Upper airway resistance syndrome
Upper airway resistance syndrome (UARS) is a sleep-disordered breathing syndrome that is very similar to obstructive sleep apnea (OSA) in that the soft tissue of the throat relaxes, reduces the size of the airway, and resulting in disturbed sleep and consequent daytime impairment, including excessive daytime sleepiness. Upper airway resistance syndrome or UARS was first named by Guilleminault in 1993 1 while investigating cases of excessive daytime sleepiness with no identified cause in adults. However, the respiratory pattern of increased upper airway resistance was previously identified in pre-pubertal children under the label “sleep-related respiratory resistive load” 2. Since this first description, many authors have attempted to describe the clinical and polysomnographic features of upper airway resistance syndrome patients based on their experience, to find a definitive way to diagnose and finally treat them. Currently, upper airway resistance syndrome is subsumed under the diagnosis of obstructive sleep apnea syndrome (OSAS) by the American Academy of Sleep Medicine 3. The term breathing-related sleep disorder refers to a spectrum of breathing anomalies ranging from chronic or habitual snoring to upper airway resistance syndrome (UARS) to frank obstructive sleep apnea (OSA) or, in some cases, obesity hypoventilation syndrome (OHS).
Upper airway resistance syndrome diagnostic criterion is still not defined and some authors believe that upper airway resistance syndrome is part of a continuum between primary snoring and obstructive sleep apnea syndrome whereas others believe that it is a distinct syndrome from obstructive sleep apnea 4. Some authors support that both upper airway resistance syndrome and obstructive sleep apnea have the same symptoms and as their pathophysiology do not significantly differ from each other, upper airway resistance syndrome is not a distinct disease 5. Nevertheless, other authors believe that upper airway resistance syndrome patients present different features than other Sleep related Breathing Disorder 6. The most frequent symptoms are excessive daytime sleepiness, fatigue and sleep fragmentation. However, upper airway resistance syndrome patients also present significantly more often with sleep-onset and sleep-maintenance insomnia, postural hypotension, headaches, gastroesophageal reflux, irritable bowel syndrome, anxiety and alpha-delta sleep 7. The proportion of women with upper airway resistance syndrome is also significantly higher than for obstructive sleep apnea 8. Besides having some different clinical presentation, it has been suggested that upper airway resistance syndrome and obstructive sleep apnea differ from each other in terms of their sleep EEG and autonomic nervous system responses. Some authors believe that upper airway resistance syndrome patients have an increase in alpha rhythm and an over-activation of the autonomic nervous system 9.
According to Pépin et al 10 upper airway resistance syndrome is defined by the occurrence of excessive daytime sleepiness unexplained by another cause and associated with more than 50% of respiratory events that are nonapneic and nonhypopneic (RERAs). Respiratory Event-Related Arousals (RERAs) are characterized by a progressive increase in respiratory effort. This may be assessed by direct measurement of esophageal pressure or by another marker of respiratory effort such as the changes in pulse transit time 10. RERAs may induce both cortical and autonomic arousals 11 and potentially lead to cardiovascular activation. Respiratory flow, when using nasal cannula or a pneumotachograph, exhibits only qualitative change named inspiratory flow limitation. This is of interest since inspiratory flow limitation results from the progressive increase in upper airway resistance 12. Flow limitation is a physiologic phenomenon that can be identified from the nasal pressure signal thus allowing noninvasive detection of RERAs 13. The time sequence of these obstructive respiratory events is close to what occurs with apneas and hypopneas, but the duration may be longer. This should be distinguished from episodes of sustained stable flow limitation occurring during slow wave sleep. This late flow-limited aspect does not lead to repeated arousals and thus differs from RERAs.
The true prevalence of upper airway resistance syndrome in the general population is unknown and pure upper airway resistance syndrome is rather rare in clinical practice. upper airway resistance syndrome patients were significantly younger, less overweight and had lower weight gain during the past 5 years compared to OSA patients 14. The female-to-male ratio seems to be highest in the upper airway resistance syndrome group 10. Upper airway resistance syndrome may also need to be considered in severely obese young patients 15. These authors reported a high prevalence of upper airway resistance syndrome (about 15 to 20% based on polysomnographic criteria).
Untreated upper airway resistance syndrome individuals can present low quality of life and cardiovascular consequences. Sleep and daytime symptoms, such as fatigue, insomnia and depressive mood, in untreated upper airway resistance syndrome usually increase over time 16. Upper airway resistance syndrome characteristic esophagic pressure negativity can cause a diastolic leftward shift of the interventricular heart septum and a consequent ventricular “collapse” 17. The longlasting flow limitation episodes can induce a small increase in end-tidal carbon dioxide that can stimulate the sympathetic nervous system activity. This could cause hypertension, and cardiovascular and metabolic consequences 6. Even an increase in inflammatory markers can happen in non-treated upper airway resistance syndrome individuals 18.
