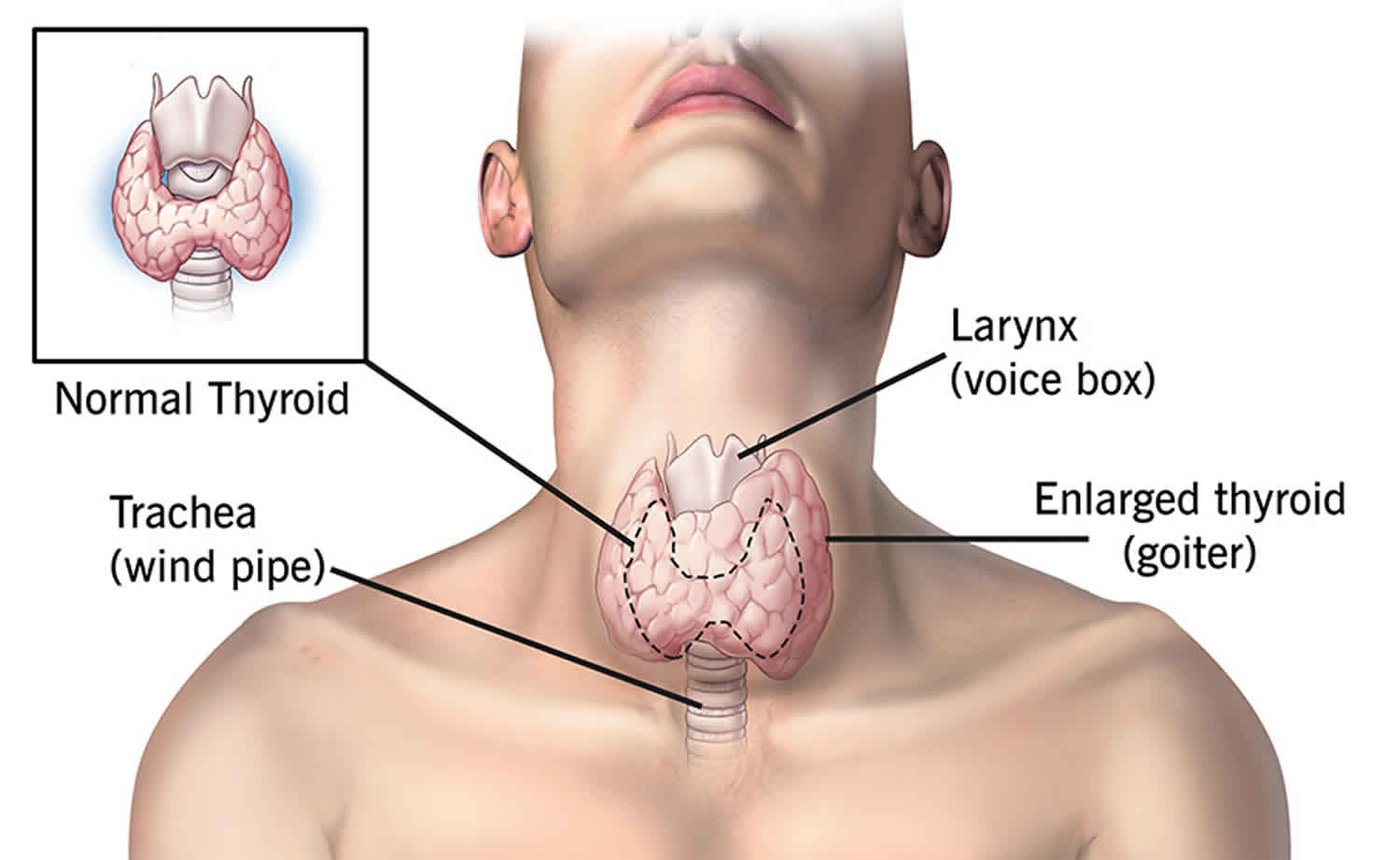
What is thyrotoxicosis
Thyrotoxicosis often called overactive thyroid or hyperthyroidism, is a condition in which your thyroid gland makes too much thyroid hormone. Thyrotoxicosis occurs in approximately 2% of women and 0.2% of men 1. Thyrotoxicosis clinical presentation varies, ranging from asymptomatic or subclinical, to life-threatening thyroid storm 2. Typical symptoms are due to the hypermetabolic state induced by excess thyroid hormones and include weight loss, heat intolerance, and palpitations. There are many different causes of thyrotoxicosis. It is important to determine the cause since treatment is based on the underlying cause. Thyrotoxicosis can lead to serious complications when not diagnosed and treated appropriately, including delirium, altered mental status, osteoporosis, muscle weakness, atrial fibrillation, congestive heart failure, thromboembolic disease, cardiovascular collapse, and death.
