What are totipotent cells
Totipotent cells are cells that are “capable of developing into a complete organism” or “differentiating into any of its cells or tissues” 1 or “an isolated cell that is able to produce a fertile adult individual”. The term totipotent describes the properties of an individual cell (not a group of cells) with the two meanings of this term roughly corresponding to the progressive restriction in potential cells exhibit during normal development 1. However, totipotency is not well defined so far and the use of this term causes some confusion in the scientific field 2. By strict definition, totipotency is the ability of a single cell to develop independently into a healthy organism in a permissive environment 2. By a less strict definition, totipotency is the potential of a cell to differentiate into any type of cells of the body as well as any cells supporting the development of a mammal including those of placenta and extra embryonic membranes 3. These loose definitions, as well as the available alternative proposals, largely overlook the genetic, epigenetic, and biochemical underpinnings of totipotency 2. With the molecular underpinnings considered, the term totipotency connotes three fundamentally different concepts: 1) genetic totipotency, referring to the genetic integrity (or contents) of a nucleus or a cell irrespective of the functional status of the genetic materials, active or inactive; 2) the epigenetic (or functional) totipotency, which is the genetic competency (or active status) of a cell with its totipotent genetic determinants active; or 3) the reprogramming capacity of a cell toward epigenetic totipotency, which is the biochemical competency of a cell independent of its genetic compositions and epigenetic status. Although some sperm proteins may have impact on development 4, the totipotent reprogramming activity is generally from the oocyte factors because an enucleated oocyte without fertilization can reprogram an implanted fully differentiated nucleus to totipotency and give birth to a healthy animal. In other words, the oocyte has the full totipotent reprogramming activity without any contribution from the sperm. For this reason, we may call the third category maternal totipotency as well. The maternal reprograming factors may be proteins and/or RNA from the oocyte reserves. Due to its independence of genetic and epigenetic components, totipotent reprogramming activity in the form of reserve proteins and/or RNA is not sustainable and cannot be captured or maintained in cell culture. The former two concepts are the nuclear features while the third one concerns mainly the cytoplasmic capacity. A genetically totipotent cell may not be so epigenetically. Totipotent reprogramming factors may be different from those for maintenance of the epigenetically totipotent status. For example, Oct4, a critical factor for the maintenance of embryonic pluripotency and possibly for totipotency, is not required for establishment of totipotency, or for induction of embryonic pluripotency 5. With the different concepts of totipotency defined above, the relevance of each of these concepts to the zygote is discussed below.
Totipotent cell examples are zygotes and some early cleavage-stage blastomeres 1. Much of the confusion surrounding the term totipotency centers on the important differences between these two definitions. A one-cell embryo (zygote) is “totipotent” in both senses; yet, some authors characterize tumors 6 and stem cells 7 as “totipotent,” based only on the second definition (ie, the ability of these cells to produce a wide range of cell types).
The difference between these two definitions is not trivial. Producing a mature organism requires the ability to both generate all the cells of the body and to organize them in a specific temporal and spatial sequence, that is, to undergo a coordinated process of development. Totipotency in this strict sense is demonstrated by the ability of an isolated cell to produce a fertile, adult individual. Consequently, a cell that is totipotent is also a one-cell embryo; that is, a cell that is capable of generating a globally coordinated developmental sequence.
While stem cells, tumors, and embryos have many molecular features in common, embryos are clearly organisms 8. Embryos develop in a predictable manner toward a species-specific adult form (human embryos do not mature into mice, monkeys, or tumors). Embryos repair injury. They adapt to changing environmental conditions. Most importantly, they show coordinated interactions between parts (molecules, cells, tissues, structures, and organs) that promote the survival, health, and continued development of the organism as a whole; that is, interactions that are characteristic of “an individual constituted to carry on the activities of life by means of organs separate in function but mutually dependent: a living being,”. In contrast, stem cells and tumors do not produce cells or structures in the functionally integrated progression that is characteristic of an organism. They are not capable of development.
