depression. Data from the Massachusetts Hispanic Elders Study showed that 30 percent of Hispanics and 28 percent of non-Hispanic whites had plasma pyridoxal 5′-phosphate (the active form of vitamin B6 used as a biomarker for vitamin B6 status) concentrations less than 30 nmol/L (indicator of inadequate status), and 11 percent of Hispanics and 16 percent of nonwhite Hispanics had concentrations less than 20 nmol/L (clinical cutoff level indicating deficient concentrations). Furthermore, pyridoxal 5′-phosphate was associated with depressive symptomatology in this population-based study of older adults 129.
- Omega-3 fatty acids. Among adults 60 years and older, the median intake of α-linolenic acid by women was above the Adequate Intake (AI), whereas the median intake by men was not 123. Omega-3 fatty acids are associated with protection against heart disease, diabetes, and cognitive decline. Low intake may be partially due to the limited sources in the diet (e.g., fatty fish, flax seeds, and walnuts).
- Dietary fiber. Fiber is important for intestinal health and protection against heart disease and metabolic syndrome; however, the median intakes of neither men nor women 60 years and older meet the AI 123.
- Vitamin D. Tucker reported that older adults’ poor vitamin D intake and status may be due to low intakes of fortified dairy foods and fatty fish, low sun exposure, reduced dermal synthesis of vitamin D3 130, and decreased capacity of kidneys to convert 25OHD into 1,25-OH2-D. A study of homebound older adults found that about 65 percent had suboptimal concentrations of 25OHD in their blood (less than 50 nmol/L) and 48 percent had intakes below 400 International Units 131. In addition to its importance to bone status, vitamin D deficiency has been associated with neurological conditions, diabetes, and other metabolic conditions. Increasingly, more nutritionists are recommending that older adults take a vitamin D supplement.
Excessive intakes
Excessive intake of some nutrients is also a concern among older adults as it is for the general population.
- Sodium. The Tolerable Upper Intake Level for sodium is 2.3 g/day; however, the 2015 Dietary Guidelines Advisory Committee recommended it should be lowered to 1.5 g/day 132 to reduce the risk of hypertension and heart disease. Men and women over the age of 70 years are exceeding both recommendations; the usual daily mean intake for men and women is 3.0 and 2.4 g, respectively 133.
- Saturated fat. The Dietary Guidelines for American’s recommendation for saturated fat intake is less than 10 percent of energy intake, with the goal of reducing that recommendation to 7 percent 132. However, most adults have intakes greater than 10 percent of their energy intake 134.
- Folic acid. Whereas some adults do not meet the recommended intake levels of folic acid (400 μg), research shows that others are at risk of exceeding the upper level of 1,000 μg per day due to intake of fortified flour and breakfast cereals, and supplement use. More research is needed but high folic acid may contribute to the progression of neurological diseases associated with vitamin B12 deficiency 135 and lead to increased risk of some cancers 136.
Food intakes
In order to determine why older adults’ nutrient intakes are inadequate, one must review their food intake patterns. The 2011 IOM report Child and Adult Care Food Programs: Aligning Dietary Guidance for All presented the mean daily food group intakes by adults ages 60 years and older as compared to the 2,000-calorie MyPyramid food group pattern. It showed that older adults are not meeting any of the MyPyramid food group recommendations and are exceeding the recommendations for daily intake of solid fats and added sugar (see Table 2).
Table 2. 2,000-Calorie MyPyramid Food Group Pattern and Mean Daily Amounts Consumed by Adults ≥ 60 Years of Age
| Food Group or Component | ≥ 19 Years | ≥ 60 Years |
|---|---|---|
| 2,000-kcal Pattern 137 | Mean Intake 134 | |
| Total fruit (cup eq) | 2 | 1.1 |
| Total vegetables (cup eq) | 2.5 | 1.7 |
| Whole grains (oz eq) | 3 | 0.86 |
| Total milk group (8 fl oz eq) | 3 | 1.3 |
| SoFAS (kcal) | 267 | 570 |
Abbreviations: eq = equivalent; fl = fluid; kcal = calories; oz = ounce; SoFAS = solid fats and and sugar.
[Source 123 ]Sarcopenia prevention
Sarcopenia prevention include the following 20:
- A healthy lifestyle, including balanced diet, adequate protein intake, and regular exercise should be encouraged in adults of all ages.
- Person‐centred physical and dietary interventions, developed with an accredited healthcare professional, are recommended for adults with health conditions known as likely to increase the risk of sarcopenia, such as frailty. It is noteworthy that anterior thigh muscles undergo atrophy earlier with ageing, and this issue is paramount for the prevention of impairments and interventions 138, 139, 140, 141. Hence, in the earlier stages, the loss of anterior thigh muscle function (e.g. mobility, sitting to standing, climbing stairs) may precede those of the other sites. Furthermore, physical activity is a powerful factor in the prevention and treatment of many health conditions in older adults 142.
Sarcopenia signs and symptoms
Sarcopenia is characterized by decreased muscle mass, strength, and physical performance in older adults 5. People with sarcopenia are often elderly, sedentary, and may present with various comorbidities or disabilities, with a subsequent decrease in function and quality of life. A patient history consistent with sarcopenia includes progressive loss of muscle mass, strength, and function to the extent that daily activities become increasingly difficult to accomplish.
Sarcopenia complications
- Fall, fracture, and nosocomial infection risk in the elderly: In a meta-analysis of 33 studies encompassing 45,926 patients, Yeung et al. 143 found that sarcopenia significantly increases both fall and fracture risk in the elderly. Additionally, sarcopenia increases the risk of nosocomial infection in elderly populations 144.
- Sarcopenia complications in medicine: Sarcopenia is associated with increased mortality in patients with end-stage renal disease (chronic kidney disease), pancreatic cancer, and chronic heart failure 145, 146. Additionally, sarcopenia is associated with increased dose-limiting toxicities (DLT) in patients undergoing chemotherapy for kidney cancer (renal cell carcinoma), liver cancer (hepatocellular carcinoma) and breast cancer 147, 148, 149
- Sarcopenia complications in surgery: Sarcopenia is associated with increased postoperative complication risk in patients undergoing general surgical procedures and liver transplant surgery 150, 151. Additionally, sarcopenia is associated with greater mortality in patients undergoing general surgical procedures and colorectal surgery 150, 152.
