Split S2
The S2 heart sound or the second heart sound (S2) is produced with the closing of the aortic and pulmonic valves in diastole 1. The S2 should split into two components when the patient inspires. The aortic valve (A2 or aortic second sound) closes sooner than the pulmonic valve (P2 or pulmonic second sound), and it is the louder component of S2; this occurs because the pressures in the aorta are higher than the pulmonary artery. The splitting of S2 occurs because inspiration brings more blood into the right ventricle. Right ventricular ejection is prolonged, and the pulmonary valve (P2) closes later. A loud, single S2 indicates either pulmonary hypertension or congenital heart disease involving one of the semilunar valves. Unlike the S1 (first heart sound, produced by the mitral and tricuspid valves closing in systole), under normal conditions, the closure sound of the aortic (A2) and pulmonic (P2) valves can be discernable, which occurs during inspiration due to the increase in venous return. The increase in volume means the right ventricle will take longer to pump out blood, which slightly delays the pressure increase in the pulmonic artery that leads to the pulmonic valve closure. So, the later sound in a physiologic split S2 is the closure of the pulmonic valve 2. S2 can provide a lot of useful clinical information. Some have referred to it as the “auscultatory anchor point” pointing to its use as a reliably discernable sound that orients the listener to the other sounds 3.
The basics of S2 (second) heart sounds
- In the normal heart:
- During inspiration:
- The S2 (second heart sound) is made of two component sounds:
- Aortic valve closure (A2) which happens first. A2 is heard widely all over the chest. So when you hear ‘S2’ at the mitral area, you are really hearing A2.
- Pulmonic valve closure (P2) which happens second. Normally, P2 is soft and only heard at the pulmonic region (left parasternal, intercostal space 2), however even in this region A2 is louder.
- The S2 (second heart sound) is made of two component sounds:
- During expiration:
- The S2 (second heart sound) is usually single. With expiration, aortic valve closure (A2) and pulmonic valve closure (P2) may be superimposed and are rarely split as much as 0.04 second 4. If the S2 is split by greater than 0.04 second on expiration, it is usually abnormal 4. Therefore, the presence of audible S2 splitting during expiration (i.e., the ability to hear two distinct sounds during expiration) is of greater significance at the bedside in identifying underlying cardiac pathology than is the absolute inspiratory increase in the A2–P2 interval.
- During inspiration:
Physiologic S2 splitting is demonstrated during inspiration in normal individuals, since the splitting interval widens primarily due to the delayed pulmonic valve closure (P2). During expiration, the A2–P2 interval is so narrow that only a single sound is usually heard.
A late systolic click of mitral valve prolapse, the opening snap of mitral stenosis, a third heart sound (S3), or a pericardial knock may be incorrectly thought to represent fixed S2 splitting 4. A systolic click may vary its location in systole with certain maneuvers that change the shape of the left ventricle. The best way to differentiate an A2–P2 from an A2–opening snap is to have the patient stand up. The A2–P2 interval remains the same or narrows, whereas the A2–opening snap interval widens. The third heart sound, which forms the S2–S3 complex, is lower in frequency than S2, is best heard at the apex, is usually not heard at the basal auscultatory area, and occurs 0.12 to 0.16 second after A2. The pericardial knock is a third heart sound that is slightly higher pitched and earlier than the usual S3 and is also best heard at the apex.
Exam technique in S2 (second) heart sounds
- Splitting best heard in the 2nd left intercostal space, close to the sternal border.
- Use the diaphragm of your stethoscope
- Second heart sounds are best heard when patients are semi-recumbent (30-40 degrees upright) and in quiet inspiration.
- The intensity of pulmonic valve closure (P2) is determined relative the aortic valve closure (A2). The intensity of P2 is considered elevated if P2 is louder than A2 at the pulmonic region (left parasternal, intercostal space 2)
Clinical assessment of S2 is best performed with the patient lying comfortably in the supine position and breathing normally.
First, attempt to palpate the aortic and pulmonic components of the second heart sound in the second right and second or third left intercostal spaces (ICS), respectively. Then begin cardiac auscultation with the stethoscope placed at the second right intercostal spaces. The second sound, like the first, is evaluated by sequentially auscultating over the second left intercostal spaces, the fourth left intercostal spaces along the left sternal border, and the cardiac apex. When listening to the heart sounds, it is essential simultaneously to palpate either the carotid artery or apex impulse to determine the onset of systole.