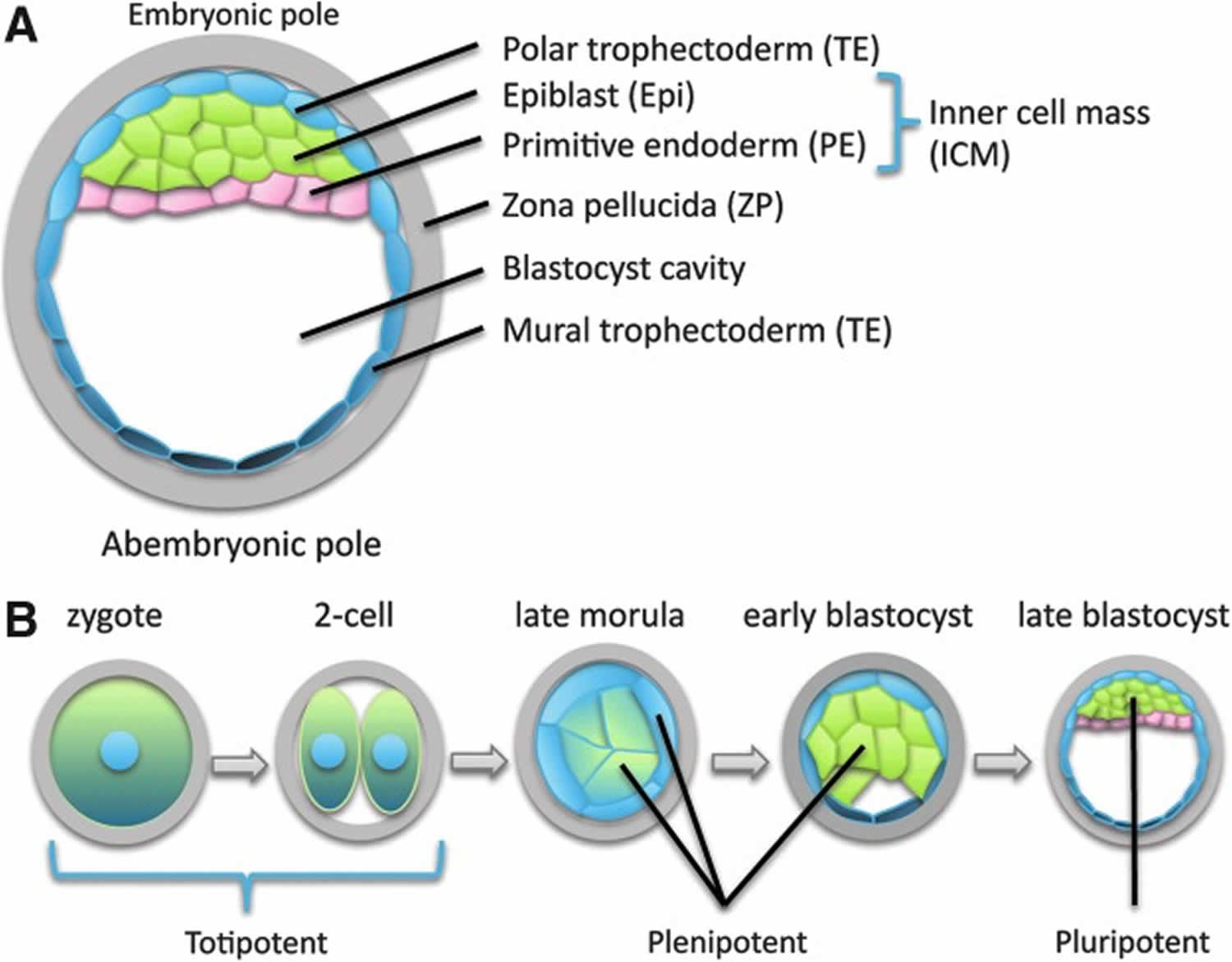
At the expanded blastocyst stage (Figure 1A), cells in specific regions of the embryo are restricted to produce only a subset of the cells observed at more-mature stages (Table 1). Restrictions in potency occur gradually as development proceeds, with the ability to independently initiate a developmental sequence (ie, totipotency in the first sense) being lost relatively early during development, and individual cells of the late morula and early blastocyst producing a wider range of derivatives than cells at the expanded blastocyst stage (Figure 1B).
Figure 1. Totipotent cells
Footnote: Cell types and potency at different developmental stages.
(A) Anatomy and cell types of the expanded blastocyst embryo (human development, ∼5–6 days; shown as a mid-sagittal section). The position of the inner cell mass [ICM] (green and pink) defines the embryonic-abembryonic axis, which has a consistent relationship to the animal-vegetal axis of the oocyte, and may be determined by both the shape of the zygote and the early cleavage patterns of the embryo. The entire embryo is surrounded by an acellular protein layer known as the zona pellucida (gray). Trophectoderm (TE) associated with the embryonic pole (light blue) induces the formation of primitive endoderm [PE] (pink), that is initially interspersed with the presumptive epiblast (green), and segregates by cell sorting. Together, epiblast and primitive endoderm [PE] constitute the inner cell mass [ICM]. The blastocyst cavity is a fluid-filled space. Polar (light blue) and mural (dark blue) trophectoderm (TE) have distinct molecular properties and distinct developmental fates.
