What is uridine
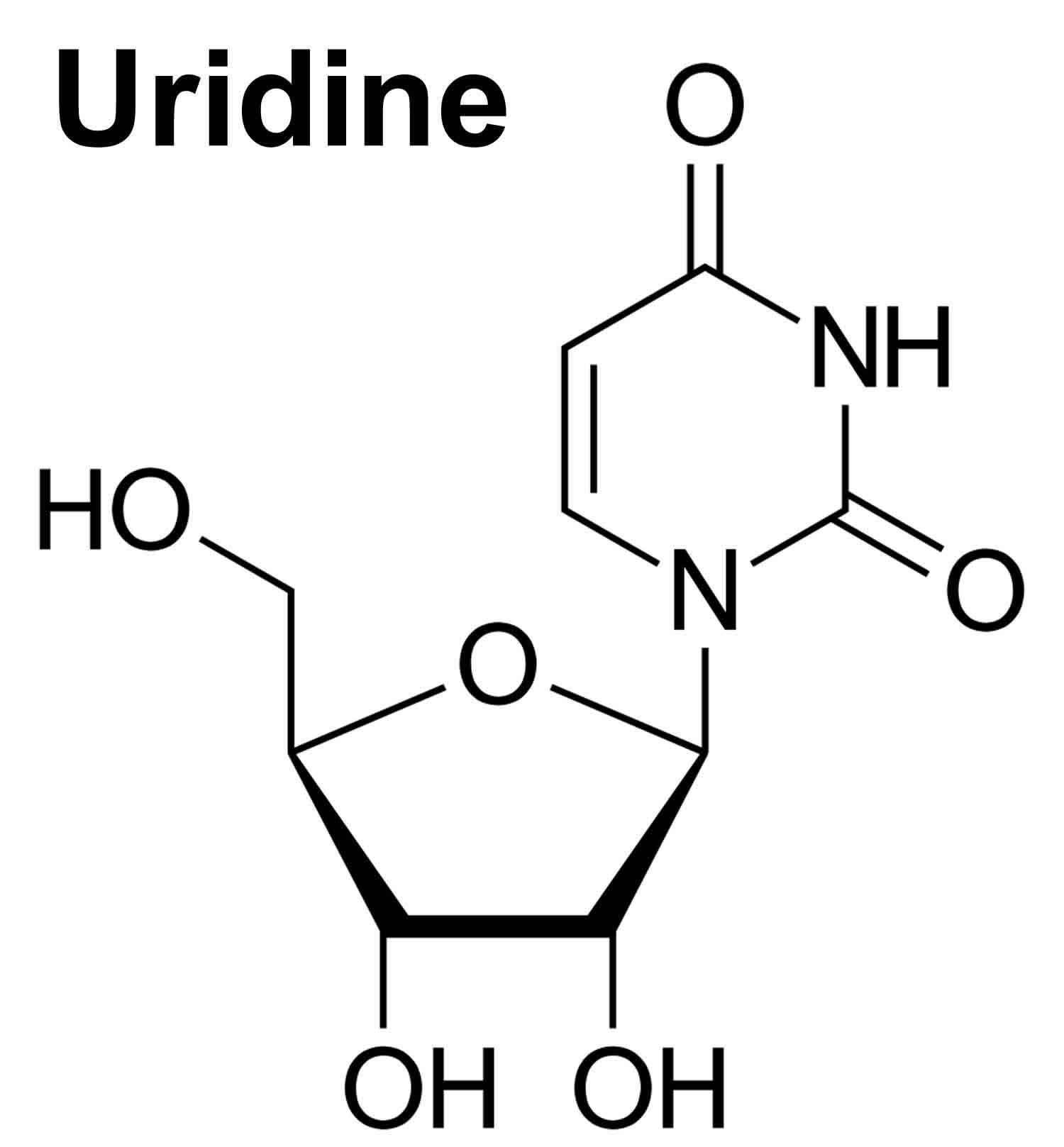
Uridine is a glycosylated pyrimidine nucleoside containing uracil attached to a ribose ring (ribofuranose) that is present at high levels in the plasma of humans that plays a critical role as a building block for ribonucleic acid (RNA) biosynthesis, glycogen synthesis, lipid deposition and many other essential cellular processes 1. Uridine also contributes to systemic metabolism, but the underlying mechanisms remain unclear 2. Uridine is intimately related to the homeostasis of the organism, regulating glucose homeostasis, lipid metabolism, amino acid metabolism, and other life processes. In addition, because uridine has nontoxic drug components, it is currently used for the treatment of hereditary disease, cancer, seizure, and central nervous system disorders. Uridine is one of the five standard nucleosides which make up nucleic acids, the others being adenosine, thymidine, cytidine and guanosine. The five nucleosides are commonly abbreviated to their one-letter codes U, A, T, C and G respectively. However, thymidine is more commonly written as ‘dT’ (‘d’ represents ‘deoxy’) as it contains a 2′-deoxyribofuranose moiety rather than the ribofuranose ring found in uridine. This is because thymidine is found in deoxyribonucleic acid (DNA) and not ribonucleic acid (RNA). Conversely, uridine is found in RNA and not DNA. The remaining three nucleosides may be found in both RNA and DNA. In RNA, they would be represented as A, C and G whereas in DNA they would be represented as dA, dC and dG.
Uridine has multi-targeted effects because it can be converted rapidly into other biologically active molecules 3. Uridine is salvaged into pyrimidine nucleotides necessary for RNA and DNA synthesis 4. Via cytidine triphosphate, uridine promotes membrane phospholipid biosynthesis. Via uridine triphosphate, uridine promotes the formation of uridine diphosphate glucose (UDPG) and uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), which are substrates for glycogen biosynthesis and protein O-linked glycosylation, respectively. Uridine catabolism produces acetyl-CoA, a substrate for protein lysine acetylation. Most interestingly, de novo pyrimidine biosynthesis is coupled to mitochondrial respiratory chain 5. The protective function of uridine on mitochondrial functions is thought to be mediated by its conversion to other pyrimidine intermediates 6.
Uridine also serves to generate pyrimidine-lipid and pyrimidine-sugar conjugates required for glycogen deposition, protein and lipid glycosylation, extracellular matrix biosynthesis, and detoxification of xenobiotics (a chemical substance found within an organism that is not naturally produced or expected to be present within the organism) 7. These anabolic reactions are critical for normal cellular function and survival. In addition, uridine catabolism by uridine phosphorylase may regulate events important to systemic metabolism, such as body temperature and circadian rhythm 8. Despite its important physiologic and pharmacological roles, uridine has received much less research emphasis than adenosine 8.
Plasma uridine concentrations are tightly regulated in humans, but the mechanisms underlying homeostasis of circulating uridine are unknown 7. The liver is considered the major organ controlling plasma uridine levels, mediating de novo biosynthesis within hepatocytes and uridine clearance via Kupffer cells 9. The liver is the predominant biosynthetic organ and contributor to plasma uridine in the fed state, whereas the adipocyte dominates uridine biosynthetic activity in the fasted state 2. The majority of plasma uridine is degraded by the liver after uptake through the portal vein. Biliary excretion is the primary mechanism for plasma uridine clearance. However, the processes by which the liver regulates uridine degradation, and the biological effects of this massive clearance process, have not been studied 2. Because nutrient intake triggers bile release, plasma uridine levels are elevated during fasting and drop rapidly in the postprandial state. The fasting-associated increase of plasma uridine elicits a hypothalamic response culminating in body temperature lowering, whereas bile-mediated uridine release promotes a decline of plasma uridine and enhances insulin sensitivity 2.
A constant supply of circulating uridine is required for a number of biological functions 10 and disruption of plasma uridine homeostasis through uridine supplementation has profound effects on systemic metabolism 11. Short-term supplementation of uridine in food improves insulin sensitivity in mice, but long-term administration causes fatty liver and promotes development of pre-diabetes 2. Thus, control of plasma uridine is tightly coupled to energy homeostasis 2.
In humans, uridine is present in plasma in considerably higher quantities than other purine and pyrimidine nucleosides, thus it may be utilized for endogenous pyrimidine synthesis 12. Uridine has a number of biological effects on a variety of organs with or without disease, such as the reproductive organs, central and peripheral nervous systems, and liver 12. In addition, it is used in clinical situations as a rescue agent to protect against the adverse effects of 5-fluorouracil. Since the biological actions of uridine may be related to its plasma concentration, it is important to examine factors that have effects on that concentration. Factors associated with an increase in plasma concentration of uridine include enhanced ATP consumption, enhanced uridine diphosphate (UDP)-glucose consumption via glycogenesis, inhibited uridine uptake by cells via the nucleoside transport pathway, increased intestinal absorption, and increased 5-phosphribosyl-1-pyrophosphate and urea synthesis. In contrast, factors that decrease the plasma concentration of uridine are associated with accelerated uridine uptake by cells via the nucleoside transport pathway and decreased pyrimidine synthesis.