The second heart sound is of shorter duration and higher frequency than the first heart sound. It has two audible components, the aortic closure sound (A2) and the pulmonic closure sound (P2), which must be separated by more than 20 msec (0.20 sec) in order to be differentiated and heard as two distinct sounds. It is clinically very important to determine the presence and degree of respiratory splitting and the relative intensities of A2 and P2.
Splitting is best identified in the second or third left intercostal spaces, since the softer P2 normally is confined to that area, whereas the louder A2 is heard over the entire precordium, including the apex. In order to appreciate splitting of S2, it may be useful to gradually move the stethoscope (“inching”) from the second right intercostal spaces to the fourth left intercostal spaces.
The influence of respiration on the second sound is extremely important. The examiner will wish to note respiratory variation both during quiet breathing and at times during exaggerated breathing. Slow, regular respirations are best for auscultation because a long deep breath may attentuate P2 by interposing lung tissue over the stethoscope, and only a single sound will be heard. The interval between the two audible components of the second heart sound normally increases on inspiration and virtually disappears on expiration. The patient’s age must be taken into consideration when assessing splitting of the second sound, since the likelihood of hearing a single S2 during both respiratory phases increases with advancing age. As with the first sound, the patient should be examined in several positions. The supine position in young patients, for example, may yield an erroneous impression of abnormally wide S2 splitting, which can be avoided by reexamining the patient in the sitting or standing position. The Valsalva maneuver may also be used to exaggerate splitting of the second sound.
When the second sound is split and both components can be heard and identified, a reliable judgment about the relative loudness (intensity) of each component can be made.
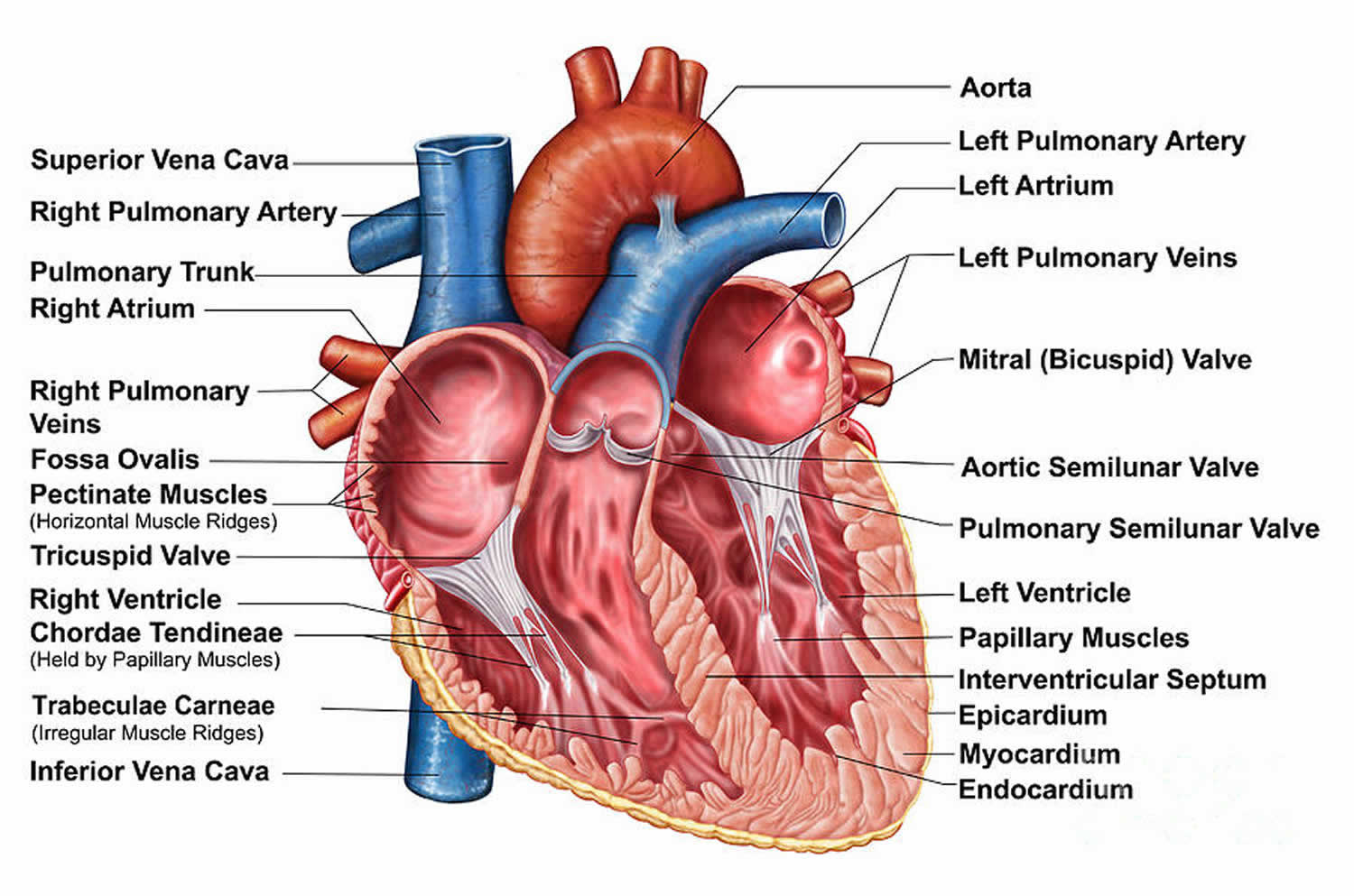
Figure 1. Heart chambers and heart valves
Split S2 causes
There are believed to be multiple causes for the physiologic splitting of S2. Both aortic valve (A2) and pulmonic valve (P2) close when the pressure above the respective valves are greater than the pressure in the ventricles below. Given the lower vascular resistance of the pulmonary artery, during inspiration, the pulmonary artery is able to tolerate more volume of blood before the pressure above the valve increases. Additionally, during inspiration, more blood fills the right ventricle leading to a slightly longer ejection time, adding to the delayed pulmonic valve closure.
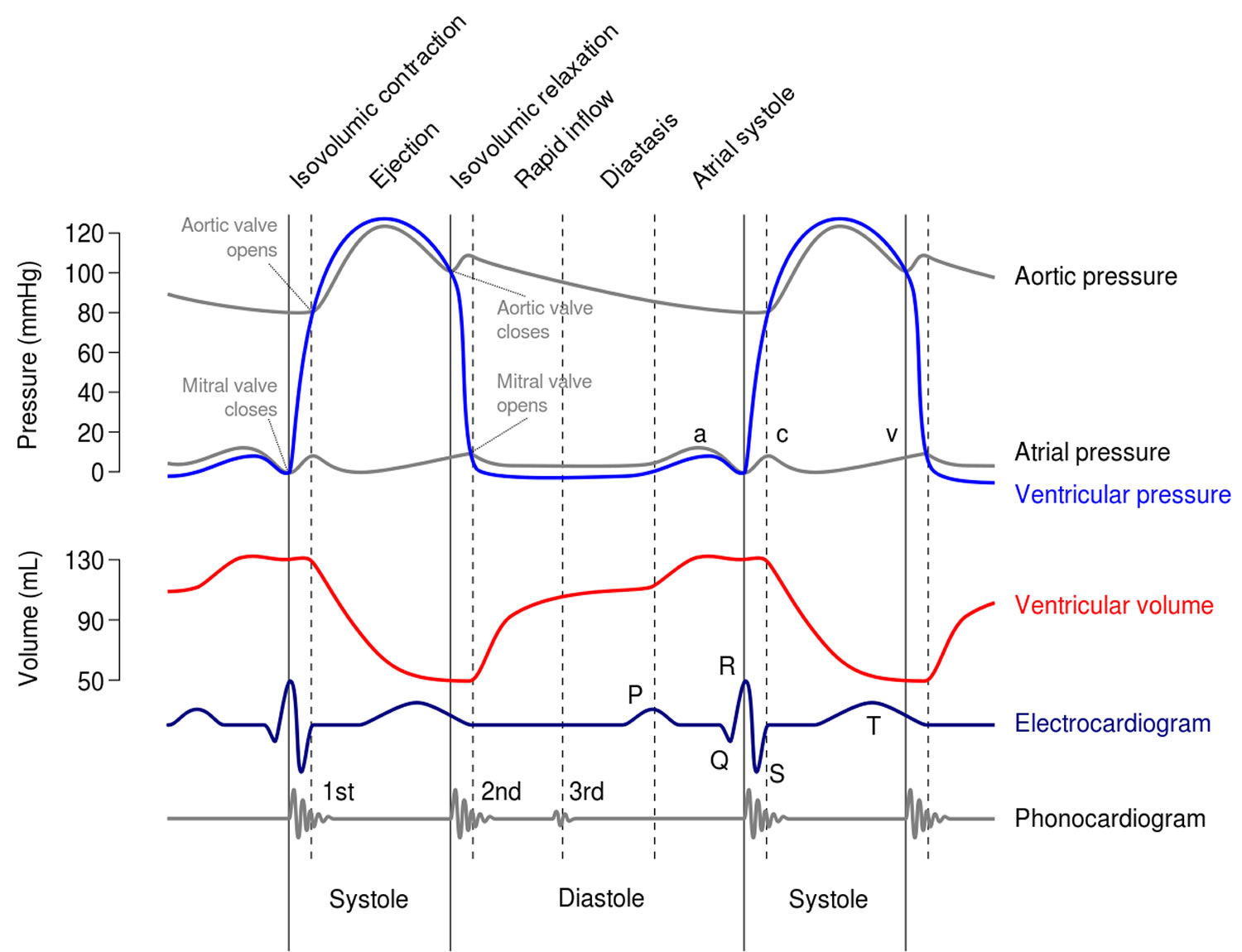
Figure 2. Cardiac cycles
Pathological findings in S2 (second) heart sounds
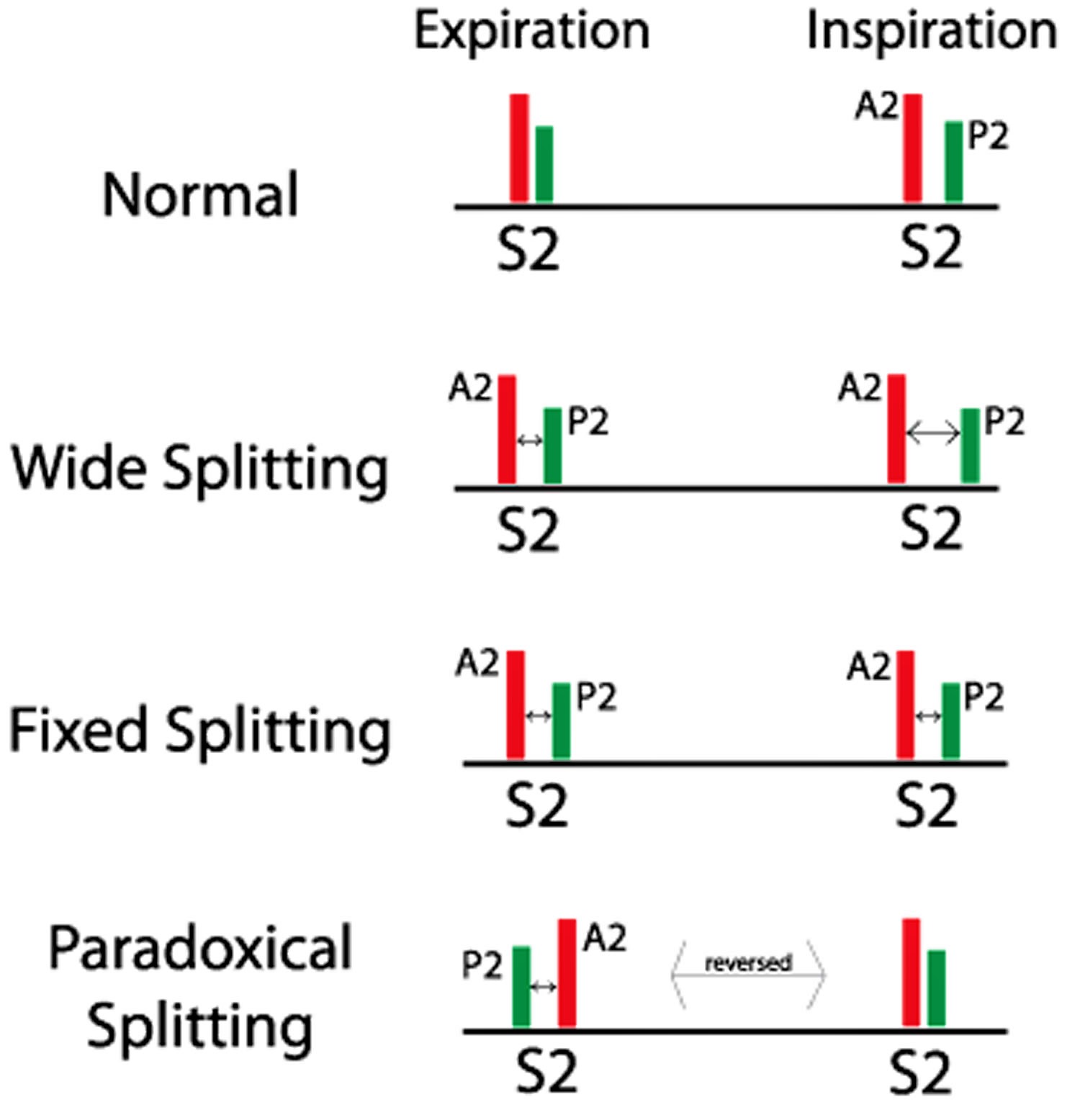
Figure 3. Split S2 heart sounds
