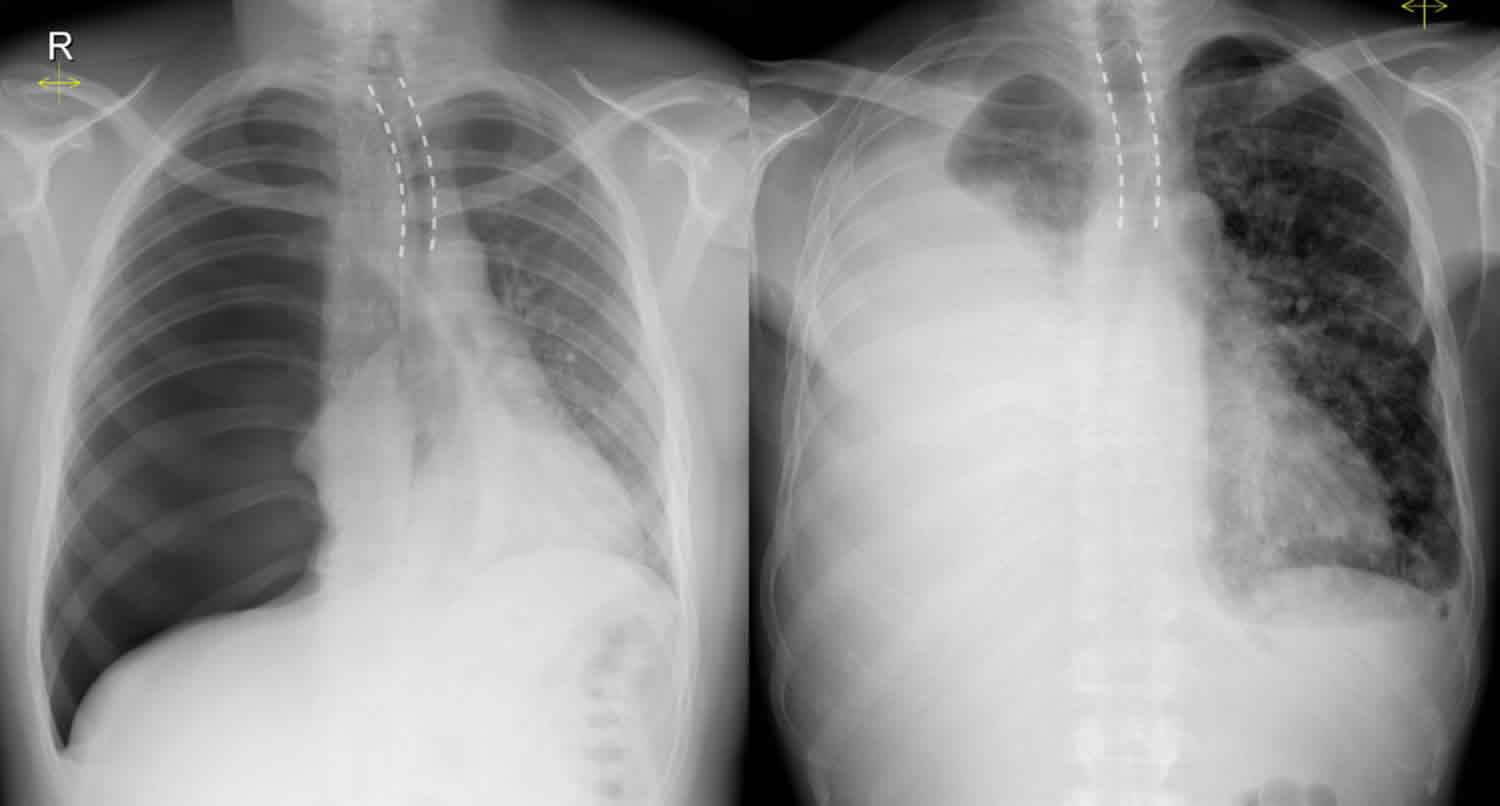
Tracheal deviation
Tracheal deviation is defined as the position of the trachea away from its normal position in the midline to one side. Normally, the trachea is located in the midline at the level of the clavicles, often deviating slightly to the right more inferiorly at the level of the aortic arch.
Tracheal deviation causes
The causes of tracheal deviation can be divided into two main groups:
- Tracheal deviation toward the cause of the problem,
- Tracheal deviation away from the cause of the problem.
Tracheal deviation TOWARDS the lung problem
This scenario occurs when the pressure in the lung and pleural cavity is less than the other side, and the trachea shifts toward the side with reduced pressure expanding it:
- Atelectasis
- Pleural effusion
- Apical lung fibrosis (for example, due to TB or prior radiotherapy)
- Collapse of one or more lung segments, for example due to bronchial obstruction by tumor
- Previous pneumonectomy
- Lung agenesis
Occasionally, in a patient with lung cancer and a large malignant pleural effusion, there will be such severe collapse of the underlying lung on the same side due to bronchial obstruction by tumor that the tracheal/mediastinal shift will, paradoxically, be towards the side of the pleural effusion.
