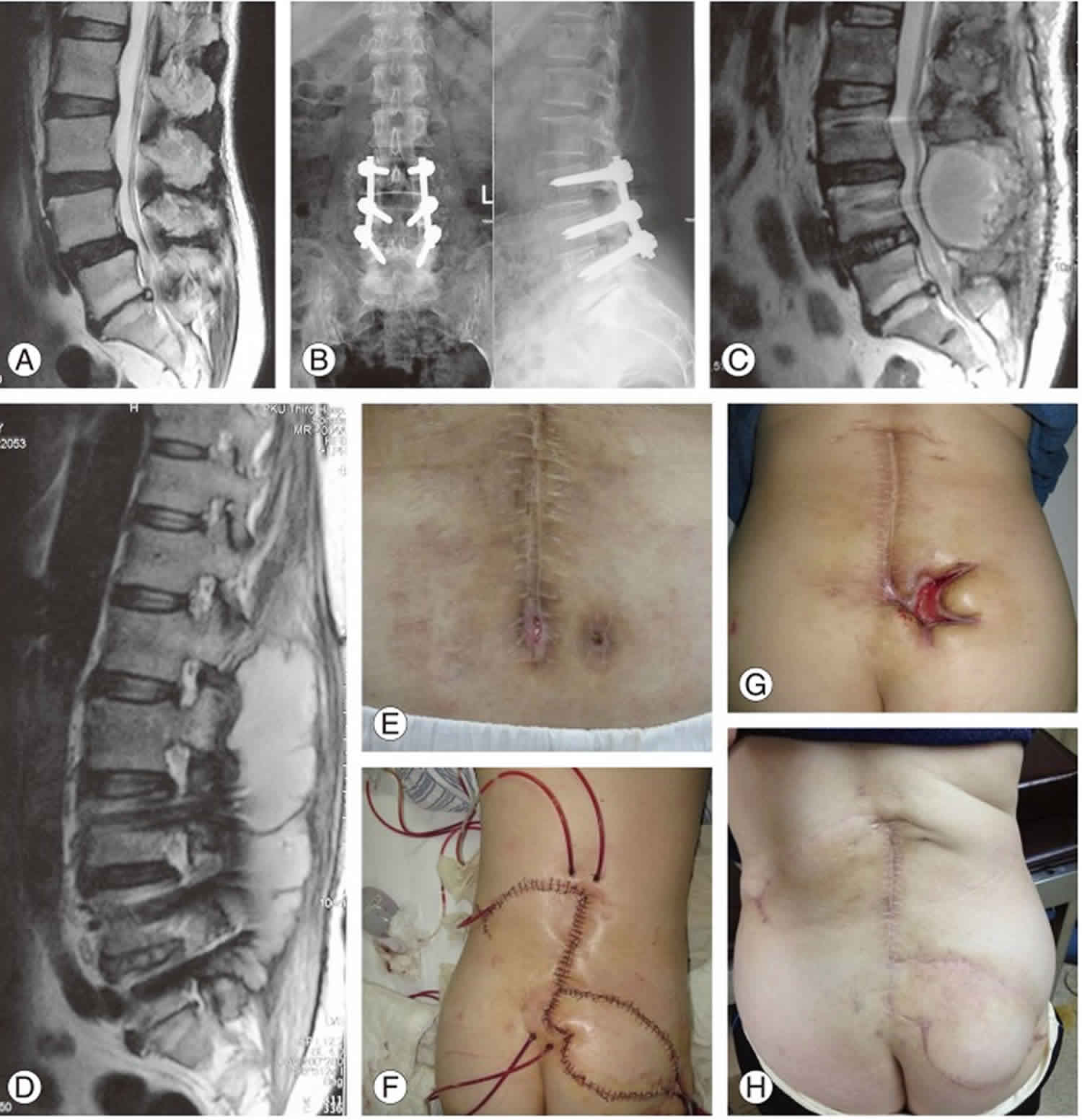
Spinal infection
Spinal infection also known as spinal osteomyelitis or spinal pyogenic osteomyelitis, is a rare condition that may be defined as an infectious disease that affects the vertebral body, the intervertebral disk, or adjacent paraspinal tissue 1. Spinal infection accounts for 2-7% of all musculoskeletal infections 2. Pyogenic vertebral osteomyelitis is the most commonly encountered form of spinal infection. It can develop from direct open spinal trauma, from infections in adjacent structures, from hematogenous spread of bacteria to a vertebra, or postoperatively. Left untreated, vertebral osteomyelitis can lead to permanent neurologic deficits, significant spinal deformity, or death 3. It can result in severe compression of the neural stuctures due to formation of an epidural abscess or due to a pathologic fracture resulting from bone softening.
Approximately 95% of pyogenic spinal infections involve the vertebral body; only 5% involve the posterior elements of the spine. This disparity has been attributed in part to the voluminous blood supply to the vertebral body and its rich, cellular marrow.
Bacteria circulating through the blood may enter a vertebra or a disk space via its arterial blood supply or via the venous system. In the typical case, bacteria enter the vertebral body through small metaphyseal arteries arising from larger primary periosteal arteries that, in turn, branch from the spinal arteries. In adults, blockage of metaphyseal arteries by septic thrombi may infarct relatively large amounts of bone. Subsequently, bacteria can readily colonize a large bony sequestrum adjacent to the disk.
In the adult, after bacterial colonization of the metaphyseal region, the avascular disk is secondarily invaded by bacteria from the endplate region. Intermetaphyseal communicating arteries allow the spread of septic thrombi from one metaphysis to the other in a single vertebral body without involvement of the midportion of the vertebra.
Although the arterial route is the usual route of bacterial spread to a vertebra, another proposed route of infection is the retrograde seeding of venous blood via the Batson plexus. During periods of increased intra-abdominal pressure, venous blood is shunted toward the vertebral venous plexus. Some authors have proposed that the venous system may be the route of bacterial spread from genitourinary tract infections.
Another possible means of infection is by the spread of contiguous infection into the vertebrae and disk (eg, from a retropharyngeal abscess or a retroperitoneal abscess), resulting in osteomyelitis and diskitis 4.
