Vasospasm
Vasospasm refers to a condition in which blood vessels spasm, leading to vasoconstriction. Vasospasm can lead to tissue ischemia and death (necrosis). Cerebral vasospasm may arise in the context of subarachnoid hemorrhage. Symptomatic vasospasm or delayed cerebral ischemia is a major contributor to post-operative stroke and death after aneurysmal subarachnoid hemorrhage. Vasospasm typically appears 4 to 10 days after subarachnoid hemorrhage.
Coronary artery vasospasm
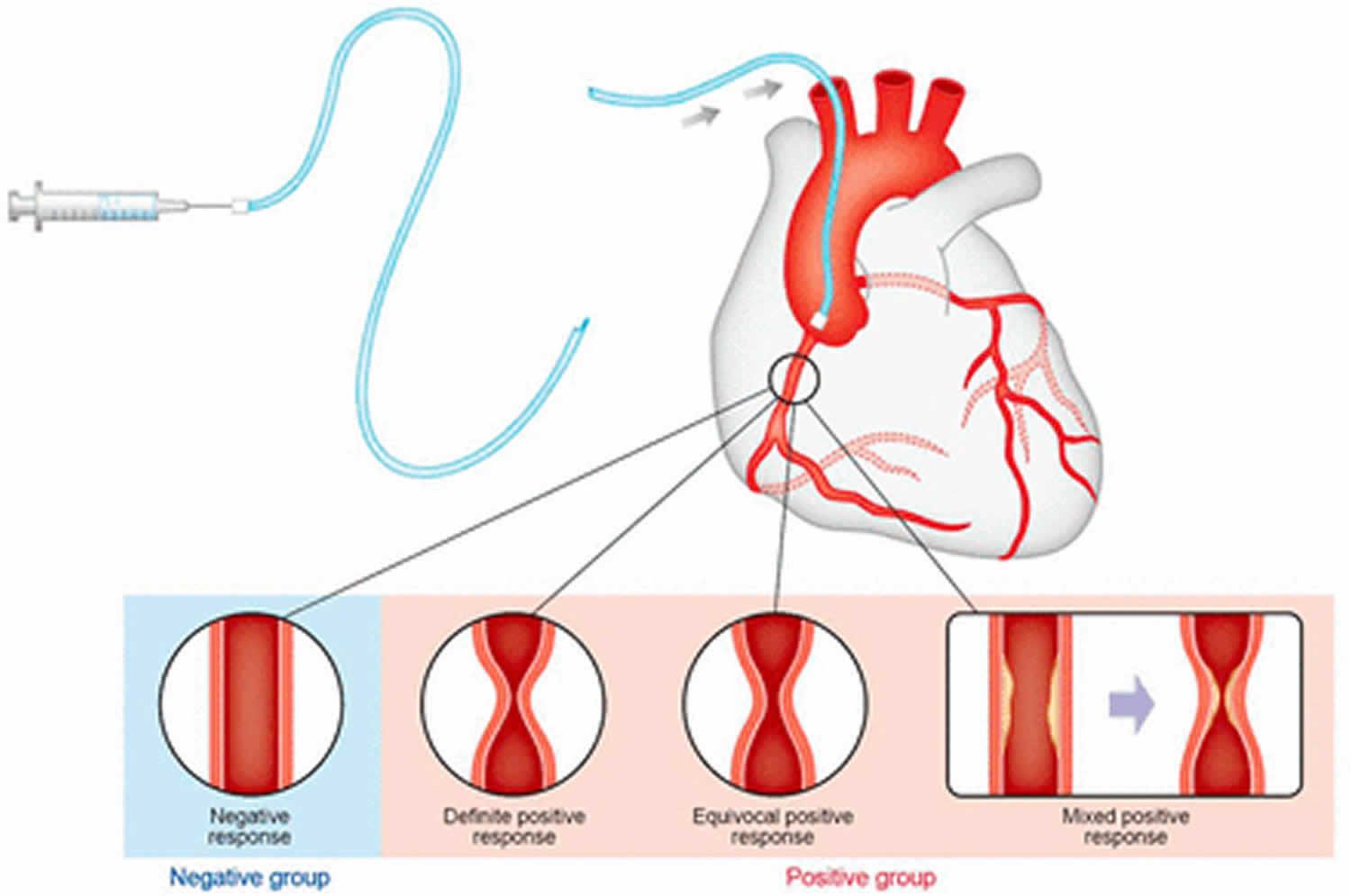
Coronary vasospasm or constriction of the coronary arteries that can cause complete or near-complete occlusion of the vessel, is a multi-factorial, transient, and abrupt reduction of luminal diameter of an epicardial coronary artery due to inappropriate constriction of coronary smooth muscle that can generate distal ischemia. This may occur spontaneously or in the context of angioplasty, particularly if denudation of the endothelium or dissection occurs. In addition, the vasospasm can either be focal or multifocal, which compromises more than one vessel. Coronary vasospasm appears to be a heterogeneous disease but does not follow the traditional risk factors in the development of coronary artery disease 1.
Coronary artery vasospasm is an important cause of chest pain syndromes that can lead to myocardial infarction (heart attack), ventricular arrhythmias, and sudden death. Coronary artery vasospasm also plays a key role in the development of atherosclerotic lesions 2.
The age at which symptoms first appear is highly variable, but on average, patients are in their 50s at symptom onset 3. The distribution is varied throughout the world, with the highest incidence noted in Japanese population when compared to the western population. Furthermore, the frequency of multiple spasms noted on provocative testing is also higher in the Japanese population (23%) than those in Caucasians (7.5%). A German study found that every fourth patient with suspected obstructive coronary artery disease (coronary heart disease) had no culprit lesion, of these tested with acetylcholine, 50% were confirmed to be due to coronary artery vasospasm.
Variant angina is believed to be more common in female patients, [19, 20] although some prognostic studies of patients with variant angina suggest a male preponderance. A 2012 study of Korean patients 4 showed that men were more likely to develop coronary artery vasospasm in response to an intracoronary acetylcholine challenge. Among women, variant angina may be relatively more common in white patients (22%) than in Japanese patients (11%).
Overall, Japanese patients are much more likely to develop coronary artery vasospasm than Caucasian patients 5. When evaluated by the same team, Japanese patients had a 3-fold higher incidence of spasm than their Caucasian counterparts even though the 2 groups of patients had similar average basal coronary tone 5.
The pathophysiologic mechanisms leading to coronary artery vasospasm are not yet completely understood. In some patients with partial vasoconstriction, symptoms can arise with activities that exceed a threshold of myocardial demand 6. In other patients, constriction may be so severe that myocardial ischemia develops at rest. Many observers use the presence of constriction-induced ischemia as the threshold for defining clinical coronary artery vasospasm 7, which has also been called vasospastic angina or variant angina.