There are some treatment studies available in the literature, but most of these are case reports and case series. Nasal continuous positive airway pressure (CPAP) is one treatment option that has been evaluated as a therapy for upper airway resistance syndrome, and the available studies demonstrated that it can improve different aspects of the condition. CPAP treatment was associated with significant improvements in the excessive daytime sleepiness, numbers of transient arousals and abnormal upper airway resistance 1. Other types of treatments evaluated included oral appliances, nasal and palatal surgeries and maxillomandibular advancement. Long-term studies to evaluate treatment response will be helpful to better define this sleep related breathing disorder.
What is the difference between upper airway resistance syndrome and obstructive sleep apnea?
One of the key differences between upper airway resistance syndrome (UARS) and obstructive sleep apnea (OSA) is that apneas (pauses in breathing) and hypopneas (decreases in breathing) are either absent or very low in patients with upper airway resistance syndrome (UARS).
Patients with obstructive sleep apnea (OSA) are often overweight or obese (although they can be of normal weight), whereas patients with upper airway resistance syndrome are often of average weight.
Obstructive sleep apnea is twice as likely to affect men as women, while upper airway resistance syndrome can affect men and women equally.
Obstructive sleep apnea is related to many more long-term health conditions as a result of apneas and hypopneas due to the decrease in blood pressure during apnea/hypopnea events, which can lead to increased risk of high blood pressure, heart disease, heart arrhythmias, stroke, and heart failure.
Patients who fail to treat upper airway resistance syndrome can end up developing obstructive sleep apnea and find themselves at risk for many of these health problems.
Upper airway resistance syndrome causes
Causes of upper airway resistance syndrome are similar to obstructive sleep apnea (OSA). It can be caused by a naturally narrowed air passage, loose fatty tissues of the throat collapsing back into the airway, or the position of the tongue (falling back) during sleep.
Patients with upper airway resistance syndrome require a greater effort in breathing to get past obstructions. Not all patients with upper airway resistance syndrome snore, and their symptoms may sound more like heavy, labored breathing during sleep. Sufferers of upper airway resistance syndrome often describe their breathing effort as “trying to breathe through a straw.”
Similar to OSA, the brain has to arouse itself from deeper stages of sleep to increase respiratory effort. When the brain is constantly being aroused from the deeper stages of sleep, it’s not able to perform other important tasks that it needs to complete so that you can feel refreshed in the morning. This can lead to symptoms of chronic fatigue and excessive daytime sleepiness, which are also present in obstructive sleep apnea.
Patients can move from snoring to upper airway resistance syndrome as a result of aging (as muscle tone decreases in the throat) and weight gain (increase of fatty tissues in the throat, which can increase material resistant to airflow). Women in their third trimester of pregnancy are also more likely to develop upper airway resistance syndrome as a result of weight gain.
Consequences of upper airway resistance syndrome:
- Frequent nocturnal awakenings
- Difficulty going to sleep/maintaining sleep
- Chronic insomnia
- Excessive daytime sleepiness
Upper airway resistance syndrome signs and symptoms
The symptoms of upper airway resistance syndrome tend to be similar to obstructive sleep apnea but may be less in severity. People with upper airway resistance syndrome usually complain of snoring, sleep fragmentation or frequent arousals from sleep, excessive daytime sleepiness, fatigue, cognitive impairment and un-refreshing sleep. Furthermore, upper airway resistance syndrome patients also present significantly more often with sleep-onset and sleep-maintenance insomnia, postural hypotension, headaches, gastroesophageal reflux, irritable bowel syndrome, anxiety and alpha-delta sleep 7. The proportion of women with upper airway resistance syndrome is also significantly higher than for obstructive sleep apnea 8. Besides having some different clinical presentation, it has been suggested that upper airway resistance syndrome and obstructive sleep apnea differ from each other in terms of their sleep EEG and autonomic nervous system responses. Some authors believe that upper airway resistance syndrome patients have an increase in alpha rhythm and an over-activation of the autonomic nervous system 9.
Owing to the lack of specificity of the reported symptoms, the question is to know whether these complaints are corroborated by objective measures. When measuring the tendency to fall asleep by multiple sleep latency tests in 137 upper airway resistance syndrome, Gold et al. 19 reported that upper airway resistance syndrome patients frequently complained about sleepiness but were objectively less sleepy compared to mild-to-moderate and severe obstructive sleep apnea syndrome patients. Powers and Frey 20 measured maintenance wakefulness tests in 19 upper airway resistance syndrome patients and found that 33% of them exhibited a mean sleep latency below 19.4 min (i.e. <2 SD below normal values). Psychomotor performances assessed by measurements of reaction time have been compared in 47 upper airway resistance syndrome matched by gender and age with 47 obstructive sleep apnea syndrome patients 21. Patients with upper airway resistance syndrome presented worse psychomotor performance on most test metrics than patients with obstructive sleep apnea syndrome. These results may suggest that patients with upper airway resistance syndrome may also present an increased risk of motor vehicle crashes as previously demonstrated in obstructive sleep apnea syndrome patients 21.