Thyroid hormone affects almost every tissue and organ system in the body by increasing basal metabolic rate and tissue thermogenesis. Thyroid hormone causes increased expression of myocardial sarcoplasmic reticulum calcium-dependent ATP, increasing heart rate and myocardial contractility with the net effect of increased cardiac output. Decreased systemic vascular resistance and decreased afterload results from arterial smooth muscle relaxation by metabolic end products, such as lactic acid, produced with increased consumption of oxygen. Decreased systemic vascular resistance leads to activation of the renin-angiotensin system, increasing reabsorption of sodium and expanding blood volume to increase preload. If left untreated, this may lead to left ventricular hypertrophy and congestive heart failure.
Thyrotoxicosis vs Thyroid storm
Thyroid storm also known as thyrotoxic crisis, is an acute, life-threatening complication of hyperthyroidism which is difficult to diagnose 3. Thyroid storm is an exaggerated presentation of thyrotoxicosis. It comes with sudden multisystem involvement. The mortality associated with thyroid storm is estimated to be 8-25% despite modern advancements in its treatment and supportive measures 4. Thus, it is very important to recognize it early and start aggressive treatment to reduce the mortality.
Thyroid storm accounts for about 1% to 2% of admissions for hyperthyroidism. As per the Japanese National Survey, the incidence of thyroid storm was 0.2 per 100,000 population per year, about 0.22% of all thyrotoxicosis patients and 5.4% of hospitalized thyrotoxicosis patients 3. The average age of people with thyroid storm was 42 to 43 years, which was similar to people with thyrotoxicosis without thyroid storm. The male to female ratio for incidence of thyroid storm was about 1:3, similar to thyrotoxicosis without storm group 5.
Thyroid storm is more common with Graves’ disease but can occur with other causes of hyperthyroidism, for example, toxic multinodular goiter and toxic adenoma of the thyroid 6.
Thyroid storm precipitating factors are:
- Abrupt discontinuation of antithyroid medicine
- Thyroid surgery
- Non-thyroid surgery
- Trauma
- Acute illness like infections, diabetic ketoacidosis, acute myocardial infarction, cardiovascular accident, cardiac failure, drug reaction
- Parturition
- Recent use of Iodinated contrast medium
- Radioiodine therapy (rare)
The pathophysiological basis for precipitation of thyroid storm in patients with thyrotoxicosis is not clear. But, a precipitating factor, as mentioned above, is always required to cause thyroid storm. Several hypotheses have been purposed. One hypothesis suggests the incidence of thyroid storm is due to the rapid increase in thyroid hormone levels, rather than the absolute hormone level that occurs during thyroid surgery, following radioactive iodine treatment, after sudden discontinuation of the antithyroid drug, or after administration of the large dose of iodine in contrast studies. The hyperactivity of sympathetic nervous system with increased response to catecholamine along with an increased cellular response to thyroid hormone during acute stress or infections, causing cytokines release and altered immunological disturbances, are other possible mechanisms of thyroid storm. Most studies have failed to relate higher thyroid hormone level as a cause of thyroid storm, except for the study by Brooks and others, reported higher free thyroid hormone among the patients with thyroid storm 7. In other words, the degree of thyroid hormone level is not directly related to higher incidence of thyroid storm 6.
