Strawberry cervix
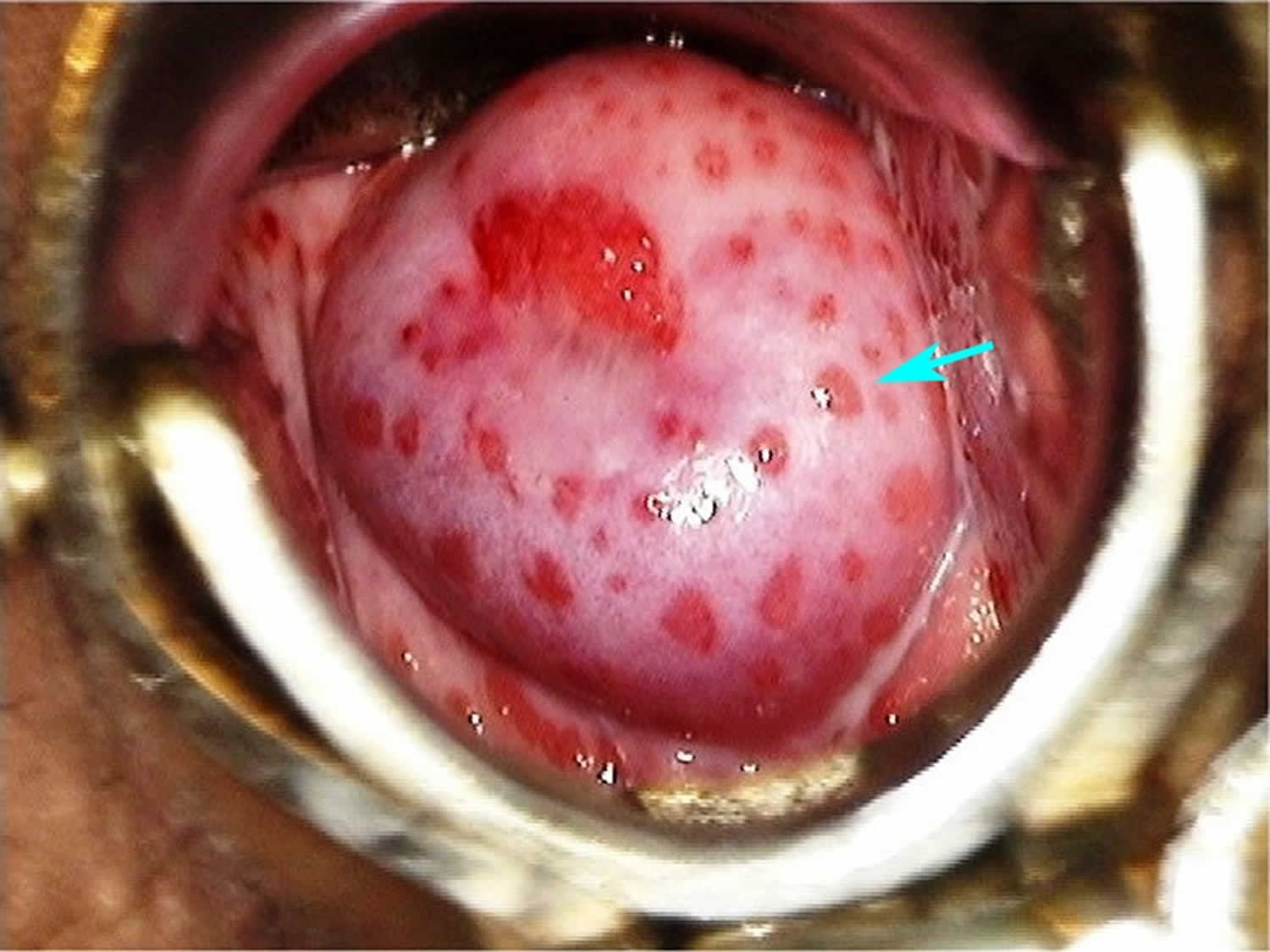
Strawberry cervix is a finding upon physical examination where the cervix has an erythematous, punctate, and papilliform appearance. Strawberry cervix is named because of the superficial similar appearance to a strawberry. As opposed to a more general inflammation of the cervix found in cervicitis, the strawberry cervix appearance is considered to be selectively associated with Trichomonas infections or Trichomoniasis 1. Strawberry cervix and spumy discharge are seen in 2% and 12% of Trichomonas vaginalis infected patients, respectively 1. A clinician discovering this finding would have a high suspicion for Trichomonas infection.