In many cases, coronary artery vasospasm can occur spontaneously without an identifiable cause. Known triggers of spasm in susceptible patients include hyperventilation, cocaine or tobacco use, and administration of provocative agents such as acetylcholine, ergonovine, histamine, or serotonin 8.
That coronary artery vasospasm can be induced through stimulation of alpha receptors 9 or intracoronary injection of the parasympathetic neurotransmitter acetylcholine 10 indicates that there are different mechanisms of action.
Acetylcholine causes coronary vasodilation in healthy coronary arteries through the release of endothelial nitric oxide (NO); however, in atherosclerotic arteries, vasoconstriction ensues instead. Patients with coronary artery vasospasm appear to have a heightened vasoconstrictor response to acetylcholine as well as an enhanced response to the vasodilator effects of nitrates, an observation that is consistent with a deficiency of endogenous nitric oxide activity 7.
Thus, nitric oxide deficiency is believed to play an important role in the development of coronary artery vasospasm. This may also explain the correlation between coronary artery vasospasm and increased intimal thickening: nitric oxide deficiency results in enhanced activity of potent vasoconstrictors and stimulators of vascular smooth muscle proliferation, such as angiotensin II and endothelin 1 7.
Several genetic polymorphisms that compromise endothelial nitric oxide production have been found to be significantly associated with coronary artery vasospasm 11. Some have even been found to have prognostic value, including the -786T/C polymorphism 12. However, additional studies showing that NO levels are not decreased at the sites of coronary artery vasospasm dispute the primacy of the role of nitric oxide 13.
Alternative or coexisting mechanisms of coronary artery vasospasm include enhanced phospholipase C activity 14. In addition, coronary artery vasospasm is associated with increased markers of oxidative stress and inflammation, including thioredoxin, C-reactive protein (CRP), and monocyte levels 7. Certain behavioral traits (eg, type A personality, panic disorder, and severe anxiety) are also described as being associated with coronary artery vasospasm 15.
Coronary artery vasospasm classification
Coronary artery vasospasm can be classified according to the location of vasoconstriction:
- Focal coronary spasm: Focal coronary spasm is limited to a localized segment of the coronary artery.
- Multifocal coronary spasm: Multifocal coronary spasm involves several localized segments of the same coronary artery.
- Multivessel coronary spasm: Multivessel coronary spasm involves several coronary arteries 16.
Coronary artery vasospasm can also be classified into either spontaneous or iatrogenic:
- Spontaneous coronary vasospasm: A subtype of epicardial coronary artery spasm is known as Prinzmetal’s angina. Prinzmetal’s angina is characterized by the sudden onset of chest pain at rest with ST elevation on ECG.
- Iatrogenic coronary vasospasm: Coronary vasospasm can be secondary to percutaneous coronary intervention.
Coronary artery vasospasm causes
The development of coronary artery vasospasm is multifactorial and can be influenced by the autonomic nervous system, inflammation, oxidative stress, endothelial dysfunction, smooth muscle cell hypercontractility, genetic predisposition, and lifestyle choices. Prinzmetal et al. published their study in 1959, which was conducted on 25 dogs where they noticed the changes that took place by occluding and releasing a large epicardial artery 17. They found clinical symptoms of pain and angina, electrocardiographic changes consistent with ischemia in the corresponding region, and systolic ballooning of the ischemic region. They postulated this as the course of a vasospastic epicardial coronary artery 18.
Coronary artery vasospasm causes in alphabetical order:
- Acute pericarditis
- Angina pectoris
- Anxiety disorders
- Cocaine toxicity
- Coronary dissection can mimic and can cause spasm
- Drugs- Naratriptan, Sumatriptan
- Esophageal motility disorders
- Esophageal spasm
- Gastroesophageal reflux disease
- Isolated coronary artery anomalies
- Myocardial infarction
- Myocardial ischemia
- Panic disorder
- Coronary thrombosis can mimic and can cause spasm
- Unstable angina
Young patients with fewer cardiovascular risk factors (with the exception of smoking) are at a higher risk for coronary vasospasm, as are noncalcified lesions and eccentric plaques.
Rotoblator cases are more prone to coronary vasospasm. The reported incidence of rotoblater cases with coronary vasospasm ranges anywhere from 4 to 36%.
Risk factors for coronary artery vasospasm
- Autoimmune diseases
- Cocaine use
- Cold exposure
- Hyperventilation
- Insulin resistance
- Japanese descent
- Smoking
Coronary artery vasospasm pathophysiology
The exact pathogenesis of coronary vasospasm is not well understood, but some causes and contributing factors are known. Coronary arterial tone varies normally via physiologic mechanisms, but the degree of vasoconstriction can range along a spectrum extending from undetectable constriction to complete arterial occlusion.
Coronary spasm can be explained by a hyperreactivity to vasoconstrictor stimuli that results from endothelial dysfunction or primary hyperreactivity of smooth muscle cells. Vasoconstrictor stimuli include changes in the autonomic nervous system, inflammation, and calcium availability in the myocardium 19.
Dysfunction of the autonomic nervous system and endothelial dysfunction can lead to chronic intermittent vasospasm, which usually occurs where a fixed, non-calcified stenosis is located.
A significant group of patients with variant angina have underlying obstructive coronary artery disease 20.
Occasionally, coronary vasospasm can be induced by angioplasty (PCI-induced), which occurs secondary to endothelial denudation and nitric oxide loss. Some cases are catheter-induced which is caused by a contact of a catheter without balloon deployment. Catheter-induced coronary vasospasm is usually short-lived. Catheter-induced coronary vasospasm is most prone to occur at the ostium of the right coronary artery. The left main is less susceptible to ostial spasm.
Coronary artery vasospasm symptoms
Patients with coronary artery vasospasm typically describe anginal symptoms, including retrosternal pain or pressure with radiation to the neck, jaw, left shoulder, or arm. This may be particularly true if there is significant coexistent atherosclerosis 21. Notably, symptoms associated with vasospastic angina often occur at rest and may exhibit a circadian pattern, with most episodes occurring in the early hours of the morning 7. In severe cases, associated arrhythmias may be present, ranging from heart block to ventricular tachycardia 22.
Distinguishing unstable angina pectoris related to coronary atherosclerosis from variant angina may be difficult and require special investigations for diagnosis, including coronary angiography. In some patients, the distinction may be an arbitrary one because it is likely that vasospasm is both a cause and a consequence of plaque rupture and thrombosis in patients with unstable angina pectoris.
In addition, many patients with variant angina have obstructive coronary artery disease. Indeed, in as many as 60% of cases, coronary artery vasospasm occurs at a site with preexisting coronary atherosclerosis 23, which suggests that underlying arterial dysfunction may be a predisposing factor for spasm.
