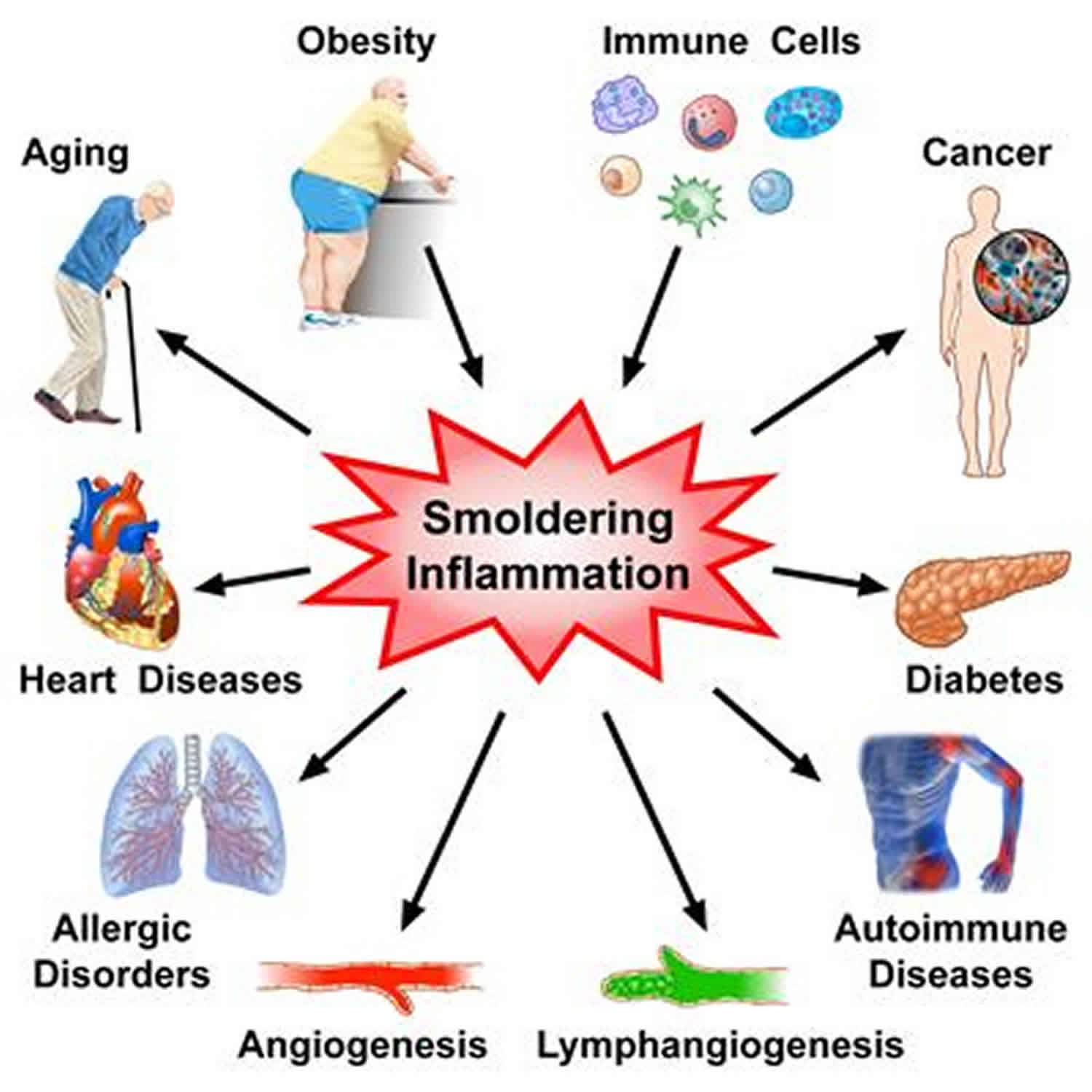
Systemic inflammation
Systemic inflammation is defined as “typical, multi-syndrome, phase-specific pathological process, developing from systemic damage and characterized by the total inflammatory reactivity of endotheliocytes, plasma and blood cell factors, connective tissue and, at the final stage, by microcirculatory disorders in vital organs and tissues” 1. Systemic inflammatory diseases or conditions include rheumatoid arthritis, allergies of different types, multiple sclerosis, cardiovascular disease, inflammatory bowel disease, chronic liver disease, diabetes, and cancer, in which pro-inflammatory cytokines are overexpressed 2. Systemic inflammation is also defined as a generalized pathologic process, which can be clinically manifested in various forms, including systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and multiple-organ dysfunction and failure 3. Systemic inflammation can lead to systemic inflammatory response syndrome (SIRS) or sepsis syndrome (when infection is suspected), recognized and defined by the presence of two of the following: tachycardia, tachypnea, fever, and abnormal white blood cell count. Increased systemic inflammation with the compensatory antiinflammatory response syndrome can further mediate severe sepsis (sepsis + single organ failure) and with immunologic dissonance, the development of multiple organ failure with acute lung injury (bilateral infiltrates with hypoxemia and a partial pressure of oxygen [Pao2]/fraction of inspired oxygen [Fio2] ratio <300) or acute respiratory distress syndrome (ARDS) (bilateral infiltrates with hypoxemia and a Pao2/Fio2 ratio <200).