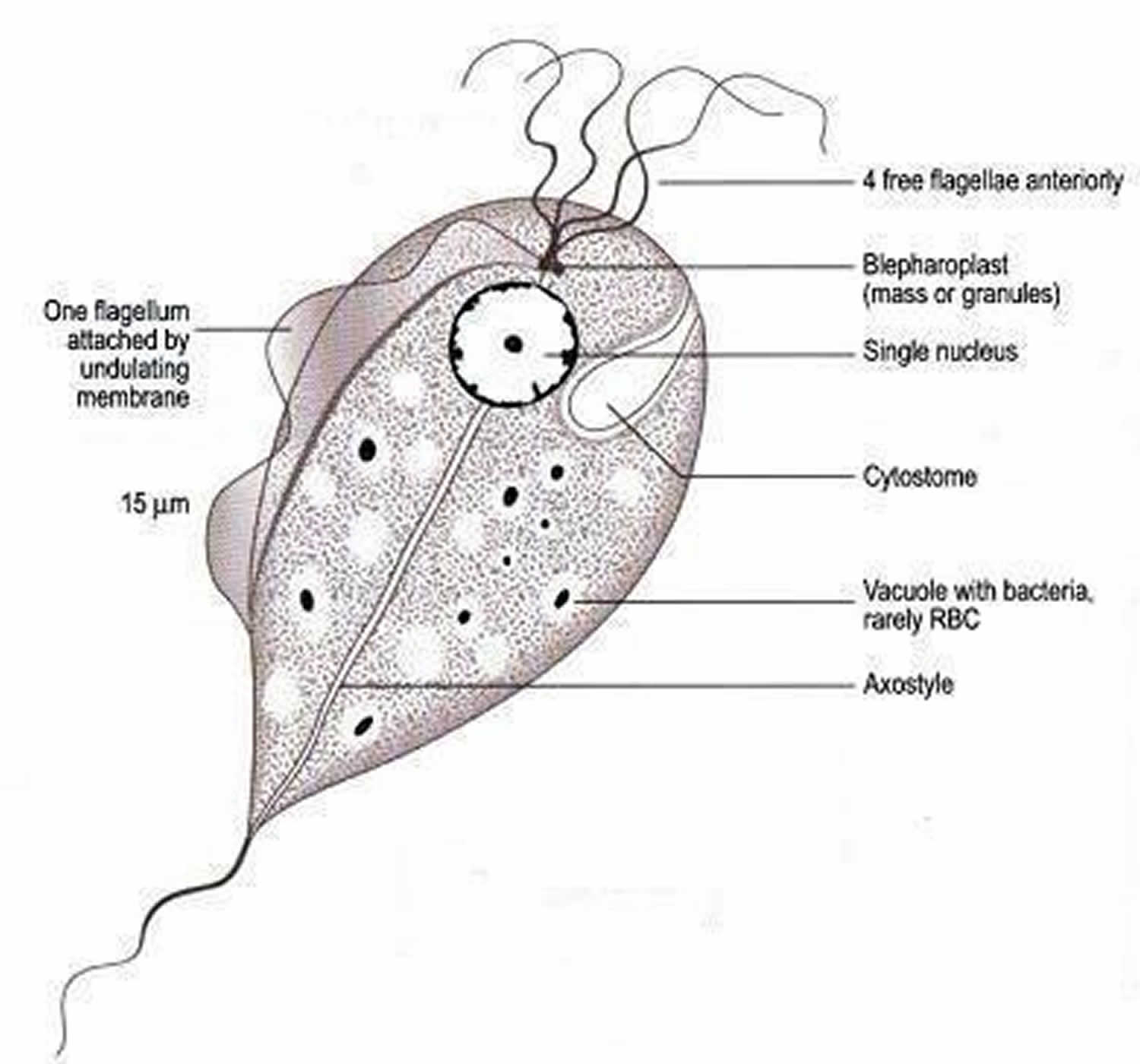
Figure 1. Trichomonas vaginalis
Figure 2. Strawberry cervix
Strawberry cervix causes
Strawberry cervix is caused by the protozoan parasite Trichomonas vaginalis infection or Trichomoniasis, which is a very common sexually transmitted disease (STD). Although symptoms of the disease vary, most people who have the parasite cannot tell they are infected. Trichomoniasis is the most common curable STD. In the United States, an estimated 3.7 million people have the Trichomonas vaginalis infection. However, only about 30% develop any symptoms of trichomoniasis. Infection is more common in women than in men. Older women are more likely than younger women to have been infected with trichomoniasis.