Although spasm is more likely to occur in the presence of atherosclerotic lesions, the absence of traditional risk factors for atherosclerotic coronary artery disease may make vasospastic angina more likely; the exception is cigarette smoking, which is a common risk factor for both clinical syndromes 24. Spasm is found more often in patients with symptoms that occur at rest (55.5%) than in those with exertional angina (27.7%) 25.
A minority of patients with variant angina may have a more systemic abnormality of vasomotor tone; this may include symptoms of migraine headache and Raynaud phenomenon 26.
No features on physical examination are specific for vasospastic angina. Signs may be absent between symptomatic episodes. During periods of angina, physical findings relating to ischemia and ventricular dysfunction may be present, including rales, jugular venous distention, peripheral edema, extra heart sounds, ectopy or other arrhythmia (eg, tachycardia or bradycardia), and murmurs such as occur with ischemic mitral regurgitation.
Coronary artery vasospasm complications
Myocardial infarction is a potential complication of variant angina, especially in the myocardial territory corresponding to the location of the electrocardiographic (ECG) changes during previous anginal attacks. The incidence of heart attack depends on diagnostic criteria, but has been reported to be as high as 30% in some series.
The incidence and prognosis of heart attack in patients with variant angina appear to be associated with the extent and severity of any underlying atherosclerotic coronary stenoses. Adverse outcomes are more likely and survival poorer in patients with multivessel atherosclerotic coronary artery disease 27.
Arrhythmias may occur with severe vasospastic angina. Both atrioventricular conduction abnormalities and ventricular arrhythmias can cause life-threatening hemodynamic deterioration and syncope. Coronary vasospasm has been identified as an important cause of out-of-hospital cardiac arrest 28. The risk of sudden death is approximately 2% and is most common in patients with multivessel spasm 29 and prior serious arrhythmia during anginal attacks. In extreme cases, defibrillator implantation may be considered 30.
Coronary artery vasospasm diagnosis
Laboratory studies
Evaluation of the standard hematology, serum chemistry, and lipid profiles is appropriate for excluding anemia, infection, primary platelet disorders, renal failure, hyperglycemia, electrolyte abnormalities, and dyslipidemia. Serial measurement of cardiac enzyme and troponin levels should be performed to assess for evidence of ischemia.
Magnesium levels may be checked; magnesium deficiency can heighten sensitivity to acetylcholine- and hyperventilation-induced spasm. Magnesium supplementation may be a potentially useful therapy 8.
Perfusion imaging and echocardiography
Myocardial perfusion imaging may be helpful in ruling out obstructive atherosclerotic disease between episodes of coronary artery vasospasm. During episodes, myocardial perfusion imaging may help identify and quantify ischemia and localize it to the culprit artery.
Standard transthoracic echocardiography should be considered to evaluate for stigmata of other causes of nonexertional chest pain (eg, pericarditis or abnormalities of the aorta). Preliminary data suggest a potential role for hyperventilation and cold-pressor stress echocardiography as a noninvasive means for detecting coronary artery vasospasm, though this method may not be as sensitive as using intracoronary acetylcholine as the provocative agent 31.
Electrocardiography and monitoring
Transient ST-segment elevation on electrocardiography (ECG) is a characteristic finding in patients with variant angina 8, but it may only be present during symptomatic episodes and typically resolves completely within minutes. However, in more severe cases, ST elevation may be followed by T-wave inversions for hours to days 32. During recovery, U-wave inversions may be present, particularly in lead V5 33.
Many episodes of coronary artery vasospasm are brief and may be asymptomatic; however, ST-segment changes may be detected by ambulatory electrocardiography, which may allow for more accurate characterization of the frequency and duration of attacks.
Severe vasospastic angina may result in heart block or potentially fatal ventricular arrhythmias. Inpatient telemetry monitoring serves an important role in acutely ill patients. In outpatients, Holter monitoring may facilitate detection of nonsustained arrhythmias.
Angiography
Coronary angiography may reveal focal spasm of a coronary artery; when coupled with typical symptoms, ECG changes, and even ventricular dysfunction, this spasm may be pathognomonic for the condition.
Most patients with variant angina and documented coronary artery vasospasm have some angiographic evidence of atherosclerotic coronary artery disease (CAD), which is typically mild. Focal spasm more commonly occurs within 1 cm of an angiographically apparent obstruction. If minimal or no angiographic evidence of coronary artery disease is found in a patient who has recently had angina at rest with transient ST-segment elevation, variant angina is the more likely diagnosis, and further testing is unnecessary.
The angiographic demonstration of a myocardial bridge may have prognostic implications as well. Not only are myocardial bridges associated with a higher likelihood of more severe coronary artery vasospasm in response to an intracoronary acetylcholine challenge, but a 2012 study suggested that these patients may have greater related morbidity and mortality 34.
Coronary artery vasospasm treatment
Patients with vasospastic angina presenting with active symptoms of ischemia often require admission. Initial evaluation should include 12-lead electrocardiography (ECG), continuous telemetry monitoring, and serial cardiac enzyme and troponin measurements. Further evaluation should include assessment for coexisting or contributory atherosclerotic coronary artery disease (CAD). This may involve stress testing with myocardial perfusion imaging or even coronary angiography.
Because atherosclerosis is common in patients with vasospastic angina, medical and lifestyle interventions for preventing or treating atherosclerosis should be implemented when appropriate.
The main goals of treating coronary vasospasm are to:
- Reverse the spontaneous abrupt luminal diameter reduction
- Reverse percutaneous transluminal coronary angioplasty induced vasospasm
- Stabilize chronic intermittent vasospasm.
Calcium channel blockers and nitrates are the mainstay of chronic therapy for coronary vasospasm.
Atropine has also been used to treat coronary vasospasm 35.
Pharmacologic therapy
Initial medical treatment should include sublingual, topical, or intravenous (IV) nitrate therapy. Nitroglycerin administered by any route (intracoronary, IV, topical, or sublingual) effectively treats episodes of angina and myocardial ischemia within minutes, and long-acting nitrate preparations reduce the frequency of recurrent events.
Until atherosclerotic coronary disease (a much more frequent cause of chest pain) is excluded, standard therapies, including antiplatelet or antithrombotic agents, statins, and beta blockers, may be administered. Statin therapy appears to improve clinical outcomes in patients with coronary spasm–induced acute myocardial infarction with nonobstructive coronary arteries 36.
Once the diagnosis of coronary artery vasospasm is made, calcium channel blockade and long-acting nitrates may be used for long-term prophylaxis.
The calcium channel blockers nifedipine, amlodipine, verapamil, and diltiazem effectively prevent coronary vasospasm and variant angina, and they should be administered in preference to beta blockers. Amlodipine may be preferable because of its long half-life 37.