Today, criteria of systemic inflammatory response syndrome (SIRS) are the following (≥2 criteria from 4):
- Body temperature ≥ 100.4 °F (≥38 °C) or ≤96.8 °F (≤36 °C);
- Frequency of heartbeat rate > = 90 beats per minute;
- Respiration rate >20 minute or hyperventilation (PaCO2≤32 mm hl);
- Blood leukocytes >12×109/ml or <4×109/l, or the number or immature forms > 10%; these criteria formalize the clinical diagnosing of sepsis if there is an inflammatory source 4.
It is clear that criteria of systemic inflammatory response syndrome (SIRS) do not directly reflect the key pathogenic phases. The wide range of existing criteria for prognosis and estimating of critical complications can be structured in individual resuscitation syndrome, for example, multiorgan dysfunction syndrome (MODS) and disseminated intravascular coagulation (DIC) 5. Development of these and other resuscitation syndromes is pathogenetically linked with the phenomena of the Systemic Inflammatory Response (SIR), which is usually associated with “Systemic Inflammation” 1.
Systemic inflammation-associated syndromes (e.g., sepsis and septic shock) often have high mortality and remain a challenge in emergency medicine. Systemic inflammation is usually accompanied by changes in body temperature: fever or hypothermia. In animal studies, systemic inflammation is often modeled by administering bacterial lipopolysaccharide, which triggers autonomic and behavioral thermoeffector responses and causes either fever or hypothermia, depending on the dose and ambient temperature. Fever and hypothermia are regulated changes of body temperature, which correspond to mild and severe forms of systemic inflammation, respectively. Mediators of fever and hypothermia are called endogenous pyrogens and cryogens; they are produced when the innate immune system recognizes an infectious pathogen. Upon an inflammatory challenge, hepatic and pulmonary macrophages (and later brain endothelial cells) start to release lipid mediators, of which prostaglandin (PG) E2 plays the key role, and cytokines. Blood PGE2 enters the brain and triggers fever. At later stages of fever, PGE2 synthesized within the blood-brain barrier maintains fever. In both cases, PGE2 is synthesized by cyclooxygenase-2 and microsomal PGE2synthase-1. Mediators of hypothermia are not well established. Both fever and hypothermia are beneficial host defense responses. Based on evidence from studies in laboratory animals and clinical trials in humans, fever is beneficial for fighting mild infection. Based mainly on animal studies, hypothermia is beneficial in severe systemic inflammation and infection.
Inflammation is part of the body’s defense mechanism. It is the process by which the immune system recognizes and removes harmful stimuli and begins the healing process. There are generally two types of inflammation: acute and chronic inflammation 6.
Acute inflammation
Tissue damage due to trauma, microbial invasion, or noxious compounds all induce acute inflammation. It starts rapidly, becomes severe in a short time and symptoms may last for a few days for example cellulitis or acute pneumonia. Subacute inflammation is the period between acute and chronic inflammation and may last 2 to 6 weeks.
Chronic inflammation
Chronic inflammation is also referred to as slow, long-term inflammation lasting for prolonged periods of several months to years. Generally, the extent and effects of chronic inflammation vary with the cause of the injury and the ability of the body to repair and overcome the damage.
Chronic inflammation can result from the following 7:
- Failure of eliminating the agent causing an acute inflammation such as infectious organisms including Mycobacterium tuberculosis, protozoa, fungi, and other parasites that can resist host defenses and remain in the tissue for an extended period.