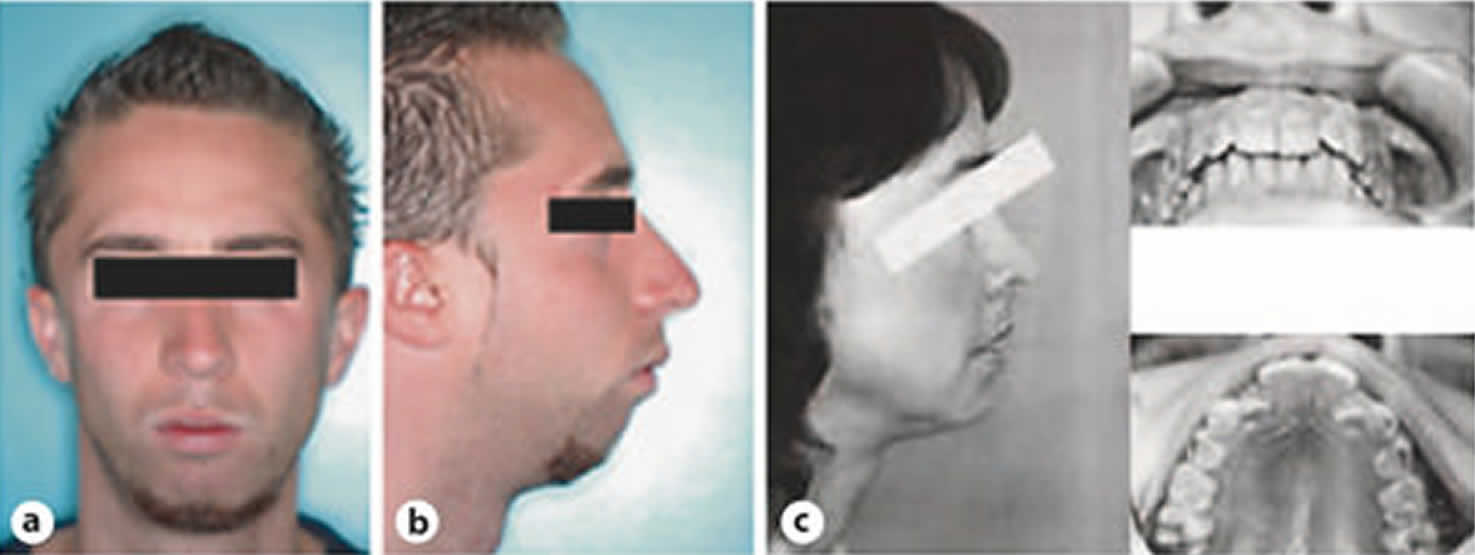
Regarding clinical examination, some craniofacial characteristics were reported as being specific to upper airway resistance syndrome 22. These patients exhibit the classical long face syndrome with a short and narrow chin and reduced mouth opening (Figure 1). There is classically a ‘click’ and a subluxation when opening the temporo-mandibular articulation, which may be evidenced by palpation. The mandible is in the back position and the palate is high and narrow. Finally, Guilleminault et al. 23 have described a low-resting arterial blood pressure or orthostatic intolerance as occurring in one fifth of subjects with upper airway resistance syndrome.
Figure 1. Typical craniofacial features in upper airway resistance syndrome patients
Footnote: Representative patients suffering from upper airway resistance syndrome with classical craniofacial abnormalities, i.e. long face syndrome with a short and narrow chin and with the mandible in the back position (a, b) and (c). During clinical examination, dental overjet and ogival hard palate are typical features of upper airway resistance syndrome.
[Source 10 ]Upper airway resistance syndrome diagnosis
The diagnosis of upper airway resistance syndrome is still controversial. Currently, upper airway resistance syndrome is considered part of Obstructive Sleep Apnea Syndrome (OSAS) by the American Academy of Sleep Medicine 3. Obstructive Sleep Apnea (OSA) is defined as the presence of more than 5 obstructive events per hour associated with symptoms or more than 15 events per hour independent of symptoms. Currently, there is no data with outcomes available defining the cut off limit for Respiratory Event-Related Arousals (RERAs) and Respiratory Disturbance Index (RDI) in sleep related breathing disorder patients, as well as, on healthy individuals 4. Respiratory index, such as, apnea-hypopnea index (AHI) and Respiratory Disturbance Index (RDI) has been used with different definitions and considering different respiratory events. There are still several issues that need to be better defined and established regarding upper airway resistance syndrome, however, most authors today agree that sleep breathing disorders cannot be limited just to OSA criteria.
To qualify as an individual disease, upper airway resistance syndrome should meet the following criteria:
- First, to exhibit specific clinical and polysomnographic diagnostic criteria;
- Second, these specific criteria should not be found in the general population;
- Third, a direct relationship should be found between this syndrome and a specific morbidity.
Upper airway resistance syndrome diagnosis is suspected in individuals with complaints of excessive daytime sleepiness or daytime tiredness, no obstructive sleep apnea (OSA) and a polysomnographic study with respiratory parameters indicative of increased upper airway resistance, such as, flow limitation during sleep. They present arousals associated with increase in respiratory effort leading to sleep fragmentation and excessive daytime sleepiness. The polysomnographic studies of these patients also showed sequences of breaths with flow limitation, which were interrupted by abrupt arousals. Arousals were defined using American Sleep Disorders Center-American Academy of Sleep Medicine 24 conventional criteria but were also described using the cyclic alternative pattern-CAP-atlas 25, including the shorter duration arousals associated with abnormal increases of “Phase A2” and “Phase A3” of the CAP scoring system and with Respiratory Event-Related Arousal (RERA) 26. The arousals associated with flow limitations were described as “respiratory event related arousals” (RERA) by American Academy of Sleep Medicine 27. The polysomnography pattern of “flow limitation,” introduced in 1991 28 was further investigated and defined particularly in New York 29 as a sign of increased upper airway resistance to airflow.