Spine anatomy
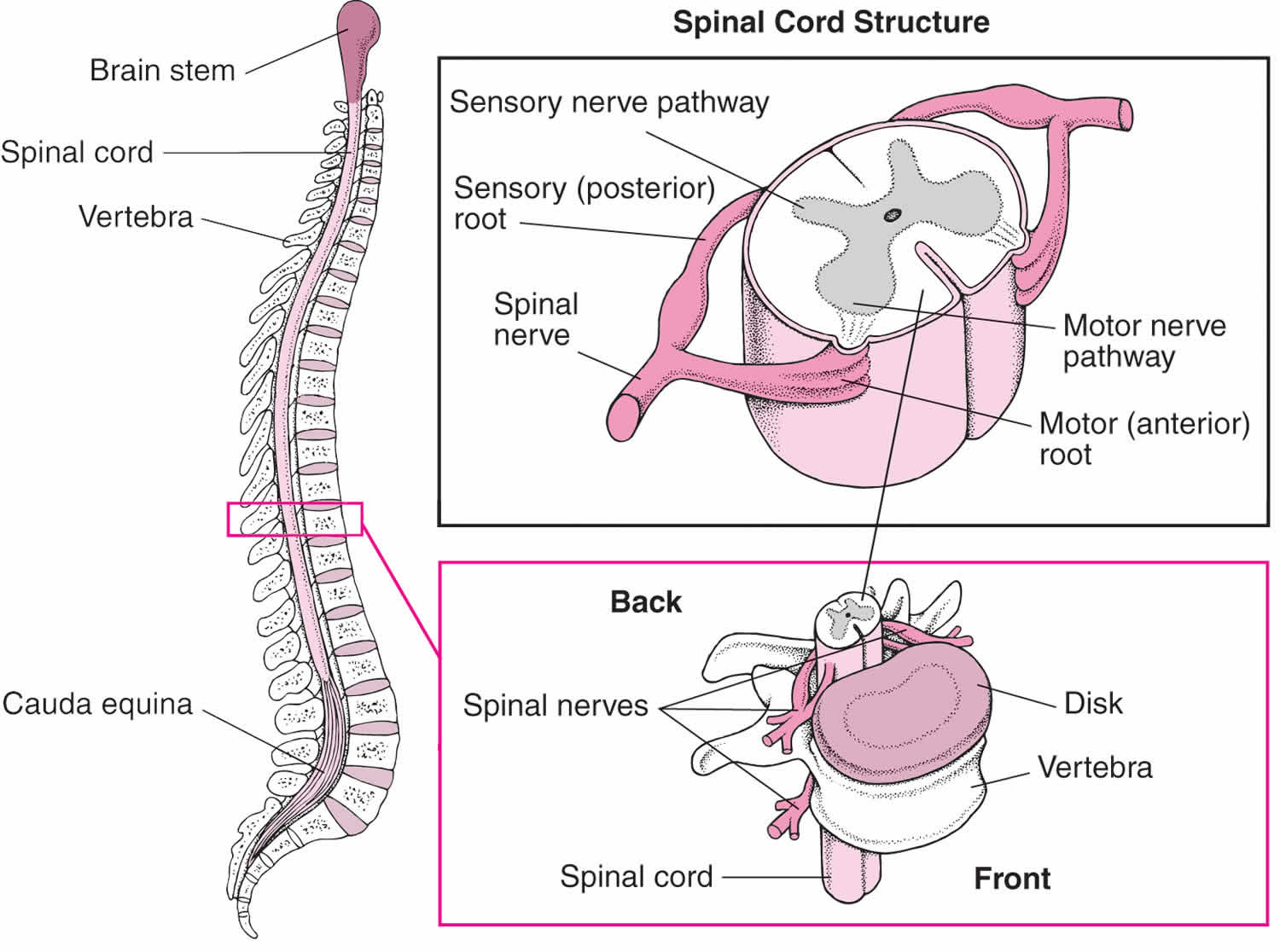
The anatomy of the spine includes the vertebral bodies, intervertebral disks, and associated joints, muscles, tendons, ligaments, and neural elements.
The intervertebral disk is a fibrocartilaginous remnant of the embryonic notochord, which provides the spine with strength, mobility, and resistance to strain. It consists of the following three parts:
- Annulus fibrosus
- Nucleus pulposus
- Cartilaginous endplates
The annulus fibrosus is made up of type I collagen fibrils, which are arranged in 15-20 concentric lamellae brought together into parallel bundles. These bundles are firmly attached to the vertebral bodies and are arranged in layers to provide strength and limit vertebral movement when the disk is compressed. The nucleus pulposus is composed of type II collagen and represents 30-60% of the disk volume. The nucleus pulposus is supplied with blood vessels through small perforations in the central cartilaginous endplates.
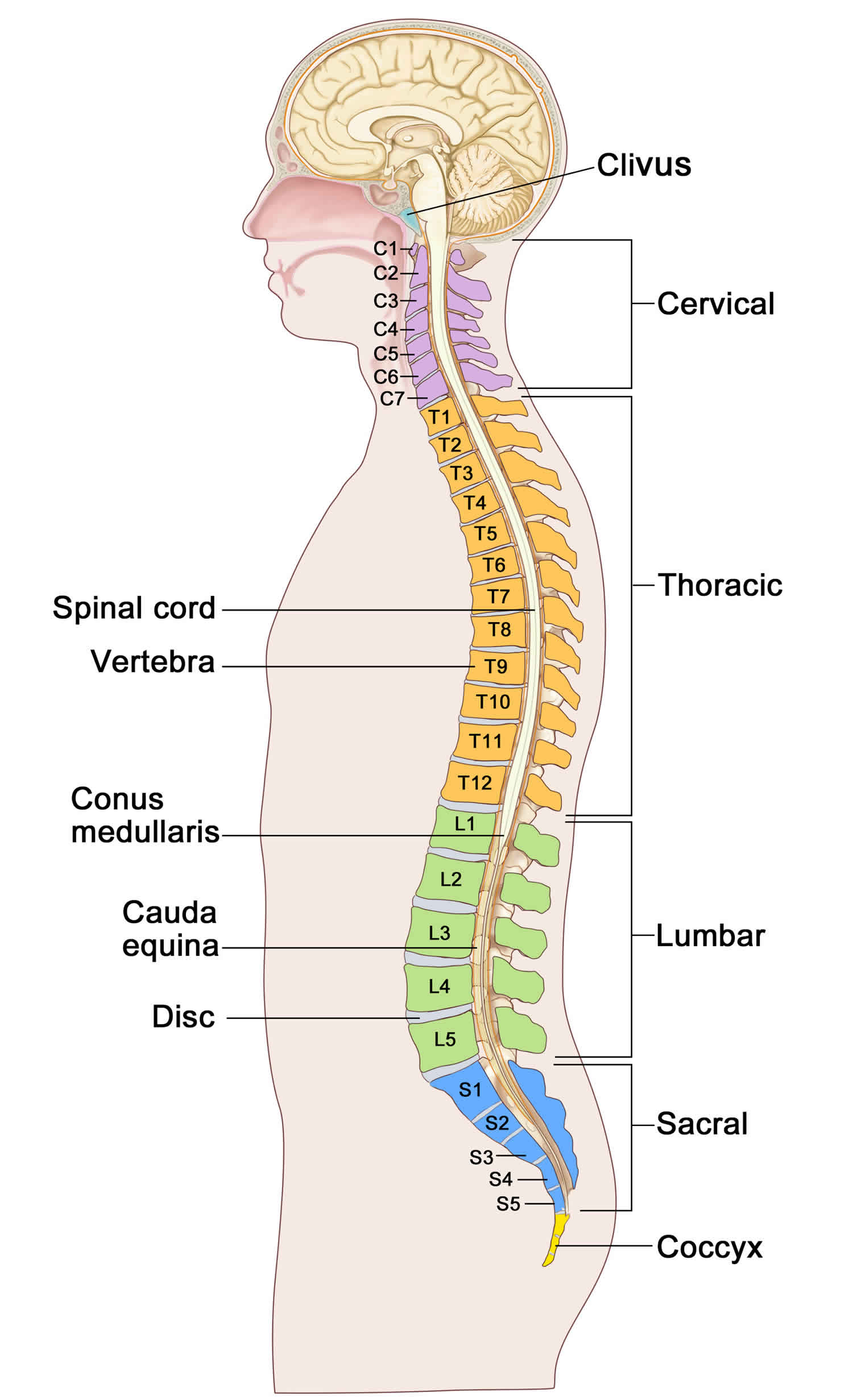
The cervical spine consists of the first seven vertebrae in the spinal column (C1-7). Typically, these vertebrae are small and possess a foramen on the transverse process for the vertebral artery. The thoracic spine consists of the next 12 vertebrae (T1-12) and is stabilized by the attached rib cage and intercostal musculature. The lumbar spine consists of a mobile segment of five vertebrae (L1-5), located between the relatively immobile segments of the thoracic and sacral segments.