Presentation of thyroid storm is an exaggerated manifestation of hyperthyroidism, with the presence of an acute precipitating factor. Fever, cardiovascular involvement (including tachycardia, heart failure, arrhythmia), central nervous system (CNS) manifestations, and gastrointestinal symptoms are common. Fever of 104 °F to 106 °F with excessive sweating (diaphoresis) is a key presenting feature. Cardiovascular manifestations include fast heart beat (tachycardia) more than 140 heart rate/minute, heart failure with pulmonary edema and peripheral edema, hypotension, arrhythmia, and death from cardiac arrest. Central nervous system (CNS) involvement includes agitation, delirium, anxiety, psychosis, or coma. Gastrointestinal (GI) symptoms include nausea, vomiting, diarrhea, abdominal pain, intestinal obstruction, and acute hepatic failure 8. A Japanese study found the central nervous system (CNS) involvement to be a poor prognostic factor for increased mortality 9.
Physical examination findings may include high temperature, tachycardia, orbitopathy, goiter, hand tremors, moist and warm skin, hyperreflexia, systolic hypertension, and jaundice.
The diagnosis of thyroid storm needs clinical suspicion based on the presentation mentioned above in a patient with hyperthyroidism or suspected hyperthyroidism.
In 1993, the following scoring system (Burch-Wartofsky Point Scale) for the diagnosis of thyroid storm was introduced 6:
- Temperature: 5 points per 1 °F above 99 °F (maximum 30 points)
- Central nervous system (CNS) dysfunction: 10 points for mild (agitation), 20 for moderate (delirium, psychosis or extreme lethargy), and 30 for severe (seizure or coma)
- Tachycardia: 5 (99-109 beats/minute), 10 (110 -119 beats/minute), 15 (120 -129 beats/minute), 20 (130 -139 beats/minute) and 25 (greater than 140 beats/minute)
- Presence of atrial fibrillation: 10
- Heart failure: 5 for mild (pedal edema), 10 for moderate (bi-basilar rales), 15 for severe (pulmonary edema)
- Gastrointestinal (GI) dysfunction: 10 for moderate (diarrhea, nausea/vomiting or abdominal pain) and 20 for severe (unexplained jaundice)
- Presence of Precipitating factor: 10 points
Diagnosis: Total score of more than 45 is highly suggestive of thyroid storm, 25 to 44 supports the diagnosis, and less than 25 makes the diagnosis unlikely 3.
The Burch-Wartofsky Point Scale scoring systems is just a guideline. The actual diagnosis is based on clinical judgment. Based on Burch-Wartofsky Point Scale scoring system, a score of 45 or more is more sensitive but less specific than Japanese Thyroid Association scoring systems TS1 or TS2 to detect thyroid storm cases. Burch-Wartofsky Point Scale score of 25 to 45 may suggest an impending storm.
Thyroid storm treatment
Treatment of thyroid storm consists of supportive measures like intravenous (IV) fluids, oxygen, cooling blankets, acetaminophen, as well as specific measures to treat hyperthyroidism. If any precipitating factors, for example, infection, are present, that needs to be taken care. Patients with thyroid storm must be admitted to the intensive care unit with close cardiac monitoring and ventilatory support if needed 6.
Specific strategic steps for thyroid storm treatment:
- Therapy to control increased adrenergic tone: Beta-blocker
- Therapy to reduce thyroid hormone synthesis: Thionamide
- Therapy to reduce release of thyroid hormone: Iodine solution
- Therapy to block peripheral conversion of T4 to T3: Iodinated radiocontrast agent, glucocorticoid, propylthiouracil (PTU), propranolol
- Therapy to reduce enterohepatic recycling of thyroid hormone: Bile acid sequestrant
After initial supportive measures, a beta blocker should be started for any case of suspected thyroid storm. Typically, propranolol 40 mg to 80 mg is given every 4 to 6 hours. Then, either a loading dose of propylthiouracil (PTU) 500 mg to 1000 mg followed by 250 mg every 4 hours or Methimazole (MMI) 20 mg every 4 to 6 hours should be given. Propylthiouracil is favored because it has a small but additional effect of blocking the peripheral conversion of T4 to T3. An hour after the administration of propylthiouracil or Methimazole, give five drops of SSKI (super saturated potassium iodide) by mouth every 6 hours. Always administer thionamide before starting iodine solution (SSKI) therapy 10. This prevents the eminent increase in thyroid hormone synthesis due to increased iodine load from super saturated potassium iodide. Hydrocortisone 100 mg IV every eight hours (or Dexamethasone 2 mg every 6 hours) should also be started. If available, oral cholestyramine 4 grams four times daily can be started for severe cases. One should look for precipitating factors and treat them accordingly. Use of aspirin should be avoided due to its potential risk of increasing free thyroid hormone levels by interfering with thyroid binding protein.