- Exposure to a low level of a particular irritant or foreign materials that cannot be eliminated by enzymatic breakdown or phagocytosis in the body including substances or industrial chemical that can be inhaled over a long period, for example, silica dust.
- An autoimmune disorder in which the immune system is sensitized to the normal component of the body and attacks healthy tissue giving rise to diseases such as rheumatoid arthritis, systemic lupus erythematosus (SLE).
- Recurrent episodes of acute inflammation. However, in some cases, chronic inflammation is an independent response and not a sequel to acute inflammation for example diseases such as tuberculosis and rheumatoid arthritis.
- Inflammatory and biochemical inducers are causing oxidative stress and mitochondrial dysfunction such as increased production of free radical molecules, advanced glycation end products (AGEs), uric acid (urate) crystals, oxidized lipoproteins, homocysteine, and others.
Chronic inflammatory diseases are the most significant cause of death in the world. The World Health Organization (WHO) ranks chronic diseases as the greatest threat to human health. The prevalence of diseases associated with chronic inflammation is anticipated to increase persistently for the next 30 years in the United States. in 2000, nearly 125 million Americans were living with chronic conditions and 61 million (21%) had more than one. In recent estimates by Rand Corporation, in 2014 nearly 60% of Americans had at least one chronic condition, 42% had more than one and 12% of adults had 5 or more chronic conditions. Worldwide, 3 of 5 people die due to chronic inflammatory diseases like stroke, chronic respiratory diseases, heart disorders, cancer, obesity, and diabetes 8. The prevalence of some specific chronic inflammation-mediated diseases are as follows:
- Diabetes: According to American Diabetes Association, 30.3 million people or 9.4% of the American population, had diabetes in 2015 and it was the 7th leading cause of death in the United States.
- Cardiovascular diseases: In line with 2017 updated report from the American Heart Association, cardiovascular diseases (CVDs) accounts for 1 out of every three deaths or approximately 800,000 deaths in the United States. Globally, CVD accounts for 31% of all deaths, and coronary heart disease (CHD) accounts for most deaths due to CVD, followed by stroke (1 of 20 deaths in the United States) and heart failure.
- Arthritis and Joint Diseases: These affect approximately 350 million people worldwide and nearly 43 million people in the United States or almost 20% of the population. This number is expected to exceed 60 million by 2020. Nearly, 2.1 million Americans suffer from rheumatoid arthritis.
- Allergies: These rank among the sixth leading cause of chronic human diseases in the United States and affect more than 50 million Americans each year. Asthma affects more than 24 million people in the United States including more than 6 million children. In 2015, 8.2% of adults and 8.4% of children were diagnosed with hay fever.
- Chronic Obstructive Pulmonary Disease (COPD): The third most common cause of death in the United States in 2014, and nearly 15.7 million Americans (6.4%) were reported to have been diagnosed with COPD.
Types of chronic inflammation
- Nonspecific proliferative: Characterized by the presence of non-specific granulation tissue formed by infiltration of mononuclear cells (lymphocytes, macrophages, plasma cells) and proliferation of fibroblasts, connective tissue, vessels and epithelial cells, for example, an inflammatory polyp-like nasal or cervical polyp and lung abscess.
- Granulomatous inflammation: A specific type of chronic inflammation characterized by the presence of distinct nodular lesions or granulomas formed with an aggregation of activated macrophages or its derived cell called epithelioid cells usually surrounded by lymphocytes. The macrophages or epithelioid cells inside the granulomas often coalesce to form Langhans or giant cells such as foreign body, Aschoff, Reed-Sternberg and Tumor giant cells. There are two types:
- Granuloma formed due to a foreign body or T-cell mediated immune response is termed as foreign body granuloma, for example, silicosis
- Granuloma that are formed from chronic infection is termed as infectious granuloma, for example, tuberculosis and leprosy.
Risk factors associated with chronic inflammation
Several risk factors promote low-level inflammatory response. These include:
- Age: Increasing age is positively correlated with elevated levels of several inflammatory molecules. The age-associated increase in inflammatory molecules may be due to mitochondrial dysfunction or free radical accumulation over time and other age-related factors like increase in visceral body fat.