Sarcopenia diagnosis
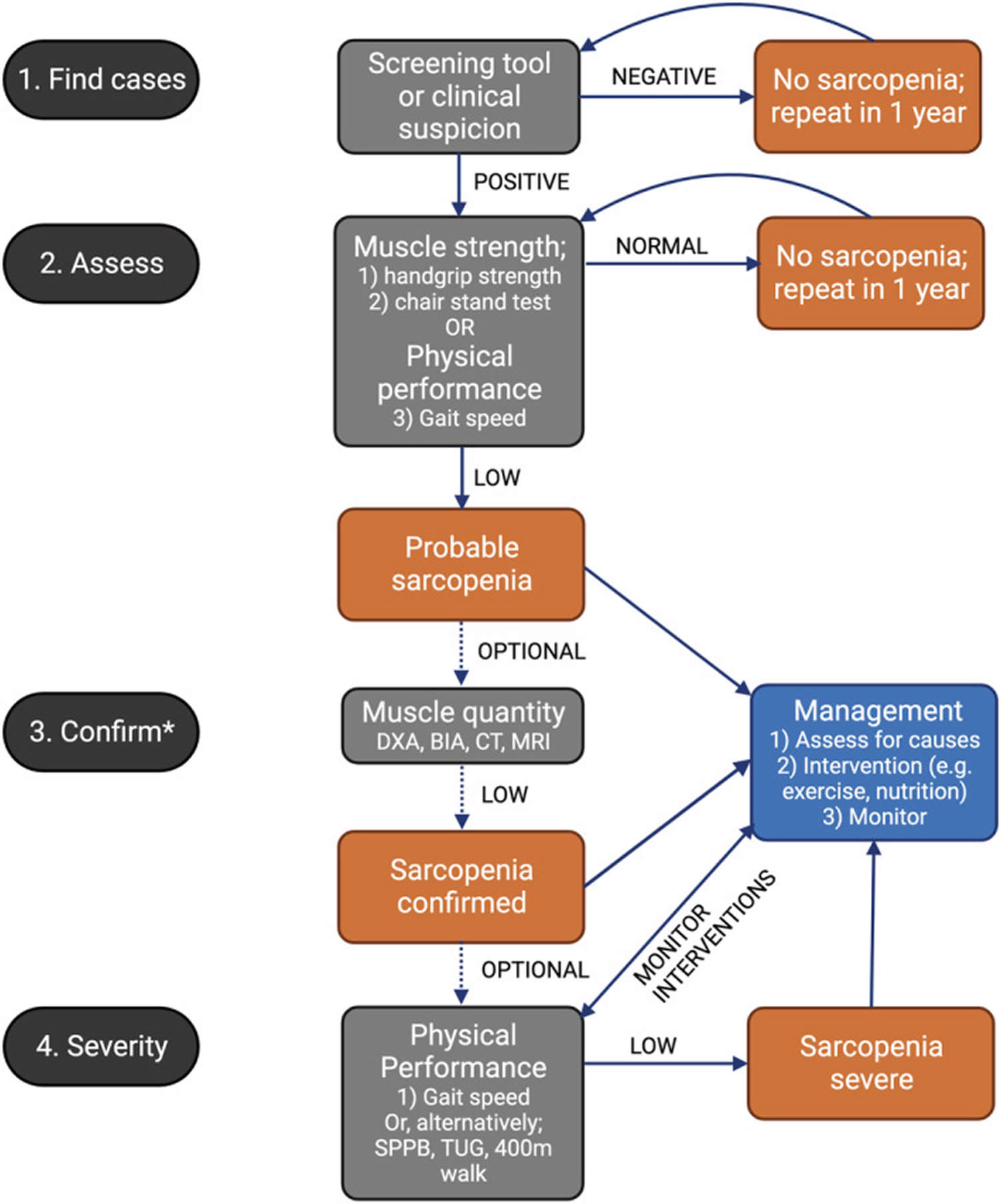
Sarcopenia diagnosis includes several modalities and screening tools, some of which are more readily available and practical than others. Sarcopenia evaluation ranges from screening questionnaires to radiographic imaging to the assessment of muscle mass cross-sectional area (CSA) 4, 153. Additionally, the European working group on sarcopenia in older people 2 (EWGSOP2) describes an algorithm that presents as follows: “find-assess-confirm-severity” or F-A-C-S 4.
Figure 5. Sarcopenia diagnostic algorithm
Abbreviations: BIA = bioelectrical impedance analysis; CT = computed tomography; DXA = dual energy X‐ray absorptiometry; MRI = magnetic resonance imaging; SPPB = Short Physical Performance Battery; TUG = Timed‐Up‐And‐Go test over 3 meters away.
Footnote: The recommendations for management of sarcopenia do not vary across sarcopenia categories (e.g., probable/confirmed/severe).
[Source 20 ]Screening Tools to Identify Probable Sarcopenia
Strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire. The SARC-F (Strength, Assistance with walking, Rising from a chair, Climbing stairs, and Falls) questionnaire is a screening tool that can be rapidly implemented by clinicians to identify probable sarcopenic patients 49. The SARC-F (Strength, Assistance with walking, Rising from a chair, Climbing stairs, and Falls) questionnaire screens patients for self-reported signs suggestive of sarcopenia, which include deficiencies in strength, walking, rising from a chair, climbing stairs, and experiencing falls 154. Each of the self-reported parameters receives a minimum and maximum score of 0 and 2, respectively, with the greatest maximum SARC-F score being 10 155. Data suggests that a SARC-F score of ≥4 best predicts the need for further, more comprehensive evaluation 4, 49.
Sarcopenia questionnaire (SARC‐F questionnaire includes scoring)
[Source 49 ]Assessing sarcopenia muscle strength
- Handgrip test: Generally, handgrip strength is one of the two methods utilized to quantify muscle strength in patients with suspected sarcopenia. Handgrip strength correlates with strength in other muscles and is therefore used as a surrogate to detect deficits in overall strength 156. Additionally, decreased handgrip strength predicts poor patient outcomes, including increased lengths of stay (LOS), functional deficits, and death 156, 157. Accurate grip strength measurement and interpretation of results rely on a calibrated dynamometer and relevant reference populations 158. The Martin Vigorimeter is a validated tool in measuring grip strength and may be used for this assessment 159. The suggested cutoff point for handgrip is <27 kg and <16 kg, for males and females, respectively 160.
- Chair stand test: The chair stand test may be used as a proxy to gauge lower extremity strength, particularly the quadriceps muscles. The chair stand test measures the number of times a patient can stand and sit from a chair, without the use of their arms, over 30 seconds 161. The chair stand test has been established as a valid indicator of lower extremity strength in community-dwelling populations 162. The suggested cutoff point for the chair stand test is >15 seconds for five rises 161.
Confirming sarcopenia
To date, there is no consensus on the most effective method for confirming sarcopenia. Each comes with their strengths and weaknesses, and often, greater accuracy presents with the cost of inconvenience. Ultimately the goal of these modalities is to determine whether patients meet the requirement of sarcopenia through quantifying total body skeletal muscle mass (SMM), appendicular skeletal muscle mass (ASM), or the cross-sectional area (CSA) of a specific muscle. Because overall body mass is correlated with total body skeletal muscle mass (SMM), appendicular skeletal muscle mass (ASM), or the cross-sectional area (CSA), it may be adjusted for height or body mass index (BMI), yielding ratios in the format of (ASM/height²), (ASM/weight), or (ASM/BMI). The recommended cutoff for appendicular skeletal muscle mass (ASM) is <20 kg and <15 kg, for males and females, respectively 163. Similarly, the recommended cutoff for ASM/height² is <7.0 kg/m² and <5.5 kg/m² for males and females, respectively 164.