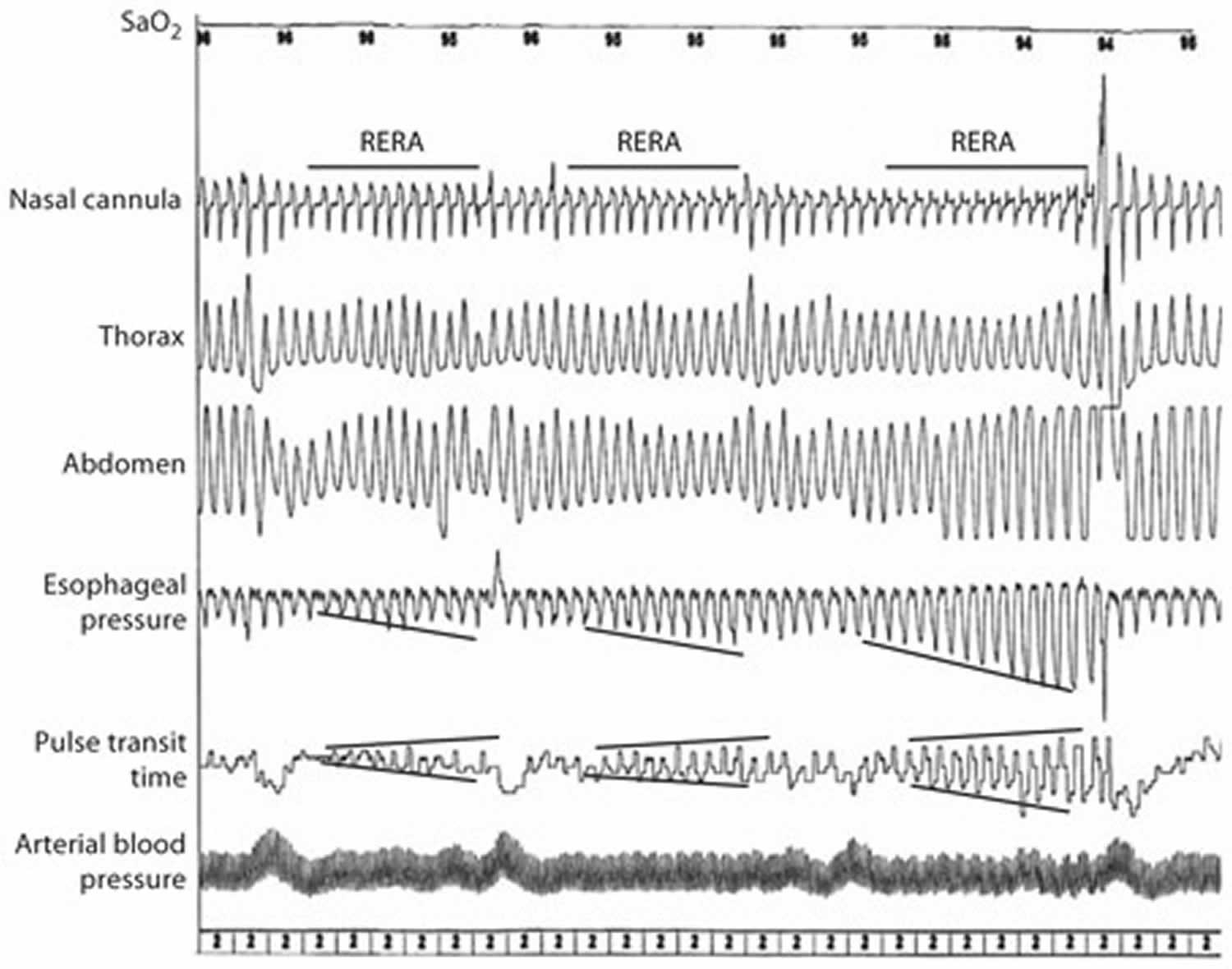
Respiratory Event-Related Arousals (RERAs) and arousal detection are the key elements during sleep monitoring (Figure 2). RERAs correspond to a sequence of breaths characterized by increasing respiratory effort leading to an arousal from sleep, but which does not meet criteria for an apnea or hypopnea in terms of flow reduction. These events must fulfill both of the following criteria:
- A pattern of progressively more negative esophageal pressure, terminated by a sudden change in pressure to a less negative level and an arousal, and
- The event lasts 10 s or longer. The number of events may be underestimated when tools used during this monitoring are not appropriate.