Deng et al 2 found that plasma uridine levels are regulated by fasting and refeeding in mice, rats, and humans. Fasting increases plasma uridine levels, and this increase relies largely on adipocytes. In contrast, refeeding reduces plasma uridine levels through biliary clearance. Elevation of plasma uridine is required for the drop in body temperature that occurs during fasting. Further, feeding-induced clearance of plasma uridine improves glucose metabolism. We also present findings that implicate leptin signaling in uridine homeostasis and consequent metabolic control and thermoregulation. Their results indicate that plasma uridine governs energy homeostasis and thermoregulation in a mechanism involving adipocyte-dependent uridine biosynthesis and leptin signaling 2.
Previous work suggests that uridine affects both body temperature and feeding behavior. For example, injection of high doses of uridine decreases body temperature in rodents 13. Co-administration of uridine and benzylacyclouridine, a compound that inhibits uridine degradation, partially prevents this temperature drop 14. In addition, elevated plasma uridine can increase brain (hypothalamic) levels of uridine diphosphate (UDP), which then promotes food intake via P2Y2-dependent activation of AgRP (Agouti-related protein) neurons 15.
Uridine monophosphate
Uridine monophosphate also known as uridine-5′-monophosphate (UMP) is a precursor of circulating circulating and brain uridine 16. Like omega-3 fatty acid docosahexaenoic acid (DHA), uridine readily crosses the blood-brain barrier (BBB) and enters brain cells 17. It is then phosphorylated by uridine-cytidine kinases to form uridine triphosphate (UTP), which can be further transformed by CTP synthetase to cytidine triphosphate (CTP), the usual rate-limiting precursor in phosphatide biosynthesis via the Kennedy cycle 18. Cytidine triphosphate (CTP), for example, can combine with phosphocholine to form cytidine-5’-diphosphocholine (CDP-choline) 19, which then combines with DAG to yield phosphatidylcholine, the major phosphatide in neuronal membranes 20. Uridine promotes neurite outgrowth in PC-12 cells treated with nerve growth factor 21, and neurotransmitter release from the rat striatum in vivo 22. Moreover, when given with DHA, uridine increases membrane phosphatides and synaptic proteins in the adult gerbil brain 23. These results suggest that uridine as well as DHA can enhance phosphatide synthesis in neuronal or synaptic membranes. Furthermore, the effects on phosphatide synthesis of giving uridine monophosphate to animals also receiving omega-3 fatty acid docosahexaenoic acid (DHA) and choline tend to be substantially greater than the sum of the increases observed after either treatment alone 23. Local application of uridine monophosphate into rats hippocampus 30 minutes prior to acquisition of the training in a Y- maze chamber reportedly improved performance, as examined 48 hours later 24. A uridine monophosphate-enriched diet can reverse the memory impairments observed among rats reared under impoverished environmental conditions 25. Consumption of uridine monophosphate along with DHA plus choline amplifies the increases in hippocampal dendritic density produced by giving choline plus DHA alone 26.
Uridine triacetate
Uridine triacetate is used to treat hereditary orotic aciduria also known as orotic aciduria type 1 (OA1). Hereditary orotic aciduria is a rare metabolic disease that is caused by uridine deficiency. Uridine triacetate was granted U.S. Food and Drug Administration (FDA) approval for treating hereditary orotic aciduria (orotic aciduria type 1 [OA1]) in 2015 27. Without treatment, children with orotic aciduria type 1 (OA1) may experience neutropenia, failure to thrive, developmental delay, and intellectual disability 28. Uridine triacetate is a pyrimidine analog that works by replacing the uridine that cannot be normally produced in patients with hereditary orotic aciduria. Hereditary orotic aciduria (orotic aciduria type 1 [OA1]) is characterized by elevated levels of orotic acid in the urine 28. Hereditary orotic aciduria typically becomes apparent in the first months of life with megaloblastic anemia, as well as delays in physical and intellectual development 29. Hereditary orotic aciduria (orotic aciduria type 1 [OA1]) is caused by changes (mutations) in the UMPS gene and inheritance is autosomal recessive. Hereditary orotic aciduria (orotic aciduria type 1 [OA1]) differs from other causes of orotic aciduria, which may include mitochondrial disorders, lysinuric protein intolerance, and liver disease 28.
Uridine triacetate is in a class of medications called pyrimidine analogs. Uridine triacetate works by blocking cell damage from certain chemotherapy medications. Uridine triacetate is used for the emergency treatment of children and adults who have either received too much of chemotherapy medications such as fluorouracil or capecitabine (Xeloda) or who develop certain severe or life-threatening toxicities within 4 days of receiving fluorouracil or capecitabine 30. Uridine triacetate is available only with your doctor’s prescription.
Uridine biosynthesis and catabolism pathway
It is acknowledged that uridine metabolism involves three pathways: de novo synthesis, salvage synthesis pathway, and catabolism 1. Within the cell, nucleotides can be synthesized from a simple metabolite by the de novo synthesis pathway or recycled by the salvage synthesis pathway 12. When endogenous supply is insufficient to maintain normal body functions, uridine is mainly exogenously supplemented by diet to maintain normal growth and cell function 31. During its catabolism, uridine is converted to beta-alanine and followed by secretion to the brain and muscle tissues 12.
Uridine biosynthesis
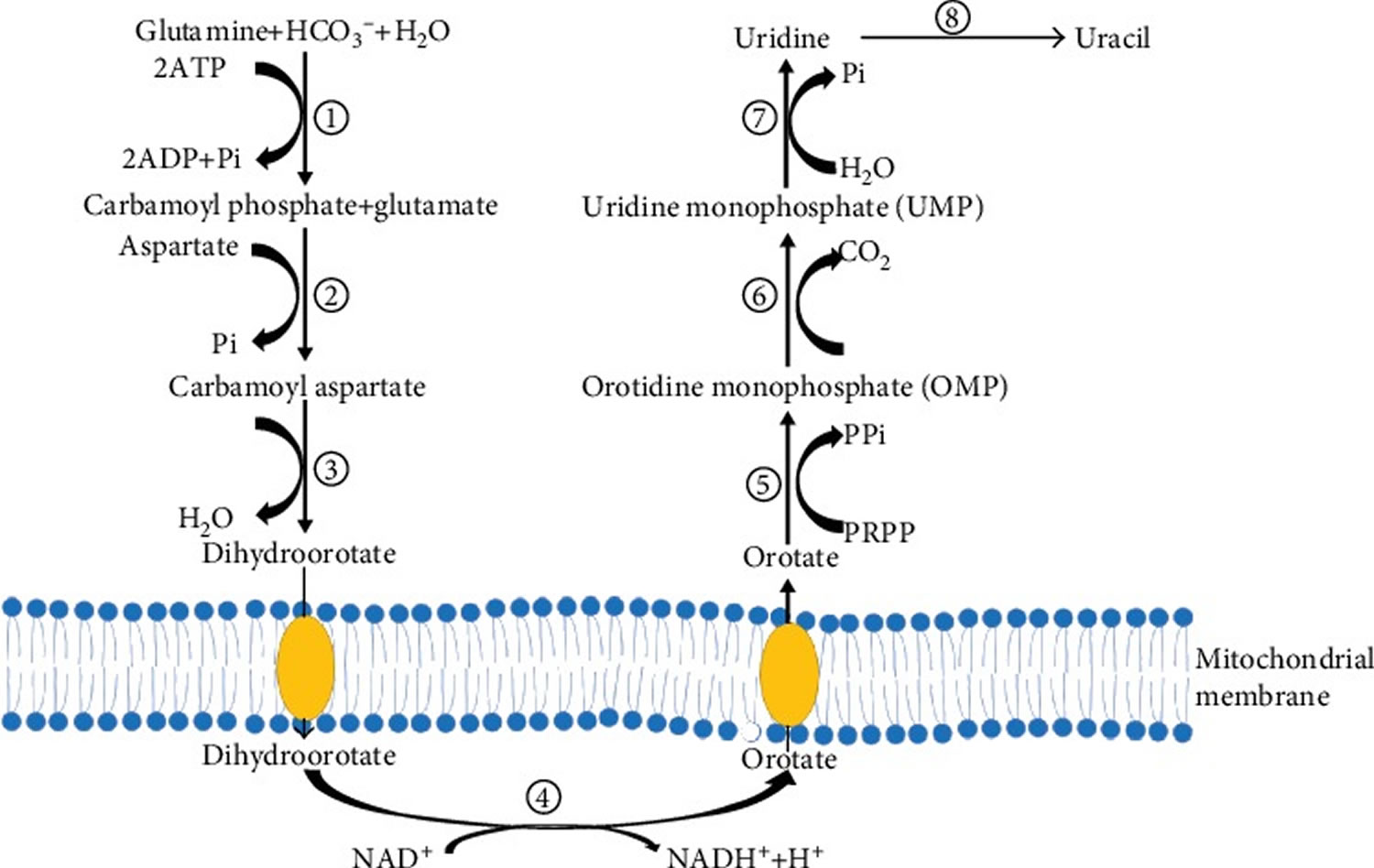
Uridine de novo synthesis originates from glutamine and is catalyzed by the CAD protein which encodes the rate-limiting enzymes during uridine biosynthesis 1. CAD is a multidomain enzyme comprised of carbamoyl phosphate synthetase (CPS II, EC=6.3.5.5), aspartic transcarbamoylase (ATCase, EC=2.1.3.2), and dihydroorotase (DHO, EC=3.5.2.3), in which CPS II is the rate-limiting enzyme, and regulation of CPS II is mediated by uridine 5′-triphosphate (UTP) feedback inhibition and phosphoribosyl pyrophosphate (PRPP) activation 31. In the de novo pathway, CAD catalyzed glutamine produces the intermediate metabolites carbamoyl phosphate, carbamoyl aspartate, and dihydroorotate (Figure 1).