The Trichomonas vaginalis parasite passes from an infected person to an uninfected person during sex. In women, the most commonly infected part of the body is the lower genital tract (vulva, vagina, cervix, or urethra). In men, the most commonly infected body part is the inside of the penis (urethra). During sex, the parasite usually spreads from a penis to a vagina, or from a vagina to a penis. It can also spread from a vagina to another vagina. It is not common for the parasite to infect other body parts, like the hands, mouth, or anus. It is unclear why some people with the infection get symptoms while others do not. It probably depends on factors like a person’s age and overall health. Infected people without symptoms can still pass the infection on to others.
Trichomoniasis prevention
The only way to avoid sexually transmitted diseases is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting trichomoniasis:
- Be in a long-term mutually monogamous relationship with a partner who has been tested and has negative STD test results;
- Use latex condoms the right way every time you have sex. This can lower your chances of getting trichomoniasis. But the parasite can infect areas that are not covered by a condom – so condoms may not fully protect you from getting trichomoniasis.
Another approach is to talk about the potential risk of STDs before you have sex with a new partner. That way you can make informed choices about the level of risk you are comfortable taking with your sex life.
If you or someone you know has questions about trichomoniasis or any other STD, talk to a health care provider.
Strawberry cervix symptoms
About 70% of infected people do not have any signs or symptoms. When trichomoniasis does cause symptoms, they can range from mild irritation to severe inflammation. Some people with symptoms get them within 5 to 28 days after being infected. Others do not develop symptoms until much later. Symptoms can come and go.
Women with trichomoniasis may notice:
- Itching, burning, redness or soreness of the genitals;
- Discomfort with urination;
- A change in their vaginal discharge (i.e., thin discharge or increased volume) that can be clear, white, yellowish, or greenish with an unusual fishy smell.
Men with trichomoniasis may notice:
- Itching or irritation inside the penis;
- Burning after urination or ejaculation;
- Discharge from the penis.
Having trichomoniasis can make it feel unpleasant to have sex. Without treatment, the infection can last for months or even years.
Trichomoniasis complications
Trichomoniasis can increase the risk of getting or spreading other sexually transmitted infections. For example, trichomoniasis can cause genital inflammation that makes it easier to get infected with HIV, or to pass the HIV virus on to a sex partner.
Pregnant women with trichomoniasis are more likely to have their babies too early (preterm delivery). Also, babies born to infected mothers are more likely to have a low birth weight (less than 5.5 pounds).
Trichomoniasis diagnosis
It is not possible to diagnose trichomoniasis based on symptoms alone. For both men and women, your health care provider can examine you and get a laboratory test to diagnose trichomoniasis.
The most common method for Trichomonas vaginalis diagnosis might be microscopic evaluation of wet preparations of genital secretions because of convenience and relatively low cost. Unfortunately, the sensitivity of wet mount is low (51%–65%) in vaginal specimens 2 and lower in specimens from men (e.g., urethral specimens, urine sediment, and semen). Clinicians using wet mounts should attempt to evaluate slides immediately because sensitivity declines as evaluation is delayed, decreasing by up to 20% within 1 hour after collection 3. When highly sensitive (e.g., nucleic acid amplification test [NAAT]) testing on specimens is not feasible, a testing algorithm (e.g., wet mount first, followed by nucleic acid amplification test [NAAT] if negative) can improve diagnostic sensitivity in persons with an initial negative result by wet mount 4. Although Trichomonas vaginalis may be an incidental finding on a Pap test, neither conventional nor liquid-based Pap tests are considered diagnostic tests for trichomoniasis, because false negatives and false positives can occur.