It is also worth noting that large mediastinal masses that extend around both sides of the trachea may allow it to remain central, however in this setting they will often cause tracheal narrowing which is also detectable on a chest X-ray.
Tracheal deviation AWAY from the lung problem
- Tension pneumothorax
- Pleural effusion
- Mediastinal neoplasm including lymphoma, lymphadenopathy, thymic tumour, germ cell tumor
- Retrosternal goiter
Tracheal deviation symptoms
Tracheal deviation symptoms depend on the cause of the tracheal deviation.
Tension pneumothorax
Any pneumothorax can become a tension pneumothorax. Tension pneumothorax is life-threatening and requires immediate intervention 1. Tension pneumothorax can develop when the pressure in the pleural space is positive throughout the respiratory cycle. This leads to decreased venous return, hypotension, and hypoxia. Findings could include hypoxia, hypotension, distended neck veins, a displaced trachea, and unilaterally decreased breath sounds 2.
Symptoms reported among case reports of patients breathing unassisted included chest pain (52.3%), dyspnea (38.4%), and shortness of breath (31.4%). Respiratory distress was described in 41.9% of cases breathing unassisted versus 8.3% receiving assisted ventilation 1.
Many (46.5%) case reports of patients breathing unassisted described tachypnea. Hypoxia or requirement for supplemental oxygen was reported among 43 (50.0%) cases who were breathing unassisted versus 89 (91.8%) receiving assisted ventilation 1. Hypoxia was also reported among 25.0% of patients breathing unassisted versus 11.1% to 50.9% receiving assisted ventilation across 2 included cohort studies 3. The median PaO2/FIO2 ratio among all included case reports was 73.0 (interquartile range, 48.4–152.6). One included cohort study of mechanically ventilated patients with tension pneumothoraces reported a mean PaO2/FIO2 ratio of 150 4. Respiratory arrest occurred in 9.3% of case reports of patients breathing unassisted.
Jugular venous distention (7.1%) and contralateral tracheal deviation (9.3%) were uncommonly reported by included case reports and were not described by any of the included cohort studies. As compared with cases who were breathing unassisted, subcutaneous emphysema was noted more often (10.5% vs 30.9%) and contralateral tracheal deviation was noted less often (17.9% vs 2.9%) among cases receiving assisted ventilation 1. In one included cohort study, subcutaneous emphysema was reported among 27.3% of patients receiving assisted ventilation 3.
Ipsilateral decreased air entry, percussion hyperresonance, and decreased thoracic excursions/mobility were the most commonly reported chest examination findings among case reports of unilateral tension pneumothoraces. Ipsilateral decreased air entry was also reported among 50.0% to 54.5% of patients receiving assisted ventilation across 2 included cohort studies 5. Hyperresonance to percussion was more commonly described among case reports of patients breathing unassisted versus receiving assisted ventilation (26.7% vs 8.3%).
Needle (46.3% vs 36.2%) and tube (46.3% vs 50%) thoracostomy were the most frequently reported initial interventions among cases who were breathing unassisted versus receiving assisted ventilation, respectively. The reported mortality of tension pneumothorax in cases breathing unassisted (6.7%) was substantially lower than that for patients receiving assisted ventilation (22.7%). One cohort study reported that development of tension pneumothorax among mechanically ventilated patients was associated with a 7.4 times increase in the adjusted hazard of mortality 4.
Atelectasis
Atelectasis is derived from the Greek words ateles and ektasis, which mean incomplete expansion 6. Atelectasis results from the partial or complete, reversible collapse of the small airways leading to an impaired exchange of CO2 and O2 – i.e., intrapulmonary shunt. The incidence of atelectasis in patient’s undergoing general anesthesia is 90% 7. Pulmonary atelectasis is one of the most commonly encountered abnormalities in chest radiographs. Recognizing an abnormality due to atelectasis on chest radiographs can be crucial to understanding the underlying pathology. Several types of atelectasis exist; each has a characteristic radiographic pattern and etiology.
The mechanism by which atelectasis occurs is due to one of three processes: compression of lung tissue (compressive atelectasis), absorption of alveolar air (resorptive atelectasis), or impaired pulmonary surfactant production or function 8.
Atelectasis can categorize into obstructive, non-obstructive, postoperative, and rounded atelectasis.
Nonobstructive atelectasis can further classify into compression, adhesive, cicatrization, relaxation, and replacement atelectasis. Compression atelectasis is secondary to increased pressure exerted on the lung causing the alveoli to collapse. In other words, there is a decreased transmural pressure gradient (transmural pressure gradient = alveolar pressure – intrapleural pressure) across the alveolus resulting in alveolar collapse. In an awake, spontaneously-ventilating patient, caudad excursion of the diaphragm during contraction causes a subsequent decrease in intrapleural pressure and alveolar pressure. The decrease in pressure allows for passive movement of air into the lungs. This process is inhibited by general anesthesia due to diaphragm relaxation. Patients lying supine have cephalad displacement of the diaphragm further decreasing the transmural pressure gradient and increasing the likelihood of atelectasis. Adhesive atelectasis is often the result of a surfactant deficiency or dysfunction as seen in acute respiratory distress syndrome (ARDS) or respiratory distress syndrome in premature neonates. Surfactant functions to decrease alveolar surface tension and prevent alveolar collapse; therefore, any alterations to surfactant production and function often manifest as an increase in the surface tension of the alveoli leading to instability and collapse. Cicatrization atelectasis is often the result of parenchymal scarring of the lung, leading to contraction of the lung. Processes that lead to cicatrization atelectasis include tuberculosis, fibrosis, and other chronic destructive lung processes. Relaxation atelectasis involves the loss of contact between parietal and visceral tissue as seen in pneumothoraces and pleural effusions. Replacement atelectasis is one of the most severe forms and occurs when all of the alveoli in an entire lobe are replaced by tumor. This is typically seen in bronchioalveolar carcinoma and results in complete lung collapse.