By definition, detection of RERAs is based on accurate flow measurement using the nasal cannula or a pneumotachograph 13 and respiratory effort measurement using either oesophageal pressure or pulse transit time 30. By using a nasal thermocouple to detect flow a shift between hypopnea to RERA will occur and an authentic OSAS will be misdiagnosed to a upper airway resistance syndrome. This has been described by Montserrat and Badia 31 as the ‘thermistance syndrome’. Pépin et al 10 investigated subjects presenting with sleep-breathing disorder and very little oxygen desaturations, thus being either mild OSAS or upper airway resistance syndrome, using the reference tools (pneumothachograph and esophageal pressure). They exhibited mainly hypopneas but we found only about 5% of true RERAs 11. RERAs must be considered as specific respiratory events expressed by patients depending on their upper airway collapsibility. To this extent, it is logical to include RERAs in the respiratory disturbance index.
Figure 2. Respiratory Event-Related Arousals (RERAs)
Footnote: Representative pattern of RERAs during a sleep study. The figure depicts the recurrence of RERAs with a progressive flow limitation aspect on nasal cannula which corresponds to an increase in respiratory effort (both esophageal pressure and pulse transit time). The consequences of these events are both sleep fragmentation (frank autonomic arousal on the first and third events) and arterial blood pressure rises.
[Source 10 ]Upper airway resistance syndrome treatment
There are several ways to treat upper airway resistance syndrome; however, treatments taken are different for adults and children.
Adults
Continuous Positive Airway Pressure (CPAP) is the most effective treatment for sleep apnea, however there are also surgical options, oral appliances, and behavioral approaches that can be used to treat obstructive sleep apnea. Weight loss, although always a good idea in reducing obesity-related conditions (e.g., hypertension, diabetes), is considered supplementary or adjunctive therapy rather than primary treatment for obstructive sleep apnea. Other underlying medical conditions, especially nasal allergies, should also be treated. A nasal steroid might help improve nasal obstruction associated with allergies as well as the obstructive sleep apnea symptoms. For a more comprehensive explanation of the treatment options for this condition, go to the Treatments Section in the Tests and Treatments Section. The same treatments that are successful for obstructive sleep apnea can be used to treat upper airway resistance syndrome. While CPAP remains the most effective treatment, this population may find it difficult to tolerate. Alternative treatments such as surgery, oral appliances, positional therapy (restricting the individual to sleeping on his/her sides), and weight loss may be effective in improving sleep disordered breathing in individuals with upper airway resistance syndrome.
Continuous Positive Airway Pressure (CPAP)
CPAP is the upper airway resistance syndrome treatment modality better investigated 32. One month of therapeutic trial with nasal CPAP can significantly change objective polysomnographic parameters 33 and subjective complaints 32. CPAP treatment was associated to decrease in transient arousals, increase in percentage of NREM stages 3 and 4 and the sleep latency at Multiple Sleep Latency Test (MSLT) 33. Subjective daytime sleepiness, fatigue 32 and snoring 34 also can improve after CPAP treatment. Nevertheless, in some studies there were patients that did not have their excessive daytime sleepiness and fatigue decreased after CPAP treatment 34. Consequently, these patients did not comply with CPAP due to the lack of beneficial effects 34. Even though CPAP may be an effective treatment for upper airway resistance syndrome, the compliance is low in this patient population. Also, in follow up studies a significant part of patients did not use the recommended nasal CPAP treatment due to refusal by the insurance companies to cover its prescriptions, on the basis that according to their policies, upper airway resistance syndrome did not meet the criteria for a CPAP prescription 16.
Auto-CPAP was indicated to treat pregnant women with severe preeclampsia and flow limitation 35. The mean overnight blood pressure was markedly reduced during the night of treatment with nasal CPAP when compared with the nontreatment night. The authors suggest that nasal CPAP may be considered as a therapy to improve blood pressure control in women with severe preeclampsia and flow limitation.
Medications
Medications that decrease sleep fragmentation could be also helpful for upper airway resistance syndrome patients. 7.5 mg Zopiclone during 1 week produced significant improvements in the sleep efficiency index and average sleep latency in Multiple Sleep Latency Test (MSLT) 36. Nevertheless, it had no effect on respiratory parameters during sleep and daytime sleepiness in patients with upper airway resistance syndrome. Despite some improvements demonstrated objectively in the polysomnography, Zopiclone use did not decrease upper airway resistance syndrome symptoms. It was concluded that medications that consolidate sleep could be an adjuvant medicine used during the main treatment of upper airway resistance syndrome patients.
Sleep fragmentation persists in many patients with insomnia despite an adequate insomnia treatment. The sleep fragmentation associated with upper airway resistance syndrome can cause daytime fatigue and enhance anxiety, factors that can increase the difficulty in treating chronic insomnia. Postmenopausal women with chronic insomnia and upper airway resistance syndrome had their daytime fatigue decreased after nasal treatment (nasal radio-frequency ablation of turbinate or septoplasty with turbinectomy) and nasal CPAP-treatment 37. This study reinforce the idea that sleep breathing disorder should be investigated in patients with chronic insomnia to guarantee an adequate insomnia treatment result.