Beta-blockers are beneficial in most patients with atherosclerotic coronary stenoses and exertional angina pectoris and are sometimes helpful in combination with the above drugs to achieve control of symptoms in these patients. However, nonselective beta blockers may be detrimental in some patients because blockade of the beta receptors, which mediate vasodilation, allows unopposed alpha receptor–mediated coronary vasoconstriction to occur and may worsen vasospastic angina in selected cases.
Other agents have been tried with variable success, including endothelin antagonists such as bosentan 38. Early experience with cilostazol has been positive but limited 39; additional research is needed to validate its clinical use.
In a study of 3349 patients diagnosed with coronary artery spasm, Choi et al 40 divided patients into 2 groups according to whether their prescriptions included renin-angiotensin system inhibitor or not, and they investigated the effect of renin-angiotensin system inhibitors on long-term clinical outcomes. Following propensity score matching analysis, two matched groups (524 pairs, n=1048 patients) were generated and their baseline characteristics were balanced. Compared with the non-renin-angiotensin system inhibitor group, the renin-angiotensin system inhibitor group had a lower incidence of recurrent angina, total death, and total major adverse cardiovascular events during the 5-year clinical follow-up.
Spontaneous remission may occur, and some patients may be able to wean or reduce their drug therapy after an initial 3-month symptom-free period.
Contraindicated medications
Coronary artery vasospasm is considered an absolute contraindication to the use of the following medications:
- Sumatriptan
Percutaneous and surgical revascularization
Up to one fifth of patients may continue to have vasospasm despite medical therapy. Mechanical revascularization has been used successfully in patients with medically resistant vasospasm. Scattered reports of coronary stenting suggest that a percutaneous strategy may be feasible in such patients 41. The results for surgical revascularization have been variable, but overall, bypass surgery appears to provide clinical benefit to less than 50% of patients 27. The efficacy of surgical treatment is greater in patients who also have significant obstructive atherosclerotic lesions. In patients without baseline obstruction, however, the risk of early graft closure is elevated.
For patients who continue to have significant symptoms or signs of coronary vasospasm despite maximally tolerated medical therapy, in whom the culprit segment can be identified, coronary stenting may be considered on a case-by-case basis. However, bypass grafting of arteries without baseline obstruction should be reserved for patients with life-threatening ischemia that is refractory to maximal medical therapy. In these patients, adding complete plexectomy to the procedure may provide additional benefit 42.
Coronary artery vasospasm prognosis
The natural history of patients undergoing medical therapy for coronary vasospasm may involve significant morbidity, but mortality is low in most cases, even on long-term follow-up 3. Patients often have 3- to 6-month clusters of recurrent attacks, separated by relatively asymptomatic periods, with a gradual reduction of symptoms in the long term 7. In a study of 59 patients followed for an average of 5.9 years, 93% experienced rest angina and 19% sustained frank heart attacks 43. However, there were no cardiac deaths.
Long-term survival is believed to be good, especially in patients who tolerate calcium antagonists and avoid smoking 3. Predictors of poorer prognosis include the presence of concurrent coronary atherosclerosis 27, ongoing smoking, intolerance of calcium antagonists, and spasm of multiple coronary arteries 44.
In patients with no or even single-vessel atherosclerosis, the prognosis is benign, with survival rates as high as 99% at 1 year and 94% at 5 years. On the other hand, survival in patients with multivessel atherosclerotic disease fell to 87% at 1 year and 77% at 5 years. Survival rates were also lower in patients with multivessel spasm 29.
A 3-year follow-up to the Coronary Artery Spasm as a Frequent Cause for Acute Coronary Syndrome (CASPAR) study concluded that patients with acute coronary syndrome (ACS) who do not have a culprit lesion have a better prognosis than patients with obstructive acute coronary syndrome 45. Persistent angina is challenging, and repeated coronary angioplasty may be required.
The Japanese Coronary Spasm Association (JCSA) derived the “JCSA risk score” to guide prognostication for patients with coronary vasospasm. Elements of the score include the following:
- History of out-of-hospital cardiac arrest (4 points)
- Smoking, angina at rest alone, organic coronary stenosis, multivessel spasm (2 points each)
- Beta blocker use, ST elevation during angina (1 point each)
Stratification of patients by score led to differentiation in their risk of major adverse cardiac events. Patients with a low score of 0-2 had a major adverse cardiac events of 2.5%. Those with an intermediate score of 3-5 had a major adverse cardiac events of 7%, and those whose scores were 6 or higher had a major adverse cardiac events of 13% 46.
Cerebral vasospasm
Cerebral vasospasm after aneurysmal subarachnoid hemorrhage is a well-described phenomenon that is defined as narrowing of the large and medium-sized intracranial arteries 47; most often, cerebral vasospasm affects the anterior circulation supplied by the internal carotid arteries. Cerebral vasospasm leading to delayed cerebral ischemia continues to be a major complication and source of morbidity in cases of aneurysmal subarachnoid hemorrhage 48.
As a consequence of the significant neurologic morbidity or mortality stemming from posthemorrhagic vasospasm, a great deal of interest and research has been focused on understanding its physiologic mechanism and developing effective preventive and therapeutic measures. However, the cause of cerebral vasospasm remains poorly understood, and no single therapeutic algorithm has shown to be uniformly effective in preventing vasospasm and its subsequent neurologic consequences 49.
Cerebral vasospasm causes
There are many postulated theories as to the cause of cerebral vasospasm after aneurysmal subarachnoid hemorrhage, but the mechanisms ultimately responsible for vasospasm are still incompletely understood. There is evidence that the normal physiologic mechanisms and signaling molecules that regulate cerebral arterial diameter are altered 50. The alteration of these regulatory processes results in excess cerebral arterial vasoconstriction. Molecules of interest in this regard include nitric oxide and endothelin.
Nitric oxide, a vasodilator produced by endothelial cells, helps maintain cerebral arterial dilation. There is some evidence that the breakdown products of red blood cells may be harmful to endothelial cells, and this harmful effect may compromise the ability of these cells to produce vasodilatory molecules such as nitric oxide 47. Endothelin, a vasoconstrictor 51, has been found to be elevated in cerebrospinal fluid (CSF) after aneurysmal subarachnoid hemorrhage and may play a role in cerebral vasospasm and delayed cerebral ischemia 52.
Research efforts are ongoing with the aim of further elucidating the mechanisms of vasospasm in the hope of developing better treatments.
Cerebral vasospasm prevention
Prevention of vasospasm after severe aneurysmal subarachnoid hemorrhage is challenging, in that vasospasm represents a natural progression of this disease process. Use of nimodipine continues to be recommended as a means to improve neurologic outcomes after aneurysmal subarachnoid hemorrhage, though it has not been shown to exert this effect by decreasing the incidence of vasospasm 53.