- Magnetic resonance imaging (MRI): Magnetic resonance imaging (MRI) is considered a “gold standard” modality for confirming sarcopenia. MRI can provide highly accurate measurements of total body muscle mass 165, 166, 167. However, MRI requires highly trained technicians, lacks portability, presents little cost-effectiveness, and is therefore rarely used in the primary care setting 166, 168.
- Computed tomography (CT): Computed tomography (CT) is also considered a “gold standard” for accurate lean muscle mass measurement. Computed tomography (CT) is rarely used in the primary care setting for similar reasons than MRI 166. However, settings in which CT is routinely performed, such as trauma or surgery, this modality presents greater value and practicability, as demonstrated in previous literature 169. More specifically, CT measured psoas cross-sectional area (PCSA) at the level of the 3rd lumbar vertebrae has consistently predicted outcomes among patient populations in the contexts of gastrointestinal cancer, heart valve surgery, orthopedic trauma, thoracolumbar surgery 170, 171, 172, 173.
- Dual-energy X-ray absorptiometry (DEXA): Dual-energy X-ray absorptiometry (DEXA) is not as accurate as either CT or MRI. However, DEXA presents with greater convenience, and is, therefore, a more widely available and practical modality for confirmation of sarcopenia 4, 174.
- Bioelectrical impedance analysis (BIA): Bioelectrical impedance analysis (BIA) is the most widely available and portable modality of muscle mass quantification. Bioelectrical impedance analysis (BIA) may also be used to confirm sarcopenia 4.
Sarcopenia severity
Once sarcopenia is confirmed through body composition assessment, the severity of the condition is determined by measuring physical performance. Suggested tests and their respective cutoff points for sarcopenia severity as recommended by the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) 4 are listed below.
- Gait speed test: Gait speed tests are simple to use in practice and predict adverse effects associated with sarcopenia 175. The “4-meter usual walking speed test” is practical and can be used to assess sarcopenia severity 176, 177. The 4-meter usual walking speed test measures time taken for a patient to travel 4 meters at their usual walking pace. A speed of ≤0.8 m/second may be indicative of severe sarcopenia 9.
- Short physical performance battery (SPPB): The short physical performance battery (SPPB) test is composed of 3 timed tasks: chair stand tests, standing balance, and walking speed. The minimal and maximal achievable scores are 0 (low performance) and 12 (high performance), respectively. A score of ≤8 is an indicator of poor physical performance and may be indicative of greater sarcopenia severity 4, 178.
- Timed-up and go test (TUG): The timed-up and go test (TUG) test observes the time taken for a patient to rise from a chair, walk 3 meters away from, and 3 meters back to the chair, terminating the test in a sitting position. Time ≥20 seconds is indicative of physical deficits, though the study used to support this recommendation failed to assess male populations and therefore, may only apply to elderly female populations 179
- The 400-meter walk test: In the 400-meter walk test, a patient attempts to walk in a series of 20, 20-meter laps as quickly as possible, with a maximum of 2 minutes rest between each lap. The inability to complete or requiring ≥6 min to complete the entire 400-meters is concerning and may suggest greater sarcopenia severity 4, 180.
Sarcopenia differential diagnosis
Considering the high probability that sarcopenia may co-exist with the below conditions and considering the overlap between these conditions, an accurate differential diagnosis may be difficult.
- Frailty: While sarcopenia and frailty present with significant overlap of symptoms, they remain indistinguishable. Frailty is defined as multi-system impairment and encompasses a broader range of dysfunction than sarcopenia, whereas sarcopenia mainly includes the musculoskeletal system. One condition may contribute directly to the other, as they often co-exist in elderly patients 181, 182.
- Malnutrition: Both malnutrition and sarcopenia can present with low muscle mass, though sarcopenia more often presents with the additional loss of function. Additionally, as a function of caloric restriction, reduced fat mass is observed in patients with malnutrition – this is often not the case with sarcopenia. Functional tests for strength and performance may be administered to rule out malnutrition 4.
- Cachexia: Cachexia is thought to have a more complex cause than sarcopenia. Cachexia is described as severe weight loss and muscle-wasting associated with conditions such as HIV, cancer, and end-stage organ failure. While cachexia and sarcopenia may co-exist, a patient with severe muscle wasting diseases such as HIV or cancer is more likely to have cachexia 183. Additionally, the Glasgow Prognostic Score can be utilized to distinguish the two conditions 184.
- Osteoarthritis of the hand: Patients with osteoarthritis of the hand may elicit a false positive test when performing the handgrip strength test. In cases where severe osteoarthritis is suspected, patients may perform methods to measure isometric torque of the lower limb to more accurately assess or rule out sarcopenia 4, 185.
Sarcopenia treatment
For the management of sarcopenia, there is a strong recommendation that individuals with sarcopenia should be enrolled in a resistance training programme 186, 187, 49. There is a reasonable amount of evidence that resistance exercise will increase both muscle mass and strength 188. The prescription of exercise should be considered, discussed, and prescribed, with progress monitored like any other medical treatment. This may be best achieved by referral to an Accredited Exercise Physiologist or similarly qualified health professional.
Additionally, increasing total protein intake through supplementation or food sources can help prevent and manage sarcopenia. The use of a protein rich diet (1 to 1.5 g/kg body weight per day) or protein supplementation received a conditional recommendation based on a small amount of evidence and a previous consensus conference 189. Specifically, consuming 20 to 35 grams of protein per meal is advised, as such amounts provide sufficient amino acid content to maximize muscle protein synthesis, thus minimizing age-related muscle loss 190. Additionally, people with sarcopenia are recommended to consume 1 to 1.2 g/kg body weight per day 191. Higher doses of protein (up to 2 g/kg body weight per day) may be appropriate in persons with severe illness or injury or when there is evidence of a pro‐inflammatory/catabolic state 192. Furthermore, the greatest effects are observed when resistance training and high protein diets are combined and appear to act synergistically 193.
Beta-hydroxy-beta-methylbutyrate (HMB) has been shown to improve muscle mass and to preserve muscle strength and function in older people with sarcopenia or frailty 194. Vitamin D supplementation specifically for sarcopenia was found to have insufficient evidence, though there is evidence that persons with low vitamin D levels may improve their strength with vitamin D supplementation 195. Similarly, while testosterone can increase muscle mass and strength in older individuals and a meta‐analysis has confirmed its safety 196, the lack of evidence in persons with sarcopenia did not lead to its integration into these recommendations. Preliminary data with anamorelin, a growth hormone secretagogue receptor type 1 (ghrelin receptor) agonist that increases muscle mass but not strength 197 and anti‐myostatin antibodies 198 were considered insufficient to make recommendations in favour of their use.
Sarcopenia prognosis
The prognosis of sarcopenia largely depends on your age, comorbidities, falls, and fractures 199. Additionally, people who have sarcopenia undergoing surgical procedures have less favorable outcomes than those without sarcopenia. These include an increased risk of postoperative complications, falls, lengths of stay in the hospital, fractures, and greater morbidity and mortality. Ultimately, the prognosis of sarcopenia alone is uncertain and not well studied, though consistent evidence demonstrates sarcopenia as an indicator of poor prognosis in several medical conditions and surgical procedures 199.