The lumbar vertebrae are particularly large and heavy in comparison with the cervical and thoracic vertebrae. The bodies are wider and have shorter and heavier pedicles, and the transverse processes project somewhat more laterally and ventrally than the other spinal segments. The laminae are shorter vertically than the bodies and are bridged by strong ligaments. The spinal processes are broader and stronger than those in the thoracic and cervical spine.
Figure 1. Spine anatomy
Spinal infection causes
Spine infections occur by three major agents: bacteria, causing pyogenic infections; tuberculosis or fungi, responsible for granulomatosis infections; or by parasites, which are the less common etiology. In the past, tuberculosis infection was the major cause of spinal infections, however, due to the success on diagnosis and treatment of lung tuberculosis, its incidence has decreased during the last 50 years. Nowadays, the majority of spinal infections are bacterial monomicrobial 5 caused by Staphylococcus aureus with an incidence between 30 and 80 % 6. Gram-negative bacteria such as Escherichia coli are responsible, in some series, for up to 25 % of spinal infections 6. Mycobacterium tuberculosis is particularly common in HIV positive patients, reaching in this susceptive group up to 60 % of identified pathogens. Anaerobic agents are also a cause of infections, especially in penetrating spine trauma 7. Despite the efforts to identify the infectious agent, one-third of these have never been identified 8. However, particular attention should be given to some endemic areas such as Eastern Europe and Mediterranean countries, where both brucellosis and tuberculosis still have a high incidence 9. Turunc et al. 10, in a prospective study including a total of 75 spondylodiscitis patients, found that 13 of them (17.3 %) were caused by tuberculosis, 32 (42.7 %) by brucellosis, and 30 (40 %) by other bacterial agents.
Classically, there are three routes of pathogen spread:
- Hematogenous,
- Direct external inoculation,
- Spread from contiguous tissues.
In children, the intraosseous arteries have extensive anastomosis with some vessels penetrating the intervertebral disc 11. For this reason, a septic embolus from hematogenous spread does not cause bone infarction, and the infection is located essentially within the disc. The adult intervertebral disc is avascular and undergoes, around the third decade of life, an involution of the intraosseous anastomosis 12. Therefore, as the adult ages, the release of septic emboli leads to the formation of extensive vascular bone infarcts and spread of infection to adjacent structures leading to the classic spondylodiscitis imaging: erosion of vertebral endplates, osteolytic lesions, and compression fractures, which can lead to spine instability, deformity, and risk of spinal cord compression 12. An infection can lead to an uncontrolled spread beyond the bone structures and access the surrounding tissues, causing paravertebral and psoas abscesses. When spreading into the spinal canal, it can cause epidural abscesses, subdural abscesses, and meningitis. Spreading to the posterior structures is very rare because of its deficit vascular supply and occurs more frequently in fungal and tuberculosis spondylodiscitis 12.
Pyogenic spondylodiscitis caused by hematogenous spread affects mainly the lumbar spine (58 %), followed by thoracic (30 %) and cervical (11 %) 13, reflecting to some extent the vascular supply of these structures. Tuberculosis lesions preferentially affect the thoracic spine, often involving more than two levels, which differentiates it from pyogenic spondylodiscitis 13. Direct inoculation pathway is frequently iatrogenic: postsurgical lumbar procedures, after lumbar puncture or epidural procedures 14. Contiguous spread is rare and may occur in the context of adjacent infection, including esophageal ruptures, retropharyngeal abscesses, or infections of aortic implants 15.
Spinal infection symptoms
Nonspecific back or neck pain are generally the first clinical features, however, up to 15 % of patients could be pain free 16. With this insidious onset, patients have constant pain that worsens at night, often associated with radicular pain to the chest or abdomen 17. Fever is less common 18 occurring in about 48 % of patients with pyogenic spondylodiscitis and in about 17 % of tuberculosis spondylitis cases. Dysphagia and torticollis are symptoms that may be caused by cervical location 19.
Symptoms associated with neurological deficits, such as leg weakness, numbness, and incontinence, are present in about one-third of patients 20. These are often associated with late diagnosis 21, cervical infection 19, presence of epidural abscess, tuberculosis infection 22, and late diagnosis. During physical examination, it is important to look for kyphosis deformities, swelling, and tumefactions, which are often associated with tuberculosis spondylitis 23. Yet, it has been recognized a frequent association of pyogenic vertebral osteomyelitis and infectious endocarditis. Pigrau et al. 21 found among 91 cases of pyogenic vertebral osteomyelitis, 28 of them (30.8 %) had infectious endocarditis. This should not be underestimated during clinical evaluation: in patients with Gram-positive infections and cardiac infection risk, or symptoms such as new heart murmur, peripheral stigmata, or other metastatic foci; it is strongly recommended to perform an echocardiography 24.