Culture was considered the gold standard method for diagnosing Trichomonas vaginalis infection before molecular detection methods became available. Culture has a sensitivity of 75%–96% and a specificity of up to 100% 4. In women, vaginal secretions are the preferred specimen type for culture, as urine culture is less sensitive (475,672,673). In men, culture specimens require a urethral swab, urine sediment, and/or semen. To improve yield, multiple specimens from men can be used to inoculate a single culture.
The use of highly sensitive and specific tests is recommended for detecting Trichomonas vaginalis. Among women, nucleic acid amplification test [NAAT] is highly sensitive, often detecting three to five times more Trichomonas vaginalis infections than wet-mount microscopy, a method with poor sensitivity (51%–65%) 5. The APTIMA Trichomonas vaginalis assay (Hologic Gen-Probe, San Diego, CA) is FDA-cleared for detection of Trichomonas vaginalis from vaginal, endocervical, or urine specimens from women. This assay detects RNA by transcription-mediated amplification with a clinical sensitivity of 95.3%–100% and specificity of 95.2%–100% 6. Among women, vaginal swab and urine have up to 100% concordance 7. As analyte-specific reagents, this assay can be used with urine or urethral swabs from men if validated per CLIA regulations. For Trichomonas vaginalis diagnosis in men, the sensitivity of self-collected penile-meatal swabs was higher than that of urine in one study (80% and 39%, respectively) 8. The BD Probe Tec TV Qx Amplified DNA Assay (Becton Dickinson, Franklin Lakes, New Jersey) is FDA-cleared for detection of Trichomonas vaginalis from endocervical, vaginal, or urine specimens from women. Although it might be feasible to perform these tests on the same specimen used for chlamydia and gonorrhea screening, the epidemiology of trichomoniasis is distinct and should not be overlooked in older adults.
Other FDA-cleared tests to detect Trichomonas vaginalis in vaginal secretions include the OSOM Trichomonas Rapid Test (Sekisui Diagnostics, Framingham, MA), an antigen-detection test using immunochromatographic capillary flow dipstick technology that can be performed at the point of care, and the Affirm VP III (Becton Dickinson, Sparks, MD), a DNA hybridization probe test that evaluates for Trichomonas vaginalis, G. vaginalis, and Candida albicans. The results of the OSOM Trichomonas Rapid Test are available in approximately 10 minutes, with sensitivity 82%–95% and specificity 97%–100% 9. Self-testing might become an option, as a study of 209 young women aged 14–22 years found that >99% could correctly perform and interpret her own self-test using the OSOM assay, with a high correlation with clinician interpretation (96% agreement) 10. The results of the Affirm VP III are available within 45 minutes. Sensitivity and specificity are 63% and 99.9%, respectively, compared with culture and TMA; sensitivity might be higher among women who are symptomatic 11. Neither the OSOM nor the Affirm VP III test is FDA-cleared for use with specimens obtained from men.
Strawberry cervix treatment
Trichomoniasis can be treated with medication (either metronidazole or tinidazole). These pills are taken by mouth. It is safe for pregnant women to take this medication. It is not recommended to drink alcohol within 24 hours after taking this medication.
Recommended regimen
- Metronidazole 2 g orally in a single dose
OR - Tinidazole 2 g orally in a single dose
Alternative regimen
- Metronidazole 500 mg orally twice a day for 7 days
Recommended Regimen for Women with HIV Infection
- Metronidazole 500 mg orally twice daily for 7 days
Alcohol consumption should be avoided during treatment with nitroimidazoles. To reduce the possibility of a disulfiram-like reaction, abstinence from alcohol use should continue for 24 hours after completion of metronidazole or 72 hours after completion of tinidazole.