- Westbury LD, Beaudart C, Bruyère O, et al. International Musculoskeletal Ageing Network. Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: Findings from population-based cohorts. J Cachexia Sarcopenia Muscle. 2023 Feb;14(1):565-575. doi: 10.1002/jcsm.13160[↩]
- Kirk B, Cawthon PM, Arai H, et al. Global Leadership Initiative in Sarcopenia (GLIS) group. The Conceptual Definition of Sarcopenia: Delphi Consensus from the Global Leadership Initiative in Sarcopenia (GLIS). Age Ageing. 2024 Mar 1;53(3):afae052. doi: 10.1093/ageing/afae052[↩][↩][↩][↩][↩][↩]
- Kara M, Kaymak B, Frontera W, Ata AM, Ricci V, Ekiz T, Chang KV, Han DS, Michail X, Quittan M, Lim JY, Bean JF, Franchignoni F, Özçakar L. Diagnosing sarcopenia: Functional perspectives and a new algorithm from the ISarcoPRM. J Rehabil Med. 2021 Jun 21;53(6):jrm00209. doi: 10.2340/16501977-2851[↩]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31. doi: 10.1093/ageing/afy169. Erratum in: Age Ageing. 2019 Jul 1;48(4):601. doi: 10.1093/ageing/afz046[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Ardeljan AD, Hurezeanu R. Sarcopenia. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560813[↩][↩][↩][↩]
- Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995 Nov 1;123(9):727-8. doi: 10.7326/0003-4819-123-9-199511010-00014[↩]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001 Apr;137(4):231-43. doi: 10.1067/mlc.2001.113504[↩]
- Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012 Jan;67(1):28-40. doi: 10.1093/gerona/glr010[↩]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010 Jul;39(4):412-23. doi: 10.1093/ageing/afq034[↩][↩]
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011 May;12(4):249-56. doi: 10.1016/j.jamda.2011.01.003[↩][↩][↩]
- Morley JE, Abbatecola AM, Argiles JM, et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011 Jul;12(6):403-9. doi: 10.1016/j.jamda.2011.04.014[↩]
- Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):584-90. doi: 10.1093/gerona/glu013[↩]
- Woo J, Arai H, Ng TP, Sayer AA, Wong M, Syddall H, et al. Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur Geriatr Med 2014;5:155–164.[↩]
- Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M; Asian Working Group for Sarcopenia. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016 Aug 1;17(8):767.e1-7. doi: 10.1016/j.jamda.2016.05.016[↩]
- Chen LK, Liu LK, Woo J, Assantachai P, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014 Feb;15(2):95-101. doi: 10.1016/j.jamda.2013.11.025[↩]
- Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016 Dec;7(5):512-514. doi: 10.1002/jcsm.12147[↩][↩]
- Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014 Dec;5(4):253-9. doi: 10.1007/s13539-014-0161-y. Epub 2014 Oct 22. Erratum in: J Cachexia Sarcopenia Muscle. 2015 Jun;6(2):192. doi: 10.1002/jcsm.12038[↩]
- von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle. 2012 Dec;3(4):213-7. doi: 10.1007/s13539-012-0089-z[↩]
- Gao L, Jiang J, Yang M, Hao Q, Luo L, Dong B. Prevalence of Sarcopenia and Associated Factors in Chinese Community-Dwelling Elderly: Comparison Between Rural and Urban Areas. J Am Med Dir Assoc. 2015 Nov 1;16(11):1003.e1-6. doi: 10.1016/j.jamda.2015.07.020[↩]
- Zanker J, Sim M, Anderson K, et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J Cachexia Sarcopenia Muscle. 2023 Feb;14(1):142-156. doi: 10.1002/jcsm.13115[↩][↩][↩]
- Zanker J, Scott D, Brennan-Olsen SL, Duque G. Sarcopenia: a deserving recipient of an Australian ICD-10-AM code. Med J Aust. 2020 Jan;212(1):45-45.e1. doi: 10.5694/mja2.50432[↩]
- van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch Gerontol Geriatr 2020;90:104125[↩]
- Phu S, Vogrin S, Zanker J, Bani Hassan E, Al Saedi A, Duque G. Agreement Between Initial and Revised European Working Group on Sarcopenia in Older People Definitions. J Am Med Dir Assoc. 2019 Mar;20(3):382-383.e1. doi: 10.1016/j.jamda.2018.11.026[↩]
- Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, Thabane L, Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019 Jan 1;48(1):48-56. doi: 10.1093/ageing/afy106[↩]
- Kirk B, Mooney K, Amirabdollahian F, Khaiyat O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University – Sarcopenia Aging Trial (LHU-SAT). Front Physiol. 2019 Apr 25;10:445. doi: 10.3389/fphys.2019.00445[↩]
- Kirk B, Mooney K, Cousins R, Angell P, Jackson M, Pugh JN, Coyles G, Amirabdollahian F, Khaiyat O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: a secondary analysis of the Liverpool Hope University-Sarcopenia Ageing Trial (LHU-SAT). Eur J Appl Physiol. 2020 Feb;120(2):493-503. doi: 10.1007/s00421-019-04293-5[↩]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-Intensity Strength Training in Nonagenarians: Effects on Skeletal Muscle. JAMA. 1990;263(22):3029–3034. doi:10.1001/jama.1990.03440220053029[↩]
- Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 Jun 18;311(23):2387-96. doi: 10.1001/jama.2014.5616[↩]
- Bernabei R, Landi F, Calvani R, et al. SPRINTT consortium. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. 2022 May 11;377:e068788. doi: 10.1136/bmj-2021-068788[↩]
- Roubenoff, R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr 54, S40–S47 (2000) doi:10.1038/sj.ejcn.1601024 https://www.nature.com/articles/1601024.pdf[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Roubenoff R, Heyms®eld SB, Kehayias JJ, Cannon JG & Rosenberg IH (1997a): Standardization of nomenclature of body composition in weight loss. Am. J. Clin. Nutr. 66, 192 ± 196.[↩]
- Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):170. Published 2016 Oct 5. doi:10.1186/s12877-016-0349-4 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5052976[↩][↩]
- Cawthon PM, Manini T, Patel SM, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J Am Geriatr Soc. 2020 Jul;68(7):1429-1437. doi: 10.1111/jgs.16517[↩]
- Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM, Stone KL, Ensrud KE, Cauley JA, Evans WJ. Muscle Mass Assessed by the D3-Creatine Dilution Method and Incident Self-reported Disability and Mortality in a Prospective Observational Study of Community-Dwelling Older Men. J Gerontol A Biol Sci Med Sci. 2021 Jan 1;76(1):123-130. doi: 10.1093/gerona/glaa111[↩]
- Morley JE. Frailty: a time for action. Eur Geriatr Med 2013;4:215–216.[↩]
- Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C. Frailty Screening in the Community Using the FRAIL Scale. J Am Med Dir Assoc. 2015 May 1;16(5):412-9. doi: 10.1016/j.jamda.2015.01.087[↩]