The fourth step in pyrimidine biosynthesis is catalyzed by dihydroorotate dehydrogenase (DHODH), to form an important intermediate, orotate. The accumulation of orotate may induce intracellular lipid accumulation 32. DHODH is a mitochondrial, respiratory chain-coupled enzyme on the outer surface of the mitochondrial inner membrane, which links pyrimidine biosynthesis to mitochondrial energy metabolism 31. Then, orotate is converted to orotidine monophosphate (OMP) and uridine monophosphate (UMP) by orotate phosphoribosyltransferase and orotidine 5′-phosphate decarboxylase, respectively. Then, uridine monophosphate (UMP) is dephosphorylated to uridine by nucleotidase or formed from cytidine by cytidine deaminase 33.
A recent study has shown that resting/low proliferating cells rely mainly on the nucleotide salvage pathway to synthesize RNA 34. Also, uridine can be obtained from uridine 5′-triphosphate (UTP) and cytosine 5′-triphosphate (CTP) via the pyrimidine salvage pathway. Uridine 5′-triphosphate (UTP) is also involved in glycogen synthesis, protein glycosylation, and membrane phospholipid biosynthesis 35. In the pyrimidine nucleotide salvage synthesis pathway, uridine-cytidine kinase 2 (UCK2) acts as a phosphorylase responsible for phosphorylating pyrimidine nucleotides (uridine and cytidine) to the corresponding monophosphates 31. Nucleoside monophosphate kinase further phosphorylates uridine monophosphate and CMP to produce uridine 5′-diphosphate (UDP) and cytosine 5′-diphosphate (CDP). UDP and CDP are further phosphorylated by the nucleoside diphosphate kinase to produce uridine 5′-triphosphate (UTP) and cytosine 5′-triphosphate (CTP). Subsequently, UTP and CTP are used only for gene duplication. During nucleic acid catabolism, some nucleoside monophosphates are released and reused in the salvage synthesis pathway by uridine-cytidine kinase 2 (UCK2) 36. In mammals, uridine-cytidine kinase 2 (UCK2) is allosterically activated by ATP to synthesize the pyrimidine nucleotides required for cellular metabolism. Nucleotide production is controlled by the feedback inhibition of uridine-cytidine kinase 2 (UCK2) by uridine 5′-triphosphate (UTP) and cytosine 5′-triphosphate (CTP) 37. Therefore, uridine-cytidine kinase 2 (UCK2) is a potential chemotherapeutic drug target 1.
Figure 1. De novo synthesis of uridine
Footnotes: (1) carbamoyl phosphate synthetase (CPS II); (2) aspartate transcarbamoylase (ATCase); (3) dihydroorotase (DHO); (4) dihydroorotate dehydrogenase (DHODH); (5) orotate phosphoribosyltransferase; (6) orotidine 5′-phosphate decarboxylase; (7) nucleotidase; (8) uridine phosphorylase.
[Source 1 ]Uridine catabolism
Uridine catabolism produces beta-alanine and acetyl-CoA, resulting in an increase in protein acetylation 38. Uridine is degraded to uracil by uridine phosphorylase (UPase) encoded by the UPP gene. Uridine phosphorylase (UPase) has two homologous forms in vertebrates: UPase1 and UPase2 39. UPase1, encoded by the UPP1 gene, regulates uridine homeostasis and is ubiquitously expressed. Inhibition of the enzymatic activity of UPase1 or UPase1 gene knockout results in elevated levels of uridine in plasma and tissues 40. UPase2 is a liver-specific protein encoded by the UPP2 gene and is indispensable for pyrimidine salvage reactions 41. Inhibition of the enzymatic activity of UPase2 increases the level of endogenous uridine in the liver, thereby protecting the liver against drug-induced lipid accumulation 41. Next, uracil is further decomposed into dihydrouracil and N-carbamoyl-β-alanine by dihydropyrimidine dehydrogenase (DPD) and hydropyrimidine hydratase (also known as dihydropyrimidinase), which is further converted to β-alanine by β-ureidopropionase 32. Beta-Alanine is excreted or enters other tissues, such as the brain and muscle.
Regulation mechanism of the uridine concentration
The plasma uridine concentration in mammals is between 3 and 8 μM, which is higher than other pyrimidine nucleotides and bases 12. Most tissues cannot synthesize uridine; thus, use plasma as a uridine source 42. Plasma uridine concentration is tightly controlled by a variety of factors in humans and rodents 2.
Feeding behavior regulates the plasma concentration of uridine
A series of recent studies indicate that the dynamic regulation of plasma uridine is related to feeding behavior 43. As the main organ in uridine biosynthesis, the liver maintains uridine homeostasis by regulating glucose metabolism during different feeding states 44. In the fed period, uridine synthesis occurs mainly in the liver tissue. However, in the fasted period, uridine synthesis depends on adipocytes, as the liver focuses on glucose production 44. Experiments to study how fasting and refeeding can cause changes in plasma uridine levels were performed in C57BL/6 mice, Sprague-Dawley rats, and healthy women. In the fasted state, adipose tissue elevated the plasma concentration of uridine via uridine biosynthesis, while plasma uridine levels decreased rapidly after refeeding 2. The reduction is caused by a decrease in uridine synthesis in adipocytes and an increase in uridine clearance in bile 45.
Uridine can be catalyzed in vivo into uracil by uridine phosphorylase. It is hypothesized that uridine phosphorylase is a direct cause of the significant increase in uracil and dihydrouracil during the fasting state 46. Endogenous plasma uracil levels are largely dependent on uridine homeostasis rather than on food intake 46. Thus, plasma uridine concentration is increased during fasting while decreased after refeeding.
Adipose tissue regulates plasma uridine homeostasis
Recent studies have shown that adipose tissue plays an indispensable role in plasma uridine homeostasis. Plasma uridine can be elevated by pyrimidine degradation induced by ATP depletion. In FAT-ATTAC mice, a model in which adipocytes can be selectively eliminated by inducing apoptosis, placed under different feeding conditions, was selected to determine if adipose tissue is involved in the regulation of plasma uridine content during fasting 2. In addition, Agpat 2 (1-acylglycerol-3-phosphate-O-acyltransferase 2) selectively silenced (a mutant in which white fat and brown fat are absent) mice was studied to confirm that adipose tissue is essential for the rapid increase of plasma uridine in the fasting state 2. To explore the mechanism by which adipose tissue regulates plasma uridine homeostasis, researchers generated an adipose tissue-specific CAD knockout mouse as a model. Results show no increase of uridine levels in the CAD knockout mice after 24 hours of fasting, indicating that the increase of plasma uridine concentration induced by fasting is mediated by the biosynthesis of uridine via adipocytes 2. These investigations demonstrate the possible molecular mechanism involving adipose tissue in the regulation of plasma uridine homeostasis.
ATP depletion increases uridine concentration
Numerous studies have shown that fructose, sucrose, ethanol, xylitol, and strenuous exercise may increase purine base (hypoxanthine, xanthine, and urate) concentration by increasing pyrimidine degradation after ATP depletion and enhancing adenine nucleotide degradation thereby increasing the concentration of uridine 47. Because UTP is produced by UDP phosphorylation using ATP as a phosphate donor, a decrease in ATP concentration leads to a decrease in phosphorylation of UDP to UTP, leading to an increase in UDP and UMP. An increase in ATP concentration thus accelerates the degradation of uracil nucleotides (UTP⟶UDP⟶UMP⟶uridine), increasing plasma uridine concentration 48.
Previous studies have demonstrated that fructose may enhance the degradation of adenine and uracil nucleotides. Fructose is rapidly phosphorylated to fructose-1-phosphate (F-1-P) using ATP as a phosphate donor 49. While ATP is derived from the phosphorylation of ADP, the phosphorylation of ADP is accelerated by inorganic phosphate (Pi), resulting in a decrease of available Pi 49. Due to the lack of Pi in the reaction, ATP is dependent on the inhibition of oxidative phosphorylation of ADP. Also, during the metabolism of fructose, AMP deaminase promotes the conversion of AMP to IMP, increasing IMP concentration, resulting in a decrease of adenine nucleotides and an increase in serum urate concentration 49. Eventually, the uridine produced by the liver passes through the hepatic vein into the blood, resulting in an increase in blood uridine concentration 50. Therefore, ingesting food and beverages that contain large amounts of fructose may help increase the plasma uridine concentration.