Psychiatric patients may also present sleep breathing disorder. A case report about a young man with treatment-resistant depression and with sleep symptoms (insomnia, fatigue and daytime sleepiness) was investigated for sleep breathing disorder 38. As his psychiatric symptoms were not well controlled despite an adequate medical treatment and he had sleep complaints he underwent a sleep study that showed upper airway resistance syndrome. The patient was submitted to rapid palatal expansion and during the two-year follow-up remained free from symptoms of depression, anxiety, sleepiness or fatigue in the absence of any psychotropic medication, although there were no statistically significant changes in the polysomnographic parameters. The article 38 reinforces the idea that a sleep study should be included and sleep breathing disorder should be investigated in psychiatric patients who have persistent sleep complaints and whose psychiatric symptoms are not controlled despite an adequate medical treatment.
UARS surgery
Upper airway surgeries have also been evaluated as upper airway resistance syndrome treatment option. Craniofacial abnormalities that may increase upper airway resistance are often present in upper airway resistance syndrome. Dental malocclusion and elevated ogival hard palate as well as a narrow posterior airway space can be frequent findings in upper airway examination 39. Surgical treatment were studied as an option for patients who cannot tolerate or are unwilling to adhere to CPAP therapy 40. Some authors studied upper airway resistance syndrome patients that preferred surgery (such as septoplasty, turbinate reduction, laser-assisted uvuloplasty – laser-assisted uvuloplasty, uvulopalatopharyngoplasty, genioglossus bone advancement, mandibular osteotomy with tongue advancement and hyoid miotomy with suspension) rather than CPAP 41. Sleep studies were performed from 3 to 6 months after treatment 41. The only outcome evaluated in these studies were subjective sleepiness 41. and snoring 42 and the follow-up was not long enough to consider surgery a long-term effective management for this group of patients.
Many authors agreed that procedures should address anatomical regions that cause upper airway obstruction and that upper airway resistance syndrome treatment consists of approaching the causes of the upper airway anatomical problems such as treatment of nasal allergies, usage of palatal soft-tissue surgeries, orthognatic surgery or use of dental devices 43. Some authors had even tried to formulate a statistical model for postoperative apnea-hypopnea index (AHI) after multilevel surgery in upper airway resistance syndrome patients 44. It was concluded that tonsillectomy had a highly significant influence on postoperative AHI.
In conclusion, few studies evaluating surgical treatment for upper airway resistance syndrome are available. Most studies about surgery treatment only consider subjective outcomes and the number of patients has been too low to lead to conclusive results. Many methodological problems in published literature did not allow a proper analysis 45. Randomized prospective protocols of surgical procedures should be conducted.
Oral devices
Another treatment option for sleep breathing disorder is the use of oral devices. Oral devices move the mandible and tongue forward in order to minimize the oropharyngeal obstruction. Patients with upper airway resistance syndrome present a narrow posterior airway space behind the base of the tongue. Oral devices increase upper airway dimensions and are often indicated for mild sleep breathing disorder. There are some case reports 46 and a prospective study 39 published about oral device as upper airway resistance syndrome treatment. Some authors noticed polysomnographic differences, such as a decrease in the total arousal index (TAI) 47, a less negative mean esophageal pressure 48, an improvement in sleep efficiency 47 and lower oxygen saturation 47. A significant increase in the mean sleep latency in the Multiple Sleep Latency Test (MSLT) 49 and an absence of sleep during the Maintenance of Wakefulness Test (MWT) was also observed 48. Oral appliance also decreased subjective daytime sleepiness 47 and snoring 50. Another study report a case of oral appliance indicated for an asthmatic patient with upper airway resistance syndrome that had poorly controlled asthma symptoms despite high-doses steroids 49. The post-treatment polysomnography showed normalization of the esophageal pressure and decrease in the total arousal index (TAI). Even the asthma symptoms were better controlled and lower doses of drugs were required after the oral device treatment.
The side effects of oral devices observed (excessive salivation and transient tooth discomfort) were minor and tolerable, and no major complications were reported 47. Objective and subjective aspects of the treatment response were evaluated during a longer follow up. The results demonstrated that oral appliance was effective in decreasing sleep fragmentation and objective and subjective daytime sleepiness and was also well tolerated.
Other types of oral devices besides the mandibular advancement device were also published as case reports but the study did not report clinical or polysomnographic changes pre- and post-treatment 46.
Nasal obstruction is another cause of flow limitation and can lead to occlusion of the pharyngeal airway. The decrease in nasal resistance might also reduce the inspiratory effort. A double-blind, randomized, controlled trial with a cross-over design study was performed to evaluate the effect of external nasal dilatation in upper airway resistance syndrome patients 51. The external nasal dilator significantly increased nasal cross-sectional area, reduced stage 1 sleep and decreased desaturation time when comparing to the placebo treatment. These were the only changes observed after treatment. There were no significant effects on the Multiple Sleep Latency Test (MSLT), apnea-hypopnea index (AHI) or total arousal index (TAI) or on the clinical complaints. The absence of significant clinical changes after treatment, despite the polysomnographic differences, demonstrated that this device, currently, cannot be recommended for the treatment of upper airway resistance syndrome. Future studies with a larger number of patients and with a longer follow-up should be conducted to better analyze this device׳s effects on upper airway resistance syndrome patients.