- Argilés JM, Muscaritoli M. The Three Faces of Sarcopenia. J Am Med Dir Assoc. 2016 Jun 1;17(6):471-2. doi: 10.1016/j.jamda.2016.03.012[↩]
- Michel JP. Sarcopenia: there is a need for some steps forward. J Am Med Dir Assoc. 2014 Jun;15(6):379-80. doi: 10.1016/j.jamda.2014.03.016[↩]
- Morley JE. Frailty screening comes of age. J Nutr Health Aging. 2014 May;18(5):453-4. doi: 10.1007/s12603-014-0457-9[↩]
- Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, Montero-Odasso M, Gunawardene P, Demontiero O, Duque G. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015 Apr;16(4):290-5. doi: 10.1016/j.jamda.2014.10.018[↩]
- Morley JE. Frailty and Sarcopenia: The New Geriatric Giants. Rev Invest Clin. 2016 Mar-Apr;68(2):59-67. https://clinicalandtranslationalinvestigation.com/files/ric_2016_68_2_059-067.pdf[↩]
- Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014 Dec;15(12):853-9. doi: 10.1016/j.jamda.2014.10.001[↩][↩]
- Liccini A, Malmstrom TK. Frailty and Sarcopenia as Predictors of Adverse Health Outcomes in Persons With Diabetes Mellitus. J Am Med Dir Assoc. 2016 Sep 1;17(9):846-51. doi: 10.1016/j.jamda.2016.07.007[↩]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127( Suppl:990S–991S.[↩]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763.[↩][↩]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896.[↩]
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004.[↩]
- Clark BC, Manini TM. Sarcopeia ≠ dynapenia. J Gerontol A Biol Sci Med Sci 2008;63:829–834.[↩]
- Bauer, J., Morley, J. E., Schols, A. M. W. J., Ferrucci, L., Cruz‐Jentoft, A. J., Dent, E., Baracos, V. E., Crawford, J. A., Doehner, W., Heymsfield, S. B., Jatoi, A., Kalantar‐Zadeh, K., Lainscak, M., Landi, F., Laviano, A., Mancuso, M., Muscaritoli, M., Prado, C. M., Strasser, F., Coats, A. J. S., and Anker, S. D. ( 2019) Sarcopenia: A Time for Action. An SCWD Position Paper, Journal of Cachexia, Sarcopenia and Muscle, 10: 956– 961. https://doi.org/10.1002/jcsm.12483[↩][↩][↩][↩][↩][↩]
- Levine JA, et al. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol. 2000;88:452–456.[↩]
- Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034[↩][↩][↩]
- Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520[↩]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x[↩]
- Studenski SA, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010[↩]
- Rush EC, Freitas I, Plank LD. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102:632–641. doi: 10.1017/S0007114508207221[↩]
- Reiss J, et al. Case finding for sarcopenia in geriatric inpatients: performance of bioimpedance analysis in comparison to dual X-ray absorptiometry. BMC Geriatr. 2016;16:52. doi: 10.1186/s12877-016-0228-z[↩]
- Buckinx F, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord. 2015;16:60. doi: 10.1186/s12891-015-0510-9[↩]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ & Roubenoff R (2000): Aging of skeletal muscle: a 12-year longitudinal study. J. Appl. Physiol. (in press).[↩]
- Winick M, ed. (1979): Hunger DiseaseÐStudies by Jewish Physicians in the Warsaw Ghetto. New York: John Wiley & Sons.[↩]
- Kotler DP, Tierney AR & Pierson RN (1989): Magnitude of body cell mass depletion and the timing of death from wasting in AIDS. Am. J. Clin. Nutr. 50, 444 ± 447.[↩]
- Tellado JM, Garcia-Sabrido JL, Hanley JA, Shizgal HM & Christou NV (1989): Predicting mortality based on body composition analysis. Ann. Surg. 208, 81 ± 87.[↩][↩]
- Ellis KJ (1990): Reference man and woman more fully characterized: variations on the basis of body size, age, sex, and race. Biol. Trace Elem. Res. 26 ± 27, 385 ± 400.[↩]
- Kehayias JJ, Fiatarone MF, Zhuang H & Roubenoff R (1997): Total body potassium and body fat: relevance to aging. Am. J. Clin. Nutr. 66, 904 ± 910.[↩][↩]
- Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423.[↩]
- Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, et al. An evidence‐based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 2014;69:584–590.[↩]
- Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101.[↩]
- Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31.[↩]
- Dent E, Morley JE, Cruz‐Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging 2018;22:1148–1161.[↩][↩][↩]
- Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom 2015;18:467–471.[↩]
- Short Physical Performance Battery (SPPB). https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb[↩]
- Bahat G, Yilmaz O, Kiliç C, Oren MM, Karan MA. Performance of SARC‐F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging 2018;22:898–903.[↩]
- Barbosa‐Silva TG, Menezes AM, Bielemann RM, Malmstrom TK, Gonzalez MC. Grupo de Estudos em Composicao corporal e Nutricao (COCONUT)Enhancing SARC‐F: Improving sarcopenia screening in the clinical practice. J Am Dir Assoc 2016;17:1136–1141.[↩]
- Locquet M, Beaudart C, Reginster JY, Petermans J, Bruyere O. Comparison of the performance of five screening methods for sarcopenia. Clin Epidemiol 2017;10:71–82.[↩]
- Nijholt W, Scafoglieri A, Jager‐Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8:702–712.[↩]
- Perkisas S, Baudry S, Bauer J, Beckwee D, De Cock A‐M, Hobbelen H, et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med 2018;9:739–757.[↩]
- Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong relation between muscle mass determined by D3‐creatine dilation. Physical performance and incidence of falls and mobility limitations I a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 2018;Jun 12 https://doi.org/10.1093/Gerona/gly129[↩]
- Shankaran M, Czerwieniec G, Fessler C, Wong PA, Killion S, Turner SM, et al. Dilution of oral D3‐creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 2018;9:540–546.[↩]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495.[↩]
- Anker SD, Coats AJ, Morley JE, Rosano G, Bernabei R, von Haehling S, et al. Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle 2014;5:1–3.[↩]
- Muscaritoli M, Lucia S, Molfino A, Cederholm T, Rossi Fanelli F. Muscle atrophy in aging and chronic diseases: is it sarcopenia or cachexia? Intern Emerg Med 2013;8:553–560.[↩][↩]
- Kouw IWK, Groen BBL, Smeets JSJ, Kramer IF, van Kranenburg JMX, Nilwik R, et al. One week of hospitalization following elective hip surgery induces substantial muscle atrophy in older patients. J Am Med Dir Assoc 2019;20:35–42.[↩]
- Wall BT, Dirks ML, Snijders T, Senden JM, Dolmans J, van Loon LJ. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf) 2014;210:600–611.[↩]