Increased intensity of pulmonic valve closure (P2)
Increased intensity of pulmonic valve closure (P2) occurs P2 louder than A2 at pulmonic region (left parasternal, intercostal space 2)
- Differential diagnosis: Pulmonary hypertension (most common), atrial septal defect will also increase P2
- Note: Since P2 is measured relative to A2, causes for lower A2 intensity should be ruled out. These include: mitral regurgitation, aortic regurgitation, low diastolic arterial pressure, severe immobile aortic valve disease
Wide S2 splitting
Wide S2 splitting is detected by presence of splitting during expiration, wider during inspiration. Persistent (audible) expiratory S2 splitting suggests an audible expiratory interval of at least 30 to 40 msec between the two sounds. Persistent S2 splitting that is audible during both respiratory phases with appropriate inspirational and expirational directional changes (i.e., further increase of the A2–P2 interval with inspiration) may occur in the recumbent position in normal children, teenagers, and young adults. If these individuals sit, stand, or perform a Valsalva maneuver, however, the second sound often becomes single on expiration. In almost all patients with heart disease and audible expiratory splitting in the recumbent position, expiratory splitting persists when the patient is examined in the sitting or standing position. Thus, the finding of audible expiratory splitting in both the recumbent and upright positions is a very sensitive screening test for heart disease.