In the first 24 hours of treatment, propylthiouracil decreases T3 level by 45%, but Methimazole drops T3 level by only 10 % to 15%. Methimazole, whereas, causes more rapid normalization of serum T3 level after few weeks of treatment and it has less hepatotoxicity compared to propylthiouracil. Therefore, after initial stabilization, we should treat with Methimazole (if propylthiouracil was started at the beginning, it should be changed to Methimazole). For patients who cannot take oral antithyroid medicine, liquid preparation (pharmacist may have to compound) can be given as enemas. Sometimes, pharmacists can prepare IV form of antithyroid medicine by dissolving the tablet 11.
Esmolol, a short-acting beta blocker, at a loading dose of 250 mcg/kg to 500 mcg/kg followed by 50 mcg/kg to -100 mcg/kg/minute can be given in ICU setting. For patients with reactive airway disease, cardioselective beta blocker like atenolol or metoprolol should be chosen. If there is a contraindication for the use of beta blocker, diltiazem is an alternative.
If thionamide therapy is contraindicated because of an allergic reaction, thyroidectomy is needed after treatment with a beta blocker, hydrocortisone, cholestyramine, and iodine solution. Plasmapheresis is the last resort if all other measures fail.
Once patients’ clinical conditions improve, the iodine solution should be stopped, glucocorticoids can be tapered and stop, and beta blocker should be adjusted. Thionamide therapy should be titrated, and if propylthiouracil is used initially, it should be switched to Methimazole. Patients should be recommended for a definitive treatment with radioiodine (RAI) therapy or thyroidectomy.
Thyrotoxicosis causes
Graves’ disease is the most common cause of thyrotoxicosis, followed by toxic multinodular goiter and toxic adenoma (autonomously functioning thyroid nodule) 2. Other causes include thyroiditis, subacute thyroiditis, painless thyroiditis, and gestational hyperthyroidism. Drug-induced thyrotoxicosis has been associated with amiodarone and iodinated contrast. Rarer causes of thyrotoxicosis include thyroid stimulating hormone (TSH)-producing adenomas, struma ovarii, gestational trophoblastic neoplasia, thyrotoxicosis factitia, activation mutations of the TSH receptor, and functional thyroid cancer metastases.
Many diseases and conditions can cause thyrotoxicosis, including:
- Increased thyroid hormone production
- Graves’ disease (most common cause of hyperthyroidism)
- Toxic multinodular goiter
- Toxic adenoma
- Type 1 amiodarone-induced thyrotoxicosis
- Metastatic thyroid cancer
- Inflammation with leakage of thyroid hormone
- Subacute thyroiditis – inflammation (thyroiditis) of the thyroid due to viral infections, some medicines, or after pregnancy (common)
- utoimmune (Hashimoto’s) thyroiditis
- Type 2 amiodarone-induced thyrotoxicosis
- Taking too much thyroid hormone (common)
- Iatrogenic thyrotoxicosis
- TSH-secreting pituitary adenoma
- Noncancerous growths of the thyroid gland or pituitary gland (rare)
- Some tumors of the testes or ovaries (rare)
- Getting medical imaging tests with contrast dye that has iodine (rare, and only if there is a problem with the thyroid)
- Eating too much of foods that contain iodine (very rare, and only if there is a problem with the thyroid).