- Purves-Smith FM, Sgarioto N, Hepple RT. Fiber typing in aging muscle. Exerc Sport Sci Rev. 2014 Apr;42(2):45-52. doi: 10.1249/JES.0000000000000012[↩]
- Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983 Oct;6(8):588-95. doi: 10.1002/mus.880060809[↩]
- Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998 May;53(3):M214-21. doi: 10.1093/gerona/53a.3.m214[↩]
- Morley JE. Hormones and the aging process. J Am Geriatr Soc. 2003 Jul;51(7 Suppl):S333-7. doi: 10.1046/j.1365-2389.2003.51344.x[↩]
- Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22(5 Suppl):110-6.[↩]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985). 2007 Mar;102(3):919-25. doi: 10.1152/japplphysiol.00627.2006[↩]
- Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013 Oct;41(4):216-23. doi: 10.1097/JES.0b013e3182a4e699[↩]
- Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: The Newcastle 85+ study. Clin Nutr. 2020 Jan;39(1):166-173. doi: 10.1016/j.clnu.2019.01.009[↩]
- Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008 Aug-Sep;12(7):452-6. doi: 10.1007/BF02982705[↩][↩]
- Hepple RT. Sarcopenia–a critical perspective. Sci Aging Knowledge Environ. 2003 Nov 19;2003(46):pe31. doi: 10.1126/sageke.2003.46.pe31[↩]
- Drey M, Grösch C, Neuwirth C, Bauer JM, Sieber CC. The Motor Unit Number Index (MUNIX) in sarcopenic patients. Exp Gerontol. 2013 Apr;48(4):381-4. doi: 10.1016/j.exger.2013.01.011[↩]
- Landi F, Onder G, Bernabei R. Sarcopenia and diabetes: two sides of the same coin. J Am Med Dir Assoc. 2013 Aug;14(8):540-1. doi: 10.1016/j.jamda.2013.05.004[↩]
- Leenders M, Verdijk LB, van der Hoeven L, Adam JJ, van Kranenburg J, Nilwik R, van Loon LJ. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc. 2013 Aug;14(8):585-92. doi: 10.1016/j.jamda.2013.02.006[↩]
- Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia Associated with Chronic Obstructive Pulmonary Disease. J Bone Metab. 2019 May;26(2):65-74. doi: 10.11005/jbm.2019.26.2.65[↩]
- Abdul Aziz SA, Mcstea M, Ahmad Bashah NS, Chong ML, Ponnampalavanar S, Syed Omar SF, Sulaiman H, Azwa I, Tan MP, Kamarulzaman A, Rajasuriar R, Kamaruzzaman SB. Assessment of sarcopenia in virally suppressed HIV-infected Asians receiving treatment. AIDS. 2018 May 15;32(8):1025-1034. doi: 10.1097/QAD.0000000000001798. Erratum in: AIDS. 2019 Mar 15;33(4):769. doi: 10.1097/01.aids.0000554204.38139.5a[↩]
- Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, Galizia G, Della-Morte D, Gargiulo G, Cacciatore F, Bonaduce D, Landi F, Abete P. Sarcopenia and Heart Failure. Nutrients. 2020 Jan 14;12(1):211. doi: 10.3390/nu12010211[↩]
- Souza VA, Oliveira Dd, Mansur HN, Fernandes NM, Bastos MG. Sarcopenia in chronic kidney disease. J Bras Nefrol. 2015 Jan-Mar;37(1):98-105. English, Portuguese. doi: 10.5935/0101-2800.20150014[↩]
- Dunne RF, Loh KP, Williams GR, Jatoi A, Mustian KM, Mohile SG. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers (Basel). 2019 Nov 25;11(12):1861. doi: 10.3390/cancers11121861[↩]
- Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019 Jul 8;12:1057-1072. doi: 10.2147/DMSO.S186600[↩]
- Friedman PJ, Campbell AJ, Caradoc-Davies TH. Prospective trial of a new diagnostic criterion for severe wasting malnutrition in the elderly. Age Ageing. 1985 May;14(3):149-54. doi: 10.1093/ageing/14.3.149[↩]
- Chindapasirt J. Sarcopenia in Cancer Patients. Asian Pac J Cancer Prev. 2015;16(18):8075-7. doi: 10.7314/apjcp.2015.16.18.8075[↩]
- Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993 Feb;123(2 Suppl):465-8. doi: 10.1093/jn/123.suppl_2.465[↩]
- Yuki A, Otsuka R, Kozakai R, Kitamura I, Okura T, Ando F, Shimokata H. Relationship between low free testosterone levels and loss of muscle mass. Sci Rep. 2013;3:1818. doi: 10.1038/srep01818[↩]
- Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996 Sep;81(9):3239-43. doi: 10.1210/jcem.81.9.8784075[↩]
- Scicchitano BM, Rizzuto E, Musarò A. Counteracting muscle wasting in aging and neuromuscular diseases: the critical role of IGF-1. Aging (Albany NY). 2009 May 13;1(5):451-7. doi: 10.18632/aging.100050[↩]
- Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999 Mar 1;107(2):123-36. doi: 10.1016/s0047-6374(98)00130-4[↩]
- Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. World J Mens Health. 2018 Sep;36(3):192-198. doi: 10.5534/wjmh.180001[↩][↩]
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008 Nov;11(6):693-700. doi: 10.1097/MCO.0b013e328312c37d[↩]
- Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008 Jun;18(5):388-95. doi: 10.1016/j.numecd.2007.10.002[↩]
- Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011 Sep;96(9):2898-903. doi: 10.1210/jc.2011-0435. Epub 2011 Jul 21. Erratum in: J Clin Endocrinol Metab. 2012 Jun;97(6):2203.[↩]
- Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab. 2005 Dec;31 Spec No 2:5S20-5S26. doi: 10.1016/s1262-3636(05)73648-x[↩]
- Boirie Y, Gachon P, Cordat N, Ritz P, Beaufrère B. Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab. 2001 Feb;86(2):638-44. doi: 10.1210/jcem.86.2.7193[↩]
- Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab. 2006 Oct;291(4):E729-36. doi: 10.1152/ajpendo.00003.2006[↩]
- Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas. 2012 Feb;71(2):109-14. doi: 10.1016/j.maturitas.2011.11.012[↩]
- Kwon YN, Yoon SS. Sarcopenia: Neurological Point of View. J Bone Metab. 2017 May;24(2):83-89. doi: 10.11005/jbm.2017.24.2.83[↩][↩]
- Thomas DR. Sarcopenia. Clin Geriatr Med. 2010 May;26(2):331-46. doi: 10.1016/j.cger.2010.02.012[↩]
- Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001;2(5):269-72. doi: 10.1186/rr67[↩]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985). 2005 Mar;98(3):911-7. doi: 10.1152/japplphysiol.01026.2004[↩]
- Londhe P, Guttridge DC. Inflammation induced loss of skeletal muscle. Bone. 2015 Nov;80:131-142. doi: 10.1016/j.bone.2015.03.015[↩]
- Institute of Medicine (US) Food and Nutrition Board. Nutrition and Healthy Aging in the Community: Workshop Summary. Washington (DC): National Academies Press (US); 2012. 2, Nutrition Issues of Concern in the Community. Available from: https://www.ncbi.nlm.nih.gov/books/NBK98453[↩]
- IOM. Child and Adult Care Food Program: Aligning Dietary Guidance for All. Washington, DC: The National Academies Press; 2011.[↩][↩][↩][↩][↩][↩]
- Lichtenstein AH, Rasmussen H, Yu WW, Epstein SR, Russell RM. Modified MyPyramid for older adults. Journal of Nutrition. 2008;138(1):5–11.[↩]
- Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Jung SL, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. American Journal of Clinical Nutrition. 2008;87(1):150–155.[↩]
- Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. American Journal of Clinical Nutrition. 2008;87(5):1562S–1566S.[↩]
- Saldeen K, Saldeen T. Importance of tocopherols beyond α-tocopherol: Evidence from animal and human studies. Nutrition Research. 2005;25(10):877–889.[↩]
- Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson PWF, Selhub J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring Study. American Journal of Clinical Nutrition. 2000;71(2):514–522.[↩][↩]
- Merete C, Falcon LM, Tucker KL. Vitamin B6 is associated with depressive symptomatology in Massachusetts elders. Journal of the American College of Nutrition. 2008;27(3):421–427.[↩]
- IOM. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011.[↩]
- Buell JS, Scott TM, Dawson-Hughes B, Dallal GE, Rosenberg IH, Folstein MF, Tucker KL. Vitamin D is associated with cognitive function in elders receiving home health services. Journal of Gerontology—Series A Biological Sciences and Medical Sciences. 2009;64(8):888–895.[↩]
- Dietary Guidelines for American https://health.gov/dietaryguidelines[↩][↩]
- IOM. Strategies to Reduce Sodium Intake in the United States. Washington, DC: The National Academies Press; 2010.[↩]
- NHANES 2001–2004, 2005–2006. https://www.cdc.gov/nchs/nhanes/index.htm[↩][↩]
- IOM. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.[↩]
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER. Folic acid for the prevention of colorectal adenomas: A randomized clinical trial. Journal of the American Medical Association. 2007;297(21):2351–2359.[↩]
- Britten P, Marcoe K, Yamini S, Davis C. Development of food intake patterns for the MyPyramid Food Guidance System. Journal of Nutrition Education and Behavior. 2006;38(6 Suppl):S78–S92.[↩]
- Kara M, Kaymak B, Ata AM, Özkal Ö, Kara Ö, Baki A, Şengül Ayçiçek G, Topuz S, Karahan S, Soylu AR, Çakır B, Halil M, Özçakar L. STAR-Sonographic Thigh Adjustment Ratio: A Golden Formula for the Diagnosis of Sarcopenia. Am J Phys Med Rehabil. 2020 Oct;99(10):902-908. doi: 10.1097/PHM.0000000000001439[↩]
- Ata AM, Kara M, Kaymak B, Özçakar L. Sarcopenia Is Not “Love”: You Have to Look Where You Lost it! Am J Phys Med Rehabil. 2020 Oct;99(10):e119-e120. doi: 10.1097/PHM.0000000000001391[↩]
- Abe T, Loenneke JP, Thiebaud RS, Ogawa M, Mitsukawa N. Age-related site-specific muscle loss in the thigh and zigzag walking performance in older men and women. Acta Physiol Hung. 2014 Dec;101(4):488-95. doi: 10.1556/APhysiol.101.2014.006[↩]
- Abe T, Kawakami Y, Kondo M, Fukunaga T. Comparison of ultrasound-measured age-related, site-specific muscle loss between healthy Japanese and German men. Clin Physiol Funct Imaging. 2011 Jul;31(4):320-5. doi: 10.1111/j.1475-097X.2011.01021.x[↩]
- Haskell WL, Blair SN, Hill JO. Physical activity: health outcomes and importance for public health policy. Prev Med. 2009 Oct;49(4):280-2. doi: 10.1016/j.ypmed.2009.05.002[↩]
- Yeung SSY, Reijnierse EM, Pham VK, Trappenburg MC, Lim WK, Meskers CGM, Maier AB. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019 Jun;10(3):485-500. doi: 10.1002/jcsm.12411[↩]
- Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006 Nov;96(5):895-901. doi: 10.1017/bjn20061943[↩]
- Kang SH, Park JW, Yoon KW, Do JY. Limb/trunk lean mass ratio as a risk factor for mortality in peritoneal dialysis patients. J Ren Nutr. 2013 Jul;23(4):315-23. doi: 10.1053/j.jrn.2012.09.004[↩]
- Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D, Honda Y, Funayama A, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Kubota I. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015 Mar;26(2):118-22. doi: 10.1016/j.ejim.2015.01.008[↩]
- Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, Ropert S, Delongchamps NB, Zerbib M, Goldwasser F. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013 Mar 19;108(5):1034-41. doi: 10.1038/bjc.2013.58[↩]
- Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, Ropert S, Vidal M, Pol S, Chaussade S, Goldwasser F. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7(5):e37563. doi: 10.1371/journal.pone.0037563[↩]
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009 Apr 15;15(8):2920-6. doi: 10.1158/1078-0432.CCR-08-2242[↩]
- Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG; Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014 Sep;156(3):521-7. doi: 10.1016/j.surg.2014.04.027[↩][↩]
- Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010 Aug;211(2):271-8. doi: 10.1016/j.jamcollsurg.2010.03.039[↩]
- Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015 Feb;261(2):345-52. doi: 10.1097/SLA.0000000000000628[↩]
- von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010 Dec;1(2):129-133. doi: 10.1007/s13539-010-0014-2[↩]
- Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016 Mar;7(1):28-36. doi: 10.1002/jcsm.12048[↩]
- Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013 Aug;14(8):531-2. doi: 10.1016/j.jamda.2013.05.018[↩]
- Leong DP, Teo KK, Rangarajan S, et al. Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015 Jul 18;386(9990):266-73. doi: 10.1016/S0140-6736(14)62000-6[↩][↩]
- Ibrahim K, May C, Patel HP, Baxter M, Sayer AA, Roberts H. A feasibility study of implementing grip strength measurement into routine hospital practice (GRImP): study protocol. Pilot Feasibility Stud. 2016 Jun 6;2:27. doi: 10.1186/s40814-016-0067-x[↩]
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011 Jul;40(4):423-9. doi: 10.1093/ageing/afr051[↩]
- Sipers WM, Verdijk LB, Sipers SJ, Schols JM, van Loon LJ. The Martin Vigorimeter Represents a Reliable and More Practical Tool Than the Jamar Dynamometer to Assess Handgrip Strength in the Geriatric Patient. J Am Med Dir Assoc. 2016 May 1;17(5):466.e1-7. doi: 10.1016/j.jamda.2016.02.026[↩]
- Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014 Dec 4;9(12):e113637. doi: 10.1371/journal.pone.0113637[↩]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999 Jun;70(2):113-9. doi: 10.1080/02701367.1999.10608028[↩][↩]
- Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, Brach JS, Tylavsky FA, Satterfield S, Bauer DC, Rubin SM, Visser M, Pahor M; Health, Aging and Body Composition Study. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009 Feb;57(2):251-9. doi: 10.1111/j.1532-5415.2008.02126.x[↩]
- Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547-58. doi: 10.1093/gerona/glu010[↩]
- Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong osteoporosis study. Calcif Tissue Int. 2014 Apr;94(4):363-72. doi: 10.1007/s00223-013-9830-7[↩]
- Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. 2013 Sep;13(3):320-8. https://www.ismni.org/jmni/pdf/53/07DEGENS.pdf[↩]
- Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016 Oct 5;16(1):170. doi: 10.1186/s12877-016-0349-4[↩][↩][↩]
- Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014 Nov;38(8):940-53. doi: 10.1177/0148607114550189. Epub 2014 Sep 19. Erratum in: JPEN J Parenter Enteral Nutr. 2016 Jul;40(5):742. doi: 10.1177/0148607116647919[↩]
- Kerr JD. MRI safety: everyone’s job. Radiol Manage. 2001 Nov-Dec;23(6):36-9.[↩]
- Zumsteg DM, Chu CE, Midwinter MJ. Radiographic assessment of sarcopenia in the trauma setting: a systematic review. Trauma Surg Acute Care Open. 2020 Mar 15;5(1):e000414. doi: 10.1136/tsaco-2019-000414[↩]
- Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, Han Y, Sui X, Wu G. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: A prospective cohort study. Clin Nutr. 2019 Dec;38(6):2881-2888. doi: 10.1016/j.clnu.2018.12.025[↩]
- Okamura H, Kimura N, Tanno K, Mieno M, Matsumoto H, Yamaguchi A, Adachi H. The impact of preoperative sarcopenia, defined based on psoas muscle area, on long-term outcomes of heart valve surgery. J Thorac Cardiovasc Surg. 2019 Mar;157(3):1071-1079.e3. doi: 10.1016/j.jtcvs.2018.06.098[↩]
- Fairchild B, Webb TP, Xiang Q, Tarima S, Brasel KJ. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015 Feb;39(2):373-9. doi: 10.1007/s00268-014-2785-7[↩]
- Bokshan SL, Han AL, DePasse JM, Eltorai AE, Marcaccio SE, Palumbo MA, Daniels AH. Effect of Sarcopenia on Postoperative Morbidity and Mortality After Thoracolumbar Spine Surgery. Orthopedics. 2016 Nov 1;39(6):e1159-e1164. doi: 10.3928/01477447-20160811-02[↩]
- Messina C, Maffi G, Vitale JA, Ulivieri FM, Guglielmi G, Sconfienza LM. Diagnostic imaging of osteoporosis and sarcopenia: a narrative review. Quant Imaging Med Surg. 2018 Feb;8(1):86-99. doi: 10.21037/qims.2018.01.01. Erratum in: Quant Imaging Med Surg. 2018 Apr;8(3):372. doi: 10.21037/qims.2018.04.07[↩]
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009 Dec;13(10):881-9. doi: 10.1007/s12603-009-0246-z[↩]
- Maggio M, Ceda GP, Ticinesi A, De Vita F, Gelmini G, Costantino C, Meschi T, Kressig RW, Cesari M, Fabi M, Lauretani F. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals. PLoS One. 2016 Apr 14;11(4):e0153583. doi: 10.1371/journal.pone.0153583[↩]
- Rydwik E, Bergland A, Forsén L, Frändin K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: a systematic review. Physiother Theory Pract. 2012 Apr;28(3):238-56. doi: 10.3109/09593985.2011.601804[↩]
- Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016 Dec 22;14(1):215. doi: 10.1186/s12916-016-0763-7[↩]
- Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, Akos R, Conzelmann M, Dick W, Theiler R. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. 2003 May;32(3):315-20. doi: 10.1093/ageing/32.3.315[↩]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006 May 3;295(17):2018-26. doi: 10.1001/jama.295.17.2018[↩]
- Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014 Jul 28;6:192. doi: 10.3389/fnagi.2014.00192[↩]
- Dodds R, Sayer AA. Sarcopenia and frailty: new challenges for clinical practice. Clin Med (Lond). 2016 Oct;16(5):455-458. doi: 10.7861/clinmedicine.16-5-455[↩]
- Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015 Apr 15;7(4):17-29. doi: 10.4251/wjgo.v7.i4.17[↩]
- Douglas E, McMillan DC. Towards a simple objective framework for the investigation and treatment of cancer cachexia: the Glasgow Prognostic Score. Cancer Treat Rev. 2014 Jul;40(6):685-91. doi: 10.1016/j.ctrv.2013.11.007[↩]
- Francis P, Toomey C, Mc Cormack W, Lyons M, Jakeman P. Measurement of maximal isometric torque and muscle quality of the knee extensors and flexors in healthy 50- to 70-year-old women. Clin Physiol Funct Imaging. 2017 Jul;37(4):448-455. doi: 10.1111/cpf.12332[↩]
- Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging. 2018;22(10):1148-1161. doi: 10.1007/s12603-018-1139-9[↩]
- Beckwée D, Delaere A, Aelbrecht S, Baert V, Beaudart C, Bruyere O, de Saint-Hubert M, Bautmans I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging. 2019;23(6):494-502. doi: 10.1007/s12603-019-1196-8[↩]
- Vlietstra L, Hendrickx W, Waters DL. Exercise interventions in healthy older adults with sarcopenia: a systematic review and meta‐analysis. Australas J Ageing 2018;37:169–183.[↩]
- Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta‐analysis. Am J Clin Nutr 2017;106:1078–1091.[↩]
- Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009 Jan;12(1):86-90. doi: 10.1097/MCO.0b013e32831cef8b[↩]
- Yanai H. Nutrition for Sarcopenia. J Clin Med Res. 2015 Dec;7(12):926-31. doi: 10.14740/jocmr2361w[↩]
- Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine‐enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double‐blind, placebo‐controlled trial. J Am Med Dir Assoc 2015;16:740–747.[↩]
- Damanti S, Azzolino D, Roncaglione C, Arosio B, Rossi P, Cesari M. Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients. 2019 Aug 23;11(9):1991. doi: 10.3390/nu11091991[↩]
- Bear DE, Langan A, Dimidi E, Wandrag L, Harridge SDR, Hart N, Connolly B, Whelan K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. Am J Clin Nutr. 2019 Apr 1;109(4):1119-1132. doi: 10.1093/ajcn/nqy373[↩]
- Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014 Nov;99(11):4336-45. doi: 10.1210/jc.2014-1742[↩]
- Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, Zou B, Borst SE, Yarrow JF. Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2018 Jun;9(3):465-481. doi: 10.1002/jcsm.12291[↩]
- Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016 Apr;17(4):519-531. doi: 10.1016/S1470-2045(15)00558-6[↩]
- Polkey MI, Praestgaard J, Berwick A, Franssen FME, Singh D, Steiner MD, et al. Activin type II receptor blockade for treatment of muscle depletion in COPD: a randomized trial. Am J Respir Crit Care Med 2018; Aug 10 https://doi.org/10.1164/rccm.201802‐0286OC[↩]
- Han A, Bokshan SL, Marcaccio SE, DePasse JM, Daniels AH. Diagnostic Criteria and Clinical Outcomes in Sarcopenia Research: A Literature Review. J Clin Med. 2018 Apr 8;7(4):70. doi: 10.3390/jcm7040070[↩][↩]