- Differential diagnosis: Anything that causes delayed conduction down the right bundle (RBBB [right bundle branch block], pre-excitation of left ventricle, pacing of left ventricle, premature left ventricular beats), pulmonary stenosis and pulmonary arterial hypertension. Right bundle branch block (RBBB) is the most common cause of the persistence of audible expiratory S2 splitting on standing.
- Other causes of persistent expiratory S2 splitting on standing may be due either to a delay in pulmonic valve closure or to early closure of the aortic valve. A delay in P2 may be secondary to the following 4:
- Delayed electrical activation of the right ventricle (e.g., left ventricular ectopic or paced beats, Wolff-Parkinson-White syndrome, and RBBB).
- Decreased impedance of the pulmonary vascular bed (e.g., atrial septal defect, partial anomalous pulmonary venous return, and idiopathic dilatation of the pulmonary artery).
- Right ventricular pressure overload lesions (e.g., pulmonary hypertension with right heart failure, moderate to severe valvular pulmonic stenosis, and acute massive pulmonary embolus).
- An early A2 may occur in patients with decreased resistance to left ventricular outflow (e.g., mitral regurgitation or constrictive pericarditis). Moderately large ventricular septal defects may also cause wide splitting of the second sound, but the aortic component is usually difficult to hear because of the loud holosystolic murmur.