Graves’ disease is an autoimmune disease comprised of antibodies that stimulate TSH receptors to cause excess secretion of thyroid hormones. This results in hyperplasia of thyroid follicular cells, causing diffuse goiter. The cause of Graves’ disease is not known, but genetic and environmental factors, such as smoking, stress, and dietary iodine play a role. The thyroid stimulating immunoglobulin (TSI) triggers the hyperthyroidism.
In toxic multinodular goiter and toxic adenoma an autonomously functioning thyroid nodule that over-secrete thyroid hormone. In thyroiditis, thyrotoxicosis is caused by the release of preformed thyroid hormone into the circulation as inflammation destroys thyroid follicles. This causes transient thyrotoxicosis that most often self-resolves. Inflammation can be precipitated by a variety of insults to the thyroid gland, including autoimmune, infectious, chemical, or mechanical insults. Gestational hyperthyroidism generally occurs in the first trimester of pregnancy, due to increased stimulation of the thyroid gland by excess human chorionic gonadotropin (hCG), which is similar in structure to thyroid stimulating hormone (TSH) and binds the TSH receptor.
Thyrotoxicosis signs and symptoms
Thyrotoxicosis common symptoms include:
- Anxiety
- Difficulty concentrating
- Fatigue
- Frequent bowel movements
- Goiter (visibly enlarged thyroid gland) or thyroid nodules
- Hair loss
- Hand tremor
- Heat intolerance
- Increased appetite
- Increased sweating
- Irregular menstrual periods in women
- Nail changes (thickness or flaking)
- Nervousness
- Pounding or racing heart beat (palpitations)
- Restlessness
- Sleep problems
- Weight loss (or weight gain, in some cases)
Patients with thyrotoxicosis most commonly present with weight loss with a normal or increased appetite, heat intolerance with increased sweating, palpitations, tremor, anxiety, proximal muscle weakness, alopecia and increased fatigability. Sinus tachycardia is the most common cardiac rhythm problem, but atrial fibrillation may occur and is more common with advanced patient age, valvular disease, and coronary artery disease. Women may present with amenorrhea or oligomenorrhea. Men may present with gynecomastia rarely. It is common for older patients to manifest fewer of the typical clinical manifestations and instead present with depression, fatigue, and weight loss.
Findings specific to Graves’ disease include ophthalmopathy resulting in proptosis, chemosis, conjunctival injection and lid lag, exposure keratitis and extra-ocular muscle dysfunction, pretibial myxedema and thyroid acropachy (clubbing). In subacute thyroiditis, patients generally present with upper respiratory symptoms followed by fever, neck pain and swelling with a firm thyroid gland. Painless thyroiditis often presents in the postpartum period, and patients frequently have a personal or family history of autoimmune or thyroid disease. Suppurative thyroiditis presents with a tender, erythematous mass in the anterior neck and patients often complain of fever, dysphagia, and dysphonia.
Occasionally, patients may present with acute muscle paralysis and severe hypokalemia, termed thyrotoxic periodic paralysis. In rare cases, patients present in thyroid storm with tachycardia, fever, altered mental status, agitation, features of cardiac failure, and impaired liver function.
On physical exam, patients are often cachectic, hyperthermic, diaphoretic, and anxious appearing. They may have goiter, tachycardia or atrial fibrillation, dyspnea, abdominal tenderness, hyperreflexia, proximal muscle weakness, tremor, and gynecomastia. Patients with Graves’ disease present with pretibial myxoedema, thyroid acropachy, and onycholysis.
Thyrotoxicosis diagnosis
Low serum thyroid stimulating hormone (TSH) (less than 0.01 mU/L) has high sensitivity and specificity for the diagnosis of thyroid disorders. If thyroid stimulating hormone (TSH) is low, elevated serum free thyroxine (T4) and triiodothyronine (T3) levels can distinguish between overt and subclinical hyperthyroidism. Usually, the increase in T3 precedes the increase in T4. Pituitary-dependent causes of hyperthyroidism may have normal or increased TSH levels and increased T4 and T3 levels with an increase in free-alpha subunit concentrations. Serum levels of antibodies to the TSH receptor diagnose Graves’ disease. Levels are 98% sensitive and 99% specific. Thyroid peroxidase antibodies are only present in about 75% of cases of Graves’ disease.