(B) During human development, only the zygote and early cleavage-stage blastomeres (possibly up to the four-cell stage) remain totipotent, that is, capable of independently initiating a developmental sequence. Cells of the late morula/early blastocyst are plenipotent; that is, they are able to produce all or most of the cells of the body, but not organize them into a coherent body plan. Cells of the epiblast at the expanded blastocyst stage (the cells from which many embryonic stem cell lines are derived) are pluripotent; that is, they are able to produce cell types found in the mature body, but are not derivatives of the trophectoderm (TE) and primitive endoderm (PE).
Abbreviations: PE = primitive endoderm; TE = trophectoderm.
[Source 1 ]Genetic totipotency is not specific to the zygote
Before animals were first cloned from nuclei of the fully differentiated cells of frogs in 1960s 9, it was posited that cells continuously lose some genetic determinants over the course of development and become permanently restricted in developmental potentials 10. Only the germline cells were thought to retain a complete set of the genetic constituents 11. In contrast, a then competing theory, the principle of nuclear equivalence, espoused the notion that a fully differentiated cell contains exactly the same genetic materials as does a blastomere or the zygote, and therefore retains the complete genetic constituents required for development to a healthy individual 12. In line with this latter principle, many animals of different species have eventually been cloned each from a fully differentiated nucleus after its transfer into an enucleated oocyte, providing clear evidence that a fully differentiated nucleus is still genetically totipotent 13. This concept has later been corroborated by induction of various somatic cells to pluripotent stem cells via ectopic expression of reprogramming factors 14 not only in the form of the sustained integrating viral vectors 15 but also in the forms of the ephemeral synthetic mRNA 16, recombinant proteins 17, or transient vectors 18.
Clearly, genetic totipotency does not apply to cells that have lost their genomes such as enucleated oocytes, enucleated zygotes, enucleated blastomeres, mature red blood cells, and platelets. Many individual genes are essential for genetic totipotency since we have seen embryonic lethality when a specific gene is knocked out. Apparently, not every gene is required for genetic totipotency because we have generated so many mice with a specific gene knocked out.
Of note, polyploid cells may be compromised in genetic totipotency, as supported by the fact that cells of a tetraploid embryo rarely contribute to the development of the embryo proper 19. A mammalian haploid genome seems deficient in genetic totipotency. First, no haploid mammals have ever been observed although haploid invertebrates are well known 20. Features of haploid ESCs also provide some insights into the totipotency of mammalian haploid cells. Mouse and monkey haploid ESCs have been established, but the haploid karyotype is very unstable 21. They undergo diploidization in the culture and during differentiation both in vitro and in vivo. Human haploid ESCs are genetically more stable in culture and during differentiation, but other factors may impair the totipotent potential of human haploid cells. These may include deficiency in parental imprinting, DNA and RNA levels (dosage imbalance), cell size, mitochondrial abundance, and skewed expression ratio of X‐linked and autosomal genes 20.
Most aneuploidy cells may lose genetic totipotency. Aneuploidy mainly originates from nondisjunction of chromosomes/chromatids during meiosis I and II 22. Their developmental potentials can be inferred from human clinical data. First of all, trisomies of all chromosomes with exception of chromosome 1 have been reported in spontaneous abortions that occur between 6 to 20 weeks of gestation, but trisomy is restricted to a few chromosomes in later stages, i.e., stillbirths and live birth 23, indicating that trisomy in most chromosomes causes early developmental arrest. Second, the incidence of aneuploidy drops significantly over developmental stages with rates of 25%, 5%, 0.34%, and 0.3% for oocytes, first trimester (5‐12 weeks), 13‐40 weeks, and after 40 weeks, respectively 23. This, again, indicates that the majority of aneuploid embryos arrest at very early stages. The genetic totipotency of monosomy appears to be impaired more severely because monosomies all abort prior to being clinically recognized. Theoretically, if monosomies and trisomies would have the same developmental potentials they should have the same incidence because they are the results of reciprocal events at meiosis 23. Interestingly, some aneuploidy may have very little impact on totipotency. For example, 0.1 to 0.2% newborn male infants have the genotype of 47, XXY 24. Many XXY individuals do not even notice their genetic differences in their entire lives.
It is now widely accepted that most cells within an individual have the same genetic makeup as that of the zygote and other early embryonic cells within the cleavage‐stage embryos. Therefore, all the different types of diploid cells of our bodies, undifferentiated or differentiated, embryonic or somatic, are genetically totipotent. Being a general feature, genetic totipotency, however, is not a characteristic that uniquely distinguishes a zygote from any normal diploid somatic cell.