In pediatric ages, clinical presentation is very nonspecific. Symptoms may include irritability, refusal to crawl, sit or walk, abdominal pain, or incontinence 25. Fever is rare in children 26, and the most frequent sign found on physical examination is the loss of lumbar lordosis 27. Development of neurological deficits is extremely rare 25.
Spinal infection diagnosis
Spinal infection diagnosis is generally difficult and requires a high level of suspicion. For this reason, a significant delay usually occurs between the first symptoms and diagnosis. This diagnosis should be supported by clinical, laboratory, and imaging findings 28.
Laboratory studies
There are several markers routinely used in clinical practice that are critical for diagnosis and further evaluation of treatment response 29. Erythrocyte sedimentation rate (ESR) is a sensitive marker of infection, yet with low specificity. Furthermore, ESR is also used as a marker of therapeutic response, for instance, Carragee et al. 29 found that a 25 % reduction of its initial value after 1 month of treatment was a good prognosis marker. However, in 9/18 (50 %) of those with no change in the ESR had good outcome 29. Thus, in patients responding to therapy, a raised ESR should not lead to unnecessary invasive procedures and/or prolonged therapy. The C-reactive protein (CRP) is also elevated in more than 90 % of spondylodiscitis cases 5, and some authors consider this marker the best monitor of treatment response, once it returns to normal after adequate treatment and faster than ESR 30. White blood cells (WBC) count is the least useful of all inflammatory markers, due to its low sensitivity 5.
Once a spinal infection is suspected, it is recommended to obtain blood and urine cultures before antibiotic initiation 31. According to the main monomicrobial pattern of pyogenic spondylodiscitis, about up to 59 % of positive blood cultures identify the causative microorganism 6. Aerobic cultures are performed routinely, while anaerobic were discouraged in the late 80s due to decreasing incidence of anaerobic bacteremia. Consequently, nowadays not all centers are capable to perform anaerobic cultures. Unfortunately, anaerobic bacteremia has reemerged as a significant clinical problem and its detection is highly recommended once it increases positive rate of blood cultures 32.
Despite a suspicious history, associated positive blood cultures, and imaging findings consistent with a clinical diagnosis of spinal infection, the definitive diagnosis only can be achieved by microscopic or bacteriological examination of the infected tissues. Several reports emphasize its importance in patients whose blood cultures were negative or inconclusive 33. However, due to the fact that biopsy is superior to blood cultures in pathogen detection, we routinely perform biopsies in suspicious cases. Gasbarrini et al. 34 published recently a comparative study where they conclude that percutaneous CT-guided needle biopsy is the mainstay of diagnosis for spinal lesions of unknown etiology and its accuracy has been reported up to 70 %. Nevertheless, the diagnostic yield of CT-guided needle biopsy is variable between centers, depending on the expertise of the radiologist, the number of samples sent, and the absence of previous antibiotic therapy; thus some authors reserve open biopsies for cases with CT-guided negative cultures 13. In cases of patients with absolute indication for surgical treatment, open biopsy is the first choice as biopsy method once allows a greater amount of tissue to be harvested. Higher tissue yield and consequently more specific results are obtained than with percutaneous CT‐guided needle biopsy. For those without criteria for surgery and given the importance of histological diagnosis, CT-guided biopsy is a true option. The role of biopsy in children is not consensual. Some authors recommend it routinely, while others defend its performance only in cases of refractory to empirical treatment or in suspected fungal or mycobacterial infection 35. The specimens should be submitted to microbiological analysis, such as Gram smear, aerobic and anaerobic cultures, and fungal culture particularly for tuberculosis infections, AFB (acid fast bacilli) smear, polymerase chain reaction, and tuberculosis culture. Once Mycobacterium tuberculosis grows slowly (6–8 weeks) 36, a valuable aid for a faster diagnosis is the use of interferon-gamma release assays (IGRA) measured from whole blood plasma, providing results in less than 24 hours. In addition, according to Kumar et al. 37, in a study with 70 patients followed for spinal TB infection, the sensitivity of the AFB smear and culture (together) was 59 % and the further addition of IGRA data resulted in a sensitivity of 88 %. Histopathology, per se, has a complementary value to microbiological culture in distinguishing pyogenic from granulomatous diseases 38 and is mandatory if tumor lesions are suspected 39.
Imaging studies
Plain radiographs should be performed in an initial evaluation for suspected pathology of the spine. Although it has low specificity (57 %) in spondylodiscitis diagnosis, it will reveal, in advanced cases, irregularity of vertebral endplates with eventual fragmentation and low intervertebral disc height 40. It is also important to identify any coronal or sagittal malalignment resulting from the disease process.
Computed tomography (CT) remains the best test for evaluation of bony changes, including early changes of vertebral endplates, the presence of bone necrosis, and pathological calcifications suggestive of tuberculosis 40. CT is also routinely used in percutaneous CT-guided needle biopsy 38.
Magnetic resonance imaging (MRI) is considered the gold standard modality for spondylodiscitis imaging diagnosis 41 due to its high sensitivity (96 %), specificity (94 %), and greater capacity to provide detailed anatomical information about surrounding soft tissues and epidural space 42. The characteristic changes consist of a hypointense signal of the disc and vertebral body on T1-weighted images and a hyperintense signal of the same structures (due to edema) on T2-weighted images. Gadolinium enhancement of the intervertebral disc, vertebral body, and surrounding soft tissues increases the accuracy of MRI, especially when other changes are subtle and also help in the differentiation of infectious lesions from degenerative (T2 hypointensity favoring Modic endplate changes) and tumor lesions (T1 hypointense relatively to normal bone marrow) 43. MRI also plays an important role in the distinction between tuberculosis spondylitis and pyogenic spondylodiscitis 44. Tuberculosis spondylitis has an extensive bone destruction pattern with relative sparing of the intervertebral disc, heterogeneous enhancement of the vertebral body, and large paravertebral abscesses. Table 1 summarizes several imaging features that can strongly support differential diagnosis of spinal infection causes. Once different appearances occur at different stages, there is no pathognomonic finding on MRI that reliably distinguishes among spinal infections etiologies or from a possible neoplasm.
Kowalski et al. 45 suggest that certain MRI findings may persist or even worsen during treatment, despite the clinical improvement, and may lead to unnecessary invasive treatments. Changes compatible to resolution of the infection appear later and consist in the loss of gadolinium uptake and restoration of bone. Therefore, despite the increasing use of follow-up MR imaging to monitor response to treatment in patients with spinal infection, the study of Kowalski does not support the routine use of follow-up MR imaging in patients who are clinically responding to therapy 45.
