Twiddler syndrome
Twiddler syndrome is an uncommon cause of pacemaker malfunction, in which twisting or rotating of the pacemaker generator device in its pocket results in lead retraction or coiling and subsequent malfunction of an implanted devices such as a pacemaker, an implantable cardioverter-defibrillator (ICD), cardiac resynchronization therapy (CRT) or a defibrillator allowing cardiac resynchronization therapy (CRT‐D) 1. Twiddler syndrome is caused by the spontaneous, accidental, deliberate or self-induced manipulation of the pacemaker pulse generator around its central axis within the pocket, resulting in retraction and dislocation of the leads, causing failure to sense or capture, or stimulation of noncardiac structures including the diaphragm or pectoral muscles 2. Twiddler syndrome has a reported frequency of around 0.07%–7%, more commonly reported among elderly females and obese patients 3. The majority of cases are diagnosed within the first year of implant 4, although it can occur at any time after device implantation. In most cases, the keys to confirming Twiddler syndrome have been loss of capture, noncardiac muscle twitching, or inappropriate implantable cardioverter-defibrillator (ICD) discharge.
Twiddler syndrome is more commonly recognized in obese patients due to the increased subcutaneous tissue, possibly providing less support from surrounding tissue to the pocket. An associated increased laxity of the subcutaneous tissues, particularly in elderly patients facilitates further dislodgement of device. Additionally, a smaller sized implanted device relative to its pocket permit their rotation within the skin pocket 5. Another factor is postimplant pocket hematoma formation. This may cause autocleavage of the original dissection plane. Once reabsorbed, the hematoma of the residual “space” allows for greater displacement of the device 6.
Elderly patients have been reported to have an increased risk of chest Twiddler syndrome since the presence of loose subcutaneous tissue allows for the rotation of the generator in its pocket 7. Furthermore, a smaller sized implanted device relative to its pocket may also present an increased risk by providing the space needed for a greater displacement of the device within the skin pocket 8. The risk of abdominal Twiddler syndrome might be similar. However, the biggest difference between abdominal implantations and chest implantations is the stability of the pacemaker pocket base. Although the chest wall, which consists of bones and muscles, is stable and the cause of chest Twiddler syndrome is patient error, the abdominal Twiddler syndrome we reported was caused by the patient’s physical elements 9.
Goldenberg, et al. 10 reported a case of Twiddler’s syndrome as a consequence of device rotation without manipulation of the pocket, thus a spontaneous “Twiddler’s” phenomenon.
The chest X-ray is the simplest and most vital diagnostic tool to diagnose Twiddler syndrome 4, as it is rapid and gives a clear image of the lead coiling and device rotation.
Figure 1. Normal pacemaker position and lead
Footnote: Fluoroscopy view taken just after implantation showing normal position of the pulse-generator and normal appearing lead without any twirling.
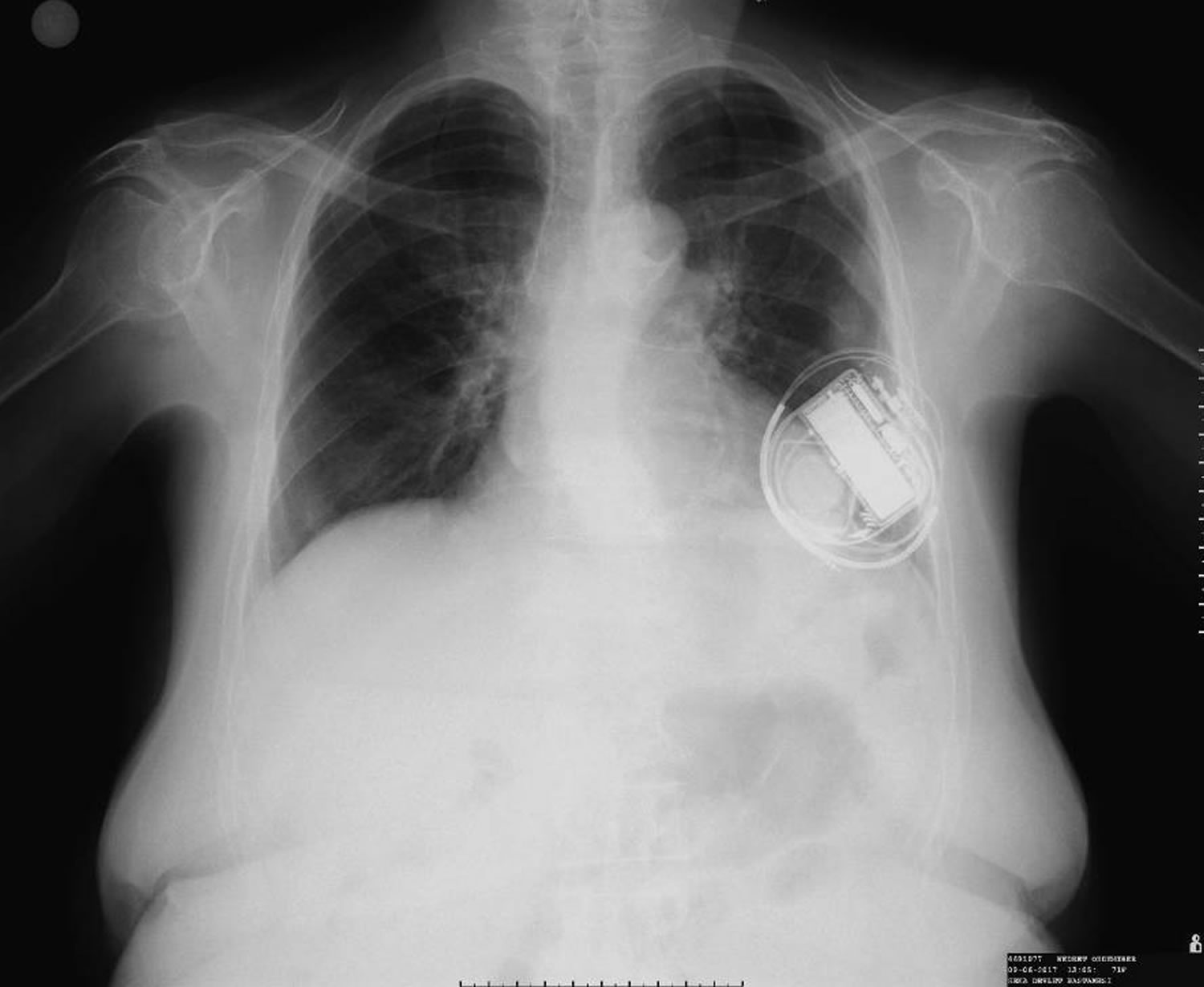
Figure 2. Twiddler syndrome
Footnote: Chest radiograph showing the coiling of the right ventricular lead around the pulse generator of an implantable cardioverter defibrillator.