Historically, strategies for preventing vasospasm emphasized triple H therapy (hypervolemia, hemodilution, and hypertension). Systematic reviews of the literature have demonstrated that studies examining triple H therapy are of mostly poor design and quality. There is relatively little compelling evidence to supporting the use of triple H therapy as an effective means of preventing vasospasm 54.
Currently, the American Heart Association and the American Stroke Association state that euvolemia should be the goal after aneurysmal subarachnoid hemorrhage and that induction of hypertension may be pursued once symptomatic vasospasm has been diagnosed 53.
Cerebral vasospasm symptoms
Cerebral vasospasm occurs in the setting of recent aneurysmal subarachnoid hemorrhage. Vasospasm most often presents within 3-7 days after aneurysmal subarachnoid hemorrhage but can occur at any time within the 21-day window following the initial hemorrhage. Vasospasm often causes delayed cerebral ischemia, which can present clinically in a number ways, depending on the severity of the vasospasm and what intracranial vessels are most affected.
The most common presentation is neurologic deterioration manifested as decreased level of consciousness or onset of new focal neurologic deficits. Patients may complain of weakness, sensory changes, new or increasing headache, visual deficit, or other neurologic symptoms. In obtunded patients (eg, those with high-grade subarachnoid hemorrhage), cerebral vasospasm may be clinically silent. Diagnosis of cerebral vasospasm in these patients requires vigilance and regular radiologic surveillance.
Physical examination may reveal a constellation of signs and symptoms of ongoing vasospasm, depending on the severity of the condition and on which intracranial vessels are most affected.
Nonlocalizing symptoms include the following:
- Lethargy
- Disorientation
- Meningismus
- New or increasing headache
Focal neurologic deficits are related to the particular vessel involved, as follows:
- Anterior cerebral artery (ACA) distribution – Disinhibition, confusion; mutism; lethargy, delayed responsiveness, abulia; leg weakness; with involvement of the recurrent artery of Heubner (a large anterior cerebral artery perforator), contralateral faciobrachial weakness without cortical findings
- Middle cerebral artery (MCA) distribution – Hemiparesis, faciobrachial weakness, monoparesis; aphasia, apractagnosia; neglect
- Posterior cerebral artery (PCA) distribution – Visual disturbance, hemianopsia
Cerebral vasospasm complications
The complications of cerebral vasospasm may consist of further clinical deterioration and poor neurologic outcome. Vasospasm leads to cerebral ischemia, and in severe cases, acute ischemic stroke from cerebral infarction can occur. This progression leads to irreversible neurologic deficits.
Neurologic outcomes can vary widely, depending on the unique circumstances of each patient. Focal deficits are likely to persist if the large intracranial arteries (eg, the anterior cerebral artery, middle cerebral artery, and posterior cerebral artery) are severely affected by vasospasm. It has been estimated that approximately 7% of patients suffering severe vasospasm following aneurysmal subarachnoid hemorrhage will have permanent neurologic deficits, and another 7% will die 55.
Cerebral vasospasm diagnosis
Various imaging modalities are employed to diagnose cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Currently, transcranial Doppler is the primary imaging technique used in screening for asymptomatic spasm 56. Transcranial Doppler is a noninvasive modality that extrapolates the likelihood of vasospasm on the basis of selective intracranial arterial blood flow velocity ratios, trends, and relations. It is less sensitive in some patients with unfavorable bony anatomy “windows” that hinder soft-tissue evaluation, and it is subject to user error or variability, which inevitably affects its clinical applicability 57.
Other imaging techniques routinely used in surveillance for vasospasm are computed tomography (CT) perfusion scanning 58 and conventional angiography, both of which have demonstrated efficacy with varying usage patterns and in different clinical settings. CT perfusion studies have been shown to be more useful for diagnosing vasospasm in patients with delayed neurologic deficits than they are in asymptomatic patients 57.
Digital subtraction angiography has been regarded as the gold standard for diagnosis and treatment of vasospasm, but outcome benefit for the treatment of angiographic vasospasm has not been demonstrated, leading to the addition of more qualitative blood flow tools 57.
Magnetic resonance angiography (MRA) has been employed in this setting as well. In a study that compared three-dimensional (3D) spin-echo-based black-blood MRA (BBMRA) with time-of-flight (TOF)-MRA for detection of cerebral vasospasm in the early posttreament period after subarachnoid hemorrhage, Takano et al found that BBMRA, owing to its contrast properties, may be superior to TOF-MRA for the evaluation of intracranial arteries 59.
New neurologic deficits caused by vasospasm may be difficult to recognize in patients who are already severely debilitated. For this reason, routine surveillance imaging studies are carried out to supplement the neurologic examination with the aim of diagnosing vasospasm before the onset of delayed cerebral ischemia. Common practice is as follows:
- Routine transcranial doppler on a daily basis
- Diagnostic cerebral angiography 1 week after aneurysm treatment
- Adjunctive imaging as clinically indicated
Other tests
In addition to clinical and radiographic evaluation for the prevention of delayed cerebral ischemia, alternative methods are being investigated, including continuous electroencephalography (EEG) 60, serum biomarker assays 61 and bedside cerebral blood oxygenation monitoring 62.
Cerebral vasospasm treatment
Medical treatment
Although there is a paucity of class A evidence regarding prevention and treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage, a number of therapeutic strategies have been studied and found to have varying degress of utility 53.
Of the management options currently used, the hemodynamic augmentation strategy known as triple H therapy, which includes hypertension, hemodilution, and hypervolemia, has been an important component. This approach was intended to improve cerebral perfusion by increasing mean arterial pressure (MAP) and reducing blood viscosity 63.
One study found that approximately 70% of patients with vasospasm experienced improvement in their symptoms after initiation of triple H therapy 64. It must be kept in mind, however, that triple H therapy can have significant complications, such as pulmonary edema and subsequent hypoxemia, which may be detrimental to an at-risk hypoperfused brain. A 2000 study by Lennihan et al 65 found that hypervolemic therapy had no significant advantage over normovolemic therapy with respect to cerebral blood flow after subarachnoid hemorrhage and that the two therapies yielded similar incidences of symptomatic vasospasm.
Increases in mean arterial pressure (MAP) alone have been shown to be beneficial to patients with vasospasm, and a number of medications have been investigated and utilized to achieve this goal 66. Phenylephrine, norepinephrine, and dopamine are the primary pressors employed in this setting 67. Phenylephrine appears to have the most evidence to support its use; several trials have suggested that is effective in patients with preserved left ventricular function 68. Other medications, such as vasopressin, may play adjunctive roles to play in management 69.