- Obesity: Many studies reported that fat tissue is an endocrine organ, secreting multiple adipokines and other inflammatory mediators. Some reports show that body mass index of an individual is proportional to the amount of pro-inflammatory cytokines secreted. Metabolic syndrome typifies this well.
- Diet: Diet rich in saturated fat, trans-fats, or refined sugar is associated with higher production of pro-inflammatory molecules, especially in individuals with diabetes or overweight individuals.
- Smoking: Cigarette smoking is associated with lowering the production of anti-inflammatory molecules and inducing inflammation.
- Low Sex Hormones: Studies show that sex hormones like testosterone and estrogen can suppress the production and secretion of several pro-inflammatory markers and it has been observed that maintaining sex hormone levels reduces the risk of several inflammatory diseases.
- Stress and Sleep Disorders: Both physical and emotional stress is associated with inflammatory cytokine release. Stress can also cause sleep disorders. Since individuals with irregular sleep schedules are more likely to have chronic inflammation than consistent sleepers, the sleep disorder is also considered as one of the independent risk factors for chronic inflammation.
Chronic inflammation pathophysiology
Most of the features of acute inflammation continue as the inflammation becomes chronic, including expansion of blood vessels (vasodilation), increase in blood flow, capillary permeability and migration of neutrophils into the infected tissue through the capillary wall (diapedesis). However, the composition of the white blood cells changes soon and the macrophages and lymphocytes begin to replace short-lived neutrophils. Thus the hallmarks of chronic inflammation are the infiltration of the primary inflammatory cells such as macrophages, lymphocytes, and plasma cells in the tissue site, producing inflammatory cytokines, growth factors, enzymes and hence contributing to the progression of tissue damage and secondary repair including fibrosis and granuloma formation, etc 9.
Chronic inflammation prevention
Chronic inflammation can have a deleterious effect on the body and is a key factor causing almost all chronic degenerative diseases. The following are some of the most effective ways to prevent chronic inflammation.
- Increase uptake of anti-inflammatory foods: It is important to avoid eating simple sugars, refined carbohydrates, high-glycemic foods, trans fats, and hydrogenated oils. Consuming whole grains, natural foods, plenty of vegetables and fruits such as avocados, cherries, kale, and fatty fish like salmon is helpful in defeating inflammation.
- Minimize intake of antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs): Use of antibiotics, antacids, and NSAIDs should be avoided as it could harm the microbiome in the gut causing inflammation in intestinal walls known as leaky gut which in turn releases toxins and triggers chronic, body-wide inflammation.
- Exercise regularly to maintain an optimum weight: It is largely known that adipose tissue in obese or overweight individuals induces low-grade systemic inflammation. Regular exercise is helpful not only in controlling weight but also decreasing the risk of cardiovascular diseases and strengthening the heart, muscles, and bones.
- Sleep longer: Overnight sleep (ideally at least 7 to 8 hours) helps stimulating human growth hormones and testosterone in the body to rebuild itself.
- Stress Less: Chronic psychological stress is linked to greater risk for depression, heart disease and body losing its ability to regulate the inflammatory response and normal defense. Yoga and meditation are helpful in alleviating stress-induced inflammation and its harmful effects on the body.
Chronic inflammation symptoms
Some of the common signs and symptoms that develop during chronic inflammation are listed below.
- Body pain
- Constant fatigue and insomnia
- Depression, anxiety and mood disorders
- Gastrointestinal complications like constipation, diarrhea, and acid reflux
- Weight gain
- Frequent infections.
Chronic inflammation complications
Although chronic inflammation progresses silently, it is the cause of most chronic diseases and presents a major threat to the health and longevity of individuals. Inflammation is considered a major contributor to several diseases.
- Cardiovascular diseases: Many clinical studies have shown strong and consistent relationships between markers of inflammation such as hsCRP and cardiovascular disease prediction. Furthermore, Atherosclerosis is a pro-inflammatory state with all the features of chronic low-grade inflammation and leads to increase cardiovascular events such as myocardial infarction, stroke, among others.
- Cancer: Chronic low-level inflammation also appears to participate in many types of cancer such as kidney, prostate, ovarian, hepatocellular, pancreatic, colorectal, lung, and mesothelioma.