Obstructive atelectasis is often referred to as resorptive atelectasis and occurs when alveolar air gets absorbed distal to an obstructive lesion. The obstruction either partially or completely inhibits ventilation to the area. Perfusion to the area is maintained; however, so gas uptake into the blood continues. Eventually, all of the gas in that segment will be absorbed and, without return of ventilation, the airway will collapse. Resorption atelectasis can be secondary to numerous pathologic processes, including intrathoracic tumors, mucous plugs, and foreign bodies in the airway. Children are especially susceptible to resorption atelectasis in the presence of an aspirated foreign body because they have poorly developed collateral pathways for ventilation.
In contrast, adults with chronic obstructive pulmonary disease (COPD) have extensive collateral ventilation secondary to airway destruction and thus are less likely to develop resorption atelectasis in the presence of an obstructing lesion (i.e., intrathoracic tumor). The use of high inspiratory oxygen concentration (high FiO2) during induction and maintenance of general anesthesia also contributes to atelectasis via absorption atelectasis. Room air is 79% nitrogen; nitrogen is slowly absorbed into the blood and therefore helps maintain alveolar patency. In contrast, oxygen is rapidly absorbed into the blood.
Postoperative atelectasis typically occurs within 72 hours of general anesthesia and is a well-known postoperative complication.
Rounded atelectasis is less common and often seen in asbestosis. The pathophysiology involves the folding of the atelectatic lung tissue to the pleura.
While all of the mechanisms mentioned above may contribute to the formation of perioperative atelectasis, absorption and compression mechanisms are the two most commonly implicated.[3]
Middle lobe syndrome involves recurrent or fixed atelectasis of the right middle lobe and lingula. Extraluminal and intraluminal bronchial obstruction can result in middle lobe syndrome. Nonobstructive causes include inflammatory processes, defects in bronchial anatomy, and collateral ventilation. Fiberoptic bronchoscopy and bronchoalveolar lavage are the treatment of choice for this syndrome. Long term consequences of chronic atelectasis include bronchiectasis. Sjogren syndrome has associations with middle lobe syndrome and treatment with glucocorticoids has been favorable.
Most symptoms and signs are determined by the rapidity with which the bronchial occlusion occurs, the size of the lung area affected, and the presence or absence of complicating infection.
Rapid bronchial occlusion with a large area of lung collapse causes pain on the affected side, sudden onset of dyspnea, and cyanosis. Hypotension, tachycardia, fever, and shock may also occur.
Slowly developing atelectasis may be asymptomatic or may cause only minor symptoms. Middle lobe syndrome often is asymptomatic, although irritation in the right middle and right lower lobe bronchi may cause a severe, hacking, nonproductive cough.
- Clinical Presentation of Patients With Tension Pneumothorax: A Systematic Review. Ann Surg. 2015 Jun;261(6):1068-78. doi: 10.1097/SLA.0000000000001073. https://journals.lww.com/annalsofsurgery/fulltext/2015/06000/Clinical_Presentation_of_Patients_With_Tension.9.aspx[↩][↩][↩][↩]
- Swierzy M, Helmig M, Ismail M, Rückert J, Walles T, Neudecker J. [Pneumothorax]. Zentralbl Chir. 2014 Sep;139 Suppl 1:S69-86; quiz S87.[↩]
- Massarutti D, Trillo G, Berlot G, et al. Simple thoracostomy in prehospital trauma management is safe and effective: a 2-year experience by helicopter emergency medical crews. Eur J Emerg Med. 2006;13:276–280.[↩][↩]
- Hsu CW, Sun SF, Lin HS, et al. Clinical characteristics, hospital outcome and prognostic factors of patients with ventilator-related pneumothorax. Minerva Anestesiol. 2014;80:29–38.[↩][↩]
- Mistry N, Bleetman A, Roberts KJ. Chest decompression during the resuscitation of patients in prehospital traumatic cardiac arrest. Emerg Med J. 2009;26:738–740.[↩]
- Grott K, Dunlap JD. Atelectasis. [Updated 2019 Aug 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545316[↩]
- Lundquist H, Hedenstierna G, Strandberg A, Tokics L, Brismar B. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol. 1995 Nov;36(6):626-32.[↩]
- Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis and management. Paediatr Respir Rev. 2000 Sep;1(3):274-8.[↩]