Radioactive iodine uptake studies or thyroid scans may be used to distinguish between causes of thyrotoxicosis besides Graves’ disease. It is recommended in all thyrotoxic patients without the clinical picture of Graves’ disease. In Graves’ disease, radioactive iodine uptake is diffuse, unless the patient also has nodules or fibrosis. In single toxic adenoma, there will be a focal uptake in the adenoma, with suppressed uptake in the surrounding thyroid tissue. Toxic multinodular goiter will show multiple areas of focal increased uptake and suppressed uptake in surrounding tissue. Radioactive iodine uptake will be near zero in patients with painless, postpartum, or subacute thyroiditis, as well as patients with ingestion of thyroid hormone or recent excess iodine exposure.
In subacute thyroiditis, inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein are frequently increased. In pregnancy, either free T3 and T4, or the total T3 and T4 with adjusted reference range 1.5 times the nonpregnant range should be used for diagnosis in addition to serum TSH levels. During the first half of pregnancy, the serum TSH levels may be lower than the nonpregnant reference range, but free T4 values should be normal. In suspected factitious hyperthyroidism, thyroglobulin levels are decreased and radioactive uptake studies due to the suppression by exogenous thyroid hormone ingestion.
Thyrotoxicosis treatment
The recommended treatment of thyrotoxicosis is dependant on the underlying cause. Beta-blocker therapy, for example, propranolol, is useful to reduce adrenergic features such as sweating, anxiety, and tachycardia. There are 3 mainstays of treatment: thionamide drugs, radioiodine, and thyroid surgery.
Thionamide drugs include propylthiouracil (PTU) and methimazole and reduce the production of thyroid hormone by acting as preferential substrates for thyroid peroxidase. In high doses, propylthiouracil (PTU) also decreases peripheral conversion of T4 to T3.
In the treatment of Graves’ disease, methimazole is used at a dose of 15 mg to 30 mg per day for 4 to 8 weeks, after which most patients become euthyroid. After patients become euthyroid, there are 2 approaches to treatment. First, in the block-replace method, the same dose of thionamide is continued to block thyroid hormone production, and levothyroxine is added to maintain euthyroidism. Alternatively, the thionamide dose may be titrated progressively to allow endogenous synthesis of thyroid hormone, maintaining a euthyroid state.
In Graves’ disease, long-term remission is achieved in about 50% of patients with thionamide drugs. A disadvantage of thionamides is the uncertainty of relapse after treatment is stopped. No advantage has been shown in remission rates with prolonged treatment beyond 18 months. Methimazole has been shown to have better efficacy and has a longer half-life, which allows once-daily dosing. Additionally, there is a higher risk of hepatotoxicity with propylthiouracil (PTU). Agranulocytosis occurs in 1 in 300 patients treated with thionamides and presents as a sore throat, mouth ulcers and high fever. It is recommended to obtain a differential white blood cell count during febrile illness and pharyngitis in all patients on thionamides. Minor side effects include pruritis, arthralgia, and gastrointestinal upset.
Radioiodine therapy is the most common therapy used for adults with Graves’ disease in the United States. It can also be used for toxic nodules and toxic multinodular goiters. Radioactive iodine is given in one oral dose. It is absorbed by the thyroid gland inducing tissue-specific inflammation that leads to thyroid fibrosis and destruction of thyroid tissue over the next several months. Hypothyroidism usually occurs within 6 to 12 months. Most patients go on to require lifelong levothyroxine treatment. There is a small risk of thyrotoxicosis exacerbation in the month after treatment due to the release of the preformed hormone. Patients with large goiters, severe thyrotoxicosis, ischemic heart disease, heart failure or arrhythmia are recommended to use thionamide pretreatment until they are euthyroid before radioiodine therapy. Radioiodine therapy is contraindicated in pregnancy and lactation and relatively contraindicated in active inflammatory Graves’ ophthalmopathy.