- Diabetes: Immune cells like macrophages infiltrate pancreatic tissues releasing pro-inflammatory molecules in diabetic individuals. Both are circulating and cellular biomarkers underscore that diabetes is a chronic inflammatory disease. Chronic complications linked with diabetes include both microvascular and macrovascular complications. Diabetes not only increases the risk of macrovascular complications like strokes and heart attacks but also the microvascular complications like diabetic retinopathy, neuropathy, and nephropathy.
- Rheumatoid arthritis: It is thought to be initiated by an infectious agent or an environmental factor like exposure to cigarette smoke which induces a local inflammatory response in joints, infiltration of immune cells and release of cytokines.
- Allergic asthma: A complex, chronic inflammatory disorder associated with inappropriate immune response and inflammation in conducting airways involving a decline in airway function and tissue remodeling.
- Chronic obstructive pulmonary disease (COPD): An obstructive lung disease, develops as a chronic inflammatory response to inspired irritants and characterized by long-term breathing problems.
- Alzheimer disease: In older adults, chronic low-level inflammation is linked to cognitive decline and dementia.
- Chronic kidney disease (CKD): Low-grade inflammation is a common feature of chronic kidney disease. It can lead to the retention of several pro-inflammatory molecules in the blood and contributes to the progression of chronic kidney disease and mortality.
- Inflammatory Bowel Disease (IBD) is a group of chronic inflammatory disorders of the digestive tract. It can develop as ulcerative colitis causing long-lasting inflammation and ulcers in the lining of large intestine and rectum or Crohn’s disease characterized by inflammation of the lining of digestive tract dispersing into affected tissues such as mouth, esophagus, stomach and the anus.
Chronic inflammation diagnosis
Unfortunately, there are no highly effective laboratory measures to assess patients for chronic inflammation and diagnoses are only undertaken when the inflammation occurs in association with another medical condition.
- The best test to confirm clinically chronic inflammation is serum protein electrophoresis which shows concomitant hypoalbuminemia and polyclonal increase in all gamma globulins (polyclonal gammopathy).
- The two blood tests that are inexpensive and good markers of systemic inflammation include high-sensitivity C-reactive protein (hs-CRP) and fibrinogen. High levels of hs-CRP indicate inflammation, but it is not a specific marker for chronic inflammation since it is also elevated in acute inflammation resulting from a recent injury or sickness. The normal serum levels for hs-CRP is less than 0.55 mg/L in men and less than 1.0 mg/L in women. The normal levels of fibrinogen are 200 to 300 mg/dl. SAA (Serum Amyloid A) can also mark inflammation but is not a standardized test.
- Detecting pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1beta), interleukin-6 (IL-6), and interleukin-8 (IL-8) is an expensive method but may identify specific factors causing chronic inflammation. Again, the assays are not standardized like hs-CRP, fibrinogen, and serum protein electrophoresis.
Chronic inflammation treatment
Many dietary and lifestyle changes may be helpful in removing inflammation triggers and reducing chronic inflammation as listed below. The most effective is weight loss.
- Low-glycemic diet: Diet with a high glycemic index is related to high risk of stroke, coronary heart disease, and type 2 diabetes mellitus. It is beneficial to limit consumption of inflammation-promoting foods like sodas, refined carbohydrates, fructose corn syrup in a diet.
- Reduce intake of total, saturated fat and trans fats: Some dietary saturated and synthetic trans-fats aggravate inflammation, while omega-3 polyunsaturated fats appear to be anti-inflammatory. Processed and packaged foods that contain trans fats such as processed seed and vegetable oils, baked goods (like soybean and corn oil) should be reduced from the diet.
- Fruits and vegetables: Blueberries, apples, Brussels sprouts, cabbage, broccoli, and cauliflower, that are high in natural antioxidants and polyphenols and other anti-inflammatory compounds, may protect against inflammation.
- Fiber: High intake of dietary soluble and insoluble fiber is associated with lowering levels of IL-6 and TNF-alpha.
- Nuts: Nuts such as almonds is associated with lowering risk of cardiovascular disease and diabetes.
- Green and black tea polyphenols: Tea polyphenols are associated with a reduction in CRP in human clinical studies.
- Curcumin: a constituent of turmeric causes significant patient improvements in several inflammatory diseases especially in animal models.
- Fish Oil: The richest source of the omega-3 fatty acids. Higher intake of omega-3 fatty acids is associated with lowering levels of TNF-alpha, CRP, and IL-6.