Children
Surgery is usually the first line of treatment for children; removing a child’s enlarged tonsils and adenoids by a tonsillectomy and adenoidectomy will often resolve the obstructive sleep apnea. However, in some children, CPAP, further surgery, or specialized orthodontic treatment may be necessary to treat the obstructive sleep apnea.
Upper airway resistance syndrome prognosis
Regarding the evolution of the disease, the severity of upper airway resistance syndrome remains stable over time 52. The progression from upper airway resistance syndrome to obstructive sleep apnea syndrome seems to be related to an increase in the body mass index (BMI) 53.
References- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781-787. doi:10.1378/chest.104.3.781
- Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring: evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139(3):165-171. doi:10.1007/BF01377349
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619. Published 2012 Oct 15. doi:10.5664/jcsm.2172
- de Godoy LB, Palombini LO, Guilleminault C, Poyares D, Tufik S, Togeiro SM. Treatment of upper airway resistance syndrome in adults: Where do we stand?. Sleep Sci. 2015;8(1):42-48. doi:10.1016/j.slsci.2015.03.001 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4608900
- Rees K., Kingshott R.N., Wraith P.K., Douglas NJ. Frequency and significance of increased upper airway resistance during sleep. Am J Respir Crit Care Med. 2000;162:1210–1214.
- Pépin J.L., Guillot M., Tamisier R., Lévy P. The upper airway resistance syndrome. Respiration. 2012;83:559–566.
- Gold A.R., Dipalo F., Gold M.S., O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123:87–95.
- Stoohs R.A., Knaack L., Blum H.C., Janicki J., Hohenhorst W. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/hypopnea syndrome. Sleep Med. 2008;9:121–128.
- Guilleminault C., Kim Y.D., Horita M., Tsutumi M., Pelayo R. Power spectral EEG findings in patients with obstructive sleep apnea and upper airway resistance syndromes. Electroencephalogr Clin Neurol. 1999;50(Suppl):S109–S112.
- Pépin JL, Guillot M, Tamisier R, Lévy P. The upper airway resistance syndrome. Respiration. 2012;83(6):559-566. doi:10.1159/000335839 https://doi.org/10.1159/000335839
- Cracowski C, Pepin JL, Wuyam B, Levy P: Characterization of obstructive nonapneic respiratory events in moderate sleep apnea syndrome. Am J Respir Crit Care Med 2001;164:944–948.
- Tamisier R, Pepin JL, Wuyam B, Smith R, Argod J, Levy P: Characterization of pharyngeal resistance during sleep in a spectrum of sleep-disordered breathing. J Appl Physiol 2000;89:120–130.
- Ayappa I, Norman RG, Krieger AC, Rosen A, O’Malley RL, Rapoport DM: Non-invasive detection of respiratory effort-related arousals (RERAs) by a nasal cannula/pressure transducer system. Sleep 2000;23:763–771.
- Kristo DA, Lettieri CJ, Andrada T, Taylor Y, Eliasson AH: Silent upper airway resistance syndrome: prevalence in a mixed military population. Chest 2005;127:1654–1657.
- Frey WC, Pilcher J: Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg 2003;13:676–683.
- Guilleminault C., Kirisoglu C., Poyares D., Palombini L., Leger D., Farid-Moayer M. Upper airway resistance syndrome: a long term outcome study. J Psychiatr. Res. 2006;40:273–279.
- Shiomi T., Guilleminault C., Stoohs R., Schnittger I. Obstructed breathing in children during sleep monitored by echocardiography. Acta Paediatr. 1993;82:863–871.
- Vassilakopoulos T., Roussos C., Zakynthinos S. The immune response to resistive breathing. Eur Respir J. 2004;24:1033–1043.
- Gold AR, Gold MS, Harris KW, Espeleta VJ, Amin MM, Broderick JE: Hypersomnolence, insomnia and the pathophysiology of upper airway resistance syndrome. Sleep Med 2008;9:675–683.
- Powers CR, Frey WC: Maintenance of wakefulness test in military personnel with upper airway resistance syndrome and mild to moderate obstructive sleep apnea. Sleep Breath 2009;13:253–258.
- Stoohs RA, Philip P, Andries D, Finlayson EV, Guilleminault C: Reaction time performance in upper airway resistance syndrome versus obstructive sleep apnea syndrome. Sleep Med 2009;10:1000–1004.
- Guilleminault C, Black JE, Palombini L: High (or abnormal) upper airway resistance (in French). Rev Mal Respir 1999;16:173–180.
- Guilleminault C, Faul JL, Stoohs R: Sleep-disordered breathing and hypotension. Am J Respir Crit Care Med 2001;164:1242–1247.
- Iber C., Ancoli-Israel S., Chesson AL, Jr., Quan SF. 1st ed. American Academy of Sleep Medicine; Westchester, IL: 2007. The American academy of sleep medicine manual for the scoring of sleep and associated events: rules, terminology and technical specifications.
- Terzano M.G., Parrino L., Sherieri A., Chervin R., Chokroverty S., Guilleminault C. Atlas, rules and recording techniques for the scoring of the cyclical alternating pattern-CAP- in human sleep. Sleep Med. 2001;2:537–553.