Total or partial thyroidectomy is a rapid and effective method of treating thyrotoxicosis. However, it is invasive and expensive, and causes permanent hypothyroidism, requiring levothyroxine treatment. It is recommended that patients be pretreated for euthyroidism before surgery to reduce the risk of worsening thyrotoxicosis and thyroid storm. Complications include hypocalcemia due to hypoparathyroidism, which is usually transient, and vocal cord paresis due to damage to the recurrent laryngeal nerve.
The treatment for thyroiditis differs in that antithyroid drugs are ineffective, since patients usually have low production of new thyroid hormone. It is usually transient, but treatment is aimed at symptom control with beta blockers. In subacute thyroiditis, non-steroidal anti-inflammatory drugs and occasionally systemic glucocorticoids may be used to help with pain and inflammation. Beta blockers are recommended for any elderly patients with symptomatic thyrotoxicosis, and any thyrotoxic patients with resting a heart rate greater than 90 bpm or cardiovascular disease.
Children with thyrotoxicosis may be treated with methimazole, radioiodine therapy or thyroidectomy. Methimazole therapy for 1 to 2 years is the first line therapy for Graves’ disease in children since some children will go into remission. Radioiodine therapy is not recommended for children younger than 5 years old. Propylthiouracil (PTU) should also be avoided in children due to the risk of hepatotoxicity.
During pregnancy, it is recommended to treat with thionamide drugs in a titrated dose regimen. Block-replace regimens increase the risk of fetal hypothyroidism and goiter. Propylthiouracil (PTU) is recommended during the first trimester of pregnancy. Propylthiouracil (PTU) is preferred during the first trimester of pregnancy due to the risk of teratogenicity associated with methimazole, including aplasia cutis and choanal or esophageal atresia.
Follow up
Serial profiles of thyroid function tests including TSH, free or total T4, and total T3 levels should be followed at regular 2-4 week intervals when treating and monitoring thyrotoxic disorders. In cases of treated hyperthyroidism, suppression of the TSH level may persist for several weeks after thyroid hormone levels have been brought under control. Treatment of post-ablative or post-surgical hypothyroidism with levothyroxine should be considered once T4 and T3 levels drop to low normal or subnormal ranges.
- Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332(7554):1369–1373. doi:10.1136/bmj.332.7554.1369 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476727[↩]
- Blick C, Jialal I. Thyrotoxicosis. [Updated 2019 Feb 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482216[↩][↩]
- Pokhrel B, Bhusal K. Thyroid Storm. [Updated 2019 Jun 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448095[↩][↩][↩]
- Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016 Oct;26(10):1343-1421[↩]
- Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani H, Teramukai S, Uehara R, Nakamura Y, Nagai M, Mori M., Japan Thyroid Association. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012 Jul;22(7):661-79[↩]
- Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol. Metab. Clin. North Am. 1993 Jun;22(2):263-77[↩][↩][↩][↩]
- Brooks MH, Waldstein SS. Free thyroxine concentrations in thyroid storm. Ann. Intern. Med. 1980 Nov;93(5):694-7[↩]
- Angell TE, Lechner MG, Nguyen CT, Salvato VL, Nicoloff JT, LoPresti JS. Clinical features and hospital outcomes in thyroid storm: a retrospective cohort study. J. Clin. Endocrinol. Metab. 2015 Feb;100(2):451-9.[↩]
- Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, Tsuboi K, Monden T, Kouki T, Otani H, Teramukai S, Uehara R, Nakamura Y, Nagai M, Mori M., Japan Thyroid Association. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012 Jul;22(7):661-79.[↩]
- Goodier CG. Endocrine Emergencies in Obstetrics. Clin Obstet Gynecol. 2019 Jun;62(2):339-346.[↩]
- Kalpakam H, Dhooria S, Agarwal R, Mukherjee S, Sehgal IS. A rare complication of bedside tracheotomy: Thyroid crisis. Lung India. 2019 Jan-Feb;36(1):77-79.[↩]