Calcium-channel antagonists have been widely investigated for prevention of vasospasm in aneurysmal subarachnoid hemorrhage 70. In particular, nimodipine has been shown to improve neurologic outcomes after aneurysmal subarachnoid hemorrhage, but it does not decrease radiographic vasospasm and does not significantly reduce mortality 71. Several studies have shown that nicardipine reduces vasospasm as determined by transcranial Doppler, but no clear improvements in outcome or mortality have been demonstrated 72.
A myriad of other drugs, including magnesium oxide, antiplatelet agents, anti-inflammatory drugs, statins, and endothelin receptor antagonists, have been studied in attempts to exploit nuances of the many theories of vasospasm physiology, including inflammatory cascade, heme breakdown product–mediated oxidative injury, spreading depolarization, and potassium channel modulation, but no clinical outcome benefit has yet been shown 73. In contrast to nimodipine, the endothelin receptor antagonist clazosentan reduces angiographic spasm but has no effect on outcome, as noted in the CONSCIOUS trials 74.
Nimodipine is currently recommended as first-line medical treatment for preventing post-aneurysmal subarachnoid hemorrhage cerebral vasospasm. It is usually given orally at a dosage of 60 mg every 4 hours for 21 days after the initial subarachnoid hemorrhage 53. Maintenance of euvolemia and normal circulating blood volume is recommended to prevent vasospasm 53. In symptomatic vasospasm, induction of hypertension is recommended to achieve increased cerebral blood flow 53. Hypervolemia is no longer recommended as a measure to prevent vasospasm 53.
In a meta-analysis of five studies that included a total of 543 patients with aneurysmal subarachnoid hemorrhage, Saber et al 75 found cilostazol, a selective inhibitor of phosphodiesterase 3, to be associated with decreased risks of symptomatic vasospasm, cerebral infarction, and poor outcome. A large multicenter trial would be needed to confirm this association.
Surgical treatment
More invasive means of treatment of vasospasm depend on the utilization of cerebral angiography and include the following:
- Intra-arterial vasodilator administration
- Balloon angioplasty
Papaverine was one of the drugs initially tried for intra-arterial injection, but it fell out of favor because of (1) the lack of evidence for its efficacy and (2) the possibility of serious side effects, including paradoxical vasospasm. Several studies suggested that intra-arterial verapamil may provide a benefit in terms of neurologic outcome, but the benefit appears not to be obtained via vasodilation 73.
Angioplasty is an accepted modality for refractory vasospasm, though to date, there have not been any good-quality clinical trials demonstrating its efficacy. Prophylactic angioplasty has not been demonstrated to yield any significant improvements in outcome 76.
Current guidelines recommend intra-arterial vasodilator therapy and balloon angioplasty as reasonable treatment modalities in symptomatic patients who are not responding to hypertensive therapy 53.
Table 1. Recommendations for endovascular treatment of cerebral vasospasm
| Degree of Radiographic Vasospasm | Clinical Findings | |
|---|---|---|
| Asymptomatic | Symptomatic | |
| Mild | No intervention | Vasodilation (rare) |
| Moderate | Vasodilation | Vasodilation ± balloon angioplasty |
| Severe | Vasodilation ± balloon angioplasty (rare) | Vasodilation + balloon angioplasty |
American Heart Association and American Stroke Association Guidelines for Post-aneurysmal subarachnoid hemorrhage Cerebral Vasospasm
In 2012, the American Heart Association and the American Stroke Association published updated evidence-based guidelines on the comprehensive management of aneurysmal subarachnoid hemorrhage, including the management of cerebral vasospasm and delayed cerebral ischemia 53. These guidelines were endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, and the Society of NeuroInterventional Surgery.
The 2012 American Heart Association and the American Stroke Association recommendations for management of cerebral vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage are as follows 53:
- Oral nimodipine should be administered to all patients with aneurysmal subarachnoid hemorrhage (class I; level of evidence, A) – It should be noted that this agent has been shown to improve neurologic outcomes but not cerebral vasospasm; the value of other calcium antagonists, whether administered orally or intravenously, remains uncertain
- Maintenance of euvolemia and normal circulating blood volume is recommended to prevent delayed cerebral ischemia (class I; level of evidence, B) – Revised recommendation from previous guidelines
- Prophylactic hypervolemia or balloon angioplasty before the development of angiographic spasm is not recommended(class III; level of evidence, B) – New recommendation
- Transcranial Doppler is reasonable to monitor for the development of arterial vasospasm (class IIa; level of evidence, B) – New recommendation
- Perfusion imaging with computed tomography (CT) or magnetic resonance imaging (MRI) can be useful to identify regions of potential brain ischemia (class IIa; level of evidence, B) – New recommendation
- Induction of hypertension is recommended for patients with delayed cerebral ischemia unless blood pressure is elevated at baseline or cardiac status precludes it (class I; level of evidence, B) – Revised recommendation from previous guidelines
- Cerebral angioplasty and/or selective intra-arterial vasodilator therapy is reasonable in patients with symptomatic cerebral vasospasm, particularly those who are not rapidly responding to hypertensive therapy (class IIa; level of evidence, B) – Revised recommendation from previous guidelines.