Sequential bone/gallium imaging and 67 Ga-SPECT are currently the radionuclide procedures of choice for spinal infections, but the observed lack of specificity have lead to an increase interest in [18F]Fluoro-2-deoxy-d-glucose (FDG) PET, as a promising technique in the absence of spinal instrumentation as degenerative changes and fractures usually do not produce intense FDG uptake 46. Despite its increasing importance, radionuclide imaging in spinal infections should be reserved for cases of uncertain diagnosis or when MRI is inconclusive.
Table 1. Imaging features that can strongly support differential diagnosis of spinal infection causes
| Pyogenic | Tuberculous | Brucellar | Fungal | |
|---|---|---|---|---|
| Spine segment | Lumbar | Thoracic/thoracolumbar junction | Lower lumbar | Lumbar |
| Vertebral body (VB) | Early stage: anterior aspect of vertebral body classically vertebral body T1 hypo- and endplate T2 hyperintensity Late stage: vertebral body destruction; T2 hyperintensity and homogeneous enhancement; Adjacent vertebral body involvement | Early stage: anterior aspect of vertebral body three patterns: para discal (more common)—discal involvement and contiguous spread to adjacent vertebral body, T1 hypointensity and T2 heterogenous hyperintensity. Anterior—anterior scalloping of vertebral body and large subligamentous abcesses Central—vertebra plana deformity; intervertebral disc not involved Late stage: T1 variable intensity with bone healing | Relatively preserved vertebral body | Involvement: serrated margins of vertebral endplates without severe vertebral body destruction |
| Disc space involvement | Present: early stage involvement T2 hyperintensity and enhancement | Variable: from disc space sparing up to severe destruction | Present | Typically spared; lack of T2 hyperintensity |
| Paraspinal/epidural space involvement | If present: inflammation and/or small abscesses with thick and irregular rim enhancement | Present: large paraspinal abscesses; thin and smooth rim enhancement | Typically not present: lack of paraspinal abscess | Present: Small paraspinal abscesses thick and irregular rim enhancement |
| Posterior elements | Typically not involved | Can be involved | Typically not involved | Can be involved Rib heads also |
| Anterior subligamentous spread | Uncommon | Present: can be more extensive than the vertebral involvement | Uncommon | Common |
| Adjacent vertebral levels involvement | Present: endplate destruction | Present: high bone destruction | Uncommon | Uncommon |
| Multilevel involvement | Uncommon | Common: skip lesions | Uncommon | Common: skip lesions |
Spinal infection treatment
The key principles for successful treatment of spinal infections are antibiotic therapy for eradication of the underlying infection; fixation of the affected segment to preserve or restore the spinal structure and stability; and debridement and decompression of the spinal canal in the presence of neurological deficits or epidural abscesses 48.
Spine infections are infrequently emergency situations at presentation and for this reason, antibiotic therapy should be initiated only after a definitive etiologic diagnosis.
In the presence of sepsis or the impossibility of an etiologic diagnosis, empirical antibiotic therapy should be considered. The antibiotic spectrum must be extended to cover S. aureus and E. coli, the commonest pathogens for pyogenic spondylodiscitis, and obviously take into account the local epidemiology and the possibility of colonization by resistant organisms 6. In cases of bacteremia caused by methicillin-resistant S. aureus (MRSA), the drug of choice is usually vancomycin; however, its efficacy is doubtful. To ensure therapeutic concentration levels in the bone, the American Society of Infectious Diseases recommends maintaining vancomycin concentrations above 15–20 mg/L.
In confirmed tuberculosis spondylitis, specific tuberculostatic therapy should be initiated. The guidelines from British infection society recommends that the treatment of all forms of central nervous system (CNS) tuberculosis should consist of four drugs (isoniazid, rifampicin, pyrazinamide, ethambutol) for 2 months followed by two drugs (isoniazid, rifampicin) for at least 10 months 49. In atypical infections, a consensual therapeutic regime has not been yet established 6.
Regarding fungal spondylodiscitis, it is generally difficult to identify the fungal agent, and antimycotic therapy is often complicated. Although there is no consensus in the literature, man authors recommend surgery as first approach 48.
The literature provides no clear guidance regarding the duration and route of administration of antibiotic therapy. Generally, an initial parenteric administration is advised, during a range of 3–8 weeks period 50. Long duration therapy has not been directly related to better outcomes. For instance, Roblot et al. 51, in a retrospective study of 120 patients, found no difference in the risk of relapse amongst patients treated for 6 weeks or longer. After this initial period, antibiotic therapy could follow on with oral administration based on individual response and type of pathogen involved. In nonspecific pyogenic spondylodiscitis, oral antibiotic therapy is recommended for an additional period of 6 weeks to 3 months 52. Given the high bioavailability and good diffusion of fluoroquinolones, clindamycin, rifampicin, and fusidic acid, some reports indicate exclusively oral antibiotherapy, avoiding the inconveniences of parenteric treatment 51. In addition, due to these facts, an early conversion from parenteric to oral therapy can be performed. In this scenario, before early oral conversion, endocarditis must be excluded 21. In tuberculosis spondylitis, the treatment should be continued for a period of 10–24 months, to allow adequate healing and prevent recurrence 6.
It has been proposed that a weekly reduction of 50 % in CRP is suggestive of a favorable evolution, and accepted criteria for discontinuing the antimicrobial treatment includes improvement or resolution of the symptoms and normalization of ESR or CRP 53.
Conservative treatment
In patients without a formal indication for surgery (neurological deficits, spine instability, and intractable pain) or with high surgical risk, conservative treatment is a real valid option 6.
The controversy arises in the presence of minor neurological deficits 21. In Pigrau et al. 21 series, only 13 % of the patients required surgery, even though 29.7 % of patients had neurologic symptoms. In our opinion, the conservative approach in this particular scenario is desirable if there is no spinal instability. Neurological symptoms are minor and expected to improve with specific antibiotic therapy.
Immobilization is one of the milestones of a successful conservative approach. The immobilization of the affected segment is necessary when pain is significant and there is no risk of instability. It also eliminates the need for prolonged bed rest. Cervical spine immobilization can be achieved using a collar or a halo-fixator. For the thoracic or lumbar spine, a thoracolumbar brace allows the load distribution to the unaffected joints and reduce the pressure on the affected vertebra 6. Known risks related to immobilization include up to 50 % non-union rate at an involved disc, which may lead to kyphosis deformity and chronic pain syndrome 28. If a patient demonstrates increasing pain and deformity in spite of improvement of laboratory indices, then surgical management should be considered.
Even with a conservative approach, the CT-guided percutaneous drainage can be effective for patients with pyogenic spondylodiscitis and a secondary psoas abscess.