- Expiratory splitting of S2 may occur in patients with severe congestive heart failure. The expiratory splitting usually disappears after satisfactory therapy of the heart failure. The high prevalence of expiratory splitting of S2 in cardiomyopathy may be explained by a combination of low cardiac output, mitral regurgitation, pulmonary hypertension, right heart failure, and bundle branch block.
Fixed S2 splitting
Fixed S2 splitting denotes absence of significant variation of the splitting interval with respiration, such that the separation of A2, and P2, remains unchanged during inspiration and expiration.
- Differential diagnosis: Atrial septal defect (due to continuous blood flow from left side to right side leading lenthened cardiac cycle on the right side of the heart), right heart failure and pulmonary hypertension.
- Atrial septal defect, with either normal or high pulmonary vascular resistance, is the classic example of fixed splitting of the second sound. The audible expiratory splitting in these patients is primarily a reflection of changes in the pulmonary vascular bed rather than selective volume overload of the right ventricle prolonging right ventricular systole. The fixed nature of the split is due to approximately equal inspiratory delay of the aortic and pulmonic components, indicating that the two ventricles share a common venous reservoir. Respiratory splitting of the second sound immediately returns to normal following surgical repair of an atrial septal defect, although the pulmonic closure sound may remain delayed for weeks or months.
- Severe right heart failure can lead to a relatively fixed split. This occurs because the right ventricle fails to respond to the increased volume produced by inspiration and because the lungs are so congested that impedance to forward flow from the right ventricle barely falls during inspiration. In anomalous pulmonary venous return without atrial septal defect, fixed splitting is not usually seen despite the simultaneous inspiratory delay in aortic and pulmonic closure.
- The Valsalva maneuver may be used to exaggerate the effect of respiration and obtain clearer separation of the two components of the second sound. Patients with atrial septal defects show continuous splitting during the strain phase, and upon release the interval between the components increases by less than 0.02 second. In normal subjects, however, splitting is exaggerated during the release phase of the Valsalva maneuver. Variation of the cardiac cycle length may also be used to evaluate splitting of S2. During the longer cardiac cycle, patients with atrial septal defect may show greater splitting as a result of increased atrial shunting and greater disparity between stroke volume of the two ventricles. In normal subjects, there is no tendency to widen the S2 splitting with longer cardiac cycles.
- Pulmonary artery hypertension causes variable effects on splitting of the second sound. Patients with ventricular septal defect who develop pulmonary hypertension may no longer have splitting of S2. Patients with atrial septal defect and associated pulmonary hypertension maintain a wide and fixed split of S2. Splitting is narrow (less than 30 msec), but remains physiologic, in patients with patent ductus arteriosus who develop pulmonary hypertension.
Paradoxical S2 splitting (reversed S2 splitting)
Paradoxical S2 splitting (reversed S2 splitting) is the reverse of normal physiology, splitting of second heart sounds during expiration, singular during inspiration. Paradoxical or reversed splitting is the result of a delay in the aortic closure sound. Therefore P2 precedes A2, and splitting is maximal on expiration and minimal or absent on inspiration. Identification of the reversed order of valve closure may be possible by judging the intensity and transmission of each component of the second sound. Often, however, the pulmonic component is as loud as the aortic component because of pulmonary artery hypertension secondary to left ventricular failure. The paradoxical narrowing or disappearance of the split on inspiration is a necessary criterion for diagnosing reversed splitting by auscultation.
- Differential diagnosis: Anything that causes delayed conduction down the left bundle (LBBB [left bundle branch block], pre-excitation of right ventricle, right ventricular pacing, premature right ventricular beats), aortic stenosis.
Paradoxical S2 splitting always indicates significant underlying cardiovascular disease and is usually due to prolongation of left ventricular activation or prolonged left ventricular emptying. The most common cause of paradoxical splitting of the second sound is left bundle branch block. Obstruction to left ventricular outflow of sufficient severity to delay aortic valve closure may also cause paradoxical splitting. In the context of aortic stenosis, such an auscultatory finding implies severe obstruction. Paradoxical splitting, however, occurs more commonly with hypertrophic cardiomyopathy than with aortic stenosis. Paradoxical splitting of the second sound may occur during the first few days after an acute myocardial infarction or secondary to severe left ventricular dysfunction.