The zygote has the capacity for reprogramming to totipotency
Oocytes are the most powerful reprogramming vehicle in nature 25. An enucleated oocyte (or with its nuclear destroyed) can reprogram an implanted fully differentiated somatic nucleus to functional totipotency and gives rise to a cloned animal 26. Reprogramming activity exists beyond oocytes. OCT4‐GFP reporter experiments using somatic cell nuclear transfer (SCNT) technology indicate that reprogramming occurs at cleavage stages 27. An enucleated zygote can still reprogram an implanted differentiated nucleus to totipotency when the right procedure is employed 28. Upon fertilization of an oocyte, the paternal and maternal chromatin starts epigenetic and transcriptional reprogramming to totipotency by the oocyte factors. This totipotent reprogramming process continues beyond the zygote; even a blastomere from a 2‐cell stage embryo retains significant capacity for totipotent reprogramming 29.
Persistence of oocyte reprogramming activity into the 2‐cell stage is additionally supported by the generation of mice after injection of a round spermatid into a haploid parthenogenote 30, which is an equivalent to a blastomere of a 2‐cell embryo. Of note, the totipotent reprogramming activity in oocyte and zygote are independent of their epigenetic status because an enucleated oocyte can reprogram into totipotency the fully differentiated implanted genomes of various origins including those from fibroblasts 31, cumulus, sertoli cells 32, T‐cell , B‐cell 33 and others. Therefore, the cellular function of a zygote is totipotent reprogramming endowed by the maternal reprogramming factors inherited from its parental oocyte although the reprogramming activity may be attenuated, but the zygote genome is not in a totipotent state transcriptionally, epigenetically, and functionally. Like in the zygote, a blastomere from a 2‐cell stage embryo may still be in the reprogramming process toward totipotency since it can reprogram an implanted nucleus to totipotency 34. Similar to zygote, the totipotency of a blastomere from the 2‐cell stage embryo may be largely maternal since it still retains a significant amount of oocyte factors.
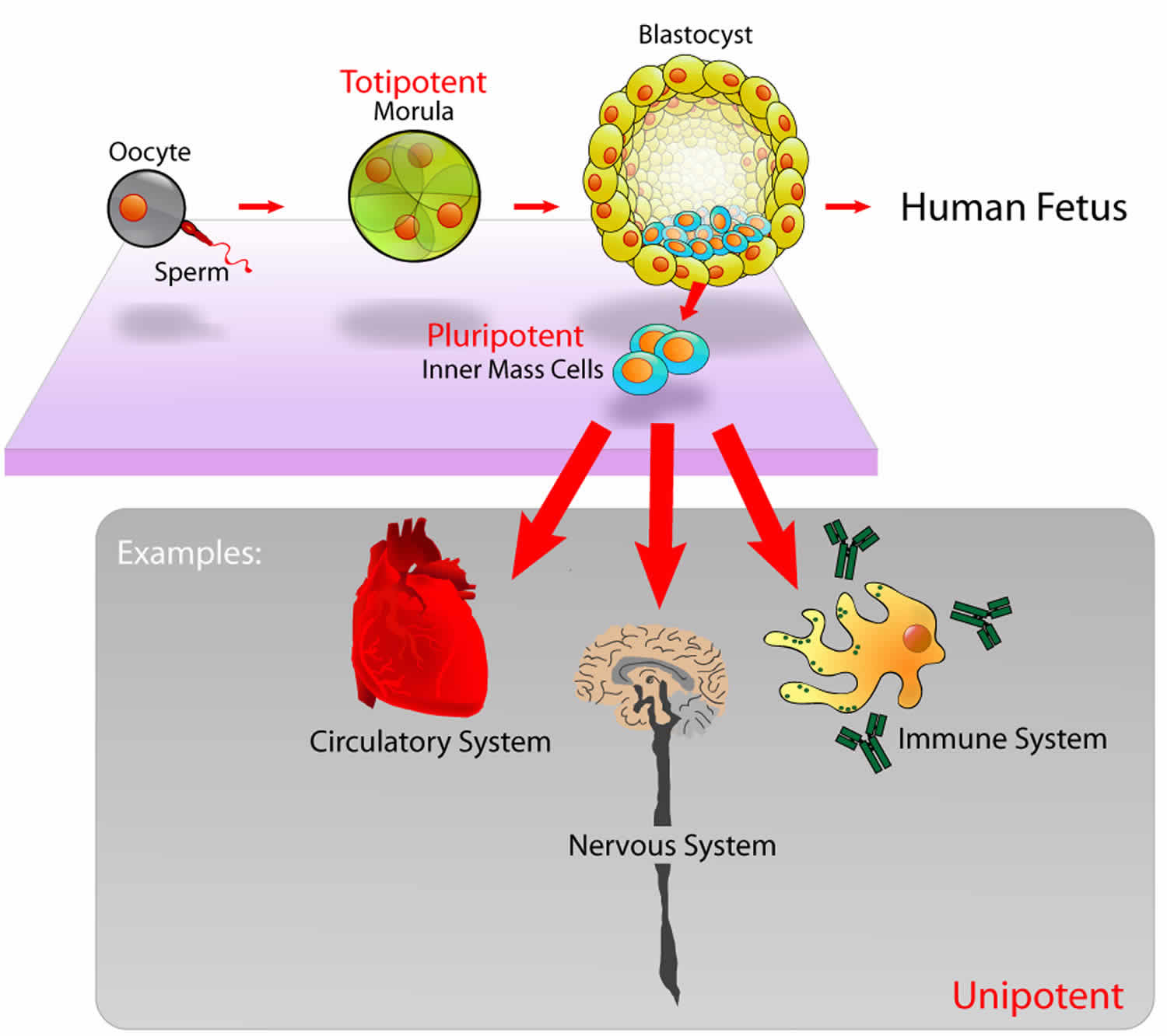
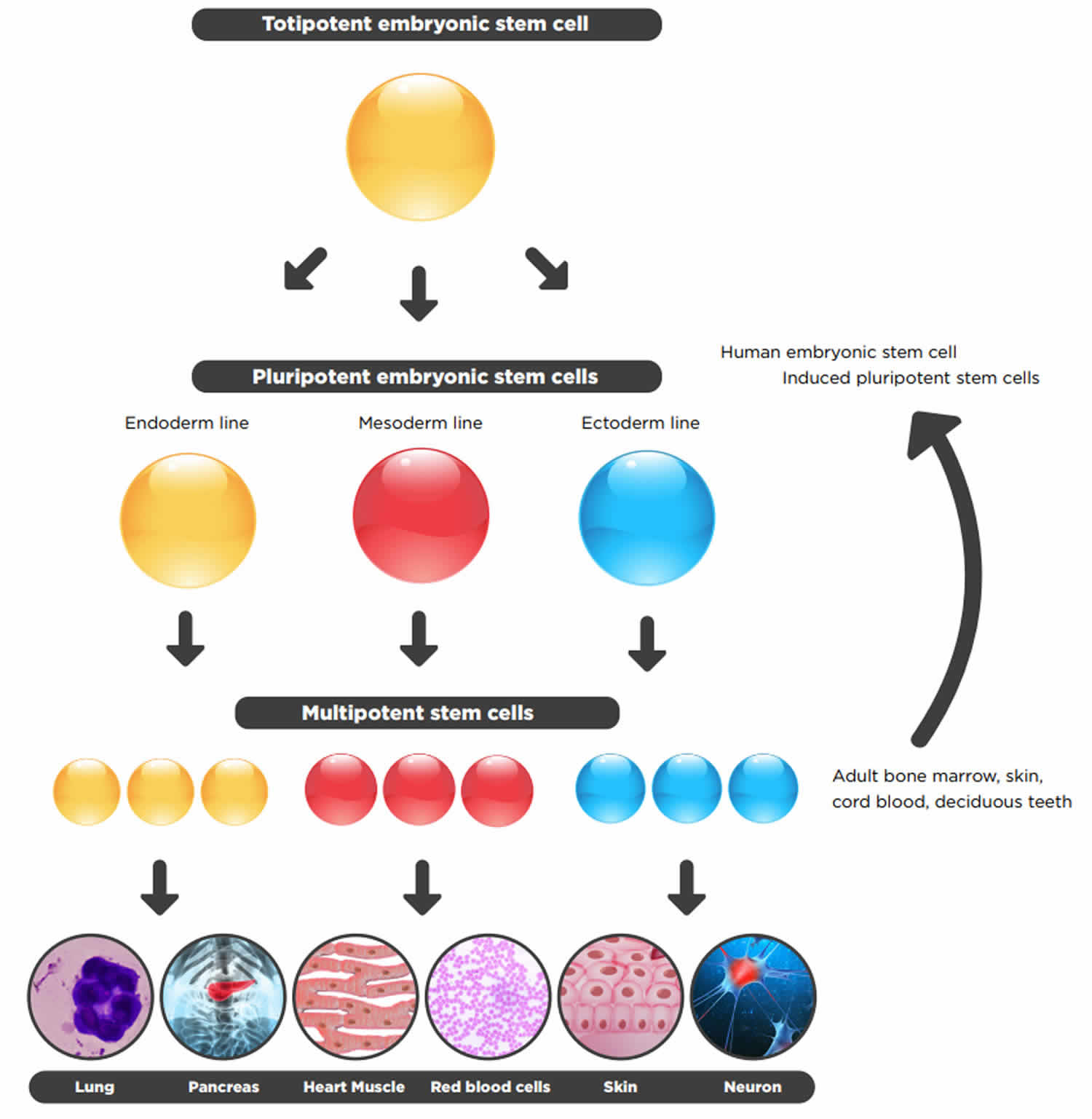
Totipotent vs Pluripotent stem cells
In humans, there are many different types of stem cells that come from different places in the body or are formed at different times in your life. These include embryonic stem cells that exist only at the earliest stages of development and various types of tissue-specific or adult stem cells that appear during fetal development and remain in your body throughout life.