Surgical management
Early surgical treatment should be performed in the presence of neurological deficits or sepsis 54. Absolute surgical indications also include spinal instability due to extensive bone destruction, severe kyphosis, intracanal spinal lesion with mass effect, unknown etiologies associated with active tumor, and in failure of conservative treatment 6. Some authors also recommend surgical treatment in the presence of epidural abscess even without associated neurological deficits, especially in the cervical and thoracic region 55. The relative indications consist of the presence of uncontrolled pain and inexistent conditions for conservative treatment 52.
Despite indication for surgery in the presence of neurological deficits, age and presence of concurrent medical conditions may affect surgical decision 8. According to Yoshimoto et al. 56 in a review of 45 cases of pyogenic spondylitis in elderly, 42 % of patients with paralysis on admission were not submitted to surgery due to poor general condition. Yet, paralysis was improved in 73 % of these patients with conservative treatment 56.
The main goals of surgical treatment of spinal infections include (1) early decompression of the spinal canal and stabilization of the involved vertebral segment, in the presence of neurological deficits 57; (2) aggressive tissue debridement, including drainage of paravertebral abscesses; and (3) sample harvesting for microbiological and histological analyses.
Regarding the surgical strategy itself, recommendations are controversial 58. Any standard approach can be used (anterior, posterior, combined, or minimally invasive approaches), whereas the choice is related mainly to the presence of neurological deficits, the location of the infection, and degree of associated bone destruction (see Table 2).
In cervical spinal infections, an anterior approach is recommended with appropriate debridement, decompression (eventual corpectomy), and fusion with bone graft, associated with anterior plate stabilization. In multilevel intervention, this should be complemented with posterior instrumentation 57. Eventually, if the involvement was mainly epidural with no severe destruction of the vertebral body, it is acceptable to proceed toward posterior decompression and fusion 57.
In the thoracic spine, as stability is maintained mostly by the rib cage and with physiologically restricted mobility, stability issues may not be a significant priority. Therefore, in the presence of an epidural involvement without anterior disc or bony destruction, a posterior approach with decompression and instrumentation is usually the first option. A purely anterior approach for decompression and fusion (using a transthoracic, posterolateral, or thoracoscopic approaches) is reserved for monosegmental lesions without involvement of posterior elements 59. Even in this situation, consideration for adjunctive posterior stabilization is often considered. In advanced anterior bone destruction and collapse, it is recommendable an anterior approach for debridement, decompression, and fusion with bone graft complemented with additional posterior instrumentation 57.
At the thoracolumbar juncture, decompression and stabilization are recommended in the presence of a neurological deficit or extensive epidural invasion. In cases of monosegmental spondylodiscitis with moderate anterior bone involvement and minimal kyphosis deformity, a posterior lumbar interbody fusion may be sufficient 60. Many surgeons prefer, however, not to invade the posterior tissues with exposure to purulent tissue and would prefer an initial anterior debridement followed by a posterior stabilization procedure.
In the presence of an extensive anterior bone destruction and collapse with segmental kyphosis, a double approach (performed in one or two stages) with anterior debridement and interbody fusion associated with posterior instrumentation results in faster fusion, improved correction of the kyphotic deformity and its maintenance, as well as earlier patient mobilization 61; yet, opinions diverge about the best option for anterior interbody fusion (see Table 2). Classically, bone grafting with tricortical iliac autograft is recognized as a safe procedure, with excellent and consistent outcomes 61. Structural bone allograft can be used as an alternative, avoiding donor site morbidity and reducing operative time 62. Furthermore, recent publications have demonstrated improved fusion rates when combining recombinant human bone morphogenic protein-2 (rhBMP-2) with structural bone graft 63. A drawback that surgeons must keep in mind when using bone allografts, although not frequent, is the risk of provoking an immune reaction or that it could become a source for infection transmission 61.
Structural bone grafting persists as a standard procedure in several centers, majorly due to the concern about risks of introducing hardware in an infected field 64 . Nevertheless, several recent publications show that metallic implants can be safely used in spinal infections (Table 2) 62. Despite the importance in distinguishing the different risks of using metallic implants in pyogenic or tuberculous infection, enthusiastic outcomes have been published on the use of hardware in both cases. Erturer et al. 65, in a series of 20 patients with tuberculous spondylitis submitted to anterior interbody fusion using titanium mesh cage, reported solid fusion in all patients with maintenance of kyphosis correction, as well as no recurrence of tuberculosis infection 65. Regarding pyogenic spondylodiscitis, Liljenqvist et al. 66 reported a 100 % fusion rate and infection eradication in a 20-patient series with destructive vertebral osteomyelitis treated by a double approach with anterior column reconstruction using an expandable titanium cage filled with morselized autologous bone graft 66.
With the advent and development of minimally invasive spine surgery, some techniques have been used successfully in the treatment of spinal infection. At the thoracic segment, thoracoscopic approach has been used in some centers with exciting results and additional advantages such as pain reduction and improved postoperative respiratory function, less damage to the soft tissues, resulting in improved esthetic results, and shorter hospital stay 59. In the lumbar segment, posterior percutaneous instrumentation is already regularly used in patients who underwent double approach.