On the other hand, exercise can also increase plasma uric acid concentration by promoting the degradation of adenine nucleotides and the production of lactic acid in muscles 51. The final degradation product of adenine nucleotide, hypoxanthine, is released from the muscle into the blood and then transported into the liver, where it is converted into uric acid under the action of xanthine dehydrogenase 49. Furthermore, plasma urate concentration is increased during and after strenuous exercise. Exercise-induced adenine nucleotide degradation is characterized by an increase in the concentrations of plasma hypoxanthine, jaundice, and uridine. The uric acid produced by the liver is released into the blood, thereby increasing the concentration of plasma urate 49.
Ethanol can also promote the degradation of uracil nucleotide by increasing the consumption of ATP, thereby increasing the concentrations of plasma urate and uridine. Purines also serve to increase the concentration of plasma uric acid 52. During alcohol metabolism, ATP is rapidly consumed and decomposed to uric acid. According to previous studies, there are two mechanisms for the acceleration of ethanol-induced uracil nucleotide degradation: one is the increase in ATP consumption, and the other is the decrease in ATP production 48. Ethanol can also increase the excretion of hypoxanthine and jaundice in urine by accelerating the degradation of adenine nucleotides and weakening the activity of xanthine dehydrogenase 52. Accordingly, it is strongly suggested that fructose, ethanol, and strenuous exercise can increase the concentration of uridine by increasing pyrimidine degradation after ATP depletion.
Uridine benefits
Uridine is an essential component of a range of cellular functions and is required for cell survival. Uridine is associated with glucose homeostasis, lipid metabolism, and amino acid metabolism by regulating enzymes and intermediates in uridine metabolism, such as UTP, DHODH, and UPase, which further are involved in systemic metabolism. The pyrimidine ring of uridine is susceptible to glycosylation 53. Uridine catabolism can also lead to an increase in cellular acetyl-CoA pool, which in turn increases protein acetylation 32. Previous studies showed that oral administration of uridine stimulated intestinal development, promoted nucleotide transport, improved growth performance, and regulated the fatty acid composition and lipid metabolism in weaned piglets 54. Scientists have demonstrated that maternal dietary uridine supplement contributed to reducing the occurrence of diarrhea by regulating cytokine secretion and intestinal mucosal barrier function of suckling pigs 55. A recent study demonstrated that uridine was able to inhibit stemness of intestinal stem cells in 3D intestinal organoids and mice 56.
Uridine has also been widely used in reducing cytotoxicity on non-cancerous cells due to the administration of anti-cancer drug 5-fluorouracil 57 and improving neurophysiological functions 58. In addition, short-term uridine coadministration with tamoxifen could suppress hepatic steatosis 41. Uridine has also been shown to suppress hepatic steatosis induced by the usage of drugs in mice including zalcitabine 59 and fenofibrate 60. Uridine mitigates lipodystrophy associated with the usage of nucleoside reverse transcriptase inhibitors for HIV treatment 61. Uridine is a nutrient critical for phosphatidylcholine biosynthesis and synapse formation 62. Uridine improves neurophysiological functions in patients with diabetic neuropathy 63. In addition, uridine triacetate (Xuriden), an orally active prodrug of uridine, has recently received an orphan drug designation by the FDA to treat hereditary orotic aciduria.
In humans, uridine is present in the blood and cerebrospinal fluid (CSF) and the content of uridine in plasma is much higher than that in other purine and pyrimidine nucleosides 42. Due to their inability to synthesize uridine, most tissues utilize plasma uridine to maintain basic cellular functions 44. Thus, plasma uridine may be used for the synthesis of endogenous pyrimidine. The biosynthesis of uridine is regulated by the liver and adipose tissues, and the excretion of uridine is mainly achieved via the kidneys or by pyrimidine catabolism in tissues 64.
Many research studies have demonstrated that uridine homeostasis is affected by a variety of factors to regulate systemic metabolism. Factors recognized for regulating the concentration of uridine include uridine phosphorylase (UPase), feeding behavior, and ATP depletion 1. In contrast, uridine can also regulate systemic homeostasis by regulating enzymes and their reaction products. Uridine is thought to regulate the mitochondrial respiratory chain via the dihydroorotate dehydrogenase (DHODH) enzyme, and since mitochondrial dysfunction is involved in many human diseases, uridine could be used as a therapeutic drug for mitochondrial diseases 32. Furthermore, the accumulation of orotate, an intermediate product in the process of uridine de novo synthesis, induces intracellular lipid accumulation 65.
It has been demonstrated that uridine evoked anti-epileptic and anti-epileptogenic effects in different models 66, in children with epileptic encephalopathy 67 and in WAG/Rij rats 68. Theoretically, as the uridine is an endogenous molecule, administration of proper doses of uridine in the treatment of epilepsy may be a safe way to evoke anti-epileptic effects without or minimal side effects and considerable risks, compared to pharmacological treatments 69. Indeed, it has been demonstrated that uridine is a well-tolerated drug with only minor toxic potential, suggesting that uridine and its analogues may be effective and safe anti-epileptic drugs in the treatment of different types of epilepsies 70.
Uridine may also exert its effect via partly the same anti-convulsant (adenosinergic and GABAergic) systems via interactions with Ado receptors and/or GABA-A receptor and putative uridine receptor 71 or by uridine-evoked increase in GABA levels 72. Moreover, it has been demonstrated that the adenosine released via nucleoside transporters preferentially bind to adenosine A1 receptors (A1Rs) 73 and uridine was active in eliciting purine (adenosine) release by means of nucleoside transporters 74. It has also been demonstrated that GABAergic and adenosinergic systems can regulate absence epileptic activity in WAG/Rij rats 68. However, excitatory Ado A2A receptors (A2ARs) and GABA-A receptors aggravated the absence epileptic activity 75 whereas A1Rs can modulate (decrease) spike-wave discharge number in WAG/Rij rats 76. Thus, these results suggest that neither GABA-A receptors nor Ado A2A receptors (A2ARs) have a role in the alleviating effect of both uridine and exogenous ketone supplements on absence epileptic activity, but A1Rs can modulate their beneficial, modulatory effects on absence epilepsy.
Uridine and thermoregulation
Temperature homeostasis is a tightly regulated process in mammals. The human body retains a set temperature of ~98.6 °F (37°C), with complications arising when the temperature increases by little more than 3°C 77. During fasting, humans manifest a reduction in body temperature, a process regulated by the sympathetic nervous system 78. Deng and colleagues findings indicate that plasma uridine is a metabolite critically involved in this process 2.
The dynamic regulation of plasma uridine is unusual for a metabolite, particularly one with the range of vital biological functions associated with uridine. Characterizing the dynamics of plasma uridine as part of the systemic response to fasting and refeeding has led scientists to propose a model that integrates feeding, carbohydrate metabolism, and thermoregulation through a novel adipo-biliary-uridine axis. In studies with leptin-deficient ob/ob mice, coupled with measurements of circulating leptin in response to uridine administration, researchers also uncovered a role for leptin signaling in the governance of uridine homeostasis and fasting-induced declines in body temperature 2.
Uridine and glucose homeostasis
As the UTP precursor, uridine can activate glycogen synthesis 58. It was found that the injection of uridine increased the levels of both uridine diphosphonate- (UDP-) glucose and uridine diphosphonate-N-acetylglucosamine (UDP-GlcNAc) in skeletal muscle. Injection of uridine also caused significant insulin resistance, suggesting that the decrease of insulin levels induced by uridine is mediated by muscle UDP-N-acetylhexosamine accumulation 12. In addition, a previous study indicated that plasma uridine is a marker of insulin resistance in patients with noninsulin-dependent diabetes mellitus (NIDDM) 12. The latest report also showed that chronic or short-term uridine supplementation in mice could lead to impairment in glucose tolerance and decreased insulin signaling 45. A study where wild-type mice fed with a high-fat diet and ob/ob mice were given uridine and glucose orally showed a significant improvement in glucose tolerance in wild-type mice, while no variation was observed in ob/ob mice 2. Furthermore, intraperitoneal injection of uridine was carried out to verify the effect of uridine on glucose metabolism, which resulted in an improvement of glucose tolerance in wild-type mice, while deterioration in ob/ob mice 2. These findings indicated that leptin might mediate the effects of uridine on glucose metabolism. Another study in aging mice with insulin resistance confirmed the role of uridine, showing that uridine administration also improved glucose tolerance in aging mice 2.
Furthermore, uridine could affect body temperature and feeding behavior through glucose metabolism. High doses of uridine may reduce body temperature in rodents, and uridine may be a driving force for thermoregulation during fasting and refeeding 2, which is another strong validation that uridine is associated with glucose homeostasis.