[Source 2 ]Figure 3. Twiddler’s syndrome
Footnote: A, The chest radiographs showing numerous loops and twists of the pacemaker leads (White arrow). B, Intraoperative image showing the cluster of tangled leads connected to the device.
[Source 9 ]Twiddler’s syndrome symptoms
Manifestations of Twiddler syndrome are depending on the final site of the dislodged lead. In most cases, the keys to confirming Twiddler syndrome have been loss of capture, stimulation of noncardiac structures or inappropriate implantable cardioverter-defibrillator (ICD) discharge. Leads that get dislodged further up may stimulate the phrenic nerves causing diaphragmatic contractions. Further coiling and withdrawal of the lead, leads to stimulation of the brachial plexus, resulting in rhythmic arm twitching 3. Recently, Sekimoto et al 11 reported a rare case of Twiddler syndrome, which was detected as an alert for intrathoracic impedance as an indicator of heart failure. The recovery of thoracic impedance after relocation in this case make the possibility that the intrathoracic impedance might have been directly affected by the length between the lead and the generator. In another case, a patient presented with a twitching in left pectoral region due to pectoral muscle stimulation by the retracted lead encircled the pulse generator 2.
Twiddler’s syndrome treatment
Treatment of diagnosed Twiddler syndrome cases include repositioning of the dislodged leads, implantation of a new lead and repositioning of the pulse generator. Avoiding pocket-implantable cardioverter-defibrillator (ICD) mismatch, correct fixation of the leads and the device with a non-absorbable suture to the pectoralis fascia and instructing patients not to manipulate their device may prevent further occurrences of Twiddler syndrome 6.
- Tonino W. A., and Winter J. B.. 2006. Images in clinical medicine. The twiddler syndrome. N. Engl. J. Med. 354:956.[↩]
- Bozyel S, Aksu T, Erdem Guler T, Serhan Ozcan K, Aktas M. Pectoral muscular twitching: a rare manifestation of spontaneous twiddler syndrome. J Geriatr Cardiol. 2017;14(8):532–533. doi:10.11909/j.issn.1671-5411.2017.08.007 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5653900[↩][↩][↩]
- Mandal S, Pande A, Kahali D, et al. A rare case of very early pacemaker twiddler’s syndrome. Heart Views. 2012;13:114–115.[↩][↩]
- DeMarco DC, Xuereb RG. ‘Twiddling’ of the pacemaker resulting in lead dislodgement. Malta Med J. 2009;21:38–41.[↩][↩]
- Fahraeus T, Hoijer CJ. Early pacemaker twiddler syndrome. Europace. 2003;5:279–281.[↩]
- Gul EE, Boles U, Haseeb S, et al. “Spontaneous twiddler’s” syndrome: the importance of the device shape. Pacing Clin Electrophysiol. 2017;40:326–329.[↩][↩]
- Nicholson WJ, Tuohy KA, Tilkemeier P. Twiddler’s syndrome. N Engl J Med. 2003;348:1726–7.[↩]
- Gul EE, Boles U, Haseeb S, et al. “Spontaneous Twiddler’s” syndrome: the importance of the device shape. Pacing Clin Electrophysiol. 2017;40:326–9.[↩]
- Higuchi S, Shoda M, Satomi N, et al. Unique abdominal twiddler syndrome. J Arrhythm. 2019;35(1):142–144. Published 2019 Jan 10. doi:10.1002/joa3.12133 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6373647[↩][↩]
- Goldenberg GR, Turner S, Singh SM. A radiographic clue to diagnose twiddler’s syndrome. J Innov Cardiac Rhythm Manag. 2016;7:2388–2389.[↩]
- Sekimoto S, Wakamatsu M, Morino A, et al. Early detection of twiddler syndrome due a congestion alert by remote monitoring. Clin Case Rep. 2017;5:950–953.[↩]