Stem cells are defined by two characteristics:
- They can make copies of themselves, or self-renew
- They can differentiate, or develop, into more specialized cells
Beyond these two things, though, stem cells differ a great deal in their behaviors and capabilities.
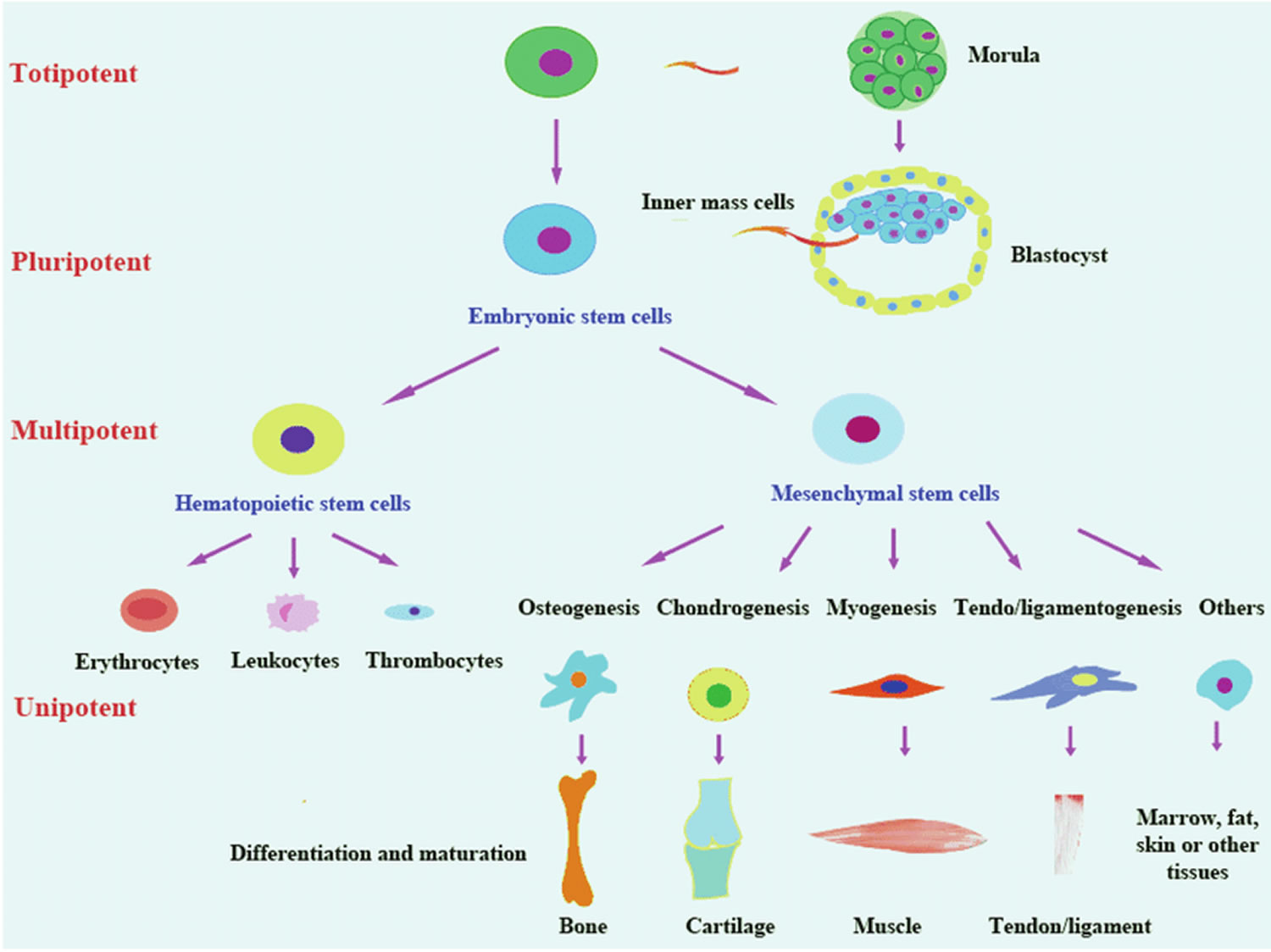
Cell potency refers to the varying ability of stem cells to differentiate into specialized cell types. Cells with the greatest potency can generate more cells types than those with lower potency.
- Totipotent Stem Cells: Totipotent (omnipotent) stem cells can give rise to any of the 220 cell types found in an embryo as well as extra-embryonic cells (placenta).
- Pluripotent Stem Cells: Pluripotent stem cells can give rise to all cell types of the body (but not the placenta).