- Mung bean: Rich in flavonoids (particularly vitexin and isovitexin). It is traditional food and herbal medicine known for its anti-inflammatory effects.
- Micronutrients: Magnesium, vitamin D, vitamin E, zinc and selenium). Magnesium is listed as one of the most anti-inflammatory dietary factors, and its intake is associated with lowering of hs-CRP, IL-6, and TNF-alpha activity. Vitamin D exerts its anti-inflammatory activity by suppressing inflammatory mediators such as prostaglandins and nuclear factor kappa-light-chain-enhancer of activated B cells. Vitamin E, zinc, and selenium act as antioxidants in the body.
Sesame Lignans: Sesame oil consumption reduces the synthesis of prostaglandin, leukotrienes, and thromboxanes and is known for its potential hypotensive activity.
Physical Exercise
In human clinical trials, it is shown that energy expenditure through exercise lowers multiple pro-inflammatory molecules and cytokines independently of weight loss.
Conventional drugs to combat chronic inflammation
- Metformin is commonly used in the treatment of type 2 diabetic patients with dyslipidemia and low-grade inflammation. The anti-inflammatory activity of Metformin is evident by reductions in circulating TNF-alpha, IL-1beta, CRP, and fibrinogen in these patients.
- Non-steroidal anti-inflammatory drugs (NSAIDs) like naproxen, ibuprofen, and aspirin acts by inhibiting an enzyme cyclooxygenase (COX) that contributes to inflammation and are mostly used to alleviate the pain caused by inflammation in patients with arthritis.
- Statins are anti-inflammatory as they reduce multiple circulating and cellular biomediators of inflammation. This pleiotropic effect appears to contribute in part to the reduction in cardiovascular events.
- Corticosteroids also prevent several mechanisms involved in inflammation. Glucocorticoids are prescribed for inflammatory conditions including inflammatory arthritis, systemic lupus, sarcoidosis, and asthma.
- Herbal supplements like ginger, turmeric, cannabis, hyssop, and Harpagophytum procumbens are shown to have anti-inflammatory properties however one should always consult with a doctor before their use and caution should be taken for using some herbs like hyssop and cannabis.
Systemic inflammation causes
Systemic inflammation is a “typical multi-syndrome, phase-specific pathological process, evolving at systemic injury and characterized by the total inflammatory reactivity of the endotheliocytes, plasma and blood cell factors, connective tissue, and, at the terminal stage—microcirculatory disorders in vital organs and tissues” 10. Thereby, the fundamental difference between classical inflammation and systemic inflammation is not the presence or absence of systemic changes, but changes in quality content, because of a shift to the system level of biologically aggressive mechanisms that were intended for local use inside the inflammatory focus. These include the following 1:
- systemic activation of endotheliocytes and vessel macrophages;
- proinflammatory transformation of structure and function of microvessels;
- microthrombosis in postcapillar venules; intravessel activation of complement and hemostasis;
- excitotoxicity—toxic accumulation of inflammatory mediators in blood;
- systemic degranulation of mastocytes, and some other mechanisms, intended for acting in the area of injury.
The transformation of stress-reaction in the hypothalamic-pituitary-adrenal system to distress-reaction is specific for systemic inflammation, but not attributive 1. Thereby, the principal distinguishing feature of systemic inflammation appears to be a transfer of program mechanisms of inflammatory focus to the systemic level in response to systemic effect of injuring factors 1. Herewith, systemic injury phenomena development is connected not only with critical changes of some homeostasis parameters, but also with coming out of microbe and endogenic molecular patterns (for example products of tissue degradation) to the system blood stream, so called—danger-associated molecular patterns (DAMP) 11. Acting power of injuring factors must overcome buffer barriers, which prevent systemic activation of proinflammatory mechanisms, genetically intended for use only in local inflammatory focus.
The initiation of proinflammatory cell-stress program is a common mechanism for activation of different injuring factors or threat of future damage, on this base complex, integral programs of different variants of cell-cooperations are forming, determining the development of one or another type of inflammatory process. Thus, the base of systemic inflammation is a mosaic development of cell-stress on the systemic level that can be characterized as two alternative variants (stages) according to the effects of injuring factors 11:
- Realization of resistance strategy—high proinflammatory activity, in prejudice of physiological functions;
- Realization of tolerance strategy—decease in cell activity, both physiological and proinflammatory.