- Beijk MA, Vlastra WV, Delewi R, van de Hoef TP, Boekholdt SM, Sjauw KD, Piek JJ. Myocardial infarction with non-obstructive coronary arteries: a focus on vasospastic angina. Neth Heart J. 2019 Jan 28[↩]
- Coronary Artery Vasospasm. https://emedicine.medscape.com/article/153943-overview[↩]
- Bory M, Pierron F, Panagides D, Bonnet JL, Yvorra S, Desfossez L. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur Heart J. 1996 Jul. 17(7):1015-21.[↩][↩][↩]
- Rha SW, Park JY, Ryu SK, et al. TCT-437 The impact of gender difference on angiographic characteristics during intracoronary acetylcholine provocation test in Korean patients. J Am Coll Cardiol. 2012 Oct 23. 60(17 Supp B):B124.[↩]
- Pristipino C, Beltrame JF, Finocchiaro ML, et al. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation. 2000 Mar 14. 101(10):1102-8.[↩][↩]
- Yasue H, Omote S, Takizawa A, Nagao M, Miwa K, Tanaka S. Circadian variation of exercise capacity in patients with Prinzmetal’s variant angina: role of exercise-induced coronary arterial spasm. Circulation. 1979 May. 59(5):938-48.[↩]
- Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm–clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008 Feb. 51(1):2-17.[↩][↩][↩][↩][↩][↩]
- Ajani AE, Yan BP. The mystery of coronary artery spasm. Heart Lung Circ. 2007 Feb. 16(1):10-5.[↩][↩][↩]
- Yasue H, Touyama M, Kato H, Tanaka S, Akiyama F. Prinzmetal’s variant form of angina as a manifestation of alpha-adrenergic receptor-mediated coronary artery spasm: documentation by coronary arteriography. Am Heart J. 1976 Feb. 91(2):148-55.[↩]
- Yasue H, Horio Y, Nakamura N, et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986 Nov. 74(5):955-63.[↩]
- Kaneda H, Taguchi J, Kuwada Y, et al. Coronary artery spasm and the polymorphisms of the endothelial nitric oxide synthase gene. Circ J. 2006 Apr. 70(4):409-13.[↩]
- Nishijima T, Nakayama M, Yoshimura M, et al. The endothelial nitric oxide synthase gene -786T/C polymorphism is a predictive factor for reattacks of coronary spasm. Pharmacogenet Genomics. 2007 Aug. 17(8):581-7.[↩]
- Egashira K, Katsuda Y, Mohri M, et al. Basal release of endothelium-derived nitric oxide at site of spasm in patients with variant angina. J Am Coll Cardiol. 1996 May. 27(6):1444-9.[↩]
- Nakano T, Osanai T, Tomita H, Sekimata M, Homma Y, Okumura K. Enhanced activity of variant phospholipase C-delta1 protein (R257H) detected in patients with coronary artery spasm. Circulation. 2002 Apr 30. 105(17):2024-9.[↩]
- Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009 May 12. 119(18):2531-4.[↩]
- Multivessel coronary vasospasm mimicking triple-vessel obstructive coronary artery disease. J Invasive Cardiol. 2007 Jul;19(7):E178-81. https://www.invasivecardiology.com/article/7471[↩]
- Swarup S, Grossman SA. Coronary Artery Vasospasm. [Updated 2019 Jan 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470181[↩]
- Lim Y, Singh D, Loh PH, Poh KK. Multivessel coronary artery spasm in pericarditis. Singapore Med J. 2018 Nov;59(11):611-613.[↩]
- Association between inflammatory markers, hemostatic markers, and traditional risk factors on coronary artery spasm in patients with normal coronary angiography. J Interv Cardiol. 2014 Feb;27(1):29-35. doi: 10.1111/joic.12086. Epub 2013 Dec 18. https://doi.org/10.1111/joic.12086[↩]
- “Variant” angina: one aspect of a continuous spectrum of vasospastic myocardial ischemia. Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol. 1978 Dec;42(6):1019-35. https://doi.org/10.1016/0002-9149(78)90691-4[↩]
- Figueras J, Cortadellas J, Gil CP, Domingo E, Soler JS. Comparison of clinical and angiographic features and longterm follow-up events between patients with variant angina and patients with ST elevation myocardial infarction. Int J Cardiol. 2006 Aug 10. 111(2):256-62.[↩]
- Previtali M, Klersy C, Salerno JA, et al. Ventricular tachyarrhythmias in Prinzmetal’s variant angina: clinical significance and relation to the degree and time course of S-T segment elevation. Am J Cardiol. 1983 Jul. 52(1):19-25.[↩]
- Bertrand ME, LaBlanche JM, et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982 Jun. 65(7):1299-306.[↩]
- Harding MB, Leithe ME, Mark DB, et al. Ergonovine maleate testing during cardiac catheterization: a 10-year perspective in 3,447 patients without significant coronary artery disease or Prinzmetal’s variant angina. J Am Coll Cardiol. 1992 Jul. 20(1):107-11.[↩]
- Sueda S, Kohno H, Fukuda H, et al. Frequency of provoked coronary spasms in patients undergoing coronary arteriography using a spasm provocation test via intracoronary administration of ergonovine. Angiology. 2004 Jul-Aug. 55(4):403-11.[↩]
- Rosamond W. Are migraine and coronary heart disease associated? An epidemiologic review. Headache. 2004 May. 44 Suppl 1:S5-12.[↩]
- Mishra PK. Variations in presentation and various options in management of variant angina. Eur J Cardiothorac Surg. 2006 May. 29(5):748-59.[↩][↩][↩]
- Kobayashi N, Hata N, Shimura T, et al. Characteristics of patients with cardiac arrest caused by coronary vasospasm. Circ J. 2013. 77(3):673-8.[↩]
- Onaka H, Hirota Y, Shimada S, et al. Prognostic significance of the pattern of multivessel spasm in patients with variant angina. Jpn Circ J. 1999 Jul. 63(7):509-13.[↩][↩]
- Hendriks ML, Allaart CP, Bronzwaer JG, Res JJ, de Cock CC. Recurrent ventricular fibrillation caused by coronary artery spasm leading to implantable cardioverter defibrillator implantation. Europace. 2008 Dec. 10(12):1456-7.[↩]
- Hirano Y, Uehara H, Nakamura H, et al. Diagnosis of vasospastic angina: comparison of hyperventilation and cold-pressor stress echocardiography, hyperventilation and cold-pressor stress coronary angiography, and coronary angiography with intracoronary injection of acetylcholine. Int J Cardiol. 2007 Apr 4. 116(3):331-7.[↩]
- Miwa K, Kambara H, Kawai C, Murakami T. Two electrocardiographic patterns with or without transient T-wave inversion during recovery periods of variant anginal attacks. Jpn Circ J. 1983 Dec. 47(12):1415-22.[↩]
- Miwa K, Murakami T, Kambara H, Kawai C. U wave inversion during attacks of variant angina. Br Heart J. 1983 Oct. 50(4):378-82.[↩]
- Im SI, Rha SW, Choi BG, et al. TCT-436 Association of myocardial bridge and acetylcholine dose response in patients with vasospastic angina. J Am Coll Cardiol. 2012 Oct. 60(17 Supp B):B124.[↩]
- Turkoglu S, Arpag U, Timurkaynak T. Spontaneous coronary vasospasm in the catheterisation laboratory: prompt resolution after atropine injection. Heart. 2007;93(2):215. doi:10.1136/hrt.2006.093187 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1861368[↩]
- Piao ZH, Jeong MH, Li Y, et al, for the Other Korea Acute Myocardial Infarction Registry (KAMIR) Investigators. Benefit of statin therapy in patients with coronary spasm-induced acute myocardial infarction. J Cardiol. 2016 Jul. 68(1):7-12.[↩]