The nitroimidazoles are the only class of antimicrobial medications known to be effective against Trichomonas vaginalis infections. Of these drugs, metronidazole and tinidazole have been cleared by FDA for the oral or parenteral treatment of trichomoniasis. Tinidazole is generally more expensive, reaches higher levels in serum and the genitourinary tract, has a longer half-life than metronidazole (12.5 hours versus 7.3 hours), and has fewer gastrointestinal side effects 12. In randomized clinical trials, recommended metronidazole regimens have resulted in cure rates of approximately 84%–98% (679-681), and the recommended tinidazole regimen has resulted in cure rates of approximately 92%–100% 13. Randomized controlled trials comparing single 2 g doses of metronidazole and tinidazole suggest that tinidazole is equivalent or superior to metronidazole in achieving parasitologic cure and resolution of symptoms 14.
Metronidazole gel does not reach therapeutic levels in the urethra and perivaginal glands. Because it is less efficacious than oral metronidazole, it is not recommended.
People who have been treated for trichomoniasis can get it again. About 1 in 5 people get infected again within 3 months after receiving treatment. To avoid getting reinfected, make sure that all of your sex partners get treated. Also, wait 7- 10 days after you and your partner have been treated to have sex again. Get checked again if your symptoms come back.
Because of the high rate of reinfection among women treated for trichomoniasis (17% within 3 months in one study) 15, retesting for Trichomonas vaginalis is recommended for all sexually active women within 3 months following initial treatment regardless of whether they believe their sex partners were treated (see Diagnostic Considerations). Testing by nucleic acid amplification can be conducted as soon as 2 weeks after treatment 16. Data are insufficient to support retesting men.
- Trichomonas vaginalis: investigation of a novel diagnostic method in urine samples using cysteine proteinase 4 gene and PCR technique. Exp Parasitol. 2010 Oct;126(2):187-90. doi: 10.1016/j.exppara.2010.04.021. Epub 2010 Apr 29. https://doi.org/10.1016/j.exppara.2010.04.021[↩][↩]
- Huppert JS, Mortensen JE, Reed JL, et al. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin Infect Dis 2007;45:194–8.[↩]
- Stoner KA, Rabe LK, Meyn LA, et al. Survival of Trichomonas vaginalis in wet preparation and on wet mount. Sex Transm Infect 2013;89:485–8.[↩]
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009;200:e181–7.[↩][↩]
- Roth AM, Williams JA, Ly R, et al. Changing sexually transmitted infection screening protocol will result in improved case finding for Trichomonas vaginalis among high-risk female populations. Sex Transm Dis 2011;38:398–400.[↩]
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011;49:4106–11.[↩]
- Hollman D, Coupey SM, Fox AS, et al. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. J Pediatr Adolesc Gynecol 2010;23:312–6.[↩]
- Dize L, Agreda P, Quinn N, et al. Comparison of self-obtained penile-meatal swabs to urine for the detection of C. trachomatis, N. gonorrhoeae and T. vaginalis. Sex Transm Infect 2013;89:305–7.[↩]
- Campbell L, Woods V, Lloyd T, et al. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J Clin Microbiol 2008;46:3467–9.[↩]
- Huppert JS, Hesse E, Kim G, et al. Adolescent women can perform a point-of-care test for trichomoniasis as accurately as clinicians. Sex Transm Infect 2010;86:514–9.[↩]
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011;49:866–9.[↩]
- Viitanen J, Haataja H, Mannisto PT. Concentrations of metronidazole and tinidazole in male genital tissues. Antimicrob Agents Chemother 1985;28:812–4.[↩]
- Prasertsawat PO, Jetsawangsri T. Split-dose metronidazole or single-dose tinidazole for the treatment of vaginal trichomoniasis. Sex Transm Dis 1992;19:295–7.[↩]
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003;2:CD000218[↩]
- Peterman TA, Tian LH, Metcalf CA, et al. High incidence of new sexually transmitted infections in the year following a sexually transmitted infection: a case for rescreening. Ann Intern Med 2006;145:564–72.[↩]
- Time from Treatment to Negative PCR Results for C. trachomatis , N. gonorrhoeae , and T. vaginalis National STD Prevention Conference; March 10-13, 2008, 2008; Chicago, IL. https://cdc.confex.com/cdc/std2008/webprogram/Paper14676.html[↩]