Uridine and lipid metabolism
Several studies have revealed the relationship between uridine and lipid metabolism 41. There are two possible mechanisms for the relationship between pyrimidine metabolism and lipid metabolism: one is inhibition of DHODH, a mitochondrial membrane-bound and respiratory chain-coupled enzyme. Inhibition of DHODH leads to microvesicular steatosis. The other mechanism is overexpression of the UPase1 enzyme, leading to the depletion of endogenous uridine levels [10]. Inhibition of DHODH may cause microvesicular steatosis, which can be alleviated after uridine supplementation, whereas uridine has no effect on DHODH enzyme activity in vitro. Other studies have also shown that supplementation with uridine induces changes in the liver’s NAD+/NADH and NADP+/NADPH ratios and in the liver’s protein acetylation profile, thereby inhibiting fatty liver development [10]. UPase1 and UPase2 catalyze the reversible conversion of uridine to uracil [1, 2, 10, 11]. UPase1 plays a significant role in the regulation of uridine homeostasis. Recently, a transgenic (knock-in) UPase1-TG mouse with a fatty liver phenotype was used to evaluate the effect of UPase1 overexpression on hepatic uridine homeostasis 32. UPase1-TG mice exhibited depleted uridine concentration in the plasma and liver, which could be reversed by dietary uridine supplementation 32. In contrast, UPase1−/− mice, with genetic knockout of the UPase1 gene, exhibited a decreased plasma and liver uridine concentration, which showed a protective effect on the multiple-drug-induced fatty liver 79. Furthermore, enhancing endogenous hepatic uridine concentration by inhibiting UPase2 could suppress liver lipid accumulation induced by drugs 41.
The treatment period of uridine is intimately related to fatty liver development. The difference between the short- and long-term effects of uridine on liver lipid accumulation can be regulated by the homeostatic control of circulating uridine levels 41. Short-term uridine treatment can prevent drug-induced hepatic lipid accumulation, while chronic uridine feeding may induce liver lipid accumulation and reduce systemic glucose intolerance 41. Chronic uridine feeding leads to inhibition of liver-specific fatty acid-binding protein FABP1 expression, while repression of the expression of FABP1 is associated with a fatty liver in mice and humans, and chronic uridine feeding may be a driver of fatty liver 80. Also, recent studies have found that dynamic oral administration of uridine affects the rhythmic fluctuations of cholesterol, bile acid, lipid, and nucleotide metabolism-related genes 81. Another study also showed that uridine supplementation at night regulated rhythmic fluctuations in cholesterol and bile acid metabolism genes by enhancing duodenal nucleotide transport and synthesis 82, which indicated that appropriate time of uridine treatment is critical to lipid metabolism. Undoubtedly, further studies are required to explore the specific mechanism of the effect of uridine on lipid metabolism.
Uridine and amino acid metabolism
Amino acids have been shown to reduce uridine concentration, possibly through nucleoside transporters present in various cell types 47. In an amino acid infusion study, amino acids did not affect hypoxanthine, xanthine, and uric acid plasma concentrations, while the content of plasma uridine decreased by 25.1% and 58.5% at 30 minutes and one hour after injection of amino acids, respectively 83. Amino acid infusion can increase uric acid excretion in urine without affecting plasma uric acid concentration and excretion of hypoxanthine and jaundice, indicating that amino acids increase renal uric acid excretion through renal transport but do not affect the degradation of sputum, where glucagon may play an important role in the process of amino acid-induced intrarenal uric acid clearance 84.
Amino acids have also been demonstrated to reduce uridine plasma concentrations directly 12. A previous study has shown that oral branched-chain amino acids (isoleucine, leucine, and valine) can reduce plasma uridine concentration without affecting plasma concentrations of purine bases (uric acid, hypoxanthine, and xanthine), glucagon, and insulin or the excretion of uridine and purines 85. Studies have shown that branched-chain amino acids play an important role in nucleoside metabolism [58]. Branched-chain amino acids may reduce the concentration of plasma uridine without altering the concentration of insulin and glucagon; thus, it is suggested that branched-chain amino acids are the direct cause of the decrease in plasma uridine concentration, rather than a consequence of insulin and glucagon levels 85. However, the specific mechanism by which amino acid induces a decrease in plasma uridine concentration is still unclear.
Furthermore, aspartate as a precursor can provide direct carbon for de novo synthesis of pyrimidine nucleotides; aspartate and glutamate also provide a nitrogen source for pyrimidine and pyrene synthesis 34. Moreover, some glucogenic amino acids, including cysteine, serine, and arginine, can produce pyruvate acid, after which pyruvate enters the glucose metabolism process and is converted to glucose-6-phosphate (6-P-G), which then enters the pentose phosphate pathway to produce ribose-5-phosphate, and catalytic synthesis 5-phosphate ribose-1-pyrophosphate by PRPP pyrophosphate to participate in nucleotide metabolism 86. PRPP is required for de novo synthesis of pyrimidine and purine nucleotides, so the acceleration of de novo synthesis of PRPP may also increase the de novo synthesis of pyrimidine nucleotides, thereby accelerating pyrimidine nucleotide biosynthesis, followed by an increase of uridine 12.
Taken together, these results suggest that amino acids can provide a carbon or nitrogen source for pyrimidine nucleotide synthesis and influence uridine concentration via different metabolic pathways.
Uridine dosage
Uridine triacetate dosage and administration
Uridine triacetate is commercially available in the US as Vistogard and Xuriden under a Restricted Distribution Program 87, 88.
- You must obtain Vistogard through a specialty pharmacy (https://www.vistogard.com/Professional/How-to-Order). Contact specialty pharmacy at 844-293-0007 (for inpatient use) or 844-374-0604 (for outpatient use and information on the case management program) or consult the Vistogard website (https://www.vistogard.com/Professional/How-to-Order) for specific ordering information.
- You must obtain Xuriden through a specialty pharmacy. Contact manufacturer Wellstat Therapeutics, Cincinnati, OH at 800-914-0071 for availability and ordering information.
Oral administration
Vistogard granules
- Begin treatment as soon as possible following overdosage or early-onset toxicity; administer ≤96 hours following the end of fluorouracil or capecitabine administration.
- Weigh pediatric doses using a scale accurate to ≤0.1 g or measure using a graduated teaspoon accurate to one-fourth teaspoonful. For patients requiring 10-g doses of uridine triacetate, contents of a full packet may be administered without weighing or measuring.
- Mix each dose with 3–4 ounces of a soft food (e.g., applesauce, pudding, yogurt) and administer orally ≤30 minutes of mixing, without regard to meals. Do not chew granules. Follow each dose with ≥120 mL (4 ounces) of water.
- If patient vomits ≤2 hours after receiving dose (either due to capecitabine or fluorouracil toxicity or slightly bitter taste of the granules), administer another full dose as soon as possible after vomiting episode; administer the next dose at the regularly scheduled time.1 7 Administration of an antiemetic (e.g., a 5-HT3 receptor antagonist) also may be helpful 89.
- If a dose is missed, administer missed dose as soon as possible and the next dose at the regularly scheduled time.
NG or Gastrostomy Tube Vistogard administration
- Prepare about 100 mL (approximately 4 fluid ounces) of food starch-based thickening product in water and stir briskly until dissolved.
- Crush contents of one full 10-g packet of Vistogard granules to a fine powder and add to the reconstituted food starch-based thickening product.
- For pediatric patients receiving doses of <10 g, prepare the mixture in a ratio not exceeding uridine triacetate 1 g to 10 mL of reconstituted food starch-based thickening product and mix thoroughly.
- Flush NG or gastrostomy tube with water after administration.
Xuriden granules
- Weigh doses using a scale accurate to ≤0.1 g or measure using a graduated teaspoon accurate to the fraction of the dose to be administered.
- For patients requiring doses of uridine triacetate in multiples of 2 g (i.e., three-fourths teaspoonful), contents of the required number of full 2-g packets may be administered without weighing or measuring.
- Mix each dose with 3–4 ounces of applesauce, pudding, or yogurt.3 Do not chew granules.3 Follow each dose with ≥120 mL (≥4 ounces) of water.
- May mix granules with 5 mL of milk or infant formula for patients receiving up to three-fourths teaspoonful (2 g).3 Consult manufacturer’s instructions for preparing doses of the drug using a medicine cup and oral syringe. Administer doses mixed in milk or infant formula using an oral syringe placed between patient’s cheek and gum at back of mouth. May follow with a bottle of milk or infant formula, if desired.
Prescribing limits
- Children
- Fluorouracil or Capecitabine Overdosage or Toxicity
- Oral 10 g/dose.
- Hereditary Orotic Aciduria
- Oral 8 g daily.
- Fluorouracil or Capecitabine Overdosage or Toxicity
- Adults
- Fluorouracil or Capecitabine Overdosage or Toxicity
- Oral 10 g/dose.
- Hereditary Orotic Aciduria
- Oral 8 g daily.
- Fluorouracil or Capecitabine Overdosage or Toxicity
Children
- Uses: Fluorouracil or capecitabine overdosage or toxicity
- Oral administrations: 6.2 g/m² (not to exceed 10 g/dose) every 6 hours for 20 doses (see Table 1).