- Taylor SH. Usefulness of amlodipine for angina pectoris. Am J Cardiol. 1994 Jan 27. 73(3):28A-33A.[↩]
- Krishnan U, Win W, Fisher M. First report of the successful use of bosentan in refractory vasospastic angina. Cardiology. 2010. 116(1):26-8.[↩]
- Yoo SY, Song SG, Lee JH, et al. Efficacy of cilostazol on uncontrolled coronary vasospastic angina: a pilot study. Cardiovasc Ther. 2013 Jun. 31(3):179-85.[↩]
- Choi BG, Jeon SY, Rha SW, et al. Impact of renin-angiotensin system inhibitors on long-term clinical outcomes of patients with coronary artery spasm. J Am Heart Assoc. 2016 Jul 21. 5(7).[↩]
- Khitri A, Jayasuriya S, Habibzadeh MR, Movahed MR. Coronary stenting in patients with medically resistant vasospasm. Rev Cardiovasc Med. 2010 Fall. 11(4):264-70.[↩]
- Bertrand ME, Lablanche JM, Rousseau MF, Warembourg HH Jr, Stankowtak C, Soots G. Surgical treatment of variant angina: use of plexectomy with aortocoronary bypass. Circulation. 1980 May. 61(5):877-82.[↩]
- Bott-Silverman C, Heupler FA Jr. Natural history of pure coronary artery spasm in patients treated medically. J Am Coll Cardiol. 1983 Aug. 2(2):200-5.[↩]
- Yasue H, Takizawa A, Nagao M, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988 Jul. 78(1):1-9.[↩]
- Ong P, Athanasiadis A, Borgulya G, Voehringer M, Sechtem U. 3-year follow-up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow-up. J Am Coll Cardiol. 2011 Jan 11. 57(2):147-52.[↩]
- Takagi Y, Takahashi J, Yasuda S, et al. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013 Sep 24. 62(13):1144-53.[↩]
- Sobey CG, Faraci FM. Subarachnoid haemorrhage: what happens to the cerebral arteries?. Clin Exp Pharmacol Physiol. 1998 Nov. 25 (11):867-76.[↩][↩]
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010 Oct. 41 (10):2391-5.[↩]
- Cerebral Vasospasm After Subarachnoid Hemorrhage. https://emedicine.medscape.com/article/2500045-overview[↩]
- Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012 Dec. 43 (12):3230-7.[↩]
- Zimmermann M, Seifert V. Endothelin and subarachnoid hemorrhage: an overview. Neurosurgery. 1998 Oct. 43 (4):863-75; discussion 875-6.[↩]
- Cheng YW, Li WJ, Dou XJ, Jia R, Yang H, Liu XG, et al. Role of endothelin‑1 and its receptors in cerebral vasospasm following subarachnoid hemorrhage. Mol Med Rep. 2018 Sep 27.[↩]
- [Guideline] Connolly ES Jr et al, American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012 Jun. 43 (6):1711-37.[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Rinkel GJ, Feigin VL, Algra A, van Gijn J. Circulatory volume expansion therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2004 Oct 18. CD000483.[↩]
- Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985 Jul-Aug. 16 (4):562-72.[↩]
- Westermaier T, Pham M, Stetter C, Willner N, Solymosi L, Ernestus RI, et al. Value of transcranial Doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care. 2014 Jun. 20 (3):406-12.[↩]
- Rawal S, Barnett C, John-Baptiste A, Thein HH, Krings T, Rinkel GJ. Effectiveness of diagnostic strategies in suspected delayed cerebral ischemia: a decision analysis. Stroke. 2015 Jan. 46 (1):77-83.[↩][↩][↩]
- Vulcu S, Wagner F, Fernandes SA, Reitmeir R, Söll N, Schöni D, et al. Repetitive CT perfusion for detection of cerebral vasospasm-related hypoperfusion in aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018 Oct 8.[↩]
- Takano K, Hida K, Iwaasa M, Inoue T, Yoshimitsu K. Three-dimensional spin-echo-based black-blood MRA in the detection of vasospasm following subarachnoid hemorrhage. J Magn Reson Imaging. 2018 Oct 4.[↩]
- Herman ST, Abend NS, Bleck TP, Chapman KE, et al, Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015 Apr. 32 (2):87-95.[↩]
- Przybycien-Szymanska MM, Ashley WW Jr. Biomarker Discovery in Cerebral Vasospasm after Aneurysmal Subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis. 2015 Jul. 24 (7):1453-64.[↩]
- Yokose N, Sakatani K, Murata Y, Awano T, Igarashi T, Nakamura S, et al. Bedside monitoring of cerebral blood oxygenation and hemodynamics after aneurysmal subarachnoid hemorrhage by quantitative time-resolved near-infrared spectroscopy. World Neurosurg. 2010 May. 73 (5):508-13.[↩]
- Origitano TC, Wascher TM, Reichman OH, Anderson DE. Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution (“triple-H” therapy) after subarachnoid hemorrhage. Neurosurgery. 1990 Nov. 27 (5):729-39; discussion 739-40.[↩]
- Egge A, Waterloo K, Sjøholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001 Sep. 49 (3):593-605; discussion 605-6.[↩]
- Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage : a randomized controlled trial. Stroke. 2000 Feb. 31 (2):383-91.[↩]
- Reynolds MR, Buckley RT, Indrakanti SS, Turkmani AH, Oh G, Crobeddu E, et al. The safety of vasopressor-induced hypertension in subarachnoid hemorrhage patients with coexisting unruptured, unprotected intracranial aneurysms. J Neurosurg. 2015 Oct. 123 (4):862-71.[↩]
- Meyer R, Deem S, Yanez ND, Souter M, Lam A, Treggiari MM. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2011 Feb. 14 (1):24-36.[↩]
- Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE, Lee K, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009 Mar. 64 (3):397-410; discussion 410-1.[↩]
- Muehlschlegel S, Dunser MW, Gabrielli A, Wenzel V, Layon AJ. Arginine vasopressin as a supplementary vasopressor in refractory hypertensive, hypervolemic, hemodilutional therapy in subarachnoid hemorrhage. Neurocrit Care. 2007. 6 (1):3-10.[↩]
- Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007 Jul 18. CD000277.[↩]
- Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, et al. Cerebral arterial spasm–a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983 Mar 17. 308 (11):619-24.[↩]
- Durrant JC, Hinson HE. Rescue therapy for refractory vasospasm after subarachnoid hemorrhage. Curr Neurol Neurosci Rep. 2015. 15 (2):521.[↩]
- Young AM, Karri SK, Helmy A, Budohoski KP, Kirollos RW, Bulters DO, et al. Pharmacologic Management of Subarachnoid Hemorrhage. World Neurosurg. 2015 Jul. 84 (1):28-35.[↩][↩]
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011 Jul. 10 (7):618-25.[↩]
- Saber H, Desai A, Palla M, Mohamed W, Seraji-Bozorgzad N, Ibrahim M. Efficacy of Cilostazol in Prevention of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. J Stroke Cerebrovasc Dis. 2018 Nov. 27 (11):2979-2985.[↩]
- Zwienenberg-Lee M, Hartman J, Rudisill N, Madden LK, Smith K, Eskridge J, et al. Effect of prophylactic transluminal balloon angioplasty on cerebral vasospasm and outcome in patients with Fisher grade III subarachnoid hemorrhage: results of a phase II multicenter, randomized, clinical trial. Stroke. 2008 Jun. 39 (6):1759-65.[↩]