- Guilleminault C., Poyares D. Arousal and Upper Airway Resistance. Sleep Med. 2002;3:S15–S20.
- EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173-184.
- Stoohs R., Guilleminault C. Snoring during NREM sleep: respiratory timing, esophageal pressure and EEG arousal. Resp Physiol. 1991;85:151–167.
- Hosselet J.J., Norman R.G., Ayappa I., Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med. 1998;157:1461–1467.
- Argod J, Pepin JL, Smith RP, Levy P: Comparison of esophageal pressure with pulse transit time as a measure of respiratory effort for scoring obstructive nonapneic respiratory events. Am J Respir Crit Care Med 2000;162:87–93.
- Montserrat JM, Badia JR: Upper airway resistance syndrome. Sleep Med Rev 1999;3:5–21.
- Ong K.C., Cheng P.P., Ong YY. Upper airway resistance syndrome–report of three cases. Ann Acad Med Singapore. 2000;29:242–245.
- Guilleminault C., Stoohs R., Clerk A., Cetel M., Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104:781–787.
- Watanabe T., Mikami A., Taniguchi M., Motonishi M., Honda H., Kyotani K. Clinical characteristics of upper airway resistance syndrome. Psychiatry Clin Neurosci. 1999;53:331–333.
- Edwards N., Blyton D.M., Kirjavainen T., Kesby G.J., Sullivan C.E. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–257.
- Lofaso F., Goldenberg F., Thebault C., Janus C., Harf A. Effect of zopiclone on sleep, night-time ventilation, and daytime vigilance in upper airway resistance syndrome. Eur Respir J. 1997;10:2573–2577.
- Guilleminault C., Palombini L., Poyares D., Chowdhuri S. Chronic insomnia, premenopausal women and sleep disordered breathing Part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosomatic Res. 2002;53:617–623.
- Miller P., Iyer M., Gold AR. Treatment resistant adolescent depression with upper airway resistance syndrome treated with rapid palatal expansion: a case report. J Med Case Rep. 2012;6:415.
- Montserrat J.M., Badia J.R. Upper airway resistance syndrome. Sleep Med Rev. 1999;3:5–21.
- Newman J.P., Clerk A.A., Moore M., Utley D.S., Terris D.J. Recognition and surgical management of the upper airway resistance syndrome. Laryngoscope. 1996;106:1089–1093.
- Lewis M.R., Ducic Y. Genioglossus muscle advancement with the genioglossus bone advancement technique for base of tongue obstruction. J Otolaryngol. 2003;32:168–173.
- Utley D.S., Shin E.J., Clerk A.A., Terris DJ. A cost-effective and rational surgical approach to patients with snoring, upper airway resistance syndrome, or obstructive sleep apnea syndrome. Laryngoscope. 1997;107:726–734.
- Guilleminault C., Los Reyes V.D. Upper-airway resistance syndrome. Handb Clin Neurol. 2011;98:401–409.
- Tschopp K., Zumbrunn T., Knaus C., Thomaser E., Fabbro T. Statistical model for postoperative apnea-hypopnea index after multilevel surgery for sleep-disordered breathing. Eur Arch Otorhinolaryngol. 2011;268:1679–1685.
- Lévy P. Management of simple snoring, upper airway resistance syndrome, and moderate sleep apnea syndrome. Sleep. 1996;19(9 Suppl):S101–S110.
- Venkat R., Gopichander N., Vasantakumar M. Four novel prosthodontic methods for managing upper airway resistance syndrome: an investigative analysis revealing the efficacy of the new nasopharyngeal aperture guard appliance. Indian J Dent Res. 2010;21:44–48.
- Yoshida K. Oral device therapy for the upper airway resistance syndrome patient. J Prosthet Dent. 2002;87:427–430.
- Rose E., Frucht S., Sobanski T., Barthlen G., Schmidt R. Improvement in daytime sleepiness by the use of an oral appliance in a patient with upper airway resistance syndrome. Sleep Breath. 2000;4:85–88.
- Guerrero M., Lepler L., Kristo D. The upper airway resistance syndrome masquerading as nocturnal asthma and successfully treated with an oral appliance. Sleep Breath. 2001;5:93–96.
- Loube D.I., Andrada T., Shanmagum N., Singer MT. Successful treatment of upper airway resistance syndrome with an oral appliance. Sleep Breath. 1997;2:98–101.
- Bahammam A.S., Tate R., Manfreda J., Kryger MH. Upper airway resistance syndrome: effect of nasal dilation, sleep stage, and sleep position. Sleep. 1999;22:592–598.
- Guilleminault C, Kirisoglu C, Poyares D, Palombini L, Leger D, Farid-Moayer M, Ohayon MM: Upper airway resistance syndrome: a long-term outcome study. J Psychiatr Res 2006;40:273–279.
- Jonczak L, Plywaczewski R, Sliwinski P, Bednarek M, Gorecka D, Zielinski J: Evolution of upper airway resistance syndrome. J Sleep Res 2009;18:337–341.