- Note: One packet of Vistogard granules contains 10 g of uridine triacetate. Dose by body surface area in this table was rounded to achieve the approximate dose. Each dose is administered every 6 hours for 20 doses. One entire 10-g packet may be used without weighing or measuring.
- Uses: Hereditary Orotic Aciduria
- Oral administration: Initially, 60 mg/kg once daily (see Table 2).
- Note: One packet of Xuriden granules contains 2 g (approximately three-fourths teaspoon) of uridine triacetate. Total daily dosages by weight category in this table were rounded to achieve the approximate dosage level. One entire 2-g packet may be used without weighing or measuring. Two entire 2-g packets may be used without weighing or measuring. Three entire 2-g packets may be used without weighing or measuring.
- May increase dosage to 120 mg/kg (not to exceed 8 g) once daily for insufficient response (see Table 3). Signs of insufficient response may include urinary concentrations of orotic acid that remain above normal or increase above the usual or expected range for the patient, worsening of laboratory parameters affected by hereditary orotic aciduria (e.g., RBC or WBC indices), or worsening of other manifestations.
Adults
- Uses: Fluorouracil or Capecitabine Overdosage or Toxicity
- Oral administration: 10 g every 6 hours for 20 doses.
- Uses: Hereditary Orotic Aciduria
- Oral administration: Initially, 60 mg/kg once daily (see Table 2).
- Note: May increase dosage to 120 mg/kg (not to exceed 8 g) once daily for insufficient response (see Table 3). Signs of insufficient response may include urinary concentrations of orotic acid that remain above normal or increase above the usual or expected range for the patient, worsening of laboratory parameters affected by hereditary orotic aciduria (e.g., RBC or WBC indices), or worsening of other manifestations.
Table 1. Uridine Triacetate (Vistogard) Pediatric Dosing Based on Body Surface Area.
| Vistogard 6.2 g/m² per dose | Vistogard 6.2 g/m² per dose | |
| Body Surface Area (m2) | Dose (g) | Dose (graduated teaspoonsful) |
| 0.34–0.44 | 2.1–2.7 | 1 |
| 0.45–0.55 | 2.8–3.4 | 1¼ |
| 0.56–0.66 | 3.5–4.1 | 1½ |
| 0.67–0.77 | 4.2–4.8 | 1¾ |
| 0.78–0.88 | 4.9–5.4 | 2 |
| 0.89–0.99 | 5.5–6.1 | 2¼ |
| 1–1.1 | 6.2–6.8 | 2½ |
| 1.11–1.21 | 6.9–7.5 | 2¾ |
| 1.22–1.32 | 7.6–8.1 | 3 |
| 1.33–1.43 | 8.2–8.8 | 3¼ |
| ≥1.44 | 10 | 1 full 10-g packet |
Table 2. Initial Uridine Triacetate (Xuriden) 60 mg/kg Daily Dosage Based on Body Weight.
| Xuriden 60 mg/kg Daily Dosage | Xuriden 60 mg/kg Daily Dosage | |
| Patient weight (in kg) | Dose (in grams using a scale) | Dose (in teaspoonsful) |
| ≤5 | 0.4 | 01/08/21 |
| 6–10 | 0.4–0.6 | ¼ |
| 11–15 | 0.7–0.9 | ½ |
| 16–20 | 1–1.2 | ½ |
| 21–25 | 1.3–1.5 | ½ |
| 26–30 | 1.6–1.8 | ¾ |
| 31–35 | 1.9–2.1 | ¾ |
| 36–40 | 2.2–2.4 | 1 |
| 41–45 | 2.5–2.7 | 1 |
| 46–50 | 2.8–3 | 1 |
| 51–55 | 3.1–3.3 | 1¼ |
| 56–60 | 3.4–3.6 | 1¼ |
| 61–65 | 3.7–3.9 | 1½ |
| 66–70 | 4–4.2 | 1½ |
| 71–75 | 4.3–4.5 | 1½ |
| >75 | 6 | 2 |
Table 3. Increased Uridine Triacetate (Xuriden) 120 mg/kg Daily Dosage for Insufficient Response Based on Body Weight.
| Xuriden 120 mg/kg Daily Dosage | Xuriden 120 mg/kg Daily Dosage | |
| Patient weight (in kg) | Dose (in grams using a scale) | Dose (in teaspoonsful) |
| ≤5 | 0.8 | ¼ |
| 6–10 | 0.8–1.2 | ½ |
| 11–15 | 1.4–1.8 | ¾ |
| 16–20 | 2–2.4 | 1 |
| 21–25 | 2.6–3 | 1 |
| 26–30 | 3.2–3.6 | 1¼ |
| 31–35 | 3.8–4.2 | 1½ |
| 36–40 | 4.4–4.8 | 1¾ |
| 41–45 | 5–5.4 | 2 |
| 46–50 | 5.6–6 | 2 |
| 51–55 | 6.2–6.6 | 2¼ |
| 56–60 | 6.8–7.2 | 2½ |
| 61–65 | 7.4–7.8 | 2½ |
| 66–70 | 8 | 2¾ |
| 71–75 | 8 | 2¾ |
| >75 | 8 | 2¾ |
Uridine side effects
Uridine triacetate may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- vomiting
- nausea
- diarrhea
Uridine triacetate may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
- Zhang, Y., Guo, S., Xie, C., & Fang, J. (2020). Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. BioMed research international, 2020, 7091718. https://doi.org/10.1155/2020/7091718[↩][↩][↩][↩][↩][↩]
- Deng, Y., Wang, Z. V., Gordillo, R., An, Y., Zhang, C., Liang, Q., Yoshino, J., Cautivo, K. M., De Brabander, J., Elmquist, J. K., Horton, J. D., Hill, J. A., Klein, S., & Scherer, P. E. (2017). An adipo-biliary-uridine axis that regulates energy homeostasis. Science (New York, N.Y.), 355(6330), eaaf5375. https://doi.org/10.1126/science.aaf5375[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Connolly GP, Duley JA. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci 1999;20(5):218–25.[↩]
- Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem 2004;279(32):33035–8.[↩]
- Loffler M, Jockel J, Schuster G, Becker C. Dihydroorotat-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol Cell Biochem 1997;174(1–2):125–9.[↩]
- Loffler M, Fairbanks LD, Zameitat E, Marinaki AM, Simmonds HA. Pyrimidine pathways in health and disease. Trends Mol Med 2005;11(9):430–7.[↩]
- Yamamoto T, Koyama H, Kurajoh M, Shoji T, Tsutsumi Z, Moriwaki Y. Biochemistry of uridine in plasma. Clin Chim Acta. 2011 Sep 18;412(19-20):1712-24. doi: 10.1016/j.cca.2011.06.006[↩][↩]
- Pizzorno G, et al. Homeostatic control of uridine and the role of uridine phosphorylase: A biological and clinical update. Biochim. Biophys. Acta. 2002;1587:133–144. doi: 10.1016/S0925-4439(02)00076-5[↩][↩]
- Liu MP, Beigelman L, Levy E, Handschumacher RE, Pizzorno G. Discrete roles of hepatocytes and nonparenchymal cells in uridine catabolism as a component of its homeostasis. Am. J. Physiol. 1998;274:G1018–G1023.[↩]
- Connolly GP, Simmonds HA, Duley JA. Pyrimidines and CNS regulation. Trends Pharmacol. Sci. 1996;17:106–107. doi: 10.1016/0165-6147(96)20001-X[↩]
- Urasaki Y, Pizzorno G, Le TT. Chronic uridine administration induces fatty liver and pre-diabetic conditions in mice. PLOS ONE. 2016;11:e0146994. doi: 10.1371/journal.pone.0146994[↩]
- Yamamoto T., Koyama H., Kurajoh M., Shoji T., Tsutsumi Z., Moriwaki Y. Biochemistry of uridine in plasma. Clinica Chimica Acta. 2011;412(19-20):1712–1724. doi: 10.1016/j.cca.2011.06.006[↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Peters GJ, et al. Uridine-induced hypothermia in mice and rats in relation to plasma and tissue levels of uridine and its metabolites. Cancer Chemother. Pharmacol. 1987;20:101–108. doi: 10.1007/BF00253962[↩]
- Peters GJ, et al. Effect of pyrimidine nucleosides on body temperatures of man and rabbit in relation to pharmacokinetic data. Pharm. Res. 1987;4:113–119. doi: 10.1023/A:1016410817898[↩]
- Steculorum SM, et al. Hypothalamic UDP increases in obesity and promotes feeding via P2Y6-dependent activation of AgRP neurons. Cell. 2015;162:1404–1417. doi: 10.1016/j.cell.2015.08.032[↩]
- Cansev M, Watkins CJ, van der Beek EM, Wurtman RJ. Oral uridine-5′-monophosphate (UMP) increases brain CDP-choline levels in gerbils. Brain Res. 2005 Oct 5;1058(1-2):101-8. doi: 10.1016/j.brainres.2005.07.054[↩]
- Cansev M. Uridine and cytidine in the brain: their transport and utilization. Brain Res Rev. 2006 Sep;52(2):389-97. doi: 10.1016/j.brainresrev.2006.05.001[↩]
- Ross BM, Moszczynska A, Blusztajn JK, Sherwin A, Lozano A, Kish SJ. Phospholipid biosynthetic enzymes in human brain. Lipids. 1997 Apr;32(4):351-8. doi: 10.1007/s11745-997-0044-x[↩]