The dynamics of systemic inflammation development is determined by the strength of the primary (initial) systemic damage, the development of secondary systemic damage phenomenon (self-developing process) and the ratio of two variants of cellular stress at the organism’s level 12. Factors of the secondary systemic damage include: impairment of a number of homeostasis parameters associated with microcirculatory disorder development; entry into the bloodstream danger-associated molecular patterns (DAMP)—products of tissue degradation and microbial endotoxins (translocation through mucosal barriers); cytotoxic effect of free radicals and proteases in the intravascular environment during the systemic activation of phagocytes 11.
Systemic inflammation signs and symptoms
Systemic inflammation is a generalized pathologic process, which can be clinically manifested in various forms, including systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and multiple-organ dysfunction and failure 3. Systemic inflammation can lead to systemic inflammatory response syndrome (SIRS) or sepsis syndrome (when infection is suspected), recognized and defined by the presence of two of the following: tachycardia, tachypnea, fever, and abnormal white blood cell count. Increased systemic inflammation with the compensatory antiinflammatory response syndrome can further mediate severe sepsis (sepsis + single organ failure) and with immunologic dissonance, the development of multiple organ failure with acute lung injury (bilateral infiltrates with hypoxemia and a partial pressure of oxygen [Pao2]/fraction of inspired oxygen [Fio2] ratio <300) or acute respiratory distress syndrome (ARDS) (bilateral infiltrates with hypoxemia and a Pao2/Fio2 ratio <200).
- Zotova NV, Chereshnev VA, Gusev EY. Systemic Inflammation: Methodological Approaches to Identification of the Common Pathological Process. PLoS One. 2016;11(5):e0155138. Published 2016 May 6. doi:10.1371/journal.pone.0155138 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4859514[↩][↩][↩][↩][↩]
- D’Mello, C., and Swain, M. G. (2017). Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr. Top. Behav. Neurosci. 31, 73–94. doi: 10.1007/7854-2016-37[↩]
- Chapter 34 – Fever and hypothermia in systemic inflammation. Handbook of Clinical Neurology Volume 157, 2018, Pages 565-597https://doi.org/10.1016/B978-0-444-64074-1.00034-3[↩][↩]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;100(6): 1644–55.[↩]
- Pierrakos C, Vincent J-L. Sepsis biomarkers: a review. Crit Care. 2010. February 9;14(1):R15 10.1186/cc8872[↩]
- Michels da Silva D, Langer H, Graf T. Inflammatory and Molecular Pathways in Heart Failure-Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int J Mol Sci. 2019;20(9):2322. Published 2019 May 10. doi:10.3390/ijms20092322 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6540104[↩]
- Pahwa R, Singh A, Jialal I. Chronic Inflammation. [Updated 2019 Dec 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493173[↩]
- Barcelos IP, Troxell RM, Graves JS. Mitochondrial Dysfunction and Multiple Sclerosis. Biology (Basel). 2019;8(2):37. Published 2019 May 11. doi:10.3390/biology8020037 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6627385[↩]
- Yousuf A, Ibrahim W, Greening NJ, Brightling CE. T2 Biologics for Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pract. 2019 May – Jun;7(5):1405-1416.[↩]
- Gusev EYu, Chereshnev VA. [Systemic inflammation: theoretical and methodological approaches to description of general pathological process model. Part I. General characteristics of the process]. Patologiya, fizioliologiya i eksperimental`naya terapiya. Pathology, Physiology and Experimental Therapy. 2012;4: 3–14. Russian.[↩]
- Gusev EYu, Chereshnev VA. [Systemic inflammation: theoretical and methodological approaches to description of general pathological process model. Part III. Prerequisites of non-syndromic approach]. Patologiya, fizioliologiya i eksperimental`naya terapiya. Pathology, Physiology and Experimental Therapy. 2013;3: 3–14. Russian.[↩][↩][↩]
- Gusev EYu, Chereshnev VA [Systemic inflammation: theoretical and methodological approaches to description of general pathological process model. Part IV. The dynamics of the process]. Patologiya, fizioliologiya i eksperimental`naya terapiya. Pathology, Physiology and Experimental Therapy. 2014;4: 4–16. Russian.[↩]