- Multipotent Stem Cells: Multipotent stem cells can develop into a limited number of cell types in a particular lineage.
Embryonic stem cells are pluripotent, meaning they can generate all of the body’s cell types but cannot generate support structures like the placenta and umbilical cord.
Other cells are multipotent, meaning they can generate a few different cell types, generally in a specific tissue or organ.
As the body develops and ages, the number and type of stem cells changes. Totipotent cells are no longer present after dividing into the cells that generate the placenta and umbilical cord. Pluripotent cells give rise to the specialized cells that make up the body’s organs and tissues. The stem cells that stay in your body throughout your life are tissue-specific, and there is evidence that these cells change as you age, too – your skin stem cells at age 20 won’t be exactly the same as your skin stem cells at age 80.
Pluripotent cell is an isolated cell that is able to produce all or most of the derivatives of the epiblast, but is unable to organize these cells into an integrated body plan or to produce derivatives of trophectoderm and/or primitive endoderm. Pluripotent cell produces all of the structures of the mature body (including germ line), with minimal contribution to the trophectoderm- and/or primitive endoderm-derived structures in a complementation assay. The cell makes substantial contributions to all three germ layers with minimal contribution to trophectoderm- and/or primitive endoderm-derived structures in a chimeric embryo/animal. Pluripotent cell is able to form type I non-yolk-sac-containing teratomas, including derivatives of all three germ layers, with minimal representation of trophectoderm- and/or primitive endoderm-derived cells. Pluripotent cell is able to differentiate in culture into cells with gene expression patterns and physiology characteristic of the derivatives of all three germ layers. Examples of pluripotent cells include epiblast cells in normal embryos (mouse embryonic stem cells) and mouse induced pluripotent cells under standard culture conditions.
Figure 2. Totipotent vs Pluripotent vs Multipotent stem cells
Table 1. Totipotent vs Pluripotent vs Multipotent Stem Cells Comparison Chart
| Totipotent Stem Cells | Pluripotent Stem Cells | Multipotent Stem Cells | |
|---|---|---|---|
| Relative potency | High | Medium | Low |
| Cell types capable of generating | Differentiate into any cell type | Differentiate into cells from any of the three germ layers | Differentiate into a limited range of cell types |
| Terminology | Toti = Whole | Pluri = Many | Multi = Several |
| Examples | Zygote, early morula | Embryonic stem cells, Induced pluripotent stem cells | Hematopoietic stem cells, neural stem cells, mesenchymal stem cells |
| Found | Early cells of fertilized egg | Inner mass cells of the blastocyst | In many tissues |
| Expression of pluripotency genes | +++ | ++ | + |
| Expression of lineage-specific genes | + | ++ | +++ |
| Pros of use in research | Easy to isolate and grow | Easy to isolate and grow | Less ethical issues, less chance of immune rejection if taken from same patient |
| Cons of use in research | Ethical issues | Ethical issues, teratoma formation | Hard to isolate, limited differentiation, scarce |
- Condic ML. Totipotency: what it is and what it is not. Stem Cells Dev. 2014;23(8):796–812. doi:10.1089/scd.2013.0364 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3991987[↩][↩][↩][↩]
- On mammalian totipotency – what is the molecular underpinning for the totipotency of zygote? Stem Cells Dev. 2019 May 24. doi: 10.1089/scd.2019.0057. [Epub ahead of print] https://www.liebertpub.com/doi/pdf/10.1089/scd.2019.0057[↩][↩][↩]
- G, L Lei and HR Scholer. (2017). Totipotency in the mouse. J Mol Med (Berl) 95:687‐694.[↩]
- Castillo J, M Jodar and R Oliva. (2018). The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum Reprod Update 24:535‐555.[↩]
- Wu G, D Han, Y Gong, V Sebastiano, L Gentile, N Singhal, K Adachi, G Fischedick, C Ortmeier, M Sinn, M Radstaak, A Tomilin and HR Scholer. (2013). Establishment of totipotency does not depend on Oct4A. Nat Cell Biol 15:1089‐1097.[↩]
- Feigin ME. and Malbon CC. (2008). OSTM1 regulates beta-catenin/Lef1 interaction and is required for Wnt/beta-catenin signaling. Cell Signal 20:949–957[↩]
- Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, Grana O, Megias D, Dominguez O, et al. (2013). Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502: 340–345[↩]
- Condic M. (2011). Pre-implantation stages of human development: the biological and moral status of early embryos. In: Is This Cell a Human Being?: Exploring the Status of Embryos, Stem Cells and Human-Animal Hybrids. Suarez A, editor; , Huarte J, editor. , eds. Springer, New York, p 209[↩]
- Gurdon JB and V Uehlinger. (1966). “Fertile” intestine nuclei. Nature 210:1240‐1241.[↩]
- Briggs R and TJ King. (1952). Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs’ Eggs. Proc Natl Acad Sci U S A 38:455‐463.[↩]
- DiBerardino MA. (1987). Genomic potential of differentiated cells analyzed by nuclear transplantation. American Zoologist 27:623‐644.[↩]
- Campbell KH. (1999). Nuclear equivalence, nuclear transfer, and the cell cycle. Cloning 1:3‐15.[↩]
- Sin T, D Kraemer, J Pryor, L Liu, J Rugila, L Howe, S Buck, K Murphy, L Lyons and M Westhusin. (2002). A cat cloned by nuclear transplantation. Nature 415:859.[↩]
- Hu K. (2014). All roads lead to induced pluripotent stem cells: the technologies of iPSC generation. Stem Cells Dev 23:1285‐1300.[↩]
- Yu J, MA Vodyanik, K Smuga‐Otto, J Antosiewicz‐Bourget, JL Frane, S Tian, J Nie, GA Jonsdottir, V Ruotti, R Stewart, Slukvin, II and JA Thomson. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917‐1920.[↩]
- Warren L, PD Manos, T Ahfeldt, YH Loh, H Li, F Lau, W Ebina, PK Mandal, ZD Smith, A Meissner, GQ Daley, AS Brack, JJ Collins, C Cowan, TM Schlaeger and DJ Rossi. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618‐630[↩]
- Zhou H, S Wu, JY Joo, S Zhu, DW Han, T Lin, S Trauger, G Bien, S Yao, Y Zhu, G Siuzdak, HR Scholer, L Duan and S Ding. (2009). Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4:381‐384.[↩]
- Hu K and I Slukvin. (2013). Generation of transgene‐free iPSC lines from human normal and neoplastic blood cells using episomal vectors. Methods Mol Biol 997:163‐176.[↩]
- Tam PP and J Rossant. (2003). Mouse embryonic chimeras: tools for studying mammalian development. Development 130:6155‐6163[↩]
- Sagi I and N Benvenisty. (2017). Haploidy in Humans: An Evolutionary and Developmental Perspective. Dev Cell 41:581‐589[↩][↩]
- Yang H, Z Liu, Y Ma, C Zhong, Q Yin, C Zhou, L Shi, Y Cai, H Zhao, H Wang, F Tang, Y Wang, C Zhang, XY Liu, D Lai, Y Jin, Q Sun and J Li. (2013). Generation of haploid embryonic stem cells from Macaca fascicularis monkey parthenotes. Cell Res 23:1187‐1200[↩]
- Hassold T and P Hunt. (2001). To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280‐291.[↩]
- Griffin DK. (1996). The incidence, origin, and etiology of aneuploidy. Int Rev Cytol 167:263‐296.[↩][↩][↩]
- Bonomi M, V Rochira, D Pasquali, G Balercia, EA Jannini, A Ferlin and NG Klinefelter Italia. (2017). Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest 40:123‐134.[↩]
- Gurdon JB. (2013). The egg and the nucleus: a battle for supremacy. Development 140:2449‐2456[↩]
- Wakayama T, AC Perry, M Zuccotti, KR Johnson and R Yanagimachi. (1998). Full‐term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394:369‐374[↩]
- Boiani M, S Eckardt, HR Scholer and KJ McLaughlin. (2002). Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev 16:1209‐1219.[↩]
- Egli D, J Rosains, G Birkhoff and K Eggan. (2007). Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 447:679‐685.[↩]
- Egli D, VM Sandler, ML Shinohara, H Cantor and K Eggan. (2009). Reprogramming after chromosome transfer into mouse blastomeres. Curr Biol 19:1403‐1409.[↩]
- Suzuki T, M Asami, M Hoffmann, X Lu, M Guzvic, CA Klein and AC Perry. (2016). Mice produced by mitotic reprogramming of sperm injected into haploid parthenogenotes. Nat Commun 7:12676.[↩]
- Wakayama T and R Yanagimachi. (1999). Cloning of male mice from adult tail‐tip cells. Nat Genet 22:127‐128[↩]
- Ogura A, K Inoue, N Ogonuki, A Noguchi, K Takano, R Nagano, O Suzuki, J Lee, F Ishino and J Matsuda. (2000). Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol Reprod 62:1579‐1584.[↩]
- Hochedlinger K and R Jaenisch. (2002). Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415:1035‐1038[↩]
- Kang E, G Wu, H Ma, Y Li, R Tippner‐Hedges, M Tachibana, M Sparman, DP Wolf, HR Scholer and S Mitalipov. (2014). Nuclear reprogramming by interphase cytoplasm of two‐cell mouse embryos. Nature 509:101‐104[↩]