- KENNEDY EP, WEISS SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193-214.[↩]
- Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24(2):69-176. doi: 10.1016/0163-7827(85)90011-6[↩]
- Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated PC12 [corrected]. Neuroscience. 2005;134(1):207-14. doi: 10.1016/j.neuroscience.2005.03.050. Erratum in: Neuroscience. 2005;135(2):657.[↩]
- Wang L, Albrecht MA, Wurtman RJ. Dietary supplementation with uridine-5′-monophosphate (UMP), a membrane phosphatide precursor, increases acetylcholine level and release in striatum of aged rat. Brain Res. 2007 Feb 16;1133(1):42-8. doi: 10.1016/j.brainres.2006.11.048[↩]
- Wurtman RJ, Ulus IH, Cansev M, Watkins CJ, Wang L, Marzloff G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006 May 9;1088(1):83-92. doi: 10.1016/j.brainres.2006.03.019[↩][↩]
- Ott T, Grecksch G, Matthies H. Retention improvement by topical application of uridine monophosphate into different brain areas. Med Biol. 1978 Jun;56(3):133-7.[↩]
- Teather LA, Wurtman RJ. Chronic administration of UMP ameliorates the impairment of hippocampal-dependent memory in impoverished rats. J Nutr. 2006 Nov;136(11):2834-7. doi: 10.1093/jn/136.11.2834[↩]
- Sakamoto, T., Cansev, M., & Wurtman, R. J. (2007). Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain research, 1182, 50–59. https://doi.org/10.1016/j.brainres.2007.08.089[↩]
- Rautio J, Karkkainen J, Sloan KB. Prodrugs – Recent approvals and a glimpse of the pipeline. European Journal of Pharmaceutical Sciences. November, 2017; 109:146-161. https://www.ncbi.nlm.nih.gov/pubmed/28782609[↩]
- Wortmann SB, Chen MA, Colombo R, et al. Mild orotic aciduria in UMPS heterozygotes: a metabolic finding without clinical consequences. J Inherit Metab Dis. May, 2017; 40(3):423-431. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5393157[↩][↩][↩]
- Nyhan WL. Disorders of purine and pyrimidine metabolism. Mol Genet Metab. 2005 Sep-Oct;86(1-2):25-33. doi: 10.1016/j.ymgme.2005.07.027[↩]
- Uridine Triacetate. https://medlineplus.gov/druginfo/meds/a616020.html[↩]
- Vincenzetti S, Polzonetti V, Micozzi D, Pucciarelli S. Enzymology of Pyrimidine Metabolism and Neurodegeneration. Curr Med Chem. 2016;23(14):1408-31. doi: 10.2174/0929867323666160411125803[↩][↩][↩][↩]
- Le T. T., Ziemba A., Urasaki Y., Hayes E., Brotman S., Pizzorno G. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. Journal of Lipid Research. 2013;54(4):1044–1057. doi: 10.1194/jlr.M034249[↩][↩][↩][↩][↩][↩]
- Greenhill C. Metabolism: Liver and adipose tissue control uridine biosynthesis. Nat Rev Endocrinol. 2017 May;13(5):249. doi: 10.1038/nrendo.2017.38[↩]
- Schoors S., Bruning U., Missiaen R., et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520(7546):192–197. doi: 10.1038/nature14362[↩][↩]
- Roach P. J., Depaoli-Roach A. A., Hurley T. D., Tagliabracci V. S. Glycogen and its metabolism: some new developments and old themes. Biochemical Journal. 2012;441(3):763–787. doi: 10.1042/BJ20111416[↩]
- Malami I., Abdul A. B. Involvement of the uridine cytidine kinase 2 enzyme in cancer cell death: a molecular crosstalk between the enzyme and cellular apoptosis induction. Biomedicine & Pharmacotherapy. 2019;109:1506–1510. doi: 10.1016/j.biopha.2018.10.200[↩]
- Huang M., Graves L. M. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cellular and Molecular Life Sciences. 2003;60(2):321–336. doi: 10.1007/s000180300027[↩]
- Urasaki Y., Pizzorno G., Le T. T. Uridine affects liver protein glycosylation, insulin signaling, and heme biosynthesis. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099728[↩]
- Deliang C., Giuseppe P. Uridine phosophorylase: an important enzyme in pyrimidine metabolism and fluoropyrimidine activation. Drugs Today. 2004;40(5):431–443.[↩]
- Cao D., Ziemba A., McCabe J., et al. Differential expression of uridine phosphorylase in tumors contributes to an improved fluoropyrimidine therapeutic activity. Molecular Cancer Therapeutics. 2011;10(12):2330–2339. doi: 10.1158/1535-7163.MCT-11-0202[↩]
- Urasaki Y., Pizzorno G., Le T. T. Chronic uridine administration induces fatty liver and pre-diabetic conditions in mice. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146994[↩][↩][↩][↩][↩][↩][↩]
- Le T. T., Urasaki Y., Pizzorno G. Uridine prevents fenofibrate-induced fatty liver. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087179[↩][↩]
- Cao D., Leffert J. J., McCabe J., Kim B., Pizzorno G. Abnormalities in uridine homeostatic regulation and pyrimidine nucleotide metabolism as a consequence of the deletion of the uridine phosphorylase gene. The Journal of Biological Chemistry. 2005;280(22):21169–21175. doi: 10.1074/jbc.M412343200[↩]
- Greenhill C. Metabolism: liver and adipose tissue control uridine biosynthesis. Nature Reviews Endocrinology. 2017;13(5):p. 249. doi: 10.1038/nrendo.2017.38[↩][↩][↩]
- Yang Q., Vijayakumar A., Kahn B. B. Metabolites as regulators of insulin sensitivity and metabolism. Nature Reviews Molecular Cell Biology. 2018;19(10):654–672. doi: 10.1038/s41580-018-0044-8[↩][↩]
- Henricks L. M., Jacobs B. A. W., Meulendijks D., et al. Food-effect study on uracil and dihydrouracil plasma levels as marker for dihydropyrimidine dehydrogenase activity in human volunteers. British Journal of Clinical Pharmacology. 2018;84(12):2761–2769. doi: 10.1111/bcp.13719[↩][↩]
- Yamamoto T., Inokuchi T., Ka T., et al. Relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides, Nucleotides & Nucleic Acids. 2010;29(4-6):504–508. doi: 10.1080/15257771003740986[↩][↩]
- Yamamoto T., Moriwaki Y., Ka T., et al. Effect of purine-free low-malt liquor (happo-shu) on the plasma concentrations and urinary excretion of purine bases and uridine–comparison between purine-free and regular happo-shu. Hormone and Metabolic Research. 2004;36(4):231–237. doi: 10.1055/s-2004-814453[↩][↩]
- Ohno M., Ka T., Inokuchi T., et al. Effects of exercise and grape juice ingestion in combination on plasma concentrations of purine bases and uridine. Clinica Chimica Acta. 2008;388(1-2):167–172. doi: 10.1016/j.cca.2007.10.032[↩][↩][↩][↩][↩]
- Kobayashi T., Inokuchi T., Yamamoto A., et al. Effects of sucrose on plasma concentrations and urinary excretion of purine bases. Metabolism. 2007;56(4):439–443. doi: 10.1016/j.metabol.2006.09.022[↩]
- Kaya M., Moriwaki Y., Ka T., et al. Plasma concentrations and urinary excretion of purine bases (uric acid, hypoxanthine, and xanthine) and oxypurinol after rigorous exercise. Metabolism. 2006;55(1):103–107. doi: 10.1016/j.metabol.2005.07.013[↩]
- Yamamoto T., Moriwaki Y., Takahashi S. Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid) Clinica Chimica Acta. 2005;356(1-2):35–57. doi: 10.1016/j.cccn.2005.01.024[↩][↩]
- Zhang Y., Knapp S. Glycosylation of nucleosides. The Journal of Organic Chemistry. 2016;81(6):2228–2242. doi: 10.1021/acs.joc.5b02852[↩]
- Xie C., Wang Q., Li G., Fan Z., Wang H., Wu X. Dietary supplement with nucleotides in the form of uridine monophosphate or uridine stimulate intestinal development and promote nucleotide transport in weaned piglets. Journal of the Science of Food and Agriculture. 2019;99(13):6108–6113. doi: 10.1002/jsfa.9850[↩]