Table 2. Summary of the most relevant features of spinal infections’ surgical treatment—clinical series published in the last 5 years
| Reference | Number of patients | Follow-up (Months) | Neurologic deficitis (%) | Etiology | Spine segment | Surgical approach | Anterior reconstruction | Mortality (%) | Neurologic improvement (%) | Union rate (%) | Loss of correction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. 2012 67 | 14 | 50.1 (42–64) | 100.0 | TB | Lumbar | Combined (two-staged) | Allograft (fresh frozen ICBG) | 0 | 100.0 | 100.0 | 1.9° |
| Shousha et al. 2012 68 | 30 | 28.4 (6–54) | 40.0 | PY | Cervical | Single anterior (17) Combined (13) | CAGE (17) Autologous ICBG (10) | 10 | 58.0 | 100.0 | (–) |
| Masuda et al. 2011 69 | 30 | 26.2 (3–60) | 90.0 | PY (19) TB (11) | TL | Combined (two-staged) | Bone graft | 0 | 56.7 | 100.0 | (–) |
| Koptan et al. 2011 70 | 30 | 66 (24–84) | 60.0 | TB | Cervical | Single anterior | CAGE (16) Autologous ICBG (14) | 0 | 94.4 | 93.0 | (–) |
| Erturer et al. 2010 65 | 20 | 52.7 (37–94) | 55 | TB | TL | Combined (single-staged) | CAGE (allgograft) | 0 | 90.9 | 100 | 0.8° |
| Chen et al. 2010 71 | 16 | 24 (–) | 31.3 | PY (10) TB (1) | Lumbar | Single anterior (12) Combined (4) | Autologous ICBG | 12.5 | 80.0 | 85.7 | 0° |
| Hempelmann et al. 2010 72 | 18 | 21 (12–42) | 38.9 | PY | Lumbar | Posterior (PLIF) | Autologous ICBG | 17 | 100.0 | 100.0 | (–) |
| Hirakawa et al. 2010 73 | 10 | 48.2 (24–101) | 60.0 | TB | Lumbar | Combined (two-staged) | Autologous bone | 0 | 66.7 | 100.0 | 5.1° |
| Okada et al. 2009 74 | 52 | 35.9 (24–120) | 80.8 | PY (25) TB (27) | Cervical TL | Single anterior (15) Posterior (7) Combined (30) | (–) | 0 | 90.4 | (–) | 1.9° PY 2.4° TB |
| Zaveri et al. 2009 75 | 15 | 41 (26–69) | 86.7 | TB | Lumbar | Posterior (PLIF) | CAGE (autologous bone) | 0 | 100.0 | 86.7 | 1° |
| Lu et al. 2009 62 | 36 | 21 (10–39) | 72.2 | PY (30) TB (6) | Cervical TL | Combined (27) Single anterior (5) Posterior (4) | CAGE (autograft or allograft) | 2.7 | 92.0 | (–) | (–) |
| Robinson et al. 2008 76 | 22 | 36 (32–47) | 22.7 | PY (12) TB (1) | Cervical TL | Single anterior (3) Combined (19) | CAGE (autologous bone) | 9 | (–) | 100.0 | 3.7° |
| Pee et al. 2008 77 | 60 | 35.8 (26–50) | 26.7 | PY | Lumbar | Combined (single-staged) | CAGE (37) Autologous ICBG (23) | 0 | 68.7 | 95.0 | (–) |
| Korovessis et al. 2008 78 | 24 | 56 (31–116) | 58.3 | PY (20) BC (4) | Cervical TL | Combined (single-staged) | CAGE (autologous bone) | 0 |
Spinal infection prognosis
With the advent of antibiotics, improved techniques of management, and early recognition, mortality associated with spinal infections has significantly decreased to <5 % in developed countries 16, and early mortality is generally related to uncontrolled sepsis. Despite mortality has declined, the most worrying outcome is the potential for a permanent neurological deficit.
Some retrospective outcome studies present distinct prognostic factors. A summary of those related to a poor outcome is shown in Table 3. Besides age and spine segment, underlying conditions that are associated with poor prognosis, the major prognostic factor was the presence of a motor deficit before treatment and if the neurological deficits are present for longer than 36 hours 52. Whenever these patients gather surgical conditions, an operative approach might greatly improve prognosis. In a series by Hadjipavlou et al. 54, 23 % of patients with paralysis on admission recovered completely after surgical decompression.
Despite the presence of neurological deficits on admission, at medium- and long-term follow-up, residual symptoms persist independently of treatment choice and this detrimental outcome is directly related with diagnosis delay 79. These sequelae are essentially the result of degenerative changes secondary to tissue destruction by the infectious process. McHenry et al. 53 reported in a 253 patients follow-up series, 14 % of patients had a recurrence of their infection of which 75 % occurred in the first year after surgery. In childhood, the prognosis is excellent 35.
Table 3. Spinal infections’ prognostic factors associated with poor outcomes
| Prognostic factor | Poor outcome |
| Age | Older patients |
| Spinal segment | Cervical/thoracic involvement |
| Underlying disease | Diabetes mellitus |
| Chronic heart disease | |
| Clinical presentation | Paralysis |
| Bowel/bladder disfunction | |
| Diagnosis | Delayed |
| Pathogen | MRSA |
| Length of time for surgery | >36 h |
- Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018 Jan 22.[↩]
- Spinal Infections. https://emedicine.medscape.com/article/1266702-overview[↩]
- Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009 Aug. 39 (1):10-7.[↩]
- Fujiyoshi T, Goto K, Shiomori T. [A case of spinal epidural abscess associated with retropharyngeal abscess]. Nippon Jibiinkoka Gakkai Kaiho. 2002 Nov. 105(11):1143-6.[↩]
- Euba G, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum. 2008;38(1):28–40.[↩][↩][↩]
- Sobottke R, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105(10):181–187.[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Lim MR, Lee JY, Vaccaro AR. Surgical infections in the traumatized spine. Clin Orthop Relat Res. 2006;444:114–119.[↩]
- Govender S. Spinal infection. J Bone Joint Surg Br. 2005;87 (B):1454–1458.[↩][↩]
- Colmenero JD, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56(12):709–715.[↩]
- Turunc T, et al. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55(2):158–163.[↩]
- Ratcliffe JF. An evaluation of the intra-osseous arterial anastomoses in the human vertebral body at different ages. Microarteriographic Study. J Anat. 1982;134(Pt 2):373–382.[↩]
- Ratcliffe JF. Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis. A microarteriographic investigation. Acta Radiol Diagn (Stockh) 1985;26(2):137–143.[↩][↩][↩]
- Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 Suppl 3:iii11–iii24.[↩][↩][↩]
- Silber JS, et al. Management of postprocedural discitis. Spine J. 2002;2(4):279–287.[↩]
- Babinchak TJ, Riley DK, Rotheram EB., Jr Pyogenic vertebral osteomyelitis of the posterior elements. Clin Infect Dis. 1997;25(2):221–224.[↩]
- Fantoni M, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):2–7.[↩][↩]
- Jensen AG, et al. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980–1990. J Infect. 1997;34(2):113–118.[↩]
- Kim CJ, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 197) 2010;35(21):E1096–E1100.[↩]
- Schimmer RC, et al. Osteomyelitis of the cervical spine: a potentially dramatic disease. J Spinal Disord Tech. 2002;15(2):110–117.[↩][↩]
- Mylona E, et al. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–17.[↩]
- Pigrau C, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118(11):1287.[↩][↩][↩][↩][↩]
- Turgut M. Spinal tuberculosis (Pott’s disease): its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurg Rev. 2001;24(1):8–13.[↩]
- Chang MC, et al. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine (Phila Pa 1976) 2006;31(7):782–788.[↩]
- Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362(11):1022–1029.[↩]