Single S2
Single S2 is either from loss of aortic valve closure (A2) or loss of pulmonic valve closure (P2).
- Differential diagnosis: Severe aortic stenosis, severe aortic regurgitation, congenital absence of pulmonary valve
- Note: in patients with difficult to hear heart sounds (obesity, emphysema, pericardial fluid), P2 may be too hard to hear causing a single (A2) heart sound
The second sound can remain single throughout the respiratory cycle due to either absence of one component or to synchronous occurrence of the two components. Since the pulmonary vascular impedance increases with age, many normal patients over age 50 have a single S2 or at most a narrow physiologic split on inspiration because P2 occurs early. A single second sound, however, is usually due to inability to auscultate a relatively soft pulmonic component. Such inability is rare in healthy infants, children, and young adults and is uncommon even in older persons under good auscultatory conditions using a rigid stethoscopic diaphragm.
Hyperinflation of the lungs is perhaps the most common cause of inability to hear the pulmonic closure sound. All the conditions causing paradoxical splitting that delay A2 may produce a single second sound when the splitting interval becomes less than 0.02 second. Inaudibility of P2 due to a true decrease in its intensity is relatively rare, however, and suggests tetralogy of Fallot or pulmonary atresia. The pulmonic component may be inaudible in chronic right ventricular failure, or the aortic component may be masked by the systolic murmur in patients with aortic stenosis.
Pulmonary closure is completely fused with aortic closure throughout the respiratory cycle only in Eisenmenger’s syndrome with a large ventricular septal defect or in cases of single ventricle, where the durations of right and left ventricular systole are virtually equal. The second sound may also be single in a variety of congenital heart defects (e.g., truncus arteriosus, tricuspid atresia, hypoplastic left heart syndrome, transposition of the great arteries, and, occasionally, corrected transposition of the great arteries).
The loudness of each component of the second heart sound is proportional to the respective pressures in the aorta and pulmonary artery at the onset of diastole. Dilatation of the aorta or pulmonary artery may also cause accentuation of the aortic and pulmonic components, respectively. The aortic component is normally of greater intensity than the pulmonic component. The aortic component, therefore, radiates widely over the chest, whereas the pulmonic component is heard mainly in the second left ICS with some radiation down the left sternal border. The greater radiation of the aortic component is probably due to the higher pressure in the aorta compared to that in the pulmonary artery. At any given level of pressure, however, the pulmonic component will be proportionately louder than the aortic component because of the closer proximity of the pulmonic valve and the pulmonary artery to the chest wall. These considerations account for the relative loudness of P2 in young patients in whom the pulmonary arteries are quite close to the chest wall. They also account for the decreased intensity of both components of the second sound in patients with emphysema in whom both arteries are displaced from the chest wall.
The pulmonic component is considered to be abnormally loud in a subject over age 20 if it is greater than the aortic component in the second left intercostal space or if it is audible at the cardiac apex. This may be due either to pulmonary artery hypertension or right ventricular dilatation, with part of the right ventricle assuming the position normally occupied by the left ventricle. A split second sound at the apex is, therefore, definitely abnormal. The loud P2 commonly heard at the apex in patients with atrial septal defect is probably due to a dilated right ventricle encroaching upon the cardiac apex.
Decreased intensity of either component of the second sound may be due to a stiff semilunar valve, decreased pressure beyond the semilunar valve, or deformity of the chest wall or lung. A decreased intensity of P2 is most common in patients with chronic obstructive lung disease or valvular pulmonic stenosis. A decreased intensity of A2 is most common in patients with valvular aortic stenosis.
- Chizner MA. Cardiac auscultation: rediscovering the lost art. Curr Probl Cardiol. 2008 Jul;33(7):326-408.[↩]
- Dornbush S, Turnquest AE. Physiology, Heart Sounds. [Updated 2019 Apr 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541010[↩]
- Shindler DM. Practical cardiac auscultation. Crit Care Nurs Q. 2007 Apr-Jun;30(2):166-80. [↩]
- Felner JM. The Second Heart Sound. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 23. Available from: https://www.ncbi.nlm.nih.gov/books/NBK341[↩][↩][↩][↩]