- Wu X., Lu-min G., Yi-lin L., et al. Maternal dietary uridine supplementation reduces diarrhea incidence in piglets by regulating the intestinal mucosal barrier and cytokine profiles. Journal of the Science of Food and Agriculture. 2020 doi: 10.1002/jsfa.10410[↩]
- Liu Y. L., Guo S. G., Xie C. Y., Niu K., De Jonge H., Wu X. Uridine inhibits the stemness of intestinal stem cells in 3D intestinal organoids and mice. RSC Advances. 2020;10(11):6377–6387. doi: 10.1039/C9RA07742A[↩]
- McEvilly M., Popelas C., Tremmel B. Use of uridine triacetate for the management of fluorouracil overdose. American Journal of Health-System Pharmacy. 2011;68(19):1806–1809. doi: 10.2146/ajhp100434[↩]
- Mironova G. D., Khrenov M. O., Talanov E. Y., et al. The role of mitochondrial KATP channel in anti-inflammatory effects of uridine in endotoxemic mice. Archives of Biochemistry and Biophysics. 2018;654:70–76. doi: 10.1016/j.abb.2018.07.006[↩][↩]
- Lebrecht D, Vargas-Infante YA, Setzer B, Kirschner J, Walker UA. Uridine supplementation antagonizes zalcitabine-induced microvesicular steatohepatitis in mice. Hepatology 2007;45(1):72–9.[↩]
- Le TT, Urasaki Y, Pizzorno G. Uridine prevents fenofibrate-induced fatty liver. PLoS ONE 2014;9(1):e87179[↩]
- McComsey GA, Walker UA, Budhathoki CB, Su Z, Currier JS, Kosmiski L, et al. Uridine supplementation in the treatment of HIV lipoatrophy: results of ACTG 5229. Aids 2010;24(16):2507–15.[↩]
- Wurtman RJ. A nutrient combination that can affect synapse formation. Nutrients 2014;6(4):1701–10.[↩]
- Gallai V, Mazzotta G, Montesi S, Sarchielli P, Del Gatto F. Effects of uridine in the treatment of diabetic neuropathy: an electrophysiological study. Acta Neurol Scand 1992;86(1):3–7.[↩]
- Ison G., Beaver J. A., McGuinn WD Jr, et al. FDA approval: uridine triacetate for the treatment of patients following fluorouracil or capecitabine overdose or exhibiting early-onset severe toxicities following administration of these rugs. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2016;22(18):4545–4549. doi: 10.1158/1078-0432.CCR-16-0638[↩]
- Löffler M., Carrey E. A., Zameitat E. Orotic acid, more than just an intermediate of pyrimidine de novo synthesis. Journal of Genetics and Genomics. 2015;42(5):207–219. doi: 10.1016/j.jgg.2015.04.001[↩]
- Zhao Q, Marolewski A, Rusche JR, Holmes GL. Effects of uridine in models of epileptogenesis and seizures. Epilepsy Res. 2006 Jul;70(1):73-82. doi: 10.1016/j.eplepsyres.2006.03.003[↩]
- Koch J, Mayr JA, Alhaddad B, Rauscher C, Bierau J, Kovacs-Nagy R, Coene KL, Bader I, Holzhacker M, Prokisch H, Venselaar H, Wevers RA, Distelmaier F, Polster T, Leiz S, Betzler C, Strom TM, Sperl W, Meitinger T, Wortmann SB, Haack TB. CAD mutations and uridine-responsive epileptic encephalopathy. Brain. 2017 Feb;140(2):279-286. doi: 10.1093/brain/aww300[↩]
- Kovács Z, Kékesi KA, Dobolyi Á, Lakatos R, Juhász G. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience. 2015 Aug 6;300:593-608. doi: 10.1016/j.neuroscience.2015.05.054[↩][↩]
- Brunner, B., Rauch, E., Ari, C., D’Agostino, D. P., & Kovács, Z. (2021). Enhancement of Ketone Supplements-Evoked Effect on Absence Epileptic Activity by Co-Administration of Uridine in Wistar Albino Glaxo Rijswijk Rats. Nutrients, 13(1), 234. https://doi.org/10.3390/nu13010234[↩]
- Koch J., Mayr J.A., Alhaddad B., Rauscher C., Bierau J., Kovacs-Nagy R., Coene K.L., Bader I., Holzhacker M., Prokisch H., et al. CAD mutations and uridine-responsive epileptic encephalopathy. Brain. 2017;140:279–286. doi: 10.1093/brain/aww300[↩]
- Kimura T, Ho IK, Yamamoto I. Uridine receptor: discovery and its involvement in sleep mechanism. Sleep. 2001 May 1;24(3):251-60. doi: 10.1093/sleep/24.3.251[↩]
- Liu P, Che X, Yu L, Yang X, An N, Song W, Wu C, Yang J. Uridine attenuates morphine-induced conditioned place preference and regulates glutamate/GABA levels in mPFC of mice. Pharmacol Biochem Behav. 2017 Dec;163:74-82. doi: 10.1016/j.pbb.2017.10.004[↩]
- Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005 Jun;1(2):111-34. doi: 10.1007/s11302-005-0649-1[↩]
- Sperlágh B, Szabó G, Erdélyi F, Baranyi M, Vizi ES. Homo- and heteroexchange of adenine nucleotides and nucleosides in rat hippocampal slices by the nucleoside transport system. Br J Pharmacol. 2003 Jun;139(3):623-33. doi: 10.1038/sj.bjp.0705285[↩]
- Lakatos RK, Dobolyi Á, Todorov MI, Kékesi KA, Juhász G, Aleksza M, Kovács Z. Guanosine may increase absence epileptic activity by means of A2A adenosine receptors in Wistar Albino Glaxo Rijswijk rats. Brain Res Bull. 2016 Jun;124:172-81. doi: 10.1016/j.brainresbull.2016.05.001[↩]
- Kovács Z, D’Agostino DP, Diamond DM, Ari C. Exogenous Ketone Supplementation Decreased the Lipopolysaccharide-Induced Increase in Absence Epileptic Activity in Wistar Albino Glaxo Rijswijk Rats. Front Mol Neurosci. 2019 Feb 28;12:45. doi: 10.3389/fnmol.2019.00045[↩]
- Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr. Physiol. 2013;3:1689–1719. doi: 10.1002/cphy.c130011[↩]
- Landsberg L, Young JB. Caloric intake and sympathetic nervous system activity. Implications for blood pressure regulation and thermogenesis. J. Clin. Hypertens. 1986;2:166–171.[↩]
- Le T. T., Urasaki Y., Pizzorno G. Uridine prevents tamoxifen-induced liver lipid droplet accumulation. Bmc Pharmacology & Toxicology. 2014;15(1) doi: 10.1186/2050-6511-15-27[↩]
- Martin G. G., Atshaves B. P., Landrock K. K., Landrock D., Schroeder F., Kier A. B. Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Archives of Biochemistry and Biophysics. 2015;580:41–49. doi: 10.1016/j.abb.2015.06.009[↩]
- Liu Y., Zhang Y., Yin J., Ruan Z., Wu X., Yin Y. Uridine dynamic administration affects circadian variations in lipid metabolisms in the liver of high-fat-diet-fed mice. Chronobiology International. 2019;36(9):1258–1267. doi: 10.1080/07420528.2019.1637347[↩]
- Zhang K., Liu Y. L., Zhang Y., et al. Dynamic oral administration of uridine affects the diurnal rhythm of bile acid and cholesterol metabolism-related genes in mice. Biological Rhythm Research. 2019;50(4):543–552. doi: 10.1080/09291016.2018.1474844[↩]
- Yamamoto T., Moriwaki Y., Takahashi S., et al. Effect of amino acids on the plasma concentration and urinary excretion of uric acid and uridine. Metabolism. 1999;48(8):1023–1027. doi: 10.1016/s0026-0495(99)90200-7[↩]
- So A., Thorens B. Uric acid transport and disease. The Journal of Clinical Investigation. 2010;120(6):1791–1799. doi: 10.1172/JCI42344[↩]
- Zhang H., Fu P., Ke B., et al. Metabolomic analysis of biochemical changes in the plasma and urine of collagen-induced arthritis in rats after treatment with Huang-Lian-Jie-Du-Tang. Journal of Ethnopharmacology. 2014;154(1):55–64. doi: 10.1016/j.jep.2014.03.007[↩][↩]
- Walker A. K. 1-Carbon cycle metabolites methylate their way to fatty liver. Trends in Endocrinology and Metabolism. 2017;28(1):63–72. doi: 10.1016/j.tem.2016.10.004[↩]
- Wellstat Therapeutics Corporation. Vistogard (uridine triacetate) oral granules prescribing information. Gaithersburg, MD; 2015 Dec.[↩]
- Wellstat Therapeutics Corporation. Xuriden (uridine triacetate) oral granules prescribing information. Gaithersburg, MD; 2015 Sep.[↩]
- McEvilly M, Popelas C, Tremmel B. Use of uridine triacetate for the management of fluorouracil overdose. Am J Health Syst Pharm. 2011 Oct 1;68(19):1806-9. doi: 10.2146/ajhp100434[↩]