- Garron E, et al. Nontuberculous spondylodiscitis in children. J Pediatr Orthop. 2002;22(3):321–328.[↩][↩]
- Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105(6):1299–1304.[↩]
- Brown R, et al. Discitis in young children. J Bone Joint Surg Br. 2001;83(1):106–111.[↩]
- Frangen TM, et al. Surgical management of spondylodiscitis. An analysis of 78 cases. Unfallchirurg. 2006;109(9):743–753.[↩][↩]
- Carragee EJ, et al. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997;22(18):2089–2093.[↩][↩][↩]
- Kang BU, et al. Surgical site infection in spinal surgery: detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010;13(2):158–164.[↩]
- Lillie P, et al. Healthcare associated discitis in the era of antimicrobial resistance. J Clin Rheumatol. 2008;14(4):234–237.[↩]
- Lassmann B, et al. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895–900.[↩]
- Karadimas EJ, et al. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008;79(5):650–659.[↩]
- Gasbarrini A, et al. Biopsy for suspected spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):26–34.[↩]
- Fucs PM, Meves R, Yamada HH. Spinal infections in children: a review. Int Orthop. 2012;36(2):387–395.[↩][↩]
- Cheng VC, et al. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. J Clin Pathol. 2004;57(3):281–285.[↩]
- Kumar R, Das RK, Mahapatra AK. Role of interferon gamma release assay in the diagnosis of Pott disease. J Neurosurg Spine. 2010;12(5):462–466.[↩]
- de Lucas EM, et al. CT-guided fine-needle aspiration in vertebral osteomyelitis: true usefulness of a common practice. Clin Rheumatol. 2009;28(3):315–320.[↩][↩]
- Delogu G, Zumbo A, Fadda G. Microbiological and immunological diagnosis of tuberculous spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):73–78.[↩]
- Jevtic V. Vertebral infection. Eur Radiol. 2004;14(Suppl 3):E43–E52.[↩][↩]
- Leone A, et al. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):8–19.[↩]
- Modic MT, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157(1):157–166.[↩]
- Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50(4):777–798.[↩]
- Anley CM, Brandt AD, Dunn R. Magnetic resonance imaging findings in spinal tuberculosis: comparison of HIV positive and negative patients. Indian J Orthop. 2012;46(2):186–190.[↩]
- Kowalski TJ, et al. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. AJNR Am J Neuroradiol. 2007;28(4):693–699.[↩][↩]
- Gemmel F, et al. Expanding role of 18F-fluoro-d-deoxyglucose PET and PET/CT in spinal infections. Eur Spine J. 2010;19(4):540–551.[↩]
- Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22(12):2787–2799. doi:10.1007/s00586-013-2850-1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3843785[↩][↩]
- Zarghooni K, et al. Treatment of spondylodiscitis. Int Orthop. 2012;36(2):405–411.[↩][↩]
- Thwaites G, et al. British infection society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–187.[↩]
- Friedman JA, et al. Spontaneous disc space infections in adults. Surg Neurol. 2002;57(2):81–86.[↩]
- Roblot F, et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007;36(5):269–277.[↩][↩]
- Butler JS, et al. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine (Phila Pa 1976) 2006;31(23):2695–2700.[↩][↩][↩]
- McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342–1350.[↩][↩]
- Hadjipavlou AG, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25(13):1668–1679.[↩][↩]
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355(19):2012–2020.[↩]
- Yoshimoto M, et al. Pyogenic spondylitis in the elderly: a report from Japan with the most aging society. Eur Spine J. 2011;20(4):649–654.[↩][↩]
- Pola E, et al. Surgical treatment of tuberculous spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):79–85.[↩][↩][↩][↩]
- Linhardt O, et al. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop. 2007;31(1):113–119.[↩]
- Muckley T, et al. The role of thoracoscopic spinal surgery in the management of pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 2004;29(11):E227–E233.[↩][↩]
- Lee JS, Suh KT. Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Joint Surg Br. 2006;88(6):765–770.[↩]
- Korovessis P, Repantis T, Hadjipavlou AG. Hematogenous pyogenic spinal infection: current perceptions. Orthopedics. 2012;35(10):885–892.[↩][↩][↩]
- Lu DC, Wang V, Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery. 2009;64(1):122–129.[↩][↩][↩]
- Rihn JA, et al. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J. 2009;18(11):1629–1636.[↩]
- Jin D, et al. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13(2):114–121.[↩]
- Erturer E, et al. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010;19(12):2209–2215.[↩][↩][↩]
- Liljenqvist U, et al. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12(6):606–612.[↩][↩]
- Zhang HQ, et al. Two-stage surgical management using posterior instrumentation, anterior debridement and allografting for tuberculosis of the lower lumbar spine in children of elementary school age: minimum 3-year follow-up of 14 patients. Arch Orthop Trauma Surg. 2012;132(9):1273–1279.[↩]
- Shousha M, Boehm H. Surgical treatment of cervical spondylodiscitis: a review of 30 consecutive patients. Spine (Phila Pa 1976) 2012;37(1):E30–E36.[↩]
- Masuda T, et al. Comparative study on the efficacy of two-staged (posterior followed by anterior) surgical treatment using spinal instrumentation on pyogenic and tuberculotic spondylitis. Arch Orthop Trauma Surg. 2011;131(6):765–772.[↩]
- Koptan W, Elmiligui Y, Elsharkawi M. Single stage anterior reconstruction using titanium mesh cages in neglected kyphotic tuberculous spondylodiscitis of the cervical spine. Eur Spine J. 2011;20(2):308–313.[↩]
- Chen LH, et al. Surgical treatment of infectious spondylitis in patients undergoing hemodialysis therapy. Eur Spine J. 2010;19(12):2223–2228.[↩]
- Hempelmann RG, Mater E, Schon R. Septic hematogenous lumbar spondylodiscitis in elderly patients with multiple risk factors: efficacy of posterior stabilization and interbody fusion with iliac crest bone graft. Eur Spine J. 2010;19(10):1720–1727.[↩]
- Hirakawa A, et al. Surgical outcome of 2-stage (posterior and anterior) surgical treatment using spinal instrumentation for tuberculous spondylitis. J Spinal Disord Tech. 2010;23(2):133–138.[↩]
- Okada Y, et al. Clinical and radiological outcome of surgery for pyogenic and tuberculous spondylitis: comparisons of surgical techniques and disease types. J Neurosurg Spine. 2009;11(5):620–627.[↩]
- Zaveri GR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009;22(4):257–262.[↩]
- Robinson Y, et al. Successful treatment of spondylodiscitis using titanium cages: a 3-year follow-up of 22 consecutive patients. Acta Orthop. 2008;79(5):660–664.[↩]
- Pee YH, et al. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine. 2008;8(5):405–412.[↩]
- Korovessis P, et al. Beneficial influence of titanium mesh cage on infection healing and spinal reconstruction in hematogenous septic spondylitis: a retrospective analysis of surgical outcome of twenty-five consecutive cases and review of literature. Spine (Phila Pa 1976) 2008;33(21):E759–E767.[↩]
- Lu CH, et al. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg. 2002;104(4):306–310.[↩]